Highlights
-
•
LG-ESS is a rare and indolent uterine tumor which has remarkable sensitivity to hormonal agents.
-
•
We presented the case of recurrent LG-ESS that was effectively and safely treated with Dienogest.
-
•
Dienogest may be useful for the treatment of LG-ESS that is resistant to other progestins.
Keywords: Dienogest, Endometrial stromal sarcoma, Hormonal therapy
Abstract
Low-grade endometrial stromal sarcoma (LG-ESS) is a rare uterine tumor that sometimes recurs and advances. Hormonal treatment, especially high-dose progestins and aromatase inhibitors (AIs), has demonstrated efficacy against these tumors. Because the standard treatment period is uncertain and hormonal treatment is effective, hormonal agents are likely to be used long-term, especially when a residual tumor is present. However, the long-term use of high-dose progestins and AIs may cause thromboembolism, as well as musculoskeletal stiffness and pain. Dienogest, a relatively new progestin, has demonstrated safety after long-term administration; it also appears to have a more favorable long-term safety profile compared with other progestins and AIs. We encountered a young patient with recurrent LG-ESS that metastasized to the liver and exhibited resistance to high-dose medroxyprogesterone acetate (MPA). The patient was successfully treated with dienogest monotherapy. This is the first report describing the efficacy of dienogest against recurrent and metastatic LG-ESS that is resistant to MPA and other agents.
1. Introduction
Low-grade endometrial stromal sarcoma (LG-ESS) is a malignant mesenchymal uterine tumor that comprises less than 1% of all uterine malignancies. According to the World Health Organization 2014 classification, ESS is categorized into four subtypes based on clinical and pathological features. LG-ESS usually occurs in premenopausal women; the lesion is associated with a slow-growing and indolent clinical course and the potential for recurrence. The JAZF1/SUZ12 fusion gene has been frequently identified in LG-ESS, and it could represent useful information for differentiating LG-ESS from other ESSs (Hrzenjak, 2016). The National Comprehensive Cancer Network published guidelines for the treatment of LG-ESS (Koh et al., 2015). Most reports found that hysterectomy with bilateral salpingectomy is appropriate for early-stage cases. The roles of postoperative radiotherapy and chemotherapy in the treatment of LG-ESS are not well established. Conversely, hormonal therapy—including high-dose progestins, aromatase inhibitors (AIs), and gonadotropin-releasing hormone (GnRH) agonists—has demonstrated efficacy in patients with advanced and recurrent lesions. Because the side effects of long-term, high-dose progestin therapy include increased risks of severe depression and thromboembolic complications, AIs have emerged as alternatives to endocrine treatment (Zang et al., 2019). However, AIs and GnRH agonists are not free of adverse effects.
Dienogest (DNG) has an extremely potent progestogenic effect in the endometrium; it also induces endometrial atrophy after prolonged use. DNG directly exerts anti-proliferative and anti-inflammatory effects on endometrial stromal cells in vitro, and the drug has been used to treat endometriosis.
In the present study, we present the case of a patient who was treated with DNG for a second relapse of LG-ESS with liver metastases; complete remission was achieved in this patient. This is the first report of the efficacy of DNG in the treatment of medroxyprogesterone acetate (MPA)-resistant recurrent LG-ESS.
2. Case presentation
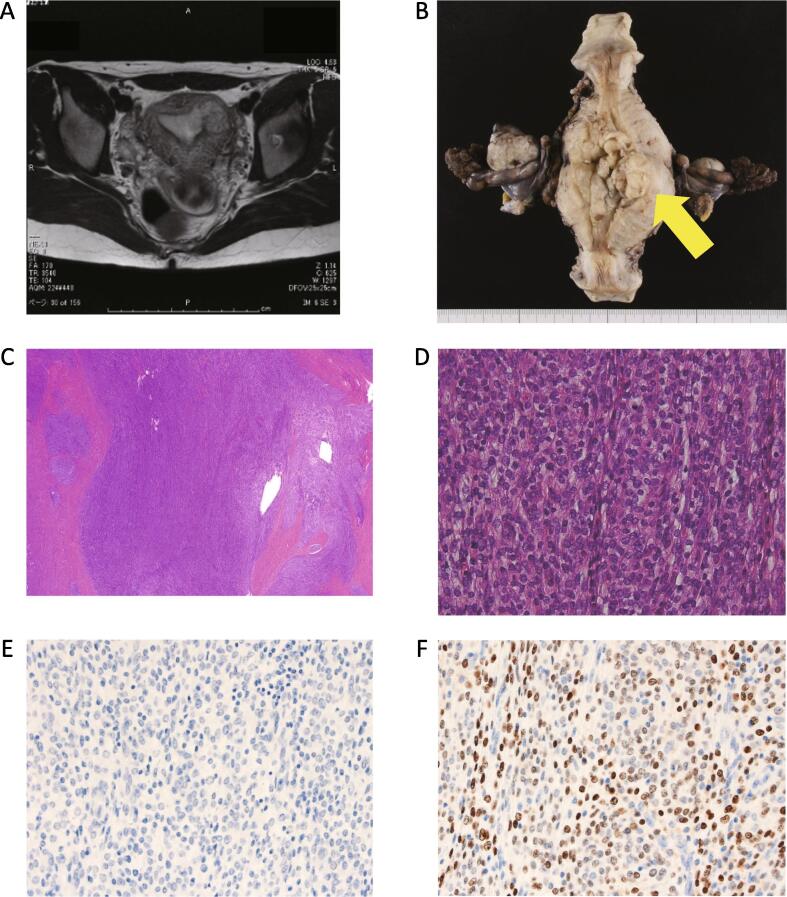
A 24-year-old woman visited a local clinic because of irregular genital bleeding. Ultrasound revealed a small endometrial tumor that grew for 3 months. The results of tumor biopsy supported a diagnosis of LG-ESS, and the patient was referred to our hospital. Computed tomography (CT) and magnetic resonance imaging (MRI) identified a 29-mm endometrial mass with myometrial invasion (Fig. 1A).
Fig. 1.
(A) Axial magnetic resonance images before the first operation. The tumor invaded the left side of the uterine myometrium. (B) Specimen excised during the first operation. The yellow arrow denotes the tumor. (C) Hematoxylin and eosin-stained section at lower magnification. The tumor infiltrated the myometrium in an irregular pattern. (D) At higher magnification, oval-to-short spindle cells with mild nuclear atypia and mitotic figures were noted in 5 of 10 high-power fields. (E) Negative immunohistochemical staining for (E) estrogen receptor. (F) Positive immunohistochemical staining for (F) progesterone receptor. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy were performed 1 month after the first examination in our hospital (Fig. 1B). Pathologic examination of the tumor revealed an irregular invasion pattern (Fig. 1C) featuring oval-to-short spindle cells with mild nuclear atypia (Fig. 1D); immunohistochemistry results were positive for progesterone receptor (PR), CD10, and smooth muscle actin, whereas they were negative for estrogen receptor (ER; Fig. 1E and F). The final diagnosis was stage Ic LG-ESS (FIGO 1988). The patient underwent hormonal therapy with MPA at a dose of 200 mg daily for 2 years. Conjugated estrogen at a dose of 0.625 mg per day was added because of the patient’s young age at onset.
Nine years after surgery, three small nodules were detected on the left side of the vaginal stump and in front of the rectum on CT. Conjugated estrogen was replaced with bazedoxifene, a third-generation selective estrogen receptor modulator, to prevent osteoporosis.
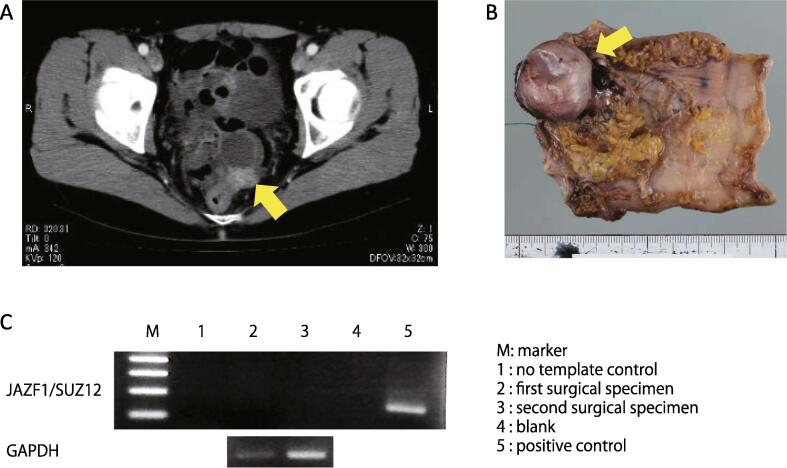
After 2.5 years, the tumors grew, becoming palpable on rectal examination. Therefore, the patient began oral dydrogesterone therapy at a daily dose of 10 mg. Nine months later, CT and MRI revealed continuous growth of the tumors (Fig. 2A). The recurrent tumors were removed via lower anterior resection of the rectum (Fig. 2B) and pathologically diagnosed as recurrent LG-ESS. The JAZF1/SUZ12 fusion gene was not detected in the first surgical specimen and recurrent tumors (Fig. 2C). There were no residual lesions, and the patient started hormonal therapy with MPA (200 mg daily).
Fig. 2.
(A) Computed tomography of the pelvis confirmed a para-rectal tumor (yellow arrow). (B) Specimen excised during the second operation. The yellow arrow denotes a metastatic tumor that was identical to the previously resected uterine tumor on histology and immunostaining. (C) Amplification of the JAZF1/SUZ12 fusion transcript in formalin-fixed, paraffin-embedded specimens. RT-PCR was performed, and the fusion transcript was not detected in either sample. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
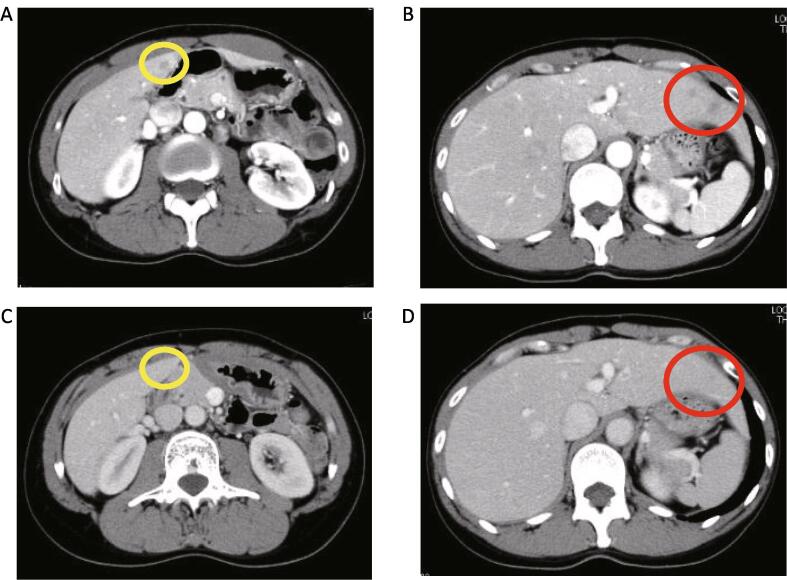
Almost 2 years after the second surgery, the recurrence of multiple nodules in the liver was detected on CT (Fig. 3A and B). Hormonal therapy was changed from MPA to DNG (2 mg daily). After 3 months, the hepatic nodules had disappeared (Fig. 3C and D) without any adverse effects. To date, the patient has been receiving DNG hormonal therapy for approximately 1 year, and she has remained alive for more than 15 years since the first surgery.
Fig. 3.
Hepatic nodules were detected on computed tomography (A and B). The nodules disappeared after 3 months of dienogest treatment (C and D).
3. Discussion
There are two important aspects in this case that are inconsistent with the current standard treatment for LG-ESS because the initial treatment was more than 15 years ago. At that time, some case series involving patients with LG-ESS demonstrated risks of lymph node metastasis. Therefore, the first difference in treatment of our patient was that we performed pelvic lymphadenectomy because it was expected to be useful for evaluating lymph node metastasis. However, the benefit of pelvic lymphadenectomy for patients with LG-ESS has since become controversial. The second difference involves hormonal therapy. Adjuvant therapy options in the National Comprehensive Cancer Network guidelines for patients with stage I LG-ESS include observation (especially if the patients have reached menopause or have a history of bilateral salpingo-oophorectomy) and estrogen blockade (Koh et al., 2015). At the time of initial surgery, remissions following treatment with progestins had been described in case reports; thus, we introduced adjuvant hormonal therapy with MPA. However, we cannot rule out the possibility that this use of adjuvant MPA might have contributed to the patient’s resistance to hormonal therapy.
LG-ESS is typically positive for ER and PR. In patients with advanced or recurrent LG-ESS, progestins (including megestrol acetate and MPA) are used as the first-line treatments. Although AIs and GnRH agonists have been successfully used in patients with LG-ESS, attention is needed concerning the safety and tolerability of the long-term administration of these agents. The adverse effects of long-term, high-dose progestin therapy include increased risks of severe depression and thromboembolic complications. Therefore, the use of combinations of anti-platelet agents should be considered. The adverse effects of GnRH agonists are associated with a lack of estrogen, and the long-term use of these drugs causes osteoporosis (Sugimoto et al., 1993). Similarly, the adverse effects of AIs are also induced by the absence of estrogen. These effects include musculoskeletal stiffness and pain, fatigue, hot flashes, and nausea. A study of patients with breast cancer who were treated with the AI letrozole for 10 years revealed that bone-related toxic effects occurred more frequently in the letrozole group than in the placebo group (Goss et al., 2016). Therefore, alternative therapeutic agents are required for long-term safety.
DNG has been used in the treatment of endometriosis, and its pharmacological effects are gradually being elucidated. Hayashi et al. suggested that DNG improves progesterone resistance in patients with endometriosis by regulating the relative expression of PR and ER isoforms (Hayashi et al., 2012). Furthermore, DNG lacks glucocorticoid activity, which has been associated with an increased risk of thromboembolism. In fact, long-term trials identified breast discomfort, nausea, and irritability as adverse effects of DNG, whereas thromboembolism was not reported (Strowitzki et al., 2015). DNG appears to be more acceptable for the treatment of uterine malignant tumors than MPA because women with these tumors are at increased risk of venous thrombosis (Satoh et al., 2008).
Katsuki et al. reported that DNG suppressed the proliferation of ER- and/or PR-positive MPA-resistant endometrial cancer cells in vivo (Katsuki et al., 1997). In a mouse model, DNG and MPA exhibited potent anti-cancer activity against endometrial neoplasms (Saito et al., 2016). No report has described the therapeutic effects of DNG against gynecologic stromal malignancies, excluding the outcomes in one patient with uterine adenosarcoma (Tasaka et al., 2013). In contrast, a case report described the use of the progestin dydrogesterone in a patient with LG-ESS that relapsed after chemotherapy; the drug was linked to tumor shrinkage and disappearance over the course of 4 years (Akashi et al., 2013).
Although the JAZF1/SUZ12 fusion gene was not detected in our case, the fusion gene is present in 45% of cases of LG-ESS; it may be useful for diagnosing difficult or unusual cases of stromal sarcoma (Hrzenjak, 2016). SUZ12 is a component of polycomb repressive complex 2 (PRC2), and it has been suggested that the fusion gene inhibits PRC2 transcriptional repression. However, the correlation between therapeutic efficacy and the presence of the fusion gene is not well understood.
Yamazaki et al. reviewed 17 studies regarding primary ESS and estimated that mortality rates in ESS have been reduced by repeated surgical resection and hormonal therapy over the past three decades (Yamazaki et al., 2015). Because LG-ESS is associated with indolence and remarkable sensitivity to hormonal agents, the adverse effects of long-term hormonal therapy represent a critical factor for drug tolerability. We anticipate that by increasing the number of hormonal drugs with low risks of side effects, both the prognosis and quality of life of patients with LG-ESS will be improved.
In conclusion, we have presented the first case of a patient with a second recurrence of MPA- and dydrogesterone-resistant LG-ESS, which was successfully and safely treated with DNG. Evident adverse effects were not observed in this case, which suggests that subsequent DNG treatment may safely control the growth of tumors that are resistant to other hormonal therapies.
Acknowledgments
Acknowledgement
We thank Joe Barber Jr., PhD, and Ryan Chastain-Gross, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript and Ms. Yoko Takeda for performing the JAZF1/SUZ12 fusion gene detection experiment.
Declaration of Competing Interest
The authors have no conflict of interest.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors’ contributions
Patient care and study conception: MO.
Manuscript writing: SM.
Acquisition of data: SM, TF, KT.
Manuscript editing and review: MO, KS, TS.
Final approval: All authors.
References
- Akashi D., Todo Y., Shimada C., Okamoto K., Minobe S., Kato H. Successful use of dydrogesterone as maintenance therapy in recurrent endometrial stromal sarcoma: a case report. Jpn. J. Clin. Oncol. 2013;43:1145–1149. doi: 10.1093/jjco/hyt142. [DOI] [PubMed] [Google Scholar]
- Goss P.E., Ingle J.N., Pritchard K.I., Robert N.J., Muss H., Gralow J., Gelmon K., Whelan T., Strasser-Weippl K., Rubin S., Sturtz K., Wolff A.C., Winer E., Hudis C., Stopeck A., Beck J.T., Kaur J.S., Whelan K., Tu D., Parulekar W.R. Extending aromatase-inhibitor adjuvant therapy to 10 years. N. Engl. J. Med. 2016;375:209–219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Tanabe A., Kawabe S., Hayashi M., Yuguchi H., Yamashita Y., Okuda K., Ohmichi M. Dienogest increases the progesterone receptor isoform B/A ratio in patients with ovarian endometriosis. J. Ovarian Res. 2012;5:1–8. doi: 10.1186/1757-2215-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrzenjak A. JAZF1/SUZ12 gene fusion in endometrial stromal sarcomas. Orphanet J. Rare Dis. 2016;11:1–8. doi: 10.1186/s13023-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki Y., Shibutani Y., Aoki D., Nozawa S. Dienogest, a novel synthetic steroid, overcomes hormone-dependent cancer in a different manner than progestins. Cancer. 1997;79:169–176. doi: 10.1002/(SICI)1097-0142(19970101)79:1<169::AID-CNCR24>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Koh W.-J., Greer B.E., Abu-Rustum N.R., Apte S.M., Campos S.M., Cho K.R., Chu C., Cohn D., Crispens M.A., Dizon D.S., Dorigo O., Eifel P.J., Fisher C.M., Frederick P., Gaffney D.K., George S., Han E., Higgins S., Huh W.K., Lurain J.R., Mariani A., Mutch D., Fader A.N., Remmenga S.W., Reynolds R.K., Tillmanns T., Valea F.A., Yashar C.M., McMillian N.R., Scavone J.L. Uterine Sarcoma, Version 1.2016. J. Natl. Compr. Cancer Netw. 2015;13:1321–1331. doi: 10.6004/jnccn.2015.0162. [DOI] [PubMed] [Google Scholar]
- Saito F., Tashiro H., Yamaguchi M., Honda R., Ohba T., Suzuki A., Katabuchi H. Development of a mouse model for testing therapeutic agents: The anticancer effect of dienogest on endometrial neoplasms. Gynecol. Endocrinol. 2016;32:403–407. doi: 10.3109/09513590.2015.1124411. [DOI] [PubMed] [Google Scholar]
- Satoh T., Matsumoto K., Uno K., Sakurai M., Okada S., Onuki M., Minaguchi T., Tanaka Y.O., Homma S., Oki A., Yoshikawa H. Silent venous thromboembolism before treatment in endometrial cancer and the risk factors. Br. J. Cancer. 2008;99:1034–1039. doi: 10.1038/sj.bjc.6604658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowitzki T., Faustmann T., Gerlinger C., Schumacher U., Ahlers C., Seitz C. Safety and tolerability of dienogest in endometriosis: Pooled analysis from the European clinical study program. Int. J. Womens. Health. 2015;7:391–401. doi: 10.2147/IJWH.S77202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto A.K., Hodsman A.B., Nisker J.A. Long-term gonadotropin-releasing hormone agonist with standard postmenopausal estrogen replacement failed to prevent vertebral bone loss in premenopausal women. Fertil. Steril. 1993;60:672–674. doi: 10.1016/S0015-0282(16)56220-7. [DOI] [PubMed] [Google Scholar]
- Tasaka N., Matsumoto K., Satoh T., Minaguchi T., Onuki M., Ochi H., Tanaka Y.O., Sakata A., Noguchi M., Yoshikawa H. Therapeutic effect of dienogest on adenosarcoma arising from endometriosis: A case report. Springerplus. 2013;2:1–6. doi: 10.1186/2193-1801-2-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Todo Y., Mitsube K., Hareyama H., Shimada C., Kato H., Yamashiro K. Long-term survival of patients with recurrent endometrial stromal sarcoma: A multicenter, observational study. J. Gynecol. Oncol. 2015;26:214–221. doi: 10.3802/jgo.2015.26.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y., Dong M., Zhang K., Gao C., Guo F., Wang Y., Xue F. Hormonal therapy in uterine sarcomas. Cancer Med. 2019;8:1339–1349. doi: 10.1002/cam4.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]