Abstract
Background
Immune checkpoint inhibitors (ICIs) are approved in relapsed classic Hodgkin lymphoma (cHL). The safety and effectiveness of allogeneic blood or marrow transplantation (alloBMT) in ICI pretreated cHL patients remain unclear. The aim of this study is to assess outcomes of cHL patients receiving ICIs before alloBMT using post-transplantation cyclophosphamide (PTCy) graft-versus-host-disease (GVHD) prophylaxis.
Methods
We performed a retrospective study of relapsed/refractory cHL patients undergoing alloBMT with PTCy at Johns Hopkins between Nov 2004 and Sept 2019. Engraftment, GVHD incidence, nonrelapse mortality (NRM), progression free survival (PFS) and overall survival (OS) were compared between patients receiving pre-alloBMT ICI or standard salvage chemotherapy.
Findings
We identified 105 consecutive relapsed/refractory cHL patients, of which 37 (35.2%) received ICIs and 68 (64.7%) received chemotherapy without ICIs (no-ICI) before alloBMT. ICI and no-ICI patients experienced a 3-year estimated OS of 94% versus 78%, [hazard ratio (HR) 0.35 (95% CI: 0.081.56), P=0.17) and a 3-year estimated PFS of 90% and 65% [HR 0.3 (95 % CI: 0.09–1), P=0.05], respectively. We observed no statically significant difference in the 12-month cumulative incidence of acute grade II-IV GVHD or in the 24-month incidence of chronic GVHD.
Interpretation
ICIs do not increase acute or chronic GVHD incidence compared to salvage chemotherapy. cHL patients receiving ICIs prior to alloBMT experienced outstanding PFS and OS. Thus ICI therapy is safe in cHL patients when undergoing alloBMT with PTCy and may improve post-alloBMT disease progression and survival.
Keywords: Immune Checkpoint Inhibitors (ICIs), Classic Hodgkin Lymphoma (cHL), Allogeneic Blood or Marrow Transplantation (alloBMT), Post-Transplantation Cyclophosphamide (PTCy), Graft-versus-host-disease (GVHD), engraftment
Introduction
Immune checkpoint inhibitors (ICIs) are associated with high response rates and progression free survivals (PFSs) in patients with relapsed or refractory classic Hodgkin lymphoma (cHL).1,2 Relapsed and refractory cHL patients responding to salvage systemic treatment including ICIs or chemotherapy are potential candidates for consolidative allogeneic blood or bone marrow transplantation (alloBMT). The utilization of non-myeloablative (NMA) conditioning alloBMT with post-transplant cyclophosphamide (PTCy) graft-versus-host-disease (GVHD) prophylaxis in cHL result in favorable outcomes with low GVHD incidence.3–5 Pre-alloBMT ICI exposure was reported to increase the incidence of immune toxicities including GVHD, veno-occlusive disease of liver and death.6–8 However, two independent retrospective studies have demonstrated that primary GVHD prophylaxis with PTCy abrogates these ICI enhanced toxicities.9,10 Previous cHL alloBMT with PTCy reports only included chemotherapy pretreated patients.3–5 Recently, however, nivolumab11 and pembrolizumab12 have been approved by the Food and Drug Administration (FDA) for relapsed cHL treatment. Hence, an analysis of the long-term influence of ICI before alloBMT in cHL is warranted. Here we report alloBMT outcomes of 105 consecutive relapsed/refractory cHL patients receiving ICI or chemotherapy without ICI before NMA alloBMT with PTCy at the Sidney Kimmel Comprehensive Cancer Center (SKCCC) at Johns Hopkins.
Methods
Patients
After Johns Hopkins Medicine Institutional Review Board approval, we queried the SKCCC transplantation database for cHL patients who received NMA alloBMT with PTCy GVHD prophylaxis between November 2004 and September 2019. Medical records including clinical notes and pathology, radiology, and laboratory reports were reviewed; data was locked in December 2019. The alloBMT treatment decision was at the discretion of the treating physician and patient, but alloBMT was generally preferred for patients with primary refractory disease or first remission lasting less than 12 months, or patients failing prior autologous transplant.
AlloBMT with PTCy
All patients received NMA conditioning consisting of fludarabine, cyclophosphamide, and 200cGy total body irradiation (TBI) as previously described.13 Patients received PTCy14 (intravenous 50 mg/kg/day) on days +3 and +4 along with mycophenolate mofetil between days +5 and +35, and tacrolimus or sirolimus between days +5 and +180 until 1/2018 (74 patients), and until day +60 after 1/2018 (31 patients), for GVHD prophylaxis.15 GVHD was treated per institutional guidelines as previously described.16,17 Our review included patients receiving alloBMT as part of research protocols and as standard of care.
Disease Status and Clinical Outcome Definitions
Overall survival (OS) was calculated as time since alloBMT until death, with censoring at the last follow-up date. PFS was calculated as time from alloBMT to disease relapse/progression or death, with censoring at the last follow-up date for relapse-free patients. Non-relapse mortality (NRM) was defined as death from causes not related to disease relapse. NRM was a competing event when estimating the cumulative incidence (CuI) of relapse and vice versa. For CuI of GVHD, graft failure was a competing event. Death without chronic (c) GVHD was an additional competing event when estimating the CuI of cGVHD. Neutrophil and platelet recovery time was the interval between allograft infusion and first of three consecutive days with more than 500 neutrophils/μl and first of three consecutive days with more than 20,000 platelets/μl, respectively. Patients were classified donor T-cell engrafted if ≥5% donor cells were detected after day +60. Engraftment failure was defined as <5% donor chimerism at any point beyond day +60. In engrafted patients, full engraftment was defined as ≥ 95% donor T-cells, while ≥5%−94% donor T-cells were designated mixed chimeras. Modified Keystone criteria and National Institutes of Health Consensus Criteria was used to diagnose and score acute (a)GVHD and cGVHD.18,19
Statistical Analysis
Patient characteristics and clinical variables were summarized via descriptive statistics. Logistic regression was utilized to assess factors associated with engraftment failure and mixed chimerism. OS, PFS and median follow-up are reported using the Kaplan-Meier and reverse Kaplan-Meier methods respectively. The cumulative incidence of aGVHD, cGVHD, relapse, and NRM outcomes were assessed with the proportional subdistribution hazard regression model for competing risks.20 Brentuximab vedotin (BV) was used as a negative exposure control21 drug to assess the potential for bias in the retrospective analysis. Early transplant patients, from 2004 to 2011, did not receive either BV or ICI. Patients received BV from 2011 onwards and ICI from 2015 onwards. Thus, pre-transplant BV exposure history was approximately similar to pre-transplant ICI exposure and BV served as a valid negative exposure control. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) and R version 3.4.4 (2018–03-15). All reported p-values were two sided. P-values less than 0.05 were considered as statistically significant.
Role of funding source
Funding sources had no role in design, analysis and reporting of the study.
Results
Patient and AlloBMT Characteristics
Between November 2004 and September 2019, 105 consecutive relapsed cHL patients underwent alloBMT at SKCCC (Table 1). The median alloBMT time period was earlier in no-ICI (2012) compared to ICI cohort (2018). The alloBMT characteristics are summarized in Table 2, and generally the ICI and non-ICI patients groups were similar. Thirty seven (35.2%) patients received ICIs and 68 (64.7%) patients received chemotherapy without ICIs before alloBMT. Among the ICI subgroup, 28 (76%) received ICIs as their last therapy before alloBMT. The median time from ICI therapy to alloBMT was 51 days (range 23–472 days). ICI patients received a median of three treatments (range: 2 – 9) including 26 (70.2%) patients who also received BV before alloBMT. Patients receiving chemotherapy without ICI (no-ICI) received a median of four treatments (range: 1 – 7), including 20 (29.4%) who received BV before alloBMT. Chemotherapy regimens included: ABVD (Doxorubicin, Bleomycin, Vinblastine, Dacarbazine); MOPP (Mechlorethamine, Vincristine, Procarbazine, Prednisone); ESHAP (Etoposide, Methylprednisolone, Cytarabine, Cisplatin); ICE (Ifosfamide, Carboplatin, Etoposide); GND (Gemcitabine, Navelbine, Doxorubicin); GDP (Gemcitabine, Dexamethasone, and Cisplatin) or single agent Gemcitabine. Primary refractory cHL was observed in 9 (24.3%) and 14 (20.5%) patients in the ICI and no-ICI subgroups, respectively. At the time of alloBMT after salvage ICIs, 14 (38%) and 21 (57%) patients were in a CR and PR, respectively, while 20 (29%) and 35 (51%) patients were in CR and PR in no-ICI sub-group. Twenty six (70%) patients in ICI subgroup and 63 (93%) patients in no-ICI subgroup received alloBMT from haploidentical donors. Other graft characteristics in ICI and no-ICI subgroups included 6 (20%) versus 12 (18%) patients receiving sex-mismatched allografts in the direction of femaledonor to a male-recipient, 6 (17%) versus 3 (4%) patients receiving major ABO mismatched products.
Table 1. Patient Characteristics.
NA=not applicable. ICIs=Immune Checkpoint Inhibitors, BV=Brentuximab vedotin. IQR=interquartile range
| All patients | ICIs | No-ICIs | P value | |
|---|---|---|---|---|
| Patients: no(%) | 105 (100%) | 37 (35.2%) | 68 (64.7%) | NA |
| Median age at alloBMT (IQR in years) | 36 (25–49) | 32 (24–46) | 36 (25–51) | 0.2 |
| AlloBMT time period: (median) range in years | (2015) 2004–19 | (2018) 2015–19 | (2012) 2004–19 | <0.001 |
|
Sex: no. (%) Male |
55 (52%) | 18 (49%) | 37 (54%) | 0.6 |
| Female | 50(48%) | 19 (51%) | 31 (46%) | |
| Median prior treatments: no. (range) | 4 (1 – 9) | 3 (2–9) | 4 (1–7) | 0.9 |
| Prior autoBMT: no. (%) | 31 (29%) | 8 (22%) | 23 (34%) | 0.19 |
| BV: no. (%) | 46 (43.8%) | 26 (70.2%) | 20 (29.4%) | <0.001 |
| ICI as last therapy: no. (%) | 28 (27%) | 28 (76%) | 0 (0%) | NA |
| Median time from ICI to alloBMT: days (range) | NA | 51 (23–472) | NA | NA |
|
Ann Arbor stage at diagnosis: no. (%) Stage I-II |
47 (45%) | 13 (35%) | 34 (50%) | 0.1 |
| Stage III-IV | 58 (55%) | 24 (65%) | 34 (50%) | |
| Primary refractory disease | 23 (22%) | 9 (24.3%) | 14 (20.5%) | 0.7 |
| Relapsed disease | 82 (78%) | 28 (75.6%) | 54 (79.4%) | |
| Pre-alloBMT remission status: no. (%) Complete remission | 56 (53%) | 21 (57%) | 35 (51%) | 0.1 |
| Partial remission | 34 (32%) | 14 (38%) | 20 (29%) | |
| Stable disease | 15 (14%) | 2 (5%) | 13 (19%) |
Table 2. Transplant Characteristics.
ICIs=Immune Checkpoint Inhibitors, NA=not applicable. IQR=interquartile range
| All patients | ICIs | No-ICIs | P value | |
|---|---|---|---|---|
|
Donor type no. (%) HLA haploidentical |
89 (85%) | 26 (70%) | 63 (93%) | 0.009 |
| HLA matched related | 5 (5%) | 3 (8%) | 2 (3%) | |
| HLA matched unrelated | 11 (10%) | 8 (22%) | 3 (4%) | |
| Median donor age (IQR in years) | 37 (IQR: 25, 52) |
27 (IQR: 22, 41) |
41 (IQR: 27, 54) |
0.008 |
|
Allograft source no. (%) Bone marrow |
94 (90%) | 31 (84%) | 63 (93%) | 0.4 |
| Peripheral Blood | 9 (9%) | 5 (14%) | 4 (6%) | |
| Cord blood | 2 (2%) | 1 (3%) | 1 (1%) | |
|
Sex mismatch no. (%) Matched |
59 (62%) | 19 (63%) | 40 (62%) | 0.9 |
| Female donor to male recipient | 18 (19%) | 6 (20%) | 12 (18%) | |
| Male donor to female recipient | 18 (19%) | 5 (17%) | 13 (20%) | |
| Major ABO mismatch no. (%) | 9 (9%) | 6 (17%) | 3 (4%) | 0.03 |
| CMV reactivation no. (%) | 36 (34%) | 9 (24%) | 27 (40%) | 0.1 |
|
Count recovery time median (range) in days Days to neutrophil count recovery |
16 (4, 751) | 16 (12, 751) | 16 (4, 64) | 0.3 |
| Days to platelet count recovery | 24 (11, 112) | 24 (11, 112) | 24 (11, 76) | 0.9 |
|
Engraftment/Chimerism at day +60 Full donor chimerism (<5% patient) |
78 (74.2%) | 22 (59.4%) | 56 (82.3%) | 0.04 |
| Mixed chimerism (5–94% patient) | 17 (16.1%) | 9 (24.3%) | 8 (11.7%) | |
| Graft Failure | 10 (9.5%) | 6 (16.2%) | 4 (5.8%) |
Engraftment and GVHD
The median neutrophil and platelet recovery times were 16 days and 24 days in both groups. The day 60 chimerism analysis revealed that full donor chimerism (≥ 95% donor) was achieved in 22 (59.4%) versus 56 (82.3%) patients in the ICI and no-ICI groups, respectively (Table 2). Graft failure (<5% donor) occurred in 6 (16.2%) ICI patients and 4 (5.8%) patients who never received ICIs (Odds Ratio [OR] 3.09 (95% CI: 0.81–11.78) P=0.09), and 9 (24.3%) versus 8 (11.7%) patients exhibited mixed chimerism (594% donor), respectively. Logistic regression showed a statistically significant risk of combined mixed chimerism and non-engraftment: OR 3.18 (95% CI: 1.29–7.91) P=0.01 (Supplementary table 1). When comparing all patients by use of BV before alloBMT, demonstrated there was no increase in graft failures [OR 0.84 (95% CI: 0.22–3.18) P=0.79] or combined graft failure and mixed chimerism [OR 1.89 (95% CI: 0.78–4.59) P=0.15] in the BV group (Supplementary table 1). However, only 2 of the 6 ICI patients who failed to achieve full donor chimerism received cytotoxic salvage therapy compared to 19/22 ICI patients who maintained engraftment (p=0.02 Fisher’s exact).
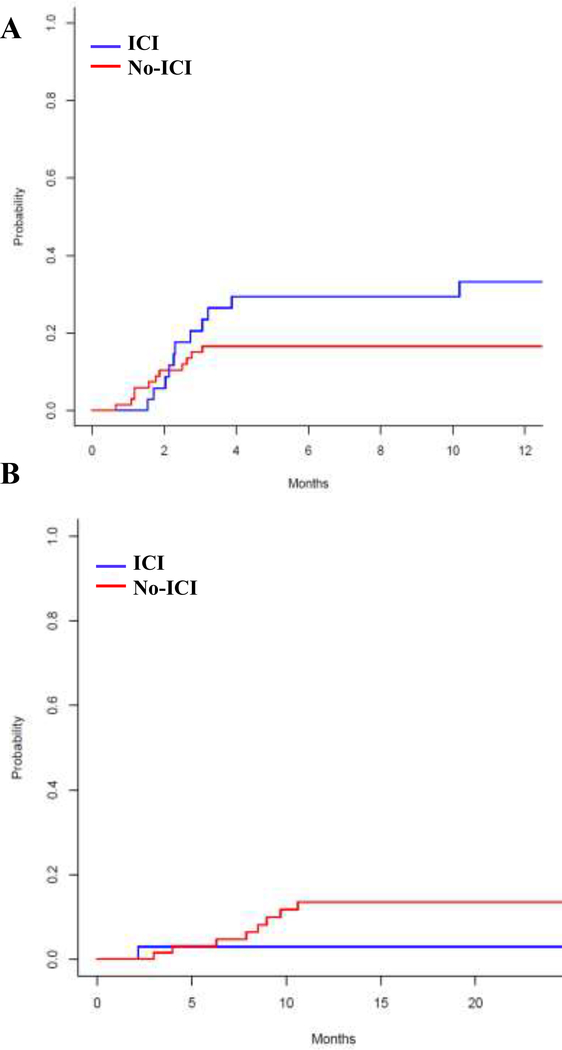
The 12-month cumulative grade II to IV aGVHD and 24-month cGVHD incidence were low among both the ICI and no-ICI cohorts: grade II-IV aGVHD 33% (95% CI: 17–50%) and 17% (95% CI: 8–26%) subdistribution hazard ratio [SDHR 1.95 (95% CI: 0.85, 4.48), P=0.11] and cGVHD 3% (95% CI: 0–9%) and 14% (95% CI: 5–22%) [SDHR 0.25 (95% CI: 0.03–2.03)], P=0.19 (Figure 1, Table 3), respectively. Female graft donors with male recipients also experienced increased risk of aGVHD [SDHR 2.66 (95% CI: 1.1–6.6), P=0.03].
Figure 1: Cumulative incidence of aGVHD (A) and cGVHD (B).
The curves were truncated at 12 months for aGVHD and 24 months for cGVHD. ICI=Immune Checkpoint Inhibitor.
Table 3. Univariate analysis for grade II-IV aGVHD and cGVHD.
ICIs=Immune Checkpoint Inhibitors. BV=Brentuximab vedotin
| Variable | aGVHD II-IV SDHR (95% CI) |
P value | cGVHD SDHR (95% CI) |
P value |
|---|---|---|---|---|
| ICI versus no-ICIs | 1.95 (0.85–4.48) | 0.11 | 0.25 (0.03–2.03) | 0.19 |
| BV versus no-BV | 1.60 (0.69–3.68) | 0.27 | 1.18 (0.32–4.35) | 0.80 |
|
Donor type HLA haploidentical versus HLA matched |
0.73 (0.25–2.1) | 0.55 | 1.35 (0.18–9.96) | 0.76 |
|
Allograft source Peripheral blood vs. Bone marrow |
1.57 (0.5–4.9) | 0.44 | Did not convg. | |
|
Female donor to male recipient Yes versus No |
2.66 (1.1–6.6) | 0.03 | 1.18 (0.3–5.4) | 0.83 |
Relapse and NRM
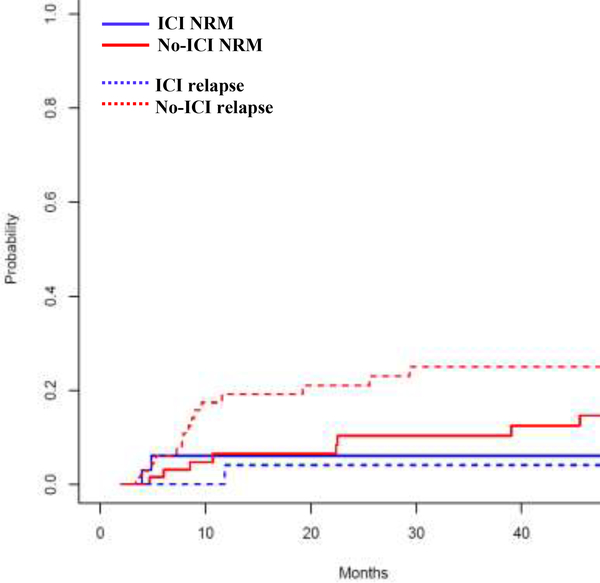
The CuI of relapse at three years was 4% (95% CI: 0–12%) versus 25% (95% CI: 14–36%) [SDHR 0.14 (95% CI: 0.02–1), P=0.05] and NRM at 3 years was 6% (95% CI: 0–14%) versus 10% (95% CI: 2–18%) SDHR 0.74 (95% CI: 0.16–3.45), P=0.7] in the ICI and no-ICI patients, respectively (Figure 2). The major causes of NRM was infection (6/12 patients), followed by refractory GVHD (3/12 patients), cardiovascular and bleeding events (2/12 patients).
Figure 2. Cumulative incidence of Relapse and NRM after alloBMT with PTCy.
ICI=Immune Checkpoint Inhibitor.
Survival
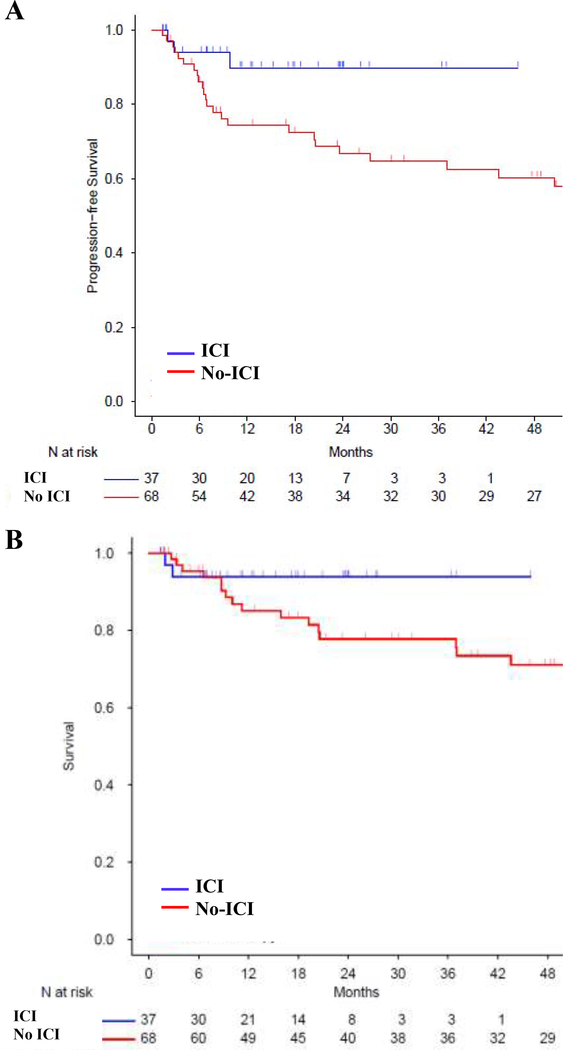
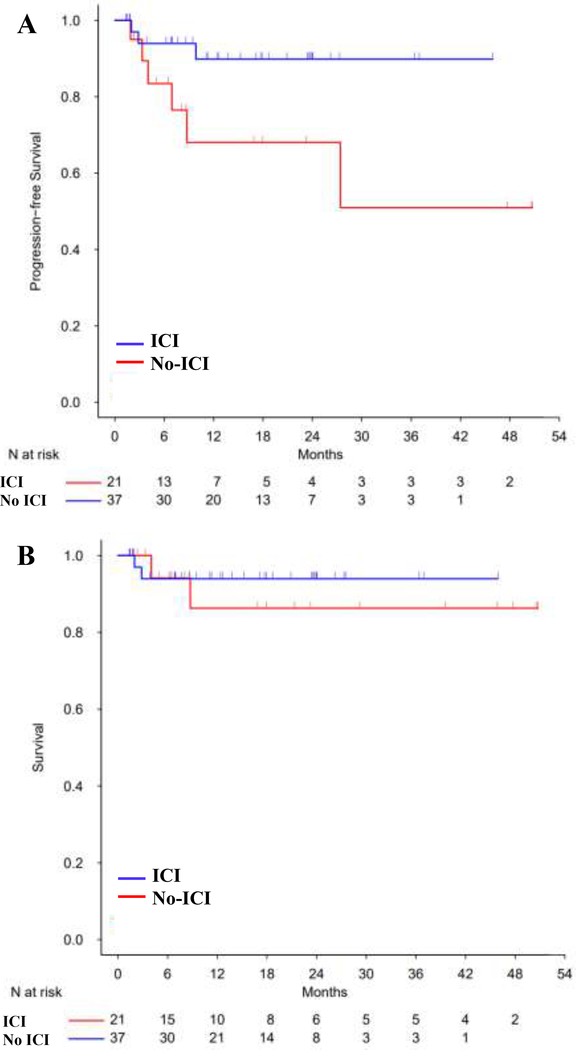
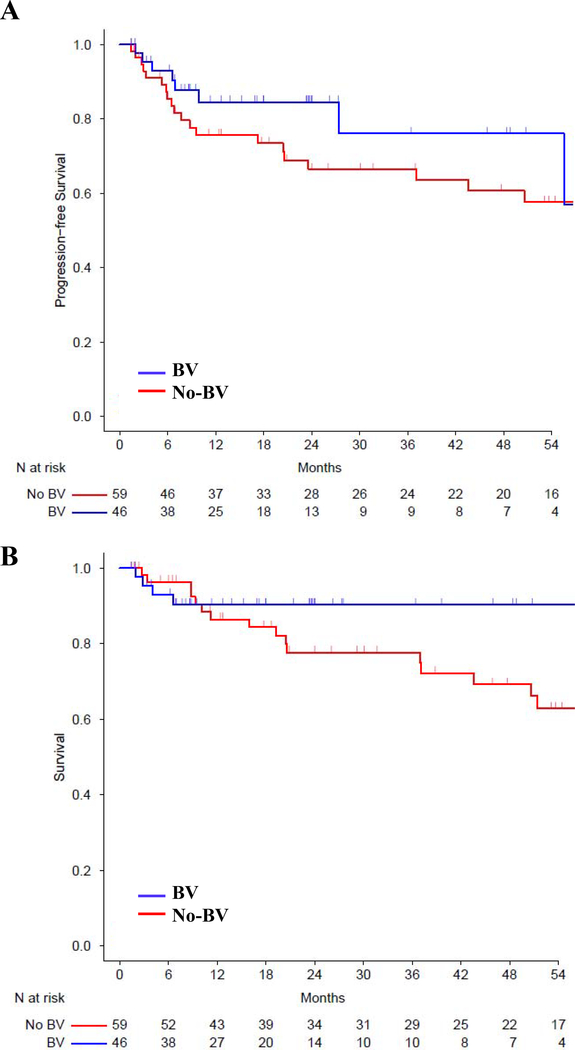
The median patient follow up was 15 months (range 1.4, 45.9) and 53 months (range 1.5, 148.7) for the ICI and no-ICI cohorts, respectively, based on reverse Kaplan-Meier method. The estimated 3-year PFS for the ICI and no-ICI cohorts was 90% (95% CI: 79–100%) versus 65% (95% CI: 53–78%) [HR ratio 0.3 (95% CI: 0.09–1, P=0.051)], and estimated 3-year OS was 94% (95% CI: 86–100%) versus 78% (95% CI: 68–89%), respectively [HR 0.35 (CI: 0.08–1.56, P=0.168)] (Figure 3). Patients received ICI before alloBMT from 2015 onwards, and we conducted a post-2015 survival analysis to account for the time bias. Since 2015, ICI and no-ICI treated patients experienced a 3-year estimated PFS of 90% and 51%, [HR 0.67 (95 % CI: 0.3–1.48), P=0.322], and a 3-year estimated OS of 94% versus 86%, [HR 0.92 (95% CI: 0.35–2.38), P=0.861] (Figure 4). The 6 ICI patients with graft failures continued to be in CR, did not require additional treatment and were disease free at last follow up (median 21 months, range 6 – 55 months). To investigate if the observed PFS benefit in ICI cohort was likely due to the ICI exposure and not due to an unexamined variable, we analyzed survival in patients receiving an unrelated drug, BV. Pre-alloBMT BV therapy compared to no BV therapy led to a 3-year estimated PFS of 76% and 67%, [HR 0.71 (95 % CI: 0.32–1.57), P=0.392], and a 3-year estimated OS of 90% versus 78%, [HR 0.59 (95% CI: 0.21–1.62), P=0.305], respectively (Figure 5). Multivariate analysis demonstrated a trend towards improved PFS with ICI [HR 0.26 (95% CI: 0.06–1.19), P=0.08] (Table 4), but the difference was not statistically significant.
Figure 3. Kaplan-Meier curve for (A) PFS (B) OS in ICI or no-ICI pre-treated alloBMT patients.
The curves are truncated at 48 months. ICI=Immune Checkpoint Inhibitor.
Figure 4. Kaplan-Meier curve for (A) PFS (B) OS in post-2015 ICI or no-ICI pre-treated alloBMT patients.
The curves are truncated at 48 months. ICI=Immune Checkpoint Inhibitor.
Figure 5. Kaplan-Meier curve for (A) PFS (B) OS in BV or no BV pre-treated alloBMT patients.
The curves are truncated at 54 months. BV=Brentuximab vedotin.
Table 4. Multivariate analysis for OS and PFS.
OS=Overall survival. PFS= Progression free survival. ICIs=Immune Checkpoint Inhibitors. BV=Brentuximab vedotin
| Variable | OS HR (95% CI) |
P value | PFS HR (95% CI) |
P value |
|---|---|---|---|---|
| ICI versus no-ICIs | 0.25 (0.03–2.09) | 0.20 | 0.26 (0.06–1.19) | 0.08 |
| BV versus no-BV | 0.83 (0.25–2.76) | 0.77 | 0.95 (0.37–2.46) | 0.92 |
|
Donor type: HLA haploidentical versus HLA matched |
0.84 (0.11–6.55) | 0.87 | 1.27 (0.17–9.68) | 0.81 |
Discussion
In ICI treated relapsed/refractory cHL patients undergoing alloBMT with PTCy, we observed a 3 year PFS and OS of 90% and 94% respectively. ICI treated patients experienced better PFS compared to patients receiving salvage treatment without ICI (ICI: 90% vs no-ICI: 65%, P=0.05). We observed a trend towards improved PFS in multivariate analysis [HR 0.26, P=0.08]. However, the study was underpowered to detect a statistically significant PFS difference in multivariate analysis due to the small sample size and low number of disease progression events. Our observed OS and PFS in the ICI cohort also compares favorably with past reports of alloBMT with PTCy after salvage chemotherapy in cHL (3-year OS: 63%, PFS: 59%3; 3-year OS: 53%, PFS: 44%4 and 2-year OS: 89%, PFS: 76%5). The PFS improvement in the ICI cohort may reflect that ICI patients were less heavily pretreated (median prior regimens 3 versus 4) and had better pre-alloBMT disease control (57%, 38% in CR, PR versus 51%, 29% in CR, PR) before alloBMT, and experienced significantly reduced disease relapse (ICI: 4% vs no-ICI 25%, P=0.05). As ICI treatment was initiated from 2015 onwards, we performed a post-2015 alloBMT patient survival analysis to adjust for time bias, and observed a similar PFS and OS between the ICI and no-ICI cohorts. We noted a trend towards improved PFS (ICI: 90% vs. no-ICI: 51%, P=0.322) but difference was not statistically significant, likely due to the reduced sample size in the post-2015 cohort.
In contrast, BV pretreatment compared to no-BV pretreatment resulted in similar alloBMT outcomes at 3 years (BV: PFS of 76% vs no-BV: 67%, P=0.392). This suggests that ICI use before alloBMT was likely driving the observed PFS benefit. Currently in relapsed/refractory cHL, BV and ICI are increasingly being incorporated in the salvage regimens. While BV is approved after failure of autoBMT or after ≥2 prior chemotherapy regimens,22 ICIs are approved after ≥3 prior regimens.23 However, the ideal places for ICI and BV in the treatment course of relapsed/refractory cHL remain under active investigation.24 The recent interim analysis of phase III Keynote-204 trial (NCT02142738), investigating ICI (pembrolizumab) or BV in relapsed/refractory cHL patients also suggest a significant PFS benefit with ICI.25 Keynote-204 is ongoing, and it remains to be seen if the observed PFS improvement is sustained and if it leads to OS benefit at study closure. Salvage ICI in combination with chemotherapy26 or BV27 provides high response rates in relapsed/refractory cHL and is safe before autoBMT. Our study highlights that salvage ICI is safe before alloBMT with PTCy GVHD prophylaxis and may lead to improvement in PFS in Hodgkin lymphoma.
Earlier studies reported that pre-alloBMT ICI therapy led to an elevated risk of aGVHD (44%,6 56%7 and 59%8) and cGVHD (41%,6 29%7 and not reported8). However, majority of patients in these reports received GVHD prophylaxis with a range of non-PTCy based regimens including: methotrexate, tacrolimus and sirolimus; tacrolimus and methotrexate; cyclosporine and methotrexate. We show that with the use of PTCy, ICIs were not associated with increased rates of aGVHD (33%) or cGVHD (3%) compared to pre-alloBMT therapy with salvage chemotherapy (aGVHD: 17%, cGVHD: 14%). However, we did observe a trend towards elevated risk of aGVHD and reduction in risk of cGVHD. The reduction in GVHD incidence with PTCy in ICI pretreated patients, compared to prior reports if ICI pre-treated patients without PTCy, is in agreement with observations in AML/MDS9 and our prior report looking at a range of hematologic malignancies.10 Animal model studies suggest PTCy mediated post-alloBMT regulatory T cell recovery ameliorates the ICI enhanced GVHD.28 A recent conference report investigating 150 pre-alloBMT ICI cHL patients with 88 patients receiving PTCy, also reported reduced cGVHD incidence in the PTCy subset (34% vs 58%).29 And a subsequent publication by some of the authors of the conference report suggests that ICI before alloBMT improves PFS in cHL.30 In view of these reports of ICI immune toxicity reduction with PTCy in alloBMT recipients, PTCy may be the preferred primary GVHD treatment in ICI pre-treated patients. The limitations of this report includes: retrospective nature of the study, fewer patients and shorter median follow up in the ICI cohort, different alloBMT time period in ICI and no-ICI cohort and dissimilar duration of tacrolimus/sirolimus GVHD prophylaxis for patients before and after 2018.
We previously reported that 3 of 14 patients receiving ICIs prior to alloBMT with PTCy experienced graft failure suggesting pre-alloBMT ICI treatment could potentiate non-engraftment.10 Our current analysis of 37 cHL patients receiving ICIs prior to alloBMT with PTCy revealed a statistically significant reduction in full donor chimerism. Such reduction in full chimerism were not reported in prior studies examining ICI use before alloBMT.6,7,9 The reasons for the reduction in full chimerism in our cohort are unclear, but we observed that these patients also received less salvage cytotoxic chemotherapy (4 out of 6 ICI patients who failed to achieve donor chimerism did not receive cytotoxic salvage therapy). The absence of cytotoxic chemotherapy before alloBMT preparative regimen is associated with poor donor engraftment.31 Importantly, all 6 graft failure patients remained disease free at last follow up, suggesting that in cHL, failure to achieve donor chimerism do not preclude long term disease free survival. Such extended PFS despite graft failures have been observed in other hematologic malignancies as well.13,16,32 The lack of full donor chimerism and even graft failure did not impact PFS. Overall, our findings suggest that ICI before NMA alloBMT is a safe treatment option when utilizing PTCy as GVHD prophylaxis and may improve post-alloBMT disease progression and survival in cHL.
Supplementary Material
Highlights.
Immune checkpoint inhibition (ICI) before allogeneic bone marrow transplantation (alloBMT) does not increase risk of graft-versus-host disease in patients receiving post-transplantation cyclophosphamide (PTCy).
ICI therapy before alloBMT with PTCy in classic Hodgkin lymphoma patients may improve disease progression and survival when compared to salvage chemotherapy before alloBMT.
Acknowledgements
The authors are thankful for the exceptional clinical care provided by nurses, physicians, staff at the bone marrow transplant coordinator’s office and the cell therapy laboratory. S.P. was supported by National Institutes of Health T32 grant 5T32CA009071-38, and the SITC-Amgen Cancer Immunotherapy in Hematologic Malignancies Fellowship. This study was supported by the National Institutes of Health, National Cancer Institute grants PO1 CA225618 and P30 CA06973 (to R.J.J.).
Funding: National Institutes of Health, National Cancer Institute grants.
Footnotes
Conflict of interest statements
N.W.J participates in advisory boards of Regeneron, ADC Therapeutics, CALIBR, Versatem, Bayer, Gilead. J.B.M has received honoraria from Incyte Corporation as a member of their Data Monitoring Committee.
Ethics committee approval
Study was approved by Johns Hopkins Medicine Institutional Review Board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, singlearm phase 2 trial. The Lancet Oncology 2016; 17(9): 1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R, Zinzani PL, Fanale MA, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017; 35(19): 2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagna L, Bramanti S, Devillier R, et al. Haploidentical transplantation with post-infusion cyclophosphamide in advanced Hodgkin lymphoma. Bone marrow transplantation 2017; 52(5): 683–8. [DOI] [PubMed] [Google Scholar]
- 4.Mariotti J, Devillier R, Bramanti S, et al. T Cell-Replete Haploidentical Transplantation with Post-Transplantation Cyclophosphamide for Hodgkin Lymphoma Relapsed after Autologous Transplantation: Reduced Incidence of Relapse and of Chronic Graft-versus-Host Disease Compared with HLA-Identical Related Donors. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(3): 627–32. [DOI] [PubMed] [Google Scholar]
- 5.Martinez C, Gayoso J, Canals C, et al. Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation as Alternative to Matched Sibling or Unrelated Donor Transplantation for Hodgkin Lymphoma: A Registry Study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017; 35(30): 3425–32. [DOI] [PubMed] [Google Scholar]
- 6.Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 2017; 129(10): 1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ijaz A, Khan AY, Malik SU, et al. Significant Risk of Graft-versus-Host Disease with Exposure to Checkpoint Inhibitors before and after Allogeneic Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2019; 25(1): 94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasamon YL, de Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R. FDA Approval Summary: Nivolumab for the Treatment of Relapsed or Progressive Classical Hodgkin Lymphoma. The oncologist 2017; 22(5): 585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oran B, Garcia-Manero G, Saliba RM, et al. Posttransplantation cyclophosphamide improves transplantation outcomes in patients with AML/MDS who are treated with checkpoint inhibitors. Cancer 2020. [DOI] [PubMed] [Google Scholar]
- 10.Schoch LK, Cooke KR, Wagner-Johnston ND, et al. Immune checkpoint inhibitors as a bridge to allogeneic transplantation with posttransplant cyclophosphamide. Blood advances 2018; 2(17): 2226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nivolumab (Opdivo) for Hodgkin Lymphoma. 2016. https://www.fda.gov/drugs/resourcesinformation-approved-drugs/nivolumab-opdivo-hodgkin-lymphoma (accessed 1/16/2020.
- 12.Pembrolizumab (KEYTRUDA) for classical Hodgkin lymphoma. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-classicalhodgkin-lymphoma (accessed 1/16/2020.
- 13.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2002; 8(7): 377–86. [DOI] [PubMed] [Google Scholar]
- 14.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010; 115(16): 3224–30. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasamon YL, Fuchs EJ, Zahurak M, et al. Shortened-Duration Tacrolimus after Nonmyeloablative, HLA-Haploidentical Bone Marrow Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(5): 1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2008; 14(6): 641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul S, Tsai HL, Lowery P, et al. Allogeneic Haploidentical Blood or Marrow Transplantation with Post-Transplantation Cyclophosphamide in Chronic Lymphocytic Leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2020; 26(3): 502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation 1995; 15(6): 825–8. [PubMed] [Google Scholar]
- 19.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015; 21(3): 389–401 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jason Fine RG. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999; 94(446): 496–509. [Google Scholar]
- 21.Arnold BF, Ercumen A. Negative Control Outcomes: A Tool to Detect Bias in Randomized Trials. JAMA 2016; 316(24): 2597–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HIGHLIGHTS OF PRESCRIBING INFORMATION ADCETRIS®(brentuximab vedotin) for injection, for intravenous use. FDA; 10/15/2019. [Google Scholar]
- 23.HIGHLIGHTS OF PRESCRIBING INFORMATION KEYTRUDA® (pembrolizumab) injection, for intravenous use Initial U.S. Approval: 2014 FDA; 01/09/2020. [Google Scholar]
- 24.Moskowitz AJ, Herrera AF, Beaven AW. Relapsed and Refractory Classical Hodgkin Lymphoma: Keeping Pace With Novel Agents and New Options for Salvage Therapy. Am Soc Clin Oncol Educ Book 2019; 39: 477–86. [DOI] [PubMed] [Google Scholar]
- 25.Merck’s KEYTRUDA® (pembrolizumab) Significantly Improved Progression-Free Survival Compared with Brentuximab Vedotin in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma (cHL). Merck; 2020. p. Press Release. [Google Scholar]
- 26.Herrera Alex F., Chen Robert W., Palmer Joycelynne, Tsai Ni-Chun, Mei Matthew, Popplewell Leslie L., Nademanee Auayporn P., Nikolaenko Liana, McBride Kathryn, Ortega Ricardo, Song Joo Y., Rosen Steven, Kwak Larry W, Forman Stephen J, Lee Hun Ju,. PET-Adapted Nivolumab or Nivolumab Plus ICE As First Salvage Therapy in Relapsed or Refractory Hodgkin Lymphoma. American Society of Hematology: American Society of Hematology; 2019. [Google Scholar]
- 27.Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018; 131(11): 1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikegawa S, Meguri Y, Kondo T, et al. PTCy ameliorates GVHD by restoring regulatory and effector T-cell homeostasis in recipients with PD-1 blockade. Blood advances 2019; 3(23): 4081–94. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merryman R CL, Corradini P et al. Safety and Efficacy of Allogeneic Hematopoietic Stem Cell Transplant after Programmed Cell Death 1 (PD-1) / Programmed Cell Death Ligand 1 (PD-L1) Blockade for Classical Hodgkin Lymphoma: Analysis of a Large International Cohort. American Society of Hematology, abstract no 704 Immunotherapies; 2019. [Google Scholar]
- 30.De Philippis C, Legrand-Izadifar F, Bramanti S, et al. Checkpoint inhibition before haploidentical transplantation with posttransplant cyclophosphamide in Hodgkin lymphoma. Blood advances 2020; 4(7): 1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattsson J, Ringden O, Storb R. Graft failure after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2008; 14(1 Suppl 1): 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitzer TR, McAfee SL, Dey BR, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation 2003; 75(10): 1748–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.