Abstract
Background
Despite high efficacy of oral antiretroviral therapy (ART), viral suppression among adolescents and young adults (AYA) living with HIV in sub-Saharan Africa (SSA) remains low. Compared to daily oral ART, bimonthly long-acting injectable ART (LA-ART) may simplify adherence, improve clinical outcomes, and decrease HIV transmission in this priority population. However, LA-ART will likely cost more than oral ART and the cost threshold at which LA-ART will be cost effective in SSA has not been evaluated.
Methods
We adapted a mathematical model of HIV transmission and progression in Kenya to include HIV acquisition and viral suppression among AYA (age 10–24). We projected the population-level health and economic impact of providing LA-ART to AYA over a 10-year time horizon assuming oral ART costs of US$233 annually and a two-month duration of viral suppression per LA-ART injection. We calculated the maximum cost at which switching from oral to LA-ART would be considered cost-effective, using thresholds of $500 and $1,508 per disability-adjusted life year averted (WHO's threshold of HIV treatment interventions and Kenya's gross domestic product per capita).
Findings
Assuming 85% of AYA switch from oral to injectable formulations, LA-ART is estimated to prevent 40,540 infections and 20,480 deaths over 10 years. The maximum increase in the annual per-person cost of receiving LA-ART is estimated to be $89 and $236 for LA-ART to be cost-effective under the thresholds of $500 and $1,508 per DALY averted, respectively. The cost threshold was lower when non-adherent oral ART AYA users were assumed to be less likely to switch to LA-ART.
Interpretation
Providing LA-ART to AYA can be cost-effective in Kenya if it is less than twice the cost of oral ART. Long-acting injectable ART for priority populations with low viral suppression has the potential to cost-effectively avert disability and death.
Funding
National Institutes of Health (R01 HD085807; PI: Kohler)
Keywords: Adolescent, Young adult, Modeling, Long-acting ART, Kenya, Cost-effectiveness
Research in context.
Evidence before this study
Stemming the HIV epidemic will require increased coverage and adherence to antiretroviral treatment (ART). Adolescents and young adults (AYA) have lower ART adherence, and long-acting ART (LA-ART) is emerging as an effective treatment tool that may increase adherence in this population.
We searched PubMed for modeling analyses of long-acting antiretroviral therapy (LA-ART) on August 28, 2019 with the terms (model*) AND (HIV) AND (LA-ART OR long-acting ART OR long-acting antiretroviral* OR long-acting injectable*), with no date or language restrictions. Of the 17 papers identified, one used mathematical modeling to investigate long-acting antiretrovirals as HIV treatment. This study investigated the cost-effectiveness of LA-ART in the U.S. by estimating reductions in morbidity and mortality in the adult HIV-positive population, and found that LA-ART would be cost-effective only for patients with multiple ART failures. We found no studies modeling LA-ART in sub-Saharan Africa or in the AYA population.
Added value of this study
Our model focuses on providing LA-ART to the AYA population in Kenya, a likely priority population to receive LA-ART due to low adherence to oral regimens. In addition to estimating health benefits and costs for HIV-positive AYA receiving LA-ART, we include the effect of LA-ART in preventing new HIV infections to estimate a comprehensive cost-effectiveness threshold for LA-ART in this population.
Implications of all the available evidence
Administering LA-ART to AYA in Kenya may be cost-effective if LA-ART is less than double the current cost of administering oral ART. Prioritizing administration further to AYA who have difficulty taking daily pills, but can adhere to LA-ART, will increase the cost-effectiveness of LA-ART.
Alt-text: Unlabelled box
1. Introduction
Adolescents and young adults (AYA) ages 10–24 continue to be disproportionately burdened by the HIV epidemic. Globally, nearly one-third of new infections occur in young adults aged 15–24 years [1]. AYA in sub-Saharan African (SSA) experience particularly high HIV prevalence, accounting for 85% of all adolescents living with HIV [2]. Despite the high efficacy of oral antiretroviral therapy (ART) in preventing HIV-related morbidity and mortality, viral suppression among AYA living with HIV in SSA remains low [3], [4], [5], [6]. Sub-optimal adherence to ART is associated with poor clinical outcomes, including increased mortality [7], drug resistance [8], and onward transmission [9,10].
AYA face a number of biological and social barriers to adherence to oral ART, including cognitive development (poor impulse control and lack of future planning) [11] and susceptibility to HIV-related stigma [12]. Adolescents may skip clinic visits to avoid missing school and possibly disclosing their HIV status to teachers and peers [13]. Further barriers to adherence include poor engagement in care, inadequate support transitioning from pediatric to adult care, and not being informed of their HIV status by caregivers [14]. Strategies to increase ART adherence among AYA are needed. Long-acting pharmaceuticals have shown increased adherence compared to daily pills in prior studies of antipsychotics [15] and contraception [16]. Long-acting ART (LA-ART) is a promising potential intervention to overcome the need for daily adherence and improve clinical outcomes in this priority population.
Results from three recent trials among adults in high-income countries have demonstrated that LA-ART is equally effective as oral regimens [17], [18], [19]. A long-acting intramuscular injectable form of cabotegravir (CAB) in combination with long-acting rilpivirine (RPV) was effective as HIV treatment at both 4-week and 8-week durations in Phase 2 of the LATTE-2 trial (94% rate of viral suppression at 96 weeks) [20]. Similarly, preliminary results from the Phase 3 FLAIR [18] and ATLAS [19] trials found that 93–94% of treatment-naïve adults were virally suppressed after 48 weeks of monthly CAB/RPV injections.
Since these trials only included adults, studies on LA-ART among adolescents have been limited to interest surveys. In a study of U.S. AYA in HIV care, nearly 90% of those surveyed indicated a willingness to try injectable ART, with greater interest at higher viral loads [21]. Interest in taking a break from daily pills was measured in the BREATHER trial, in which some adolescents were randomized to a regimen that included weekends off from taking ART [22]. Adolescents expressed a strong preference for the weekends off regimen, with 98% of participants agreeing to participate in a two-year follow-up study [22].
Policymakers need to weigh the projected cost and benefits of LA-ART in order to make programmatic decisions. LA-ART will likely cost more than oral ART due in part to logistical challenges such as maintaining the transportation and storage cold chain, additional provider training and laboratory monitoring, and ensuring a steady supply [23]. The threshold at which LA-ART would be considered cost-effective has not been evaluated in SSA. To inform these decisions, we used a mathematical model to simulate HIV acquisition, transmission, and viral suppression among AYA in Kenya to assess the cost threshold at which switching from oral to LA-ART would be considered cost-effective. Our model, among the first adapted to an AYA population in SSA, addresses an urgent need to evaluate new interventions to increase ART adherence in this population.
2. Methods
2.1. Mathematical model
We adapted a previously published dynamic compartmental model of HIV transmission and progression to the setting of Kenya [24] as part of a cost-effectiveness evaluation of an intervention to improve AYA retention in care [25]. The population includes all Kenyans aged 0–59 years and is stratified by sex, five-year age group, sexual activity (low, medium, and high), and circumcision status. HIV natural history is modeled through five stages of viral load and five stages of CD4 count.
Sexual behavior is assumed to change both as individuals age and over time as the epidemic progresses. The model estimates the force of HIV infection as a function of sexual mixing (by age and sexual activity), proportion of HIV infected individuals, ART use, circumcision, and HIV transmission probability (based on number of sex acts per year and the probability of transmission per sex act). Although the LA-ART intervention is targeted to the AYA population, the full population (ages 0–59) is included in the model to account for the effects of sexual mixing between AYA and other age groups and to capture the population-level benefits of implementing LA-ART. Young adults (ages 15–24) are assumed to have the highest levels of sexual activity and are therefore at high risk of acquiring and transmitting HIV. The model allows HIV risk to be modified by male circumcision, pregnancy, and sexually transmitted disease coinfection. For further details, see the Supplemental Appendix.
The proportion of HIV-positive individuals receiving ART (oral or LA-ART) was assumed to increase from an average of 75% in 2017 [26] to an average of 81% by 2029, with no change in suppression rates over time. Individuals adherent to ART have a 96% reduction in transmission risk [27] and are assumed to have the same mortality rates as those who are HIV uninfected. Individuals who are non-adherent to ART are assumed to not be virally suppressed. Rates of non-adherence vary by age, sex, and CD4 count, with AYA having lower adherence than adults [28]. Of AYA currently on oral ART, on average 75% are estimated to be virally suppressed, compared to roughly 85% of adults aged 25 and older [28].
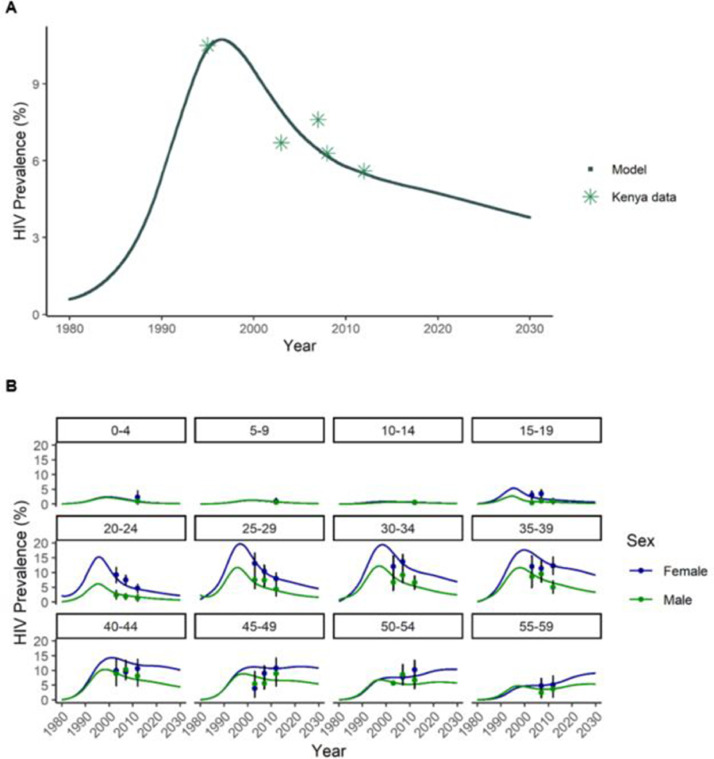
Changes in the population over time are estimated using a system of ordinary differential equations, approximated using discrete 0•05-year intervals. Before projecting the impact of the LA-ART intervention, the model was calibrated to fit the age-specific and overall HIV prevalence from Kenya by adjusting sexual activity and probability of HIV transmission per sex act (Fig. 1). Table 1 includes major inputs into the model; additional details about the model, parameters, and calibration results are available in the Supplemental Appendix. All analyses were conducted in R v3•5•1 [29].
Fig. 1.
(A) Comparison of modeled HIV prevalence for ages 15–49 to observed Kenya prevalence (for observed sources, see Supplemental Appendix). (B) Model calibration: Comparison of modeled age-specific HIV prevalence to the observed 2012 Kenya AIDS Indicator Survey (KAIS) prevalence (including restatement of 2003 and 2007 prevalence). KAIS error bars represent 95% confidence intervals.
Table 1.
Key model parameters.
| Proportion of PLHIV receiving ART[26] | |
| 2017 | 75% |
| 2029 | 81%a |
| Proportion on ART who are virally suppressed by age group[28] | |
| 0–3 | 56•9% |
| 3–10 | 65•5% |
| 10–20 | 63•4% |
| 20–30 | 81•4% |
| 30–60 | 86•7% |
| Costs of ART provision (per person-year) | |
| Antiretroviral drug cost [30] | $72 |
| Non-antiretroviral cost [31] | $161 |
| Costs of health-care for pre-ART patients (per person-year)[31] | |
| Non-antiretroviral cost | $155 |
| Disability Weights for HIV-positive[43] | |
| CD4 count >350 cells per μL (untreated)b | 0•078 |
| CD4 count >200–350 cells per μL (untreated) | 0•274 |
| CD4 count ≤200 cell per μL (untreated) | 0•582 |
| On antiretroviral therapy | 0•078 |
Assumes UNAIDS target of 90% aware of status, 90% on ART is reached by 2029.
HIV infection with a CD4 count of 350 cells per μL or greater was assumed to cause the same disability (0•078) as those receiving antiretroviral therapy.
2.2. Scenarios
The health and economic impact of switching from oral ART to LA-ART was evaluated under two scenarios (Table 2) with different proportions of AYA on ART who switch to LA-ART. Both scenarios assume 94% of LA-ART users are virally suppressed based on the LATTE-2 Phase II trial results [20]. Based on a U.S. survey [21], the “base” scenario assumes 85% of AYA on oral ART would switch to LA-ART with a two-month duration of viral suppression per injection. The second “lower uptake” scenario considers a 20-week oral ART induction period similar to those required in two of the current clinical trials of long-acting CAB/RPV [18,20]. If AYA who are currently non-adherent to their oral regimens are less likely to be virally suppressed at the end of the induction period, they will not be able to switch to LA-ART at the same rate. This “lower uptake” scenario assumes 85% of currently adherent AYA switch to LA-ART, but only 30% of non-adherent AYA successfully complete the induction period and switch, resulting in a total switch proportion of 71%.
Table 2.
Health Impacts and Maximum Cost-Effective LA-ART Administration Costa.
| Base | Lower Uptake | |
|---|---|---|
| Proportion of AYA on ART who switch to LA-ARTb | 85% | 71%c |
| Viral suppression of AYA on LA-ARTd | 94% | 94% |
| Overall (oral and long-acting) viral suppression of AYA ART users | 91% | 78% |
| HIV infections averted (% of estimated new infections) | 40,540 (4•5%) | 6807 (0•8%) |
| Deaths averted (% of all deaths) | 20,480 (0•9%) | 4178 (0•2%) |
| DALYs averted | 122,081 | 25,173 |
| Maximum incremental annual cost ($500 threshold) | $89 | $20 |
| Maximum incremental annual cost ($1508 threshold) | $236 | $56 |
Costs and health outcomes are captured over 10-year time horizon. Incremental costs and DALYs associated with each scenario are discounted at 3% annually. LA-ART intervention is added to current ART expansion. Costs are in 2017 USD.
85% of AYA on ART desiring to switch to LA-ART based on LA-ART interest survey in U.S.21.
30% of AYA who are not virally suppressed under oral ART, 85% who are suppressed under oral ART.
94% viral suppression based on LATTE-2 phase 2b trial20.
2.3. Cost-threshold analysis
We estimated the maximum incremental cost of LA-ART administered to AYA which would be considered cost-effective (i.e., having an incremental cost-effectiveness ratio less than the threshold). We assessed population-level effects of LA-ART over a 10-year time horizon. Health benefits and costs were discounted annually at 3%. Costs were inflated to 2017 U.S. dollars using the Kenya GDP deflator (ratio of current to constant price GDP) [26]. Total incremental LA-ART drug and administration costs were divided by the number of person-years on LA-ART under each scenario to determine the incremental cost of LA-ART per person-year. Oral ART drug costs were assumed to be $72 USD per year [30] (all costs for this study reported in 2017 USD). Non-drug costs including HIV-related health care for pre- and post-ART patients were taken from a study of comprehensive HIV treatment costs in Kenya [31]. The total annual per-person cost of ART administration, drug cost plus clinical services, was estimated to be $233.
For both scenarios, we calculated the maximum incremental cost of LA-ART compared to oral ART that would be considered cost-effective. We calculated the incremental cost-effectiveness ratio (ICER) per disability-adjusted life year (DALY) averted associated with LA-ART as the additional cost divided by the additional health benefit of LA-ART compared to oral ART only. We utilized two thresholds or cost-effectiveness: 1) $500 per DALY averted as recommended by the World Health Organization (WHO) for high priority HIV interventions [32], and 2) Kenya's 2017 gross domestic product (GDP) per capita ($1508) per DALY averted [33], following health economic convention.
2.4. Sensitivity analyses
We conducted one-way sensitivity analyses on the base scenario to determine the impact of key parameters on the maximum incremental cost. We varied the growth in the proportion of the HIV-positive population receiving ART by 2029 from 0% (75% coverage) to 15% (90% coverage); base case assumes 81% coverage. We adjusted the percentage of AYA who are non-adherent to oral ART by 0•8 to 1•2 times the base case non-adherence rates, which vary by age, and we tested the effects of lowering or raising the number of sexual partnerships in the AYA population. While keeping overall adherence levels the same, we varied oral and long-acting ART adherence by sexual risk group, with lowest adherence in the high-risk group. We extended the model time horizon from ten to 20 years. Two assumptions directly related to LA-ART coverage were also tested; we varied the rate of viral suppression under LA-ART from 85% to 100% and varied the percentage of AYA who switch to LA-ART from 40% to 100%.
Finally, as dolutegravir (DTG) usage expands, it may have an impact on ART adherence and average oral ART drug cost. Since DTG has better drug tolerance and a lower resistance profile [34], we conducted a sensitivity analysis to determine the effect on cost thresholds if current oral ART non-adherence rates decrease across the population with scale-up of more tolerable drug. In addition to evaluating a 20% decrease in oral ART dropout starting in 2019, we evaluated the equivalent increase. If scale-up of DTG does not meet expectations, costs may be higher, and if two-drug formulations are approved for use, costs may be lower. We therefore varied annual oral ART drug costs from $60 to $90.
2.5. Reporting guidelines
Our reporting of this study follows Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guidelines [35].
2.6. Role of the funding source
The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
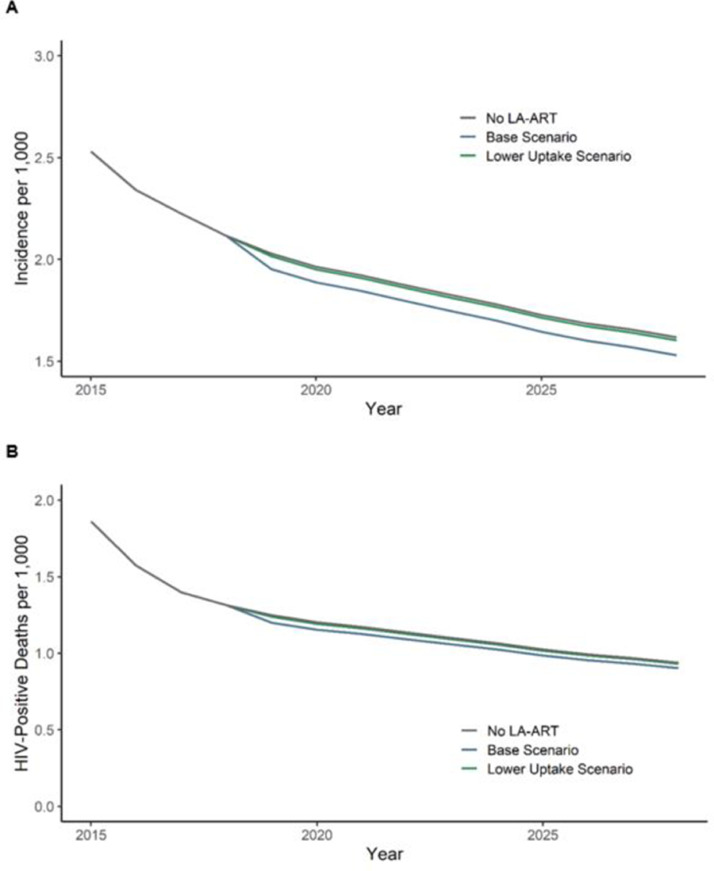
Our base scenario assumed 85% of AYA on oral ART switch to LA-ART, with a 94% viral suppression rate, resulting in an overall (oral and injectable) AYA viral suppression increase from 75% with only oral ART to 91% including LA-ART. In this scenario, we projected that LA-ART would avert 40,540 HIV infections and 20,480 HIV-related deaths over ten years compared to standard of care (oral ART) (Table 2, Fig. 2). To have an ICER below Kenya's per capita GDP, the annual per-person cost of LA-ART, including both drug injections six times per year and administration costs, can be at most $236 higher than oral ART administration ($233 per year). Using a cost-effectiveness threshold of $500, the maximum annual cost can be at most $89 higher than oral ART.
Fig. 2.
(A) Estimated reduction in new HIV infections per 1000 person-years due to LA-ART introduction under base and lower uptake scenarios. (B) Estimated reduction in mortality rate among HIV-positive persons over ten years due to LA-ART introduction under base and lower uptake scenarios.
In the lower uptake scenario, AYA who are not virally suppressed under oral regimens switch to LA-ART at a lower rate than those who are virally suppressed (30% vs. 85%) due to the requirement to maintain a 20-week oral ART induction period before starting LA-ART. In this scenario, overall AYA viral suppression would increase only slightly from 75% to 78%. To have an ICER below Kenya's per capita GDP in this lower uptake scenario, the annual per-person cost of LA-ART can be at most $56 higher than oral ART administration, or $289. Using a cost-effectiveness threshold of $500, the maximum annual cost can be at most $20 higher than oral ART, or $253.
3.1. Sensitivity analyses
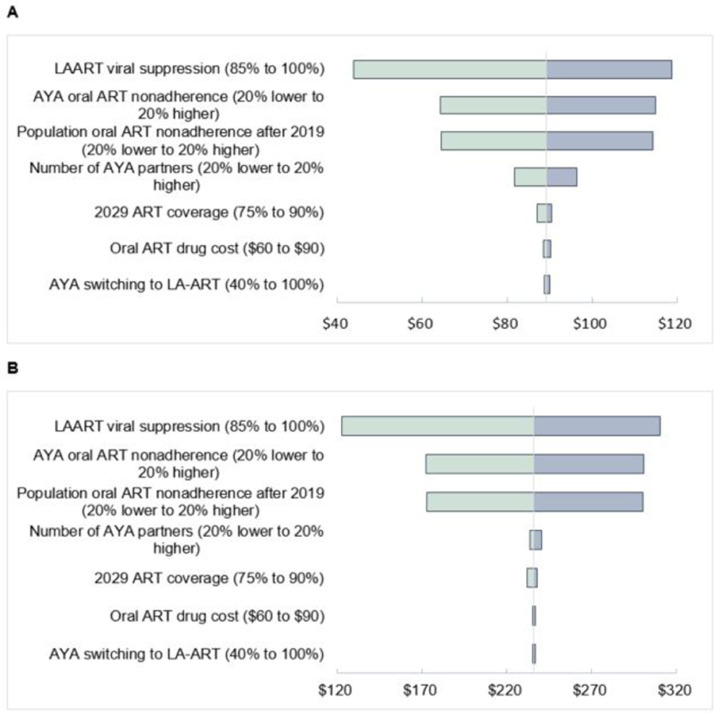
Fig. 3 shows tornado diagrams of the sensitivity of the maximum incremental LA-ART implementation cost for the $500 (3a) and $1508 (3b) ICERs to changes in the proportion of AYA who switch to LA-ART, oral ART drug cost, growth of oral ART coverage in the population, number of AYA sexual partnerships, proportion of population non-adherent to oral ART, proportion of AYA who are non-adherent to oral ART, and viral suppression rates under LA-ART. Maximum incremental costs were sensitive to LA-ART viral suppression and oral ART non-adherence rates, although results were qualitatively similar to the base scenario. Increasing the time horizon of LA-ART administration to 20 years roughly doubled the maximum incremental LA-ART cost to $185 and $461 under the $500 and $1508 ICER scenarios, respectively (not shown). Maximum LA-ART costs were robust to changes in the population-level increase in ART coverage by 2029, number of AYA sexual partnerships, or the percentage of AYA that switch to LA-ART, as changes in costs were mostly offset by changes in health benefits. Incremental LA-ART costs were also robust to changes in oral ART drug cost and variations in adherence by risk group (not shown).
Fig. 3.
(A) Sensitivity of annual LA-ART administration cost considered cost-effective using an ICER of US$500. Base case annual cost is US$89. (B) Sensitivity of annual LA-ART administration cost considered cost-effective using an ICER of $1508. Base case annual cost is US$236.
4. Discussion
This study uses a novel mathematical model to investigate one of the emerging questions about the new long-acting antiretroviral formulation. Our model-based analysis shows that providing LA-ART to AYA has the potential to be cost-effective for reducing HIV burden in Kenya if it is less than double the cost of oral ART. Assuming 85% of AYA on ART switch to LA-ART and 94% maintain viral suppression, the annual cost of LA-ART and administration could be approximately 25% higher than the cost for oral ART and still be considered cost-effective under a $500/DALY threshold. If the threshold for cost-effectiveness is Kenya's per capita GDP, LA-ART could be considered cost-effective at a cost of up to double the cost for oral ART.
Prior to this study, few mathematical models have been specifically tailored to the AYA population. LA-ART has only recently been developed and the projected impact has not been widely studied using mathematical modeling. A major strength of our study is that our model allows more precise investigations of questions relevant to AYA, assessing the circumstances under which LA-ART would be considered cost-effective. We note that to date, all clinical trials of LA-ART have been conducted in adult populations. Additional clinical studies are needed to establish efficacy and safety of LA-ART among AYA before LA-ART can be administered to this population.
We assumed 94% adherence for AYA taking LA-ART from the LATTE-2 Phase II trial conducted in high-income countries [20]. An increase from 75% viral suppression under oral ART to 94% viral suppression under LA-ART assumes that the requirement for daily adherence to pills is a main driver of nonadherence for AYA in SSA. If factors, including not being informed of one's HIV status, difficulty attending clinic visits due to stigma, inability to leave school or work, cost of travel, or poor engagement in care play a substantial role in nonadherence, then our analysis may overestimate the maximum cost at which LA-ART would be cost-effective. However, current studies for reaching persons with low adherence to oral ART include strategies to increase engagement in care and overcome barriers [36].
Results were most sensitive to assumptions about whether AYA who are not currently adherent to oral ART will be able to complete the oral induction period successfully to switch to LA-ART. For LA-ART to have maximum impact, administration should be prioritized for populations who struggle with adherence to oral ART mainly due to the requirement for daily pills. These populations may have difficulty completing the induction period on oral ART. Adequate drug supply, kind providers, transportation vouchers, and counseling during the induction period could improve the likelihood that AYA are able to successfully transition to LA-ART [37]. After the induction period, adherence counseling should be adapted to address barriers to LA-ART adherence. Spacing LA-ART injections with school break schedules could also improve adherence in the AYA population. Our results were sensitive to the assumed rate of viral suppression for AYA on LA-ART; as the rate approaches current oral ART suppression rates, the benefit of LA-ART declines. Although the cost threshold for LA-ART was not impacted by the proportion of AYA on ART who switched from oral to LA regimens, the clinical impact of LA-ART would be reduced if fewer AYA switched to LA-ART. If LA-ART is scaled up in Kenya, future surveys should monitor the number of AYA who are willing to switch treatment regimens.
A previous study examined the maximum drug cost of LA-ART considered cost-effective for adult HIV patients in the United States [38]. Using a willingness to pay threshold of $100,000 per quality-adjusted life-year (QALY) and assuming 91% LA-ART viral suppression, LA-ART would be considered cost-effective if it were roughly double the current oral ART regimen cost for those with multiple ART failures. In order for LA-ART to be considered cost-effective as a first- or second-line regimen, the cost would need to be similar to current oral ART costs. However, this model did not take into account the effect of LA-ART on averting HIV transmission, so the QALYs gained and the maximum cost were likely an underestimate. Our model captures the dynamics of HIV transmission, and therefore captures additional health benefits from preventing HIV infections.
Our study has several limitations. We did not simulate drug resistance due to non-adherence to ART regimens, and this should be evaluated in future studies. However, with the scale-up of DTG-based regimens, it is possible that drug resistance will greatly decline. Further, since AYA adherence is expected to be higher under LA-ART [12], it is possible that drug resistance using LA-ART will be lower than oral regimens. However, when long-acting ART is stopped, it is followed by a long tail of subtherapeutic drug levels which could lead to drug resistance. In order to combat this, individuals who discontinue LA-ART may need to take daily oral ART until the LA-ART drug concentration falls [39]. This could decrease the proportion of individuals who adopt LA-ART as their primary therapy strategy, as well as increase drug resistance for those who do not take oral ART after discontinuation of LA-ART. To our knowledge, no LA-ART trials are currently planned in AYA populations. We administered LA-ART only to AYA because we predicted that they may receive the most benefit. Although other age groups may also experience declines in morbidity and mortality from LA-ART, the cost-threshold for AYA is likely lower than other age groups. LA-ART administration may therefore be rolled out to this priority population first, reflecting our model assumptions. Our model results are specific to Kenya, but we expect our overall findings can be applicable to other settings in SSA with generalized HIV epidemics. Lastly, we have presented the results from the programmatic perspective, i.e. the Ministry of Health, and included only costs incurred or averted by the health system. However, the societal perspective would capture client preferences and the trade-offs they would be willing to make for the convenience of LA-ART. LA-ART will likely reduce costs to patients who will require fewer clinical visits, and using a societal perspective would therefore increase the cost threshold of LA-ART for AYA in Kenya.
When LA-ART becomes available in the market, it may be formulated for one-month or three-month durations, rather than the two-month duration we modeled. AYA have shown more interest in a three-month option compared to two-month [21], and in Kenya this would be better aligned with school break schedules and the shift to differentiated care [40]. This would increase the proportion of currently non-adherent AYA that switch to LA-ART and allow a higher maximum incremental cost. In addition to injectables, other long-acting formulations such as implants or microarray patch are possible that may have different durations and acceptability to patients [41,42]. A once-weekly formulation of oral ART may be a cost-effective alternative to injectable ART, but there is a lack of clinical data on adherence and programmatic costs. Future studies should consider these additional ART formulations in development once more research is available on adherence and effectiveness.
In summary, administering LA-ART to AYA in Kenya has the potential to avert thousands of HIV infections and HIV-related deaths. Costs may increase due to provider training and clinic logistical needs in addition to drug cost and transportation. For LA-ART to be cost-effective in the AYA population, it needs to be relatively low-cost (double current oral ART costs or less) Administration should be prioritized to those who are currently non-adherent to oral ART due to the need to take daily pills.
Author contributions
J.C., M.S., D.A.R., and R.V.B developed the mathematical model. J.C. ran all analyses and wrote the first draft of the paper. All authors discussed the results, contributed to the final manuscript, and approved the final version of the article for submission.
Declaration of Competing Interest
P.K.K. and J.C. report grants from NIH/NICHD during the conduct of the study.
Acknowledgments
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (R01 HD085807; PI: Kohler)
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100453.
Appendix. Supplementary materials
References
- 1.United Nations Joint Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2019. Published online 2019. Accessed May 1, 2020. https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf_aidsinfo.unaids.org.
- 2.Idele P., Gillespie A., Porth T. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(SUPPL. 2):S144–S153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 3.Ryscavage P.A., Anderson E.J., Sutton S.H., Reddy S., Taiwo B. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr. 2011;58(2):193–197. doi: 10.1097/QAI.0B013E31822D7564. [DOI] [PubMed] [Google Scholar]
- 4.Ferrand R.A., Briggs D., Ferguson J. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Trop Med Int Health. 2016;21(3):325–333. doi: 10.1111/tmi.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherutich P., Kim A.A., Kellogg T.A. Detectable HIV Viral Load in Kenya: data from a Population-Based Survey. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0154318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukui I.N., Ng'Ang'A L., Williamson J. Rates and predictors of non-adherence to antiretroviral therapy among HIV-positive individuals in Kenya: results from the second Kenya AIDS indicator survey, 2012. PLoS ONE. 2016;11(12) doi: 10.1371/journal.pone.0167465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García de Olalla P., Knobel H., Carmona A., Guelar A., López-Colomés J.L., Caylà J.A. Impact of Adherence and Highly Active Antiretroviral Therapy on Survival in HIV-Infected Patients. JAIDS J Acquir Immune Defic Syndr. 2002;30(1):105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 8.Sethi A.K., Celentano D.D., Gange S.J., Moore R.D., Gallant J.E. Association between Adherence to Antiretroviral Therapy and Human Immunodeficiency Virus Drug Resistance. Clin Infect Dis. 2003;37(8):1112–1118. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 9.Cohen M.S., Chen Y.Q., McCauley M. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanser F., Bärnighausen T., Grapsa E., Zaidi J., Newell M.L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal. Science (80-) 2013;339(6122):966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartley C.A., Somerville L.H. The neuroscience of adolescent decision-making. Curr Opin Behav Sci. 2015;5:108–115. doi: 10.1016/j.cobeha.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettifor A., Stoner M., Pike C., Bekker L-G. Adolescent lives matter. Curr Opin HIV AIDS. 2018;13(3):1. doi: 10.1097/coh.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanoni B.C., Sibaya T., Cairns C., Haberer J.E. Barriers to Retention in Care are Overcome by Adolescent-Friendly Services for Adolescents Living with HIV in South Africa: a Qualitative Analysis. AIDS Behav. 2019;23(4):957–965. doi: 10.1007/s10461-018-2352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adejumo O.A., Malee K.M., Ryscavage P., Hunter S.J., Taiwo B.O. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review. J Int AIDS Soc. 2015;18(1) doi: 10.7448/IAS.18.1.20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilon D., Tandon N., Lafeuille M.-.H. Treatment Patterns, Health Care Resource Utilization, and Spending in Medicaid Beneficiaries Initiating Second-generation Long-acting Injectable Agents Versus Oral Atypical Antipsychotics. Clin Ther. 2017;39(10) doi: 10.1016/J.CLINTHERA.2017.08.008. 1972-1985.e2. [DOI] [PubMed] [Google Scholar]
- 16.Winner B., Peipert J.F., Zhao Q. Effectiveness of long-acting reversible contraception. Obstet Gynecol Surv. 2012;67(9):552–553. doi: 10.1097/01.ogx.0000421455.21771.a1. [DOI] [PubMed] [Google Scholar]
- 17.Margolis D.A., Gonzalez-Garcia J., Stellbrink H.J. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 18.Orkin C., Keikawus A., Hernandez-Mora M.G. Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI) 2019. Long-Acting Cabotegravir + Rilpivirine for HIV Maintenance: FLAIR Week 48 Results.http://www.croiconference.org/sessions/long-acting-cabotegravir-rilpivirine-hiv-maintenance-flair-week-48-results [Google Scholar]
- 19.Swindells S., Andrade-Villanueva J.-.F., Richmond G.J. Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI). 2019. Long-Acting Cabotegravir + Rilpivirine as maintenance Therapy: ATLAS Week 48 Results.http://www.croiconference.org/sessions/long-acting-cabotegravir-rilpivirine-maintenance-therapy-atlas-week-48-results [Google Scholar]
- 20.Margolis D.A., Gonzalez-Garcia J., Stellbrink H.-.J. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 21.Weld E.D., Rana M.S., Dallas R.H., et al. Interest of Youth Living with HIV in Long-Acting Antiretrovirals. JAIDS J Acquir Immune Defic Syndr. Published online 2018:1. doi:10.1097/QAI.0000000000001896 [DOI] [PMC free article] [PubMed]
- 22.Butler K., Inshaw J., Ford D. BREATHER (PENTA 16) short-cycle therapy (SCT) (5 days on/2 days off) in young people with chronic human immunodeficiency virus infection: an open, randomised, parallel-group phase II/III trial. Health Technol Assess (Rockv) 2016;20(49):1–107. doi: 10.3310/hta20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havlir D., Gandhi M. Implementation challenges for long-acting antivirals as treatment. Curr Opin HIV AIDS. 2015;10(4):282–289. doi: 10.1097/COH.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma M., Farquhar C., Ying R. Modeling the Cost-Effectiveness of Home-Based HIV Testing and Education (HOPE) for Pregnant Women and Their Male Partners in Nyanza Province, Kenya. JAIDS J Acquir Immune Defic Syndr. 2016;72:S174–S180. doi: 10.1097/QAI.0000000000001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson K.S., Mugo C., Bukusi D. Simulated patient encounters to improve adolescent retention in HIV care in Kenya: study protocol of a stepped-wedge randomized controlled trial. Trials. 2017;18(1):619. doi: 10.1186/s13063-017-2266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Bank Development Indicators. Accessed December 16, 2017. https://data.worldbank.org/country/kenya
- 27.Cohen M.S., Chen Y.Q., McCauley M. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mwau M., Syeunda C.A., Adhiambo M. Scale-up of Kenya's national HIV viral load program: findings and lessons learned. PLoS ONE. 2018;13(1) doi: 10.1371/journal.pone.0190659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Core Team R. R: a Language and Environment for Statistical Computing. Published online. 2019 https://www.r-project.org/ [Google Scholar]
- 30.Clinton Health Access Initiative. HIV Market Report.; 2018. Accessed December 11, 2018. https://clintonhealthaccess.org/content/uploads/2018/09/2018-HIV-Market-Report_FINAL.pdf
- 31.U.S. Centers for Diseases Control and Kenya Ministry of Health. The Cost of Comprehensive HIV Treatment in Kenya. Report of a Cost Study of HIV Treatment Programs in Kenya.; 2013.
- 32.Phillips A.N., Venter F., Havlir D. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6(2):e116–e127. doi: 10.1016/S2352-3018(18)30317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization; 2001. Macroeconomics and health investing in health for economic development : report of the commission on macroeconomics and health. [Google Scholar]
- 34.WHO. WHO recommends dolutegravir as preferred HIV treatment option in all populations. Published2019. https://www.who.int/news-room/detail/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations
- 35.Husereau D., Drummond M., Petrou S. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Heal. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 36.The LATITUDE Study: Long-Acting Therapy to Improve Treatment SUccess in Daily LifE. Accessed August 21, 2019. https://clinicaltrials.gov/ct2/show/NCT03635788
- 37.Cluver L., Pantelic M., Toska E. STACKing the odds for adolescent survival: health service factors associated with full retention in care and adherence amongst adolescents living with HIV in South Africa. J Int AIDS Soc. 2018;21(9) doi: 10.1002/jia2.25176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross E.L., Weinstein M.C., Schackman B.R. The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis. 2015;60(7):1102–1110. doi: 10.1093/cid/ciu1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boffito M., Jackson A., Owen A., Becker S. New approaches to antiretroviral drug delivery: challenges and opportunities associated with the use of long-acting injectable agents. Drugs. 2014;74(1):7–13. doi: 10.1007/s40265-013-0163-7. [DOI] [PubMed] [Google Scholar]
- 40.Grimsrud A., Bygrave H., Doherty M. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression: the. J Int AIDS Soc. 2016;19(1) doi: 10.7448/IAS.19.1.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovarova M., Benhabbour S.R., Massud I. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun. 2018;9(1):4156. doi: 10.1038/s41467-018-06490-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mc Crudden M.T.C., Larrañeta E., Clark A. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J Control Release. 2018;292:119–129. doi: 10.1016/j.jconrel.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salomon J.A., Haagsma J.A., Davis A. Disability weights for the Global Burden of Disease 2013 study. Artic Lancet Glob Heal. 2015;3:712–735. doi: 10.1016/S2214-109X(15)00069-8. www.thelancet.com/lancetgh Accessed July 10, 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.