Highlights
-
•
We report here a case of JC virus granule cell neuronopathy associated with Ruxolitinib
-
•
It is worthwhile considering the possibility of JCV-GCN in myelofibrosis patients receiving ruxiolitinib, who present with progressive cerebellar symptoms and cerebellar atrophy.
-
•
Combination therapy using mefloquine and mirtazapine may be an effective treatment.
Keywords: Progressive multifocal leukoencephalopathy, Granule cell neuronopthy, JC virus, Myelofibrosis, Ruxolitinib
Dear editor.
JC virus (JCV) is a human polyomavirus. After a primary asymptomatic infection, JCV remains latent in the kidney and lymphoid organs. However, it can reactivate in the setting of immunosuppression. Rearrangement of JCV into neurotropic variants causes several JCV-associated central nervous system (CNS) diseases, including progressive multifocal leukoencephalopathy (PML), JCV granule cell neuronopathy (JCV-GCN), JCV encephalopathy, and JCV meningitis. Ruxolitinib is an orphan drug that has been approved for the treatment of myeloproliferative disorders [1]. Here, we report a case of JCV-GCN in a patient with post-essential thrombocythemia (ET) myelofibrosis who was receiving ruxolitinib.
1. Case report
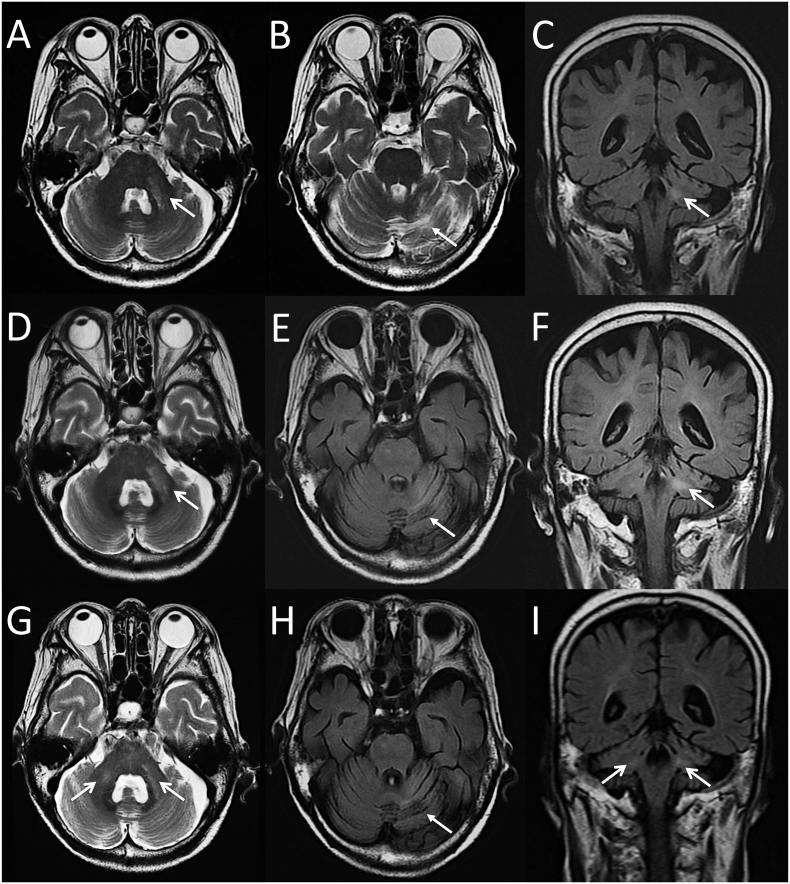
A 73-year-old woman was diagnosed as having ET in 2002. The platelet count was reduced by administration of ranimustine (nitrosourea alkylating agent) or anagrelide (phospholipase A2 inhibitor that inhibits megakaryocyte proliferation), but her anemia gradually progressed. In November 2017, a bone marrow biopsy was performed, after which she was diagnosed with CALR-mutated post-ET myelofibrosis. Thereafter, ruxolitinib was introduced at a dose of 5 mg twice daily. One year later, she presented with slowly progressive gait inability and left-sided intention tremor. Five months after initial presentation, she developed gradually progressive dysarthria. Moreover, she had difficulty in walking and needed a cane to walk. A brain magnetic resonance imaging (MRI) showed multiple hyperintense lesions in the left middle cerebellar peduncle (MCP), left cerebellar hemisphere, and brainstem on T2 weighted and fluid-attenuated inversion recovery (FLAIR) images (Fig. 1A-C). Thereafter, she was admitted to our department approximately 7 months after the onset of symptoms. Neurological examination revealed ataxic dysarthria, slight left hemiparesis, left-sided limb ataxia, and gait ataxia. The scale for the assessment and rating of the ataxia (SARA) score was 19 points. Laboratory examination revealed anemia (Hb 9.5 g/dL) and thrombocytopenia (4.6 × 104 /μL). Lymphocyte counts were normal, but CD4+ T cell counts were decreased (170 cells/μL). The CD4/CD8 ratio was 0.35, and an HIV antibody test was negative. Serum levels of blood urea nitrogen (39 mg/mL), creatinine (1.99 mg/mL), and ferritin (2099 ng/mL) were increased. Tests for auto-antibodies including anti-nuclear, anti-SS-A, anti-SS-B, anti-double-stranded DNA, and anti-neutrophil cytoplasmic antibodies were all negative. Soluble interleukin-2 receptor was normal. A cerebrospinal fluid (CSF) analysis showed elevated levels of protein (48 mg/dL) without pleocytosis. No oligoclonal IgG band or elevated myelin basic protein was found. The IgG index was 0.58. Repeat MRI revealed extension of the hyperintense lesions (Fig. 1D-F). CSF polymerase chain reaction (PCR) for JCV was positive (109,000 copies/mL). The JCV genome had deletion in the region D within the non-coding control region, which is characteristic of PML-type (prototype) JCV, as judged by multiplex real-time PCR [2]. Based on these results, the diagnosis of infratentorial PML was made; and ruxolitinib was discontinued. Treatment with mirtazapine (30 mg/day) was started, and then mefloquine (375 mg/day for 3 days followed by 375 mg/week) was added [3]. The clinical symptoms slightly improved, and her SARA score decreased to 17 points. The copy numbers of JCV DNA also decreased to 23,400 copies/mL at 10 months after the onset of symptoms. Nevertheless, a follow-up MRI revealed progressive cerebellar atrophy with a new lesion in the right MCP (Fig. 1G-I). Furthermore, hypointense cerebellar cortical lesions on FLAIR images became prominent (Fig. 1H). She developed slightly right-sided limb ataxia, but her SARA score remained stable at 17 points. The copy numbers of JCV DNA further decreased to 1304 copies/mL at 16 months after initial symptoms. We finally diagnosed our case as JCV-GCN.
Fig. 1.
Brain MRI Findings A-C: At 6 months after initial presentation, axial T2-weighted images (T2WI) and coronal fluid-attenuated inversion recovery (FLAIR) images show multiple hyperintense lesions in the left middle cerebellar peduncle (MCP, open arrow), left cerebellar hemisphere (closed arrow), and brain stem. D-F: Two months later, axial T2WI, axial and coronal FLAIR images reveal extension of the hyperintense lesions in the left MCP (open arrow) and mild cortical atrophy in the left cerebellar hemisphere (closed arrow). G-I: Nine months later, axial T2WI and coronal FLAIR images demonstrate a new hyperintense lesion in the right MCP (open arrow) and loss of cerebellar volume with enlargement of the fourth ventricule. Axial FIALR image shows the progression of cerebellar atrophy (closed arrows).
2. Discussion
Janus kinase (JAK) plays an essential role in the regulation of cytokine signaling. Deregulation of JAK signaling has been associated with the pathogenesis of hematological malignancies, rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Recently, several JAK inhibitors have been developed as new therapies for these patients. Ruxolitinib is the first JAK1/2 inhibitor that has been approved for the treatment of myelofibrosis, since it causes a reduction in spleen size and improves debilitating symptoms [1]. However, the drug suppresses the immune system through the inhibition of the actions of IFN-γ or TNF-α. Furthermore, ruxolitinib inhibits CD4+ T cell activation and differentiation [4]. As a result, its use can lead to serious and opportunistic infections. CD4+ cells appear to play a central role in the control of JCV CNS infections [5]. It is known that IFN-γ suppresses JCV replication and propagation by causing down-regulation of the major regulatory protein, the T antigen, of this virus [6]. Therefore, this drug might be involved in an increased susceptibility to a JCV CNS infection.
To date, a few cases suggesting an association between JCV-related diseases and ruxolitinib have been reported [[7], [8], [9]], but no cases of JCV-GCN have been described. JCV-GCN is a rare disease caused by a selective infection of the cerebellar granule cells, leading to slowly progressive cerebellar ataxia. This disease is thought to be a distinct entity caused by mutations in the VP1 gene of JCV, leading to a shift in viral tropism from oligodendroglial cells to granule cells [10]; but it has also been reported that wild-type JCV can cause JCV-GCN. The most characteristic MRI feature of JCV-GCN is cerebellar atrophy, which often occurs in combination with changes in the infratentorial white matter, particularly that in the MCP and pons [11]. Our case showed slowly progressive cerebellar symptoms 1 year after initiation of ruxolitinib therapy, suggesting that the clinical picture of our patient was consistent with JCV-GCN. The initial brain MRI revealed white matter abnormalities in the left MCP and in the periphery of left cerebellar hemisphere, as well as small punctate T2 and FLAIR hyperintense lesions in the brainstem. Subsequently, our case showed progression of already existing cerebellar atrophy with new lesions in the right MCP. Although we have no histopathological data showing infected granule cell neurons to confirm our suspicion of JCV-GCN, the obtained clinical and radiological findings were highly suggestive of JCV-GCN rather than infratentorial PML.
In conclusion, this is the first report of a possible association between ruxolitinib therapy and JCV-GCN. JCV-GCN should be considered in myelofibrosis patients receiving ruxiolitinib who present with progressive cerebellar symptoms and cerebellar atrophy. Combination therapy using mefloquine and mirtazapine may be an effective treatment, but further studies are needed to determine the effectiveness of this therapy in non-AIDS-drug- induced JCV-associated CNS diseases.
Consent for publication
Written informed consent was obtained from the patients for publication.
Declaration of competing interest
The authors declare there is no conflict of interest.
Acknowledgements
This work was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (to K. N., Grant Number 17 K09768) and by a Grant-in-Aid for the Research Committee of Prion Disease and Slow Virus Infection, Research on Policy Planning and Evaluation for Rare and Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (to M. S., Grant Number H29-Nanchitou (Nan)-Ippan-036).
References
- 1.Verstovsek S., Mesa R.A., Gotlib J., Levy R.S., Gupta V., DiPersio J.F., Catalano J.V., Deininger M., Miller C., Silver R.T., Talpaz M., Winton E.F., Harvey J.H., Jr., Arcasoy M.O., Hexner E., Lyons R.M., Paquette R., Raza A., Vaddi K., Erickson-Viitanen S., Koumenis I.L., Sun W., Sandor V., Kantarjian H.M. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2013;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryschkewitsch C.F., Jensen P.N., Major E.O. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. J Clin Virol. 2013;57:243–248. doi: 10.1016/j.jcv.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epperla N., Medina-Flores R., Mazza J.J., Yale S.H. Mirtazapine and mefloquine therapy for non-AIDS-related progressive multifocal leukoencephalopathy. WMJ. 2014;113:242–245. [PubMed] [Google Scholar]
- 4.Parampalli Yajnanarayana S., Stübig T., Cornez I. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol. 2015;169:824–833. doi: 10.1111/bjh.13373. [DOI] [PubMed] [Google Scholar]
- 5.Pavlovic D., Patel M.A., Patera A.C., Peterson I. Progressive multifocal leukoencephalopathy consortium. T cell deficiencies as a common risk factor for drug associated progressive multifocal leukoencephalopathy. Immunobiology. 2018;223:508–517. doi: 10.1016/j.imbio.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 6.De-Simone F.I., Sariyer R., Otalora Y.L. IFN-gamma inhibits JC virus replication in glial cells by suppressing T-antigen expression. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wathes R., Moule S., Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N Engl J Med. 2013;369:197–198. doi: 10.1056/NEJMc1302135. [DOI] [PubMed] [Google Scholar]
- 8.Ballesta B., González H., Martín V., Ballesta J.J. Fatal ruxolitinib-related JC virus meningitis. J Neurovirol. 2017;23:783–785. doi: 10.1007/s13365-017-0558-4. [DOI] [PubMed] [Google Scholar]
- 9.Reoma L.B., Trindade C.J., Monaco M.C. Fatal encephalopathy with wild-type JC virus and ruxolitinib therapy. Ann Neurol. 2019;86:878–884. doi: 10.1002/ana.25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dang X., Vidal J.E., Oliveira A.C. JC virus granule cell neuronopathy is associated with VP1 C terminus mutants. J Gen Virol. 2012;93:175–183. doi: 10.1099/vir.0.037440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijburg M.T., van Oosten B.W., Murk J.L., Karimi O., Killestein J., Wattjes M.P. Heterogeneous imaging characteristics of JC virus granule cell neuronopathy (GCN): a case series and review of the literature. J Neurol. 2015;262:65–73. doi: 10.1007/s00415-014-7530-5. [DOI] [PubMed] [Google Scholar]