Summary
Identifying the most effective therapeutic intervention in patients with NAFLD is challenging. Precise stratification in clinical trials is key to ensuring the inclusion of patients who will benefit (and not those who will be harmed) and/or in whom the natural history can be improved. Clinical trials in NAFLD can provide useful information about the individual components that underlie this complex metabolic disorder and the concomitant medications that could interfere with responses to an experimental intervention. However, to date, clinical trial reporting for NAFLD has been suboptimal, limiting our understanding. Frequently dysmetabolic comorbidities and/or daily habits are not reported or adequately accounted for. Herein, we suggest new strategies to integrate the spectra of comorbidities usually present in patients with NAFLD, accounting for the impact of lifestyle, to develop personalised therapeutic approaches. First, the mechanism of action of the drug being explored should be considered. Second, the same proportion of patients with relevant metabolic comorbidities should be maintained from phase II to III clinical trials, if such comorbidities are expected to impact on the treatment response. Third, innovative trial designs, such as the adaptative, umbrella or basket strategies, could be used to increase the efficiency of clinical trials, potentially benefiting patients while reducing costs and enhancing the likelihood of finding a real benefit of the therapy being studied. Finally, alcohol intake and daily exercise should be assessed objectively not only in the screening period but also during follow-up.
Keywords: NAFLD, Clinical trial, Stratification, Comorbidities, Alcohol, Lifestyle
Abbreviations: NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; OR, odds ratio
Key points.
-
•
An adequate stratification is essential to ensure the reliability of clinical trials.
-
•
Structured information should be reported and homogenised in each NAFLD clinical trial.
-
•
Considering the mechanism of action of the drug being explored is relevant because treatments that act directly on the main NAFLD trigger could achieve better response rates.
-
•
Maintaining the same proportion of patients with some relevant metabolic comorbidity from phase II to III clinical trials is crucial because it might impact on the treatment response.
-
•
The standard design of the clinical trials might not consider the heterogeneity of NAFLD, so innovative designs should be considered to overcome the challenges inherent to research in this field.
-
•
Objective measurements of lifestyle should be incorporated into clinical trials because they are common confounders in clinical trials for NAFLD.
Current challenges in NAFLD clinical trials
Non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent liver diseases worldwide.1 It ranges from simple steatosis and non-alcoholic steatohepatitis (NASH) to cirrhosis and hepatocellular carcinoma,2 so a practical therapeutic approach is necessary to halt its natural history. However, no licensed drugs are currently approved. We are now in a race to identify the first drug able to prevent NAFLD or reverse the disease course in patients at more advanced stages.3
Clinical trials for NAFLD pose many challenges.4 On the one hand, some problems relate to recruitment. Individuals enrolling in NAFLD clinical trials are usually required to have a NAFLD activity score (NAS) ≥4 and a fibrosis stage from F1 to cirrhosis by liver biopsy. However, up to half of the screened individuals are failing to meet these eligibility criteria.5 Notably, previous studies have reported that 20–40% of the NAFLD population do not display a definitive histological diagnosis of NASH,6 which makes these individuals ineligible for enrollment.
On the other hand, there are also challenges directly related to drug efficacy. Firstly, selecting the appropriate endpoint and duration of therapy are essential for assessing the efficacy of a drug in clinical trials.7 The reversal of NASH (with no worsening of fibrosis) or improvement of fibrosis (with no further deterioration of NASH) are the endpoints for pre-cirrhotic patients, while the main goal in the cirrhotic population is to avoid decompensated cirrhosis, hepatocellular carcinoma, liver transplant and mortality (beyond the regression of NAS or fibrosis). Secondly, patients given placebo have significant histologic, radiologic, and biochemical responses in NAFLD clinical trials,8 probably owing to the Hawthorne effect.9 Thus, the placebo response must be taken into account as it can confuse the results and interfere with the calculation of sample sizes and the definition of treatment endpoints.10
The stratification of patients appears to be another significant and relevant pitfall in NAFLD clinical trials, particularly regarding the distribution of baseline features.11 In this review, we analyse the existing literature on the impact of metabolic comorbidities and lifestyle on treatment response, how this is reported in the different published clinical trials, and how we can use this information to optimise stratification.
Impact of comorbidities and lifestyle on treatment response
Among the experimental drugs, a plethora of pathways and target mechanisms are being tested, such as metabolic dysfunction, glucose homeostasis, lipid metabolism, or bile acid signalling.12 In this scenario, it has been hypothesised that the presence of a NAFLD-related comorbidities could influence response rates depending on the mechanism of action.
Type 2 diabetes mellitus represents an excellent example of the influence of a comorbidity on response rates. Pioglitazone and liraglutide are antidiabetic drugs that have shown significant results in both diabetic and non-diabetic patients. However, the effect of these drugs was stronger in patients suffering from diabetes mellitus, which could indicate that this entity was primarily responsible for NAFLD.13 Sanyal et al. included 80 patients receiving pioglitazone for 96 weeks,14 while Cusi et al. enrolled 50 patients taking the same drug for 72 weeks.15 Regarding baseline features, the main difference between these trials was that Sanyal et al. did not include any patient with type 2 diabetes mellitus. In contrast, all the patients included by Cusi et al. had diabetes or insulin resistance (in addition to having higher triglyceride levels). Both trials showed a benefit of pioglitazone over placebo, with the primary endpoint achieved in 34% of patients compared to 19% in the study by Sanyal et al., and in 58% vs. 17% in the study by Cusi et al. Despite these positive results, the treatment difference between pioglitazone and placebo was very different between these studies. Thus, the treatment difference in achieving the primary endpoint was 41% for the clinical trial led by Cusi et al. and 15% for the study performed by Sanyal et al. (p = 0.007). Of note, Cusi et al. assessed a stricter endpoint (a reduction of ≥2 points in the NAS score in 2 histologic categories without worsening of fibrosis) than Sanyal et al. (mainly, an improvement by ≥1 point(s) in ballooning without an increase in the fibrosis score). Considering all the individual components of NASH, steatosis, lobular inflammation, and, notably, ballooning were decreased more frequently in patients with diabetes or insulin resistance. Meanwhile, the LEAN trial demonstrated that liraglutide was able to achieve the primary endpoint (resolution of definite NASH with no worsening in fibrosis) in up to 39% of patients, while placebo did so in only 9%.16 Despite the low number of patients enrolled, these results were significant (p = 0.019). Interestingly, no diabetic patients treated with placebo achieved the primary endpoint, while this percentage was 14% in non-diabetic patients. However, the percentage of patients that reached the primary endpoint on liraglutide was very similar irrespective of the presence of type 2 diabetes mellitus (38% vs. 40%). Consequently, the relative risk for the primary endpoint was superior for diabetic (4.7 [95% CI 0.3–75]) than non-diabetic patients (3.4 [95% CI 0.8–14]) for liraglutide.
Obesity could be the paradigm of a complex syndrome encompassing different metabolic comorbidities. Although BMI is usually reported, the impact of obesity on treatment response has not been widely evaluated. The FLINT trial demonstrated that patients with BMI >35 had a better response to obeticholic acid (44% vs. 8% placebo; odds ratio [OR] 8.7; 95% CI 2.4–32.1) than those with BMI <35 (46% vs. 27% placebo; OR 2.3; 95% CI 1.1–4.8). While the proportion of patients achieving the primary outcome was similar for obeticholic acid irrespective of BMI, it is possible that the obesity-dependent variation in placebo response was responsible for this difference.
Assessing the impact of lifestyle (e.g., exercise, dietary habits, and alcohol intake) on response rates is a challenge because of the difficulty obtaining accurate information. The benefit of lifestyle intervention, consisting of diet and exercise, on NASH and fibrosis is well documented.17,18 However, both dietary habits and exercise are usually recorded by baseline questionnaires, instead of objective tools, which limits the usefulness of this information enormously and prevents optimal stratification. For instance, BMI is typically used to monitor lifestyle changes during follow-up and is considered a covariate for the response rate. Alcohol intake is an exclusion criterion for NAFLD clinical trials (>20 g and >30 g per day in men and women, respectively), although its baseline assessment is suboptimal, and the benefits or harms of its intake are controversial.19 Irrespective of baseline parameters, patients receiving placebo show significant histologic, radiologic, and biochemical responses.8 This means that patients change their behaviour after enrolment (Hawthorne effect), indirectly reflecting the impact of lifestyle on response rates (not only on the placebo but also on the experimental group). Thus, new NAFLD clinical trials should include an objective assessment of lifestyle, including exercise, and dietary and alcohol habits, in order to control this cofounding factor adequately.
Information reported about comorbidities and lifestyle in clinical trials
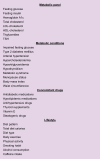
Knowing the baseline features of patients included in clinical trials is essential to understand, at least partially, the current suboptimal response rates achieved with experimental drugs. Besides, this information would allow for comparisons between clinical trials. The publication of trials should include relevant and essential information about metabolic comorbidities and lifestyle.11 We can structure this information at 4 levels: i) a metabolic panel consisting predominantly of variables associated with glucose and lipid metabolism; ii) the presence of comorbidities related to metabolic syndrome; iii) the use of concomitant medication for the dysmetabolic comorbidities that could influence the efficacy of the experimental drug; iv) information about lifestyle, including daily dietary habits, exercise, and alcohol consumption (Box 1).
Box 1.
Authors' recommendations of metabolic and lifestyle factors to be reported in clinical trials in NASH.
|
NASH, non-alcoholic steatohepatitis.
We have analysed 10 phase II clinical trials published to date,5,16,[20], [21], [22], [23], [24], [25], [26], [27] to determine whether they provide information on essential metabolic comorbidities, concomitant drugs and daily lifestyle habits (Table 1). All studies reported the number of patients with type 2 diabetes mellitus and the mean BMI, so comorbidities appear to be considered more important by the study authors. However, dyslipidaemia and arterial hypertension were reported in 7 and 5 studies, respectively. Finally, only 2 out of the 10 clinical trials provided information regarding how many patients had metabolic syndrome. The information about concomitant drugs was also suboptimal. Patients with medications for dyslipidaemia, type 2 diabetes mellitus, and arterial hypertension were reported in 5, 4 and 2 clinical trials, respectively. Surprisingly, only 1 trial reported information on dietary habits, alcohol consumption (to fulfil the inclusion criteria) and smoking, and none reported on exercise.
Table 1.
Information reported in published phase II clinical trials.
| Essential metabolic comorbidities and daily lifestyle | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Drug | T2DM | HOMA | AHT | Dyslipidaemia | BMI | Metabolic syndrome | Alcohol | Dietary habits | Smoking | Exercise |
| Neuschwander-Tetri, 2014 | Obeticholic acid | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Friedman, 2018 | Cenicriviroc | Yes | No | No | Yes | Yes | Yes | No | No | No | No |
| Ratziu, 2016 | Elafibranor | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Armstrong, 2016 | Liraglutide | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No |
| Loomba, 2018 | Selonsertib | Yes | No | No | No | Yes | No | No | No | No | No |
| Sanyal, 2018 | Pegbelfermin | Yes | No | No | Yes | Yes | No | No | No | No | No |
| Harrison, 2018 | NGM282 | Yes | No | Yes | Yes | Yes | No | No | No | No | No |
| Traussnigg, 2019 | norUDCA | Yes | No | Yes | No | Yes | No | No | No | No | No |
| Loomba, 2018 | GS-0976 | Yes | Yes | No | No | Yes | No | No | No | No | No |
| Harrison, 2020 | Emricasan | Yes | No | No | Yes | Yes | Yes | No | No | No | No |
| Concomitant drugs | |||||||
|---|---|---|---|---|---|---|---|
| Author | Drug | T2DM therapy (%) | T2DM therapy (individualised) | AHT therapy (%) | AHT therapy (individualised) | Dyslipidaemia therapy (%) | Dyslipidaemia therapy (individualised) |
| Neuschwander-Tetri, 2014 | Obeticholic acid | Yes | Yes | No | No | Yes | No |
| Friedman, 2018 | Cenicriviroc | No | No | No | No | No | No |
| Ratziu, 2016 | Elafibranor | Yes | Yes | No | No | Yes | Yes |
| Armstrong, 2016 | Liraglutide | Yes | Yes | Yes | No | Yes | No |
| Loomba, 2018 | Selonsertib | No | No | No | No | No | No |
| Sanyal, 2018 | Pegbelfermin | No | No | No | No | Yes | Yes |
| Harrison, 2018 | NGM282 | Yes | Yes | Yes | No | Yes | Yes |
| Traussnigg, 2019 | norUDCA | No | No | No | No | No | No |
| Loomba, 2018 | GS-0976 | No | No | No | No | No | No |
| Harrison, 2020 | Emricasan | No | No | No | No | No | No |
This information has been obtained from the manuscripts and corresponding supplementary material.
The information reported by the clinical trials is also relevant to compare the response rates of experimental drugs objectively. Looking at the baseline features, we found that the percentage of patients with diabetes mellitus included in the trial testing selonsertib was significantly higher (about 70–80%) than in the trial of norursodeoxycholic acid (10–15%). Similarly, the prevalence of arterial hypertension varied considerably between the studies testing NGM282 (65–75%) and norursodeoxycholic acid (40%), while that of dyslipidemia varied between the trials of obeticholic acid (60%) and pegbelfermin (25–30%) (Table 2). Consequently, the distribution of metabolic comorbidities should be considered when we perform non-head-to-head comparisons between different experimental drugs.
Table 2.
Proportion of patients showing metabolic comorbidities in the clinical trials, according to the experimental drug vs. placebo.
| Author | Drug | Type 2 diabetes mellitus |
Arterial hypertension |
Dyslipidaemia |
|||
|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | ||
| Neuschwander-Tetri, 2014 | Obeticholic acid | 53% | 52% | 62% | 60% | 62% | 61% |
| Friedman, 2018 | Cenicriviroc | 57% | 44% | Not reported | 48% | 49% | |
| Ratziu, 2016 | Elafibranor | 42% | 36% | 62% | 47% | 49% | 54% |
| Armstrong, 2016 | Liraglutide | 35% | 31% | 58% | 54% | 35% | 27% |
| Loomba, 2018 | Selonsertib | 66% | 80% | Not reported | Not reported | ||
| Sanyal, 2018 | Pegbelfermin | 33% | 42% | Not reported | 25% | 31% | |
| Harrison, 2018 | NGM282 | 61% | 63% | 64% | 78% | 54% | 30% |
| Traussnigg, 2019 | norUDCA | 6% | 16% | 39% | 41% | Not reported | |
| Loomba, 2018 | GS-0976 | 65% | 58% | Not reported | Not reported | ||
| Harrison, 2020 | Emricasan | 52% | 49% | Not reported | 56% | 61% | |
In summary, it would be desirable to follow standard reporting recommendations for NAFLD clinical trials in order to provide additional transparency and facilitate the understanding and comparison of different therapeutic approaches. Besides, considering the different proportions of metabolic comorbidities between studies, specific statistical adjustments, and interpretations of the outcome measures should be made when comparing results across clinical trials.
Managing the stratification of metabolic comorbidities
One of the main aims of the stratified randomisation strategy in NAFLD clinical trials is to reduce the influence of some potential confounders that could affect study outcomes, complicating the analysis of an experimental drug's efficacy (Table 3). In particular, the presence of metabolic comorbidities (such as obesity, diabetes mellitus, or arterial hypertension), which are very common in patients with NAFLD,28 may influence treatment response.
Table 3.
Recommendations about the management of confounders in NAFLD clinical trials.
| Metabolic and lifestyle confounders on NAFLD clinical trials | Recommendations |
|---|---|
| Current or recent history (<5 years) of significant alcohol consumption before the screening. |
|
| Engagement in an active weight loss program or taking weight loss medication. |
|
| Changes in the lifestyle due to the enrolment (Hawthorne effect). |
|
| Patients with decompensated diabetes (HbA1c >9.0%). |
|
| Taking drugs known to have potential therapeutic activity on NASH prior to entry into the study (e.g., vitamin E, GLP-1 agonists). |
|
| Taking drugs that can induce steatosis/steatohepatitis (e.g., steroids). |
|
Metabolic comorbidities from phase II to phase III clinical trials
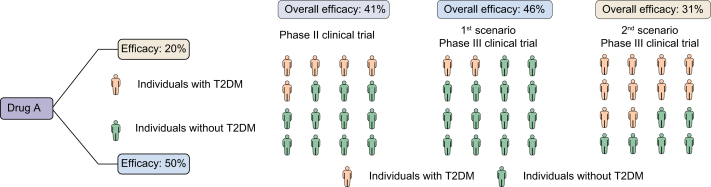
The CENTAUR study demonstrated that 20% of patients receiving cenicriviroc achieved an improvement in fibrosis of ≥1 stage and no worsening of NASH compared to 10.4% of patients receiving placebo (OR 2.20; p = 0.023) in the overall cohort.21 However, cenicriviroc was not superior to placebo in the presence of diabetes (23% [17/74] vs. 18% [10/57]; OR 1.40; 95% CI 0.59–3.35; p = 0.52), while the experimental drug was very effective in the absence of it (23% [12/52] vs. 7% [5/69]; OR 3.84; 95% CI 1.26–11.7; p = 0.017). It must be noted that the percentage of patients with type 2 diabetes mellitus was significantly higher in the group of patients taking cenicriviroc (57.2% [82/145] vs. 44.4% [64/144]; p = 0.039). Therefore, we speculate that if the ongoing phase III clinical trial includes a lower proportion of diabetic patients (e.g., 30%), the effect of cenicriviroc could be greater. However, if the proportion of diabetic patients is higher (e.g., 75%), the experimental drug could have worse response rates (Fig. 1).
Fig. 1.
Hypothetical scenario demonstrating the variation in the efficacy of one drug depending on the proportion of a comorbidity able to influence it.
Another example comes from a secondary analysis of the FLINT trial.29 In this study, the authors found that the presence of baseline hypertriglyceridemia (triglyceride levels >154 mg/dl) impacted negatively on the effect of obeticholic acid (p = 0.020); up to 58.9% (43/73) of patients with histological response had triglyceride levels less than 154 mg/dl (vs. 45.7% 58/127 in those with hypertriglyceridemia). Again, we could expect different response rates depending on the proportion of patients with hypertriglyceridemia in the ongoing phase III clinical trial of obeticholic acid.
Given that the presence of particular comorbidities could influence treatment response, we think it is essential to maintain their proportion from phase II to phase III clinical trials. Otherwise, we cannot directly compare the results from this transition because the efficacy of the drug could be affected by the proportion of baseline comorbidities more than the experimental therapy itself. In this case, trial designs should be refined and improved by learning the lessons of earlier phases. However, the occurrence of metabolic disturbances30 in patients included in NAFLD clinical trials must be closely assessed.
Role of concomitant medications
Many drugs are involved in the management of patients with NAFLD, including those used to treat metabolic comorbidities (such as statins or antidiabetic medications), or those directly prescribed for fatty liver (like vitamin E, although its use is off-label). These therapies could play different roles in NAFLD by targeting elements of the metabolic syndrome. Given the potential confounding influence on outcomes, we think that concomitant medications should be completely stratified by comparison group (experimental drug vs. placebo), and that these proportions should be maintained when moving from phase II to phase III trials. Additionally, it is essential to have stable doses of such medications for at least 3 months before recruitment, and any dose changes should be recorded during the trial.
Obeticholic acid is the only drug that has had preliminary positive results in the ongoing phase III clinical trial (an 18-month interim analysis), although only 1 of the 2 primary endpoints was achieved (improvement of fibrosis with no worsening of NASH).31 Despite most of the baseline features being similar between the phase II and III trials, some elements could impact on the results. Both trials included patients taking vitamin E, but the percentage was significantly higher in the phase II trial (21% vs. 10% [p = 0.002] for the treatment group and 23% vs. 14% [p = 0.019] for the placebo group). In the FLINT trial, the primary outcome was a decrease ≥2 points in the NAS score with no worsening in fibrosis, which was achieved in 45% of patients taking obetichloic acid compared to 21% of patients taking placebo (response rate 1.9; 95% CI 1.3–2.8; p = 0.0002). Although it was not the primary outcome, the same endpoint was assessed in the interim analysis of the phase III trial, but the response rate was lower (36% vs. 24%; response rate 1.5; 95% CI 1.2–1.9; p = 0.0012). The number of patients receiving vitamin E could influence this endpoint, as the PIVENS trial demonstrated that vitamin E was associated with a significantly higher rate of NASH improvement.14
New designs for clinical trials
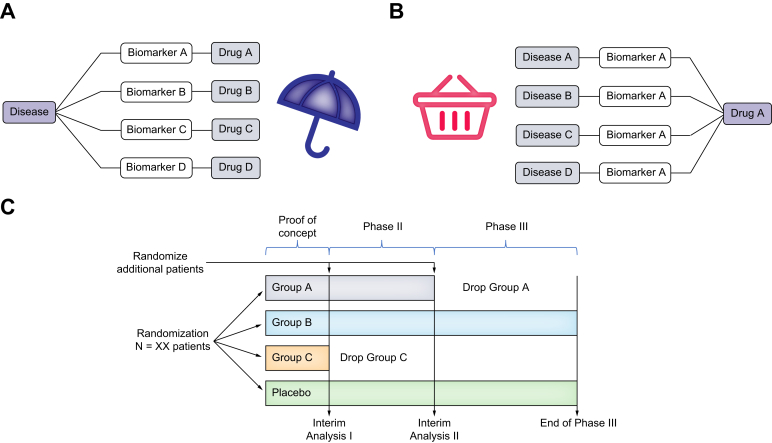
The wide variety of NAFLD phenotypes is a crucial element to bear in mind when designing clinical trials. The standard clinical trial design does not adequately reflect disease heterogeneity and may not represent the best option for this entity. Over time, innovative trials, such as umbrella, basket, and adaptive designs, have been suggested to overcome the aforementioned challenges32 (Fig. 2).
Fig. 2.
Innovative clinical trial designs that could be used for NAFLD.
(A) Umbrella trial. (B) Basket trial. (C) Adaptative trial, combining phase II and phase III. NAFLD, non-alcoholic fatty liver disease.
Umbrella trial designs aim to assess multiple targeted treatments for a single disease (e.g., NAFLD), depending on a previous stratification into subgroups based on molecular biomarkers (e.g., metabolic vs. inflammatory patterns). From a pragmatic point of view, an umbrella trial simultaneously represents small trials within one large trial.33
Basket trials include designs to evaluate a specific therapy for multiple diseases that share similar genetic abnormalities (e.g., NAFLD and type 2 diabetes mellitus). Thus, this approach focuses on testing one treatment against a specific target, irrespective of the disease.34
The adaptive design provides flexibility so that prospectively planned adaptions can be made based on early findings. The adaptative trial may reduce the number of patients by allowing patients with promising results (e.g., first interim analysis assessing safety and futility criteria based on non-invasive biomarkers) to move to the next phase (e.g., biopsy-driven endpoints), enrolling additional patients and maintaining the exposure to long-term follow-up. By contrast, arms with poor or no efficacy could be stopped. In an adaptative trial, the later stages could enrol more diabetic patients if the experimental drug showed efficacy only in the presence of diabetes at the first interim analysis. This approach is particularly relevant in NAFLD clinical trials because many patients are not willing to have multiple liver biopsies. Besides, it does not compromise the quality of the evidence needed to establish the efficacy and safety of therapeutic agents. However, the adaptative strategy adds substantial complexity to data interpretation.35
Managing the stratification of lifestyle habits
Alcohol consumption
The quantification of alcohol intake is challenging. Although it is important to screen patients for alcohol intake prior to inclusion in clinical trials for NAFLD, its assessment is not standardized during patient follow-up. Usually, all the information obtained about alcohol consumption is self-reported by the patient through questionnaires, such as AUDIT, instead of relying on biomarkers (e.g., urinary ethyl glucuronide, phosphatidyl ethanol).36 However, the use of self-reported questionnaires detected mainly heavy drinkers. From a clinical point of view, laboratory findings, such as aspartate to alanine aminotransferase ratio or gamma glutamyltransferase values, show low sensitivity and specificity for identifying the consumption. Anyhow, mild to moderate alcohol consumption is relatively frequent in patients with NAFLD, so the strict exclusion of such individuals might limit the application of trial results to routine practice. Therefore, we must collect some additional information about alcohol intake routinely, irrespective of the estimated levels of consumption. At least, we should consider collecting and stratifying by the age at onset of drinking, patterns of recent drinking, preferred beverages, and frequency of binge drinking.37 These data must be additionally incorporated into the final analyses, in order to improve the stratification of patients into clinical trials, to evaluate more precisely the role of the experimental drug on outcomes.
Diet and exercise
Lifestyle intervention significantly improved NAFLD as previously reported,18 so its monitoring is essential for the optimal stratification of patients. A placebo arm with a hidden lifestyle intervention could become a hidden active arm. Considering that the Hawthorne effect influences NAFLD, it is mandatory to assess diet (mainly western diet vs. the rest) together with physical activity and sedentary behaviour at baseline and to continue monitoring this during therapy and follow-up in both active and placebo arms. Body weight loss monitoring, if not part of the primary endpoint, could uncover a hidden intervention. Given that a growing body of evidence supports the role of the number of daily steps,38 a technology-based approach (e.g., smartphone applications or pedometers) to calculate them would be a simple and an easy-to-perform tool to stratify the results. Besides, it allows researchers to deliver an intervention and monitor physical activity remotely. Diet should also be recorded at baseline, together with anthropometric measures like weight, BMI, and waist circumference. Changes in body weight and physical activity must be recorded and included in the final analysis to avoid potential biases.39
Recommendations on diet and physical activity should be provided to both arms and included in protocols.39 However, given that clinical trials are distributed over different geographical regions, cultural and regional differences could limit the ability to standardise lifestyle for diverse populations. Thus, participants should adopt lifestyle modifications based on specific recommendations according to location, but with an emphasis on standardised protocols, for instance, eliminating processed foods and sugar-sweetened beverages.
Conclusions
The goal of stratifying metabolic comorbidities in NAFLD clinical trials is to pave the way for personalised medicine. Adequate stratification is essential to ensure the reliability of clinical trials. In trials for NAFLD, the same drug is often given to patients with different underlying comorbidities, leading to a wide variety of treatment responses which must be considered and managed with specific strategies. To date, the reporting of clinical trials has been suboptimal, with much data related to metabolic comorbidities and concomitant medications lacking. Structured information should be reported and homogenised for each NAFLD clinical trial. Innovative trial designs should be considered, as these may help to address some of the current pitfalls of NAFLD trials. Finally, objective measurements of lifestyle should be incorporated into clinical trials as they may lead to significant confounding. Addressing some of these issues should lead to clearer trial outcomes and expedite drug development for NAFLD.
Financial support
This project has been partially funded by the “Consejería de Salud de la Junta de Andalucía” (PI-0075-2014), and the “Spanish Ministry of Economy, Innovation and Competition, Instituto de Salud Carlos III” (PI19/01404, PI16/01842, PI17/00535 and GLD19/00100). ∗The funders have not had any role in the design, analysis, writing, or interpretation of this project.
Authors' contributions
Guarantor of the article: JA. Drafting the manuscript: JA. Critical review of the manuscript: MRG. All authors approved the final version of the article, including the authorship list.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100148.
Supplementary data
References
- 1.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Ampuero J., Sánchez-Torrijos Y., Aguilera V., Bellido F., Romero-Gómez M. New therapeutic perspectives in non-alcoholic steatohepatitis. Gastroenterol Hepatol. 2018;41:128–142. doi: 10.1016/j.gastrohep.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Rinella M.E., Tacke F., Sanyal A.J., Anstee Q.M. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol. 2019;71:823–833. doi: 10.1016/j.jhep.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R., Lawitz E., Mantry P.S., Jayakumar S., Caldwell S.H., Arnold H. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67:549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai R.K., Kleiner D.E., Hart J., Adeyi O.A., Clouston A.D., Behling C.A. Standardising the interpretation of liver biopsies in non-alcoholic fatty liver disease clinical trials. Aliment Pharmacol Ther. 2019;50:1100–1111. doi: 10.1111/apt.15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui M.S., Harrison S.A., Abdelmalek M.F., Anstee Q.M., Bedossa P., Castera L. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology. 2018;67:2001–2012. doi: 10.1002/hep.29607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M.A.T., Altayar O., Hamdeh S., Takyar V., Rotman Y., Etzion O. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:616–629.e26. doi: 10.1016/j.cgh.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 9.McCambridge J., Witton J., Elbourne D.R. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enck P., Klosterhalfen S. Placebos and the placebo effect in drug trials. Handb Exp Pharmacol. 2019;260:399–431. doi: 10.1007/164_2019_269. [DOI] [PubMed] [Google Scholar]
- 11.Patel Y.A., Imperial J.C., Muir A.J., Anstee Q.M., DeBrota D., Dimick-Santos L. Baseline parameters in clinical trials for nonalcoholic steatohepatitis: recommendations from the liver Forum. Gastroenterology. 2017;153:621–625.e7. doi: 10.1053/j.gastro.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black D., Brockbank S., Cruwys S., Goldenstein K., Hein P., Humphries B. The future R&D landscape in non-alcoholic steatohepatitis (NASH) Drug Discov Today. 2019;24:560–566. doi: 10.1016/j.drudis.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Bell L.N., Wang J., Muralidharan S., Chalasani S., Fullenkamp A.M., Wilson L.a. Relationship between adipose tissue insulin resistance and liver histology in NASH: a PIVENS follow-up study. Hepatology. 2012;233:2–22. doi: 10.1002/hep.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus a randomized trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 17.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378.e5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Romero-Gómez M., Zelber-Sagi S., Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Petroni M.L., Brodosi L., Marchignoli F., Musio A., Marchesini G. Moderate alcohol intake in non-alcoholic fatty liver disease: to drink or not to drink? Nutrients. 2019;11:3048. doi: 10.3390/nu11123048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman S.L., Ratziu V., Harrison S.A., Abdelmalek M.F., Aithal G.P., Caballeria J. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 24.Sanyal A., Charles E.D., Neuschwander-Tetri B.A., Loomba R., Harrison S.A., Abdelmalek M.F. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2018;392:2705–2717. doi: 10.1016/S0140-6736(18)31785-9. [DOI] [PubMed] [Google Scholar]
- 25.Traussnigg S., Schattenberg J.M., Demir M., Wiegand J., Geier A., Teuber G. Norursodeoxycholic acid versus placebo in the treatment of non-alcoholic fatty liver disease: a double-blind, randomised, placebo-controlled, phase 2 dose-finding trial. Lancet Gastroenterol Hepatol. 2019;4:781–793. doi: 10.1016/S2468-1253(19)30184-0. [DOI] [PubMed] [Google Scholar]
- 26.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155:1463–1473.e6. doi: 10.1053/j.gastro.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison S.A., Goodman Z., Jabbar A., Vemulapalli R., Younes Z.H., Freilich B. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72:816–827. doi: 10.1016/j.jhep.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Ampuero J., Aller R., Gallego-Durán R., Banales J.M., Crespo J., García-Monzón C. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48:1260–1270. doi: 10.1111/apt.15015. [DOI] [PubMed] [Google Scholar]
- 29.Loomba R., Sanyal A.J., Kowdley K.V., Terrault N., Chalasani N.P., Abdelmalek M.F. Factors associated with histologic response in adult patients with nonalcoholic steatohepatitis. Gastroenterology. 2019;156:88–95.e5. doi: 10.1053/j.gastro.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ampuero J., Aller R., Gallego-Durán R., Crespo J., Calleja J.L., García-Monzón C. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol. 2020;73:17–25. doi: 10.1016/j.jhep.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 32.Mandrekar S.J., Dahlberg S.E., Simon R. Improving clinical trial efficiency: thinking outside the box. Am Soc Clin Oncol Educ Book. 2015:e141–e147. doi: 10.14694/EdBook_AM.2015.35.e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirakawa A., Asano J., Sato H., Teramukai S. Master protocol trials in oncology: review and new trial designs. Contemp Clin Trials Commun. 2018;12:1–8. doi: 10.1016/j.conctc.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garralda E., Dienstmann R., Piris-Giménez A., Braña I., Rodon J., Tabernero J. New clinical trial designs in the era of precision medicine. Mol Oncol. 2019;13:549–557. doi: 10.1002/1878-0261.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filozof C., Chow S.-C., Dimick-Santos L., Chen Y.-F., Williams R.N., Goldstein B.J. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: facilitating development approaches for an emerging epidemic. Hepatol Commun. 2017;1:577–585. doi: 10.1002/hep4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piano S., Marchioro L., Gola E., Rosi S., Morando F., Cavallin M. Assessment of alcohol consumption in liver transplant candidates and recipients: the best combination of the tools available. Liver Transpl. 2014;20:815–822. doi: 10.1002/lt.23881. [DOI] [PubMed] [Google Scholar]
- 37.Crabb D.W., Bataller R., Chalasani N.P., Kamath P.S., Lucey M., Mathurin P. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 2016;150:785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuoka Y., Haskell W., Lin F., Vittinghoff E. Short- and long-term effects of a mobile phone app in conjunction with brief in-person counseling on physical activity among physically inactive women: the mPED randomized clinical trial. JAMA Netw Open. 2019;2:e194281. doi: 10.1001/jamanetworkopen.2019.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass O., Filozof C., Noureddin M., Berner-Hansen M., Schabel E., Omokaro S.O. Standardization of diet and exercise in clinical trials of NAFLD-NASH: recommendations from the Liver Forum. J Hepatol. 2020;73(3):680–693. doi: 10.1016/j.jhep.2020.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.