Abstract
Background
The COMBO stent is a biodegradable-polymer sirolimus-eluting stent with endothelial progenitor cell capture technology for faster endothelialization.
Objective
We analyzed COMBO stent outcomes in relation to bleeding risk using the PARIS bleeding score.
Methods
MASCOT was an international registry of all-comers undergoing attempted COMBO stent implantation. We stratified patients as low bleeding-risk (LBR) for PARIS score ≤ 3 and intermediate-to-high (IHBR) for score > 3 based on baseline age, body mass index, anemia, current smoking, chronic kidney disease and need for triple therapy. Primary endpoint was 1-year target lesion failure (TLF), composite of cardiac death, myocardial infarction (MI) not clearly attributed to a non-target vessel or clinically-driven target lesion revascularization (TLR). Bleeding was adjudicated using the Bleeding Academic Research Consortium (BARC) definition. Dual antiplatelet therapy (DAPT) cessation was independently adjudicated.
Results
The study included 56% (n = 1270) LBR and 44% (n = 1009) IHBR patients. Incidence of 1-year TLF was higher in IHBR patients (4.1% vs. 2.6%, p = 0.047) driven by cardiac death (1.7% vs. 0.7%, p = 0.029) with similar rates of MI (1.8% vs. 1.1%, p = 0.17), TLR (1.5% vs. 1.6%, p = 0.89) and definite/ probable stent thrombosis (1.2% vs. 0.6%, p = 0.16). Incidence of 1-year major BARC 3 or 5 bleeding was significantly higher in IHBR patients (2.3% vs. 0.9%, p = 0.0094), as was the incidence of DAPT cessation (29.3% vs. 22.8%, p < 0.01), driven by physician-guided discontinuation.
Conclusions
Patients with intermediate-to-high PARIS bleeding risk in the MASCOT registry experienced greater incidence of 1-year TLF, major bleeding and DAPT cessation than LBR patients, without significant differences in stent thrombosis.
Keywords: PARIS bleeding risk score, COMBO stent, Endothelial progenitor cell capture, Dual therapy stent
Clinicaltrials.gov identifier: NCT02183454
1. Introduction
Drug eluting stents (DES) usually require a mandated duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) to prevent stent related complications, mainly stent thrombosis (ST) [1]. Novel DES types have included variations in polymer type, drug elution profiles, reduced strut thickness and added pro-healing layers to minimize the rate of incomplete strut coverage and ST towards shorter DAPT durations [2], [3]. Biodegradable polymer DES may be preferred with a view to decreasing risk of late and very late ST. The COMBO stent is a novel sirolimus eluting biodegradable polymer DES with polymer resorption within 3 months [4], [5]. In addition, this stent combines a circumferential pro-healing antibody layer which attracts circulating endothelial progenitor cells (EPCs) that mature in to healthy endothelial cells for faster coverage and more homogeneous endothelialization [5], [6]. The global MASCOT registry recently demonstrated a low rate of 1-year target lesion failure (TLF) and definite ST after COMBO stent PCI [7]. Given the stent’s unique properties for healthy strut coverage, and the possibility of short DAPT duration, the efficacy and safety of the COMBO stent in all-comer patients at increased bleeding risk are of interest. In this pre-specified analysis from the MASCOT registry, we sought to compare clinical outcomes and DAPT cessation events in low (LBR) versus intermediate-high bleeding risk (IHBR) groups defined using the Patterns of non-adherence to anti-platelet regimens in stented patients (PARIS) major bleeding risk score [8].
2. Methods
The study design and primary results of the MASCOT registry have been described elsewhere [7]. In brief, this was a prospective international post-marketing registry of all comers undergoing attempted PCI with the COMBO stent as part of routine clinical care. The exclusion criteria were: PCI for treatment of ST, concurrent participation in another drug or device trial where routine angiographic follow up was planned, life expectancy <12 months, high probability for non-adherence to follow-up due to social, psychological or medical reasons and refusal to participate in the registry. The study included 2,614 patients from 60 sites in Europe, Asia, the Middle East and South America with 1-year follow. DAPT duration was recommended per societal guidelines for a period of 6–12 months in all patients, and 12 months in patients with acute coronary syndrome (ACS). Follow-up was conducted by telephone or clinic visit at 1, 6 and 12-months post-PCI. The study was managed by the Clinical Coordinating Center at the Icahn School of Medicine at Mount Sinai with rigorous monitoring during the study period. All clinical events and DAPT cessation events were adjudicated by an independent clinical events committee (CEC). The study was conducted in keeping with principles of the Declaration of Helsinki and Good Clinical Practice. All sites received local ethics committee approval or a waiver to participate in the registry and all patients provided written informed consent. The study was registered on clinicaltrials.gov (identifier NCT02183454).
2.1. Study device
The OrbusNeich COMBO sirolimus-eluting stent is composed of a 316L stainless steel alloy in a double helix strut design with a strut thickness of 100 µm. The COMBO stent is abluminally coated with a biodegradable polymer containing sirolimus, with a time release profile of the drug similar to Cypher DES. The total sirolimus drug content of the COMBO Stent is 5 μg/mm stent length, and the drug is fully eluted from the stent within 30 days. The biodegradable polymer is fully resorbed within 90 days. Covalently attached to the stent matrix is a unique circumferential layer of murine monoclonal anti-human CD34 antibody, which targets circulating CD34 + EPCs towards faster endothelialization [4], [5].
2.2. Endpoints and definitions
The primary endpoint of the study was 1-year TLF, defined as a composite of cardiac death, non-fatal myocardial infarction (MI) not clearly attributable to a non-target vessel or ischemia driven target lesion revascularization (TLR) by PCI or coronary artery bypass grafting (CABG). Secondary endpoints included the individual components of TLF, ST, major adverse cardiac events (MACE) (composite of all-cause death, MI or ischemia driven revascularization), bleeding and DAPT cessation.
Death, ST and ischemia driven TLR were adjudicated using the Academic Research Consortium definitions [9], and MI was adjudicated using the third universal definition [10]. Bleeding was classified using the Bleeding Academic Research Consortium (BARC) definitions [11]– major bleeding as BARC 3 or 5, minor bleeding as BARC 2 and nuisance bleeding as BARC 1. DAPT cessations were categorized as discontinuation, disruption and interruption using the PARIS definitions [12]; discontinuation was defined as physician-guided permanent cessation, disruption as non-recommended cessation, and interruption as temporary cessation for less than 14 days.
The PARIS bleeding risk score was derived from a large registry in all comers undergoing PCI [8], and has been validated in other PCI populations [8], [13]. For this analysis, patients undergoing COMBO PCI were grouped by calculated PARIS bleeding score, comprising 6 baseline variables: age, body mass index (BMI), anemia, current smoking, chronic kidney disease (CKD) and need for triple therapy [8]. All variables required for calculation of the PARIS bleeding score were available in 2,279 out of 2,614 (87.2%) study patients. Supplementary Fig. 1 shows the histogram displaying the frequency of patients for each value of the PARIS bleeding score. Due to the small number of patients with a high bleeding score of ≥ 8 (n = 71, 3.1%), we dichotomized the study sample into low bleeding risk (LBR) for score ≤ 3 and intermediate-high (IHBR) bleeding risk for score >3.
2.3. Statistical analysis
Categorical data are reported as numbers and frequencies and compared using the chi-square test. Continuous data are reported as means and standard deviations, and compared using the Student’s t-test. Clinical and DAPT cessation events between the LBR and IHBR groups were analyzed in a time to event manner using Kaplan-Meier methods and represented using time to event curves. Follow-up was censored to last known follow-up or 12-months, whichever came first. In a sensitivity analysis we examined for trends in 1-year TLF and major bleeding from low to high bleeding risk groups. Two-sided p-values of < 0.05 were considered significant. Statistical analyses were conducted using SAS version 9.4 (Durham, North Carolina) and Stata version 14.0 (College Station, Texas).
3. Results
The study included 56% (n = 1270) LBR and 44% (n = 1009) IHBR patients. Table 1 shows the baseline characteristics of the groups. Per the bleeding score definition, the IHBR group included older patients (70.5 ± 10.3 years vs. 61.4 ± 10.1 years, p < 0.01) with lower BMI and higher prevalence of current smokers, CKD, anemia and atrial fibrillation. Almost 47.9% IHBR patients had BMI < 25 kg/m2 compared to 8.5% low bleeding risk patients (p < 0.01). Despite lower BMI, fewer Asians compared to Caucasians were noted in the IHBR group. IHBR patients included more women (28.5% vs. 19.8%, p < 0.01) and also had higher prevalence of peripheral arterial disease, prior stroke and heart failure. Interestingly there were no differences between groups in prior MI, PCI or surgical revascularization. With respect to indication for index PCI, IHBR patients presented more often with non-ST segment elevation MI (NSTEMI) and less often with unstable angina. The incidence of STEMI and stable angina presentations were not different between the groups.
Table 1.
Baseline characteristics between Low and Intermediate/High bleeding risk groups.
| Low Bleeding Risk N = 1270 |
Intermediate to High Bleeding Risk N = 1009 |
P-value | |
|---|---|---|---|
| Age, years | 61.4 ± 10.1 | 70.5 ± 10.3 | <0.0001* |
| Females | 251(19.8%) | 288(28.5%) | <0.0001* |
| Race | 0.011 | ||
|
263(21.1%) | 188(18.9%) | |
|
7(0.6%) | 2(0.2%) | |
|
59(4.7%) | 26(2.6%) | |
|
919(73.6%) | 779(78.3%) | |
| BMI (kg/m2) | 28.8 ± 3.6 | 27.1 ± 5.8 | <0.0001* |
| BMI < 25 kg/m2 | 108(8.5%) | 483(47.9%) | <0.0001* |
| Diabetes mellitus | 398(31.3%) | 320(31.7%) | 0.85 |
|
84(22.5%) | 88(29.0%) | 0.06 |
| Current smoker | 250(19.7%) | 391(38.8%) | <0.0001* |
| Hypercholesterolemia | 762(60.0%) | 588(58.3%) | 0.41 |
| Hypertension | 900(70.9%) | 726(72.0%) | 0.57 |
| Congestive heart failure | 69(5.4%) | 102(10.1%) | <0.0001* |
| Chronic renal failure | 11(0.9%) | 140(13.9%) | <0.0001* |
| Peripheral arterial disease | 47(3.8%) | 85(8.6%) | <0.0001* |
| Previous stroke | 43(3.4%) | 62(6.1%) | 0.0018* |
| Previous MI | 312(24.6%) | 228(22.6%) | 0.27 |
| Previous PCI | 325(25.6%) | 270(26.8%) | 0.53 |
| Previous CABG | 66(5.2%) | 63(6.2%) | 0.28 |
| Atrial fibrillation | 42(3.4%) | 130(13.2%) | <0.0001* |
| Medications on Admission | |||
|
747(58.8%) | 577(57.2%) | 0.43 |
|
735(57.9%) | 555(55.0%) | 0.17 |
|
23(1.8%) | 108(10.7%) | <0.0001* |
|
921(72.5%) | 663(65.7%) | 0.0005* |
|
422(33.2%) | 400(39.6%) | 0.0015* |
|
886(69.8%) | 642(63.6%) | 0.0020* |
| Discharge medications | |||
|
957(75.4%) | 745(73.8%) | 0.41 |
|
977(76.9%) | 754(74.7%) | 0.22 |
|
38(3.0%) | 151(15.0%) | <0.001 |
|
1226(96.5%) | 940(93.2%) | 0.0002 |
|
589(46.4%) | 570(56.5%) | <0.001 |
|
1262(99.4%) | 989(98.0%) | 0.004 |
|
0.0001 | ||
|
830(65.4%) | 737(73.0%) | |
|
0(0.0%) | 4(0.4%) | |
|
52(4.1%) | 24(2.4%) | |
|
385(30.3%) | 243(24.1%) | |
|
3(0.2%) | 1(0.1%) |
Values are shown as n (%) or mean ± standard deviation (SD). ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafts; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention.
Table 2 shows the procedural characteristics between the groups. The rates of radial access PCI were similar (69.6% vs 73.0%, p = 0.31). The use of glycoprotein 2b/3a inhibitors was 10.6% overall without differences between the groups. IHBR patients had a higher prevalence of complex lesions mainly ACC/AHA type B2 or C lesions, and heavy lesion calcification. The rates of in-stent restenosis and bypass graft PCI were similar, and chronic total occlusions were less frequent in IHBR compared to LBR patients. IHBR patients more often underwent PCI to the left coronary system, and received rotational atherectomy (1.9% vs. 1.0%, p = 0.03) and post-dilation of stents compared to LBR patients. IHBR patients more often required use of optical coherence tomography (3.7% vs. 2.0%, p = 0.009). There were no differences in total implanted stent length or implanted stent diameter between groups.
Table 2.
Procedural characteristics between Low and Intermediate/High bleeding risk groups.
| Procedure level characteristics | Low Bleeding Risk N = 1270 | Intermediate to High Bleeding Risk N = 1009 | P-value |
|---|---|---|---|
| Radial access | 910(73.0%) | 688(69.6%) | 0.31 |
| Vascular closure device successfully deployed | 215(19.4%) | 177(19.4%) | 0.95 |
| Indication for PCI | 0.0007* | ||
|
164(12.9%) | 185(18.3%) | |
|
268(21.1%) | 216(21.4%) | |
|
112(8.8%) | 93(9.2%) | |
|
453(35.7%) | 353(35.0%) | |
|
271(21.4%) | 162(16.1%) | |
| Extent of CAD: | 0.06 | ||
|
640(50.4%) | 464(46.0%) | |
|
391(30.8%) | 322(31.9%) | |
|
239(18.8%) | 223(22.1%) | |
| Left main disease >= 50% | 115(9.1%) | 109(10.8%) | 0.16 |
| Any procedural complication | 27(2.1%) | 28(2.8%) | 0.32 |
| Lesion level characteristics | N = 1536 | N = 1241 | P- value |
| Location of lesion | 0.0048* | ||
|
705(45.9%) | 581(46.8%) | |
|
320(20.8%) | 265(21.4%) | |
|
18(1.2%) | 36(2.9%) | |
|
492(32.1%) | 359(28.9%) | |
| Bypass Graft | 24(1.6%) | 17(1.4%) | 0.37 |
| AHA/ACC lesion type | 0.0075* | ||
|
144(9.5%) | 112(9.1%) | |
|
563(37.1%) | 387(31.4%) | |
|
550(36.2%) | 515(41.8%) | |
|
261(17.2%) | 217(17.6%) | |
|
811 (52.8%) | 732 (59.0%) | 0.0013 |
| Heavy calcification | 246(16.0%) | 247(19.9%) | 0.0079* |
| Chronic total occlusions | 76(5.3%) | 42(3.6%) | 0.0364* |
| Fractional flow reserve testing | 47(3.2%) | 27(2.3%) | 0.15 |
| Intravascular ultrasound | 69(4.7%) | 68(5.7%) | 0.24 |
| Optical coherence tomography | 30(2.0%) | 44(3.7%) | 0.0088* |
| Rotational atherectomy | 14(1.0%) | 23(1.9%) | 0.0333* |
| Post dilatation | 767(49.9%) | 700(56.4%) | 0.0007* |
| Total length of Combo stents implanted (mm) | 23.02 ± 11.68 | 22.41 ± 10.90 | 0.17 |
| Diameter of largest Combo stent (mm) | 3.13 ± 0.43 | 3.11 ± 0.44 | 0.13 |
Values are shown as n (%) or mean ± standard deviation (SD). ACC, American College of Cardiology; AHA, American Heart Association; CAD, coronary artery disease; NSTEMI, Non-ST segment myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST segment myocardial infarction.
At discharge, IHBR patients were more likely to receive clopidogrel (73% vs. 65.4%), oral anticoagulation (15.0% vs. 3.0%, p < 0.001) and proton pump inhibitors (56.5% vs 46.4%, p < 0.001), but they were less likely than LBR patients to receive statins (93.2% vs. 96.5%, p = 0.0002).
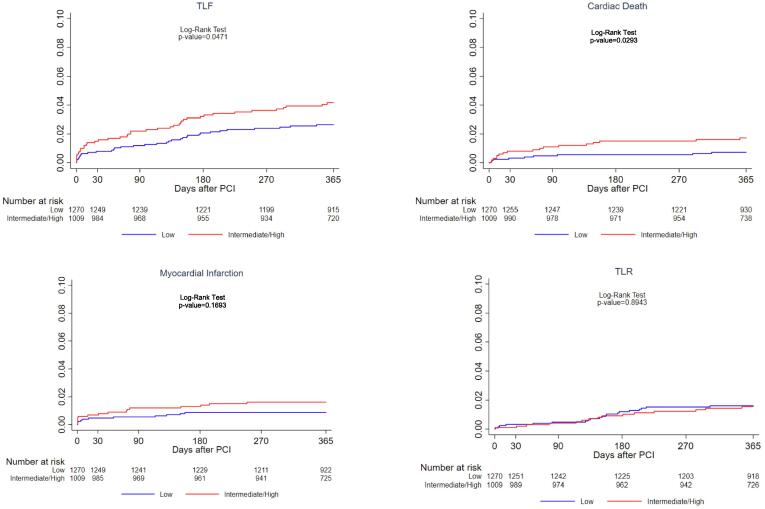
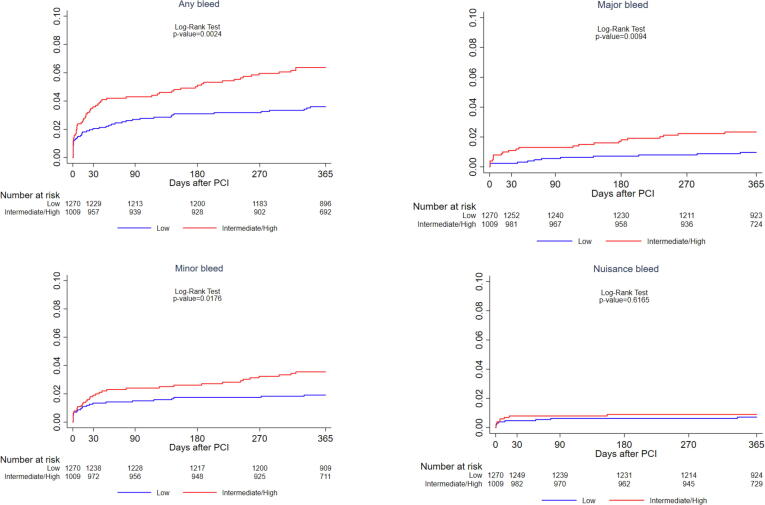
One-year clinical outcomes are shown in Supplementary Table 1 and Fig. 1. One-year TLF was higher in IHBR vs. LBR patients, 4.1% vs. 2.6% (p = 0.047) driven by higher cardiac death (1.7% vs. 0.7%, p = 0.029) despite similar rates of MI, TLR and definite ST (0.5% vs. 0.5%, p = 0.94). The rate of definite or probable ST was not statistically different between the groups (1.2% vs. 0.6%, p = 0.16). In contrast, the incidence of bleeding outcomes was significantly higher in IHBR patients through 1-year follow up (Fig. 2). At 1-year incidence of any bleeding events was 6.2% in IHBR compared to 3.5% in LBR patients (p = 0.0024). Similarly, BARC 3 or 5 major bleeding occurred in 2.3% vs. 0.9% patients of the two groups (p = 0.0094). In a sensitivity analysis we examined the trend in the crude hazard of 1-year TLF and major bleeding from low to high bleeding risk categories. A significant trend was noted for increasing TLF from low to high risk groups (p-trend = 0.011). Greater risk was attributed to the high bleeding risk group (HR 3.43, 95% CI 1.44–8.18, p = 0.006) followed by the intermediate risk group (HR 1.45, 95% CI 0.90–2.33, p = 0.13) compared to low risk patients (reference group). Similarly, the hazard associated with major bleeding increased from the low risk to high risk patients (p-trend < 0.001).
Fig. 1.
Cumulative incidence of 1-year ischemic events in Low versus Intermediate to high bleeding risk patients.
Fig. 2.
Cumulative incidence of 1-year bleeding events in Low versus Intermediate to high bleeding risk patients.
With respect to DAPT cessation, at 1-year IHBR patients demonstrated higher rates of overall DAPT cessation (29.3% in IHBR vs. 22.8% in LBR patients) and physician recommended discontinuation (26.0% vs. 20.2%), p < 0.01 for both. There were no statistically significant differences in the rates of 1-year DAPT interruptions (0.9% in both groups, p = 0.89) or disruption (2.5% vs. 1.7%, p = 0.16). Supplementary Fig. 2 provides an overview of DAPT cessations over 1-year follow up between IHBR and LBR patients. At each follow up time point, aspirin discontinuation was significantly greater in IHBR compared to LBR patients, without differences in P2Y12 inhibitor discontinuation. At 12 months, aspirin discontinuation was noted in 7.5% IHBR vs. 1.9% LBR patients, p < 0.01. There were no differences between groups for P2Y12 inhibitor discontinuation at 12 months (18.4% vs. 18.3%, p = 0.89) (Supplementary Figure 3).
4. Discussion
This is the first analysis of 1-year COMBO stent outcomes in all comers stratified by predicted bleeding risk prior to PCI. The main findings are as follows: 1. Intermediate to high bleeding risk patients included higher prevalence of several comorbidities per the PARIS bleeding score definition and they were more often women compared to low bleeding risk patients. Procedurally, IHBR patients had several complex lesion characteristics; 2. at 1-year the incidence of TLF was higher among IHBR patients driven by significantly higher rates of cardiac death without differences in ST and other ischemic outcomes; 3. the incidence of any bleeding and BARC 3 or 5 major bleeding was greater in IHBR patients. Accordingly, the rate of DAPT cessations was higher in this group, mainly due to more physician recommended discontinuation; 4. At each follow-up time point, the rate of ASA but not P2Y12 inhibitor discontinuation was greater in IHBR than LBR patients.
Marked improvements in DES have improved clinical outcomes for all patient groups with low rates of stent related adverse outcomes [2]. An important clinical question to address is whether specific stent types are more suitable to special groups such as high bleeding risk patients. Prior studies have shown that these patients can be at high risk of both ischemic and bleeding outcomes [14], [15], [16], [17]. The novel COMBO stent combines low dose sirolimus elution from a biodegradable polymer with anti CD34 coating for EPC capture and more optimal endothelialization [4], [5]. The unique aspects of this stent technology suggest the feasibility of shorter DAPT duration [18]. Recent data from the Japan-USA HARMONEE study demonstrate that COMBO stent PCI in short lesions were associated with more homogeneous coverage at 12 months compared to durable polymer everolimus eluting stents [6].
However, there may be several patient factors contributing to stent failure and recurrent ischemic events including systemic risk factors, complex anatomy and non-compliance to DAPT in the early time period [19], which may be more pronounced in IHBR patients. Complex PCI is well known to be associated with greater risk of ischemic outcomes, as well as higher risk of bleeding due to underlying patient characteristics [20]. Despite PCI to more complex lesions, IHBR patients in this study were less likely to receive not only potent antiplatelet therapy but also statins at the time of discharge, which could have impacted clinical outcomes.
We noted that IHBR patients had a 1.8% absolute increase in 1-year TLF compared to LBR patients. This was largely driven by cardiac death which did not appear to be a function of ST, MI or TLR events. Given that IHBR patients were a decade older with double the prevalence of heart failure and greater prevalence of atrial fibrillation compared to LBR patients, we can hypothesize that arrhythmias and heart failure may have contributed to the higher cardiac death rate. IHBR patients had numerical differences in stent related outcomes of target vessel MI and definite or probable ST but these differences were not statistically significant compared to LBR patients. Other studies have shown that high bleeding risk patients have greater risk of ST, [14], [17], [21], albeit differences in the definitions of high bleeding risk compared to our study may in part be responsible for discrepancies with our results. Indeed, the rate of 1-year definite ST was very low at 0.5%, irrespective of bleeding risk in the current study.
Conversely as can be expected, there were significant differences in bleeding outcomes between the two groups. Although major bleeding was low in both groups with nearly 70% undergoing radial access PCI and 19% undergoing successful arterial closure after transfemoral PCI, IHBR patients included more women and had a 1.4% absolute increase in major bleeding events. This was on a background of greater clopidogrel use rather than potent antiplatelet therapy. Given that the study did not mandate shorter DAPT, rather the recommendation was for DAPT per local institutional policy and societal guidelines, we noted that early cessation was infrequent and nearly 75% study patients continued DAPT to 12 months, in keeping with guidelines at the time of study enrollment. However, aligned with more bleeding events in IHBR patients, the incidence of any DAPT cessation was higher in this group. Reassuringly this was driven by physician recommended discontinuation. The PARIS registry has previously shown that physician recommended discontinuation is safe whereas disruption due to non-compliance is adversely linked with post-PCI outcomes [12], [16]. Although greater DAPT disruption may be anticipated in high bleeding risk patients, the overall low bleeding rates and close patient follow-up may have minimized opportunities for unsupervised DAPT cessation.
The current analysis demonstrates that IHBR patients undergoing PCI with the COMBO stent experienced low rates of 1-year TLF and ST, however operators should remain vigilant regarding pharmacotherapy strategies and DAPT duration. Results from the REDUCE trial in young ACS patients demonstrate the potential for short DAPT for 3 months after COMBO PCI, where risk of bleeding outweighs benefit of ongoing DAPT [18]. Our study demonstrates that overall differences in COMBO stent related outcomes (MI, ST) were not greater in increased bleeding risk patients when guided by physician monitored DAPT cessation. The higher observed rates of ASA but not P2Y12 inhibitor discontinuations in IHBR patients suggest a real-world preference for ASA free antithrombotic strategies, further supported by recent randomized trial data [22], [23]. Nevertheless, short term DAPT mandates utmost attention to optimal approaches to PCI, with careful attention to stent post-dilation and judicious use of adjuvant technologies [24], [25].
The current study had some limitations. This was a pre-specified subgroup analysis from an observational study. The frequency of truly high bleeding risk patients was small, thus, we analyzed intermediate and high bleeding risk patients as a single group. However, we demonstrated a significant trend for greater hazard of 1-year TLF with increasing PARIS risk of bleeding. Although the findings show the efficacy of COMBO stents in patients with elevated bleeding risk, randomized data are needed to confirm safety outcomes coupled with short DAPT duration. Compliance to DAPT was patient reported and not verified by pill count or pharmacy assessments. We did not have information on adherence to other secondary prevention medications. For example, the rate of statin use was lower in IHBR patients at discharge. However, since statins are believed to increase the rate of circulating EPCs [26], increased adherence may be of both technical and clinical relevance after COMBO stenting.
5. Conclusions
Patients with PARIS intermediate to high bleeding risk undergoing COMBO stent PCI experienced higher 1-year TLF rates driven by cardiac death, without differences in other ischemic outcomes. DAPT discontinuation rates were significantly greater in IHBR patients in keeping with greater major bleeds. A preference for ASA rather than P2Y12 inhibitor discontinuation was noted in IHBR patients during the first year after PCI.
CRediT authorship contribution statement
Jaya Chandrasekhar: Conceptualization, Methodology, Writing - original draft. Usman Baber: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Samantha Sartori: Data curation, Formal analysis, Writing - review & editing. Melissa B. Aquino: Data curation, Formal analysis, Writing - review & editing. Petr Hájek: Investigation, Project administration, Writing - review & editing. Borislav Atzev: Investigation, Project administration, Writing - review & editing. Martin Hudec: Investigation, Project administration, Writing - review & editing. Tiong Kiam Ong: Investigation, Project administration, Writing - review & editing. Martin Mates: Investigation, Project administration, Writing - review & editing. Borislav Borisov: Investigation, Project administration, Writing - review & editing. Hazem M. Warda: Investigation, Project administration, Writing - review & editing. Peter den Heijer: Investigation, Project administration, Writing - review & editing. Jaroslaw Wojcik: Investigation, Project administration, Writing - review & editing. Andres Iniguez: Investigation, Project administration, Writing - review & editing. Zdeněk Coufal: Investigation, Project administration, Writing - review & editing. Ahmed Khashaba: Investigation, Project administration, Writing - review & editing. Muhammad Munawar: Investigation, Project administration, Writing - review & editing. Robert T. Gerber: Investigation, Project administration, Writing - review & editing. Bryan P. Yan: Investigation, Project administration, Writing - review & editing. Paula Tejedor: Investigation, Project administration, Writing - review & editing. Petr Kala: Investigation, Project administration, Writing - review & editing. Houng Bang Liew: Investigation, Project administration, Writing - review & editing. Michael Lee: Supervision, Writing - review & editing. Deborah N. Kalkman: Conceptualization, Writing - review & editing. George D. Dangas: Conceptualization, Supervision, Writing - review & editing. Robbert J. Winter: Conceptualization, Investigation, Methodology, Supervision, Writing - review & editing. Antonio Colombo: Conceptualization, Supervision, Writing - review & editing. Roxana Mehran: Conceptualization, Methodology, Funding acquisition, Project administration, Supervision, Writing - review & editing.
Acknowledgments
Acknowledgements
The authors thank all patients for their participation in the included studies. All interventional cardiologists, catheterization laboratory staff and research teams are acknowledged for their contributions.
Funding
OrbusNeich Medical (Ft.Lauderdale, Florida, USA) was the sponsor of the MASCOT registry.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100605.
Appendix A. MASCOT investigators
Steering committee
Antonio Colombo, MD (Principal Investigator)
Roxana Mehran, MD
Tiong Kiam Ong, MD
Michael Lee, MD
Andres Iniguez, MD, PhD
Stephen Rowland, PhD
Clinical events Committee:
Newsha Ghodsi, MD (Chair)
Steven Marx, MD
Douglas DiStefano, MD
Jesse Weinberger, MD
Shing Chiu Wong, MD
Bruce Darrow, MD
David Kaufman, MD
Mark Milstein, MD
Clinical Coordinating Center:
Roxana Mehran, MD (Director)
Jaya Chandrasekhar, MBBS, MS
Usman Baber, MD, MS
Melissa Aquino, MS
Samantha Sartori, PhD
Clayton Snyder, BS
Theresa Franklin-Bond, PA, MS
Jin Young Cha, BSc.
Lynn Vandertie
Emma Whittaker
Kate Allen
Birgit Vogel, MD
Serdar Farhan, MD
Sabato Sorrentino, MD, PhD
Zhen Ge, MD
Site investigators:
Marc Carlier, MD
Suzanne Pourbaix, MD
Borislav Borisov, MD
Borislav Atzev, MD
Dobrin Vasilev, MD
Christos Christou, MD
Ladislav Pešl, MD
Zdeněk Coufal, MD
Petr Kala, MD & Petr Jeřábek, MD
Petr Hájek, MD, Ph.D
Alexander Schee, MD & Roman Ondrejcak, MD
Vladimír Rozsíval, MD & Jan Matějka, MD
Martin Mates, MD
Ahmed Khashaba, MD
Hazem Warda, MD
Sulev Margus, MD
Bryan Yan, MD
Cheuk Sum Lam, MD
Tak Sun Chung, MD
Li Wah Tam, MD
Alan Ka Chun Chan, MD
Ping Tim Tsui, MD
Kin Lam Tsui, MD
Teguh Santoso, MD
Muhammad Munawar, MD
Muhammad Syukri, MD
Dasdo Antonius Sinaga, MD
Alessandro Lupi, MD
Carlo Briguori, MD
Antonio Colombo, MD
Oteh Maskon, MD
Tiong Kiam Ong, MD
Sazzli Kazim, MD
Chuey Yan Lee, MD
Houng Bang Liew, MD
Rosli Mohammad Ali, MD
Ramesh Singh, MD
Peter Den Heijer, MD
Jaroslaw Wojcik, MD
Vladan Vukčević, MD
Martin Hudec, MD
Anton Farkaš, MD
Stanislav Juhás, MD & Monika Jankajová, MD
Milan Dragula, MD
Carlos Cuellas Ramon, MD
Andres Iniguez, MD, PhD
Paula Tejedor, MD
Fernando Lozano Ruiz-Poveda, MD
Alfonso Torres Bosco, MD
Essia Boughzela, MD & Medhi Slim, MD
Habib Haouala, MD & Dhaker Lahidheb, MD
Mohamed Rachid Boujnah, MD
Grahame K. Goode, MD
Sukhbir Dhamrait, MD
Robert Gerber, MD
Piers Clifford, MD
Timothy Kinnaird, MD
Gabriel Varnagy, MD
Pedro J. Aguiar R., MD
Thuong Van Huynh, MD
Appendix B. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Levine G.N., Bates E.R., Bittl J.A. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–155. doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekhar J., Martin K., Mehran R. Role of coronary drug-eluting stents in current clinical practice. Clin. Pharm. 2016 doi: 10.1211/CP.2016.20201885. [DOI] [Google Scholar]
- 3.Bangalore S. The Elusive Late Benefit of Biodegradable Polymer Drug-Eluting Stents. Circulation. 2019;139:334–336. doi: 10.1161/CIRCULATIONAHA.118.038378. [DOI] [PubMed] [Google Scholar]
- 4.Granada J.F., Inami S., Aboodi M.S. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ. Cardiovasc. Interv. 2010;3:257–266. doi: 10.1161/CIRCINTERVENTIONS.109.919936. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa G., Granada J.F., Alviar C.L. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc. Interv. 2010;3:68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Saito S., Krucoff M.W., Nakamura S. Japan-United States of America Harmonized Assessment by Randomized Multicentre Study of OrbusNEich's Combo StEnt (Japan-USA HARMONEE) study: primary results of the pivotal registration study of combined endothelial progenitor cell capture and drug-eluting stent in patients with ischaemic coronary disease and non-ST-elevation acute coronary syndrome. Eur. Heart J. 2018;39:2460–2468. doi: 10.1093/eurheartj/ehy275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo A., Chandrasekhar J., Aquino M. Safety and efficacy of the COMBO bio-engineered stent in an all-comer PCI cohort: 1-Year final clinical outcomes from the MASCOT post-marketing registry. Int. J. Cardiol. 2019;283:67–72. doi: 10.1016/j.ijcard.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Baber U., Mehran R., Giustino G. Coronary Thrombosis and Major Bleeding After PCI With Drug-Eluting Stents: Risk Scores From PARIS. J. Am. Coll. Cardiol. 2016;67:2224–2234. doi: 10.1016/j.jacc.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip D.E., Windecker S., Mehran R. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K., Alpert J.S., Jaffe A.S. Third universal definition of myocardial infarction. Eur. Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 11.Mehran R., Rao S.V., Bhatt D.L. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R., Baber U., Steg P.G. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. doi: 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 13.Sorrentino S., Claessen B., Mehran R., Kandzari D., Morice M.C., Leon M., Gersh B., Ben-Yehuda O., Redfors B., Dressler O., Pocock S., Kappetein A.P., Sabik J., Serruys P., Stone G. Validation of PARIS Risk Scores in Patients Treated With Everolimus-Eluting Stents for Left Main Coronary Artery Disease: Analysis From the EXCEL Trial. J. Am. Coll. Cardiol. 2018;72(13) doi: 10.1016/j.jacc.2018.08.2078. Supplement. [DOI] [Google Scholar]
- 14.Jensen C.J., Naber C.K., Urban P. Two-year outcomes of high bleeding risk patients with acute coronary syndrome after Biolimus A9 polymer-free drug-coated stents: a LEADERS FREE substudy. EuroIntervention. 2018;13:1946–1949. doi: 10.4244/EIJ-D-17-00720. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekhar J, Mehran, R., Genereux, P., Dangas, G., Zhang, Y., McAndrew, T., Kirtane, A., Stone, G. Effect of thrombotic risk and bleeding risk by platelet reactivity status on 2-year mortality: A report from the ADAPT DES study. Journal of the American College of Cardiology March 21, 2017, 69 (11 Supplement) 1012; DOI: 10.1016/S0735-1097(17)34401-7.
- 16.Sorrentino S., Sartori S., Baber U. Bleeding Risk, Dual Antiplatelet Therapy Cessation, and Adverse Events After Percutaneous Coronary Intervention: The PARIS Registry. Circ. Cardiovasc. Interv. 2020;13(4) doi: 10.1161/CIRCINTERVENTIONS.119.008226. [DOI] [PubMed] [Google Scholar]
- 17.Sorrentino S., Baber U., Claessen B.E. Determinants of Significant Out-Of-Hospital Bleeding in Patients Undergoing Percutaneous Coronary Intervention. Thromb. Haemost. 2018;118:1997–2005. doi: 10.1055/s-0038-1673687. [DOI] [PubMed] [Google Scholar]
- 18.De Luca G., Damen S.A., Camaro C. Final results of the Randomised Evaluation of short-term DUal antiplatelet therapy in patients with acute Coronary syndromE treated with a new generation stent (REDUCE) trial. EuroIntervention. 2019;15(11):e990–e998. doi: 10.4244/EIJ-D-19-00539. [DOI] [PubMed] [Google Scholar]
- 19.Kirtane A.J., Stone G.W. How to minimize stent thrombosis. Circulation. 2011;124:1283–1287. doi: 10.1161/CIRCULATIONAHA.110.976829. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekhar J., Baber U., Sartori S. Associations Between Complex PCI and Prasugrel or Clopidogrel Use in Patients With Acute Coronary Syndrome Who Undergo PCI: From the PROMETHEUS Study. Can. J. Cardiol. 2018;34:319–329. doi: 10.1016/j.cjca.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Vogel B, Claessen, B., Baber, U., Sorrentino, S., Zhao, W., Krucoff, M., Kozuma, K., Ge, J., Seth, A., Makkar, R., Bangalore, S., Bhatt, D., Angiolillo, D., Saito, S., Neumann, FJ., Hermiller, J. and Valgimigli, M. Clinical outcomes in high bleeding risk patients undergoing complex PCI with the Xience everolimus eluting stent: a patient-level pooled analysis from four Xience post-approval trials. Journal of the American College of Cardiology. Volume 72, Issue 13 Supplement, September 2018. DOI: 10.1016/j.jacc.2018.08.1723.
- 22.Watanabe H., Domei T., Morimoto T. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehran R., Baber U., Sharma S.K. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019;381(21):2032–2042. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- 24.Jeremias A, Davies JE, Maehara A. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention: The DEFINE PCI Study. JACC Cardiovasc Interv. 2019;12(20):1991–2001. doi: 10.1016/j.jcin.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekhar J., Allada C., O'Connor S. Efficacy of non-compliant balloon post-dilation in optimization of contemporary stents: A digital stent enhancement study. Int. J. Cardiol. Heart Vessel. 2014;3:43–48. doi: 10.1016/j.ijchv.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hibbert B., Simard T., Ramirez F.D. The effect of statins on circulating endothelial progenitor cells in humans: a systematic review. J. Cardiovasc. Pharmacol. 2013;62:491–496. doi: 10.1097/FJC.0b013e3182a4027f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.