Abstract
The global pandemic COVID-19, caused by novel coronavirus SARS-CoV-2, has emerged as severe public health issue crippling world health care systems. Substantial knowledge has been generated about the pathophysiology of the disease and possible treatment modalities in a relatively short span of time. As of August 19, 2020, there is no approved drug for the treatment of COVID-19. More than 600 clinical trials for potential therapeutics are underway and the results are expected soon. Based on early experience, different treatment such as anti-viral drugs (remdesivir, favipiravir, lopinavir/ritonavir), corticosteroids (methylprednisolone, dexamethasone) or convalescent plasma therapy are recommended in addition to supportive care and symptomatic therapy. There are several treatments currently being investigated to address the pathological conditions associated with COVID-19. This review provides currently available information and insight into pathophysiology of the disease, potential targets, and relevant clinical trials for COVID-19.
Keywords: COVID-19, SARS-Cov-2, Pathophysiology, Pharmacotherapy, Clinical trials
1. Introduction
Since its emergence in December 2019 from the Wuhan city of China, the outbreak of novel coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 virus has infected over 21.2 million people including 761,000 deaths as of August 16, 2020 (World Health Organization, 19//2020). COVID-19 has proven to be very contagious with significantly higher rate of transmission compared to earlier outbreaks from closely related viruses, SARS (2002-03) and MERS (2012 to the present) (Fauci et al., 2020; Liu et al., 2020c). The patients with mild symptoms generally recover after one week. However, in many cases it has progressed to severe life threatening or fatal conditions (Adhikari et al., 2020). The patients with older age, pre-existing comorbidities and compromised immunity are considered at higher risk of developing fatal conditions (Arentz et al., 2020; Jordan et al., 2020). However, many young patients with no known pre-existing conditions have also developed severe symptoms (Liu et al., 2020a; Livingston and Bucher, 2020; Oxley et al., 2020). The mortality in severe cases is attributed to hypoxemia and cardiovascular complications resulting from abnormal blood clotting (Xie et al., 2020). In severe cases, disseminated intravascular coagulopathy is a major complication evident from strokes, kidney injury, cardiac injury, and ecchymosis (Li et al., 2020b; Zhou et al., 2020a). The activation of pulmonary endothelium and consequently the micro-thrombosis in pulmonary vasculature leads to acute respiratory distress syndrome and hypoxemia (Luks and Swenson, 2020). The underlying mechanism responsible for hypercoagulation is associated with inflammatory state and cytokine storm resulting from host defense system. The objective of this review is to present findings related to pathological complications in COVID-19 and potential therapies under investigation.
Several treatments are being investigated based on the pathophysiology of the disease and earlier experiences and similarities with SARS-CoV-1 and MERS (Petrosillo et al., 2020; Prompetchara et al., 2020). The search term “COVID-19” on Clinical trials.gov reported 2226 clinical trials as of June 23, 2020, which included studies ranging from evaluation of small molecule pharmacotherapies, mesenchymal stem cells or T-cell-based therapies, convalescent plasma therapies, immunoglobulins to medical devices in the treatment of COVID-19. To restrict discussion of present review to small molecules and monoclonal antibodies-based pharmacotherapies, studies pertaining only to such pharmacotherapies are summarized. We have attempted to categorize these trials depending upon the clinical features of COVID-19 and possible mechanism of action of the therapeutics in the disease.
1.1. Virus characteristics and clinical manifestations
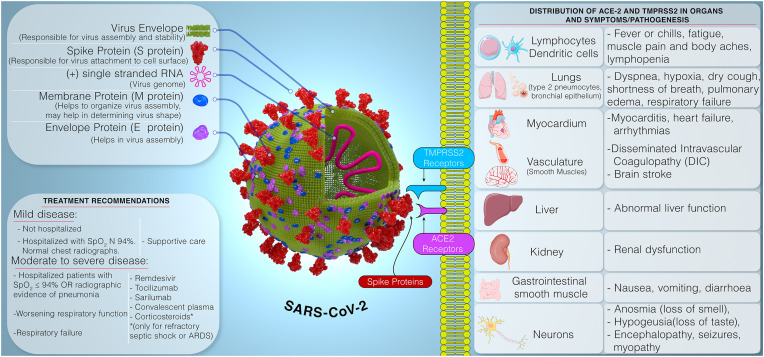
Like other coronaviruses, SARS-CoV-2 virus is spherical shaped virus containing genetic material inside phospholipid envelope (Fig. 1 ). The spike proteins protruding from the surface helps the virus to attach to the cell surface receptors followed by fusion and transfer of genes to the host cell (Venkatasubbaiah et al., 2020). Current evidence indicates an initial animal-to-human transmission from wild animals traded at the Huanan seafood market in Wuhan. As the pandemic is progressing, person-to-person transmission through (1) respiratory droplets released via coughing or sneezing, (2) aerosol generated during clinical procedures, and (3) mucosal membrane contact with fomites remains the main mode of spread (Adhikari et al., 2020; Zaim et al., 2020). The long incubation period (~10–14 days) and transmission of virus by the asymptomatic carriers (Bai et al., 2020) have resulted in alarmingly high transmission rate of the SARS-CoV-2 (Liu et al., 2020c).
Fig. 1.
Representation of Structure of SARS-CoV-2, clinical symptoms, pathogenesis, and currently recommended treatment of COVID-19.
SARS-CoV-2 primarily affects the lower respiratory tract. The clinical manifestations range from asymptomatic or mild disease to critical illness with rapid deterioration of health, and death (Mehta et al., 2020). The commonly reported symptoms in mild cases (81%) are fever or chills, cough, runny nose, headache, myalgia, fatigue, loss of smell and taste, gastrointestinal disturbance, occasional diarrhea, confusion, conjunctivitis and shortness of breath, which are reported to recover after 1 week (Adhikari et al., 2020; Centers for Disease Control and Prevention, 2020; Grant et al., 2020a). Severe cases (14%) display dyspnea, hypoxia (blood oxygen saturation [SpO2] ≤93%), or >50% lung involvement on imaging. The critical cases (5%) shows respiratory failure, shock, or multiorgan system dysfunction (Centers for Disease Control and Prevention, 2020). Older age, smoking and several pre-existing conditions such as hypertension, coronary artery disease, diabetes, end-stage renal disease, or immunosuppression are considered as risk factors for developing severe disease, poor prognosis and possibly death with COVID-19 (Mehta et al., 2020; Zhou et al., 2020b). SARS-CoV-2 virus uses angiotensin converting enzyme 2 (ACE-2) receptors in concert with transmembrane protease serine 2 protease (TMPRSS2) to enter the host cells. The SARS-CoV-2 spike (S) protein binds ACE2, and with the help of host proteases, principally TMPRSS2, promotes cellular entry of the virus. The copresence of these two entities in tissues to a large extent explains tropism of viral proliferation. ACE2 and TMPRSS2 are coexpressed in lungs, heart, gut smooth muscle, liver, kidney, neurons, and immune cells. Their distribution in the tissue may help to explain the symptoms and pathogenesis of COVID-19 (Fig. 1) (Liu et al., 2020b; Ziegler et al., 2020).
The diagnosis of COVID-19 involves detection of virus nucleic acid by quantitative reverse transcription PCR (RT-qPCR) in nasal swab (Singhal, 2020). The patients are monitored for increase in D-dimer levels, prolonged prothrombin time and reduced platelet count and fibrinogen levels, IL-6 levels, and viral load. Chest CT scans are examined at regular intervals in order to detect progression of the disease (Zhou et al., 2020a). Histopathological studies showed that when compared to normal lung tissue, COVID-19 affected lung tissue showed edema, proteinaceous exudates as large protein globules, vascular congestion combined with inflammatory clusters of fibrinoid material and multinucleated giant cells and hyperplasia of pneumocytes (Cascella et al., 2020).
The current recommendation for treating mild to moderate disease is supportive care while for severe disease with worsening respiratory function or respiratory failure, interleukin inhibitors, convalescent plasma or remdesivir is recommended. Corticosteroids are used only in refractory septic shock (Mehta et al., 2020). As of July 07, 2020, remdesivir is the only drug product that has been granted the emergency use authorization (EUA) by the U.S. Food and Drug Administration (USFDA). Remdesivir has also been recommended by the European Medicines Agency (EMA) for compassionate use in COVid-19 patients. It has been approved by the Ministry of Health, Labour and Welfare, Japan (MHLW) for the treatment of COVID-19. The EUA of chloroquine or hydroxychloroquine for moderate disease has been revoked by the USFDA on June 15, 2020 (National Institute of Health, 2020). Several potential treatments are under investigation and is discussed in this review by summarizing the ongoing clinical trials.
1.2. SARS-CoV-2 replication cycle
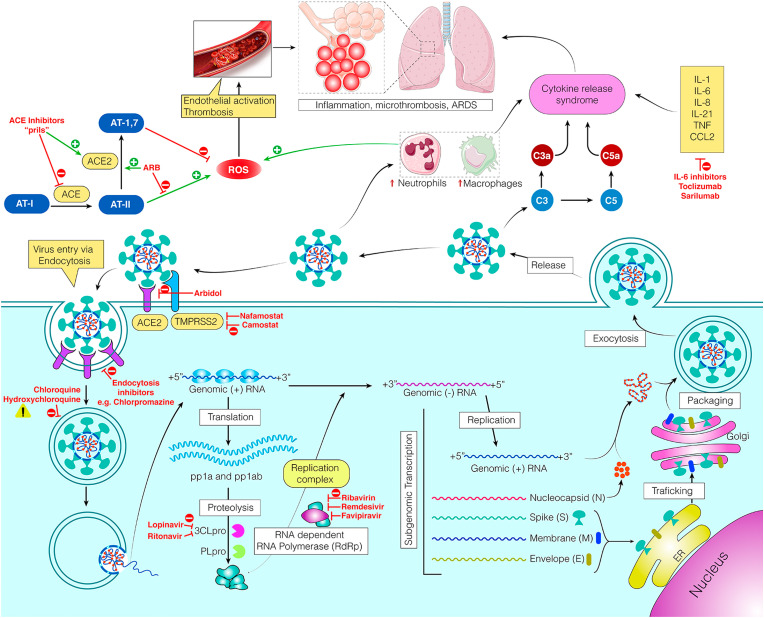
The SARS-CoV-2 viruses are enveloped, single-stranded positive-sense RNA viruses. The viral envelope projects spike proteins (S proteins) composed of two subunits (S1 and S2), which facilitate viral entry into the host cell by attachment to host cell receptors. SARS-CoV-2 uses angiotensin-I converting enzyme-2 (ACE-2) receptors for entering the pulmonary cells. As shown in Fig. 2 , after host cell attachment, the protease enzymes from the host cells cleave the S protein, thereby fusing the virus to cell membrane (Cascella et al., 2020). The process has been shown to be dependent on S protein priming by a serine protease (TMPRSS2) in many coronavirus models (Hoffmann et al., 2020a; Valencia, 2020). Once the genomic RNA is released into the cytoplasm, the viral genome is translated into polyprotein 1a/1 ab (pp1a/pp1ab) and structural proteins (spike [S], membrane [M], envelope [E], and nucleocapsid [N] proteins) via open reading frames (ORFs) of the genome. The production of polyproteins is facilitated by virally encoded chymotrypsin-like protease (3CLpro) or main protease (Mpro) (Cascella et al., 2020). Cleavage of polyproteins leads to formation of non-structural proteins for the RNA replicase-transcriptase complex. Upon viral replication and transcription, viral nucleocapsids are assembled with structural proteins manufactured in the lumen of the endoplasmic reticulum Golgi intermediate compartment. These nucleocapsids form new virions after encasing viral RNA and released from the cell via exocytosis (Valencia, 2020).
Fig. 2.
Schematic representation of replication cycle of SARS-CoV-2 Virus and potential therapeutic targets. Complex interactions involving renin angiotensin system, oxidative state, endothelial interaction, and immune activation leads to alveola edema, lung inflammation, microvascular thrombosis, and acute respiratory distress syndrome. Potential targets have been identified for possible pharmacotherapies for the prevention, treatment, or management COVID-19. (Abbreviations: ACE-Angiotensin-Converting Enzyme, ARDS-Acute Respiratory Distress Syndrome, AT-Angiotensin, C3, C5-Complement proteins, IL-Interleukins, 3CLpr-PLpro- Proteases, ROS-Reactive Oxygen Species, TMPRSS- Transmembrane protease, serine 2) (Adapted with permission from (Cascella et al., 2020a; Valencia, 2020)).
2. Pharmacotherapies in clinical trails
Based on the pathological conditions, several targets are identified to prevent, treat or mange COVID-19. The therapeutic targets are presented in a manner to represent the events in COVID-19 (i.e. virus entry and replication, virus multiplication and events occurring subsequent to that). The potential targets are discussed below, and ongoing clinical trials are summarized in the relevant tables.
2.1. Targeting virus entry into the host cell
At present, it is widely believed that SARS-CoV-2 enters the host cell mainly via endocytosis after attachment to ACE-2 receptors present on the surface of the host cell. Although some evidence suggests that autophagy (non-endocytosis) may also contribute to virus entry, endocytosis is being considered primary mechanism for infection (Yang and Shen, 2020). The endocytosed virus subsequently releases the viral genome in cytoplasm and begins reproducing. Two types of interventions are being investigated to affect this process (1) prevention of endocytosis (2) prevention of release of the viral genome in the cytoplasm. Currently, camostat mesilate, nafamostat mesilate, arbidol (umifenovir) and chlorpromazine are being investigated to prevent endocytosis while chloroquine or hydroxychloroquine is being investigated to prevent release of the viral genome in cytoplasm. Table 1 summarizes the ongoing clinical trials of potential drugs that interfere with, prevent, or reduce viral entry in the host cells.
Table 1.
Therapies targeted to prevent virus entry in host cell.
| Drug Name | Mechanism of Action/Proposed mechanism in COVID-19 | Clinical Trail No. |
|---|---|---|
| Camostat Mesilate | Inhibition of transmembrane protease serine 2 (TMPRSS2) which is essential for coronavirus spike protein (S protein) activation and virus entry into the host cell via ACE-2 binding. | NCT04353284, NCT04321096, NCT04338906, NCT04435015 |
| Nafamostat Mesilate | Inhibition of transmembrane protease serine 2 (TMPRSS2) which is essential for coronavirus spike protein (S protein) activation and virus entry into the host cell via ACE-2 binding. | NCT04352400, NCT04418128 |
| Arbidol (Umifenovir) | Broad spectrum antiviral drug with both direct viricidal effect and host-targeting abilities; inhibits clathrin mediated endocytosis. | NCT04286503, NCT04273763, NCT04260594, NCT04254874, NCT04350684 |
| Chlorpromazine | Inhibits clathrin-mediated endocytosis. | NCT04366739, NCT04354805 |
| Chloroquine/Hydroxychloroquine | Increases endosomal pH. | Approximately 160 clinical trials, some of the examples are: |
| NCT04359095, NCT04347915, NCT04329611, NCT04359615, NCT04321993, NCT04359316, NCT04343768, NCT04329832, NCT04364022, NCT04350684, NCT04330586, NCT04361318, NCT04389359, NCT04390594, NCT04328285, NCT04359953, NCT04352933, NCT04369742 | ||
| DAS181 | A sialidase fusion protein that inhibits attachment of parainfluenza virus to respiratory cells. | NCT04324489, NCT04354389, NCT04298060 |
| LY3819253 | A human antibody against SARS-CoV-2 spike protein. It is derived from B cells from convalescent patients. It is designed to block viral attachment and entry into human cells. | NCT04427501, NCT04411628 |
2.1.1. Endocytosis inhibitors
The coronavirus spike protein (S protein) is activated by the enzyme transmembrane protease serine 2 (TMPRSS2), which is essential for viral entry into the host cell. camostat mesilate and nafamostat are serine protease inhibitors, approved for the treatment of pancreatitis in Japan. Both of these molecules have shown to inhibit the host cell enzyme transmembrane protease-serine 2 (TMPRSS2), thereby block COVID-19 entry into the cell (Bittmann et al., 2020; Blaising et al., 2013). In an in-vitro study, nafamostat showed potent inhibitory effect on virus entry via inhibition of SARS-CoV-2 entry into the human lung cells (Hoffmann et al., 2020b). Chlorpromazine, widely used drug for the treatment of psychotic disorders such as schizophrenia, has been shown to inhibit clathrin dependent endocytosis (Monpara et al., 2019; Singh et al., 2012). Chlorpromazine is reported to prevent endocytosis of SARS CoV-1 in Vero-E6 and HEK293 cell lines (Wang et al., 2008), and therefore it may potentially inhibit the endocytosis and entry of SARS-CoV-2. Arbidol (umifenovir) is a broad-spectrum antiviral agent shown to inhibit clathrin-mediated endocytosis by impeding dynamin-2-induced membrane scission (Blaising et al., 2013). Zhu et al., 2020. reported patients treated with arbidol had a shorter duration of positive RNA test compared to those in the lopinavir/ritonavir treatment (Zhu et al., 2020).
2.1.2. Chloroquine/hydroxychloroquine
Another avenue is the release of the viral genome in the cytoplasm by affecting the endosomal osmolarity. Chloroquine and hydroxychloroquine have been the center of discussion from the very start of pandemic as potential agents to prevent the disease. Chloroquine and hydroxychloroquine are widely used in the treatment of malaria, rheumatoid arthritis, and systemic lupus erythematosus. The optimism of effective treatment of COVID-19 with chloroquine or hydroxychloroquine with or without the combination of macrolide antibiotics such as azithromycin arose mainly from two lines of evidence (1) in-vitro data suggesting inhibition of SARS and SARS-CoV-2 possibly by increasing the endosomal pH thereby negatively affecting the process of release of the viral genome from the endosomes (Vincent et al., 2005; Wang et al., 2020a; Yao et al., 2020) and (2) an open-label non-randomized clinical trial showing significant reduction of the viral carriage at day six-post inclusion compared to controls. The study showed that addition of azithromycin to the treatment significantly improved the outcomes. The study generated unusual degree of attention. Several experts have raised concerns over small sample size (ntreatment = 20, ncontrol = 16, ntotal = 36) and serious methodological limitations of the study (Juurlink, 2020). Despite of concerns from scientific advisors regarding lack of randomized controlled trial to support efficacy of drugs in the population, USFDA, on March 28, 2020, authorized clinicians to prescribe chloroquine and hydroxychloroquine for patients admitted to hospital with covid-19. The agency acknowledged that the approval was based on “limited in vitro and anecdotal clinical data.” (Lenzer, 2020). Indian Council of Medical Research COVID-19 National Task Force later advocated use of hydroxychloroquine in prophylaxis for health care workers (Juurlink, 2020). The World Health Organization (WHO) announced SOLIDARITY clinical trial with five arms involving (1) standard of care (2) Remdesivir (3) combination of lopinavir/ritonavir (4) combination of lopinavir/ritonavir/interferon beta and (5) hydroxychloroquine. Thereafter, series of reports showed that chloroquine/hydroxychloroquine (with or without azithromycin) was not effective at all in Covid-19 and rather it caused serious harm due to cardiac arrhythmia (QT prolongation).
A study reported by Mehra et al. created controversies around use of chloroquine in COVID-19. The study claimed that a multinational registry analysis of 96,032 patients (14,888 patients in the treatment group and [81,144 patients in the control group] from 671 hospitals in six continents showed significantly high mortality, increased risk of de novo ventricular arrhythmia and decreased in-hospital survival in the treatment group (Mehra et al., 2020). Three days later WHO suspended hydroxychloroquine arm of the SOLIDARITY trial. The study was retracted after several researchers pointed out that there was no data or code sharing and implausible numbers (Mahase, 2020). However, the evidence of ineffectiveness of chloroquine/hydroxychloroquine in hospitalized COVID-19 are continuously being reported. Geleris et al. examined the association between hydroxychloroquine use and intubation or death at a large medical center in New York City. The authors assessed the association between hydroxychloroquine use and a composite endpoint of intubation or death over a median follow-up of 22.5 days in 1376 consecutive patients. The authors reported that there was no evidence of a substantial difference in the rate of the composite endpoint compared to the control group (Geleris et al., 2020; Paliani and Cardona, 2020). Other studies also reported that in hospitalized patients, hydroxychloroquine was not useful and was perhaps even harmful (Mahévas et al., 2020; Mercuro et al., 2020). Indeed, caution should be used while using hydroxychloroquine + azithromycin combination as they have very serious cardiotoxicity and other side effects such as widened QRS complex, atrioventricular heart block, QT interval prolongation as well as U waves from hypokalemia and refractory seizures (Erickson et al., 2020; Moore, 2020). In addition to cardiac side effects, the drug has other side effects such as hypoglycemia, neuropsychiatric effects (agitation, insomnia, confusion, mania, hallucinations, paranoia, depression, catatonia, psychosis and suicidal ideation), hematologic toxicities, drug-drug interactions and immunologically mediated adverse reactions (Stevens-Johnson syndrome) (Juurlink, 2020).
Following the scientific evidence on potential harm related to use of chloroquine/hydroxychloroquine alone or in combination, USFDA, on June 15, 2020, revoked the emergency use authorization (EUA) that allowed for chloroquine phosphate and hydroxychloroquine sulfate to treat certain hospitalized patients with COVID-19 when a clinical trial was unavailable, or participation in a clinical trial was not feasible. Currently, Indian Council of Medical Research still recommends prophylactic use of hydroxychloroquine in healthcare workers and the police. The National Institute of Allergy and Infectious Diseases announced a trial to assess whether hydroxychloroquine, given with azithromycin, can prevent admission to hospital or death from covid-19 in people who have tested positive (Mahase, 2020). However, the question on the effectiveness of chloroquine/hydroxychloroquine can only be answered through well designed clinical trials. There are more than 160 clinical trials underway for evaluating efficacy of chloroquine or hydroxychloroquine alone or in combination of azithromycin and zinc for COVID-19.
2.2. Targeting virus replication
There are two major targets to halt virus replication cycle (1) proteolysis of pp1a and pp1ab by protease (3CLpro and PLpro) which forms nonstructural proteins required for replicase complex and (2) replication complex (complex between nonstructural proteins and RNA-dependent RNA polymerase). Antivirals such as lopinavir and ritonavir inhibit protease (3CLpro), thereby inhibiting formation of non-structural proteins while remdesivir and favipiravir inhibits RNA-dependent RNA polymerase, thereby inhibiting formation of the viral genome and structural proteins. Other antivirals such as reverse transcriptase inhibitors and DNA polymerase inhibitors are also being investigated for COVID-19. Currently, ongoing clinical trials for the potential therapies affecting SARS-CoV-2 replication are enlisted in Table 2 . Some of the potential candidates to prevent viral replication are discussed below.
Table 2.
Therapies under investigation to inhibit replication of COVID-19 in the host cells.
| Drug Name |
Mechanism of Action/Proposed mechanism in COVID-19 |
Clinical Trail No. |
|---|---|---|
| Anti-viral drugs | ||
| Remdesivir | Nucleoside analog that inhibits RNA dependent RNA polymerase, thereby reducing viral multiplication. | NCT04280705, NCT04365725, NCT04292899, NCT04292730, NCT04410354, NCT04431453, NCT04409262 |
| Favipiravir | Purine nucleoside analog that inhibits RNA dependent RNA polymerase, thereby reducing viral multiplication. | NCT04336904, NCT04359615, NCT04358549, NCT04349241, NCT04387760, NCT04333589, NCT04351295, NCT04346628, NCT04434248, NCT04425460, NCT04359615, NCT04411433, NCT04303299, NCT04402203, NCT04392973, NCT04310228, NCT04376814, NCT04373733, NCT04400682, NCT04407000 |
| Lopinavir/ritonavir | Lopinavir is HIV type 1 aspartate protease inhibitor, with potential for repurposing for COVID-19. Ritonavir is combined with lopinavir to increase its plasma half-life by inhibition of cytochrome P450. | NCT04321993, NCT04350671, NCT04346147, NCT04366245, NCT04295551, NCT04255017, NCT04321174, NCT04261907, NCT04275388, NCT04276688, NCT04386876, NCT04403100, NCT04425382 |
| Galidesivir | Nucleoside analog that inhibits RNA dependent RNA polymerase, thereby reducing viral multiplication. | NCT03891420 |
| Tenofovir | Reverse transcriptase inhibitor. | NCT04334928 |
| Emtricitabine | Reverse transcriptase inhibitor. | NCT04334928 |
| Oseltamivir | Inhibitor of neuraminidase enzyme. It prevents virus entry into the host cell. | NCT04371601, NCT04255017, NCT04261270 |
| Danoprevir | Danoprevir is an inhibitor of NS3/4A protease. NS3/4A protease is involved in viral replication and suppressing host cell response to viral infection. | NCT04345276, NCT04291729 |
| Darunavir | Protease inhibitor currently approved for use in HIV. | NCT04252274 |
| Clevudine | Blocks viral replication by blocking enzymes DNA polymerase and reverse transcriptase. Currently approved for Hepatitis B with potential for repurposing. | NCT04347915 |
| Azvudine | Reverse transcriptase inhibitor. | NCT04425772 |
| ABX464 | Inhibits viral replication by affecting the biogenesis of viral RNA. Binds to the cap binding complex at the 5′-end of the pre-mRNA thereby inhibits transcription. It has strong anti-inflammatory activity in addition to antiviral activity. | NCT04393038 |
| AT-527 | Inhibits viral RNA polymerase. | NCT04396106 |
| ASC09F | A viral protease inhibitor. Investigated for HIV. | NCT04261270 |
| Anthelmintic drugs | ||
| Ivermectin | Anthelmintic agent being investigated in combination with Nitazoxanide. Exact mechanism not known. | NCT04360356, NCT04381884, NCT04374279, NCT04390022, NCT04373824, NCT04406194, NCT04405843, NCT04429711, NCT04407130, NCT04438850, NCT04425707, NCT04343092, NCT04407507, NCT04390022, NCT04392713, NCT04403555, NCT04351347, NCT04399746, NCT04425863, NCT04425850 |
| Levamisole and Isoprinosine combination | Levamisole is an Anthelmintic drug with immunoregulatory activity probably via biomimicry of thymopoietin. Isoprinosine is antiviral agent having immunomodulatory activity. The combination is investigated for synergistic action. | NCT04383717 |
| Niclosamide | Anti-parasitic drug. It has inhibitory effect on multiple signaling pathways such as IL-6-JAK1-STAT3, mTORC1 signaling, etc. Primarily investigated for anticancer effect. | NCT04436458 |
2.2.1. Antiviral agents
Remdesivir, an anti-viral drug, has emerged as promising candidate for the treatment of COVID-19. It is an adenosine nucleoside analog drug which gets incorporated into nascent viral RNA chains by the RNA-dependent RNA Polymerase resulting in pre-mature termination of RNA elongation. Remdesivir has shown significant antiviral activity against wide array of RNA viruses (including SARS/MERS-CoV) in cultured cells, mice, and non-human primates. Remdesivir showed significant potency against SARS-CoV-2 infection in Vero E6 cell line [half maximal effective concentration (EC50) = 0.77 μM; half-maximal cytotoxic concentration (CC50) > 100 μM; selectivity index (SI) > 129.87) (Wang et al., 2020a). Administration of remdesivir 24h prior to infection (preventive) in MERS-CoV infected rhesus monkeys completely prevented viral replication, while administering 12 h after inducing infection also showed clinical benefits such as reduction of lung lesions, viral replication, and other symptoms (Cao et al., 2020b). In vitro studies by Wuhan virus research institute suggested that remdesivir is the most effective and fastest acting antiviral agent for COVID-19, with IC50 = 0.069 μM against SARS-CoV-2 and IC50 = 0.074 μM against MERS-CoV (Cao et al., 2020b). A randomized, double-blind, placebo-controlled study in 237 patients showed that although remdesivir was not associated with a difference in time to clinical improvement, patients receiving remdesivir had a numerically faster time to clinical improvement than those receiving placebo (Wang et al., 2020). Another double-blind, randomized, placebo-controlled trial conducted by Beigel et al. showed that amongst 1059 patients (538 assigned to remdesivir and 521 to placebo), patients who received remdesivir had a median recovery time of 11 days compared to 15 days in patients who received placebo. The Kaplan-Meier estimates of mortality by 14 days were 7.1% with remdesivir and 11.9% with placebo. Serious adverse events (acute respiratory failure, hypotension, viral pneumonia, and acute kidney injury) were reported for 114 of the 541 patients in the remdesivir group who underwent randomization (21.1%) and 141 of the 522 patients in the placebo group who underwent randomization (27.0%) (Beigel et al., 2020). Zhu et al., analyzed published trials for remdesivir and emphasized that remdesivir significantly increased the recovery rate, decreased mortality, and decreased the risk of serious adverse events (Zhu et al., 2020).
Favipiravir is an RNA-dependent RNA Polymerase inhibitor approved by the USFDA and MHLW for the treatment of influenza. Favipiravir has been used for the treatment of Ebola infection (Venkatasubbaiah et al., 2020). A detailed review by Du and Chen describes the mechanism of action, pharmacological profile, dosing of favipiravir (Du and Chen, 2020). Briefly, it is recognized as a purine nucleotide analog by the RNA-dependent RNA polymerase and incorporated into a nascent RNA strand which prevents RNA elongation (Takashita, 2020). Favipiravir has shown significant efficacy against influenza viruses. It was recently demonstrated that favipiravir effectively inhibits SARS-CoV-2 infection in Vero-E6 cells (EC50 = 61.88 μmol·L−1, CC50 > 400 μmol·L−1, SI > 6.46). The potency was significantly low compared to remdesivir [(EC50 = 0.77 μM; CC50 > 100 μM; SI > 129.87)] in reported in-vitro study. However, favipiravir has been shown to be 100% effective in protecting mice against Ebola virus challenge despite of low potency in-vitro (EC50 = 67 μM), which suggest that an in-vivo studies are required for better understanding of efficacy of favipiravir (Wang et al., 2020a). An Open-Label Control Study comparing oral favipiravir plus interferon (IFN)-α by aerosol inhalation verses lopinavir plus IFN-α by aerosol inhalation showed that favipiravir arm improved chest CT scans, reduced viral clearance time and reduced side effects (Cai et al., 2020). In another independent, randomized, prospective study in 240 adult patients, favipiravir showed significant improvement of latency to pyrexia and cough observed on day 7 of treatment (Chen et al., 2020a). Although favipiravir is well tolerated, it has limitations in its use for pregnant and potentially pregnant women due to its potential for both teratogenicity and embryotoxicity in humans (Dong et al., 2020).
Lopinavir-Ritonavir combination, approved for treatment of human immunodeficiency virus (HIV), is being evaluated as potential therapy for COVID-19. Lopinavir is a potent HIV specific protease inhibitor. Co-administration of low-dose ritonavir has shown to increase plasma concentration of lopinavir. Therefore, these drugs are usually co-administered (Oldfield and Plosker, 2006). A randomized, controlled, open-labeled clinical trial was conducted on 199 adults who received a combination of lopinavir-ritonavir (400 mg–100 mg respectively) with standard care versus standard care alone. In this trial, no benefit was observed with lopinavir-ritonavir treatment as compared to the standard care in terms of time to clinical improvement and mortality at 28 days (Cao et al., 2020a). Improvement in the course of COVID-19 was studied in a prospective, phase 2 clinical trial. Patients were randomized in two groups, a combination of lopinavir and ritonavir (400 mg–100 mg respectively) alone versus lopinavir + ritonavir (same dose) in combination with interferon beta-1b (3 doses of 8 million international units). The combination group had a significantly shorter median time from start of study treatment to negative nasopharyngeal swab (7 days) than the control group (12 days). Although, only the combination of lopinavir-ritonavir with interferon significantly reduced the time to negative nasopharyngeal swab, it was seen that both the treatments were safe and efficacious (Hung et al., 2020). Lopinavir/ritonavir treatment arm was included in WHO's Solidarity Trial. However, in addition to hydroxychloroquine arm, the lopinavir/ritonavir arm was discontinued on July 04, 2020 due to little or no reduction in the mortality of hospitalized COVID-19 patients when compared to standard of care in the interim trial results. The WHO stated that “This decision applies only to the conduct of the Solidarity trial in hospitalized patients and does not affect the possible evaluation in other studies of hydroxychloroquine or lopinavir/ritonavir in non-hospitalized patients or as pre- or post-exposure prophylaxis for COVID-19.” (World Health Organization, 2020).
2.2.2. Anthelmintic drugs
Anthelmintic drugs such as ivermectin and levamisole have potential immunomodulatory and antiviral properties. The exact mechanism of virus inhibition is not reported yet, but it is speculated that inhibition of importin α/β1 mediated transport of viral proteins in and out of the nucleus may be responsible for the antiviral activity (Gupta et al., 2020). Recently, Caly et al. reported that ivermectin caused ~5000-fold reduction in viral RNA in Vero-hSLAM cells at 48 h after 2 h of incubation with 5 μM solution of ivermectin. The IC50 (50% inhibition) was found to be 2.4 μM, 2.5 μM and for 2.2 μM for supernatant E-gene, cell associated virus RdRp gene and supernatant RdRp gene, respectively (Caly et al., 2020). Despite of significant inhibition of SARS-CoV-2 virus, concerns have been raised over the required plasma concentrations for the activity. Schmith et al. reported that the IC50 value reported by Caly et al. is more than 35 times higher than the reported maximum plasma concentration (Cmax) [0.05 μM (46.6 ng/mL)] after oral administration of the approved dose (~200 μg/kg). Ivermectin is highly bound to serum albumin (93%) and it is unlikely that the unbound ivermectin will reach required plasma concentration even with 10 times high oral dose (Schmith et al., 2020). Caly et al. also emphasize on the possible requirement of improved dosing regimen based on pharmacokinetic data (Caly et al., 2020). A retrospective study reported by David Scheim showed that the mortality rate was 40% less in patients treated with ivermectin (n = 173, mortality rate 15%) compared to the control group (n = 107, mortality rate = 25.2%). Amongst 75 patients with severe pulmonary disease (receiving oxygen at FiO2 ≥ 50% or ventilation), the ivermectin treatment group (n = 46) had 38.8% mortality compared to 80.7% mortality in the control group (n = 29). The author emphasized that higher doses of ivermectin could result in greater clinical benefits. The author suggested that the dose-response gains could be attributed to the shielding of SARS-CoV-2 spike proteins by ivermectin. It is believed that in addition to ACE-2 receptors, SARS-CoV-2 also bind to the CD147 transmembrane receptor via spike proteins. The CD147 are found abundantly on the red blood cells (RBCs) and SARS-CoV-2 is hypothesized to cause clumping of RBCs impending the blood flow. The author also hypothesized that ivermectin could prevent the RBC clustering by shielding the spike proteins (Scheim, 2020). These studies are still at initial stage of investigation and more evidence are needed to establish the efficacy of ivermectin in COVID-19. The Anthelmintic drugs currently investigated in the clinical trials are listed in Table 2.
2.3. Acute respiratory distress syndrome (ARDS) and viral sepsis
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection (Singer et al., 2016). The severe cases of influenza reflect a combination of pathological processes, including the spread of viral infection from upper to lower respiratory tract, mucosal surface injury and possibly bacterial superinfection, and compromised pulmonary function due to effect of host inflammatory response causing alveolar injury. The mechanism by which influenza virus infection causes acute respiratory distress syndrome is poorly understood. It is believed that release of pro-inflammatory cytokines and infiltration of mononuclear cells and neutrophils leads to exacerbation in tissue damage. In addition to damage to the alveoli, and leakage of lung alveolocapillary membrane, the influenza virus interferes with the function of the epithelial sodium channel (ENaC), which regulates fluid absorption from the alveolar space, causing edema and respiratory distress (Armstrong et al., 2013). Li et al. reported that many severe or critically ill patients of COVID-19 developed typical clinical manifestations of shock, including cold extremities and weak peripheral pulses, even in the absence of hypotension. The patients showed severe metabolic acidosis, impaired liver, and kidney function in addition to severe lung injury. These clinical manifestations met the criteria for sepsis and septic shock. However, 76% sepsis patients had the blood and lower respiratory tract specimen negative for bacteria and fungi. This makes viral sepsis more appropriate to describe the clinical manifestations of OVID-19 (Li et al., 2020a). The immune response to influenza virus shares many common pathways with the response to bacteria (Kalil and Thomas, 2019; Remy et al., 2020).
The immune response to severe COVID-19 is associated with hyper-cytokinemia (Giamarellos-Bourboulis et al., 2020). In vitro studies have shown that respiratory epithelial cells, dendritic cells, and macrophages release high levels of pro-inflammatory cytokines (interleukin [IL]-1β, IL-6, tumor necrosis factor) and chemokines (Mcgonagle et al., 2020b; Ye et al., 2020). Significantly high levels of proinflammatory cytokines and chemokines including tumour necrosis factor-α, interleukin 1β (IL-1β), IL-6, granulocyte colony-stimulating factor, interferon gamma-induced protein-10, monocyte chemo attractant protein-1, and macrophage inflammatory proteins 1-α were observed in severe COVID-19 patients. In response to the infection, the alveolar macrophages or epithelial cells produce inflammatory cytokines and chemokines attracting monocytes and neutrophils leading to uncontrolled inflammation (Li et al., 2020a). Additionally, in severe cases, profound lymphopenia (low absolute lymphocyte counts) to the level seen in septic shock was found uniformly and correlated with higher secondary infections and mortality. The level of all lymphocyte subsets, including CD8+ and natural killer cells (important antiviral roles), and B cells (essential for making antiviral antibodies) were reduced significantly (Remy et al., 2020). The innate and adaptive immune response due to lymphopenia results in uncontrolled virus infection and vicious cycle of macrophage infiltration leading to worsening of lung injury. The systemic cytokine storm and microcirculation dysfunction could be the key contributor to the vascular endothelium damage, leakage of fluid in alveolar space, pulmonary edema resulting in acute respiratory distress syndrome and viral sepsis (Li et al., 2020a). Martin C. and Merad M. have reviewed the role of activated monocytes and macrophages in pathological inflammation in COVID-19 comprehensively (Merad and Martin, 2020). The readers are redirected to excellent reviews on role of cytokine storm in COVID-19 for further reading (Cao, 2020; Jose and Manuel, 2020; Ye et al., 2020).
In addition to host inflammatory response, abnormal coagulation has been observed as major clinical features in severe cases. McGonagle et al. have discussed the role of immune activation in pulmonary intravascular coagulopathy (Mcgonagle et al., 2020a). Studies have shown that 71.4% of non-survivors of COVID-19 had disseminated intravascular coagulation (grade ≥5). Whether SARS-CoV-2 induced abnormal coagulation and sepsis could be attributed to its ability to attack vascular endothelial cells is an important question remained to be answered yet. Additionally, the question of how SARS-CoV-2 spreads to extrapulmonary organs remains an enigma (Li et al., 2020a). One possible explanation could be virus gaining access to systemic circulation after apoptosis of infected endothelial cells and attacking organs with high ACE-2 expression. The alveolar epithelium and vascular endothelium are in very close proximity. The alveolocapillary is just over 1 μm thick with barrier thickness as low as 100–200 nm in some regions. It is plausible explanation that apoptosis of infected epithelial cell can expose the endothelial cells to new virion particles which may infect the exposed endothelial cells (Armstrong et al., 2013). This is particularly relevant for SARS-CoV-2 virus infection since the receptors for virus attachment (ACE-2) are highly expressed on the endothelial cells. Therefore, effective antiviral therapy and immunomodulatory therapies are expected to improve outcomes in severe COVID-19 cased involving viral sepsis.
2.4. Addressing the cytokine storm in COVID-19
Multiple strategies aimed at reducing the pro-inflammatory cytokines such as JAK-STAT inhibitors, granulocyte colony-stimulating factor (GM-CSF) inhibitors, and IL-6 and IL-1β inhibitors are being investigated in addition to classical immunomodulators such as interferons and corticosteroids. Studies have shown that preventing cytokine release can improve clinical outcomes, however, the timing of the immune modulation is critical for benefiting the patient. Table 3 shows clinical trials for potential therapies, including monoclonal antibodies, immunosuppressant and anti-arthritic drugs and anti-leukemic agents that are expected to inhibit cytokine storm in CIVID-19.
Table 3.
Therapeutic strategies under investigation to address the cytokine storm syndrome during COVID-19 infection.
| Drug Name |
Mechanism of Action/Proposed mechanism in COVID-19 |
Clinical Trail No. |
|---|---|---|
| Immunomodulators and rheumatoid arthritis drugs | ||
| Colchicine | It is an alkaloid anti-inflammatory drug known to non-selectively inhibit NLRP3 inflammasome, and hence may play a role in preventing COVID-19 related complications. NLRP3 inflammasome is a multimeric protein complex that triggers the release of pro-inflammatory interleukins (IL-1beta and IL-18). | NCT04375202, NCT04355143, NCT04360980, NCT04350320, NCT04328480, NCT04326790, NCT04322565, NCT04363437, NCT04322682, NCT04392141, NCT04403243, NCT04367168 |
| Leflunomide | Immunomodulatory drug that can prevent excessive immune reaction related to COVID-19. | NCT04361214 |
| Dexamethasone | Potent corticosteroid that inhibits inflammatory cells and suppresses expression of inflammatory mediators. | NCT04325061, NCT04360876, NCT04344730, NCT04327401, NCT04347980, NCT04395105 |
| Hydrocortisone | Glucocorticosteroid agonist, suppresses immune and inflammatory responses. | NCT04366115, NCT04359511, NCT04348305 |
| Prednisone | Glucocorticosteroid agonist, suppresses immune and inflammatory responses. | NCT04344288 |
| Methylprednisolone | Inhibits pro-inflammatory cytokine production, thereby mitigates cytokine storm associated with COVID-19. | NCT04244591, NCT04355247, NCT04273321, NCT04341038, NCT04377503, NCT04263402, NCT04323592, NCT04374071, NCT04343729, NCT03852537 |
| Tacrolimus | Immunosuppressant that inhibits interleukin-2 transcription in a calcium dependent fashion, thereby mitigates cytokine storm associated with COVID-19. | NCT04341038 |
| Sirolimus | Inhibits IL-2 and other cytokine dependent signal transduction mechanisms, thereby mitigates cytokine storm associated with COVID-19. | NCT04341675, NCT04371640 |
| Ciclesonide | Anti-inflammatory drug used for treating obstructive airway diseases. It interferes with mediators of inflammatory response and may help to combat ARDS associated with COVID-19. | NCT04381364, NCT04377711, NCT04330586, NCT04435795, |
| Piclidenoson | It is Adenosine Receptor agonist. It reduces inflammatory response. | NCT04333472 |
| Isotretinoin | It causes down regulation of ACE-2 receptors that play a role in viral entry into the host cell. | NCT04361422, NCT04353180, NCT04389580 |
| Interferon-β-1a | Affects multiple signaling pathways leading to immune modulation. Induction of ribonuclease and protein kinase leading to mRNA degradation and inhibition of protein synthesis, respectively. | NCT04343768, NCT04350671 |
| Interferon-λ-1A | Affects multiple signaling pathways leading to immune modulation. Induction of ribonuclease and protein kinase leading to mRNA degradation and inhibition of protein synthesis, respectively. | NCT04331899, NCT04388709, NCT04354259, NCT04343976, NCT04344600 |
| Ozanimod | It has sphingosine-1-phosphate (S1P) receptor agonistic activity which leads to lymphocyte sequestration in lymph nodes. | NCT04405102 |
| Opaganib | Inhibits SK2, a lipid kinase that catalyzes formation of the lipid signaling molecule sphingosine 1-phosphate (S1P). | NCT04414618 |
| NP-120 (Ifenprodil) | N-methyl-d-aspartate (NDMA) receptor -type subunit 2B antagonist. The subunit receptor is primarily expressed on neutrophils and T cells. | NCT04382924 |
| Rintatolimod | Synthetic mismatched double-stranded RNA toll-like receptor (TLR) agonist, having specificity for TLR-3. | NCT04379518 |
| N-803 (formerly known as ALT-803) | IL-15 agonist fusion protein. Affects the maintenance, function, and development of natural killer and T- cells. | NCT04385849 |
| Monoclonal Antibodies | ||
| Tocilizumab | Inhibits IL-6 signaling by blocking IL-6 receptors, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04345445, NCT04317092, NCT04330638, NCT04331795, NCT04377659, NCT04346355, NCT04320615, NCT04372186, NCT04359667, NCT04377750, NCT04335305, NCT04335071, NCT04377503, NCT04349410, NCT04363853, NCT04363853, NCT04356937, NCT04361032, NCT04333914, NCT04339712, NCT04310228, NCT04306705, NCT04370834, NCT04361552, NCT04347031, NCT04331808, NCT04412772, NCT04363736, NCT04332913, NCT04435717, NCT04424056, NCT04315480, NCT04423042 |
| Sarilumab | Inhibits IL-6 signaling by blocking IL-6 receptors, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04315298, NCT04357808, NCT04386239, NCT04357860, NCT04327388, NCT04324073, NCT04345289, NCT04359901 |
| Clazakizumab | Anti- IL-6, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04381052, NCT04343989, NCT04348500, NCT04363502 |
| Siltuximab | Anti- IL-6, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04330638, NCT04329650, NCT04322188 |
| Olokizumab | Anti- IL-6, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04380519 |
| Levilimab (BCD-089) | Anti- IL-6, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04397562 |
| Sirukumab | Anti- IL-6, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04380961 |
| Anakinra | Blocks biological activity of IL-1 alpha and beta (competitive inhibitor), thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04361214, NCT04362111, NCT04364009, NCT04357366, NCT02735707, NCT04341584, NCT04366232, NCT04324021 |
| Canakinumab | Anti- IL-1β, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04348448, NCT04365153, NCT04362813 |
| Astegolimab | Anti-IL1, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04386616 |
| Ravulizumab | Complement component 5 (C5) inhibitor, binds to C5 and prevents its cleavage to C5a (pro-inflammatory anaphylatoxin) and C5b (responsible for complement complex activation) thereby potentially preventing inflammatory responses associated with COVID-19. | NCT04369469, NCT04390464 |
| Eculizumab | Anti- C5 (Complement component C5 Drives complement- mediated cell death through formation of membrane attack complex). | NCT04346797 |
| Avdoralimab | Anti-C5aR, reduces biological activity of C5a, thereby potentially reducing COVID-19 associated inflammation. | NCT04371367 |
| Leronlimab | Anti- CCR5, CCR5 is a helical protein that plays a role in IL-6 activation, thereby helps to prevent or control COVID-19 associated cytokine response. | NCT04343651, NCT04347239 |
| Lenzilumab | Anti- GM- CSF, potential drug for COVID-19 associated ARDS. | NCT04351152 |
| Gimsilumab | Anti- GM- CSF, potential drug for COVID-19 associated ARDS. | NCT04351243 |
| Otilimab | Anti- GM- CSF, potential drug for COVID-19 associated ARDS. | NCT04376684 |
| Pembrolizumab | Programmed cell death protein 1 (PD-1) receptor antagonist, inhibits cytokine production associated with COVID-19 | NCT04335305 |
| Nivolumab | PD-1 receptor antagonist, inhibits cytokine production associated with COVID-19 | NCT04343144, NCT04356508, NCT04413838 |
| Emapalumab | Anti- IFN-γ, plays a role in neutralizing cytokine activity | NCT04324021 |
| Meplazumab | Anti-CD147 antibody, antagonist of IL-5, thereby helps to prevent or control cytokine storm associated with COVID-19. | NCT04275245 |
| Infliximab | TNF-α inhibitor. | NCT04425538 |
| Pamrevlumab | It inhibits the activity of connective tissue growth factor (CTGF), a critical mediator in the progression of fibrosis. | NCT04432298 |
| Crizanlizumab | Binds to P-selectin on the surface of activated endothelial cells and platelets, blocks interactions among endothelial cells, platelets, red blood cells, and white blood cells. | NCT04435184 |
| Lanadelumab | Targets plasma kallikrein (pKal) in order to promote prevention of angioedema. | NCT04422509 |
| Mavrilimumab | Selectively blocks GM-CSF which is involved in the activation, differentiation, and survival of neutrophils and macrophages. | NCT04397497 |
| Bevacizumab | Vascular endothelial growth factor (VEGF) inhibitor. | NCT04275414 |
| Axatilimab | High affinity antibody targeting the colony stimulating factor 1 receptor (CSF-1R) thereby preventing macrophage expansion and infiltration | NCT04415073 |
| IFX-1 | Binds to the soluble human complement split product C5a and blocks C5a-induced biological effects | NCT04333420 |
| Anti-leukemia drugs | ||
| Duvelisib | Phosphatidylinositol 3-kinase (PI3K) inhibitor. PI3K plays an important role in eliciting immune response. | NCT04372602 |
| Ruxolitinib | JAK1/JAK2 inhibitors. Both JAK1 and JAK2 play a role in cytokine signaling. | NCT04362137, NCT04348071, NCT04354714, NCT04377620, NCT04334044, NCT04331665, NCT04366232, NCT04338958, NCT04361903, NCT04374149, NCT04348695, NCT04359290, NCT04414098, NCT04338958 |
| Baricitinib | JAK1/JAK2 inhibitors. Both JAK1 and JAK2 play a role in cytokine signaling. | NCT04340232, NCT04321993, NCT04362943, NCT04358614, NCT04320277, NCT04345289, NCT04421027, NCT04373044, NCT04393051, NCT04399798 |
| Acalabrutinib | Bruton's tyrosine kinase (BTK) inhibitor. BTK is a molecule in cytokine signaling pathway, thus may potentially help to combat COVID-19 associated cytokine storm. | NCT04380688, NCT04346199 |
| Zanubrutinib | Bruton's tyrosine kinase (BTK) inhibitor, BTK is a molecule in cytokine signaling pathway, thus may potentially help to combat COVID-19 associated cytokine storm. | NCT04382586 |
| Ibrutinib | Bruton's tyrosine kinase (BTK) inhibitor, BTK is a molecule in cytokine signaling pathway, thus may potentially help to combat COVID-19 associated cytokine storm. | NCT04375397 |
| Tofacitinib | JAK1/JAK3 inhibitor. Both JAK1 and JAK2 play a role in cytokine signaling, thus may potentially help to combat COVID-19 associated cytokine storm. | NCT04390061, NCT04390061, NCT04412252, NCT04415151, NCT04332042 |
| Nintedanib | Tyrosine kinase inhibitor, helps combating pulmonary fibrosis | NCT04338802 |
| Imatinib | Kinase inhibitor. | NCT04346147, NCT04394416, NCT04422678, NCT04357613 |
| Ibrutinib | Bruton's tyrosine kinase inhibitor. | NCT04375397, NCT04439006 |
| Lenalidomide | Thalidomide analogue with immunomodulatory actions. | NCT04361643 |
2.4.1. Corticosteroids
Timely administration of corticosteroids in SARS-1 during 2003 resulted in clinical improvements such as reduction in fever and improved oxygenation. However, studies have shown that administration of corticosteroid therapy during COVID-19 infection led to adverse consequences. The timing and dosage of glucocorticoids administration are very important in the disease prognosis. Early administration of glucocorticoids has shown to inhibit the initiation of the body's immune defense mechanism. This immunosuppression increases the viral load and ultimately leads to adverse consequences (Sanders et al., 2020). Therefore, glucocorticoids are used mainly in critically ill patients suffering from cytokine storm (Ye et al., 2020). In a retrospective study on 46 patients in a hospital in Wuhan, China, distributed in two groups with similar age, sex, comorbidities, one group received low-dose methylprednisolone (1–2 mg/kg/day for 5–7 days) and the other did not. It was observed that the time to clinical recovery was significantly shorter for the patients that received methylprednisolone (Wang et al., 2020b). The WHO currently recommends against the routine use of corticosteroids in the treatment of patients with COVID-19, due to the potential for delayed viral clearance and other adverse effects such as avascular necrosis and psychosis and should be used only in certain situations such as refractory septic shock or severe acute respiratory distress syndrome (Mehta et al., 2020, 2020).
2.4.2. Interferons
Type I interferon (IFNαβ) have antiviral effects but can also activate immune cells that lead to tissue pathology. Conversely, type III interferon (also known as IFNλ) mainly targets epithelial cells as well as a restricted pool of immune cells, inducing a potent antiviral effect without promoting tissue inflammation (Merad and Martin, 2020). Interferon-λ reduces the mononuclear macrophage-mediated pro-inflammatory activity of IFN-αβ and inhibits the recruitment of neutrophils to the sites of inflammation (Mcgonagle et al., 2020b) thereby inhibiting tissue-damaging events, such as ROS production and degranulation (Zanoni et al., 2017). Pegylated Interferon-λ is under investigation for use in COVID-19 (Table 3).
2.4.3. Interleukin inhibitors
Monoclonal antibodies (mAb) targeted to inhibit these cytokines are potentially useful to alleviate COVID-19-related inflammatory reactions. Tocilizumab, a mAb against IL-6, has been evaluated as a potential drug to treat patients with COVID-19 with a risk of cytokine storm. In a single center study, of 15 patients (2 moderately ill, six seriously ill and seven critically ill), tocilizumab in combination with methylprednisolone was used in eight patients. The researchers showed that tocilizumab appeared to be an effective treatment strategy and must be administered repeatedly to patients with elevated IL-6 levels (Luo et al., 2020). In an independent study, 20 patients received tocilizumab with standard therapy, and it was observed that their clinical symptoms improved, requirements of oxygen therapy decreased in 15 out of 20 patients and the C-reactive protein concentrations and blood lymphocyte counts decreased, and no obvious adverse effects were observed (Xu et al., 2020). Several other monoclonal antibodies to inhibit cytokines are being investigated in clinical trials (Table 3).
Another repurposed drug, colchicine, used for the treatment of gout is also being evaluated. Colchicine is an alkaloid anti-inflammatory drug known to non-selectively inhibit NLRP3 inflammasome resulting in decreased release of pro-inflammatory interleukins (IL-1beta and IL-18), and hence may play a role in preventing COVID-19-related complications.
2.5. Hypercoagulation in COVID-19
COVID-19 has been shown to elicit a pro-inflammatory and hypercoagulable state with marked elevations in lactate dehydrogenase, ferritin, C-reactive protein, D-dimer, and interleukin levels (Casey et al., 2020; Terpos et al., 2020). Multiple mechanisms are responsible for COVID-19 associated hyper coagulopathy, which includes endothelial dysfunction, Von Willebrand factor elevation, Toll-like receptor activation, and tissue-factor pathway activation (Giannis et al., 2020). Additionally, disruption of the thrombo-protective state of the vascular endothelial cells leads to microvascular thrombosis and disseminated intravascular coagulation (Connors and Levy, 2020; Lillicrap, 2020). The patients commonly develop thrombocytopenia along with elevated D-dimer levels (Giannis et al., 2020). A meta-analysis by Lippi et al. identified significantly lower platelet count in patients with severe disease. The study showed that thrombocytopenia was associated with five times higher risk of severe disease (Lippi et al., 2020). Both thrombocytopenia and elevated D-dimer (20 - 2000-fold) can be explained by the excessive activation of the coagulation cascade and platelets. The elevated D-dimer levels even in early stage in ambulatory patients indicate thrombosis is very serious complication in COVID-19 central to severity of the disease (Giannis et al., 2020). The hypercoagulation seems to be one of the major causes of death in COVID-19.
A cohort study by Wichmann et al. on autopsy findings on 12 victims of COVID-19 revealed that massive pulmonary embolism was the cause of death in four victims while in three cases fresh deep venous thrombosis (DVT) was present in the absence of pulmonary embolism. Nonetheless, in all 12 cases, the cause of death was found to be related to pathologies in the lungs or the pulmonary vascular system (Wichmann et al., 2020). The authors emphasized that the pulmonary vascular changes in COVID-19 are distinct from classical thromboembolism. In COVID-19, the cause of thrombi in pulmonary vasculature is thrombosis within the pulmonary microvasculature. This can be attributed to the fact that the alveolar type 2 cells and the endothelium in the lung both express high levels of ACE-2 receptors resulting in diffuse alveolar damage (Mcgonagle et al., 2020a; Wichmann et al., 2020). The ACE-2 receptors are also expressed highly in endothelial cells, heart and kidney (Danilczyk and Penninger, 2006; Varga et al., 2020). Varga et al. has demonstrated endothelial cell involvement across vascular beds of different organs in patients died with COVID-19 (Varga et al., 2020). In the post-mortem analysis by electron microscopy, viral inclusion structures in endothelial cells were found in the transplanted kidney. Inflammatory cells associated with endothelium, as well as apoptotic bodies were accumulated in heart, small bowel, and lungs (Varga et al., 2020). The involvement of general endothelium might be the reason for stroke in small as well as large vessels, even in younger population and in absence of comorbidities (Oxley et al., 2020). Multiple organ dysfunction/failure including cardiac injury, kidney failure, liver and intestinal damage has been associated with increased mortality with COVID-19 (Liu et al., 2020a; Tang et al., 2020).
2.5.1. Anticoagulants
International Society on Thrombosis and Hemostasis (ISTH) guidance document described the rationale for using anticoagulants in COVID-19 and recommended early initiation of unfractionated heparin in patients without significant bleeding risks (Barrett et al., 2020). Anticoagulant therapy by heparin has been shown to mitigate the coagulopathy associated with COVID-19. A retrospective study was conducted in 499 patients diagnosed with COVID-19 of which 99 received low molecular weight heparin for 7 days or longer. It was observed that the 28-day mortality in heparin group was lower than non-heparin group (Tang et al., 2020). Low molecular weight heparin (LMWH) can also protect critically ill patients against venous thromboembolism, and the anti-inflammatory properties are an added benefit for COVID-19 complications (Thachil et al., 2020). An overview of ongoing clinical trials for therapies to combat COVID-19 associated hypercoagulation are summarized in Table 4 .
Table 4.
Anticoagulant therapies under investigation for treating COVID-19 associated hypercoagulation.
| Drug Name | Mechanism of Action/Proposed mechanism in COVID-19 | Clinical Trail No. |
|---|---|---|
| Low Mol. Wt. Heparin (LMWH), Heparin | Natural anticoagulant. Reversibly binds to Antithrombin-III (ATIII), ATIII inactivates coagulation enzymes thrombin and factor Xa. | NCT04344756, NCT04393805, NCT04397510, NCT04401293 |
| Fondaparinux | Antithrombotic activity as a result of ATIII mediated deactivation of factor Xa. | NCT04359212, NCT04367831, NCT04372589 |
| Enoxaparin | Enoxaparin is a low molecular weight heparin, with identical mechanism to heparin. | NCT04359277, NCT04366960, NCT04377997, NCT04367831, NCT04354155, NCT04373707, NCT04345848, NCT04427098, NCT04400799 |
| Tranexamic acid | Antifibrinolytic agent, that competitively inhibits activation of plasminogen to plasmin, an enzyme that degrades fibrin clots. | NCT04338074, NCT04338126 |
| Rivaroxaban | Oral anticoagulant that directly inhibits factor Xa. | NCT04333407 |
| Clopidogrel | Pro-drug of platelet inhibitor, that irreversibly binds to P2Y12 ADP receptors on platelets, thereby preventing platelet aggregation and embolism. | NCT04333407, NCT04368377 |
| Aspirin | Inhibits COX-1 and COX-2 enzymes non-selectively. Inhibition of COX-1 results in inhibition of thromboxane production and thus interference with normal platelet aggregation. | NCT04333407, NCT04365309, NCT04363840 |
| Alteplase | Thrombolytic drug that binds to fibrin-rich clots via fibronectin finger like domain and Kringle 2 domain. The protease domain cleaves Arg/Val bond in plasminogen to plasmin. Plasmin then degrades fibrin matrix in the thrombus. | NCT04357730 |
| Tissue-Plasminogen Activator (rt-PA) | Thrombolytic drug that binds to fibrin-rich clots via fibronectin finger like domain and Kringle 2 domain. The protease domain cleaves Arg/Val bond in plasminogen to plasmin. Plasmin then degrades fibrin matrix in the thrombus. | NCT04356833 |
| Dociparstat | Glycosaminoglycan derived from porcine heparin. | NCT04365309, NCT04389840 |
2.6. Hypoxemia and coagulation abnormalities in COVID-19
Extensive immune cell infiltration in lungs leads to increased capillary permeability, impaired surfactant production and function. Reabsorption of alveolar fluids is sometimes inhibited, and apoptosis is induced by various pathways, often leading to alveolar flooding, reduced lung compliance, ventilation-perfusion mismatch, pulmonary shunts, and impaired gas exchange (Luks and Swenson, 2020). Severe and critically ill patients had relatively normal pulmonary ventilation function and an inefficient oxygen uptake (Chen et al., 2020b). In some COVID-19 patients, the oxygen saturation level can be significantly lower than 85%, sometimes even below 30% (normal blood-oxygen saturation is at least 95%) (Chen et al., 2020b). Despite of such a low oxygen level the apnea is not apparent, clinicians call it “happy hypoxia” (Couzin-Frankel, 2020). In addition to pulmonary insufficiency, the inflammatory response also causes diffuse alveolar damage and endothelial cell activation resulting in local thrombosis and hypoxia. There is a possibility that the diffused nature of inflammation may lead to initial pulmonary intravascular coagulopathy which ultimately extends to disseminated intravascular coagulopathy (Mcgonagle et al., 2020a). Recent observations suggest that the respiratory failure is not driven only by the acute respiratory distress syndrome, but also by the microvascular thrombosis. The microvascular thrombosis has a major role in the resulting hypoxemia and overall disease severity (Oudkerk et al., 2020).
Lang et al. reported that in COVID-19 patients, CT imaging of lungs showed considerable proximal and distal pulmonary vessels dilation and tortuosity, predominately within, or surrounding, areas of lung opacities which might be due to relative failure of normal, physiological hypoxic pulmonary vasoconstriction in the setting of overactivation of a regional vasodilatation cascade. The perfusion abnormalities, combined with the pulmonary vascular dilation suggest intrapulmonary shunting toward areas where gas exchange is impaired, leading to worsening of clinical hypoxia by ventilation-perfusion mismatch (Lang et al., 2020). Therefore, attempts to improve the hypoxic conditions should be chosen wisely. Chen et al. have found that hyperbaric oxygen therapy (HBOT) might be useful in correcting hypoxia associated with COVID-19. In five severely ill patients, the SpO2 level improved from 70% to normal after HBOT treatment. Improved oxygenation and consequently aerobic metabolism had positive effect on other markers of COVID-19. The number of lymphocytes in each patient was elevated after HBOT treatments (P < 0.05). Fibrinogen was significantly declined, and D-Dimer levels were decreased. Chest CT showed significantly improved imaging status of lung lesions in each patient (Chen et al., 2020b).
2.6.1. Vasodilators
Systemic administration of vasodilators such as calcium channel blockers (nifedipine) and phosphodiesterase-5 inhibitors (sildenafil and tadalafil) should be avoided as these medications may worsen oxygenation by increasing perfusion of poorly ventilated area and thereby exacerbating the already abnormal ventilation-perfusion mismatching in injured regions of the lungs. On the other hand, vasodilators given by inhalation such as epoprostenol and nitric oxide are expected to improve hypoxia by selective vasodilation in these areas and not affecting the unventilated regions (Luks and Swenson, 2020).
Another potential treatment to overcome hypoxia is inhaled nitric oxide. Nitric oxide causes vascular and nonvascular smooth muscle relaxation, and inhibition of platelet function. Nitric oxide is a reactive free radical that can covalently modify protein function and can also scavenge reactive oxygen species. Moreover, in an in vitro study, NO donors (i.e., S-nitroso-N-acetyl penicillamine) greatly increased the survival rate of SARS-CoV-1 infected eukaryotic cells by inhibiting viral protein and RNA synthesis, suggesting direct antiviral effects (Ignarro, 2020). There are ongoing clinical trials to assess the efficacy of inhaled nitric oxide (NCT04306393, NCT04305457) in COVID-19.
α-1 adrenergic receptor antagonists (α-blockers) such as Prazosin have been shown to prevent cytokine storm syndrome and death in mice (Vogelstein et al., 2020). A retrospective analysis by Vogelstein et al. showed that in patients with acute respiratory distress or pneumonia; the patients who were taking α-blockers had a reduced risk of requiring ventilation compared to patients who were not taking α-blockers (Vogelstein et al., 2020). Another vasodilator, a combination of vasoactive intestinal peptide (VIP) and phentolamine mesylate (Aviptadil®), is also being evaluated for overcoming hypoxemia in COVID-19. VIP is a naturally occurring 28-amino acid neurotransmitter having potent vasodilatory effect. Phentolamine mesylate is a competitive nonselective α 1- and α 2-adrenoceptor blocker. VIP has a potent effect on the veno-occlusive mechanism, but little effect on arterial inflow, while Phentolamine increases arterial blood flow without showing any impact on the veno-occlusive mechanism (W. Wallace Dinsmore and Michael G. Wyllie, 2008). The combination of VIP with Phentolamine is expected to complement each-other resulting in improved arterial flow and oxygenation. The proposed therapeutic strategies to improve COVID-19 associated hypoxia and relevant clinical trials are summarized in Table 5 .
Table 5.
Therapeutic strategies to improve blood oxygenation and overcoming COVID-19 related hypoxemia.
| Drug Name | Mechanism of Action/Proposed mechanism in COVID-19 | Clinical Trail No. |
|---|---|---|
| Hyperbaric Oxygen Therapy | Improves SpO2 | NCT04343183, NCT04332081, NCT04344431, NCT04358926, NCT04327505 |
| Conventional Oxygen | Improves SpO2 | NCT04344730, NCT04368923, NCT04378712, NCT04371601, NCT04366089, NCT04312100, NCT04333251, NCT04336462 |
| Inhaled Nitric Oxide | Increases intracellular levels of cyclic-guanosine 3′,5′-monophosphate, which then leads to vasodilation. | NCT04383002, NCT04388683, NCT04338828, NCT04305457, NCT04312243, NCT04337918, NCT04306393, NCT03331445 |
| Prazosin | Selective alpha 1-adrenergic blocking agent. Produces vasodilation and reduces peripheral resistance. | NCT04365257 |
| Sildenafil citrate | Enhances the effect of nitric oxide (NO) by inhibiting phosphodiesterase type 5 (PDE5). | NCT04304313 |
| Aviptadil (VIP + Phentolamine) | vasoactive intestinal polypeptide produces selective venous dilation. Phentolamine is a short-acting alpha-adrenoceptor antagonist produces arteriolar dilation. |
NCT04311697, NCT04360096 |
| PB1046 | A long-acting, sustained release human VIP analogue. | NCT04433546 |
2.7. Involvement of Angiotensin-Converting Enzyme 2 in prognosis of the disease
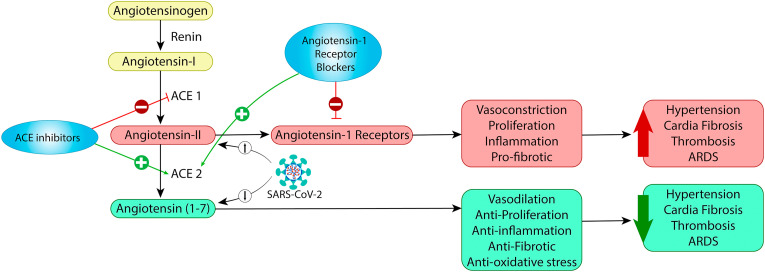
Angiotensin-I converting enzyme and ACE2 are homologues with distinct important functions in the renin-angiotensin system. As shown in Fig. 3 , the renin-angiotensin system is composed of two, mutually antagonistic pathways that are responsible for maintaining homeostasis. The first pathway is represented by Angiotensin-I converting enzyme/angiotensin-II/angiotensin-I which promotes inflammation, cell proliferation and vasoconstriction. Angiotensin-II signaling increases oxidative stress, reactive oxygen species, inflammation, and migration of endothelial cells resulting in atherosclerosis (Cheng et al., 2020). The second pathway comprises ACE-2/angiotensin (1-7)/MAS receptors, an anti-growth pathway inhibiting inflammation and promoting vasodilation. Angiotensin 1–7, by binding to the MAS receptor, is known to promote vasodilation, antioxidant, and anti-inflammatory effects (Hamdi and Abdaldayem, 2020; Sriram and Insel, 2020). When ACE-2 is activated, angiotensin 1–7 is increased instead of angiotensin II. Since the receptors are endocytosed along with SARS-CoV-2 virus, there is a reduction in availability of ACE-2 and therefore a decrease in ACE-2-derived peptides (Angiotensin [1-7]) in the COVID-19 infection. This may not only increase inflammatory, oxidative stress and remodeling events but also potentially induce myocardial injury due to increased myocardial oxygen demand in presence of severe hypoxia (Sriram and Insel, 2020; Tan and Aboulhosn, 2020). The readers are redirected to in-depth discussion on role of renin angiotensin system and ACE-2 in pathophysiology of COVID-19 in published literature (D'Ardes et al., 2020; Guo et al., 2020; Park et al., 2020; Tolouian et al., 2020).
Fig. 3.
Renin-Angiotensin System (RAS) and SARS-Cov-2. In classis RAS system, ACE convert angiotensin I to angiotensin II. Angiotensin II is converted to Angiotensin 1-7 by ACE-2. SARS-Cov-2 binds with ACE-2 on the cell surface and internalizes via endocytosis carrying ACE-2 along, thereby essentially decreases ACE-2 availability. Decreases availability in ACE-2 strikes imbalance between Angiotensin-II and Angiotensin 1-7. ACE inhibitors or Angiotensin receptor blockers may have value in reversing the imbalance (Adapted from (Guo et al., 2020)).
The SARS-CoV-2 primarily affects lungs, heart, and gastrointestinal tract (the tissues which highly express ACE-2) (Terpos et al., 2020). There are concerns over theoretical increase in ACE-2 receptors and thereby increasing the risk of infection, however, the clinical evidence is not available (Guo et al., 2020; Park et al., 2020; South et al., 2020). Conversely, there is evidence that RAS inhibitors may actually have protective effect against severe COVID-19. Experimental models have suggested that angiotensin receptor blockers may mitigate angiotensin-II mediated acute lung injury in COVID-19 (South et al., 2020). Meta-analysis of clinical data of 511 COVID-19 patients showed that among the elderly (age>65) COVID-19 patients with existing hypertension, patients who were on angiotensin receptor blockers prior to Covid-19 had lower risk of severe disease compared to patients who were not on angiotensin receptor blockers (Liu et al., 2020d). With this observation, it is possible that increasing angiotensin-I through administration of recombinant version of angiotensin-I or by administration of angiotensin-I converting enzyme inhibitors or angiotensin receptor blockers might be beneficial in COVID-19. Table 6 enlists potential treatments affecting RAS and ongoing clinical trials of the same.
Table 6.
Therapeutics affecting RAS system.
| Drug Name | Mechanism of Action/Proposed mechanism in COVID-19 | Clinical Trail No. |
|---|---|---|
| Recombinant Bacterial ACE2 receptors -like enzyme | Attenuates acute lung failure | NCT04375046 |
| ACE inhibitor/Angiotensin receptor blocker | Upregulation of AT1,7; downregulation of AT-II; prevents virus entry into the host cell | NCT04353596, NCT04379310, NCT04330300, NCT04367883, NCT04345406, NCT04322786, NCT04364984 |
| Ramipril | ACE inhibitor, prevents virus entry into the host cells. | NCT04366050 |
| Captopril | ACE inhibitor, prevents virus entry into the host cells. | NCT04355429 |
| Losartan | Angiotensin receptor blocker, prevents virus entry into the host cells. | NCT04335123, NCT04312009, NCT04311177, NCT04340557, NCT04312009, NCT04311177, NCT04340557 |
| Telmisartan | Angiotensin receptor blocker, prevents virus entry into the host cells. | NCT04360551, NCT04355936 |
| Angiotensin 1-7 | Protective effect on endothelium, prevents virus entry into the host cells. | NCT04332666, NCT04375124, NCT04335136 |
2.8. Reactive oxygen species and oxidative stress in COVID-19
Increased influx of neutrophils and macrophages lead to increase in oxygen consumption at the site of infection. Combined with low oxygen supply (hypoxia), the imbalance in demand and supply immensely increases the number of reactive oxygen species at the site of infection (Zeitouni et al., 2016). This is further amplified by depletion of critical antioxidants such reduced glutathione (GSH) and NADPH (Erol, 2020; Spearow and Copeland, 2020). Increased oxidative stress is central to inflammation and thrombosis. The reactive oxygen species can lead to endothelial activation and subsequent formation of von Willebrand bodies which enhances platelet activation aggregation. The reactive oxygen species also reduces availability of nitric oxide (Krötz et al., 2004).
2.8.1. Antioxidants
Studies have shown that treatments that increase GSH level including N-acetyl cysteine (NAC) and bioavailable GSH preparations can reduce the severity of some influenza and coronavirus infections (Spearow and Copeland, 2020). Vitamin-C is a known antioxidant and cofactor for physiological reactions. High dose of Vitamin-C is being advocated to reduce the oxidative stress in COVID-19 (Erol, 2020). Similarly, Quercetin, a flavonoid found in fruits and vegetables, has been shown to have potent antioxidant effect (Anjaneyulu and Chopra, 2004), and may be useful to reduce oxidative stress.
Recently, Vitamin-D supplement has been proposed for the treatment of COVID-19 based on the observation that Vitamin-D supplement is safe and could be beneficial in preventing acute respiratory tract infections (Martineau et al., 2017; McCartney and Byrne, 2020). Moreover, Vitamin-D deficiency has been associated with higher mortality rate in COVID-19 patients (Ebadi and Montano-Loza, 2020). In a mice study, Vitamin-D deficiency was linked to o hepatic inflammation, at least in part, due to IL-6 (Labudzynskyi et al., 2016). Vitamin-D can also enhance the cellular immunity and promote anti-inflammatory cytokines by inducing regulatory T cells (Treg) (Grant et al., 2020b). While there is strong evidence that antioxidant and Vitamin-D supplements can be beneficial in COVID-19, the results for ongoing clinical trials should provide concrete information. The ongoing clinical trials of antioxidants and supplements have been summarized in Table 7 .
Table 7.
Antioxidant supplement in Covid-19.
| Drug Name | Mechanism of Action/Proposed mechanism in COVID-19 | Clinical Trail No. |
|---|---|---|
| N-acetylcysteine | Mucolytic agent and antioxidant precursor to glutathione (γ-glutamylcysteinylglycine; GSH). | NCT04374461, NCT04370288, NCT04279197 |
| Ergocalciferol, Vitamin D3 | Prophylactic, protective effect against respiratory infections. | NCT04385940, NCT04351490, NCT04366908, NCT04344041, NCT04372017, NCT04386850, NCT04334005, NCT04407286, NCT04394390 |
| Vitamin-C | Prophylactic, protective effect against respiratory infections. | NCT04334967, NCT04323514, NCT04363216, NCT04357782, NCT04344184, NCT04342728, NCT04347889, NCT04264533 |
| Quercetin | Prophylactic, antioxidant. | NCT04377789 |
3. Conclusion
Although new knowledge pertaining to COVID-19 is emerging every day, it has become clear that COVID-19 is complex disease involving interactions of respiratory system, cardiovascular system, and other major organs. The hyper inflammation and hypercoagulation associated with COVID-19 are major contributing factors toward the disease severity and mortality. Extensive research is being carried out by the research institutes and pharmaceutical industries evident from the number of ongoing clinical trials. As we await more data on the efficacy of ongoing clinical studies, hope remains that we overcome this pandemic and based on the lessons learned in COVID-19 pandemic, we prepare ourselves to prevent any future pandemic.
Authors contributions
All authors contributed equally to the preparation of the manuscript.
Declaration of competing interest
Authors declare no conflict of interest.
References
- Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., Sun C., Sylvia S., Rozelle S., Raat H., Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):1–12. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjaneyulu M., Chopra K. Quercetin, an antioxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2004;31(4):244–248. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S.M., Mubareka S., Lee W.L. The lung microvascular endothelium as a therapeutic target in severe influenza. Antivir. Res. 2013;99(2):113–118. doi: 10.1016/j.antiviral.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett C.D., Moore H.B., Yaffe M.B., Moore E.E. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: a Comment. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.-D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittmann S., Luchter E., Weissenstein A., Villalon G., Moschuring-Alieva E. TMPRSS2-Inhibitors play a role in cell entry mechanism of COVID-19: an insight into camostat and nefamostat. J Regen Biol Med. 2020;2(2):1–3. [Google Scholar]
- Blaising J., Lévy P.L., Polyak S.J., Stanifer M., Boulant S., Pécheur E.-I. Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antivir. Res. 2013;100(1):215–219. doi: 10.1016/j.antiviral.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (beijing) 2020. [DOI] [PMC free article] [PubMed]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.-C., Deng Q.-X., Dai S.-X. Travel Med Infect Dis; 2020. Remdesivir for Severe Acute Respiratory Syndrome Coronavirus 2 Causing COVID-19: an Evaluation of the Evidence; p. 101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R., editors. StatPearls. Treasure Island. StatPearls Publishing; (FL: 2020. Features, evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- Casey K., Iteen A., Nicolini R., Auten J. COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html accessed 26 June 2020.
- Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J. medRxiv; 2020. Favipiravir versus Arbidol for COVID-19: a Randomized Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Zhong X., Tang Y., Liang Y., Li B., Tao X., Liao C. 2020. The Outcomes of Hyperbaric Oxygen Therapy to Severe and Critically Ill Patients with COVID-19 Pneumonia. [Google Scholar]
- Cheng H., Wang Y., Wang G.-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. The mystery of the pandemic's ‘happy hypoxia’. Science. 2020;368(6490):455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- Danilczyk U., Penninger J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006;98(4):463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- D'Ardes D., Boccatonda A., Rossi I., Guagnano M.T., Santilli F., Cipollone F., Bucci M. COVID-19 and RAS: unravelling an unclear relationship. Int. J. Mol. Sci. 2020;21(8) doi: 10.3390/ijms21083003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Du Y.-X., Chen X.-P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- Ebadi M., Montano-Loza A.J. Perspective: improving vitamin D status in the management of COVID-19. Eur. J. Clin. Nutr. 2020 doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson T.B., Chai P.R., Boyer E.W. Chloroquine, hydroxychloroquine and COVID-19. Toxicology Communications. 2020;4(1):40–42. doi: 10.1080/24734306.2020.1757967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A. Orthomolecular Medicine News Service; 2020. High-dose Intravenous Vitamin C Treatment for COVID-19. [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N. Engl. J. Med. 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M.C., Geoghegan L., Arbyn M., Mohammed Z., McGuinness L., Clarke E.L., Wade R.G. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PloS One. 2020;15(6):e0234765. doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7):e016219. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D., Sahoo A.K., Singh A. Ivermectin: potential candidate for the treatment of Covid 19. Braz. J. Infect. Dis. 2020 doi: 10.1016/j.bjid.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi H., Abdaldayem E. The human-COVID-19 tango: connecting the dots. Preprints. 2020 doi: 10.20944/preprints202004.0160.v2. [DOI] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 2020;64(6):e00754–20. doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M., Yan W.-W., Chan W.-M., Chan J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L.J. Inhaled nitric oxide and COVID-19. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15085. [DOI] [Google Scholar]
- Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet Respiratory Medicine. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink D.N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ (Can. Med. Assoc. J.) 2020;192(17):E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A.C., Thomas P.G. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit. Care. 2019;23(1):258. doi: 10.1186/s13054-019-2539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krötz F., Sohn H.-Y., Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004;24(11):1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- Labudzynskyi D., Shymanskyy I., Veliky M. Role of vitamin D3 in regulation of interleukin-6 and osteopontin expression in liver of diabetic mice. Diabetes. 2016;37(2.12):22.1–24.4. [PubMed] [Google Scholar]
- Lang M., Som A., Mendoza D.P., Flores E.J., Reid N., Carey D., Li M.D., Witkin A., Rodriguez-Lopez J.M., Shepard J.-A.O. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzer J. Covid-19: US gives emergency approval to hydroxychloroquine despite lack of evidence. BMJ. 2020;369:m1335. doi: 10.1136/bmj.m1335. [DOI] [PubMed] [Google Scholar]
- Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg. Microb. Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemostasis. 2020;18(4):786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Trav. Med. 2020;27(2) doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Huang F., Xu J., Yang P., Qin Y., Cao M., Wang Z., Li X., Zhang S., Ye L., Lv J., Wei J., Xie T., Gao H., Xu K.-F., Wang F., Liu L., Jiang C. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.03.20.20039586. 2020.03.20.20039586. [DOI] [Google Scholar]
- Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- Luks A.M., Swenson E.R. Ann Am Thorac Soc; 2020. COVID-19 Lung Injury and High-Altitude Pulmonary Edema: A False Equation with Dangerous Implications. [DOI] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Hydroxychloroquine for covid-19: the end of the line? BMJ. 2020;369:m2378. doi: 10.1136/bmj.m2378. [DOI] [PubMed] [Google Scholar]
- Mahévas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C., Fois E., Lepeule R., Szwebel T.-A., Lescure F.-X., Schlemmer F., Matignon M., Khellaf M., Crickx E., Terrier B., Morbieu C., Legendre P., Dang J., Schoindre Y., Pawlotsky J.-M., Michel M., Perrodeau E., Carlier N., Roche N., Lastours V. de, Ourghanlian C., Kerneis S., Ménager P., Mouthon L., Audureau E., Ravaud P., Godeau B., Gallien S., Costedoat-Chalumeau N. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Stelmach I., Kumar G.T., Urashima M., Camargo C.A. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney D.M., Byrne D.G. Optimisation of vitamin D status for enhanced immuno-protection against covid-19. Ir. Med. J. 2020;113:58. [PubMed] [Google Scholar]
- Mcgonagle D., O’donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. The Lancet Rheumatology. 2020 doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehta N., Mazer-Amirshahi M., Alkindi N., Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monpara J., Velga D., Verma T., Gupta S., Vavia P. Cationic cholesterol derivative efficiently delivers the genes: in silico and in vitro studies. Drug Delivery and Translational Research. 2019;9(1):106–122. doi: 10.1007/s13346-018-0571-z. [DOI] [PubMed] [Google Scholar]
- Moore N. Chloroquine for COVID-19 infection. Drug Saf. 2020;1–2 doi: 10.1007/s40264-020-00933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/ accessed 26 June 2020. [PubMed]
- Oldfield V., Plosker G.L. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2006;66(9):1275–1299. doi: 10.2165/00003495-200666090-00012. [DOI] [PubMed] [Google Scholar]
- Oudkerk M., Büller H.R., Kuijpers D., van Es N., Oudkerk S.F., McLoud T.C., Gommers D., van Dissel J., Cate H. ten, van Beek E.J. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the national institute for public health of The Netherlands. Radiology. 2020:201629. doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., Leacy R.A. de, Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of covid-19 in the young. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliani U., Cardona A. COVID-19 and hydroxychloroquine: is the wonder drug failing? Eur. J. Intern. Med. 2020 doi: 10.1016/j.ejim.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee H.Y., Cho E.J., Sung K.C., Kim J., Kim D.-H., Ihm S.-H., Kim K.-i., Sohn I.-S., Chung W.-J., Kim H.C., Ryu S.K., Pyun W.B., Shin J. Is the use of RAS inhibitors safe in the current era of COVID-19 pandemic? Clin Hypertens. 2020;26(1):1–5. doi: 10.1186/s40885-020-00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Remy K.E., Brakenridge S.C., Francois B., Daix T., Deutschman C.S., Monneret G., Jeannet R., Laterre P.-F., Hotchkiss R.S., Moldawer L.L. Immunotherapies for COVID-19: lessons learned from sepsis. The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheim D. 2020. Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion. Available at SSRN 3636557. [Google Scholar]
- Schmith V.D., Zhou J.J., Lohmer L.R.L. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S. The third international consensus definitions for sepsis and septic shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Michel D., Chitanda J.M., Verrall R.E., Badea I. Evaluation of cellular uptake and intracellular trafficking as determining factors of gene expression for amino acid-substituted gemini surfactant-based DNA nanoparticles. J. Nanobiotechnol. 2012;10:7. doi: 10.1186/1477-3155-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020 doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearow J.L., Copeland L. OSF Preprints; 2020. Review: Improving Therapeutics for COVID-19 with Glutathione-Boosting Treatments that Improve Immune Responses and Reduce the Severity of Viral Infections. [DOI] [Google Scholar]
- Sriram K., Insel P.A. A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashita E. Influenza polymerase inhibitors: mechanisms of action and resistance. Cold Spring Harb Perspect Med. 2020 doi: 10.1101/cshperspect.a038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int. J. Cardiol. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020 doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemostasis. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolouian R., Zununi Vahed S., Ghiyasvand S., Tolouian A., Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J. Ren. Inj. Prev. 2020;9(2):e19. doi: 10.34172/jrip.2020.19. e19. [DOI] [Google Scholar]
- Valencia D.N. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12(3):e7386. doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubbaiah M., Dwarakanadha Reddy P., Satyanarayana S.V. Literature-based review of the drugs used for the treatment of COVID-19. Curr Med Res Pract. 2020;10(3):100–109. doi: 10.1016/j.cmrp.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein J.T., Powell M., Koenecke A., Xiong R., Fischer N., Huq S., Khalafallah A.M., Caffo B., Stuart E.A., Papadopoulos N., Kinzler K.W., Vogelstein B., Zhou S., Bettegowda C., Konig M.F., Mensh B., Athey S. Alpha-1 adrenergic receptor antagonists for preventing acute respiratory distress syndrome and death from cytokine storm syndrome. 2020. https://arxiv.org/pdf/2004.10117
- Wallace Dinsmore W., Wyllie Michael G. Vasoactive intestinal polypeptide/phentolamine for intracavernosal injection in erectile dysfunction. BJU Int. 2008;102(8):933–937. doi: 10.1111/j.1464-410X.2008.07764.x. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P., Dong N., Tong Q. medRxiv; China: 2020. Early, Low-Dose and Short-Term Application of Corticosteroid Treatment in Patients with Severe COVID-19 Pneumonia: Single-Center Experience from Wuhan. [Google Scholar]
- Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., Heer G. de, Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., Weerth A. de, Paschen H.-R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Weekly epidemiological update - 1. 19/August/2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports accessed 19 August, 2020.
- World Health Organization WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. 2020. https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 accessed 6 July 2020.
- Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., Kara T., Somers V.K. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin. Proc. 2020 doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Shen H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020;16(10):1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020;45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I., Granucci F., Broggi A. Interferon (IFN)-λ takes the helm: immunomodulatory roles of type III IFNs. Front. Immunol. 2017;8:1661. doi: 10.3389/fimmu.2017.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitouni N.E., Chotikatum S., Köckritz-Blickwede M. von, Naim H.Y. The impact of hypoxia on intestinal epithelial cell functions: consequences for invasion by bacterial pathogens. Mol Cell Pediatr. 2016;3(1):14. doi: 10.1186/s40348-016-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Lu J., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Teng Z., Yang L., Xu S., Liu J., Teng Y., Hao Q., Zhao D., Li X., Lu S., Zeng Y. 2020. Efficacy and Safety of Remdesivir for COVID-19 Treatment: an Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. [Google Scholar]
- Ziegler C.G., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020 doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]