Abstract
Introduction
In the Mayo Imaging Classification (MIC) for autosomal dominant polycystic kidney disease (ADPKD), the height-adjusted total kidney volume (HtTKV) growth rate is estimated for classification. Estimated HtTKV slope, termed as eHTKV-α, is calculated by the equation [HtTKV at age t] = K(1+α/100)(t-A), where K = 150 and A = 0 are used in MIC. If eHTKV-α is nearly stable during a standard-of-care period, the change in eHTKV-α from baseline can be used for estimation of the treatment effect on the HtTKV slope.
Methods
The constancy of eHTKV-α (A = 0 and K = 150) was evaluated using 453 placebo-assigned subjects in the Tolvaptan Efficacy and Safety in Management of ADPKD and Its Outcomes (TEMPO) 3:4 trial. A and K were sought out respectively by a converged pattern of regression lines of log10(HtTKV) plotted against age for subgroups divided according to MIC, and by change in eHTKV-α from baseline. A total of 239 standard-of-care patients from the Kyorin University Cohort (KUC) served as validation. Changes in eHTKV-α from baseline were evaluated in 809 tolvaptan-treated subjects in TEMPO 3:4.
Results
In placebo-assigned subjects, eHTKV-α (A = 0 and K = 150) changed significantly from baseline at the third year. As regression lines of placebo-assigned subgroups converged around age 0, A was set as 0, which was confirmed by KUC. K = 130 was selected because of minimal change in eHTKV-α from baseline. The KUC validated the constancy of eHTKV-α (A = 0 and K = 130) but not that of eHTKV-α (A=0 and K=150). In tolvaptan-treated subjects, eHTKV-α remained significantly lower than baseline for 3 years.
Conclusions
eHTKV-α (A = 0 and K = 130) was nearly stable from baseline through follow-up in standard-of-care adults. Treatment effects on the HtTKV slope can be estimated by changes in eHTKV-α from baseline.
Keywords: autosomal dominant polycystic kidney disease (ADPKD), biomarker, height-adjusted total kidney volume (HtTKV), total kidney volume (TKV), tolvaptan
See Commentary on Page 1383
ADPKD is a common hereditary kidney disease characterized by the formation and enlargement of renal cysts and deterioration in kidney function, leading to end-stage renal disease in half of patients by age 60 years.1 As a result of cyst development, total kidney volume (TKV) increases continuously, and TKV enlargement typically precedes decline in renal function.2, 3, 4, 5, 6 Hence, TKV has been approved by the European Medicines Agency and the US Food and Drug Administration as a prognostic biomarker in ADPKD7 and is used as an outcome measure in clinical trials conducted in this population.8, 9, 10
Change in TKV is usually calculated by capturing measurements at 2 or more time points. The TKV growth rate estimated by using 1 TKV observation and age is more stable than the TKV growth rate estimated by using 2 TKV observations, because the TKV measurement error is spread to 20 or more years in the former case but the error is spread to only 1 or 2 years in the latter case.11
In the prospective, placebo-controlled TEMPO 3:4 trial, a mixed-model repeated-measures analysis (MMRM) was applied to reveal a treatment benefit in reducing the TKV growth rate.8 This study aimed to estimate TKV growth rate using only 1 TKV observation and the age when the TKV was observed. In medical practice, it is necessary to estimate a patient’s TKV growth rate when the patient comes to a visit with a TKV observation, and patients do not need to wait half a year or more to measure the baseline TKV growth rate. Treatment effect is estimated conventionally by the difference of the TKV growth rate between baseline and postbaseline that are estimated by each single measurement of TKV.
The MIC was developed as a prediction model for renal prognosis in adult patients with ADPKD.12 Patients are stratified by HtTKV–estimated annual growth rate α (%/yr, termed eHTKV-α) which is derived from an equation HtTKVt =150(1+α/100)t, where HtTKVt is HtTKV at age t.
In the MIC, a classification chart is applied to determine the eHTKV-α in any individual at an arbitrary adult age. The rationale for this method is based on the assumption that eHTKV-α is individually stable. If eHTKV-α is stable in untreated patients, the change in eHTKV-α from baseline can be used for the estimation of individual treatment effects on HtTKV. However, eHTKV-α used in the MIC has not yet been shown to be stable over years.
Mathematical analysis of the pattern of cyst development by the Consortium of Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) indicated that the total cyst volume increases at an exponential-like rate as a summative reflection of the formation and enlargement of individual cysts.4 Other mathematical growth models, such as the logistic and Gompertz curve models, simulate limited or sigmoid growth patterns. Because the observed TKV growth pattern is exponential and unlimited,2,4,6 limited growth models are not adopted in the MIC and are not considered in this study.
An exponential TKV growth model is expressed by the following equation: HtTKVt = K(1+α/100)(t-A), in which K is the initial HtTKV and A is the age at the start of HtTKV growth.
Using imaging data from subjects assigned to placebo in TEMPO 3:4, constancy of eHTKV-α derived from A = 0 and K = 150 used in the MIC was examined. As eHTKV-α (A = 0 and K = 150) was not stable, appropriate equation parameters A and K were sought. The KUC was used for validation of A and K. Changes in eHTKV-α from baseline to postbaseline were evaluated to examine the treatment effect on the HtTKV growth rate for tolvaptan-treated subjects in TEMPO 3:4.
Materials and Methods
Study Design
This retrospective analysis was designed to examine the constancy of eHTKV-α used in the MIC model. If eHTKV-α was found to be unstable, appropriate equation parameters A and K were sought out in the equation HtTKVt = K(1+α/100)(t-A).
The equation parameters A and K were determined to make eHTKV-α stable from baseline through the follow-up years using data from subjects assigned to placebo in TEMPO 3:4 (development set) and were validated using the KUC (validation set) (Figure 1). The eHTKV-α derived from the equation developed in this study was applied to tolvaptan-treated subjects in TEMPO 3:4 (application set).
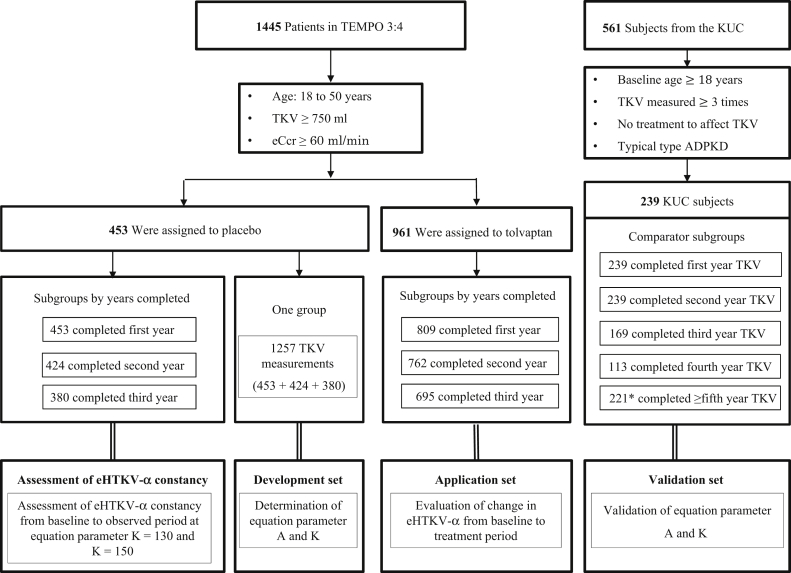
Figure 1.
The constancy of estimated height-adjusted total kidney volume growth rate (α) (eHTKV-α) (A = 0 and K = 150) was examined using 3 subgroups of 453 placebo-assigned subjects in the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 trial. A total of 1257 total kidney volume (TKV) measurements from 453 placebo-assigned subjects were used as a development set for eHTKV-α equation parameters A and K. A total of 239 patients from the Kyorin University Cohort (KUC) served as a validation set. The eHTKV-α derived from the present study was applied to tolvaptan-treated subjects in TEMPO 3:4. ADPKD, autosomal dominant polycystic kidney disease; eCcr, estimated creatinine clearance. ∗Number of TKV measurements.
The Kyorin University Institutional Review Board approved the study, and all participants provided informed consent.
Study Participants
The TEMPO 3:4 trial was a prospective, randomized, double-blind, placebo-controlled, 3-year trial in 1445 patients with ADPKD of typical presentation, aged 18 to 50 years, with TKV >750 ml and estimated creatinine clearance >60 min/min. Subjects were assigned to tolvaptan (961 patients), a V2-receptor antagonist, or placebo (484 patients). Tolvaptan dosing started at a daily split dose of 45 mg/15 mg and increased weekly to 60/30 mg and 90/30 mg as tolerated. After the titration period, patients were asked to remain on the highest tolerated dose, with dose change permitted if not tolerated.8,13
A total 239 patients were selected from 561 subjects registered to the KUC by the end of 2018. Exclusion criteria were baseline age <18 years, TKV measured ≤2 times, treatment with tolvaptan, or surgical intervention that would affect kidney volume, and ADPKD with atypical presentation.
Data Collection and Measurements
In TEMPO 3:4, evaluations were performed at baseline, randomization, week 3 during the titration phase, every 4 months during treatment, and twice after completion of treatment at 36 months. The TKV was assessed using a standardized protocol for magnetic resonance imaging without the use of contrast medium at baseline and months 12, 24, and 36 (±2 weeks) or at early withdrawal (±2 weeks).13 In the KUC, TKV was measured approximately once a year by magnetic resonance imaging using the same standard method since 2007.6
Development of Exponential Equation Parameters
Based on the observed exponential and unlimited TKV growth pattern,2,4,6 the MIC model and this study hypothesized that the equation HtTKVt = K x (1+α/100) (t-A) describes the relationship between HtTKVt, age (t) and eHTKV-α (α). For calculation of eHTKV-α, age was measured to the second decimal place.
Determination of A
The equation assumes that HtTKV starts to increase at age A. As there is not enough data of HtTKV growth pattern before adolescence, and as the HtTKV growth pattern is a mixture of heterogeneous HtTKV growth rate,12 age A is sought by the following method. The development set was divided into subgroups according to the MIC. The point at which log10(HtTKV) regression lines converge for the most subgroups was regarded as an approximate common starting age of HtTKV growth.
Determination of K
After determining parameter A, eHTKV-α was calculated for a different K using 1257 TKV measurements from the development set. An appropriate K for the equation was determined at the value that resulted in the smallest changes in eHTKV-α from baseline to postbaseline. In addition, an appropriate K was calculated differently using changes in eHTKV-α from baseline to postbaseline of 3-year subgroups, and the mean of 3 K-values was compared with the K-value determined by 1257 TKV measurements as 1 group.
Validation of A, K, and Constancy of eHTKV-α
The validation set of the KUC was divided into subgroups according to the MIC. The convergence pattern of regression lines of log10(HtTKV) against age was analyzed to validate A.
K was validated using a validation set by a method similar to what with which K was determined.
K-values derived from changes in eHTKV-α from the beginning to the end of each year of the development set and the KUC validation set were compared with determined appropriate K.
After validation of A and K, constancy of eHTKV-α from baseline to postbaseline years was validated using 5 comparator subgroups of the KUC (Figure 1).
Application of eHTKV-α
Changes in eHTKV-α, calculated using developed equation parameters A and K, from baseline to post-baseline years were analyzed by paired t test using data from tolvaptan-treated subjects of TEMPO 3:4 (Figure 1).
In addition, eHTKV-α was compared between known risk factor groups (i.e., male vs. female, and hypertensive vs. normotensive), using baseline data of 1445 participants of TEMPO 3:4.
Statistical Analyses
Normally distributed variables are expressed as mean ± SE. Differences between groups were tested using the χ2 test for categorical variable and a general linear model with covariates (year, dose, and MIC subgroup) as factors for continuous variables. Changes in eHTKV-α from baseline within individual patients were derived from the paired t test. Changes in eHTKV-α from baseline to different follow-up years were compared using MMRM with fixed effects of visit as a factor.
Analyses on TEMPO 3:4 and KUC data were performed using SAS 9.4 (SAS Institute, Cary, NC) and JMP Pro 14.3.0 (SAS Institute), respectively. A 2-sided P value of <0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics of Subjects in TEMPO 3:4 and KUC
The TEMPO 3:4 analysis population consisted of 453 placebo-assigned and 809 tolvaptan-treated subjects who completed TKV measurements (Table 1). The KUC subjects consisted of 239 subjects with mean TKV-measuring period (±SD) 4.11 ± 2.59 years (95% upper and lower of mean value were 4.44 and 3.78 years, respectively) (Table 2).
Table 1.
Baseline clinical characteristics of participants of TEMPO 3:4 trial
| Characteristic | 3 Tolvaptan subgroups divided by follow-up years |
|||
|---|---|---|---|---|
| Placebo group |
First year completed |
Second year completed |
Third year completed |
|
| (n = 453) | (n = 809) | (n =762) | (n = 695) | |
| Male sex, n (%) | 234 (51.7) | 421 (52.0) | 405 (53.2) | 365 (52.5) |
| Age, yr | 39.08 ± 0.34 | 38.82 ± 0.24 | 38.89 ± 0.25 | 38.90 ± 0.26 |
| Height, cm | 173.43 ± 0.45 | 173.50 ± 0.36 | 173.70 ± 0.38 | 173.55 ± 0.39 |
| Weight, kg | 77.94 ± 0.83 | 79.22 ± 0.63 | 79.36 ± 0.65 | 78.80 ± 0.67 |
| Systolic blood pressure, mm Hg | 128.46 ± 0.63 | 128.36 ± 0.48 | 128.43 ± 0.49 | 128.25 ± 0.52 |
| Diastolic blood pressure, mm Hg | 82.48 ± 0.44 | 82.32 ± 0.34 | 82.37 ± 0.36 | 82.28 ± 0.38 |
| TKV, ml | 1677.0 ± 41.6 | 1718.6 ± 32.1 | 1715.5 ± 32.8 | 1683.8 ± 32.9 |
| HtTKV, ml/m | 963.6 ± 23.0 | 987.3 ± 18.0 | 984.0 ± 18.3 | 967.3 ± 18.4 |
| eHTKV-α, %/yra | 5.196 ± 0.078 | 5.246 ± 0.054 | 5.221 ± 0.055 | 5.190 ± 0.058 |
| eGFR, ml/min per 1.73 m2b | 81.04 ± 1.06 | 76.02 ± 0.74 | 76.19 ± 0.76 | 76.14 ± 0.79 |
eGFR, estimated glomerular filtration rate; eHTKV-α, estimated HtTKV growth rate (α); HtTKV, height-adjusted TKV; TEMPO, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes; TKV, total kidney volume.
Data for continuous variables are expressed as mean ± SE.
The eHTKV-α was calculated using K = 130 ml/m and A = 0 year.
The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation adjusted for race.
Table 2.
Baseline clinical characteristics of the Kyorin University Cohort
| Characteristic | Comparator subgroups |
||||
|---|---|---|---|---|---|
| From baseline to first year |
From baseline to second year |
From baseline to third year |
From baseline to fourth year |
From baseline to after fourth year |
|
| (n = 239) | (n = 239) | (n = 169) | (n = 113) | (N = 221)a | |
| Male sex, n () | 98 (41.0) | 98 (41.0) | 63 (37.3) | 40 (35.4) | 71 (32.1) |
| Age, yr | 46.06 ± 0.85 | 46.06 ± 0.85 | 46.29 ± 1.01 | 47.24 ± 1.24 | 46.18 ± 0.89 |
| TKV measurement interval after baseline, yr | 1.12 ± 0.07 | 2.06 ± 0.07 | 3.05 ± 0.08 | 4.21 ± 0.10 | 6.55 ± 0.07 |
| Height, cm | 163.98 ± 0.57 | 163.87 ± 0.57 | 162.85 ± 0.68 | 162.28 ± 0.84 | 162.24 ± 0.60 |
| Weight, kg | 60.26 ± 0.77 | 60.26 ± 0.77 | 59.14 ± 0.92 | 58.47 ± 1.12 | 58.75 ± 0.80 |
| Hypertension present, n (%) | 176 (73.6 ) | 176 (73.6) | 128 (75.7) | 90 (79.7) | 178 (80.5) |
| Anti-hypertension treatment, n (%) | 170 (71.1) | 171 (71.1) | 125 (74.0) | 89 (78.8) | 175 (79.2) |
| TKV, ml | 1493 ± 55 | 1493 ± 55 | 1475 ± 65 | 1473 ± 79 | 1299 ± 57 |
| HtTKV, ml/m | 908 ± 33 | 908 ± 33 | 904 ± 40 | 907 ± 48 | 799 ± 35 |
| eHTKV-α, %/yrb | 4.169 ± 0.095 | 4.169 ± 0.095 | 4.118 ± 0.114 | 4.035 ± 0.139 | 3.930 ± 0.099 |
| eGFR, ml/min per 1.73 m2c | 66.13 ± 1.71 | 66.13 ± 1.71 | 65.84 ± 2.04 | 64.11 ± 2.49 | 65.63 ± 1.78 |
eGFR, estimated glomerular filtration rate; HtTKV, height-adjusted TKV; eHTKV-α, estimated HtTKV growth rate (α); TKV, total kidney volume.
Data for continuous variables are expressed as mean ± SE.
N = 221 is the total number of subjects (equal to the total number of TKV measurements).
The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation adjusted for race.
The eHTKV-α was calculated using K = 130 ml/m and A = 0 year.
Assessment of eHTKV-α (A = 0 Year and K = 150 ml/m) Constancy From Baseline
Changes in the eHTKV-α (A = 0 and K = 150) from baseline to the year of follow-up were assessed using the placebo-assigned subjects in TEMPO 3:4. The eHTKV-α changed significantly (P = 0.0016) from baseline to the third year (Table 3).
Table 3.
Changes in eHTKV-α (% per year) from baseline to each year of follow-up
| Follow-up year | n | eHTKV-α |
Change in eHTKV-α from baseline |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Follow-up year |
Mean | SE | P valuea | ||||
| Mean | SE | Mean | SE | |||||
| Results of A = 0 and K = 150 using placebo-assigned subjects in TEMPO 3:4 | ||||||||
| First year | 453 | 4.794 | 0.075 | 4.79 | 0.075 | –0.004 | 0.011 | 0.7021 |
| Second year | 424 | 4.763 | 0.076 | 4.788 | 0.076 | 0.025 | 0.012 | 0.0705 |
| Third year | 380 | 4.781 | 0.08 | 4.839 | 0.081 | 0.057 | 0.018 | 0.0016 |
| Results of A = 0 and K = 130 using placebo-assigned subjects in TEMPO 3:4 | ||||||||
| First year | 453 | 5.196 | 0.078 | 5.181 | 0.078 | –0.016 | 0.012 | 0.1801 |
| Second year | 424 | 5.164 | 0.079 | 5.169 | 0.079 | 0.004 | 0.014 | 0.8009 |
| Third year | 380 | 5.182 | 0.084 | 5.208 | 0.084 | 0.026 | 0.018 | 0.1480 |
| Validation of A = 0 and K = 150 using KUC | ||||||||
| First year | 239 | 3.819 | 0.095 | 3.845 | 0.095 | 0.026 | 0.014 | 0.0655 |
| Second year | 239 | 3.819 | 0.096 | 3.847 | 0.096 | 0.027 | 0.017 | 0.1025 |
| Third year | 169 | 3.774 | 0.111 | 3.814 | 0.111 | 0.04 | 0.021 | 0.054 |
| Fourth year | 113 | 3.693 | 0.133 | 3.773 | 0.133 | 0.081 | 0.033 | 0.0165 |
| After fourth year | 221b | 3.58 | 0.086 | 3.641 | 0.09 | 0.062 | 0.03 | 0.0400 |
| Validation of A = 0 and K = 130 using KUC | ||||||||
| First year | 239 | 4.173 | 0.099 | 4.19 | 0.098 | 0.019 | 0.014 | 0.2632 |
| Second year | 239 | 4.173 | 0.099 | 4.168 | 0.099 | 0.013 | 0.017 | 0.584 |
| Third year | 169 | 4.124 | 0.115 | 4.138 | 0.114 | 0.02 | 0.022 | 0.4747 |
| Fourth year | 113 | 4.035 | 0.137 | 4.084 | 0.137 | 0.048 | 0.033 | 0.1418 |
| After fourth year | 221b | 3.93 | 0.09 | 3.943 | 0.093 | 0.013 | 0.03 | 0.6647 |
| Application of A = 0 and K = 130 using tolvaptan-treated subjects in TEMPO 3:4 | ||||||||
| First year | 809 | 5.246 | 0.054 | 5.066 | 0.054 | –0.18 | 0.008 | <0.0001 |
| Second year | 762 | 5.221 | 0.055 | 5.021 | 0.054 | –0.2 | 0.011 | <0.0001 |
| Third year | 695 | 5.19 | 0.058 | 5.01 | 0.057 | –0.18 | 0.014 | <0.0001 |
eHTKV-α, estimated height-adjusted total kidney volume growth rate (α); KUC, Kyorin University Cohort; TEMPO 3:4, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4.
P value was derived from paired t test.
Represents number of TKV measurements.
Determination of Equation Parameter A
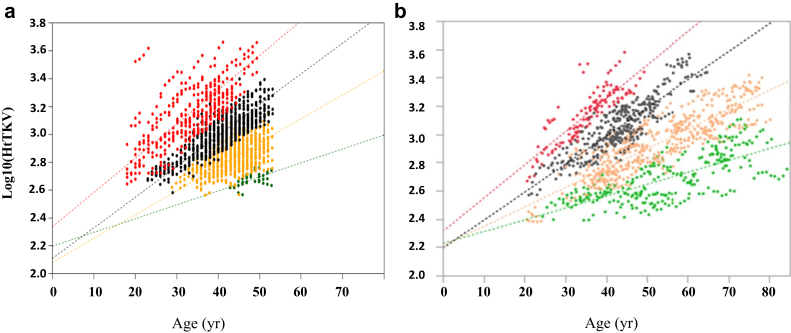
Log-converted HtTKV was plotted against age for MIC subgroups 1B through 1E in the development set (Figure 2a). Most of the regression lines (1B to 1D) converged in a relatively narrow intersection area of age −5.8 to 16.1 (Table 4), and 0 was selected as a parameter A. The intercept and slope of subgroup 1E were higher than those of the other 3 subgroups, and the intersection of subgroup 1E with other regression lines shifted to a negative age range.
Figure 2.
Log-converted height-adjusted total kidney volume (HtTKV) was plotted against age for each Mayo Imaging Classification (MIC) subgroup, 1B (green dots), 1C (yellow dots), 1D (black dots), and 1E (red dots), with limits defined based on the estimated height-adjusted total kidney volume growth rate (α) (eHTKV-α) equation calculated using A = 0 and K = 130. Given that subjects classified as 1A were absent from the development set and consisted of only 3 patients in the Kyorin University Cohort (KUC), subgroup 1A was deleted from the analysis. (a) Development set. Regression lines for the 1B, 1C, and 1D subgroups converged in an age range from −5.8 to 16.1 years; accordingly, 0 was selected as the parameter A. The regression line for 1E converged with the regression lines for the other subgroups in a negative age range (Table 4). (b) Validation set (KUC). Regression lines showed patterns similar to those for the development set. The regression lines for 1B, 1C, and 1D converged in a small area (2.9 years), which supported defining A as 0.
Table 4.
Age, log10(HtTKV), and HtTKV of the intersections of regression lines for MIC subgroups
| MIC | Development set |
Validation set (KUC) |
||||
|---|---|---|---|---|---|---|
| Age | Log10(HtTKV) | HtTKV | Age | Log10(HtTKV) | HtTKV | |
| 1B and 1C | 16.10 | 2.36 | 229 | 2.91 | 2.25 | 178 |
| 1B and 1D | 7.23 | 2.27 | 186 | 2.91 | 2.25 | 178 |
| 1C and 1D | –5.80 | 1.98 | 95 | 2.90 | 2.25 | 178 |
| Mean (SD) | 5.84 (11.02) | 2.20 (0.20) | 170 (68.42) | 2.91 (0.01) | 2.25 (0) | 178 (0) |
| Upper and lower 95% of mean | 33.2 and –21.5 | 2.70 and 1.71 | 340 and 0.04 | 2.92 and 2.89 | 2.25 and 2.25 | 178 and 178 |
| 1E and 1B | –9.78 | 2.10 | 126 | –5.74 | 2.18 | 151 |
| 1E and 1C | –35.03 | 1.48 | 30 | –10.48 | 2.07 | 117 |
| 1E and 1D | –92.78 | 0.06 | 1 | –32.84 | 1.54 | 35 |
| Mean (SD) | –45.86 (42.55) | 1.21 (1.05) | 52.3 (65.4) | –16.35 (14.47) | 1.93 (0.34) | 101 (59.6) |
| Upper and lower 95% of mean | 59.83 and –151.56 | 3.81 and –1.38 | 214 and –110 | 19.60 and –52.31 | 2.78 and 1.08 | 249.1 and –47.1 |
HtTKV, height-adjusted total kidney volume; KUC, Kyorin University Cohort; MIC, Mayo Imaging Classification.
Intersections were derived from regression lines (data not shown). Intersection parameters of the development set distributed widely in comparison to the validation set, probably because of the small number of subjects in subgroup 1B.
Determination of Equation Parameter K
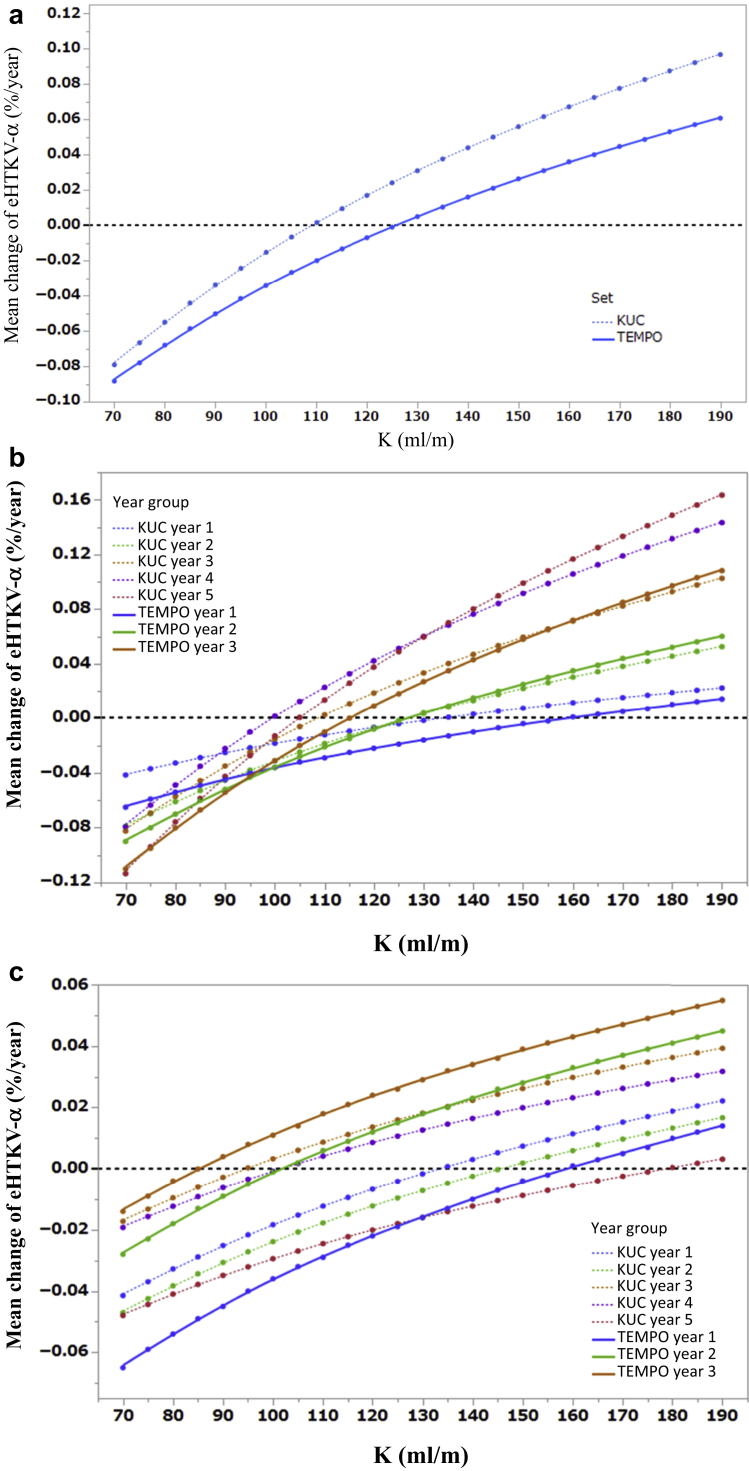
The mean change in eHTKV-α from baseline to postbaseline, calculated using A = 0, was plotted against K using 1257 TKV measurements from development set (Figure 3a, solid line). The plotted line crossed 0 at K = 130.1 ml/m; therefore, 130 was selected as K. The K-values at the minimal mean change in eHTKV-α were 158.3, 126.7, and 115 ml/m, in the first to the third year of development set, respectively (Figure 3b, solid line). The mean of 3 K-values was 133 ml/m, and the small difference from 130 ml/m was thought to be due to the difference in the observed numbers of the subgroups.
Figure 3.
(a) Mean change in estimated height-adjusted total kidney volume growth rate (α) (eHTKV-α) from baseline was plotted for different K using 1257 total kidney volume (TKV) measurements from the development set (solid line) and 981 TKV measurements from validation set (broken line). An appropriate K was determined at the value at which the plotted line crossed 0 mean change in eHTKV-α. (b) Mean change in eHTKV-α from baseline was plotted for different K using 3-years subgroups from the development set (3 solid lines) and 5-years subgroups from the validation set (5 dotted lines). The KUC year 5 subgroup data excluded TKV measurement data after the fifth year for comparison purpose. (c) Mean changes in eHTKV-α from beginning to end of each year were plotted for different K using 3-years subgroups from the development set (3 solid lines) and 5-years subgroups from the validation set (5 dotted lines). The KUC year 5 subgroup excluded TKV measurement data after the fifth year.
Validation of Equation Parameters
The log-converted HtTKV of the validation set was plotted against age by the MIC subgroup (Figure 2b). As subjects categorized as 1A were absent from the development set and consisted of only 3 in the KUC, this subgroup was deleted from the regression analysis. The convergence pattern of the regression lines of the validation set was similar to that of the development set (Figure 2a and b). Subgroups 1B through 1D intersected at age 2.9 years in the KUC (Table 4), close to an A of 0. The similarity of the regression parameters and its converted pattern between the development and validation sets (Figure 2, Table 4) were in accordance with the assumption that kidney volume started to increase approximately at age 0 in most MIC subgroups.
In that validation set using all data as one group, mean changes from baseline eHTKV-α crossed 0 at K = 117.2 ml/m (Figure 3a, blue dotted line). The difference in the appropriate K-value between the development and validation sets (130 vs. 117 ml/m) is explained by lower appropriate K-values in the fourth- and fifth-year subgroups of the validation set (Figure 3b). The appropriate K-values derived from mean changes in eHTKV-α from the beginning to the end of each year, which represent random variation because of similar measurement intervals, were 115.0 and 130.7 in the development and validation set, respectively (Figure 3c, Table 5).
Table 5.
Appropriate K-values by follow-up years subgroups and comparison methods
| Comparison methods | Follow-up years subgroups |
P value | |
|---|---|---|---|
| TEMPO (n = 3) | KUC (n = 5) | ||
| From-baseline methoda | 133.3 ± 22.4 | 114.6 ± 14.7 | 0.1957 |
| Each-year methodb | 115 ± 38.4 | 130.7 ± 34.2 | 0.5698 |
| Combined | 124.2 ± 29.9 (n = 6) | 122.6 ± 26.2 (n = 10) | 0.9146 |
| Follow-up years subgroups | Comparison methods |
P value | |
|---|---|---|---|
| From-baseline methoda | Each-year methodb | ||
| TEMPO (n = 3) | 133.3 ± 22.4 | 115.0 ± 38.4 | 0.5149 |
| KUC (n = 5) | 114.6 ± 14.7 | 130.7 ± 34.2 | 0.3619 |
| Combined (n = 8) | 121.6 ± 19.0 | 124.8 ± 34.0 | 0.8202 |
eHTKV-α, estimated height-adjusted total kidney volume growth rate (α); KUC, Kyorin University Cohort; TEMPO, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes.
Data are presented as mean ± SD. Data derive from 3-year subgroups of TEMPO 3:4 and 5-year subgroups of KUC.
From-baseline method indicates that appropriate K-value was determined at a point of minimal change in eHTKV-α from baseline to comparison years (Figure 3b).
Each-year method, appropriate K-value was determined at a point of minimal change in eHTKV-α from beginning to end of each year (Figure 3c).
Changes in the eHTKV-α from baseline to year of follow-up were significant at the fourth and after the fourth year at K = 150 ml/m, but not significant at K = 130 ml/m from the first through after the fourth year (Table 3).
In summary regarding validation, the eHTKV-α remained roughly stable for 5 years at A = 0 and K = 130.
Application of eHTKV-α to Tolvaptan-Treated Subjects and High-Risk Groups
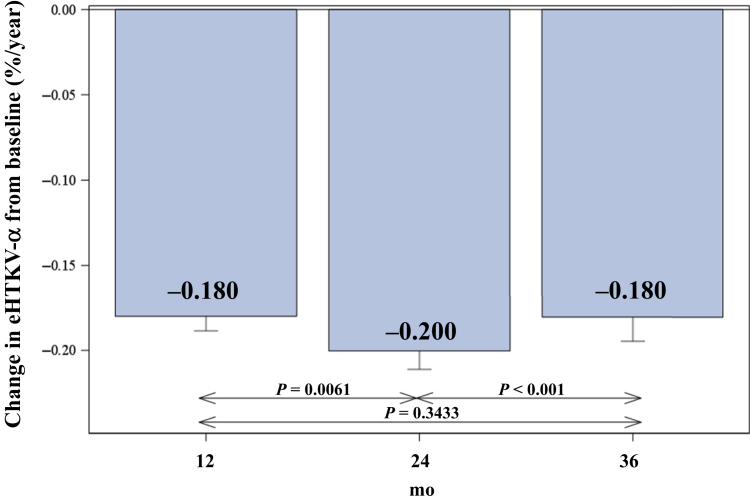
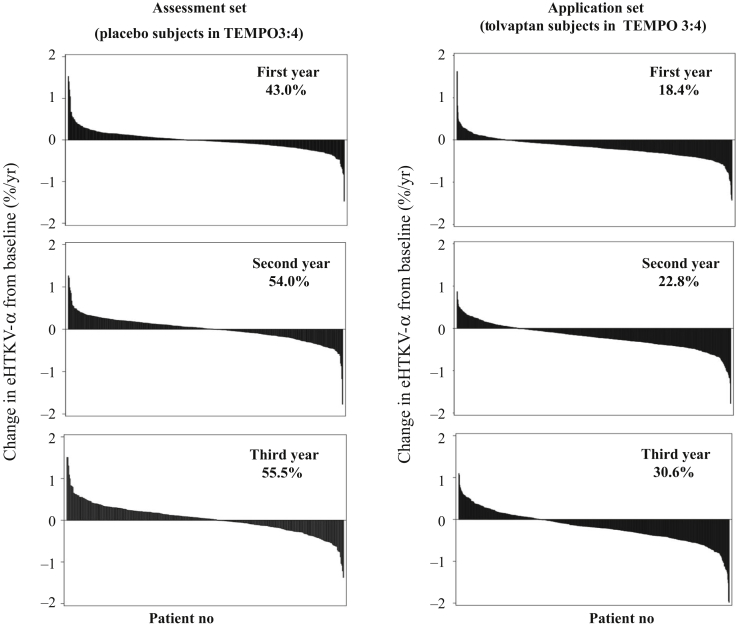
The eHTKV-α decreased significantly from baseline during follow-up in subjects treated with tolvaptan (Table 3, Figures 4 and 5). Changes in eHTKV-α from baseline to 3 treatment-years were all significant (P < 0.0001, derived from the paired t test). The mean change in the eHTKV-α from baseline remained within a narrow range from –0.18% to –0.20% per year, suggesting persistent treatment benefit of tolvaptan for 3 years.
Figure 4.
Mean changes (±SE) in estimated height-adjusted total kidney volume growth rate (α) (eHTKV-α) from baseline during 3 follow-up years in tolvaptan-treated subjects in TEMPO 3:4 were significant and negative (P < 0.0001 by paired t test (Table 3). P values for comparisons between years were derived from a mixed-model repeated-measures analysis with fixed effects of visit as a factor.
Figure 5.
Waterfall plots of individual change in estimated height-adjusted total kidney volume growth rate (α) (eHTKV-α) from baseline to comparison year. eHTKV-α was calculated using A = 0 and K = 130. Percentage represents patients with positive change.
Changes in the eHTKV-α from baseline in tolvaptan-treated subjects were evaluated by the MIC subgroups (Table 6). The changes became larger from MIC 1B through 1E over 3 years (bP in Table 6).
Table 6.
Changes in eHTKV-α from baseline to each follow-up year in tolvaptan-treated subjects in TEMPO 3:4 according to MIC subclassification
| MIC subclassification |
Change in eHTKV-α (%/yr) from baseline to follow-up year (mean ± SE) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to first year |
Baseline to second year |
Baseline to third year |
||||||||
| (%/yr) | N | (n = 809) | aP value | n | (n = 762) | aP value | n | (n = 695) | aP value | |
| 1B | (1.5–3.0) | 27 | –0.149 ± 0.0372 | 0.0005 | 26 | –0.130 ± 0.047 | 0.0112 | 24 | –0.126 ± 0.054 | 0.0306 |
| 1C | (3.0–4.5) | 246 | –0.150 ± 0.011 | <0.0001 | 233 | –0.170 ± 0.015 | < 0.0001 | 221 | –0.140 ± 0.020 | <0.0001 |
| 1D | (4.5–6.0) | 325 | –0.181 ± 0.012 | <0.0001 | 309 | –0.198 ± 0.016 | < 0.0001 | 273 | –0.175 ± 0.021 | <0.0001 |
| 1E | (>6.0) | 211 | –0.219 ± 0.023 | <0.0001 | 194 | –0.250 ± 0.028 | < 0.0001 | 177 | –0.248 ± 0.036 | <0.0001 |
| bP value | 0.0210 | 0.0265 | 0.0309 | |||||||
eHTKV-α, estimated height-adjusted total kidney volume growth rate (α); MIC, Mayo Imaging S eHTKV-α, estimated height-adjusted total kidney volume growth rate (α); MIC, Mayo Imaging Classification; TEMPO 3:4, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4.
Baseline eHTKV-α (A = 0 and K = 130 ml/m) was used for MIC subclassification.
P value derived from paired t test between baseline and follow-up year.
P value derived from analysis of variance with MIC subclassification as a factor.
Of 809 tolvaptan-treated subjects, 556 maintained a constant dose for the first year. Within this group, changes in the eHTKV-α from baseline to the first year were not different among the 3 dose subgroups (Table 7).
Table 7.
Comparison of changes in eHTKV-α from baseline to the first year by tolvaptan dose
| Tolvaptan dose |
n | Baseline eHTKV-α |
Year 1 eHTKV-α |
Change in eHTKV-α |
Comparison of dose groups |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/d) | Mean | SE | Mean | SE | Mean | SE | P valuea | Comparison | P valueb | |
| 60 | 39 | 5.126 | 0.219 | 4.913 | 0.210 | –0.213 | 0.044 | <0.0001 | 60 mg vs. 90 mg | 0.7766 |
| 90 | 25 | 5.451 | 0.460 | 5.257 | 0.456 | –0.195 | 0.054 | 0.0015 | 60 mg vs. 120 mg | 0.4178 |
| 120 | 492 | 5.318 | 0.070 | 5.139 | 0.070 | –0.179 | 0.011 | <0.0001 | 90 mg vs. 120 mg | 0.7618 |
eHTKV-α, estimated height-adjusted total kidney volume growth rate (α).
The eHTKV-α was calculated using A = 0 year and K = 130 ml/m. Subjects who maintained the same dose for the first year were analyzed.
P value derived from paired t test.
P value derived from the general linear model with the dose as a factor.
In 1445 participants of TEMPO 4:3, baseline eHTKV-α was higher in the high-risk groups (men and hypertensive patients) than in the low-risk groups (women and normotensive patients), irrespective of the K-value (Table 8).
Table 8.
Comparison of eHTKV-α between the male and female patients and between hypertensive and normotensive patients using baseline data of all participants to TEMPO 3:4
| Characteristics and eHTKV-α | Male | Female | P valuea | Hypertensiveb | Normotensivec | P valuea |
|---|---|---|---|---|---|---|
| All subjects, n | 746 | 699 | — | 1192 | 253 | — |
| Subjects with hypertension, n (%) | 652 (87.4) | 540 (77.3) | <0.0001 | — | — | — |
| Male, n (%) | — | — | — | 652 (54.7) | 94 (37.2) | <0.0001 |
| Baseline age, yr | 38.3 ± 7.1 | 39.1 ± 7.1 | 0.0315 | 39.1 ± 6.9 | 36.7 ± 7.6 | <0.0001 |
| Baseline eGFR, ml/min per 1.73 m2 | 76.5 ± 22.3 | 80.1 ± 20.9 | 0.0022 | 75.9 ± 21.3 | 89.3 ± 20.1 | <0.0001 |
| Baseline TKV, ml | 1899 ± 1023 | 1471 ± 694 | <0.0001 | 1786 ± 948 | 1250 ± 459 | <0.0001 |
| Baseline HtTKV, ml/m | 1054 ± 566 | 884 ± 410 | <0.0001 | 1023 ± 527 | 733 ± 272 | <0.0001 |
| Baseline eHTKV-α (K = 130), %/yr | 5.481 ± 1.634 | 4.999 ± 1.529 | <0.0001 | 5.334 ±1.644 | 4.842 ± 1.311 | <0.0001 |
| Baseline eHTKV-α (K = 150), %/yr | 5.070 ± 1.574 | 4.598 ± 1.460 | <0.0001 | 4.933 ± 1.580 | 4.413 ± 1.238 | <0.0001 |
eGFR, estimated glomerular filtration rate; eHTKV-α, estimated HtTKV growth rate; HtTKV, height-adjusted TKV; TKV, total kidney volume.
Data for continuous variables are expressed as mean ± SD.
P value was derived from χ2 test for the categorical variables and from t test for the continuous variables.
Hypertensive is defined by diastolic blood pressure >89 mm Hg or systolic blood pressure >139 mm Hg or with anti-hypertensive therapy.
Normotensive is defined by diastolic blood pressure ≤89 mm Hg and systolic blood pressure ≤139 mm Hg and without antihypertensive therapy.
Discussion
Systematic measurement of TKV in the CRISP study identified an exponential-like TKV growth pattern at an individually quantifiable growth rate.2, 3, 4 The concept of HtTKV reduced bias derived from variability in body size and enhanced role of TKV to predict renal prognosis.14 The MIC integrated an age concept with the HtTKV, using an exponential HtTKV growth model, and further improved the role of TKV for renal prognosis.12
The stable eHTKV-α with equation parameters A = 0 and K = 130 (Table 3) is in accordance with the hypothetical background for the equation HtTKVt =K(1+α/100)(t-A).
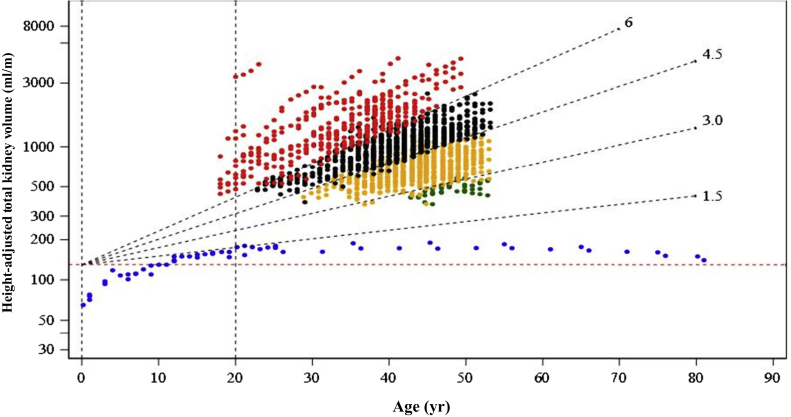
The HtTKV has been reported to increases before adolescence in non-ADPKD subjects,15, 16, 17 and this HtTKV growth pattern certainly takes place in younger ADPKD patients (Figure 6). In the equation to calculate the eHTKV-α of adult patients, the period before adolescence was included. However, as the HtTKV growth pattern in younger patients is not identified, application of the eHTKV-α equation should be limited to adult patients.
Figure 6.
Changes in height-adjusted total kidney volume (HtTKV) in placebo-assigned subjects in Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 and non−autosomal dominant polycystic kidney disease (non-ADPKD) subjects. 1E (red dots), 1D (black dots), 1C (yellow dots), and 1B (green dots) are subgroups according to Mayo Image Classification (MIC) of ADPKD12 (using K = 130 ml/m). Data of non-ADPKD subjects (blue dots) are from MacKay,15 Yoshimura et al.,16 and the Planning and Investigation Committee of the Japanese Society of Legal Medicine.17 Change pattern in non-ADPKD subjects is totally different from that in ADPKD subjects. Broken red line indicates HtTKV = 130 ml/m.
Four subgroups of the development set (Figure 2A) were defined according to MIC12 using A = 0 and K = 130. Subgroups were stratified using the eHTKV-α equation, but regression lines themselves were derived from log-converted HtTKV and age, which were the factors independent of the eHTKV-α equation. Regression pattern and parameters were similar between the development and validation sets (Figure 2, Table 4), and the intersections of the regression lines for validation set subgroups 1B through 1D were close to 0 (Figure 2, Table 4). These results support A = 0, but do not necessarily indicate that HtTKV starts to enlarge at age 0. The eHTKV-α equation merely mathematically assumes age 0 as a starting point of growth for most MIC subgroups, and the actual HtTKV growth pattern prior to adolescence is unknown. In fact, the regression line of the 1E subgroup had a higher intercept than those of the other subgroups (Figure 2). Cysts of the 1E subgroup might potentially develop at exuberant rates in utero.18
The stability of eHTKV-α was compared between K = 130 and K = 150. The KUC validation set confirmed that the eHTKV-α derived from the equation using K = 130 was more stable than that derived from the equation using K = 150 (Table 3). Subjects in KUC have different ethnic and clinical profiles from participants in TEMPO 3:4, strengthening the reliability of the validation.
Subgrouping using K = 150 does not decrease the role of predicting the rate of estimated glomerular filtration rate decline and enriching the design in clinical trials, because a small change in eHTKV-α does not affect subgrouping limits seriously.11,12,19,20 However, because eHTKV-α (K = 150) changed significantly from baseline as age advanced in placebo-assigned and standard-of-care subjects (Table 3), it will affect estimations of change in the HTKV growth rate (eHTKV-α) from baseline to postbaseline.
In tolvaptan-treated subjects, changes in the eHTKV-α from baseline to postbaseline were –0.18, –0.200, and –0.180 in the first through third years, respectively (all P < 0.0001) (Table 3, Figure 4). If the effect of tolvaptan decreased, the difference in eHTKV-α between baseline and postbaseline became smaller. The present findings indicated a stable benefit of tolvaptan treatment for 3 years. A previous MMRM analysis of TEMPO 3:4 data showed that changes from baseline in the ratio of geometric TKV means (tolvaptan treatment effect) were 0.940, 0.925, and 0.922 from the first through third years, respectively, in comparison with those in subjects who received placebo.8 The tolvaptan effects seemed to decrease yearly. The difference in tolvaptan effects between the 2 analyses might be due to the use of different controls and different analytical methods.
The 0.2%/yr decrease in eHTKV-α seems to be a small effect of tolvaptan. However, for example, the eHTKV-α of a subject with HtTKV= 1 491 ml/m at age 50 years is calculated as 5.0%/yr. That individual’s HtTKV will increase to 1726 ml/m at age 53 years at eHTKV-α = 5.0%/yr. If eHTKV-α decreases to 4.8%/yr by tolvaptan treatment for 3 years, HtTKV will be 1560 ml/m, and the reduction rate of 9.6% will not be small.
The change in eHTKV-α from baseline in the second year was larger than during the first and third years in tolvaptan-treated subjects (Table 3, Figure 4). Tolvaptan reduced TKV enlargement by rapid inhibition of chloride secretion into the cyst cavity and long-lasting inhibition of cellular proliferation of cyst epithelium.21, 22, 23 The initial net effects of tolvaptan might be complicated, and longer clinical observation might be necessary to interpret the unequal effects seen during the initial 3 years.
The effect of tolvaptan on the TKV slope was reported to be greater in MIC subgroups 1C to 1E than in 1B.19 The changes in eHTKV-α significantly increased from 1B to 1E in each of 3 years of follow-up (Table 6) and are compatible with a larger treatment effect as higher eHTKV-α.
Dose-dependency of tolvaptan might be observed in the subgroup of patients with higher plasma vasopressin. As a vasopressin V2 receptor antagonist, tolvaptan might be more effective in the presence of higher vasopressin levels.24,25 The absence of dose-effect in this analysis (Table 7) might be explained by imbalances in subject numbers and inadequately characterized clinical backgrounds.
The eHTKV-α was higher in male than in female participants and in hypertensive than in normotensive subjects (Table 8). It was reported that eHTKV-α (K = 150) was higher in subjects with PKD1 mutation than in subjects with PKD2 mutation.11 An association of a higher growth rate of HtTKV with higher risk of disease progression can be identified using eHTKV-α.
A limitation of this study is a relatively short follow-up interval in the development set; however, subjects in the validation set were observed for up to 5 years or more. The appropriate K-value of the fifth-year KUC subgroup is not farther from 130 ml/m than that of the fourth-year subgroup (Figure 3B). This and the K-distribution in the each-year method (Table 5) might indicate that the appropriate K-value for the equation remains around 115 to 135 ml/m for at least several years.
Several GFR estimating equations were developed according to biomarkers used, patient ages, and chronic kidney disease stages.26 Similarly, a specific HtTKV growth rate estimating equation might be developed according to disease severity, age, and ethnicity with accumulated data in the future. For estimation of treatment effects, this equation might be a useful and convenient method at least after several years from the start of treatment.
In conclusion, the minimal and nonsignificant fluctuation in the eHTKV-α from baseline using the present equation is in accordance with a proposed exponential HtTKV growth model.2, 3, 4 Because the eHTKV-α is calculated using a single measurement of TKV, the individual treatment effect or loss of treatment effect is conveniently estimated by changes in the eHTKV-α from baseline for adult patients with ADPKD.
Disclosure
EH was a member of the Steering Committee of the TEMPO 3:4 trial. JO and JL are employees of Otsuka Pharmaceutical Development and Commercialization (OPDC, Rockville, MD). EH, SM, and SH have received research funding from Otsuka Pharmaceuticals Co., Ltd. (Tokyo, Japan). All the other authors declared no competing interests.
Acknowledgments
The TEMPO 3:4 trial was funded by Otsuka Pharmaceuticals Co., Ltd., Tokyo, Japan and Otsuka Pharmaceutical Development and Commercialization, Inc., Rockville, MD. This work is supported by Grant-in-Aid for Scientific Research (16K10996 and 16K11058 to SH). This paper is dedicated to the memory of Dr. Jared J. Grantham, who led the CRISP study and developed core thoughts on the relationship between kidney volume and function. We owe this manuscript to him. We thank Ms. Ayako Hirao for secretarial assistance.
Author Contributions
EH, KN, SM, and SH planned this study. EH, HF, KN, MT, TY, ST, SK, and IM contributed to data collection. JO, JL, and EH performed statistical analyses. EH prepared the manuscript.
References
- 1.Chebib F.T., Torres V.E. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis. 2016;67:792–810. doi: 10.1053/j.ajkd.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.JJ1 Grantham, Torres V.E., Chapman A.B. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 3.Grantham J.J., Chapman A.B., Torres V.E. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–157. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
- 4.Grantham J.J., Cook L.T., Torres V.E. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–116. doi: 10.1038/sj.ki.5002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grantham J.J., Mulamalla S., Swenson-Fields K.I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556–566. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- 6.Higashihara E., Nutahara K., Okegawa T. Kidney volume and function in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2014;18:157–165. doi: 10.1007/s10157-013-0834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrone R.D., Mouksassi M.S., Romero K. A drug development tool for trial enrichment in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep. 2017;2:451–460. doi: 10.1016/j.ekir.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serra A.L., Poster D., Kistler A.D. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 10.Walz G., Budde K., Mannaa M. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 11.Higashihara E., Yamamoto K., Kaname S. Age- and height-adjusted total kidney volume growth rate in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2019;23:100–111. doi: 10.1007/s10157-018-1617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irazabal M.V., Rangel L.J., Bergstralh E.J. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres V.E., Meijer E., Bae K.T. Rationale and design of the TEMPO3/4 study, tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes. Am J Kidney Dis. 2011;57:692–699. doi: 10.1053/j.ajkd.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Chapman A.B., Bost J.E., Torres V.E. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKay E.M. Kidney weight, body size and renal function. Arch Intern Med. 1932;50:590–594. [Google Scholar]
- 16.Yoshimura M., Furutani A., Konishi S. Normal weight of the heart, liver and kidneys in Japanese (1988–1992) Med J Kinki Univ. 1994;19:297–302. [Google Scholar]
- 17.Planning and Investigation Committee of the Japanese Society of Legal Medicine Tokyo: Weights and sizes of internal organs measured in forensic autopsy cases from 2009 to 2013 in Japan, 2017. http://www.jslm.jp/problem/zouki.pdf Available at:
- 18.Grantham J.J., Cook L.T., Wetzel L.H. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol. 2010;5:889–896. doi: 10.2215/CJN.00550110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irazabal M.V., Blais J.D., Perrone R.D. Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the TEMPO 3:4 clinical trial. Kidney Int Rep. 2016;1:213. doi: 10.1016/j.ekir.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irazabal M.V., Abebe K.Z., Bae K.T. Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the HALT-PKD clinical trial. Nephrol Dial Transplant. 2017;32:1857–1865. doi: 10.1093/ndt/gfw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irazabal M.V., Torres V.E., Hogan M.C. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80:295–301. doi: 10.1038/ki.2011.119. [DOI] [PubMed] [Google Scholar]
- 22.Devuyst O., Torres V.E. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:459–470. doi: 10.1097/MNH.0b013e3283621510. [DOI] [PubMed] [Google Scholar]
- 23.Boertien W.E., Meijer E., de Jong P.E. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65:833–841. doi: 10.1053/j.ajkd.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Devuyst O., Chapman A.B., Gansevoort R.T. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2017;28:1592–1602. doi: 10.1681/ASN.2016040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gansevoort R.T., van Gastel M.D.A., Chapman A.B. Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease. Kidney Int. 2019;96:159–169. doi: 10.1016/j.kint.2018.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz G.J., Work D.F. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]