Abstract
Expanded polyglutamine-containing proteins in neurons intrinsically contributes to neuronal dysfunctions and neuronal cell death in polyglutamine (polyQ) diseases. In addition, an expanded polyQ-containing protein in microglia also leads to apoptosis of neurons. However, detailed morphological analysis of neurons exposed to conditioned medium (CM) derived from polyQ-containing microglia has not been essentially carried out. Here, we introduced aggregated peptide with 69 glutamine repeat (69Q) into BV2 microglial cells. The 69Q-containing BV2 cells showed shorter branches. The CM from 69Q-containing microglia (69Q-CM) induced neurite retraction and fewer number of branch point of neurites of differentiated PC12 cells. Likewise, the 69Q-CM induces disturbed differentiation of PC12 cells with shorter total length of neurites and fewer number of branch point of neurites. Thus, the factor(s) released from polyQ-containing microglia affect both differentiation and degeneration of neuron-like cells.
Keywords: Neurite, Polyglutamine, Neuronal death, Molecular neuroscience, Neuroanatomy, Cell culture, Cell differentiation
Neurite, Polyglutamine, Neuronal death, Molecular neuroscience, Neuroanatomy, Cell culture, Cell differentiation
1. Introduction
The symptoms of patients with polyglutamine (polyQ) diseases include motor dysfunction such as ataxia and cognitive impairment that result in compromised daily living capacity [1, 2]. The neurological symptoms are brought by degeneration of neurons in various regions of the central nervous system (CNS) such as the cerebellum, basal ganglia, cerebral cortex, and brainstem as well as the spinal cord [3]. So far, no effective treatments have been presented for reversing the symptoms of polyQ diseases. The pathogenesis of the polyQ disease is expansions of CAG trinucleotide repeats in the causative genes that encode expanded polyQ tracts in the causative proteins [4, 5, 6].
Although polyQ in neurons plays a pivotal role in neurodegeneration, recent findings suggest non-negligible contribution of microglia, the ramified brain-resident phagocytes, to neuronal dysfunctions in polyQ diseases [7, 8]. In addition to phagocytosis, microglia plays vital roles in homeostasis of CNS by perpetually scanning the CNS [9, 10]. Dysregulation of the sentinel can give rise to neurological disease [10]. Multiple types of spinocerebellar ataxia (SCA) are inherited and belong to polyQ diseases [11, 12, 13]. SCA type 1 (SCA1)-model mice revealed that microglia are activated very early in the absence of neuronal death even when mutant ataxin 1 (ATXN1) expression was restricted to cerebellar Purkinje neurons, indicating microglial activation stimulated by signals from dysfunctional neurons in non-cell autonomous manner.
Huntington's disease (HD) is also a polyQ disease which brings a plethora of neuropsychiatric behavior [14]. Huntingtin protein (HTT) is the causative molecule of HD and is expressed in both neurons and various non neuronal cells [15]. Notably, nuclear mutant HTT inclusions were found in microglia in the frontal cortex of adult-onset HD and in the frontal cortex and striatum of juvenile-onset HD [16].
An influence of polyQ-containing microglia on neurons was studied in mice. Addition of mutant Htt knock-in microglia (Q175/Q175) induced apoptosis of embryonic stem cell-derived neurons cultured on a substrate of wild type primary astrocytes [17]. However, the apoptosis was not essentially observed in a case of wild type (Q7/Q7) microglia [17]. Even in vivo experiment, mice expressing mutant HTT specifically in microglia using Cx3cr1-driven Cre recombinase resulted in higher incidence of neuron death under sterile inflammation condition than the control littermates [17]. These observations suggest that HTT having expanded polyQ in microglia leads to neuron death.
Given the findings, we sought to study detailed morphological changes of neuron-like cells by expanded polyQ-containing microglia because neuronal dysfunctions occur prior to neuronal cell death. In this study, we prepared a synthetic polyQ peptide with 69 glutamine repeats (69Q) without flanking sequences of any causative proteins as an expanded polyQ. We also used 15Q as a non-expanded polyQ. These peptides were introduced into microglial cells and conditioned medium (CM) of the cells was collected. Then, the CM was added to differentiated neuron-like PC12 cells and retraction of neurites was analyzed. We also did same experiments using PC12 cells before differentiation to see neurite elongation in the presence of the CM.
2. Materials and methods
2.1. PolyQ peptides
TAMRA-labeled synthetic 15Q and 69Q were purchased from Bio-Synthesis Inc. (Lewisville, TX). The sequences of 15Q and 69Q are 5,6-TAMRA-KKQQQQQQQQQQQQQQQKK-CONH2 and 5,6-TAMRA-KKQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQQKK-CONH2, respectively. The purity of these peptides were more than 95%
2.2. Collection of CM
BV2 microglial cell was kindly provided by Dr. Choi (Korea University) and SH-SY5Y cell was purchased from ATCC. The two cells were cultured in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution at 37 °C with 5% CO2. BV2 cells were plated on micro cover glass coated with laminin (30 μg/ml) (WAKO, Osaka, Japan) in 6-well plates, while SH-SY5Y cells were plated in 24-well plates without cover glass.
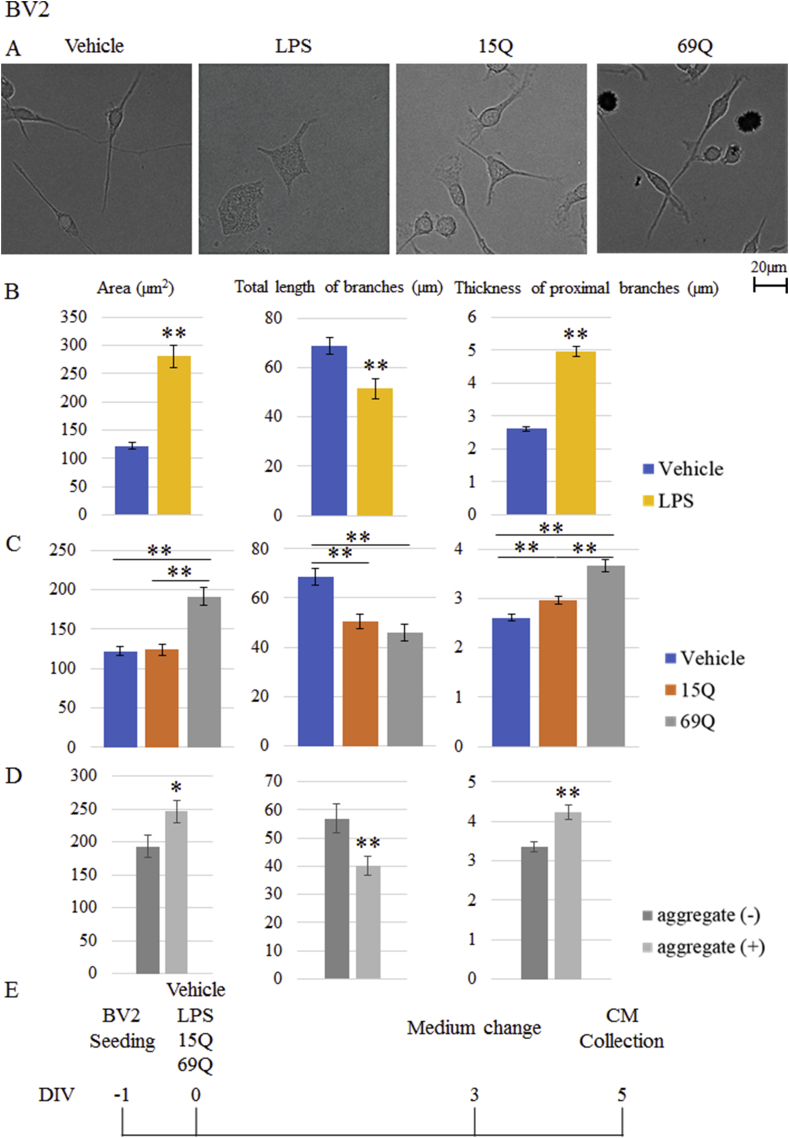
Vehicle, LPS (5 μg/ml), 15Q (10 μg/ml) or 69Q (10 μg/ml) was added to these cells in DMEM containing 1% FBS and cultured for 3 days. After the 3 days culture, these cells were washed in PBS and were cultured in the medium without vehicle, polyQ or LPS. Two days later, CM from BV2 and SH-SY5Y cells were collected (Figure 2E).
Figure 2.
Morphological changes of BV2 microglia by addition of polyQ. (A, B, C) Quantification of the area of cell body, total length of branches and thickness of proximal branches of BV2 microglia with vehicle (A, B, C), LPS (A, B), 15Q (A, C) or 69Q (A, C) (n = 99 cells, each from 4 experiments). Representative images are shown in (A). (D) Comparison of the area of cell body, total length of branches and thickness of proximal branches between BV2 cells with visible 69Q aggregates (n = 60 cells from 4 experiments) and the cells without the visible aggregates (n = 60 cells from 4 experiments) in 69Q-treated cells. (E) Time schedule of addition of vehicle, LPS, 15Q or 69Q to BV2 cells and collection of CM. Error bars represent SE. ANOVA, ∗p < 0.05; ∗∗p < 0.01. Scale bar, 20 μm.
2.3. PC12 cell culture
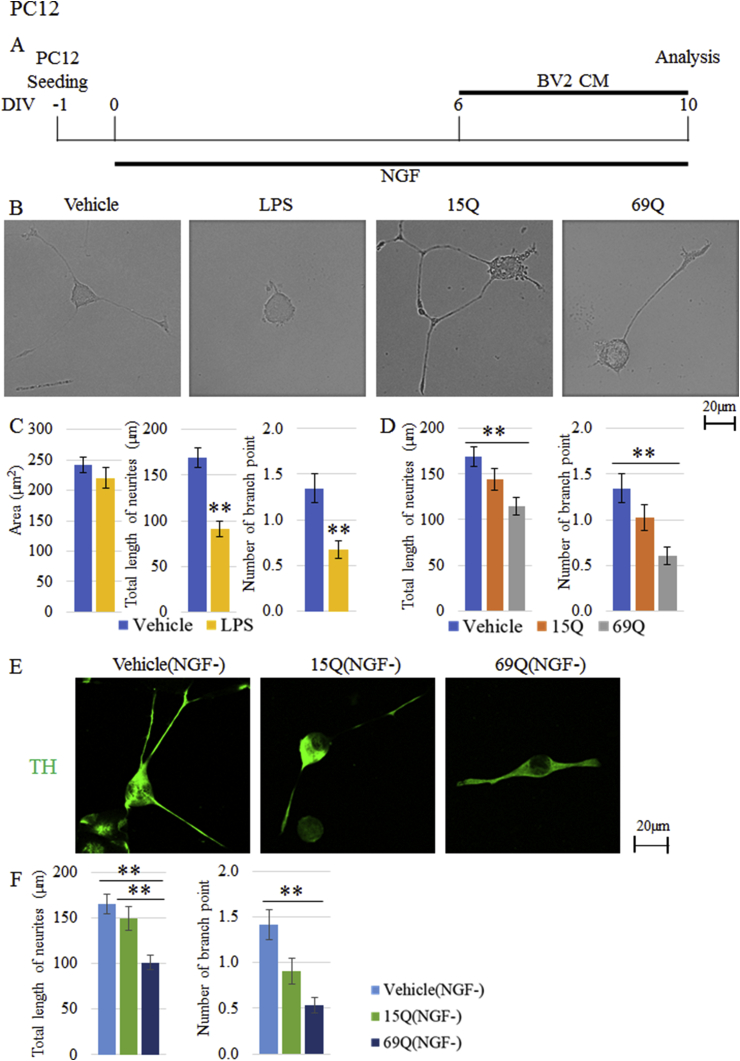
PC12 cells were purchased from RIKEN BRC. PC12 cells were first plated on laminin-coated (30 μg/ml) micro cover glass (density of 1.0×104) in 24 well plates. When CM from BV2 or SH-SY5Y cells was added to PC12 cells after differentiation, PC12 cells were differentiated in DMEM containing 1% FBS, 0.25% BSA and 50 ng/ml NGF for 6 days followed by replacement of the medium into CM (1% FBS) with 0.25% BSA and 50 ng/ml NGF. Then, PC12 cells were further cultured for 4 days for morphological analysis. When CM was added to PC12 cells at the onset of differentiation, the cells were cultured in CM (1% FBS) with 0.25% BSA and 50 ng/ml NGF for 4 days. The protocol of PC12 cell culture is almost same as our previous paper [18]. In the previous paper, we had to place PC12 cells in room temperature without 5% CO2 for 30 min for irradiation of free electron laser. In contrast, we could keep the cells in 37 °C with 5% CO2 in this study. Therefore, the neurite length is 1.5 times longer in this study than that in the previous study.
2.4. Quantification of cultured cells
The visible and fluorescence signals were observed with a fluorescence microscope (Keyence, Osaka, Japan) or a confocal microscope, LSM880 (ZEISS, Oberkochen Germany).
For BV2 cells, quantification of the area of cells was carried out by measuring the area of cell body but not branches. Total length of branches was calculated by adding length of long axis of an individual branch. The thickness of the proximal branch is the length of short axis at the base of a branch. For PC12 cells, number of branch point of the neurites were also measured. When a primary neurite branches multiple times, all branch points were counted. The measurement of the area of the cell body and total length of neurites were essentially same as those for BV2 cells. The quantification was done using Image J software by two observers. Because 1.0 μm proved the shortest limit to detect using Image J software, the branches longer than 1.0 μm were selected.
2.5. Immunofluorescence staining
Immunofluorescence staining was done essentially according to a previous literature [18] using Iba1 (GeneTex, Irvine, CA) and tyrosine hydroxylase (TH) (Merck Millipore, Burlington, MA) antibodies. Nuclear staining with DAPI was also included. The fluorescence signals were detected with a confocal microscope, LSM880 (ZEISS, Oberkochen Germany).
2.6. Statistical analysis
The data were expressed as the mean (SE). Statistical analysis was done using ANOVA followed by Games-Howell post hoc test. P values smaller than 0. 05 were assumed as statistically significant.
3. Results
3.1. PolyQ-induced morphological changes of BV2 microglial cells
We sought to investigate polyQ-induced morphological changes of BV2 microglia. RAW264.7 macrophage cells given with LPS and IFN-γ can be changed into M1-type with larger cell size [19]. Therefore, we quantified the area of cell body. Likewise, an observation that galectin-3-knockdown microglia had short and long thick branches [20] motivated us to measure the total length of branches and thickness of proximal branches of BV2 microglial cells.
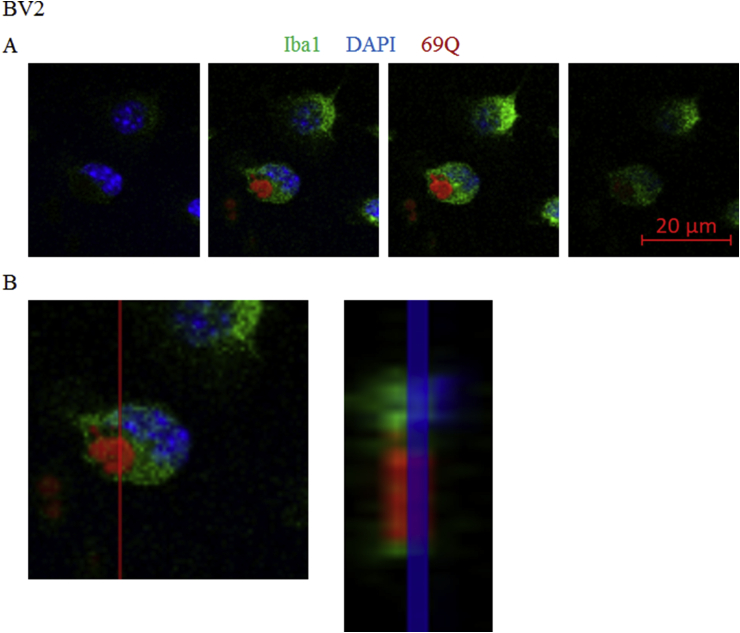
We have previously shown that fluorescence-labeled 69Q forms aggregation in aqueous solution [21] and internalization of 69Q into SH-SY5Y cells is observed [18]. We checked if that is also the case in BV2 cells. 69Q were added to the medium of BV2 microglial cells and the cells were stained with Iba1 antibody, a marker of microglia, and DAPI. The fluorescence z-stack images were taken at every 1 μm (Figure 1A) and the ortho image of the z-stacks with YZ-axis cross section verified presence of the 69Q signals between the Iba1 signals (Figure 1B). The result suggests that 69Q in the culture medium was taken up by BV2 cells. Large 69Q aggregates were found inside the cells in approximately 30% of the cells. However, small 69Q aggregates that cannot be detected by fluorescence microscope might also be present in some of the remaining 70% of the cells.
Figure 1.
69Q spontaneously enter BV2 cells. (A) Fluorescence z-stack images of 69Q-treated BV2 microglial cells (Iba1, green; 69Q, red; DAPI, blue) taken at every 1 μm using confocal laser scanning microscopy. (B) Ortho image of z-stacks taken at every 1 μm using confocal laser scanning microscopy. The left panel shows a cell with the view of XY-axis plane and the right panel is the magnified YZ-axis cross section image along the red line in the left panel. Scale bar, 20 μm.
Since LPS induces microglia having amoeboid shape, which is characterized by the expansion of the cell body and a loss of ramifications [22], we first compared the morphology between vehicle-treated BV2 cells and LPS-treated BV2 cells. BV2 cells were exposed to vehicle or LPS for 3 days. Then, the cells were further cultured for 2 days without vehicle or LPS and the morphological analysis was employed. ANOVA with posthoc test verified larger area of cell body, shorter total length of branches and thicker proximal branches in LPS-treated BV2 cells (Figure 2A, B).
Using same morphological parameters, we then assessed morphology of polyQ-treated BV2 cells. The area of cell body was larger in 69Q-treated BV2 cells compared to vehicle-treated cells (Figure 2A, C). Furthermore, the area of cell body in 69Q-treated BV2 cells was larger than 15Q-treated BV2 cells. Likewise, total length of branches was significantly shorter in both 15Q-treated and 69Q-treated BV2 cells than vehicle-treated cells (Figure 2A, C). Proximal branches were also thicker in 69Q-treated BV2 cells than both vehicle-treated and 15Q-treated cells (Figure 2A, C). Those of 15Q-treated cells was also significantly thicker than vehicle-treated cells (Figure 2A, C).
We then distinguished BV2 cells with visible 69Q aggregates from the cells without the visible aggregates in 69Q-treated cells (Figure 2D). The area of cell body was significantly larger in cells having visible 69Q aggregates than the cells without visible aggregates. Likewise, total length of branches and thickness of proximal branches were significantly shorter and thicker in cells having visible 69Q aggregates, respectively.
3.2. CM derived from polyQ-treated BV2 microglia induces neurite retraction in differentiated PC12 cell
The shorter branches of polyQ-treated microglia partly reflect the morphological character of activated amoeboid microglia. Thus, the factor(s) released from polyQ-treated microglia might have toxic or beneficial effects on the structure of neurites of neuron-like cells. PolyQ-treated BV2 cells were cultured for 3 days. After exclusion of polyQ from the medium, CM was collected 2 days later (Figure 2E). Majority of the phenotypes and morphological abnormalities of neurons seen in polyQ diseases become apparent after aging in both patients and model animals. As a model of mature neurons in vitro, PC12 cells were fully differentiated into neuron-like cells characterized by the presence of neurites by an addition of NGF [18]. To determine the time point when PC12 cells are fully differentiated, we initially checked area, length of neurite and number of branch point of PC12 cells on Day 6 and Day 10 in culture, and found that all the parameters were not different between the two time points (data not shown). Thus, we set the time point for full differentiation on Day 6.
We recently reported that 69Q directly introduced into differentiated PC12 cells induces neurite retraction of PC12 cells as exemplified by shorter total length of neurites [18]. We applied total length of neurites as an index of neurite retraction by the effect of BV2 CM on PC12 cells. In addition, we used number of branch point of neurites as an another morphological index because NGF-stimulated PC12 cells showed an increase in branch point of neurites by active Rac [23].
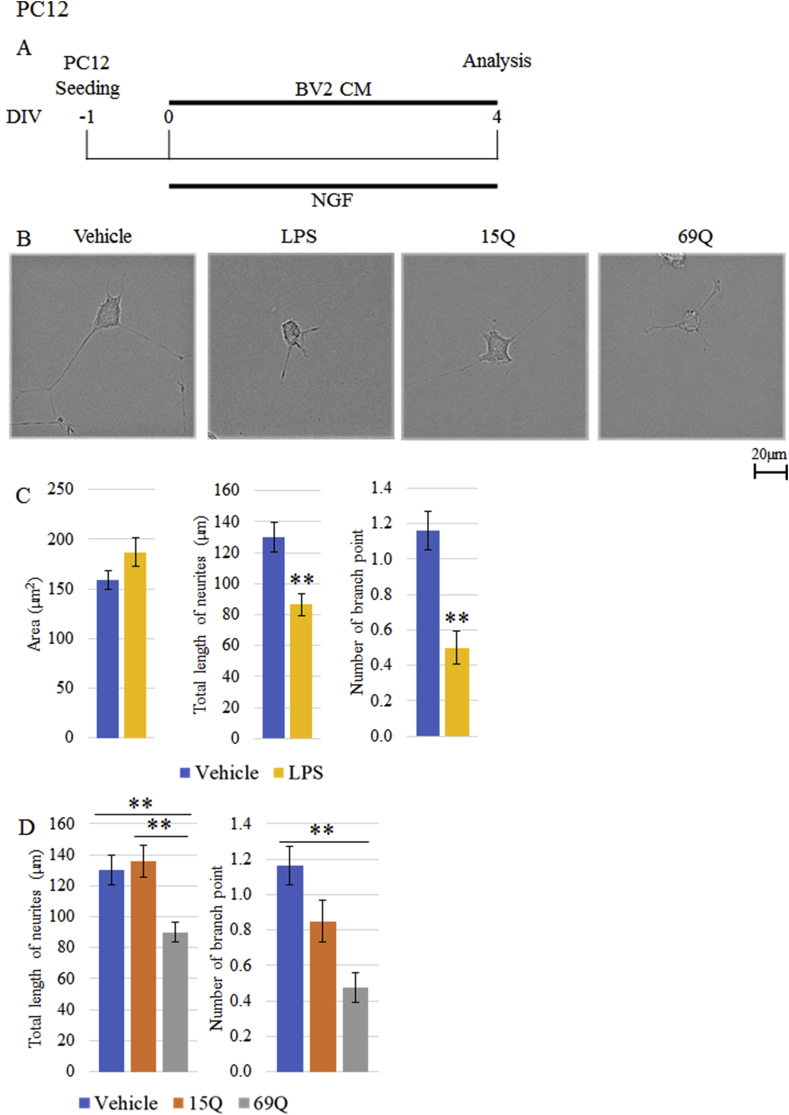
As an initial experiment, CM from BV2 cells treated with vehicle or LPS were added to PC12 cells on day 6 and morphological analysis was done 4 days later (Figure 3A). As shown in Figure 3B, C, shorter total length of neurites and fewer number of branch point of neurites were seen in LPS-CM-treated PC12 cells compared to vehicle-CM-treated cells. However, area of cell body was not different between the two groups (Figure 3B, C).
Figure 3.
The morphological effects of CM derived from BV2 cells treated with polyQ on PC12 cell after differentiation. (A) Time schedule of differentiation of PC12 cells and treatment with CM from BV2 cells treated with vehicle, LPS, 15Q or 69Q. (B, C, D) Quantification of the area of cell body, total length of neurites and/or number of branch point of the neurites of PC12 cells after differentiation in the presence of CM from BV2 cells treated with vehicle (B, C, D), LPS (B, C), 15Q (B, D) or 69Q (B, D) (n = 99 cells, each from 3 experiments). Representative images of PC12 cells are shown in (B). (E, F) Quantification of total length of neurites and number of branch point of the neurites of PC12 cells after differentiation without NGF. NGF was excluded on day 6, and then PC12 cells were cultured for 4 days in the presence of CM from BV2 cells treated with vehicle, 15Q or 69Q (n = 75 cells, each from 3 experiments). TH-stained representative images of PC12 cells are shown in (E). Error bars represent SE. ANOVA, ∗∗p < 0.01. Scale bars, 20 μm.
Then, we assessed the effects of polyQ-CM on PC12 cells. Total length of neurites was significantly shorter by addition of 69Q-treated CM than that of vehicle-treated CM (Figure 3B, D). However, 15Q-treated CM was ineffective, indicating that the effect of polyQ CM is repeat-length dependent. Likewise, number of branch point of the neurites was also significantly fewer in 69Q-treated CM but not in 15Q-treated CM (Figure 3B, D).
To further confirm the retraction of neurites by polyQ-CM, we excluded NGF on day 6, and then cultured PC12 cells for 4 days in the presence of CM. Both total length of neurites and number of branch point were significantly different in 69Q-CM treatment compared to vehicle-CM group (Figure 3E, F). The total length of neurite was also significantly shorter in 69Q-CM-treated PC12 cells than in 15Q-CM-treated PC12 cells (Figure 3E, F).
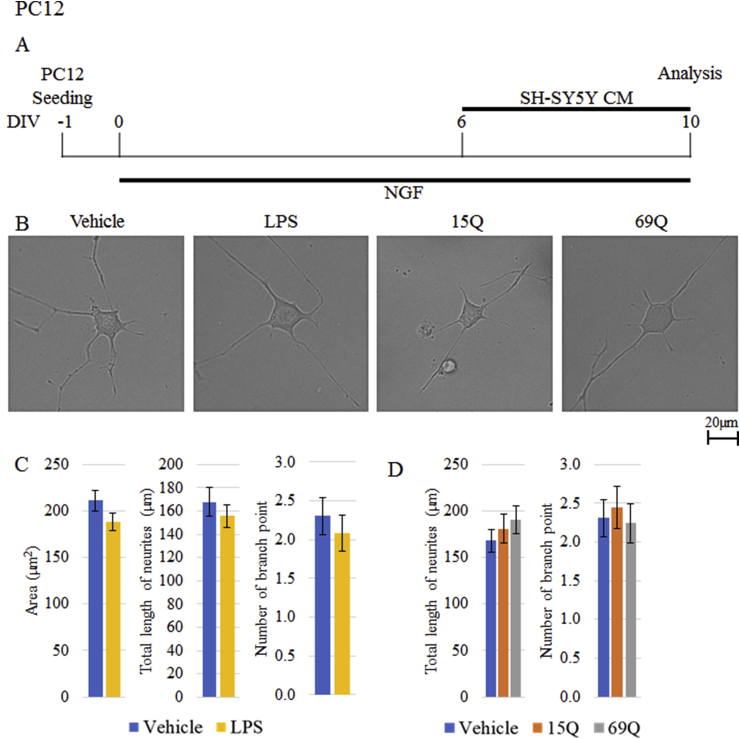
To rule out the possibility that small amount of polyQ in CM rather than factor(s) released from polyQ-treated microglia might directly affect PC12 cells, we carried out same experiment using CM from polyQ-treated SH-SY5Y cell, a non-microglial cell. There were no significant differences in all the parameters examined by addition of either 15Q-treated CM or 69Q-treated CM (Figure 4). Thus, the neurite retraction of PC12 cells depends on the factor(s) released from polyQ-treated microglia.
Figure 4.
The morphological effects of CM derived from SH-SY5Y cells treated with polyQ on PC12 cell after differentiation. (A) Time schedule of differentiation of PC12 cells and treatment with CM from SH-SY5Y cells treated with vehicle, LPS, 15Q or 69Q as Figure 3. (B, C, D) Quantification of the area of cell body, total length of neurites and/or number of branch point of neurites of PC12 cells after differentiation in the presence of CM from SH-SY5Y cells treated with vehicle (n = 95 cells) (B, C, D), LPS (n = 89 cells) (B, C), 15Q (n = 89 cells) (B, D) or 69Q (n = 75 cells) (B, D) (each from 3 experiments). Representative images of PC12 cells are shown in (B). Error bars represent SE. Scale bars, 20 μm.
3.3. CM derived from polyQ-treated BV2 microglia inhibits neurite outgrowth of differentiating PC12 cell
Although expanded polyQ leads to defects in aged neurons, a few reports suggest its role during the stage of neural development [24]. We next focused on the stage of neural development to test the effects of polyQ-treated CM. The CM was added to PC12 cells at the onset of differentiation with NGF, and the cells were cultured for 4 days (Figure 5A). As shown in Figure 5B, C, the total length of neurites is shorter and the number of branch point of the neurites were fewer in LPS-treated CM than in vehicle-treated CM (Figure 5B, C). However, area of the cell body was not different between the two groups (Figure 5B, C).
Figure 5.
The morphological effect of CM derived from BV2 cells treated with polyQ on PC12 cell during differentiation. (A) Time schedule of differentiation of PC12 cells and treatment with CM from BV2 cells treated with vehicle, LPS, 15Q or 69Q during differentiation. (B, C, D) Quantification of the area of cell body, total length of neurites and/or number of branch point of the neurites of PC12 cells during differentiation in the presence of CM from BV2 cells treated with vehicle (n = 99 cells) (B, C, D), LPS (n = 70 cells) (B, C), 15Q (n = 99 cells) (B, D) or 69Q (n = 99 cells) (B, D) (each from 3 experiments). Representative images of PC12 cells are shown in (B). Error bars represent SE. ANOVA, ∗∗p < 0.01. Scale bar, 20 μm.
We next measured total length of neurites and the number of branch point in polyQ-CM groups because these two parameters were changed in LPS-CM group. Both total length of neurites and the number of branch point were significantly different from vehicle-treated CM group in 69Q-treated CM group but not in 15Q-treated CM group (Figure 5B, D). In addition, total length of neurites in 69Q-treated CM group was significantly shorter than 15Q-treated CM group (Figure 5B, D). Again, addition of either 15Q-treated CM or 69Q-treated CM from SH-SY5Y cells did not have any effects on the differentiation of PC12 cells (Figure 6). Taken together, the factor(s) released from polyQ-treated microglia disturbed neurite extension of PC12 cells.
Figure 6.
The morphological effects of CM derived from SH-SY5Y cells treated with polyQ on PC12 cell during differentiation. (A) Time schedule of differentiation of PC12 cells and treatment with CM from SH-SY5Y cells treated with vehicle, LPS, 15Q or 69Q as Figure 5. (B, C, D) Quantification of the area of cell body, total length of neurites and/or number of branch point of neurites of PC12 cells during differentiation in the presence of CM from SH-SY5Y cells treated with vehicle (n = 95 cells) (B, C, D), LPS (n = 95 cells) (B, C), 15Q (n = 75 cells) (B, D) or 69Q (n = 95 cells) (B, D) (each from 3 experiments). Representative images of PC12 cells are shown in (B). Error bars represent SE. Scale bars, 20 μm.
4. Discussion
Microglia is a resident immune cell in the brain. The cells maintain homeostasis and also contribute to normal brain functions (reviewed in [25]). Under pathological conditions such as injuries and diseases, microglia is activated. Indeed, activated microglial cells were detected close to lesion sites in neurodegenerative disorders such as Alzheimer's disease, multiple sclerosis, Parkinson's disease and muscular amyotrophic lateral sclerosis [26]. The activated microglial cells occasionally secret anti-inflammatory neuroprotective factors for tissue repair and wound healing (reviewed in [25]). The neuroprotective aspect of microglia in a neurodegenerative disorder is closely correlated with a motor dysfunction in an inducible model mouse for amyotrophic lateral sclerosis [27]. In the mice, reactive microglia selectively cleared neuronal TDP-43 and blockade of microgliosis during the early recovery phase failed to regain full motor function. In contrast, over activated microglia may exacerbate some neurodegenerative diseases by secretion of proinflammatory factors that lead to cytotoxicity [26].
The activation of microglia is parallel to the morphological changes. The resting and activated microglial cells show a highly ramified morphology and an amoeboid shape, respectively [25, 28]. In the present study, we demonstrated morphological changes of microglia by addition of polyQ. We observed larger area of cell body, shorter length of branches and thicker proximal branches in 69Q-treated microglia. The morphological character in 69Q-treated microglia is partially similar but lesser extent to the amoeboid shape seen in the LPS-activated microglia. Thus, the morphological changes of 69Q likely reflect activated state.
The shorter length of branches and thicker proximal branches were also seen in 15Q-treated BV2 cells. These observations are not surprising because even short 15Q peptide led to shorter total length of neurites in SH-SY5Y cells [18].
We showed that CM from 69Q-treated BV2 microglia induces neurite retraction in differentiated PC12 cells. Given the observation that nuclear mutant HTT inclusions were found in microglia in HD [16], extended polyQ-containing proteins other than HTT in microglia might have some effects on neurons. The Purkinje cell dendrites become shorter [29] and axons of spinal cord become thinner [30] in SCA1 model mice with aging before neuronal cell death. The degeneration of neuronal processes in SCA1 model mice was correlated with electrophysiological and behavioral abnormalities [29, 30]. Accordingly, it is interesting to study electrophysiological properties of neurons in the presence of polyQ-introduced microglia. In contrast to CM from 69Q-treated BV2 microglia, CM from 15Q-treated BV2 microglia did not bring any morphological changes of PC12 cells, suggesting that the extent of activation might not be high enough in 15Q-treated BV2 cells, as compared to 69Q-treated BV2 cells.
We also demonstrated that CM from polyQ-treated BV2 microglia inhibits neurite outgrowth of differentiating PC12 cells. As an evidence of a contribution of polyQ in cells other than neurons to development, proliferation of neural progenitor cells was decreased in a cell autonomous fashion in SCA1 mice [24], which potentially leads to compromised neurogenesis in the adult. Our results also reveal a potential contribution of expanded polyQ in non-neuronal cells to the differentiation of neuron-like cells.
As for the candidate factor(s) released from BV2 cells that led to neurite retraction and disturbed differentiation of PC12 cells, multiple cytokines can be raised. IL-1β level was increased in post-mortem studies using HD brains. Similarly, elevated levels of some molecules such as TNFα and IL-6 were seen in the plasma from HD patients (reviewed in [25]).
The data derived from model mice for polyQ diseases raised a possibility that higher mRNA of these cytokines leads to the elevated levels of the cytokines. Quantitative RT-PCR using mRNA from the cerebellum of transgenic ATXN1 [82Q] mice of 12 weeks old showed higher levels of TNFα, MCP-1 and IL-6 [7]. In SCA1154Q/2Q mice of 8 weeks old, RNA levels of TNFα and MCP-1 were significantly higher, whereas, that of IL-6 showed trend for increase [7]. Likewise, expression of mutant HTT in microglia induced cell-autonomous pro-inflammatory transcriptional activation [17]. This activation was brought by increase in the expression and transcriptional activities of PU.1 and C/EBPs [17]. We have not identified the factor(s) that led to neurite retraction and disturbed differentiation of PC12 cells. Given the morphological similarities between 69Q-treated BV2 and LPS-treated BV2, these factor(s) might be molecule(s) regulated by stimulation with LPS. Whatever the case, additional experiments will be needed to identify the responsible factors by using either siRNA or neutralizing antibodies.
Declarations
Author contribution statement
Ryuji Owada: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Saaya Awata: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Kazutomo Suzue, Hiroyasu Kanetaka, Yohei Kakuta: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Kazuhiro Nakamura: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Kazuhiro Nakamura was supported by Japan Society for the Promotion of Science (19K07242).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Dr. Choi in Korea University for kindly providing us with BV2 microglial cells.
Contributor Information
Yohei Kakuta, Email: m1620020@gunma-u.ac.jp.
Kazuhiro Nakamura, Email: knakamur@gunma-u.ac.jp.
References
- 1.Harding A.E. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1(8334):1151–1155. doi: 10.1016/s0140-6736(83)92879-9. [DOI] [PubMed] [Google Scholar]
- 2.Podvin S., Reardon H.T., Yin K., Mosier C., Hook V. Multiple clinical features of Huntington's disease correlate with mutant HTT gene CAG repeat lengths and neurodegeneration. J. Neurol. 2019;266(3):551–564. doi: 10.1007/s00415-018-8940-6. [DOI] [PubMed] [Google Scholar]
- 3.Robitaille Y., Schut L., Kish S.J. Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA-1) mutation define a unique phenotype. Acta Neuropathol. 1995;90(6):572–581. doi: 10.1007/BF00318569. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman A.P., Shakkottai V.G., Albin R.L. Polyglutamine repeats in neurodegenerative diseases. Annu. Rev. Pathol. 2019;14:1–27. doi: 10.1146/annurev-pathmechdis-012418-012857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orr H.T., Zoghbi H.Y. Trinucleotide repeat disorders. Annu. Rev. Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 6.Paulson H.L., Shakkottai V.G., Clark H.B., Orr H.T. Polyglutamine spinocerebellar ataxias - from genes to potential treatments. Nat. Rev. Neurosci. 2017;18(10):613–626. doi: 10.1038/nrn.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cvetanovic M., Ingram M., Orr H., Opal P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience. 2015;289:289–299. doi: 10.1016/j.neuroscience.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olejniczak M., Urbanek M.O., Krzyzosiak W.J. The role of the immune system in triplet repeat expansion diseases. Mediat. Inflamm. 2015;2015:873860. doi: 10.1155/2015/873860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris G.P., Clark I.A., Zinn R., Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol. Learn. Mem. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Nayak D., Roth T.L., McGavern D.B. Microglia development and function. Annu. Rev. Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manto M.U. The wide spectrum of spinocerebellar ataxias (SCAs) Cerebellum. 2005;4(1):2–6. doi: 10.1080/14734220510007914. [DOI] [PubMed] [Google Scholar]
- 12.Taroni F., DiDonato S. Pathways to motor incoordination: the inherited ataxias. Nat. Rev. Neurosci. 2004;5(8):641–655. doi: 10.1038/nrn1474. [DOI] [PubMed] [Google Scholar]
- 13.Matilla-Duenas A., Goold R., Giunti P. Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum. 2008;7(2):106–114. doi: 10.1007/s12311-008-0009-0. [DOI] [PubMed] [Google Scholar]
- 14.Pandey M., Rajamma U. Huntington's disease: the coming of age. J. Genet. 2018;97(3):649–664. [PubMed] [Google Scholar]
- 15.Trottier Y., Devys D., Imbert G., Saudou F., An I., Lutz Y., Weber C., Agid Y., Hirsch E.C., Mandel J.L. Cellular localization of the Huntington's disease protein and discrimination of the normal and mutated form. Nat. Genet. 1995;10(1):104–110. doi: 10.1038/ng0595-104. [DOI] [PubMed] [Google Scholar]
- 16.Jansen A.H., van Hal M., Op den Kelder I.C., Meier R.T., de Ruiter A.A., Schut M.H., Smith D.L., Grit C., Brouwer N., Kamphuis W., Boddeke H.W., den Dunnen W.F., van Roon W.M., Bates G.P., Hol E.M., Reits E.A. Frequency of nuclear mutant huntingtin inclusion formation in neurons and glia is cell-type-specific. Glia. 2017;65(1):50–61. doi: 10.1002/glia.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crotti A., Benner C., Kerman B.E., Gosselin D., Lagier-Tourenne C., Zuccato C., Cattaneo E., Gage F.H., Cleveland D.W., Glass C.K. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat. Neurosci. 2014;17(4):513–521. doi: 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohara M., Kawasaki T., Owada R., Imai T., Kanetaka H., Izumi S., Tsukiyama K., Nakamura K. Restoration from polyglutamine toxicity after free electron laser irradiation of neuron-like cells. Neurosci. Lett. 2018;685:42–49. doi: 10.1016/j.neulet.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann H., Hoehne K., Rist E., Louw A.M., Schlosshauer B. miR-124 disinhibits neurite outgrowth in an inflammatory environment. Cell Tissue Res. 2015;362(1):9–20. doi: 10.1007/s00441-015-2183-y. [DOI] [PubMed] [Google Scholar]
- 20.Reichert F., Rotshenker S. Galectin-3 (MAC-2) controls microglia phenotype whether amoeboid and phagocytic or branched and non-phagocytic by regulating the cytoskeleton. Front. Cell. Neurosci. 2019;13:90. doi: 10.3389/fncel.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki T., Ohori G., Chiba T., Tsukiyama K., Nakamura K. Picosecond pulsed infrared laser tuned to amide I band dissociates polyglutamine fibrils in cells. Laser Med. Sci. 2016;31(7):1425–1431. doi: 10.1007/s10103-016-2004-x. [DOI] [PubMed] [Google Scholar]
- 22.Kaewmool C., Kongtawelert P., Phitak T., Pothacharoen P., Udomruk S. Protocatechuic acid inhibits inflammatory responses in LPS-activated BV2 microglia via regulating SIRT1/NF-kappaB pathway contributed to the suppression of microglial activation-induced PC12 cell apoptosis. J. Neuroimmunol. 2020;341:577164. doi: 10.1016/j.jneuroim.2020.577164. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi M., Onishi K., Masuyama N., Gotoh Y. The phosphatidylinositol-3 kinase (PI3K)-Akt pathway suppresses neurite branch formation in NGF-treated PC12 cells. Gene Cell. Devoted Mol. Cell. Mech. 2003;8(8):657–669. doi: 10.1046/j.1365-2443.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- 24.Cvetanovic M., Hu Y.S., Opal P. Mutant ataxin-1 inhibits neural progenitor cell proliferation in SCA1. Cerebellum. 2017;16(2):340–347. doi: 10.1007/s12311-016-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subhramanyam C.S., Wang C., Hu Q., Dheen S.T. Seminars in Cell & Developmental Biology. 2019. Microglia-mediated neuroinflammation in neurodegenerative diseases. [DOI] [PubMed] [Google Scholar]
- 26.Xu L., He D., Bai Y. Microglia-Mediated inflammation and neurodegenerative disease. Mol. Neurobiol. 2016;53(10):6709–6715. doi: 10.1007/s12035-015-9593-4. [DOI] [PubMed] [Google Scholar]
- 27.Spiller K.J., Restrepo C.R., Khan T., Dominique M.A., Fang T.C., Canter R.G., Roberts C.J., Miller K.R., Ransohoff R.M., Trojanowski J.Q., Lee V.M. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat. Neurosci. 2018;21(3):329–340. doi: 10.1038/s41593-018-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreutzberg G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 29.Matsuura S., Shuvaev A.N., Iizuka A., Nakamura K., Hirai H. Mesenchymal stem cells ameliorate cerebellar pathology in a mouse model of spinocerebellar ataxia type 1. Cerebellum. 2014;13(3):323–330. doi: 10.1007/s12311-013-0536-1. [DOI] [PubMed] [Google Scholar]
- 30.Takechi Y., Mieda T., Iizuka A., Toya S., Suto N., Takagishi K., Nakazato Y., Nakamura K., Hirai H. Impairment of spinal motor neurons in spinocerebellar ataxia type 1-knock-in mice. Neurosci. Lett. 2013;535:67–72. doi: 10.1016/j.neulet.2012.12.057. [DOI] [PubMed] [Google Scholar]