Abstract
Background
Little evidence exists regarding the clinical value of synbiotics in the management of post-treatment complications of breast cancer especially breast cancer-related lymphedema (BCRL). This study aimed to investigate the effects of synbiotic supplementation along with calorie restriction on quality of life and edema volume in patients with BCRL.
Methods
This randomized, placebo-controlled, clinical trial was conducted on 135 overweight and obese women with BCRL aged 18–65 years old. Participants were randomly allocated to receive a calorie-restricted diet plus 109 CFU synbiotic supplement (CRS group; n = 45) or placebo (CRP group; n = 45), daily for 10 weeks. Also, a control group (n = 45) with no intervention was included in the trial. All of the participants received Complete Decongestive Therapy for lymphedema treatment. The quality of life score, edema volume and body mass index (BMI) were measured at baseline and end of the trial.
Results
A total of 121 subjects completed the trial. CRS group showed a significant decrease in the total quality of life score (P = 0.004), and it’s psychosocial (P = 0.022) and functional (P = 0.002) domain scores, as well as edema volume (P = 0.002) and BMI (P < 0.001) in comparison to the control. However, there were no significant differences in changes in trial outcomes between the CRS and CRP groups.
Conclusion
Synbiotic supplementation along with a low-calorie diet was effective in quality of life, edema volume, and BMI improvement; mostly due to low-calorie diet. It seems that adding a dietitian consultation on the lymphedema management strategy may provide a better result in lymphedema control.
Keywords: Breast cancer, Lymphedema, Synbiotics, Calorie restriction, Quality of life
1. Introduction
Breast cancer is the second most common cancer and the fifth leading cause of cancer deaths worldwide [1]. In 2018, 2.1 million new cases of breast cancer were identified worldwide, which this figure is anticipated to reach about 3.2 million by 2050 [2,3]. Notably, there has been an increasing trend in the survival of patients with breast cancer over recent years as a 5-year survival rate is reported at 89% between 2005 and 2011 [4]. However, the complications related to the treatment, particularly the lymphedema remains a challenge for patients with breast cancer.
Breast cancer-related lymphedema (BCRL) occurs as a result of obstruction of lymphatic vessels that drain fluid from tissues throughout the body, accumulation of protein-rich fluid in the interstitial space, and consequently swelling of the arm, shoulder, hand, breast, and/or trunk [5]. This complication is estimated to affect about 20% of patients following the treatment, accounting for more than one in five breast-cancer survivors [6]. It can exert an adverse impact on various aspects of quality of life including physical wellbeing (i.e. severity of pain, arm tightness, and heaviness associated with lymphedema and impaired swollen arm/limb function), psychosocial wellbeing (i.e. the person’s ability in the perception of feelings, and socializing with others), and functional wellbeing (i.e. the person’s ability to perform the daily activities such as eating, dressing, etc.) [7].
Besides the treatment-related risk factors (e.g. extensive lymph node dissection and adjuvant chemo/radiation therapy), several other factors such as overweight and obesity have been suggested to could increase the risk of development of BCRL [8]. The mechanisms behind the pathogenesis of BCRL are still under investigation. However, the accumulating evidence suggests that chronic inflammation might be a key contributor to the development of BCRL through the formation of collateral lymphatic vessels, which is regulated by inflammatory cytokines and growth factors [9]. Therefore, using adjuvant therapies that target the inflammation, might be a good approach in the management of BCRL. Earlier studies have well established the role of dietary modifications such as restricted-calorie diets, focusing on weight loss/maintenance as a major component of BCRL treatment [10,11]. Also, another factor that has recently been highlighted in the literature is synbiotics.
Synbiotics refer to the combination of pro- and prebiotics in the form of synergism [12]. Probiotics are certain viable microorganisms, that can confer health benefits on the host when administered in adequate dosage [13]. In comparison, prebiotics is nonviable food components that can modulate the composition of gut microbiota resulting in the host’s wellbeing [14]. Together, the stimulation of probiotics with prebiotics could exert a superior health effect in comparison to the single administration [12]. Recent studies show that the microbiota of women with breast cancer differs from that of healthy women, indicating that certain bacteria may be associated with different responses to therapy [[15], [16], [17]]. Also, some evidence has shown that synbiotic supplementation is accompanied by favourable changes on markers of inflammation and oxidative stress and also, increasing the activity of antioxidant enzymes in BCRL patients [18,19]. This might be due to their ability to maintain the balance of the intestinal microbiota, regulate the immunomodulatory responses as well as suppression of the potential pathogens, and the inhibition of carcinogenesis [12,16,17]. Since elevated inflammation and oxidative stress are associated with impairment of quality of life [[20], [21], [22], [23], [24]], thus, using synbiotics might be promising in improving the quality of life in patients with BCRL.
Current evidence regarding the efficacy of synbiotic supplementation on quality of life in patients with cancer, especially breast cancer survivors is limited. With this regard, the present clinical trial aimed to investigate the effect of synbiotic supplementation along with a low-calorie diet on the quality of life in BCRL patients. We hypothesized that co-administration of synbiotic and low-calorie diet may exert a synergic effect and could contribute to a more considerable improvement in the quality of life in comparison to the low-calorie diet alone.
2. Methods
2.1. Study Design and subjects
This parallel, randomized, placebo-controlled, clinical trial was performed on 121 BCRL women. Inclusion criteria were age 18–65 years old with body mass index (BMI) between 25 and 35 kg/m2, who had been diagnosed with unilateral arm lymphedema at stage I or II, at least six months passed from surgery and adjuvant therapy completion (except hormone therapy/aromatase inhibitors) and were treated by Complex Decongestive Therapy (CDT) in the Seyed-Khandan Lymphedema Clinic (Tehran, Iran), between October 2017 and February 2018. CDT was achieved in two phases. In the “intensive phase,” the swollen arm was decongested daily by a Lymphotherapist for at least two weeks, using the manual lymph drainage and multi-layer compression bandaging in the clinic. The “maintenance phase” was achieved by the patient and family at home to stabilize edema reduction [25]. This study was performed in the maintenance phase to evaluate the effect of the interventions on changing arm volume.
Subjects were not included in the trial if they had at least one of the following conditions: a history of any chronic or acute diseases including cardiovascular, renal, pulmonary, and inflammatory diseases as well as diabetes, autoimmune, thyroid and psychological disorders; a history of smoking or alcohol consumption; taking any nutritional supplements and/or probiotic supplements over the three months before the intervention; following the weight-loss diets during the last six months; having lymphedema due to other cancers or diseases. Moreover, the exclusion criteria during the intervention were as follows: breast cancer recurrence or metastasis; intolerance or allergy to synbiotic or placebo supplements or reporting any unexpected adverse effects; the presence of any illness during the study, which requires the special treatments; compliance to the supplementation less than 80% at the end of trial; intake of any probiotic- and/or synbiotic-fortified foods during the intervention; and unwillingness to continue the study.
2.2. Sample size
In 2017, the mean value of Quality of Life impairment in Iranian lymphedema patients had been reported by 0.38 ± 0.22 [26]. Considering the standard deviation of 0.22, type I error of 5%, type II error of 20%, and effect size of 0.16 due to synbiotic treatment, the sample size was calculated 29 patients in each group. To study between three groups’ correlation, the correction coefficient of 1.4 was multiplied by the estimated sample size, and it was increased to 41 patients. Thus, the researchers decided to recruit 45 patients in each group to compensate for the probability of a 10% loss to follow up.
2.3. Study design
At the time of enrollment, all participants were advised to avoid from consumption of any food or supplements containing probiotic/synbiotic in 2 week run-in period and during the entire study.
Participants were assigned to receive either a calorie-restricted diet plus a synbiotic (CRS group) or a calorie-restricted diet plus a placebo (CRP group) for ten weeks. Also, a control group that received no intervention was included in the study. To avoid control contamination, all recruited patients were randomly allocated to CRS and CRP groups using the balanced block randomization method with a block size of 4. The allocation was performed using software-generated random numbers, which was provided by an academic member who was not associated with the study. After completion of the sample size in two intervention groups, all participants recruited into the control group.
Patients in the CRS and CRP groups were instructed to take one capsule of either synbiotic or placebo per day, preferably after the main meal. Synbiotic capsules consisted of 109 CFU/g of seven probiotic strains including Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophiles, and 38.5 mg fructooligosaccharides (as prebiotic). While, the placebo capsule was comprised of lactose and was equal with the synbiotic capsule in terms of appearance, color, shape, size, smell, taste, and packaging. All capsules were produced by Zist Takhmir Co., Tehran, Iran, and were approved by the Food and Drug Administration. Participants were instructed to keep the box of capsules in the refrigerator and take them daily after meals for ten weeks. Both the study participants and personnel were blinded to the type of supplementation that patients received. Compliance with the consumption of capsules was monitored once a week through phone calls. After five weeks of the study, participants were revisited, and their diet, study compliance, and adverse events were evaluated.
Furthermore, a low-calorie diet plan was prescribed for each patient in the CRS and CRP groups, that based on age, baseline weight, height, and the subject food records (using 24-h dietary recalls for three days) they would have about 0.5–1 kg weight loss per week. No participant was recommended a daily intake <1200 kcal and their diets were changed after five weeks of the study.
The energy requirement of participants was calculated based on the Mifflin formula and the recommended energy content for each macronutrient-based on American Cancer Society guidelines. It consisted of 55%–65% of calories from carbohydrates, 10%–15% from proteins, and 20%–35% from fats [27].
2.4. Outcomes
The primary outcome of this study was differences in quality of life score following the synbiotic supplementation along with a low-calorie diet, while the differences in edema volume and BMI were the secondary outcomes. All measurements were conducted at baseline and end of the trial.
2.4.1. Assessment of quality of life
Lymphedema Life Impact Scale (LLIS) questionnaire was used to assess the quality of life in the lymphedema patient. The questionnaire comprises 18-item, which evaluates the physical (pain, heaviness, tightness, strength …), psychosocial (body image, socializing, intimate relations …) and functional (duties at home, duties at work, recreational activities …) concerns related to lymphedema. Physical, psychosocial, and functional subscales consist of 8, 4, and 6 items, respectively. Items are scored based on a five-point Likert scale ranging from 1 (no impairment) to 5 (severe impairment), and the total score is obtained by summing up all items’ scores. The total and each subscale scores are a percentage ranging from 0 to 100, in which higher percent of impairment indicates the lower quality of life due to lymphedema. The validity and reliability of the Persian version of LLIS were reported to be acceptable in BCRL patients in Iran [28].
2.4.2. Assessment of edema volume
The volume of edema was measured using the water displacement method (Submerging the affected limb after the healthy limb in a water tank up to 2 cm below the armpit) [29]. Water overflow was measured in milliliters in a graduated cylinder [30]. The volume of the limb was measured as the volume difference between affected and healthy arms.
2.4.3. BMI measurement
Weight was measured using a Seca scale (Seca co., Hamburg, Germany) as the patients were minimally clothes, barefoot, and in standing position. Height was measured by a Seca stadiometer (Seca co., Hamburg, Germany). Body mass index (BMI) was calculated as weight (kg) divided by height squared (cm2).
2.4.4. Assessment of diet and physical activity
Dietary intake of participants was collected using 24-h dietary recalls for three nonconsecutive days (2 weekdays and one weekend day) at baseline and after ten weeks of intervention. The intake of energy, macro- and micro-nutrients were analyzed using Nutritionist IV software (First Data Bank; Hearst Corp, San Bruno, CA, USA). Also, physical activity level was determined by the International Physical Activity Questionnaire (IPAQ), and it was reported as metabolic equivalent per minute per week (MET-minutes/week) [31].
2.5. Statistical analysis
In the descriptive analysis, data with normal distribution were reported as mean and standard deviation, while those with non-normal distribution were presented as the median and interquartile range (IQR). The outcome of interests was quality of life score, BMI, and edema volume. Kolmogorov–Smirnov test showed non-normal distribution in quality of life score and edema volume variables, and So, One-way analysis of variance (ANOVA) and analysis of covariance (ANCOVA) was applied for comparison of those variables between the trial arms. BMI variable had normal distributions, so its difference between groups was studied by the Kruskal–Wallis test.
In the case of BMI and quality of life score variables, which showed significant differences (P < 0.05), Bonferroni correction was performed to detect the significance of changes between the study groups (two-sided α = 0.05/3 = 0.016). Also, the Chi-square test or Fisher’s exact test was used for between-group comparison of the categorical data such as chemotherapy, radiotherapy, breast cancer stage at diagnosis, surgery type, estrogen status, progesterone status, HER2 status, lymphedema stage, homogeneity of hands. Furthermore, the within-group changes of outcomes were assessed using the paired sample t-test or its non-parametric equivalent (Wilcoxon signed-rank) test. Those statistical analyses were performed using SPSS version 24 (IBM SPSS, Armonk, NY: 2016).
Finally, the marginal modelling with generalized estimating equations (GEE) approach was employed to compare the mean trend of quality of life, edema volume, and BMI between the intervention arms and control group, adjusting for covariates (baseline BMI, percentage change BMI, and edema volume). Also, to compare the mean trend of outcomes between two intervention arms (CRS and CRP groups), a contrast analysis was used. The marginal modelling process was performed using the SAS software, version 9.4.
3. Results
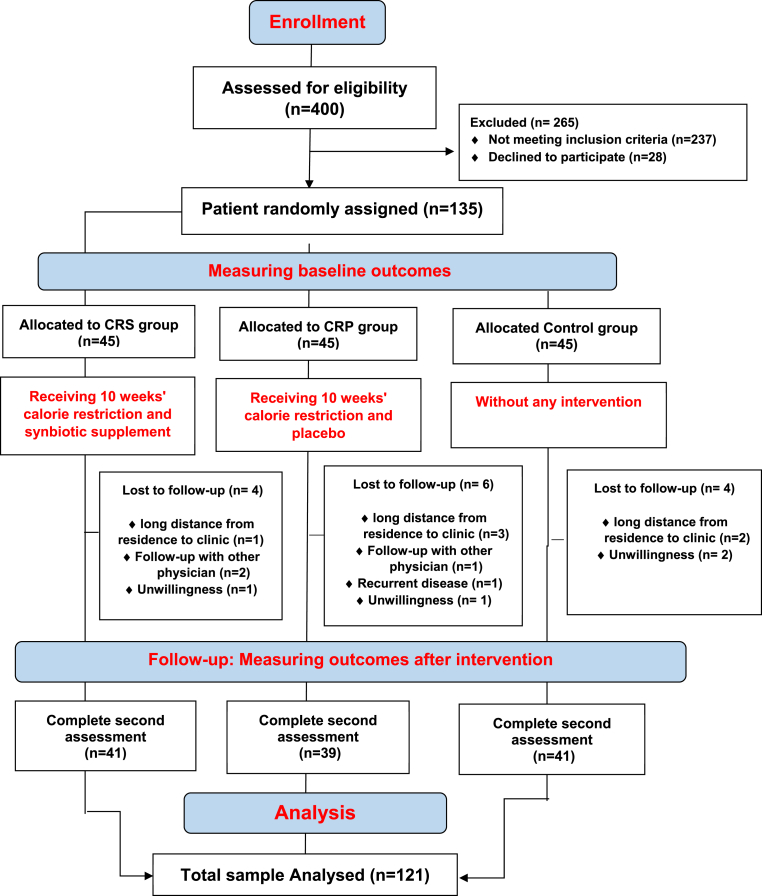
As it is shown in the study flow diagram (Fig. 1), Of the total of 135 subjects that were randomly allocated to the interventions and control groups, 14 patients were excluded during the follow-up interval due to the following reasons: long distance from residence to the clinic (n = 6); recurrent disease (n = 1); follow-up with other physicians (n = 3); and unwillingness to continue the study (n = 4). Thus, data on 121 subjects were included in the analysis. No adverse effect was reported by study participants following the intake of synbiotic and placebo capsules.
Fig. 1.
CONSORT diagram for randomized controlled dietary intervention.
The baseline clinical characteristics of the study subjects have been shown in Table 1. The study groups had a nearly similar distribution in terms of the most demographic and clinical features except the proportion of patients with a history of radiotherapy which was higher in the CRS group rather than the other two groups (P = 0.019). Because radiation therapy does not have a direct correlation with studied outcomes, it was not adjusted in GEE analysis. However, the distribution of other clinical features was not significantly different between the study groups.
Table 1.
Baseline clinical characteristics of study participants.
| Study groups |
P-value | |||
|---|---|---|---|---|
| CRS (n = 41) | CRP (n = 39) | Control (n = 41) | ||
|
Mean ± SD |
Mean ± SD |
Mean ± SD |
||
| Age (years) | 53.80 ± 1.42 | 52.41 ± 1.19 | 53.24 ± 1.5 | 0.736a |
| Physical activity (MET-minutes/week) | 369.42 ± 194.37 | 342.24 ± 187.45 | 426.19 ± 234.40 | 0.182a |
| Time since cancer treatment (years) | 5.43 ± 1.09 | 3.34 ± 3.7 | 3.66 ± 7.5 | 0.401a |
| Tumor size (cm) | 3.19 ± 03 | 3.24 ± 0.34 | 2.65 ± 0.19 | 0.527a |
| CDT course numbers |
15.66 ± 0.5 |
15.09 ± 0.5 |
14.24 ± 0.5 |
0.207a |
|
Number (%) |
Number (%) |
Number (%) |
||
| Chemotherapy | 39 (%95.1) | 37 (%94.9) | 40 (%97.6) | 0.847b |
| Radiotherapy | 41 (%100) | 35 (%89.7) | 34 (%82.9) | 0.026b |
| Homogeneity of hands | 19 (%46.3) | 22 (%56.4) | 21 (%51.2) | 0.566b |
| Lymphedema stage | 0.690b | |||
| I | 6 (%14.6) | 8 (%20.5) | 7 (%17.1) | |
| II | 35 (%85.4) | 31 (%79.5) | 33 (%80.5) | |
| Surgery type | 0.331b | |||
| Mastectomy | 26 (%63.4) | 26 (%66.7) | 20 (%48.8) | |
| Lumpectomy | 15 (%36.6) | 13 (%33.3) | 21 (%51.2) | |
| Breast cancer stage at diagnosis | 0.616b | |||
| I | 3 (%7.3) | 4 (%10.3) | 1 (%2.4) | |
| II | 32 (%78) | 28 (%71.8) | 31 (%75.6) | |
| III | 6 (%14.6) | 7 (%17.9) | 9 (%22) | |
| Estrogen status | 0.700b | |||
| Positive | 30 (%73.2) | 31 (%79.5) | 31 (%75.6) | |
| Negative | 11 (%26.8) | 8 (%20.5) | 10 (%24.4) | |
| Progesterone status | 0.648b | |||
| Positive | 31 (%75.6) | 27 (%69.2) | 28 (%68.3) | |
| Negative | 10 (%24.4) | 12 (%30.8) | 13 (%31.7) | |
| HER2 status | 0.752b | |||
| Positive | 9 (%22) | 7 (%17.9) | 6 (%14.6) | |
| Negative | 32 (%78) | 32 (%82.1) | 35 (%85.6) | |
Note: CRS group refers to the subjects received a calorie-restricted diet plus synbiotic, while CRP group followed a calorie-restricted diet plus placebo.
Abbreviations SD, standard deviation; MET, metabolic equivalent; CDT, complete decongestive therapy; HER2, human epidermal growth factor receptor 2.
Obtained from one-way ANOVA.
Obtained from Chi-square or Fisher exact test.
Dietary energy and nutrients intake have been summarized in Table 2. The mean intakes of total energy and sodium were significantly different between the study groups at baseline (P = 0.039; P < 0.001, respectively), which was adjusted for baseline values of variables in ANCOVA analysis. Also, there were significant differences in the intake of energy (0.024), carbohydrates (P = 0.026), protein (P = 0.008), and sodium (P < 0.001) between the study groups at the end of the ten weeks intervention.
Table 2.
Dietary intakes of participants throughout the trial.
| Study groups |
P-value a | ||||
|---|---|---|---|---|---|
| CRS (n = 41) |
CRP (n = 39) |
Control (n = 41) |
|||
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Energy (kcal/d) | Baseline | 2941.81 ± 349.09 | 3013.97 ± 329.68 | 2651.75 ± 183.88 | 0.058 |
| End of trial | 2537.39 ± 429.39 | 2562.89 ± 468.33 | 2779.02 ± 395.26 | 0.024 | |
| P-value b | <0.001 | <0.001 | 0.076 | ||
| Carbohydrate (g/d) | Baseline | 348.84 ± 57.99 | 349.81 ± 75.23 | 347.49 ± 40.26 | 0.984 |
| End of trial | 308.82 ± 54.29 | 325.67 ± 58.73 | 340.88 ± 45.16 | 0.026 | |
| P-value b | <0.001 | <0.001 | 0.135 | ||
| Protein (g/d) | Baseline | 118.66 ± 37.77 | 132.63 ± 45.54 | 120.27 ± 12.81 | 0.134 |
| End of trial | 100.08 ± 21.35 | 110.88 ± 44.03 | 120.88 ± 17.24 | 0.008 | |
| P-value b | <0.001 | 0.236 | 0.662 | ||
| Fat (g/d) | Baseline | 135.73 ± 204.20 | 143.15 ± 103.90 | 112.53 ± 20.60 | 0.123 |
| End of trial | 124.41 ± 52.68 | 122.50 ± 85.74 | 122.78 ± 34.83 | 0.872 | |
| P-value b | 0.659 | 0.247 | 0.571 | ||
| Sodium (mg/d) | Baseline | 1627.44 ± 793.14 | 1430.79 ± 509.92 | 1696.68 ± 181.47 | <0.001 |
| End of trial | 1523.15 ± 815.81 | 1379.86 ± 490.69 | 2035.96 ± 193.06 | <0.001 | |
| P-value b | 0.336 | 0.002 | 0.011 | ||
| Potassium (mg/d) | Baseline | 4828.65 ± 1728.52 | 5240.36 ± 1710.64 | 4846.07 ± 918.61 | 0.359 |
| End of trial | 4561.09 ± 1626.52 | 4986.15 ± 1572.50 | 4902.14 ± 871.15 | 0.353 | |
| P-value b | 0.242 | 0.260 | 0.039 | ||
Note: CRS group refers to the subjects received a calorie-restricted diet plus synbiotic, while CRP group followed a calorie-restricted diet plus placebo for ten weeks.
Abbreviations: SD, standard deviation.
Obtained from ANCOVA test adjusted for baseline values of variables.
Obtained from Paired sample t-test.
Table 3 shows the changes in LLIS, edema volume, and BMI between baseline measurements and after ten weeks of intervention in CRS, CRP, and control groups. There were no significant differences in these variables between the study groups at baseline, except in BMI (P = 0.014) and Psychosocial LLIS (0.029). Even though Bonferroni correction of Post hoc analysis showed no significant difference between the study groups for BMI and Psychosocial LLIS at baseline, BMI was considered an important confounder of outcomes, and its effect was adjusted in GEE analysis.
Table 3.
The effects of the intervention on main variables before and after study.
| Study groups |
P-VALUE | post-hoc P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| CRP (n = 39) | CRS (n = 41) | Control (n = 41) | CRP/CRS | CRP/Control | CRS/Control | |||
|
Mean ± SD |
Mean ± SD |
Mean ± SD |
||||||
| BMI (Kg/m2) | Before | 31.22 ± 3.88 | 31.28 ± 3.74 | 28.64 ± 5.78 | 0.014c | 0.99 | 0.05 | 0.04 |
| After | 30.53 ± 3.79 | 30.32 ± 3.68 | 29.00 ± 5.94 | 0.273c | – | – | – | |
| %change | −2.15 ± 2.78 | −3.01 ± 3.46 | +1.18 ± 2.30 | <0.001c | 0.42 | <0.001∗ | <0.001∗ | |
|
Pa |
<0.001 |
<0.001 |
0.005 |
|||||
|
Median (IQR) |
Median (IQR) |
Median (IQR) |
||||||
| Edema Volume (mm3) | Before | 440 (460) | 440 (560) | 450 (400) | 0.967b | – | – | – |
| After | 400 (440) | 320 (420) | 480 (580) | 0.147b | – | – | – | |
| % change | −28.57 (46) | −29.72 (42.31) | −22.72 (27.72) | 0.531b | – | – | – | |
| Pa | 0.001 | <0.001 | 0.225 | |||||
| Total LLIS | Before | 0.24 (0.23) | 0.24 (0.22) | 0.28 (0.19) | 0.126b | – | – | – |
| After | 0.15 (0.16) | 0.12 (0.21) | 0.25 (0.18) | <0.001b | 0.84 | 0.001∗ | 0.001∗ | |
| %change | −28.57 (56.6) | −39.53 (50.2) | −1.58 (11.42) | 0.002b | 0.436 | 0.011∗ | 0.001∗ | |
| Pa | 0.225 | 0.005 | 0.259 | |||||
| Physical LLIS | Before | 0.22 (0.15) | 0.25 (0.28) | 0.31 (0.19) | 0.052b | – | – | – |
| After | 0.13 (0.15) | 0.19 (0.19) | 0.22 (0.22) | 0.003b | 0.186 | 0.034 | <0.001∗ | |
| %change | −40.90 (45.64) | −42.10 (62.5) | −19.23 (83.72) | 0.026b | 0.534 | 0.031 | 0.005∗ | |
| Pa | <0.001 | <0.001 | 0.051 | |||||
| Psycho social LLIS | Before | 0.19 (0.28) | 0.13 (0.25) | 0.25 (0.25) | 0.029b | 0.791 | 0.04 | 0.041 |
| After | 0.06 (0.25) | 0.06 (0.19) | 0.25 (0.25) | <0.016b | 0.447 | <0.001∗ | 0.001∗ | |
| %change | −21.8 (72.21) | −23.53 (60.31) | 36.22 (107.56) | 0.056b | – | – | – | |
| Pa | 0.001 | 0.010 | 0.705 | |||||
| Functional LLIS | Before | 0.25 (0.3) | 0.25 (0.24) | 0.40 (0.3) | 0.121b | – | – | – |
| After | 0.15 (0.15) | 0.15 (0.29) | 0.35 (0.3) | 0.003b | 0.692 | 0.001∗ | 0.006∗ | |
| %change | −25 (66.63) | −36.36 (60) | −10 (63.28) | 0.007b | 0.487 | 0.014∗ | 0.005∗ | |
| Pa | 0.260 | <0.001 | 0.260 | |||||
∗Significant pairwise comparison in Post hoc analysis (According to Bonferroni correction, the P-value will be significant if α is lower than 0.016).
% Change was calculated as [absolute change]/[baseline value]∗100.
Wilcoxon signed-rank test.
Kruskal-Wallis test.
one-way ANOVA.
After ten weeks intervention period, the median values of the total (P = 0.001), physical (P < 0.001), psychosocial (P = 0.001), and functional (P = 0.006) LLIS scores showed a significant difference between CRS and control group. Also, the CRP group was significantly different from the control group in total (P = 0.001), psychosocial (P < 0.001), and functional (P = 0.001) LLIS Scores at the end of the study.
The P-values of total, physical, psychosocial, and functional LLIS percent changes between the three groups were 0.002, 0.026, 0.056, and 0.007, respectively. Post hoc analysis showed that the significant correlation in physical subscales was due to the median differences between CRS and control groups (−42.10 vs −19.23 P = 0.005). Percent change of functional LLIS than the control in the CRP (−25% vs. −10%; P = 0.014) and CRS (−36.36% vs. −10%; P = 0.005) groups decreased significantly. Also total quality of life impairment compared to the control in CRS (−39.53% vs. −1.58% P = 0.001) and CRP (−28.57% vs. −1.58% P = 0.011) groups reduced significantly.
There was a significant difference between groups in BMI value before the intervention (P = 0.014), and its changes during the study (P < 0.001). Post hoc analysis showed that this significant difference was due to a higher BMI decrease in CRP (2.15%) and CRS (3.01%) compared to a 1.18% increase of BMI in the Control group. Edema volume decreased significantly in CRP (−28.57%; P = 0.001) and CRS (−29.72%; P < 0.001) groups compared to the beginning of the study. But there was no significant difference between the study groups. (P = 0.531).
As mentioned before, because of the longitudinal nature response data, we used the marginal modelling methodology with the GEE approach to compare the mean trend of edema volume, BMI, and LLIS in intervention and control groups (Table 4). In studying the trend of quality of life during the intervention time in three groups, the effect of baseline BMI, the percentage change of BMI, and edema volume were adjusted. For each response variable, if there was no statistically significant difference among the three groups at the baseline, the group (intervention) variable was not included in the marginal model. The results of this analysis demonstrated that the CRS and CRP groups had a significant difference compared to the control group regarding the mean total (P = 0.004 for CRS; P = 0.012 for CRP), psychosocial (P = 0.022 for CRS; and P = 0.009 for CRP), and functional (P = 0.002 for CRS, and P = 0.003 for CRP) LLIS scores. The edema volume had a higher decrease in the CRS group compared to the control (P = 0.002). In addition, a greater reduction in BMI could be observed in CRS (P < 0.001) and CRP groups (P < 0.001) compared to the control group (Table 4).
Table 4.
The GEE results for comparing the mean trend of Edema Volume, BMI, LLIS and its subscales in intervention and control groups.
| Dependent variable | Independent Variable | Beta | Standard Error | P-value |
|---|---|---|---|---|
| Edema volumea | Intercept | 606.92 | 38.44 | <0.001 |
| Time | −50.76 | 37.20 | 0.172 | |
| CRP∗time | −92.24 | 56.10 | 0.105 | |
| CRS∗time | −135.13 | 45.31 | 0.002 | |
| BMI | Intercept | 28.64 | 0.89 | <0.001 |
| Time | 0.36 | 0.10 | <0.001 | |
| CRP∗time | −1.04 | 0.17 | <0.001 | |
| CRS∗time | −1.32 | 0.20 | <0.001 | |
| Total LLIS b | Intercept | 0.26 | 0.08 | 0.001 |
| Time | −0.02 | 0.03 | 0.362 | |
| CRP∗time | −0.07 | 0.03 | 0.012 | |
| CRS∗time | −0.08 | 0.03 | 0.004 | |
| Physical LLIS b | Intercept | 0.30 | 0.02 | <0.001 |
| Time | −0.12 | 0.03 | <0.001 | |
| CRP∗time | 0.03 | 0.04 | 0.356 | |
| CRS∗time | 0.07 | 0.04 | 0.058 | |
| Psychosocial LLIS b | Intercept | 0.23 | 0.09 | 0.009 |
| Time | 0.03 | 0.03 | 0.394 | |
| CRP∗time | −0.09 | 0.04 | 0.009 | |
| CRS∗time | −0.08 | 0.04 | 0.023 | |
| Functional LLIS b | Intercept | 0.50 | 0.11 | <0.001 |
| Time | −0.15 | 0.02 | <0.001 | |
| CRP∗time | 0.10 | 0.03 | 0.003 | |
| CRS∗time | 0.10 | 0.03 | 0.003 |
GEE analysis compares the changes of study outcomes in each intervention arm (intervention arm ∗ time interaction) with the control (as the reference group), adjusted for variable factors.
Significant correlations (P < 0.05) are shown in bold text.
Abbreviations: GEE, generalized estimating equations; LLIS, lymphedema life impact scale.
Adjusted for % BMI change.
Adjusted for baseline BMI, % BMI change, and % edema volume change.
Furthermore, the results of contrast analysis showed no significant difference between the CRS and CRP groups regarding the mean trend of BMI (P = 0.39) and edema volume (P = 0.389) as well as the mean total (P = 0.682), physical (P = 0.343), psychosocial (P = 0.698), and functional (P = 0.917) LLIS scores.
4. Discussion
To the best of our knowledge, the present study is the first randomized clinical trial investigating the effect of synbiotic supplementation along with a low-calorie diet in patients with BCRL. The results indicate that a low-calorie diet supplemented with synbiotics significantly decreased the quality of life impairment as well as edema volume and BMI. Furthermore, a more considerable improvement in trial outcomes was observed following a low-calorie diet plus synbiotics compared to those that had followed a low-calorie diet alone. However, the differences between the two intervention groups were not statistically significant.
The present study was performed on 121 women with breast cancer-induced lymphedema. There was no statistical difference in demographic and clinical characteristics between the study groups except for those receiving chemotherapy. This data insists on the strength of the study in eliminating assignment bias of participants to groups by randomization. Coincidentally, the BMI showed a significant difference between the study groups in the baseline, which adjusted its effect on the analysis.
Quality of life improved significantly by 39.53%, 28.57%, and 1.5% in CRS, CRP, and control groups, respectively. indicating that the intervention, which was mostly low-calorie diet, was able to improve the total quality of life of these patients significantly.
Although there was no significant difference in improving the quality of life between CRS and CRP groups (P = 0.84), the effect was higher in the group receiving synbiotic supplementation along with a low-calorie diet compared to the group receiving the low-calorie diet alone (39.53% vs 28.57%). The higher dose of synbiotic supplement or longer follow-up duration may affect this difference of estimation and can be evaluated in future researches. Due to ethical issues and limited studies in this field [32,33], we could not include a separate group that had just received synbiotic supplementation, which could clarify the independent effect of synbiotic on quality of life. According to the results of this study and lack of side effects, it seems that the effect of synbiotics alone can be assessed in the next study.
The results of this study demonstrated that the edema volume decreased by 29.72% in the CRS group and 28.57% in the CRP group. However, this difference between the study groups was not significant. But, by adjusting the effect of BMI changes as a confounder in edema volume, we found that the edema volume in the group who received synbiotic supplementation along with a low-calorie diet decreased significantly compared to the control group. Since lymphedema is an inflammatory disease [34,35], synbiotics by exerting their anti-inflammatory effects [36,37] can reduce inflammation and subsequently reduce the volume of edema in these people.
Furthermore, BMI was significantly reduced by 3.01% in the CRS group and 2.15% in the CRP group compared to the control, and this may correlate with a better quality of life. Arikawa [38] and wahnefried [39] in different studies approved the improved quality of life in breast cancer survivors following a weight loss. It seems that weight loss is so effective in these patients that it is better to be included in the lymphedema treatment protocols. Also, since the lymphatic system moves fluid, macromolecules, and formed elements from within the interstitial spaces into initial lymphatics and to pre collectors and collectors, the liquids intake in weight loss diets is very important.
After adjusting the interaction effect of intervention and time on the study outcomes, we observed that the total quality of life and its Psychosocial and functional subgroups improved significantly compared to the control group in both groups of receiving a low-calorie diet with or without synbiotic supplementation. Adjusting important confounders of this correlation persists in the marginal effect of a low-calorie diet on the quality of life of lymphedema patients.
The possible mechanisms underlying the favourable effects of synbiotics on quality of life in patients with cancer are not fully understood yet. Synbiotics could modulate the gut microbiota, inhibit the production of proinflammatory cytokines and cell proliferation. Besides, it exerts cytotoxic effect against cancer cells [40,41], which consequently may lead to amelioration of the physical and functional impairments related to the BCRL. Furthermore, it has been shown that synbiotics could stimulate the production of molecules with neuroactive properties, including short-chain fatty acids (SCFAs) and brain-derived neurotrophic factor (BDNF). They may influence behavior, emotion, and cognition through the stimulatory effect on neurogenesis, the biosynthesis of serotonin, and neuronal survival and differentiation; while they could suppress the neuroinflammation [42,43]. Due to such neuroprotective properties, they might relieve psychological symptoms related to the lymphedema and contribute to the improved quality of life in BCRL patients.
We assumed that the quality of life in these patients might have improved due to BMI and edema volume changes, so we adjusted for these two important confounders of quality of life in lymphedema, and surprisingly we found that quality of life in both intervention groups compared to control remained significant. This indicates the importance of low-calorie diet in these patients because these are often involved in weight gain, so, if we reduce the BMI, we can improve the quality of life in these patients. Since the most critical goal of lymphedema treatment is to decrease the patients’ quality of life disturbance, including a dietary consultant in their edema reduction protocol, can improve the outcome.
This study adds novel and relevant findings to the scientific body of knowledge regarding the efficacy of a combination of synbiotic and low-calorie diet as the adjuvant therapy in the management of post-treatment complications of breast cancer.
5. Conclusion
In summary, this randomized, placebo-controlled clinical trial showed that daily supplementation of synbiotics plus a low-calorie diet for ten weeks caused a significant improvement in the quality of life, edema volume, and BMI. Therefore, adding low-calorie diet along with synbiotics supplement to the treatment protocols used is recommended for overweight and obese breast cancer survivors with lymphedema.
Ethical consideration
The present study was conducted according to the principals of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol of the study was approved by the Ethical Committee of Iran University of Medical Sciences, Tehran, Iran (IR.IUMS.REC1396.31819). All participants were clarified about the study objectives and procedures and signed written informed consent. Also, this study was registered in the Iranian Registry of Clinical Trials (IRCT20180226038874N1).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
We would like to gratefully thank the participants for their support in the study. We also thank the Iran University of Medical Science for supporting the run of the project.
Contributor Information
Mitra Zarrati, Email: zarrati_ms@yahoo.com.
Shahpar Haghighat, Email: sha_haghighat@yahoo.com, haghighat@acecr.ac.ir.
References
- 1.Verdam F.J., Fuentes S., de Jonge C., Zoetendal E.G., Erbil R., Greve J.W. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21(12):E607–E615. doi: 10.1002/oby.20466. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Tao Z., Shi A., Lu C., Song T., Zhang Z., Zhao J. Breast cancer: epidemiology and etiology. Cell Biochem Biophys. 2015;72(2):333–338. doi: 10.1007/s12013-014-0459-6. [DOI] [PubMed] [Google Scholar]
- 4.Rojas K., Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016;59(4):651–672. doi: 10.1097/GRF.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 5.Sayegh H.E., Asdourian M.S., Swaroop M.N., Brunelle C.L., Skolny M.N., Salama L. Diagnostic methods, risk factors, prevention, and management of breast cancer-related lymphedema: past, present, and future directions. Curr Breast Cancer Rep. 2017;9(2):111–121. doi: 10.1007/s12609-017-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiSipio T., Rye S., Newman B., Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 7.Shaitelman S.F., Cromwell K.D., Rasmussen J.C., Stout N.L., Armer J.M., Lasinski B.B. Recent progress in the treatment and prevention of cancer-related lymphedema. Ca - Cancer J Clin. 2015;65(1):55–81. doi: 10.3322/caac.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu R., Huang X., Dong X., Zhang H., Zhuang L. Obese patients have higher risk of breast cancer-related lymphedema than overweight patients after breast cancer: a meta-analysis. Ann Transl Med. 2019;7(8):172. doi: 10.21037/atm.2019.03.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ly C.L., Kataru R.P., Mehrara B.J. Inflammatory manifestations of lymphedema. Int J Mol Sci. 2017;18(1) doi: 10.3390/ijms18010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeely M.L., Peddle C.J., Yurick J.L., Dayes I.S., Mackey J.R. Conservative and dietary interventions for cancer-related lymphedema: a systematic review and meta-analysis. Cancer. 2011;117(6):1136–1148. doi: 10.1002/cncr.25513. [DOI] [PubMed] [Google Scholar]
- 11.Shaw C., Mortimer P., Judd P.A. Randomized controlled trial comparing a low-fat diet with a weight-reduction diet in breast cancer-related lymphedema. Cancer. 2007;109(10):1949–1956. doi: 10.1002/cncr.22638. [DOI] [PubMed] [Google Scholar]
- 12.Markowiak P., Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganguly N., Bhattacharya S., Sesikeran B., Nair G., Ramakrishna B., Sachdev H. ICMR-DBT guidelines for evaluation of probiotics in food. Indian J Med Res. 2011;134(1):22. [PMC free article] [PubMed] [Google Scholar]
- 14.Pineiro M., Asp N.-G., Reid G., Macfarlane S., Morelli L., Brunser O. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42:S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 15.Fernández M.F., Reina-Pérez I., Astorga J.M., Rodríguez-Carrillo A., Plaza-Díaz J., Fontana L. Breast cancer and its relationship with the microbiota. Int J Environ Res Publ Health. 2018;15(8) doi: 10.3390/ijerph15081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Douglass J., Prasath V., Neace M., Atrchian S., Manjili M.H. The microbiome and breast cancer: a review. Breast Canc Res Treat. 2019;178(3):493–496. doi: 10.1007/s10549-019-05407-5. [DOI] [PubMed] [Google Scholar]
- 17.Parida S., Sharma D. The power of small changes: comprehensive analyses of microbial dysbiosis in breast cancer. Biochim Biophys Acta Rev Canc. 2019;1871(2):392–405. doi: 10.1016/j.bbcan.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navaei M., Haghighat S., Janani L., Vafa S., Saneei Totmaj A., Raji Lahiji M. The effects of synbiotic supplementation on antioxidant capacity and arm volumes in survivors of breast cancer-related lymphedema. Nutr Canc. 2019:1–12. doi: 10.1080/01635581.2019.1616781. [DOI] [PubMed] [Google Scholar]
- 19.Vafa S., Haghighat S., Janani L., Totmaj A.S., Navaei M., Amirinejad A. The effects of synbiotic supplementation on serum inflammatory markers and edema volume in breast cancer survivors with lymphedema. Excli j. 2020;19:1–15. doi: 10.17179/excli2019-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Almeida Roediger M., de Fátima Nunes Marucci M., Duim E.L., Santos J.L.F., de Oliveira Duarte Y.A., de Oliveira C. Inflammation and quality of life in later life: findings from the health, wellbeing and aging study (SABE) Health Qual Life Outcome. 2019;17(1):26. doi: 10.1186/s12955-019-1092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faugere M., Micoulaud-Franchi J.A., Faget-Agius C., Lancon C., Cermolacce M., Richieri R. Quality of life is associated with chronic inflammation in depression: a cross-sectional study. J Affect Disord. 2018;227:494–497. doi: 10.1016/j.jad.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 22.Imai R., Hori H., Itoh M., Lin M., Niwa M., Ino K. Relationships of blood proinflammatory markers with psychological resilience and quality of life in civilian women with posttraumatic stress disorder. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-54508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes C.S., Maes M., Roomruangwong C., Moraes J.B., Bonifacio K.L., Vargas H.O. Lowered quality of life in mood disorders is associated with increased neuro-oxidative stress and basal thyroid-stimulating hormone levels and use of anticonvulsant mood stabilizers. J Eval Clin Pract. 2018;24(4):869–878. doi: 10.1111/jep.12918. [DOI] [PubMed] [Google Scholar]
- 24.Wilkins J., Ghosh P., Vivar J., Chakraborty B., Ghosh S. Exploring the associations between systemic inflammation, obesity and healthy days: a health related quality of life (HRQOL) analysis of NHANES 2005–2008. BMC Obesity. 2018;5(1):21. doi: 10.1186/s40608-018-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeffs E., Ream E., Taylor C., Bick D. Clinical effectiveness of decongestive treatments on excess arm volume and patient-centered outcomes in women with early breast cancer-related arm lymphedema: a systematic review. JBI Database Syst. Rev. Implement. Rep. 2018;16(2):453–506. doi: 10.11124/JBISRIR-2016-003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haghighat S.H., Zayeri F., Montazeri A.L.I., Ebrahimi M. Factor validity of Persian version of the lymphedema life impact scale (LLIS) questionnaire in breast cancer induced lymphedema. Iran. Q. J. BREAST Dis. 2017;10(2):27–37. [Google Scholar]
- 27.Rock C.L., Doyle C., Demark-Wahnefried W., Meyerhardt J., Courneya K.S., Schwartz A.L. Nutrition and physical activity guidelines for cancer survivors. Ca - Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 28.Haghighat S., Montazeri A., Zayeri F., Ebrahimi M., Weiss J. Psychometric evaluation of the Persian version of the Lymphedema Life Impact Scale (LLIS, version 1) in breast cancer patients. Health Qual Life Outcome. 2018;16(1):132. doi: 10.1186/s12955-018-0958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kootstra J.J., Hoekstra-Weebers J.E.H.M., Rietman J.S., de Vries J., Baas P.C., Geertzen J.H.B. A longitudinal comparison of arm morbidity in stage I-II breast cancer patients treated with sentinel lymph node biopsy, sentinel lymph node biopsy followed by completion lymph node dissection, or axillary lymph node dissection. Ann Surg Oncol. 2010;17(9):2384–2394. doi: 10.1245/s10434-010-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrek J.A., Heelan M.C. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83(12 Suppl American):2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 31.Vasheghani-Farahani A., Tahmasbi M., Asheri H., Ashraf H., Nedjat S., Kordi R. The Persian, last 7-day, long form of the International Physical Activity Questionnaire: translation and validation study. Asian J Sports Med. 2011;2(2):106–116. doi: 10.5812/asjsm.34781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monshikarimi A., Ostadrahimi A., Jafarabadi M.A., EivaziZiaei J., Barzeghari A., Esfahani A. Does combination of Lactobacillus rhamnosus Heriz I and beta glucan improve quality of life in women with breast cancer receiving chemotherapy? Nutr Food Sci. 2019;50(3) [Google Scholar]
- 33.Lee J.Y., Chu S.H., Jeon J.Y., Lee M.K., Park J.H., Lee D.C. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis. 2014;46(12):1126–1132. doi: 10.1016/j.dld.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Ly C.L., Kataru R.P., Mehrara B.J. Inflammatory manifestations of lymphedema. Int J Mol Sci. 2017;18(1):171. doi: 10.3390/ijms18010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockson S.G. The lymphatics and the inflammatory response: lessons learned from human lymphedema. Lymphatic Res Biol. 2013;11(3):117–120. doi: 10.1089/lrb.2013.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon S.S., Sun J. Probiotics, nuclear receptor signaling, and anti-inflammatory pathways. Gastroenterol. Res. Pract. 2011;2011:971938. doi: 10.1155/2011/971938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLoughlin R.F., Berthon B.S., Jensen M.E., Baines K.J., Wood L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106(3):930–945. doi: 10.3945/ajcn.117.156265. [DOI] [PubMed] [Google Scholar]
- 38.Arikawa A.Y., Kaufman B.C., Raatz S.K., Kurzer M.S. Effects of a parallel-arm randomized controlled weight loss pilot study on biological and psychosocial parameters of overweight and obese breast cancer survivors. Pilot Feasibility Stud. 2017;4(1):17. doi: 10.1186/s40814-017-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demark-Wahnefried W., Colditz G.A., Rock C.L., Sedjo R.L., Liu J., Wolin K.Y. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast Canc Res Treat. 2015;154(2):329–337. doi: 10.1007/s10549-015-3627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madempudi R.S., Ahire J.J., Neelamraju J., Tripathi A., Nanal S. Randomized clinical trial: the effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci Rep. 2019;9(1):12210. doi: 10.1038/s41598-019-48554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranjbar S., Seyednejad S.A., Azimi H., Rezaeizadeh H., Rahimi R. Emerging roles of probiotics in prevention and treatment of breast cancer: a comprehensive review of their therapeutic potential. Nutr Canc. 2019;71(1):1–12. doi: 10.1080/01635581.2018.1557221. [DOI] [PubMed] [Google Scholar]
- 42.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 43.Haghighat N., Rajabi S., Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci. 2019:1–10. doi: 10.1080/1028415X.2019.1646975. [DOI] [PubMed] [Google Scholar]