Highlights
-
•
Patients with ovarian cancer were treated with intraperitoneal submicron particle paclitaxel after debulking surgery.
-
•
Following surgery, patients received IV chemotherapy without evidence of enhanced systemic toxicity.
-
•
By RECIST 1.1 criteria, 66% of patients had progression free survival at 6 months and 1-year following surgery.
Keywords: Ovarian cancer, Paclitaxel, Intraperitoneal therapy, NanoPac, Submicron particle paclitaxel
Abstract
Submicron particles (~800 nm) of paclitaxel (SPP) contain 1–2 billion molecules of pure drug that release tumoricidal levels of paclitaxel over many weeks. This study compared two dose-levels of SPP instilled into the peritoneal cavity (IP) in 200 ml of saline post-cytoreductive surgery. Eligible patients with primary (n = 6) or recurrent (n = 4) epithelial ovarian cancer who underwent complete cytoreductive surgery were enrolled to receive a single instillation of IP SPP followed by standard IV carboplatin and paclitaxel. Endpoints were PFS and evaluation of treatment emergent adverse events. Clinical response was determined by symptoms, physical exams, CT scans, and serum CA-125 measurements. Of the 24 subjects screened, 10 were enrolled and received treatment: seven patients received 100 mg/m2 and three received 200 mg/m2. Seven subjects completed the 12-month follow-up period. Six patients were evaluable due to one subject who had unevaluable scans throughout the follow-up period and was thus excluded from PFS determination. Upon completion of planned chemotherapy post-SPP instillation, the PFS at 6 months was 66% (4/6) and at 12-months 66% (4/6) using RECIST 1.1. One subject had a complete response at the end of IV treatment but died (unrelated to study treatment) before PFS evaluation. There was one case of incision dehiscence and one case of vaginal cuff leakage after surgery. This pilot study supports further evaluation of IP SPP to treat peritoneal carcinomas.
1. Introduction
The goal of intraperitoneal (IP) chemotherapy is to treat cancer confined to the peritoneal cavity with higher concentrations of drug for longer periods of time than is possible with IV therapy (Goldberg et al., 2002). IP therapy is designed to maximize drug delivery to the tumor while reducing systemic toxicities. Although a number of Phase II and III trials demonstrated feasibility and potential clinical benefit of IP therapy in treating ovarian cancer (NCI, 2006), other studies have not found IP therapy to provide benefits beyond IV therapy in addition to added toxicity (Monk and Chan, 2017).
Markman’s study of IP Taxol encouraged development of paclitaxel formulations for IP treatment of ovarian cancer (Markman et al., 1992). Availability of nab-paclitaxel provides a cremophor-free drug which has activity as IP therapy in treatment of ovarian cancer. IP nab-paclitaxel + IV regimens for treatment of advanced malignancies (including ovarian cancer) suggested improved progression free survival (PFS); however, this regimen increased systemic toxicity (Cristea et al., 2019). Thus, novel paclitaxel formulations for IP administration with prolonged residence and reduced systemic toxicity are of interest.
Submicron particles of paclitaxel (SPP), were manufactured using a Precipitation with Compressed Antisolvent (PCA) technique that employs supercritical carbon dioxide and acetone to generate particles within a narrow distribution (Niu et al., 2006). Scanning electron microscopy showed native paclitaxel particles were rod-shaped while PCA paclitaxel particles were irregular with an increased surface area (800 nm with a volume mean of 1400 nm).
Initial Phase I trial of IP SPP (50–275 mg/m2) was performed in patients with solid tumors predominantly confined to the peritoneal cavity for whom no other curative systemic therapy treatments were available (Williamson et al., 2015). IP administration of SPP via catheter did not induce toxicity over that associated with IV paclitaxel. The peritoneal levels of paclitaxel rose during the two days after dosing to concentrations 450–2900 times the peak plasma paclitaxel concentrations and remained elevated. Tumor response assessed by RECIST 1.0 was 25% (5 subjects with stable disease and 15 with progressive disease). In the Phase I study, many patients who had failed other therapies survived >4-years. Given these findings, this Phase II study was conducted to evaluate the safety and potential benefit of SPP administered at the time of surgical debulking in patients with ovarian cancer (Williamson et al., 2015).
2. Methods
2.1. Subject population
Subjects with epithelial ovarian cancer appropriate for IV platinum and paclitaxel undergoing cytoreductive surgery were included. Epithelial ovarian cancer was limited to the abdominal cavity but could include pleural effusion. If the subject had recurrence, disease must have been platinum-sensitive. Study also required minimal or non-symptomatic ascites. Subjects who, in the investigator's judgement, would benefit from neoadjuvant IV carboplatin and paclitaxel chemotherapy strategy in advance of cytoreductive surgery, were also eligible for participation.
Exclusion criteria included anticipated use of concomitant chemotherapy (other than the protocol-specified drugs), immunotherapy, or radiation therapy. Subjects treated with a prior investigational agent within 30 days of planned IP SPP were excluded with the exception of subjects participating in PARP inhibitor trials. These subjects must have discontinued investigational agent prior to surgery. History of prior malignancy other than ovarian not in remission for >5 years were excluded. Ileostomy or hepatic resection during cytoreductive surgery was an exclusion.
The study (NCT03029585) received Institutional Review Board approval at each institution. All subjects provided informed consent per institution and federal guidelines.
2.2. Treatment
Investigational product was supplied to the site in kits with a 60 cm3 vial containing 306 mg SPP powder (CritiTech, Lawrence, KS) and a smaller vial with 7 ml of reconstitution solution (1% polysorbate 80 in 0.9% sodium chloride for injection, USP). This study compared two dose-levels of SPP (100 mg/m2 and 200 mg/m2) instilled IP in 200 ml of lactated Ringer’s solution or phosphate buffered saline post-cytoreductive surgery.
Eligible subjects who underwent cytoreductive surgery received a single instillation of IP SPP prior to closing the peritoneum/fascia in the normal fashion. Final methodology for instillation of the SSP suspension was at the surgeon’s discretion, usually by direct administration to surgical sites using a syringe. All subjects were expected to initiate IV carboplatin and IV paclitaxel treatment 2–6 weeks following surgery. IV carboplatin and IV paclitaxel were administered, either every 21 days or on a dose-dense schedule for six cycles (SOC). Bevacizumab could be added to the SOC regimen at the investigator’s discretion, if it was initiated at least 6 weeks post-surgery. Dosing and dose adjustments were as per institutional standards. A Safety Monitoring Committee (SMC) was in place for study duration.
2.3. Response evaluation
Subjects were monitored post-surgery through the treatment period for safety based on adverse events and laboratory values. Imaging was performed prior to initiation of post-surgery IV chemotherapy to establish baseline tumor burden, after cycle 3, and at the end-of-treatment (EOT) visit. Baseline CA-125 serum levels were established prior to post-surgery cycle 2, and subsequent CA-125 testing was performed at each cycle and following the last cycle.
During the minimum 12-month follow-up period, subjects returned to the clinic every 3 months for CA-125 and clinical evaluation of the subject for signs or symptoms of disease progression. Imaging was performed when a subject developed symptoms, had a doubling of CA-125, or every 6 months, whichever was sooner. After 12 months of follow-up, subjects were contacted every 6 months until disease progression occurred in 50% of subjects or 12 months after the last subject completed their IV chemotherapy, whichever came first.
Endpoints were PFS at 6 months and evaluation of treatment-emergent adverse events. Clinical response was determined by radiological imaging and serum CA-125 measurements.
2.4. Statistical design
Data was summarized descriptively by dose level and relationship to safety, e.g., frequency of pre-specified AEs. Efficacy data (e.g., CA-125 and/or RECIST, PFS and survival) was evaluated in a similar manner without statistical testing.
In addition, the subjects entered a post-treatment follow-up phase and were monitored for >12 months post-chemotherapy to determine short-term PFS and survival.
3. Results
3.1. Subject characteristics
Ten subjects were enrolled at six institutions between September 2017 and May 2018 (Table 1). The median age was 67 years. All subjects were of non-Hispanic ethnicity. All subjects had high grade serous cell type tumors. Six subjects had primary disease and four subjects had recurrent disease. Four subjects received neoadjuvant chemotherapy. Seven subjects received IP SPP (100 mg/m2) and 3 subjects received IP SPP (200 mg/m2).
Table 1.
Patient demographic and baseline characteristics.
| IP SPP (100 mg/m2) + SOC (N = 7) |
IP SPP (200 mg/m2) + SOC (N = 3) |
Total (N = 10) |
|
|---|---|---|---|
| Age (years) | |||
| Median (range) | 68 (50–72) | 62 (56–71) | 67 (50–72) |
| Race | |||
| Not Hispanic, Black or African American | 1 (14.3%) | 0 (0.0%) | 1 (10.0%) |
| Not Hispanic, White | 6 (85.7%) | 3 (100.0%) | 9 (90.0%) |
| BMI (kg/m2) | |||
| Median (range) | 26.6 (20.0–40.6) | 32.3 (24.1–35.0) | 29.45 (20.0–40.6) |
| ECOG Status | |||
| 0 | 5 (71.4%) | 2 (66.7%) | 7 (70.0%) |
| 1 | 2 (28.6%) | 1 (33.3%) | 3 (30.0%) |
| Disease Status | |||
| Primary | 4 (57.1%) | 2 (66.7%) | 6 (60.0%) |
| Recurrent | 3 (42.9%) | 1 (33.3%) | 4 (40.0%) |
| Status of Ovarian Cancer at Screening | |||
| IIIA2 | 0 (0.0%) | 1 (33.3%) | 1 (10.0%) |
| IIIB | 2 (28.6%) | 0 (0.0%) | 2 (20.0%) |
| IIIC | 4 (57.1%) | 1 (33.3%) | 5 (50.0%) |
| IVB | 1 (14.3%) | 1 (33.3%) | 2 (20.0%) |
| Cell Type | |||
| High Grade Serous | 7 (100%) | 3 (100%) | 10 (100%) |
| Number of Completed IV Chemotherapy Cycles | |||
| 1 | 1 (14.3%) | 1 (33.3%) | 2 (20.0%) |
| 3 | 1 (14.3%) | 2 (66.7%) | 3 (30.0%) |
| 5 | 1 (14.3%) | 0 (0.0%) | 1 (10.0%) |
| 6 | 3 (42.9%) | 0 (0.0%) | 3 (30.0%) |
| 8 | 1 (14.3%) | 0 (0.0%) | 1 (10.0%) |
| Completion of 12 Month Follow-Up | |||
| Yes | 5 (71.4%) | 2 (66.7%) | 7 (70.0%) |
| No | 2 (28.6%) | 1 (33.3%) | 3 (30.0%) |
| Reason 12 Month Follow-Up Not Completed* | |||
| Withdrawal by Subject | 1 (50.0%) | 0 (0.0%) | 1 (33.3%) |
| Death** | 1 (50.0%) | 1 (100.0%) | 2 (66.7%) |
Percentages for 'Reason 12 Month Follow-Up Not Completed' are based on the subjects not completing the 12-month follow-up in that arm.
One subject death due to leptomeningeal carcinomatosis and one subject death due to respiratory arrest. Both deaths considered unrelated to study treatment.
3.2. Treatment response
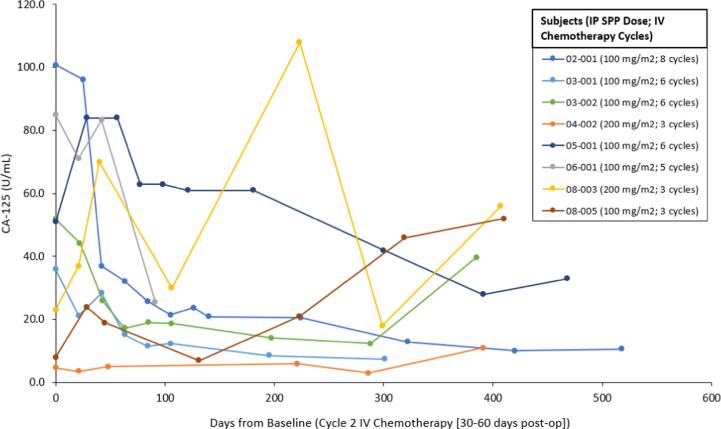
For analysis, 7 of 10 subjects were evaluable (1 subject withdrew consent shortly after receiving SPP due to travel constraints, 1 died unrelated to study drug during IV treatment and 1 was unevaluable). At completion of IV chemotherapy post-SPP instillation, PFS at 6 months was 66% (4/6) and at 12 months 66% (4/6) using RECIST 1.1. One subject had a complete response at the end of IV treatment, however died (unrelated to study treatment) before PFS evaluation. CA-125 levels typically fell and remained less than baseline (Fig. 1).
Fig. 1.
CA-125 Levels by Subject.
3.3. Safety
There was one case of incision dehiscence and one case of vaginal cuff leakage after surgery, both of which were considered possibly related to IP SPP. Treatment emergent adverse events that occurred in at least 3 subjects can be found in Table 2a. Treat emergent adverse events related to study treatment that occurred in at least 2 subjects can be found in Table 2b.
Table 2a.
Treatment emergent adverse events that occurred in at least 3 subjects.
| IP SPP (100 mg/m2) + SOC (N = 7) |
IP SPP (200 mg/m2) + SOC (N = 3) |
Total (N = 10) |
|
|---|---|---|---|
| Nausea | 3 (42.9%) | 3 (100%) | 6 (60.0%) |
| Procedural pain | 4 (57.1%) | 2 (66.7%) | 6 (60.0%) |
| Vomiting | 2 (28.6%) | 2 (66.7%) | 4 (40.0%) |
| Fatigue | 3 (42.9%) | 1 (33.3%) | 4 (40.0%) |
| Leukocytosis | 2 (28.6%) | 1 (33.3%) | 3 (30.0%) |
| Constipation | 2 (28.6%) | 1 (33.3%) | 3 (30.0%) |
| Urinary tract infection | 2 (28.6%) | 1 (33.3%) | 3 (30.0%) |
| Hypokalaemia | 2 (28.6%) | 1 (33.3%) | 3 (30.0%) |
Table 2b.
Treatment emergent adverse events related to study treatment that occurred in at least 2 subjects.
| IP SPP (100 mg/m2) + SOC (N = 7) |
IP SPP (200 mg/m2) + SOC (N = 3) |
Total (N = 10) |
|
|---|---|---|---|
| Leukocytosis | 2 (28.6%) | 1 (33.3%) | 3 (30.0%) |
| Fatigue | 3 (42.9%) | 0 (0.0%) | 3 (30.0%) |
| Nausea | 1 (14.3%) | 1 (33.3%) | 2 (20.0%) |
| Vomiting | 1 (14.3%) | 1 (33.3%) | 2 (20.0%) |
| Hypokalaemia | 2 (28.6%) | 0 (0.0%) | 2 (20.0%) |
4. Discussion
IV chemotherapy commonly utilizes dose-schedules based on systemic toxicity in contrast to maximal dose-response determinations of tumor response. Local treatment of solid tumors has the potential to overcome limitations of IV chemotherapy including rapid diffusion away from the administration site resulting in short tumor-dwell time and reduced systemic toxicities. Local tumor treatment would make drug available to tumor cells over multiple cell-division cycles and result in minimal systemic toxicity, thus providing tumoricidal benefits without compromising the patient’s wellbeing (Goldberg et al., 2002).
Local treatment of solid tumors with SPP has shown reduced levels of drug in the peripheral circulation, leading to minimal systemic toxicity with higher and sustained drug concentrations in the lungs and peritoneal cavity in both preclinical (Verco et al., 2018) and clinical settings (Othman et al., 2019, Lo et al., 2019), respectively. In this current study, upon completion of planned chemotherapy post-SPP instillation, we observed a PFS at 6 months of 66% (4/6) and at 12-months 66% (4/6) using RECIST 1.1.
The toxicities associated with SOC chemotherapy for ovarian cancer that often limit dosing and clinical benefit have encouraged the development of alternative therapies (Ledermann, 2017, Fujiwara et al., 2019, Lee et al., 2019, Armstrong and Walker, 2019, Trimble et al., 2008). Results from this study using IP SPP given at the time of surgical debulking as a local drug-delivery depot of paclitaxel are encouraging. As there are theoretical concerns that the prolonged half-life of paclitaxel associated with IP administration may delay wound healing and thus contribute to the 2 patients who developed wound healing problems, a larger Phase IIb randomized, placebo-controlled trial, administering 50 mg/m2 of SPP instilled at the time of surgical debulking is required to asses overall safety and efficacy in this difficult to treat population.
CRediT authorship contribution statement
Sally Mullany: Investigation, Writing - review & editing. David Scott Miller: Investigation, Writing - review & editing. Katina Robison: Investigation, Writing - review & editing. Kimberly Levinson: Investigation, Writing - review & editing. Yi-Chun Lee: Investigation, Writing - review & editing. S. Diane Yamada: Investigation, Writing - review & editing. Joan Walker: Conceptualization, Methodology, Investigation, Writing - review & editing. Maurie Markman: Conceptualization, Methodology, Writing - original draft, Visualization. Alyson Marin: Conceptualization, Formal analysis, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Peter Mast: Formal analysis, Resources, Data curation, Writing - original draft, Visualization, Supervision, Project administration. Gere diZerega: Conceptualization, Methodology, Resources, Writing - original draft, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
Sally Mullany: nothing to disclose
David Scott Miller: non-financial support from US Biotest.
Katina Robison: nothing to disclose
Kimberly Levinson: nothing to disclose
Yi-Chun Lee: nothing to disclose
S. Diane Yamada: non-financial support from US Biotest.
Joan Walker: nothing to disclose
Maurie Markman: personal fees from NanOlogy, outside the submitted work.
Gere diZerega: personal fees and non-financial support from NanOlogy and US Biotest.
Alyson Marin: personal fees from US Biotest.
Peter Mast: personal fees from US Biotest.
Acknowledgments
The authors would like to thank CritiTech, Inc. for investigational product supply and NanOlogy, LLC for funding the studies. Additional thanks to Leanne Drummond for assistance with study design and initiation.
References
- Armstrong D.K., Walker J.L. Role of intraperitoneal therapy in the initial management of Ovarian cancer. J. Clin. Oncol. 2019;37:2416–2419. doi: 10.1200/JCO.19.00671. [DOI] [PubMed] [Google Scholar]
- Cristea M.C., Frankel P., Synold T., Rivkin S., Lim D., Chung V. A phase I trial of intraperitoneal nab-paclitaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Cancer Chemother. Pharmacol. 2019;589–98 doi: 10.1007/s00280-019-03767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Hasegawa K., Nagao S. Landscape of systemic therapy for ovarian cancer in 2019: Primary therapy. Cancer. 2019;125(Suppl 24):4582–4586. doi: 10.1002/cncr.32475. [DOI] [PubMed] [Google Scholar]
- Goldberg E.P., Hadba A.R., Almond B.A., Marotta J.S. Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery. J. Pharm. Pharmacol. 2002;54:159–180. doi: 10.1211/0022357021778268. [DOI] [PubMed] [Google Scholar]
- Ledermann J.A. Front-line therapy of advanced ovarian cancer: new approaches. Ann. Oncol. 2017;28:viii46–viii50. doi: 10.1093/annonc/mdx452. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Minasian L., Kohn E.C. New strategies in ovarian cancer treatment. Cancer. 2019;125(Suppl 24):4623–4629. doi: 10.1002/cncr.32544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S., Hendifar A., Sharma N., Othman M., Mendoza-Ladd A., Verco S. A Novel EUS-Guided Intratumoral Delivery of Submicron Particle Paclitaxel (SPP) for the Treatment of Locally Advanced Pancreatic Cancer (LA-PAC): A Prospective Safety, Tolerability and Preliminary Efficiency Study: 6. Am. J. Gastroenterol. 2019;114:S3–S4. doi: 10.14309/01.ajg.0000589556.41054.b3. [DOI] [Google Scholar]
- Markman M., Rowinsky E., Hakes T., Reichman B., Jones W., Lewis J.L., Jr Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J. Clin. Oncol. 1992;10:1485–1491. doi: 10.1200/JCO.1992.10.9.1485. [DOI] [PubMed] [Google Scholar]
- Monk B.J., Chan J.K. Is intraperitoneal chemotherapy still an acceptable option in primary adjuvant chemotherapy for advanced ovarian cancer? Ann. Oncol. 2017;28:viii40–viii45. doi: 10.1093/annonc/mdx451. [DOI] [PubMed] [Google Scholar]
- NCI Clinical Announcement on Intraperitoneal Chemotherapy in Ovarian Cancer. Available from https://ctep.cancer.gov/highlights/20060105_ovarian.htm2006.
- Niu F., Roby K.F., Rajewski R.A., Decedue C. Paclitaxel nanoparticles: production using compressed CO2 as antisolvent, characterization and animal model studies. Polym. Drug Delivery II. 2006;924 doi: 10.1021/bk-2006-0924.ch017. [DOI] [Google Scholar]
- Othman M., Tabash A., Verco S., Verco J., Wendt A., diZerega G. NanoPac-2017-01 mid-study report: safety, tolerability, and preliminary efficacy of intracystic submicron particle paclitaxel (SPP) for the treatment of mucinous cysts: 69. Am. J. Gastroenterol. 2019;114:S42. doi: 10.14309/01.ajg.0000589808.63032.ba. [DOI] [Google Scholar]
- Trimble E.L., Thompson S., Christian M.C., Minasian L. Intraperitoneal chemotherapy for women with epithelial ovarian cancer. Oncologist. 2008;13:403–409. doi: 10.1634/theoncologist.2007-0058. [DOI] [PubMed] [Google Scholar]
- Verco J., Johnston W., Baltezor M., Kuehl P.J., Gigliotti A., Belinsky S.A. Pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac((R))) in a rodent model. J. Aerosol. Med. Pulm. Drug Deliv. 2018 doi: 10.1089/jamp.2018.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S.K., Johnson G.A., Maulhardt H.A., Moore K.M., McMeekin D.S., Schulz T.K. A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax(R)) in patients with peritoneal malignancies. Cancer Chemother. Pharmacol. 2015;75:1075–1087. doi: 10.1007/s00280-015-2737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]