Abstract
Low density lipoprotein receptor-related protein 6 (LRP6), a Wnt co-receptor, induces multiple functions in various organs. We recently reported cardiac specific LRP6 deficiency caused cardiac dysfunction in mice. Whether cardiomyocyte-expressed LRP6 protects hearts against ischemic stress is largely unknown. Here, we investigated the effects of cardiac LRP6 in response to ischemic reperfusion (I/R) injury. Tamoxifen inducible cardiac specific LRP6 overexpression mice were generated to build I/R model by occlusion of the left anterior descending (LAD) coronary artery for 40 min and subsequent release of specific time. Cardiac specific LRP6 overexpression significantly ameliorated myocardial I/R injury as characterized by the improved cardiac function, strain pattern and infarct area at 24 h after reperfusion. I/R induced-apoptosis and endoplasmic reticulum (ER) stress were greatly inhibited by LRP6 overexpression in cardiomyocytes. LRP6 overexpression enhanced the expression of heat shock transcription factor-1(HSF1) and heat shock proteins (HSPs), the level of p-glycogen synthase kinase 3β(GSK3β)(S9) and p-AMPK under I/R. HSF1 inhibitor deteriorated the apoptosis and decreased p-GSK3β(S9) level in LRP6 overexpressed –cardiomyocytes treated with H2O2. Si-HSF1 or overexpression of active GSK3β significantly attenuated the increased expression of HSF1 and p-AMPK, and the inhibition of apoptosis and ER stress induced by LRP6 overexpression in H2O2-treated cardiomyocytes. AMPK inhibitor suppressed the increase in p-GSK3β (S9) level but didn’t alter HSF1 nucleus expression in LRP6 overexpressed-cardiomyocytes treated with H2O2. Active GSK3β, but not AMPK inhibitor, attenuated the inhibition of ubiquitination of HSF1 induced by LRP6-overexpressed-cardiomyocytes treated with H2O2. LRP6 overexpression increased interaction of HSF1 and GSK3β which may be involved in the reciprocal regulation under oxidative stress. In conclusion, cardiac LRP6 overexpression significantly inhibits cardiomyocyte apoptosis and ameliorates myocardial I/R injury by the crosstalk of HSF1 and GSK3β signaling.
Keywords: Ischemic reperfusion, Apoptosis, LRP6, HSF1, GSK3β
Abbreviations
- LRP6
low density lipoprotein receptor-related protein 6
- I/R
ischemic reperfusion
- LAD
left anterior descending
- ID
intercalated disc
- AMPK
adenosine 5‘-monophosphate (AMP)-activated protein kinase
- α-MHC-MCM
α-myosin heavy chain Mer-Cre-Mer
- TTC
evan’s blue and triphenyltetrazolium chloride
- EF
ejection fraction
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- IA/AAR
infarct area/area at risk
- AAR/LV
area at risk /left ventricle
- ER
endoplasmic reticulum
- HSF1
heat shock transcription factor-1
- GSK3β
glycogen synthase kinase 3β
1. Introduction
Myocardial ischemia is the leading cause of morbidity and mortality in the world. The mainstay therapy for myocardial ischemia currently focuses on timely reperfusion. However, additional reperfusion approaches render the myocardium to be more vulnerable to severe injury, known as myocardial ischemia reperfusion (I/R) injury, which restricts the development of reperfusion therapy [1]. Cardiomyocyte apoptosis is a critical component of myocardial I/R injury. During I/R, oxidative stress causes a burst of reactive oxygen species (ROS), leading to necrotic or apoptotic cell death [2]. However, the endogenous defensive molecular mechanisms in myocardial I/R injury are incompletely understood.
We recently reported that low density lipoprotein receptor-related protein 6 (LRP6) is downregulated in heart tissue from patients with dilated cardiomyopathy, and cardiomyocyte-specific deletion of LRP6 causes dilated cardiomyopathy owing to mitochondria and fatty acid metabolism dysfunction in mice [3,4]. LRP6, a wnt co-receptor, belonging to the low density lipoprotein (LDL) receptor-related family, is a transmembrane cell surface protein to induce canonical Wnt signaling pathway [5]. Upon binding of Wnts to its receptor Frizzled, LRP6 is recruited to form a complex with Wnt-Frizzled eventually leading to the activation of downstream β-catenin (named as β-catenin stabilization), the increased β-catenin enters into nucleus and binds Tcf/Lef to promote target genes transcription [5]. The molecular mechanisms are involved multiple biological process including cancer development, immune response or cell metabolism [6,7]. Mutations in LRP6 relate to early onset hypercholesterolemia, atherosclerosis, and osteoporosis in human [8,9]. We also observed LRP6 is a critical protein in intercalated disc (ID) and regulates the components of IDs [10]. In Li J’s work that LRP6 acts as a scaffold protein in cardiac gap junction assembly [11]. However, whether cardiomycyte-expressed-LRP6 exerts the protective role against myocardial ischemia-reperfusion injury needs to be explored.
This study aimed to investigate the roles of cardiomyocyte-expressed- LRP6 under I/R injury and explore the potential mechanisms by building I/R model with tamoxifen inducible cardiomyocytes specific LRP6 overexpression mice. It will indicate a novel role of LRP6 in ischemic myocardium, and regulating LRP6 may become a new therapy target of myocardial I/R.
2. Materials and methods
2.1. Tamoxifen inducible cardiac specific LRP6 overexpression mice
The transgenic construct CAG-CAT consists of CAG promoter followed by a lox P-flanked cassette of the chloramphenicol acetyltransferase (CAT) resistance fusion gene and a triple poly A transcription termination signal (STOP) [12]. The full-length human LRP6 coding sequence (from LRP6-pCS2-VSVG; Addgene 27282#) was inserted into the downstream of CAG-CAT to construct CAG-CAT-LRP6. After purified and linearized, the construct was introduced by pronuclear microinjection of fertilized blastocysts of FVBN mice to generate LRP6CAG mice. The presence of “STOP” signal blocks LRP6 expression in LRP6CAG mice. The mice were bred with α-myosin heavy chain (α-MHC) Mer-Cre-Mer Tg mice (α MHC-MCM, termed MCM) crossed to generated LRP6CAG/MCM mice. After tamoxifen injection, the Cre enzyme was activated to cleave “STOP” sequence, so the CAG promoter will drive high level of LRP6 expression in cardiomyocytes. In the present study, 6–8 weeks old male LRP6-CTG mice were injected with tamoxifen (30 mg/kg i. p.) for 3 consecutive days to mediate cardiac LRP6 overexpression for further analysis. To avoid the transient influence of tamoxifen, we performed ischemic-reperfusion at 2 weeks after tamoxifen injection.
2.2. Echocardiography analysis
Echocardiography was performed on the day before and second day after I/R surgery. Mice were anaesthetized by using the inhalation of isoflurane and M-mode images were acquired with a RMV 2100 scan head on the Vevo 2100 (VisualSonics Inc., Toronto, Canada). Ejection fraction (EF) and fraction shortening (FS) were assessed. Heart rate (HR) was maintained at more than 400bpm. The parasternal long-axis views of heart were obtained by placing the transducer in a vertical fashion and transducer pointing towards the head of the animal was used as the marker. Radial strain analysis and longitudinal strain analysis were obtained by using a speckle-tracking algorithm with VevoStrain analysis Software. All the B-mode images were of good echogenicity without any image artifacts. The endocardial borders traced using the semi-automated tracing mode were obtained from the VevoStrain imaging software for at least three consecutive cycles. The left ventricle was divided into 6 standard anatomic segments. The anterior apex section was the infarcted area and the post base and post mid were the non-infarcted area. Left ventricle dys-synchrony was measured by the maximum opposite wall delay [13].
2.3. Western blot analysis
RIPA buffer was used to lyse mouse heart tissues or cells to acquire total protein. Nuclear and cytoplasmic protein were obtained by using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime China). The protein concentration was measured by BCA protein assay (Thermo Scientific). Equal amounts of samples were separated by SDS-PAGE gel according to the molecular weight of target proteins and then transferred to PVDF membranes (Millipore, USA), the membranes were incubated in 5% BSA blocking buffer for 60 min and then incubated with specific primary antibodies (Table S1). The immunoreactive bands were detected by digital chemiluminescence system (Biorad USA). Related signals were quantified using the Image J software.
2.4. Build cardiac ischemia/reperfusion model
Adult male mice 8–10 weeks of age were subjected to cardiac I/R surgery as followed description. Briefly, mice were anesthetized with inhalation of isoflurane. Next, an oblique incision was made from the left sternal border to visualize the third and fourth intercostal space. Mosquito forceps was used to open the intercostal space and then push the heart out. With the left anterior descending (LAD) coronary artery exposed, a ligature was placed 2 mm below the tip of the left atrial appendage using a 6-0 silk suture. Electrocardiogram showed that ST segment elevated, which meant ischemia model was successfully built. After 40 min of coronary occlusion, the silk suture was removed and the ligation was released to allow for tissue reperfusion. The animals were then analyzed at specific time-points. Tissues were frozen in liquid nitrogen and stored at −80 °C for further analysis.
2.5. Evan’s blue and triphenyltetrazolium chloride (TTC) staining
We investigate the infarct area (IA) by calculating the percentage of myocardium that underwent infarction compared to the area at risk (AAR) using the double staining technique with Evan’s blue and triphenyltetrazolium chloride (TTC). We occluded LAD with 6-0 silk suture again, followed by injection of Evan’s blue until the dye was visible in the cardiac veins. The heart was then excised and sliced into six slices. The slices were incubated in a TTC at 37 °C for 20 min and then transferred into formalin for fixation overnight. A digital camera was used to take pictures. AAR and IA were quantified by Image J software (National Institutes of Health, Bethesda, MD, USA). The myocardial infarct size was showed as a percentage of area at risk. The area at risk was showed as a percentage of the left ventricular.
2.6. Flow cytometry
To quantify cell death and apoptosis, cells were harvested by digesting with 0.25% trypsin without EDTA after washing in cold PBS. Proteolysis was then neutralized with fetal bovine serum, and the lysates were concentrated and resuspended in 100 μL PBS. Annexin V-FITC and PI staining solution (Beyotime China) were added. After incubated in the solution for 10–20 min at room temperature away from light, the cell suspension was analyzed for apoptosis by flow cytometry.
2.7. Culture of cardiac myocytes
Human cardiomyocytes cell line (AC16 cells) were kindly provided from the laboratory of Dr. Mercy Davidson [14]. Neonatal rat ventricular myocytes (NRVM) were isolated from 1 to 3 day-old Sprague-Dawley rat hearts. After decapitation, hearts were isolated and atria were removed. Tissue was minced to small pieced and incubated with 0.25% in Trypsin solution at 37 °C. Cells were filtered over a 100 μm cell strainer and centrifuged for 5’ at 100×g. The cell pellet was resuspended in plating medium (DMEM/F12 containing 10% FBS and 1% antibiotics) and plated for 1.5 h in a culture flask to let fibroblastes attach. Non-attached cardiomyocytes were collected, diluted, and plated at a density of 1 × 106 cells per well in 6-well plates.
NRVM and AC16 cells were cultured with DMEM/F12 containing 10% FBS and 1% antibiotics for 1–2days, then the culture media was added with 200 μM H2O2 for 3 or 24 h to mimic oxidative stress. H2O2 was diluted in PBS, and PBS treatment was as control.
2.8. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Cardiomyocytes apoptosis was measured by TUNEL assay (Beyotime China) according to manufacturer’s protocols. The samples was fixed by 4% paraformaldehyde for 30min and then treated by 0.3%Triton for 5min followed by incubating with Goat Serum for 1 h at room temperature. And then, all these samples were incubated with anti-cardiac troponin I (Abcam Cambridge, ab47003) at 4 °C overnight. Next day, they were incubated with TUNEL reaction liquid and goat anti-rabbit IgG (H + L) secondary antibody, Alexa Fluor 488(Invitrogen U.S.A.) for 1 h at 37 °C. After washed with PBS for 3 times, fluorescence images were obtained and analyzed using a confocal microscope (Zeiss, Germany). The cells with TUNEL-positive nucleus were regarded as apoptotic cells, and DAPI labelled all the nuclei of cells. The apoptotic cells ratio was calculated as the number of apoptotic cells vs. total cells.
2.9. Adenovirus construction and transduction
To engineer adenovirus overexpressing LRP6, the cDNA was cloned into pHBAd-Shuttle to constructed Ad-LRP6 plasmids. HEK-293A cells were cultured and transfected with Ad-LRP6 plasmids using the conventional CaCl2 transfection method. After 2–3days incubation, cells and cultured medium were harvested to be thawed and frozen. These cells and medium were added to HEK-293A cells again, which was repeated several times. The mild virus existed in cultured medium was purified by CsCl-gradient sedimentations. The purified viruses were stored at −80 °C. GSK3β(S9)-CA adenovirus was constructed using the same method. Cardiomyocytes were exposed to adenovirus for 6 h (MOI 3–10), followed by restoration of standard cultured medium. Protein overexpression can be detected after 48–72 h transfection.
2.10. Real-time PCR analysis
Total RNA was extracted from AC16 cell lines using TRIzol (Sangon Biotech China), and 300–500 ng of total RNA was reversed to cDNA using PrimeScriptTM II 1st Strand cDNA Synthesis Kit (TaKaRa Japan). qRT-PCR was performed by Takara SYBR Premix Ex TaqTM (TaKaRa Japan) on CFX96 Touch (Bio-Rad U.S.A.). The fold change of relative mRNA expression was calculated using the 2-ΔΔCt method with GAPDH serving as internal control. Sequences for qRT-PCR primers are shown as follows: human GAPDH forward:5′-GGAGCGAGATCCCTCCAAAAT-3′, reverse:5′-GGCTGTTGTCATA.
CTTCTCATGG-3’; human HSF1 forward 5′ GCCAAGGAGGTGCTGCCCAAG TAC-3′, reverse 5′-GGCCGCCCTGCTCGATGTG-3’.
2.11. SiRNA transfection
The siRNAs targetted to human HSF1 were designed and synthesized by GenePharma (Shanghai, China). HSF1 siRNA was transfected into cells using siPORTTM NeoFXTM Transfection Agent (Ambion Inc, Texas, U.S.A.). 100 nM HSF1 siRNA diluted in 100 μl opti-DMEM and 5 μl transfection agent diluted in 100 μl opti-DMEM were mixed and incubated at room temperature for 10 min. Next, the mixture were added into cultured cardiomyocytes.
2.12. Immunoprecipitation and immunoblot analysis
Total protein (500–1000 μg) from whole-cell lysate was immunoprecipitated. The extract incubated for 12 h at 4 °C with HSF1 antibody and GSK3β antibody respectively (Cell Signaling Technology U.S.A.) followed by addition of protein G beads and incubated further for 6 h at 4 °C. The beads were centrifuged at 2500 rpm for 5 min and washed with PBS for 3 times. The beads were re-suspended with protein loading buffer, and then incubated at 95 °C for 5 min. The supernatant was collected to analyzed by sodium dodecyl sulfate polyacrylamide gel immunoblotting. The immunoreactive bands were incubated with anti-ubiquitin antibody, phospho-GSK-3β (Ser9) antibody, GSK-3β antibody and HSF1antibody respectively for 10 h at 4 °C and detected by digital chemiluminescence system (Biorad USA).
2.13. Statistical analysis
Date are expressed as mean ± standard error (SEM). The comparison between the means of two groups was analyzed by unpaired Student’s t tests. The comparison among the multiple groups was analyzed by one-way ANOVA with Tukey post-test or two-way ANOVA with a Bonferroni post hoc test. Cartogram and statistical analyses were carried out by using Prism 6 (GraphPad). P value < 0.05 was considered statistically significant.
3. Results
3.1. Phosphorylation of LRP6 increases following myocardial I/R at early phase
To determine whether LRP6 is stimulated in I/R-stressed cardiomyocytes, C57 BL/6 mice were subjected to 40 min ischemia by ligating the left anterior descending coronary artery (LAD), followed by reperfusion of different time points (15min, 30min, 1 h), cardiac p-LRP6 and LRP6 were monitored via western blotting. Compared with sham-operated mice, p-LRP6 expression began to increase as early as 15 min after reperfusion, matained high levels at 30min and then recovered at 1 h following reperfusion. LRP6 expression showed no increase in left ventricle at any time-points after reperfusion (Fig. 1A and B). GSK3β, a crucial protein in Wnt signaling pathway, inhibiting β-catenin phosphorylation, is known to be the key event in Wnt/β-catenin signaling [15], p-GSK3β(S9) was increased from 15min to 1 h while p-GSK3α was transiently increased at 15min and 30min in heart tissue following I/R (Fig. 1A and D). Similar results were observed in the levels of p-AKT (T308 or S473) (Fig. 1A and C), which are involved in the process of myocardial I/R injury [[16], [17], [18]]. These results suggest that the activation of LRP6 at early period of reperfusion may protect heart against myocardial ischemia-reperfusion injury.
Fig. 1.
LRP6 is involved in myocardial ischemia reperfusion. Western blot analysis of LRP6, p-LRP6, p-AKT(T308), p-AKT(S473), p-GSK3α and p-GSK3β(S9) expression in ischemia area of heart tissue from mice subjected to I/R surgery at different reperfusion time points (15min, 30min, 1 h) after ischemia for 45min. A. Representative images. B. Quantitative analysis of p-LRP6 and LRP6 level. C. Quantitative analysis of p-AKT(T308) and p-AKT(S473) level. D. Quantitative analysis of p-GSK3α and p-GSK3β(S9) level. Values are the means ± S.E.M. N = 3/group. *p < 0.05, **p < 0.005.
3.2. Inducible cardiac specific LRP6 overexpression protects heart against ischemia reperfusion injury
To explore the potential role of LRP6 following I/R injury, we constructed tamoxifen-inducible cardiac specific LRP6 overexpression mice (LRP6-CTG mice) to build I/R model (see method section). Experiment protocol was showed in Fig. 2A. 6–8 weeks male LRP6CAG/MCM were intraperitoneally injected with tamoxifen (30 mg/kg) for 3 continuous days to induce cardiac LRP6 overexpression (LRP6-CTG), MCM mice treated with tamoxifen (marked MCM) were used as control group, 2 weeks later, these mice were subjected to I/R surgery or sham operation. Cardiac LRP6 overexpression was evidenced by western blot analysis (Fig. 2B, Fig.S1). Transthoracic echocardiography was employed to assess cardiac function after 24 h reperfusion following myocardial ischemia for 40min. When compared with sham operation group, ejection fraction (EF) and fractional shortening (FS), indices of systolic fraction, were decreased in MCM mice following I/R. The effects were significantly improved in LRP6-CTG mice subjected to I/R (Fig. 2C). We also performed strain and strain-rate analysis. Radial strain analysis showed LRP6 overexpression induced a significant increase globally and in infarcted areas as compared to MCM mice after I/R. Maximum opposite-wall delay showed a significant decrease in time for LRP6-CTG mice as compared to MCM mice (Fig. 2D). Longitudinal strain analysis revealed the similar results as in the radial strain analysis (Fig. S2).
Fig. 2.
Cardiac specific LRP6 overexpression protects heart from I/R injury. A. The overall experimental protocol of in vivo to explore the effects of cardiac LRP6 overexpression in mice subjected to I/R. 6–8 weeks age male LRP6-CTG or MCM mice were intraperitoneally injected with tamoxifen (30 mg/kg) for 3 continuous days respectively, 2 weeks later, these mice (LRP6-CTG mice and MCM mice) were subjected to I/R surgery or sham operation. The MCM mice were used as control group. B. The expression of LRP6 and p-LRP6 in MCM mice and LRP6-CTG mice were analyzed by western blot. N = 3/group. C. Echocardiographic analysis at 24 h after I/R surgery or sham operation in MCM and LRP6-CTG mice. Enjection fraction (EF) and fraction shortening (FS) were quantified at 24 h after I/R injury. N = 4–8/group. D. Radial strain analysis in MCM mice and LRP6-CTG mice at 24 h after I/R were obatined from VevoStrain analysis software. Upper lane: Representative images of radial strain curves. Colored lines represent 6 standard myocardial regions; 7th black line calculates average (global) strain at each time point. Down lane: Quantitative analysis of radial strain both globally and in infarct area (anterior apex), and maximal opposite wall delay in time. N = 6/group. E. Representative TTC staining of the heart sections from Evans blue perfused hearts was shown for each group. The bar = 500 μm. The ratio of infarct area (IA) to area at risk (AAR), and area at risk/left ventricle (AAR/LV) were quantified at 24 h after I/R injury, as detailed in the Methods. N = 12–15/group. Values are means ± S.E.M. *p < 0.05, **p < 0.005,***p < 0,001 and ****p < 0.0001 vs. MCM group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Myocardial infarction area was measured by TTC staining. As shown in Fig. 2E, I/R 24 h induced significant myocardial injury as indicated by the infarcted area of mice. Cardiac overexpression of LRP6 greatly decreased the ratio of infarct area/area at risk (IA/AAR) by about 17% compared with control group (27.2% ± 1.98% vs 41.69% ± 2.85%, P < 0.05; Fig. 2E). There was no significant difference in the ratio of area at risk/left ventricle (AAR/LV) (Fig. 2E). These data indicate that cardiac LRP6 overexpression protects heart against I/R injury.
3.3. Cardiac specific LRP6 overexpression inhibits cardiomyocytes apoptosis following myocardial I/R
Given the pivotal role of apoptosis in pathogenesis of myocardial I/R injury, we detected apoptosis in frozen heart tissue by TUNEL staining. About 60% TUNEL positive cardiomyocytes was obviously observed in ischemic area of MCM mice, and the effect was attenuated in LRP6- CTG mice after I/R (Fig. 3A). Further analysis revealed I/R induced the activation of caspase 3 and c-Jun N terminal kinases (JNK), and the effects were significantly suppressed by LRP6 overexpression (Fig. 3B). The disruption of endoplasmic reticulum (ER) protein homeostasis, known as ER stress, has been involving myocardial I/R injury including apoptosis [19]. Compared with sham mice, I/R injury increased the expression of caspase12, the marker of ER stress, while LRP6 overexpression attenuated the effect (Fig. 3C).
Fig. 3.
Cardiac specific LRP6 overexpression inhibits cardiomyocytes apoptosis following I/R. A. Terminal deoxynucleotidyl transferase-mediated dUTP (deoxyuridine triphosphate)-biotin nick-end labeling (TUNEL) in heart tissue from MCM and LRP6-CTG mice at 24 h reperfusion following 45min ischemia. Scale bar, 50 μm. The percentage of TUNEL positive cells was calculated. N = 4/group. B–C. Western blot analysis of apoptosis-related proteins (cleaved-caspase3; p-JNK; JNK) expression and an ER stress marker protein (caspase12) in the ischemia zone of hearts from MCM and LRP6-CTG mice at 24 h reperfusion after 45min ischemia. N = 3/group. In vitro, neonatal rat cardiomyocytes were cultured and transfected Ad-LRP6 (LRP6 OVER) or control adenovirus (CON), 72 h later, 200 μM H2O2 was treated to induce oxidative stress, and PBS treatment was as control. D. TUNEL staining analysis of control or LRP6 overexpressed-cardiomyocytes treated with H2O2 for 24 h, scale bar, 200 μm. The percentage of TUNEL positive cells (apoptosis) was calculated. N = 11/group. E. Western blot analysis for apoptosis-related proteins (cleaved-caspase3; p-JNK; JNK) expression and ER stress marker protein (caspase12) expression in control or LRP6 overexpressed-cardiomyocytes treated with H2O2 or PBS for 24 h. N = 3/group. Values are means ± S.E.M. *p < 0.05, **p < 0.005; ***p < 0.001. and ****p < 0.0001.
We subsequently cultured neonatal rat ventricular myocytes and treated with 200 μm H2O2 for 24 h to mimic oxidative stress in vitro. Adenoviral LRP6 was transfected into cultured cardiomyocytes to induce LRP6 overexpression. H2O2 treatment induced about 40% TUNEL positive cardiomyocytes, and the ratio was decreased to ~25% in cardiomyocytes transfected with LRP6 adenovirus (Fig. 3D). Similarly, LRP6 overexpression greatly inhibited the activation of caspase3 and JNK, and attenuated the elevated expression of caspase12 (an ER stress marker) in H2O2-treated-cardiomyoctes (Fig. 3E).
Overall, in vivo and in vitro, LRP6 overexpression can protect cardiomyocyte against apoptosis and ER stress induced by I/R injury.
3.4. Cardiac specific LRP6 overexpression promotes nucleus accumulation of HSF1 and phosphorylation of GSK3β and AMPK following myocardial I/R
We examined the activation of p-Drp1(ser616), which participates in myocardial I/R injury [20], and mediates cardiac dysfunction in LRP6 knockout hearts in our recent study [3]. I/R induced the increased p-Drp1(ser616) level, but LRP6 overexpression didn’t affect the activation of Drp1 in I/R hearts (Fig. 4A). GSK3β mediates LRP6 phosphorylation, leading to stabilization of β-catenin and its translocation into nucleus which initiates T cell factor(TCF)/lymphoid enhancer factor (LEF) related genes expression [21], and the phosphorylation of GSK3β(S9) has been reported to protect myocardial I/R injury [18]. At 3 h after I/R, p-GSK3β(S9) level was recovered to baseline, while active-β-catenin was decreased in the ischemic area of MCM hearts. LRP6 overexpression promoted the phosphorylation of GSK3β(S9) but didn’t affect the activation of β-catenin in ischemic hearts (Fig. 4A and B). IWP-2, the inhibitor of Wnts secretion, greatly inhibited apoptosis in control and LRP6-overexpressed-cardiomyocytes treated with H2O2 (Fig S3). The data suggest oxidative stress may promotes secretion of Wnts to induce cardiomyocytes apoptosis, while β-catenin activation isn’t involved in cardiac protection induced by LRP6 overexpression.
Fig. 4.
Cardiac specific LRP6overexpression promotes HSF1 nucleus translocation and phosphorylation of GSK3β and AMPK after I/R. A. Western blot analysis of LRP6, p-LRP6, p-AKT(T308), p-AKT(S473), AKT, p-GSK3β(S9), GSK3β, p-ERK, ERK in the ischemia zone of hearts from MCM and LRP6-CTG mice at 3 h reperfusion following 45min ischemia. N = 3/group. B–C. Western blot analysis of the active β-catenin and p-AMPK level in the ischemia zone of hearts from MCM and LRP6-CTG mice at 3 h reperfusion following 45min ischemia. N = 3/group. In vitro, human cardiomyocytes (AC16 cell line) were cultured and transfected LRP6 adenovirus (Ad-LRP6) or control adenovirus (Ad-CON), 72 h later, 200 μM H2O2 was treated to induce oxidative stress, and PBS treatment was as control. D. Western blot for p-AMPK, AMPK, p-GSK3β and GSK3β in control or LRP6 overexpressed-cardiomyocytes treated with 200 μM H2O2 or PBS for 3 h. N = 3/group. E. Western blot analysis of HSF1 in nucleus and cytoplasm lysates from the ischemia zone of hearts of MCM and LRP6-CTG mice at 3 h reperfusion following 45min ischemia. N = 3/group. F. Western blot analysis of HSF1 in nucleus and cytoplasm lysates from control or LRP6 overexpressed-cardiomyocytes treated with 200 μM H2O2 or PBS for 3 h. N = 3/group. Values are means ± S.E.M. *p < 0.05, **p < 0.005,***p < 0.001 and ****p < 0.0001.
P-AKT and p-ERK are involved in the phosphorylation of GSK3β (S9) in myocardial I/R injury [17,18]. In the present study, I/R induced the increase in p-AKT (S473 and T303) and p-ERK in MCM hearts, but the effects weren’t affected by LRP6 overexpression. AMPK and GSK3β has reciprocal regulation under oxidative condition [22,23]. P-AMPK level showed the similar expression profile to the p-GSK3β(S9) in MCM or LRP6 overexpression mice subjected to I/R or sham operation (Fig. 4C). In vitro, H2O2 treatment induced the increased p-GSK3β (S9) and p-AMPK level in cultured cardiomyocytes, and the effects were promoted by LRP6 overexpression in cardiomyocytes (Fig. 4D). These data suggest that LRP6 overexpression enhances the phosphorylation of GSK3β (S9) and AMPK to protect heart aganist I/R injury.
Heat shock transcription factor 1 (HSF1) protects cardiomyocytes from death following myocardial I/R [24]. We sought to whether LRP6 regulates HSF1 to activate GSK3β or AMPK to protect heart from I/R injury. At 3 h after I/R, the expression HSF1 was increased in nucleus but decreased in cytoplasm in MCM heart tissues, and LRP6 overexpression promoted the HSF1 expression in nucleus and cytoplasm in I/R hearts (Fig. 4E). In vitro in cultured cardiomyocytes, H2O2 treatment induced the increased HSF1 expression in nucleus or cytoplasm and the effects were promoted by LRP6 overexpression in cardiomyocytes (Fig. 4F). HSF1 nuclear accumulation activates transcription of heat shock proteins (HSP) genes which are involved in cardiac protection under stress conditions [25]. The present data showed that the expression of HSP70 and HSP27 was higher in LRP6 overexpression hearts than those in control group after myocardial I/R (Fig. S4).
These data revealed cardiac LRP6 overexpression promoted HSF1 nucleus accumulation, phosphorylation of AMPK and GSK3β, which may participate in the cardiac protection of LRP6 against I/R injury.
3.5. Active GSK3β overexpression restrains the increased level in nuclear HSF1 and p-AMPK, and the inhibition of cardiomyocytes apoptosis mediated by LRP6 overexpression in cardiomyocytes under oxidative stress
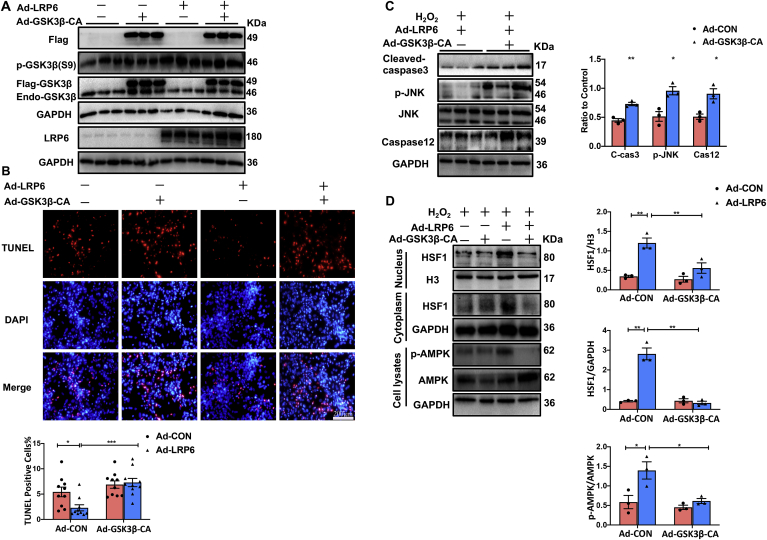
GSK3β, a Ser/Thr protein kinase, is inactivated by phosphorylation, which promotes cell survival under stress conditions [26]. We transfected adenovirus GSK3β(S9)-CA (a active form of GSK3β, tagged with flag, 49 KDa) into cardiomyocytes with LRP6 overexpression or not to observe the expression of HSF1 and p-AMPK, and apoptosis under oxidative stress. The proteins were extracted from nucleus or cell lysates of cardiomyocytes treated with 200 μm H2O2 for 3 h. Adenovirus GSK3β (S9)-CA transfection induced the effective overexpression of active GSK3β(49 KDa) but didn’t affect the endogenous p-GSK3β(S9) level (46 KDa) (Fig. 5A). We then examined apoptosis by TUNEL Staining. Samely, TUNEL positive cardiomyocytes was significant decreased in LRP6 overexpressed-cardiomyocytes compared with control group after H2O2 treatment, and the effect was attenuated by GSK3β(S9)-CA overexpression (Fig. 5B). GSK3β(S9)-CA overexpression also attenuated the decreased expression of p-JNK level and cleaved-caspase3 induced by LRP6 overexpression in H2O2 treated cardiomyocytes (Fig. 5C). The expressions of caspase12, an ER stress marker, was increased by GSK3β(S9)-CA overexoression in LRP6 overexpressed-cardiomyocytes treated with H2O2 (Fig. 5C). As expected, LRP6 overexpression induced the increase in HSF1 nuclear accumulation and p-AMPK level in H2O2-treated-cardiomyocytes, and the effects were reversed by overexpression of GSK3β(S9)-CA (Fig. 5D).
Fig. 5.
Active GSK3β overexpression restrains the increased level of HSF1 and p-AMPK, and the inhibition of cardiomyocytes apoptosis mediated by LRP6 overexpression in cardiomyocytes under oxidative stress. In vitro, neonatal rat cardiomyocytes were cultured and transfected LRP6 adenovirus (Ad-LRP6) and/or GSK3β-CA adenovirus (Ad-GSK3β-CA tagged with flag) or control adenovirus (Ad-CON), 72 h later, 200 μM H2O2 was treated to induce oxidative stress. A. Western blot analysis for the expression of flag, p-GSK3β(S9), GSK3β and LRP6 in cultured cardiomyocytes (grouped as above) treated with H2O2 for 24 h. N = 3/group. B. TUNEL staining of cultured cardiomyocytes (grouped as above) treated with H2O2 for 24 h. scale bar, 200 μm. The percentage of TUNEL positive cells was calculated. N = 10/group. C. Western blot analysis for p-JNK, JNK, cleaved-caspase3, and caspase12 in cultured LRP6 overexpressed-cardiomyocytes transfected with GSK3β-CA adenovirus (Ad-GSK3β-CA) or control adenovirus (Ad-CON), and these cardiomyocytes were treated with 200 μM H2O2 for 24 h. N = 3/group. D. Western blot for HSF1 in nucleus and cytoplasm, p-AMPK and AMPK in whole cell lysates from GSK3β-CA adenovirus (Ad-GSK3β-CA) and/or LRP6 adenovirus (Ad-LRP6) transfected cardiomycoytes treated with 200 μM H2O2 for 3 h. N = 3/group. Values are means ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
These data indicated that LRP6 overexpression inhibited the activity of the GSK3β to suppress apoptosis by regulation of HSF1 and AMPK signaling in cardiomyocytes under oxidative stress.
3.6. Si-HSF1 blunts the increase in p-GSK3β(S9) and p-AMPK level and attenuates the inhibition of apoptosis induced by LRP6 overexpression in cardiomyocytes under oxidative stress
To further investigate the effects of HSF1 on the apoptosis and phosphorylation of AMPK or GSK3β, we transfected si-HSF1 into cultured cardiomyocytes to knockdown HSF1 expression and observe the phosphorylation of GSK3β and AMPK in LRP6 overexpressed- or control cardiomyocytes under oxidative stress. Si-HSF1 transfection effectively decreased HSF1 expression in cultured cardiomyocytes (Fig. 6A). Cardiomyocyte apoptosis was determined by flow cytometry analysis for annexin V- and propodium iodide(PI)-staining. Annexin V+PI− and Annexin V+PI+ are considered early apoptosis and late apoptosis respectively. In H2O2-treated-cardiomyocytes, we observed that a decreased early apoptosis level in LRP6 overexpression group comparing to control group, the effect was reversed by knockdown of HSF1 (Fig. 6B). Oxidative stress induced-elevation of p-AMPK and p-GSK3β (S9) were conferred by LRP6 overexpression, the effects was abolished in si-HSF1-cardiomyocytes treated with 200 μm H2O2 for 3 h (Fig. 6C).
Fig. 6.
Si-HSF1 blunts the increase in p-GSK3β(S9) and p-AMPK level and attenuates the inhibition of apoptosis induced by LRP6 overexpression in cardiomyocytes during oxidative stress. A. Western blot analysis for HSF1 expression level in cultured cardiomyocytes with transfection of si-Scramble (si-Scram) or si-HSF1. N = 3/group. B. The apoptosis was assessed by flow cytometry analysis for annexin-V and propidium iodide (PI) staining in cultured control or LRP6 overexpressed-cardiomyocytes treated with 200 μM H2O2 for 24 h, and these cardiomyocytes were pre-transfected into si-scramble (si-Scram) or si-HSF1. N = 3–6/group. C. Western blot analysis for p-AMPK, AMPK, p-GSK3β (S9) and GSK3β in cultured control or LRP6 overexpressed-cardiomyocytes treated with 200 μM H2O2 for 24 h, and these cardiomyocytes were pre-transfected into si-Scramble or si-HSF1. N = 3/group. A-C, Human cardiomyocytes (AC16 cells) were transfected control adenovirus (Ad-CON) or LRP6 adenovirus (Ad-LRP6) as control or LRP6 overexpressed-cardiomyocytes respectively. D. Western blot analysis for HSP70, p-GSK3β (S9) and GSK3β in cultured control or LRP6 overexpressed-cardiomyocytes (NRCMs) treated with 200 μM H2O2 for 3 h, and these cardiomyocytes were pre-treated with DMSO or KRIBB11(20 μM) for 48 h. N = 3/group. E. TUNEL staining of cultured cardiomyocytes (grouped as above) treated with H2O2 for 24 h. scale bar, 200 μm. The percentage of TUNEL positive cells was calculated. N = 4/group.Values are means ± S.E.M. *p < 0.05, **p < 0.005,***p < 0.001 and ****p < 0.0001.
To explore the relationship between HSF1-induced-HSPs expression and LRP6 overexpression-induced-cardiac protection, we used KRIBB11, an inhibitor of HSF1, which can effectively inhibit the expression of HSPs [27], to detect apoptosis and p-GSK3β level in H2O2-treated-cardiomyocytes with LRP6 overexpression or not. The data showed that the inhibitor greatly suppressed the HSP70 expression in control or H2O2-treated-cardiomyocytes (Fig. 6D). KRIBB11 deteriorated the apoptosis and inhibited p-GSK3β level in LRP6 overexpressed-cardiomyocytes treated with H2O2 (Fig. 6D and E). These data suggest that HSF1-induced-HSPs expression may be involved in the regulation of GSK3β activity and cardiac protection in LRP6 overexpressed-cardiomyocytes.
These data indicated that LRP6 overexpression increased HSF1 level to protect cardiomyocytes against oxidative stress by regulation of AMPK and GSK3β signaling.
3.7. AMPK inhibitor suppresses the increase in p-GSK3β(S9) level but not HSF1 in nucleus induced by LRP6 overexpression in cardiomyocytes during oxidative stress
To determine whether the activation of AMPK is involved in LRP6-mediated HSF1 nucleus accumulation and the increased p-GSK3β (S9) level under oxidative stress, we provided compound C, an inhibitor of AMPK into LRP6 overexpressed- or control cardiomyocyte treated with H2O2. As expected, AMPK phosphorylation was inhibited by compound C treatment in LRP6 overexpressed- or control cardiomyocytes under oxidative stress (Fig. 7A). Likewise, elevation of p-GSK3β (S9) mediated by LRP6 overexpression also was attenuated by AMPK inhibition in H2O2 treated cardiomyocytes. But the inhibitor didn’t affect the increased HSF1 expression in nucleus and cytoplasm induced by LRP6 overexpression in cardiomyocytes under oxidative stress (Fig. 7B).
Fig. 7.
AMPK inhibitor suppresses the increased level in p-GSK3β(S9) but not HSF1 induced by LRP6 overexpression in cardiomyocytes during oxidative stress. Human cardiomyocytes (AC16 cells) were transfected control adenovirus (Ad-CON) or LRP6 adenovirus (Ad-LRP6) as control or LRP6 overexpressed-cardiomyocytes respectively. A. Western blot analysis for LRP6, p-AMPK, AMPK, p-GSK3β (S9), GSK3β in cultured control or LRP6 overexpressed-cardiomyocytes treated with 200 μM H2O2 for 3 h, and these cardiomyocytes were pre-treated with PBS or Compound C (20 μM) for 24 h. N = 3/Group. B. Western blot analysis of HSF1 in nucleus and cytoplasm in cultured control or LRP6 overexpressed-cardiomyocytes treated with 200 μM H2O2 for 3 h, and these cardiomyocytes were pre-treated with PBS or Compound C (20 μM) for 24 h. N = 3/group. Values are means ± S.E.M. *p < 0.05, **p < 0.005, ***p < 0.001.
These data suggest LRP6 overexpression induced AMPK activation which promotes phosphorylation of GSK3β (S9) but didn’t affect HSF1 level in nucleus of cardiomyocytes under oxidative stress.
3.8. LRP6 overexpression inhibits the ubiquitination of HSF1 by regulation of GSK3β signaling in cardiomyocytes during oxidative stress
LRP6 overexpression didn’t affect the increased HSF1 mRNA level in cardiomyocytes induced by H2O2 treatment (Fig. 8A). We then explore whether LRP6 overexpression inhibits the ubiquitination to promote HSF1 nucleus stabilization in cardiomyocytes under oxidative stress. The ubiquitination of HSF1 was analyzed by the ubiquetination assay, and the proteasome inhibitor MG132 was used to block the function of the proteasome. As expected, LRP6 overexpression inhibited the increased ubiquitination of HSF1 by H2O2 treatment in cardiomyocytes (Fig. 8B). To exlore whether GSK3β or AMPK signaling is involved in the process, we transfected adenovirus GSK3β-CA or added Compound C, an AMPK inhibitor, into cardiomyocytes in response to oxidative stress. GSK3β-CA overexpression greatly attenuated the inhibition of ubiquitination of HSF1 mediated by LRP6 overexpression in the H2O2-treated cardiomyocytes (Fig. 8C), but Compound C didn’t affect the effect (Fig. 8D).
Fig. 8.
LRP6 overexpression inhibits the ubiquitination of HSF1 by regulation of GSK3β signaling in cardiomyocytes under oxidative stress. A. Real-time PCR analysis of HSF1 mRNA level in cultured cardiomyocytes treated with 200 μM H2O2 for 3 h. N = 3/Group. Values are means ± S.E.M. ***p < 0.001. B. Analysis of the ubiquitination of HSF1 in cardiomyocytes. Human cardiomyocytes (AC16 cells) were pre-transfected with control adenovirus (Ad-CON) or LRP6 adenovirus (Ad-LRP6), and then treated with 200 μM H2O2 or PBS for 3 h. C. The ubiquitination of HSF1 was analyzed in neonatal rat cardiomyocytes. These cardiomyocytes were pre-transfected with GSK3β-CA adnovirus (GSK3β-CA) and/or control adenovirus (Ad-CON) or LRP6 adenovirus (Ad-LRP6), and treated with 200 μM H2O2 for 3 h. D. The ubiquitination of HSF1 was analyzed in cardiomyocytes (AC16 cells). These cardiomyocytes were pre-transfected with control adenovirus (Ad-CON) or LRP6 adenovirus (Ad-LRP6). After incubation of Compund C (20 μM) or PBS for 24 h, these cardiomyocytes were treated with 200 μM H2O2 for 3 h. B–D: Whole-cell lysates (WCL) were immunoprecipitated using an anti-HSF1 antibody, and the ubiquitin levels were determined by western blotting using an anti-ubiquitin antibody. Ubi, ubiquitin. The experiments were repeated at least 3 times. E. The interaction between HSF1 and GSK3β was investigated by co-immunoprecipitation. HSF1 and GSK3β was precipitated from AC16 cell lysate with anti-HSF1 antibody and blotted with anti-GSK3β and p-GSK3β antibody, and vice versa. The experiments were repeated at least 3 times.
We further examined whether LRP6 induces the interaction of GSK3β and HSF1 to regulate GSK3β activity or HSF1 ubiquitination. IP analysis revealed that LRP6 overexpression increased the interaction of HSF1 and GSK3β or p-GSK3β in cardiomyocytes under oxidative stress (Fig. 8E).
These data indicated that LRP6 overexpression inhibited the ubiquitination of HSF1 to promote nucleus stabilization by regulation of GSK3β signaling in cardiomyocytes during oxidative stress, and the interaction of GSK3β and HSF1 may be involved in the effects.
4. Discussion
In this study, we demonstrated cardiomyocyte-expressed-LRP6 inhibited endoplasmic reticulum stress (ER stress) and cardiomyocyte apoptosis aganist myocardial I/R injury by the crosstalk of HSF1 and GSK3β, and AMPK activation is involved in the process. Cardiac LRP6 phosphorylation was increased at early phase in I/R mice. Cardiomyocyte LRP6 specific overexpression improved heart function, reduced cardiac infarction area, inhibited cardiomyocyte apoptosis and ER stress aganist I/R injury. SiHSF1 or active GSK3β attenuated the activation of AMPK, the inhibition of cardiomyocytes apoptosis and ER stress while AMPK inhibitor suppressed p-GSK3β (S9) but not HSF1 level in LRP6-overexpressed cardiomyocytes under oxidative stress (Fig. 9).
Fig. 9.
The summary of the study. The present study indicated that cardiomyocyte-expressed-LRP6 inhibited ER stress and apoptosis by the crosstalk of HSF1 and GSK3β pathway which protects heart against myocardial I/R injury, and AMPK may be involved the process.
We recently reported that cardiac specific LRP6 deletion induced lethal heart failure [3]. The present data revealed that p-LRP6 level transiently increased and then recovered after I/R injury. Cardiac specific LRP6 overexpression improved cardiac function and decreased infarcted area. Cardiac specific LRP6 deletion inhibited autophagic degradation and cardiac dysfunction [3]. Considering the cardiac dysfunction in baseline of LPR6 knockout mice, we didn’t use the knockout mice to build I/R model. In the present study, cardiac LRP6 overexpression showed few effects on autophagy (data not shown), while the overexpression inhibited cardiomyocyte apoptosis under I/R injury. Endoplasmic reticulum stress (ER stress) is one of critical factors for cell apoptosis during I/R [1,19,28,29]. We also observed LRP6 overexpression inhibited ER stress induced by I/R. It suggests that the overexpression of LRP6 protects heart by inhibition of ER stress and apoptosis.
LRP6 is the single-pass type I membrane protein. It functions as a co-receptor, together with members of the seven-pass transmembrane receptors of the Frizzled family, in the canonical Wnt/β-catenin signaling cascade [30]. However, LRP6 overexpression didn’t affect the inhibition of active β-catenin induced by I/R, suggesting LRP6 protects cardiomyocyte from apoptosis independently of Wnt canonical signaling. Under oxidative stress, LRP6 overexpression promoted the increased phosphorylation of GSK3β(S9) and AMPK, and HSF1 level in cardiomyocytes. Inhibition of GSK3β activity is involved in the protective effects during I/R injury [31]. The present study revealed LRP6 overexpression promoted the phosphorylation of GSK3β at S9, which inhibits the GSK3β activity. It is possible LRP6 overexpression increased the p-LRP6 level which negatively regulated GSK3β activity [32]. P-AKT and p-ERK are involved in the phosphorylation of GSK3β (S9) in myocardial I/R injury [17,18]. AMPK and GSK3β has reciprocal regulation under oxidative condition [22,23]. In the present study, LRP6 overexpression didn’t affect the activation of AKT and ERK, but promoted the phosphorylation of AMPK in I/R hearts. AMPK, a cellular metabolic stress sensor, plays a critical role in maintaining cellular energy homeostasis [33]. The activation of AMPK protects cardiomyocytes from apoptosis via multiple mechanism under myocardial I/R injury [[34], [35], [36]]. Our data indicated that AMPK may be involved in the regulation of GSK3β by LRP6 in cardiomyocytes under oxidative stress. LRP6 overexpression also increased nucleus localization of HSF1 and its activity evidenced by the increased HSPs expression under I/R injury. HSF1, is a transcriptional factor mediating the heat shock response. Under normal physiological conditions, mammalian HSF-1 is distributed in both the cytoplasm and nucleus in a latent, constitutively phosphorylated monomeric form [37]. Upon heat shock, ischemia or other stress, HSF1 translocates into nucleus, forms trimmers in hyperphosphorylated form, and then enhances its DNA binding and transcriptional activity, to upregulate the expression of targeted genes including HSPs, which is important to protect organisms and cells from injury including apoptosis [37]. By these mechanisms, HSF1 protects cardiomyocytes against myocardial I/R injury, and HSF1- induced-HSPs are involved the effects [24]. Thus, the increased HSF1 expression and the subsequent HSPs expression may contribute to the protective effects of LRP6 overexpression in cardiomyocytes under oxidative stress. LRP6 overexpression didn’t change the increased mRNA level of HSF1 but inhibited ubiquitylation of HSF1, suggesting LRP6 attenuates the degradation of HSF1 in cardiomyocytes under oxidative stress.
Active GSK3β overexpression, knockdown or inhibition of HSF1 attenuated the protective effects in LRP6 overexpressed cardiomyocytes under oxidative stress. Active GSK3β overexpression promoted the ubiquitylation and inhibited the increased HSF1 level, and knockdown or inhibition of HSF1 suppressed the increased p-GSK3β(S9) level induced by LRP6 overexpression in cardiomyocytes under oxidative stress. Several evidences revealed GSK3β and ERK negatively regulates DNA-binding and transcriptional activity of HSF1 in cell lines in response to heat [[38], [39], [40]]. Kourtis et al. reported ubiquitin ligase FBXW7α ubiquitylates HSF1 and inhibits its nuclear stabilization dependently of a conserved motif phosphorylated at Ser 307 and Ser 303 by GSK3β and ERK1 [41]. We speculate LRP6 overexpression inhibits the GSK3β activity to suppress the phosphorylation of HSF1 which results in the decreased ubiquitylation of HSF1 in cardiomyocytes under oxidative stress. We also found LRP6 induced the interaction of HSF1 and GSK3β or p-GSK3β, suggesting LRP6 promotes phosphorylation of GSK3β and subsequently mediates the interaction of GSK3β and HSF1, which results in the inhibition of HSF1 ubiquitylation under oxidative stress. Our data suggest that LRP6 overexpression promotes reciprocally regulation GSK3β and HSF1 in cardiomyocytes which inhibits apoptosis under I/R injury, and HSF1-induced-HSPs may be involved the effects. In addition, CaMKII-δB promotes phosphorylation of HSF1 at Ser230 to enhance nuclear translocation which contributes to cardiac protection under oxidative stress [42]. Whether LRP6 mediates phosphorylation of HSF1 at Ser230 to protect heart in response to stress needs further study.
By sensing the decreased adenosine monophosphate (AMP)/ATP level, AMPK was activated through Thr172 phosphorylation of the α‐subunit under multiple stress conditions, such as ischemic stress [33]. AMPK negatively regulates HSF1 activation via phosphorylation in tumor cells which inhibits the invasion and metastasis of pancreatic cancer [43,44]. In the present study, overexpression of active GSK3β and knockdown of HSF1 both attenuated the activation of AMPK in LRP6 overexpressed cardiomyocytes under oxidative stress. Inhibition of AMPK suppressed the increase in p-GSK3β level but showed few effects on HSF1 ubiquitylation and expression in LRP6 overexpressed-cardiomyocytes under oxidative stress. GSK3β-dependent-inhibition of AMPK potentiates inflammatory activation during LPS-induced-lung injury or liver I/R injury [23,45]. Our data suggest that AMPK activation is induced by the p-GSK3β (S9) through HSF1 activation in LRP6 overexpressed-cardiomyocyte under oxidative stress, and we postulated the regulation of AMPK on the activation of HSF is cell-specific. However, we couldn’t exclude other mechanisms that promote the activation and amplification of HSF1 in LRP6-overexpressed-cardiomyocytes under oxidative stress. In addition, HSF1 activity is promoted by acetylation through the regulation of SIRT1 during stress [46], whether LRP6 overexpression regulates the acetylation of HSF1 waits to be eliminated.
5. Conclusions
The present study indicated that cardiomyocyte-expressed-LRP6 inhibited ER stress and apoptosis by the crosstalk of HSF1 and GSK3β pathway which protects heart against myocardial I/R injury, and AMPK may be involved the process. Our finding is helpful to provide a novel therapeutic strategies for improve cardiac function under myocardial I/R injury.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81770239, 81730009 and 81521001).
Declaration of competing interest
We declare that there are no competing interests.
Acknowledgement
We thank Dr. Yunlong Huo (Shanghai Jiaotong University, China) for strain analysis support, and Dr. Mercy Davidson (Columbia University, New York, USA) for generously providing the AC16 cells.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101699.
Contributor Information
Yunzeng Zou, Email: zou.yunzeng@zs-hospital.sh.cn.
Hui Gong, Email: gonghui2005@fudan.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Abbate A., Narula J. Role of apoptosis in adverse ventricular remodeling. Heart Fail. Clin. 2012;8:79–86. doi: 10.1016/j.hfc.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z., Li Y., Wang Y., Qian J., Ma H., Wang X. Cardiomyocyte-restricted low density lipoprotein receptor-related protein 6 (LRP6) deletion leads to lethal dilated cardiomyopathy partly through Drp1 signaling. Theranostics. 2018;8:627–643. doi: 10.7150/thno.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Yin C., Chen Z., Li Y., Zou Y., Wang X. Cardiac-specific LRP6 knockout induces lipid accumulation through Drp1/CPT1b pathway in adult mice. Cell Tissue Res. 2020;380:143–153. doi: 10.1007/s00441-019-03126-3. [DOI] [PubMed] [Google Scholar]
- 5.Niehrs C., Shen J. Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. : CMLS. 2010;67:2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C.C., Prior J., Piwnica-Worms D., Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong W., Yang L., Li P.P., Kong Q.Q., Wang H.Y., Han G.X. MiR-381-3p inhibits proliferation, migration and invasion by targeting LRP6 in papillary thyroid carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3804–3811. doi: 10.26355/eurrev_201806_15264. [DOI] [PubMed] [Google Scholar]
- 8.Mani A., Radhakrishnan J., Wang H., Mani A., Mani M.A., Nelson-Williams C. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R., Smith E., Fathzadeh M., Liu W., Go G.W., Subrahmanyan L. Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Hum. Mutat. 2013;34:1221–1225. doi: 10.1002/humu.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Zou Y., Li Y., Chen Z., Yin C., Wang Y. Lipoprotein receptor-related protein 6 is required to maintain intercalated disk integrity. Gene Cell.: devoted to molecular & cellular mechanisms. 2019;24:789–800. doi: 10.1111/gtc.12727. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Li C., Liang D., Lv F., Yuan T., The E. LRP6 acts as a scaffold protein in cardiac gap junction assembly. Nat. Commun. 2016;7:11775. doi: 10.1038/ncomms11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schönig K., Weber T., Frömmig A., Wendler L., Pesold B., Djandji D. Conditional gene expression systems in the transgenic rat brain. BMC Biol. 2012;10:77. doi: 10.1186/1741-7007-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler M., Hohmann J.D., Searle A.K., Abraham M.K., Nandurkar H.H. A single-chain antibody-CD39 fusion protein targeting activated platelets protects from cardiac ischaemia/reperfusion injury. Eur. Heart J. 2018;39:111–116. doi: 10.1093/eurheartj/ehx218. [DOI] [PubMed] [Google Scholar]
- 14.Davidson M.M., Nesti C., Palenzuela L., Walker W.F., Hernandez E., Protas L. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2005;39:133–147. doi: 10.1016/j.yjmcc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Wu D., Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda Y., Shirakabe A., Maejima Y., Zhai P., Sciarretta S., Toli J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 17.Breivik L., Helgeland E., Aarnes E.K., Mrdalj J., Jonassen A.K. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res. Cardiol. 2011;106:135–145. doi: 10.1007/s00395-010-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min J., Wei C. Hydroxysafflor yellow A cardioprotection in ischemia-reperfusion (I/R) injury mainly via Akt/hexokinase II independent of ERK/GSK-3β pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;87:419–426. doi: 10.1016/j.biopha.2016.12.113. [DOI] [PubMed] [Google Scholar]
- 19.Murphy E., Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong S.B., Subrayan S., Lim S.Y., Yellon D.M., Davidson S.M., Hausenloy D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 21.Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J., Ma X., Cui Y., Song Y., Yao L., Liu Y. Methyleugenol protects against t-BHP-triggered oxidative injury by induction of Nrf2 dependent on AMPK/GSK3β and ERK activation. J. Pharmacol. Sci. 2017;135:55–63. doi: 10.1016/j.jphs.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H., Wang H., Ni M., Yue S., Xia Y., Busuttil R.W. Glycogen synthase kinase 3β promotes liver innate immune activation by restraining AMP-activated protein kinase activation. J. Hepatol. 2018;69:99–109. doi: 10.1016/j.jhep.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou Y., Zhu W., Sakamoto M., Qin Y., Akazawa H., Toko H. Heat shock transcription factor 1 protects cardiomyocytes from ischemia/reperfusion injury. Circulation. 2003;108:3024–3030. doi: 10.1161/01.CIR.0000101923.54751.77. [DOI] [PubMed] [Google Scholar]
- 25.Gray C.C., Amrani M., Yacoub M.H. Heat stress proteins and myocardial protection: experimental model or potential clinical tool? Int. J. Biochem. Cell Biol. 1999;31:559–573. doi: 10.1016/s1357-2725(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 26.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 27.Yoon Y.J., Kim J.A., Shin K.D., Shin D.S., Han Y.M., Lee Y.J. KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J. Biol. Chem. 2011;286:1737–1747. doi: 10.1074/jbc.M110.179440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Xu L., Gillette T.G., Jiang X., Wang Z.V. The unfolded protein response in ischemic heart disease. J. Mol. Cell. Cardiol. 2018;117:19–25. doi: 10.1016/j.yjmcc.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W., Paschen W. Unfolded protein response in brain ischemia: a timely update. J. Cerebr. Blood Flow Metabol. Off. J. Int. Soc. Cerebr. Blood Flow Metabol. 2016;36:2044–2050. doi: 10.1177/0271678X16674488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massink M.P., Créton M.A., Spanevello F., Fennis W.M., Cune M.S., Savelberg S.M. Loss-of-Function mutations in the WNT Co-receptor LRP6 cause autosomal-dominant oligodontia. Am. J. Hum. Genet. 2015;97:621–626. doi: 10.1016/j.ajhg.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez L., Paillard M., Thibault H., Derumeaux G., Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 32.Beagle B., Mi K., Johnson G.V. Phosphorylation of PPP(S/T)P motif of the free LRP6 intracellular domain is not required to activate the Wnt/beta-catenin pathway and attenuate GSK3beta activity. J. Cell. Biochem. 2009;108:886–895. doi: 10.1002/jcb.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014;276:543–559. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell R.R., Li J., Coven D.L., Pypaert M., Zechner C., Palmeri M. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim A.S., Miller E.J., Wright T.M., Li J., Qi D., Atsina K. A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2011;51:24–32. doi: 10.1016/j.yjmcc.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing Y., Musi N., Fujii N., Zou L., Luptak I., Hirshman M.F. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J. Biol. Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 37.Baler R., Dahl G., Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol. Cell Biol. 1993;13:2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xavier I.J., Mercier P.A., McLoughlin C.M., Ali A., Woodgett J.R., Ovsenek N. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J. Biol. Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- 39.He B., Meng Y.H., Mivechi N.F. Glycogen synthase kinase 3beta and extracellular signal-regulated kinase inactivate heat shock transcription factor 1 by facilitating the disappearance of transcriptionally active granules after heat shock. Mol. Cell Biol. 1998;18:6624–6633. doi: 10.1128/mcb.18.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu B., Soncin F., Price B.D., Stevenson M.A., Calderwood S.K. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J. Biol. Chem. 1996;271:30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- 41.Kourtis N., Moubarak R.S., Aranda-Orgilles B., Lui K., Aydin I.T., Trimarchi T. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat. Cell Biol. 2015;17:322–332. doi: 10.1038/ncb3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng W., Zhang Y., Zheng M., Cheng H., Zhu W., Cao C.M. Cardioprotection by CaMKII-deltaB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ. Res. 2010;106:102–110. doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen K., Qian W., Li J., Jiang Z., Cheng L., Yan B. Loss of AMPK activation promotes the invasion and metastasis of pancreatic cancer through an HSF1-dependent pathway. Molecular oncology. 2017;11:1475–1492. doi: 10.1002/1878-0261.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai S., Tang Z., Cao J., Zhou W., Li H., Sampson S. Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 2015;34:275–293. doi: 10.15252/embj.201489062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park D.W., Jiang S., Liu Y., Siegal G.P., Inoki K., Abraham E. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L735–L745. doi: 10.1152/ajplung.00165.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westerheide S.D., Anckar J., Stevens S.M., Sistonen L., Morimoto R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.