Abstract
The present study aimed to determine the toxic effect of malathion pesticide on root growth, cell division and the chromosomal abnormalities frequency using the L. culinaris test. Initially, the lentil seeds were subjected to different doses of malathion (0.0 0.5, 1, 2.5, 5, 10, 15, 20, 25 and 30 mgL-1) and during 24, 48, and 72 h, the root length was measured. Subsequently, at 72h, the mitotic index, mitotic inhibition, and cellular abnormalities were calculated for all treatments. According to the obtained results, it was visualized that the root growth was inversely proportional to the concentration of malathion at all times of exposure. After 72h of exposure, the lowest values of the mitotic index and inhibition were presented at malathion concentrations 20, 25 and 30 mgL-1. Additionally, micronuclei cell abnormalities, metaphase sticky chromosomes, split chromosomes, nuclear lesions, irregular anaphase, anaphase bridges, binucleated cells, absence of nucleus and telophase bridge were observed. Finally, Malathion induced mitodepressive and cytotoxic effects in the meristematic cells of the L. culinaris root tip. A high frequency of abnormality was found in the micronuclei, which represented an indicator of a high degree of toxicity at the cellular level.
Keywords: Ecology, Environmental chemistry, Environmental engineering, Environmental toxicology, Plant biology, Systems biology, Mitotic index, Lentil, Cytotoxic, Genotoxic, Relative abnormality rate
Ecology; Environmental chemistry; Environmental engineering; Environmental toxicology; Plant biology; Systems biology; Mitotic index; Lentil; Cytotoxic; Genotoxic; Relative abnormality rate
1. Introduction
Pesticides play an important role in pest control, in agriculture, and in non-agricultural settings. They are applied in high concentrations and their residues cause contamination to the environment, representing risks to organisms and human health (Ryberg and Gilliom, 2015; Willison et al., 2019; Elfikrie et al., 2020; Meftaul et al., 2020). Malathion (diethyl (dimethoxythiophosphorylthio) succinate or S-1,2-bis (ethoxycarbonyl) ethyl O, O-dimethyl phosphorodithioate) is one of the most widely used organophosphate pesticides worldwide (Singh and Roy, 2017), as an acaricide and insecticide, it is commonly applied to stored fruit, vegetable and grain crops (Climent et al., 2019). When ingested or inhaled, it quickly passes into the bloodstream, interfering with the nervous system by inhibiting the enzyme cholinesterase, whose function is to inactivate the acetylcholine neurotransmitter in synapses (Bavcon et al., 2003; Wu et al., 2011; Houbraken et al., 2017). Likewise, malathion has been found in the children's nutritional diet of Japan by 4% (Kawahara et al., 2007). Interviewing with several farmers, they described negative health symptoms after pesticide application, including vomiting, headache and eye irritation, and skin conditions (Sumon et al., 2016). Furthermore, according to Mendoza et al. (2015), exposure to Malathion could cause damage to organs such as kidneys and heart. In rats, the mean lethal dose is 1200 mg/kg, via oral (Gallo and Lawryk, 1991), producing an increase in the activities of catalase, superoxide dismutase, as well as the concentration of malondialdehyde (MDA) in liver and erythrocytes (Akhgari et al., 2003).
According to Bujagić et al. (2019); Radović et al. (2015), there are high concentrations of malathion found in the sediments of Danube and Tisza rivers in Serbia at 5–10 cm depth (2.9 and 69 ng g −1, respectively). The previous concentrations are significantly higher than the minimum levels known (6.7 × 10−3 ng g−1), which negatively affects benthic organisms (Fisher et al., 1993). In surface waters they have found malathion doses of 0.193 μg·L−1 and 25 ng/g, which represents a negative impact on aquatic ecosystems, especially fish, invertebrates and algae (Köck-Schulmeyer et al., 2013; Houbraken et al., 2017; Picó et al., 2018; Triassi et al., 2019). Additionally, it has been found in some amphibian tissues at doses of 0.68 ppm and in soils of 13.06 ppm, affecting the amphibian's liver metabolomics (Climent et al., 2019). Likewise, at malathion concentrations of 0.1 and 1.0 mg/L, larval activity decreases in anuran species (Relyea and Edwards, 2010). In tomato plants specifically in the fruit, they have found malathion doses higher than 1,000 μg/kg in the pericarp, in cabbage of 1000 ng/g and in Chinese cabbage of 330 ppb (Reiler et al., 2015; Wanwimolruk et al., 2015; Picó et al., 2018), which represents a high risk of consuming these foods without being washed and processed after harvest, particularly for children. In this sense, it is important to educate farmers to use less dangerous pesticides, in order to guarantee public and ecosystem health (Reiler et al., 2015).
Searching for bioindicators, plants that can detect toxic substances due to their high sensitivity to environmental changes have been used (Salazar and Maldonado, 2019). These have similar characteristics to mammalian chromosomes and are inexpensive to perform various toxicity tests (Abdelsalam et al., 2018; Salazar-Mercado et al., 2019). The plants mostly used as bioindicators in research related to cytotoxic and genotoxic tests are Allium cepa L. (Martins et al., 2016; Haq et al., 2017; de Souza et al., 2017; Silveira et al., 2017; Braga et al., 2018; Verma and Srivastava, 2018; Heikal et al., 2019; García-Medina et al., 2020; Salazar and Quintero, 2020), Pisum sativum L. (Salazar-Mercado et al., 2019), Lactuca sativa L. (Andrade-Vieira et al., 2018), Zea mays L. (Reynoso et al., 2015), Triticum aestivum L. (Abdelsalam et al., 2018) and in recent studies Lens culinaris Medik (Shahwar et al., 2019; Salazar and Maldonado, 2020; Salazar et al., 2020a).
Trials conducted by Singh and Roy (2017), who evaluated Malathion-induced cytogenetic effects using the A. cepa test. They found that, at malathion doses of 50, 125, 250, and 375 mgL-1 at different exposure periods, it inhibited root growth, reduced the mitotic index, and produced different chromosomal aberrations. In the literature up till now, no research has been conducted on the malathion toxic effect on L. culinaris cells. As stated above, this study aims to determine the toxic effect of the malathion pesticide on root growth, cell division and the frequency of chromosomal abnormalities using the L. culinaris test.
2. Material and methods
2.1. Plant material and treatments
Malathion was diluted in distilled water at doses of 0.5, 1, 2.5, 5, 10, 15, 20, 25 and 30 mgL-1 and control of distilled water. For L. culinaris seeds germination, it was carried out in Petri dishes with cotton and filter paper under controlled environmental conditions (26 ± 2 in the dark). The seeds were subjected to darkness for three days as described by Salazar and Botello (2018); Salazar et al. (2020b); Salazar et al. (2020c) and root growth was measured at 24, 48 and 72 h. Subsequently, the mitotic index and the frequency of cellular abnormalities were determined.
2.2. Mitotic index and inhibition
After 72 h, the root tips were cut 4 mm approximately, washed with running water and subjected to toluidine blue metachromatic dye for 30 min. Subsequently, the roots were taken to the slide, the coverslip was placed and the squash technique was performed, applying uniform pressure with the fingertip of the thumb to disperse the cells, making each phase of the cell cycle visible (Salazar and Maldonado, 2019). Samples were visualized under the Leica DME 500 compound microscope at 40X and 100X magnification. To determine the mitotic index, 1000 cells per repetition (5000 cells per treatment) were analyzed, using the formula Mitotic index (MI): number of dividing cells/number of total cells x100. Continuedly, the mitosis inhibition was found according to Salazar-Mercado et al. (2019).
Cellular abnormalities are alterations that occur in the structure and number of chromosomes and depend on the concentration and type of cytotoxic substance (Fatma et al., 2018). Therefore, the following formula used by Salazar and Quintero (2020) was applied in this research: Frequency of chromosomal anomalies (%): Total number of abnormal cells/Total number of cells observed x100.
2.3. Statistical analysis
For root development, 25 L. culinaris roots were used per treatment with 5 replications. 1000 cells per repeat (5000 cells per treatment) were analyzed for the mitotic index and cellular abnormalities. The obtained data were evaluated using the analysis of variance (ANOVA). Subsequently, the averages of each treatment were compared by applying the HSD multiple range test (Tukey's Honestly Significant Difference) (P ≤ 0.05). Using the InfoStat program.
3. Results and discussion
3.1. Root length
Exposing lentil seeds to hydration stimulates cell growth, elongating their meristematic cells. Furthermore, when the roots are subjected to chemical substances, variations in their morphology and coloration occur (Khanna and Sharma, 2013). The degree of affectation depends on the chemical substance and the time of exposure (Salazar-Mercado et al., 2019).
The results, as shown in Table 1, indicate that at 24, 48 and 72 h the highest root growth was achieved at the control treatment and the lowest growth was found at the 30 mgL−1dose, this proves that the root growth inhibition was greater, with the increase of the malathion concentration. At 24h, the doses of 20, 25 and 30 mgL-1, root growth did not show statistically significant differences, whereas, at 48 and 72 h, the treatments at 25 and 30 mgL-1 were homogeneous. However, at 24h (0.5, 1, 2.5 mgL-1) and 48h (0.5, 1, 2.5, 5 mgL-1) the doses of malathion did not differ from the control treatment. At 72h, only the control treatment was significantly related to the 0.5 mgL-1 dose.
Table 1.
L. culinaris root growth subjected to different doses of Malathion.
| Dose: Malathion (mgL−1) | Root length (cm) |
||
|---|---|---|---|
| 24h | 48 h | 72 h | |
| T1: Control | 2.36 ± 0.23a | 4 ± 0.7a | 5 ± 0.04a |
| T2: 0.5 | 1.86 ± 0.18a,b,c | 3.66 ± 0.61a | 4.34 ± 0.42a,b |
| T3: 1 | 2.0 ± 0.7a,b | 3.64 ± 0.65a | 4.04 ± 0.4b |
| T4: 2.5 | 1.76 ± 0.11a,b,c | 3.22 ± 0.68a,b | 3.98 ± 0.04b,c |
| T5: 5 | 1.52 ± 0.3b,c,d | 3.02 ± 0.17a,b,c | 3.64 ± 0.46b,c,d |
| T6:10 | 1.54 ± 0.35b,c,d | 2.54 ± 0.37b,c | 3.14 ± 0.49c,d,e |
| T7: 15 | 1.38 ± 0.4b,c,d | 2.3 ± 0.44b,c | 2.92 ± 0.57d,e |
| T8: 20 | 1.16 ± 0.15d,e | 2.14 ± 0.35b,c | 2.66 ± 0.46e,f |
| T9: 25 | 0.94 ± 0.08d,e | 1.58 ± 0.34c,d | 2.22 ± 0.34f,g |
| T10: 30 | 0.54 ± 0.23e | 1.04 ± 0.11d | 1.34 ± 0.39g |
The means ± SD values with different letter indicate statistically significant differences, according to Tukey (P ≤ 0.05). SD: Standard deviation; cm: centimeter.
These results are similar to those reported by Singh and Roy (2017), where malathion had a negative effect on A. cepa root growth at all concentrations for 3 days. For this reason, root inhibition is a parameter that can be used to measure the toxicity of malathion. This agrees with studies carried on by Salazar and Maldonado (2019), who submitted different doses of chlorpyrifos organophosphate pesticide (0, 1, 3, 5, 7, 8, 10 and 15 mgL-1) in L. culinaris seeds, affecting root growth significantly at 24, 48 and 72h of exposure. The inhibition of root growth caused by the malathion pesticide might be due to the disturbance of the Reactive oxygen species (ROS) in the plant since, according to Mhamdi and Van Breusegem (2018), when ROS homeostasis is altered, numerous processes are affected, from seed germination to root development, because ROS interact with hormones (auxin and cytokinin) associated with plant development.
3.2. Mitotic index and inhibition
In the evaluation of the mitotic index (5000 cells per treatment) after 72 h of exposure to malathion, it shows that the control had a higher MI with statistically significant differences with respect to the different doses of the pesticide (18.4%; Table 2). The lowest MI values were found at 20, 25 and 30 mgL-1 concentrations (11.4%, 10.6% and 8.8%, respectively). This indicates that the mitotic index decreased depending on the doses of malathion. According to results presented by Singh and Roy (2017), when subjecting A. cepa to 125 mgL-1 of malathion for 18h, they found a mitotic index of 8.77%. Likewise, in Vicia faba roots subjected to 320 mgL-1 of malathion, the mitotic index was 8.99% (Adam et al., 2014).
Table 2.
Mitotic index and Percentage of mitosis inhibition of L. culinaris root tip cells, submitted to different doses of Malathion.
| Dose: Malathion (mg L−1) | Mitotic index (MI: %) |
Inhibition of mitosis (%) |
|---|---|---|
| L. culinaris | L. culinaris | |
| T1: Control | 18.4 ± 1.5a | ---- |
| T2: 0.5 | 14.2 ± 1.3b | 22.8 |
| T3: 1 | 14.4 ± 1.1b | 21.7 |
| T4: 2.5 | 14.6 ± 0.7b | 20.6 |
| T5: 5 | 13.6 ± 1.1b,c | 26.1 |
| T6:10 | 13.2 ± 1.6b,c,d | 28.2 |
| T7: 15 | 12.8 ± 0.8b,c,d | 30.4 |
| T8: 20 | 11.4 ± 1.5b,d,e | 38 |
| T9: 25 | 10.6 ± 10.6d,e | 42.3 |
| T10: 30 | 8.8±1e | 52.1 |
The means ± SD values with different letter indicate statistically significant differences, according to Tukey (P ≤ 0.05). SD: Standard deviation. MI: Mitotic index.
In a study, carried out by Srivastava and Singh (2020) in A. cepa root cells, exposed to different malathion concentrations (50, 130, 260, 390 and 520 mgL-1) and exposure time (4, 8 and 18 h). They found that the application of malathion affected the MI as the concentration and the treatment periods increased, reducing the growth rate and cell division in the A. cepa roots. In the previous study, the 50 mgL-1 dose of malathion had an MI of approximately 12%. In contrast, in this research the concentration of 30 mgL-1, the mitotic index was 8.8%. According to the above, the L. culinari method is more sensitive to malathion than the A. cepa test. This is corroborated by studies related to other pesticides, where the mitotic index has been higher in A. Cepa, in relation to the L. culinaris test in equal doses (Salazar et al., 2020a). The mitotic index is a measurement to quantitatively evaluate the cell division of an individual (Lessa and Cariello, 2017). Therefore, if the MI is low compared to the control treatment, the pesticide is affecting mitosis. According to Rosales (2015), organophosphate pesticides cause damage to deoxyribonucleic acid (DNA) at any stage of the cell cycle.
Regarding mitotic inhibition, the concentration that achieved data greater than 50% cell cycle inhibition was 30 mgL-1 dose (52.1%: Table 2), evidencing the mitodepressive activity of malathion. In contrast, lower doses of mitotic inhibition were found at doses of 0.5, 1, and 2.5 mgL-1 (22.8%, 21.7% and 20.6%, respectively). These results differ from those reported by Salazar and Quintero (2020), where 30 mgL-1 of glyphosate in A. cepa, obtained a mitotic inhibition index of 90.8%. Likewise, in L. culinaris roots at 15 mgL-1 of chlorpyrifos, an inhibition of 72.41% was found for 72h (Salazar and Maldonado, 2019).
3.2.1. Cellular abnormalities and abnormality rate
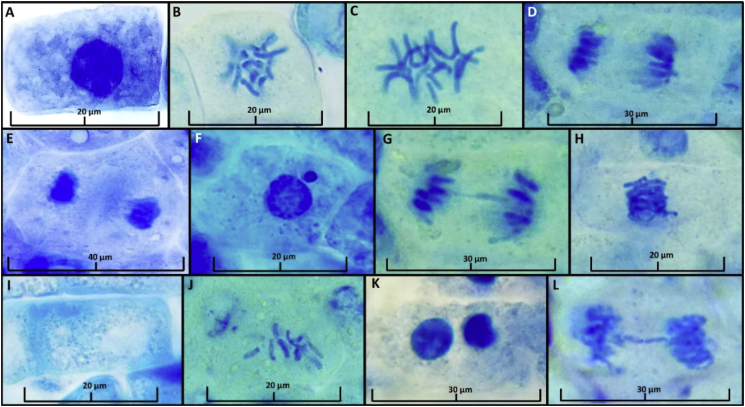
As a reference indicator, Figure 1 shows the normal phases of the cell cycle (interface, prophase, metaphase, anaphase and telophase) and the cellular anomalies found in meristematic cells from L. culinaris roots. Nine cellular abnormalities were found at all malathion concentrations, varying according to each treatment, except in control treatment (Figure 1; Table 3). Micronuclei, metaphase sticky chromosomes, split chromosomes, nuclear lesions, and irregular anaphase were visualized in all the doses that contained malathion, with the nuclear lesions being the mostly found at 30 mgL-1 dose (116.6 ± 16). Likewise, the anaphase bridges, binucleated cells, absence of nuclei and telophase bridges anomalies were not observed in some treatments (Table 3). In the same way, at 0.5 mgL-1 of malathion, fewer anomalies were found.
Figure 1.
Cell cycle phases and cellular abnormalities in L. culinaris root cells treated with doses of Malathion. A = Interphase: T1. B= Prophase: T1. C = Metaphase: T1. D = Anaphase: T1. E = Telophase: T1. F = Micronuclei: T10 G = Anaphase bridge: T9. H= Sticky chromosomes at metaphase: T8. I = Absence of nucleus: T7. J = Chromosome break: T6. K= Binucleate cells: T6. L = Telophase bridge: T10.
Table 3.
Frequency of chromosomal anomalies in L. culinaris treated with different doses of Malathion.
| Dose: Malathion (mg L−1) | Frequency of Chromosomal Anomalies (Mean ± SD) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | AB | SCM | AN | CB | BC | TB | NL | IA | |
| T1: Control | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| T2: 0.5 | 1.2 ± 0.44a | 0a | 0.4 ± 0.1a | 0a | 0.6 ± 0.8a | 0a | 0a | 3.6 ± 0.8a | 0.2 ± 0.08a |
| T3: 1 | 4.0 ± 1.2a,b | 0a | 1.8 ± 0.8a | 0.2 ± 0.1a | 3.0 ± 1.3a | 0a | 0a | 7 ± 1.5a | 0.2 ± 0.01a |
| T4: 2.5 | 4.4 ± 1.5a | 0a | 3.8 ± 1.7a | 0.6 ± 0.8a | 3.2 ± 0.8a | 0a | 0a | 10 ± 0.9a | 0.2 ± 0.07a |
| T5: 5 | 5.8 ± 0.8a,b | 0a | 4.2 ± 2.1a | 3.4 ± 1.1a | 3.6 ± 1.4a | 0a | 0a | 12.2 ± 1.9a | 0.4 ± 0.18a |
| T6:10 | 13 ± 1.3a,b | 0a | 4.4 ± 1.4a | 5.4 ± 0.8a | 5.8 ± 1.5a,b | 0.6 ± 0.25a,c | 0a | 15.8 ± 5.7a | 0.4 ± 0.14a |
| T7: 15 | 13.6 ± 3.5a,b | 0.6 ± 0.1a | 4.8 ± 1.7a | 8.4 ± 1.1a | 8 ± 2.5a,b | 1 ± 0.4a | 0a | 20.2 ± 3.1a | 0.6 ± 0.12a |
| T8: 20 | 16.8 ± 2.1b | 0.6 ± 0.12a | 5.8 ± 1.1a,c | 25.2 ± 3.2b | 16.6 ± 5.5 | 1.2 ± 0.43a,c,d | 0.2 ± 0.01a,b | 44.8 ± 14.7b | 0.6 ± 0.1a |
| T9: 25 | 37.6 ± 9.8c | 2.6 ± 0.9b | 11 ± 6.2a,c | 35 ± 5.5b | 17.4 ± 5.9b | 1.2 ± 1.7a,c,d | 2.2 ± 1.7b | 87.4 ± 18c | 0.8 ± 0.2a |
| T10: 30 | 47 ± 9.4c | 2.8 ± 0.8b | 16 ± 7.4a,c | 36±5b | 24 ± 7.1 | 8.0 ± 3.1b,d | 2.2 ± 1.1b | 116.6 ± 16d | 2.6 ± 0.5b |
SD: Standard deviation. Mn: Micronuclei. AB: anaphase bridge. SCM: Sticky chromosomes at metaphase. AN: Absence of nucleus. CB: Chromosome break. BC: Binucleate cells. TB: Telophase bridge. NL: Nuclear lesions. IA: Irregular anaphase.
The means ± SD values with different letter indicate statistically significant differences, according to Tukey (P ≤ 0.05).
These results contrast with the study carried out by Singh and Roy (2017) in the A. cepa test, where 50, 125, 250 and 375 mgL-1 of malathion doses, produced various chromosomal aberrations (anaphase and telophase bridging, multipolarity, chromosomal breakage (sticky and lagging chromosomes, nuclear lesions and binucleated cells)), but no micronuclei were found in A. cepa. However, in Vicia faba roots subjected to 80, 160 and 320 mgL-1 of malathion, micronuclei were found in all treatments (Adam et al., 2014). According to Doherty et al. (2016), the micronuclei are fragments of DNA that are separated from the main nucleus and have originated from eccentric chromosomes or fragments of chromatic, can result from alterations of structural type (clastogenic effect) or numerical alterations (aneugenic effect). They are used as a high degree of toxicity parameter (Bhatia and Kumar, 2013). In this investigation, micronuclei were observed in all doses, being in greater quantity in 30 mgL-1 concentration (47 ± 9.4; Table 3). Worrisome data since, it is evident that malathion induces genetic instability in L. culinaris, because it is a cytotoxic and genotoxic agent.
Table 4 compares the obtained results from the abnormality rate in treatments with malathion in L. culinaris. It is observed that at the concentration of 30 mgL-1, a higher rate of relative abnormality (25.5) was found, indicating that the formation of abnormal cells in L. culinaris roots increases as the dose of malathion is increased. These results also agree with the observations made by Salazar and Maldonado (2019); Salazar et al. (2020a), by subjecting L. culinaris to different concentrations of chlorpyrifos and propanil. Likewise, according to Singh and Roy (2017), pesticides alter ROS homeostasis and cause intracellular oxidative stress (Wu et al., 2017). Consequently, the tubulin polymerization, the mitotic spindle, the assembly of the phragmoplast, the dynamics of the nuclear envelope, the separation and movement of the chromosomes, and the formation of cell plates are affected, delaying the stages of cell division (Livanos et al., 2012). Furthermore, according to Singh and Roy (2017), the toxic and genotoxic activity of malathion is possibly associated with an overproduction of pro-oxidant agents and oxidative stress due to the significant increase in concentrations of malondialdehyde (MDA) caused by malathion in the plant. These products of lipoperoxidation reactions are also associated with damage to lysosomal membranes and DNA structure (Cortesía et al., 2015).
Table 4.
Relative abnormality rate for each doses of Paraquat.
| Dose: Malathion (mg L−1) | Relative abnormality rate |
|---|---|
| T1: Control | 0 |
| T2: 0.5 | 0.6 |
| T3: 1 | 1.66 |
| T4: 2.5 | 2.36 |
| T5: 5 | 2.9 |
| T6:10 | 4.8 |
| T7: 15 | 5.56 |
| T8: 20 | 11.1 |
| T9: 25 | 19.38 |
| T10: 30 | 25.5 |
4. Conclusion
To conclude, the present investigation determined the toxic effect of malathion on root growth, cell division and formation of cellular abnormalities, producing mitodepressive and cytotoxic effects on meristematic cells at L. culinaris root tip. The presence of changes at the cellular level and damage to the chromosomes was observed at 0.5 to 30 mgL-1 of malathion; being the 30 mgL-1 dose the one that produced a greater cellular inhibition and relative abnormality rate. In addition, a high frequency of abnormality was found in the micronuclei, which represents an indicator of a high degree of toxicity at the cellular level. Accordingly, the use of less toxic and environmentally friendly pesticides is suggested to farmers. According to the above, it is suggested that farmers use less toxic and environmentally friendly pesticides since the repeated and common use of this molecule can lead to problems in the performance and crop yield, thanks to physiological abnormalities that are harmful to development of the agricultural exploitation.
Declarations
Author contribution statement
Seir Antonio Salazar Mercado: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Jesús David Quintero Caleño: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
To Universidad Francisco de Paula Santander for their valuable collaboration.
Contributor Information
Seir Antonio Salazar Mercado, Email: seirantoniosm@ufps.edu.co, salazar663@hotmail.com.
Jesús David Quintero Caleño, Email: jesusdavidqc@ufps.edu.co.
References
- Abdelsalam N., Megeed A., Ali H.M., Salem M.Z.M., Al-Hayali M., Elshikh M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018;155:76–85. doi: 10.1016/j.ecoenv.2018.02.069. [DOI] [PubMed] [Google Scholar]
- Adam Z., Mikhael E., El-Ashry Z., Ehsan N., Ali R. Comparative cytogenetic and ultra-structural effects of storing dusted seeds of Vicia faba with insecticide malathion 1% and two insecticidal active plant products. World Appl. Sci. J. 2014;32(7):1423–1436. [Google Scholar]
- Akhgari M., Abdollahi M., Kebryaeezadeh A., Hosseini R., Sabzevari O. Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003;22(4):205–211. doi: 10.1191/0960327103ht346oa. [DOI] [PubMed] [Google Scholar]
- Andrade-Vieira L., Bernardes P., Ferreira M. Mutagenic effects of spent potliner and derivatives on Allium cepa L. and Lactuca sativa L.: a molecular approach. Chemosphere. 2018;208:257–262. doi: 10.1016/j.chemosphere.2018.05.186. [DOI] [PubMed] [Google Scholar]
- Bavcon M., Trebse P., Zupancic-Kralj L. Investigations of the determination and transformations of diazinon and Malathion under environmental conditions using gas chromatography coupled with a flame ionisation detector. Chemosphere. 2003;50:595–601. doi: 10.1016/s0045-6535(02)00643-4. [DOI] [PubMed] [Google Scholar]
- Bhatia A., Kumar Y. Cancer cell micronucleus: an update on clinical and diagnostic applications. Actapathologica, microbiologica, etimmunologica Scandinavica. 2013;(121):569–581. doi: 10.1111/apm.12033. [DOI] [PubMed] [Google Scholar]
- Braga A., Melo A., de Oliveira Santos J., Reis A., Torres de Lima T. Toxicogenetic study of omeprazole and the modulatory effects of retinol palmitate and ascorbic acid on Allium cepa. Chemosphere. 2018;204:220–226. doi: 10.1016/j.chemosphere.2018.04.021. [DOI] [PubMed] [Google Scholar]
- Bujagić I., Grujić S., Laušević M., Hofmann T., Micić V. Emerging contaminants in sediment core from the iron gate I reservoir on the Danube river. Sci.Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.01.205. [DOI] [PubMed] [Google Scholar]
- Climent M.J., Coscollà C., Lopez A., Barra R., Urrutia R. Legacy and current-use pesticides (CUPs) in the atmosphere of a rural area in central Chile, using passive air samplers. Sci. Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.01.302. [DOI] [PubMed] [Google Scholar]
- Cortesía C., Marcano L., Marcano Elena, Zapata-Vívenes Edgar. Inmunotoxicidad de malatión y clorpirifos en la lombriz de tierra Eisenia sp. (Annelida: Oligochaeta). Saber. Revista Multidisciplinaria del Consejo de Investigación de la Universidad de Oriente. 2015;27(4):530–536. [Google Scholar]
- de Souza R., de Souza C., Bueno O., Fontanetti S. Genotoxicity evaluation of two metallic-insecticides using Allium cepa and Tradescantia pallida: a new alternative against leaf-cutting ants. Chemosphere. 2017;168:1093–1099. doi: 10.1016/j.chemosphere.2016.10.098. [DOI] [PubMed] [Google Scholar]
- Doherty A., Bryce S.M., Bemis J.C. The in vitro micronucleus assay. Gen. Toxicol. Test. 2016:161–205. [Google Scholar]
- Elfikrie N., Ho Y., Zaidon S., Juahir H., Tan E. Occurrence of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers in Tengi River Basin, Malaysia. Sci. Total Environ. 2020;712:136540. doi: 10.1016/j.scitotenv.2020.136540. [DOI] [PubMed] [Google Scholar]
- Fatma F., Verma S., Kamal A., Srivastava A. Monitoring of morphotoxic, cytotoxic and genotoxic potential of mancozeb using Allium assay. Chemosphere. 2018;195:864–870. doi: 10.1016/j.chemosphere.2017.12.052. [DOI] [PubMed] [Google Scholar]
- Fisher S.W., Lydy M.J., Barger J., Landrum P.F. Quantitative structure-activity relationships for predicting the toxicity of pesticides in aquatic systems with sediment. Environ. Toxicol. Chem. 1993;12:1307–1318. [Google Scholar]
- Gallo M., Lawryk N. Organic phosphorus pesticides. In: Hayes W.J., Laws E.R., editors. Handbook of Pesticide Toxicology. Academic Press; New York: 1991. pp. 5–13. [Google Scholar]
- García-Medina S., Galar-Martínez M., Gómez-Oliván L., Torres-Bezaury R., Islas-Flores H. The relationship between cyto-genotoxic damage and oxidative stress produced by emerging pollutants on a bioindicator organism (Allium cepa): the carbamazepine case. Chemosphere. 2020;253 doi: 10.1016/j.chemosphere.2020.126675. [DOI] [PubMed] [Google Scholar]
- Haq I., Kumar S., Raj A., Lohani M., Satyanarayana G. Genotoxicity assessment of pulp and paper mill effluent before and after bacterial degradation using Allium cepa test. Chemosphere. 2017;169:642–650. doi: 10.1016/j.chemosphere.2016.11.101. [DOI] [PubMed] [Google Scholar]
- Heikal Y.M., Şuţan N.A., Rizwan M., Elsayed A. Green synthesized silver nanoparticles induced cytogenotoxic and genotoxic changes in Allium cepa L. varies with nanoparticles doses and duration of exposure. Chemosphere. 2019;125430 doi: 10.1016/j.chemosphere.2019.125430. [DOI] [PubMed] [Google Scholar]
- Houbraken M., Habimana V., Senaeve D., López-Dávila E., Spanoghe P. Multi-residue determination and ecological risk assessment of pesticides in the lakes of Rwanda. Sci. Total Environ. 2017;576:888–894. doi: 10.1016/j.scitotenv.2016.10.127. [DOI] [PubMed] [Google Scholar]
- Kawahara J., Yoshinaga J., Yanagisawa Y. Dietary exposure to organophosphorus pesticides for young children in Tokyo and neighboring area. Sci. Total Environ. 2007;378(3):263–268. doi: 10.1016/j.scitotenv.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Khanna N., Sharma S. Allium cepa root chromosomal aberration assay: a review. Indian J. Pharm. Biol. 2013;1(3):105–119. [Google Scholar]
- Köck-Schulmeyer M., Villagrasa M., López de Alda M., Céspedes-Sánchez R., Ventura F., Barceló D. Occurrence and behavior of pesticides in wastewater treatment plants and their environmental impact. Sci. Total Environ. 2013;458–460:466–476. doi: 10.1016/j.scitotenv.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Lessa L., Cariello F. Adsorção do paracetamol em carvão ativado: regressão da citotóxicidade e mutagênicidade no sistema Allium cepa. HÓRUS. 2017;12(1):44–54. [Google Scholar]
- Livanos P., Apostolakos P., Galatis B. Plant cell division. Plant Signal. Behav. 2012;7(7):771–778. doi: 10.4161/psb.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M., Ventura de Souza V., da Silva T. Cytotoxic, genotoxic and mutagenic effects of sewage sludge on Allium Cepa. Chemosphere. 2016;148:481–486. doi: 10.1016/j.chemosphere.2016.01.071. [DOI] [PubMed] [Google Scholar]
- Meftaul I., Venkateswarlu K., Dharmarajan R., Annamalai P., Megharaj M. Pesticides in the urban environment: a potential threat that knocks at the door. Sci. Total Environ. 2020;711:134612. doi: 10.1016/j.scitotenv.2019.134612. [DOI] [PubMed] [Google Scholar]
- Mendoza E., González-Ramírez C., Martínez-Saldaña M., Avelar-González F.J., Valdivia-Flores A., Aldana-Madrid M., Rodríguez-Olibarría G., Jaramillo-Juárez F. Estudio de exposición a malatión y cipermetrina y su relación con el riesgo de daño renal en habitantes del municipio de Calvillo Aguascalientes. México Revista Mexicana de Ciencias Farmacéuticas. 2015;46(3):62–72. [Google Scholar]
- Mhamdi A., Van Breusegem F. Reactive oxygen species in plant development. Development. 2018;145(15):dev164376. doi: 10.1242/dev.164376. [DOI] [PubMed] [Google Scholar]
- Picó Y., Alvarez-Ruiz R., Alfarhan A.H., El-Sheikh M.A., Alobaid S.M., Barceló D. Uptake and accumulation of emerging contaminants in soil and plant treated with wastewater under real-world environmental conditions in the Al Hayer area (Saudi Arabia) Sci. Total Environ. 2018 doi: 10.1016/j.scitotenv.2018.10.224. [DOI] [PubMed] [Google Scholar]
- Radović T., Grujić S., Petković A., Dimkić M., Laušević M. Determination of pharmaceuticals and pesticides in river sediments and corresponding surface and ground water in the Danube River and tributaries in Serbia. Environ. Monit. Assess. 2015;187 doi: 10.1007/s10661-014-4092-z. [DOI] [PubMed] [Google Scholar]
- Reiler E., Jørs E., Bælum J., Huici O., Alvarez Caero M.M., Cedergreen N. The influence of tomato processing on residues of organochlorine and organophosphate insecticides and their associated dietary risk. Sci. Total Environ. 2015;527–528:262–269. doi: 10.1016/j.scitotenv.2015.04.081. [DOI] [PubMed] [Google Scholar]
- Relyea R., Edwards K. What doesn't kill you makes you sluggish: how sublethal pesticides alter predator–prey interactions. Copeia. 2010;2010:558–567. [Google Scholar]
- Reynoso M.S., Alvarez C., De la Cruz L., Escoto M., Sánchez J. Evaluation of the genotoxic activity of dicamba and atrazine herbicides in several Mexican and South American varieties of sweetcorn (Zea mays L.) Genet. Mol. Res. 2015;14(4):16585–16593. doi: 10.4238/2015.December.11.5. [DOI] [PubMed] [Google Scholar]
- Rosales J. Uso de marcadores genotoxicológicos para la evaluación de agricultores expuestos a plaguicidas organofosforados. An. Fac. Med. 2015;76(3):247–252. [Google Scholar]
- Ryberg K.R., Gilliom R.J. Trends in pesticide concentrations and use for major rivers of the United States. Sci. Total Environ. 2015;538:431–444. doi: 10.1016/j.scitotenv.2015.06.095. [DOI] [PubMed] [Google Scholar]
- Salazar S., Botello E. Viabilidad de semillas de Glycine max (l.) Utilizando la prueba de tetrazolio. RIAA. 2018;9(2):89–98. [Google Scholar]
- Salazar S., Maldonado H. Evaluation of cytotoxic potential of chlorpyrifos using Lens culinaris Med as efficient bioindicator. Ecotoxicol. Environ. Saf. 2019;183:109528. doi: 10.1016/j.ecoenv.2019.109528. [DOI] [PubMed] [Google Scholar]
- Salazar S., Maldonado H. Evaluation of the cytotoxic potential of sodium hypochlorite using meristematic root cells of Lens culinaris Med. Sci. Total Environ. 2020;701:134992. doi: 10.1016/j.scitotenv.2019.134992. [DOI] [PubMed] [Google Scholar]
- Salazar S., Quintero J. Cytotoxic evaluation of glyphosate, using Allium cepa L as bioindicator. Sci. Total Environ. 2020;700 doi: 10.1016/j.scitotenv.2019.134452. [DOI] [PubMed] [Google Scholar]
- Salazar S., Quintero J., Rojas J. Cytogenotoxic effect of propanil using the Lens culinaris Med and Allium cepa L test. Chemosphere. 2020;249 doi: 10.1016/j.chemosphere.2020.126193. [DOI] [PubMed] [Google Scholar]
- Salazar S., Quintero J., Botello E. Optimización de la prueba de tetrazolio para evaluar la vialidad en semillas de Solanum lycopersicum L. Ciencia Y Tecnología Agropecuaria. 2020;21(3) [Google Scholar]
- Salazar S., Quintero J., Bustos V. Implementación de la prueba de tetrazolio en las semillas de Raphanus sativus L. Revista Facultad De Ciencias Básicas. 2020;15(2):7–15. [Google Scholar]
- Salazar-Mercado S.A., Torres-León C.A., Rojas-Suárez J.P. Cytotoxic evaluation of sodium hypochlorite, using Pisum sativum L as effective bioindicator. Ecotoxicol. Environ. Saf. 2019;173:71–76. doi: 10.1016/j.ecoenv.2019.02.027. [DOI] [PubMed] [Google Scholar]
- Shahwar D., Ansari M., Choudhary S. Induction of phenotypic diversity in mutagenized population of lentil (Lens culinaris Medik) by using heavy metal. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira G., Lima M., dos Reis G., Palmieri M., Andrade-Vieria L. Toxic effects of environmental pollutants: comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere. 2017;178:359–367. doi: 10.1016/j.chemosphere.2017.03.048. [DOI] [PubMed] [Google Scholar]
- Singh D., Roy B.K. Evaluation of malathion-induced cytogenetical effects and oxidative stress in plants using Allium test. Acta Physiol. Plant. 2017;39:92. [Google Scholar]
- Srivastava A.K., Singh D. Assessment of malathion toxicity on cytophysiological activity, DNA damage and antioxidant enzymes in root of Allium cepa model. Sci. Rep. 2020;10:886. doi: 10.1038/s41598-020-57840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumon K.A., Rico A., Ter Horst M.M.S., Van den Brink P.J., Haque M.M., Rashid H. Risk assessment of pesticides used in rice-prawn concurrent systems in Bangladesh. Sci. Total Environ. 2016;568:498–506. doi: 10.1016/j.scitotenv.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Triassi M., Nardone A., Giovinetti M.C., De Rosa E., Canzanella S., Sarnacchiaro P., Montuori P. Ecological risk and estimates of organophosphate pesticides loads into the Central Mediterranean Sea from Volturno River, the river of the “Land of Fires” area, southern Italy. Sci. Total Environ. 2019;678:741–754. doi: 10.1016/j.scitotenv.2019.04.202. [DOI] [PubMed] [Google Scholar]
- Verma S., Srivastava A. Morphotoxicity and cytogenotoxicity of pendimethalin in the test plant Allium cepa L. - a biomarker based study. Chemosphere. 2018;206:248–254. doi: 10.1016/j.chemosphere.2018.04.177. [DOI] [PubMed] [Google Scholar]
- Wanwimolruk S., Kanchanamayoon O., Phopin K., Prachayasittikul V. Food safety in Thailand 2: pesticide residues found in Chinese kale (Brassica oleracea), a commonly consumed vegetable in Asian countries. Sci. Total Environ. 2015;532:447–455. doi: 10.1016/j.scitotenv.2015.04.114. [DOI] [PubMed] [Google Scholar]
- Willison S.A., Daniel Stout I.I., Mysz A., Starr J., Tabor D., Wyrzykowska-Ceradini B., Snyder E.G. The impact of wipe sampling variables on method performance associated with indoor pesticide misuse and highly contaminated areas. Sci. Total Environ. 2019;655:539–546. doi: 10.1016/j.scitotenv.2018.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Zhang R., Liu J., Guo Y., Ma E. Effects of malathion and chlorpyrifos on acetylcholinesterase and antioxidant defense system in Oxya chinensis (Thunberg) (Orthoptera: acrididae) Chemosphere. 2011;83(4):599–604. doi: 10.1016/j.chemosphere.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Wu H., Zhang Y., Shi X., Zhang J., Ma E. Overexpression of Mn-superoxide dismutase in Oxya chinensis mediates increased malathion tolerance. Chemosphere. 2017;181:352–359. doi: 10.1016/j.chemosphere.2017.04.087. [DOI] [PubMed] [Google Scholar]