Abstract
With standard therapies for patients with acute myeloid leukemia (AML), many patients either do not achieve complete response (CR) or relapse after CR. There are a scarcity of real-world data on outcomes of unselected patients with relapsed/refractory acute myeloid leukemia (RR-AML). We retrospectively evaluated treatment patterns and survival outcomes of unselected patients aged ≥18 years diagnosed with RR-AML identified from the Alberta Cancer Registry, Alberta, Canada, between January 2013 and December 2016. We included 199 patients who met predefined criteria for RR-AML. Following RR-AML diagnosis, 23% of patients received intensive therapy (IT), 33% non-intensive therapy (NIT), and 44% best supportive care (BSC). The unadjusted median overall survival (OS) of the study cohort was 5.3 months from the time of RR-AML diagnosis, with a 5-year OS rate of 12.6% (95% confidence interval 7.5-21.1). According to treatment intensity after RR-AML, the median OS outcomes were 13.6, 9.4, and 2.0 months for IT, NIT, and BSC groups, respectively (P<0.001). Patients who received treatment (IT or NIT) had better survival than those who received only BSC. This study emphasizes the need for newer therapy options for patients with RR-AML.
Keywords: Acute myeloid leukemia, relapsed, refractory, overall survival, real-world data, registry
Introduction
The standard therapy for fit patients with acute myeloid leukemia (AML) consists of induction chemotherapy with an anthracycline plus cytarabine (3+7 regimen). For patients achieving complete response (CR), consolidation chemotherapy is usually administered, and some patients undergo allogeneic stem cell transplantation (alloSCT). Although 70-80% of patients achieve CR, many patients are refractory to, or relapse, following such treatment [1,2]. For patients who are unfit for intensive chemotherapy, non-intensive treatment (NIT), using either a hypomethylating agent (HMA) or low-dose cytarabine (LDAC), with or without another chemotherapy agent, is generally used. However, most patients do not achieve CR with such treatments and relapse is virtually inevitable for those who do achieve CR [3-5].
For patients with relapsed/refractory AML (RR-AML), options include re-induction with intensive therapy (IT), usually using a different chemotherapy regimen such as FLAG-IDA (fludarabine, high-dose cytosine arabinoside, idarubicin, and granulocyte colony-stimulating factor) [6] or MEC (mitoxantrone, etoposide, and cytarabine) [7]. If a second complete response (CR2) is achieved, this may be followed by alloSCT. If refractory to IT, or if the patient is not considered a candidate for IT, palliative NIT or best supportive care (BSC) alone may be used. However, outcomes are generally poor in these patients; apart from those who undergo alloSCT after CR2, cures are rare in this setting [8-10].
Recently, a number of novel targeted agents have been approved or are under evaluation in clinical trials, in patients with RR-AML; these include FLT3 inhibitors (quizartinib, gilteritinib, crenolanib, and sorafenib) [11-14], IDH inhibitors (enasidenib and ivosidenib) [15,16] and others such as gemtuzumab ozogamicin, venetoclax, idasanutlin, and vosaroxin [17-20]. Although these agents have clearly demonstrated antileukemic activity in these patients and have shown improved survival in some randomized trials, responses are usually not durable. Moreover, most trials have rigorous selection criteria, and it remains unclear how results from these studies compare with those from patients who are treated in real-world settings where such selection criteria do not apply. While real-world registry-based data are available for patients who are newly diagnosed with AML [21,22], there are a scarcity of real-world data to evaluate the outcomes of unselected relapsed/refractory (RR) patients.
To address this deficiency, we evaluated the treatment patterns and outcomes of unselected adult patients with RR-AML, identified by registry data in a single jurisdiction (Alberta, Canada) within a specified timeframe.
Methods
Search strategy
The Alberta Cancer Registry collects comprehensive lists of all cancer patients by diagnosis within the province (population approximately 4 million). From this registry, we compiled a complete list of all patients aged ≥18 years with a diagnosis of AML according to International Classification of Diseases codes, over a 4-year period from January 2013 to December 2016. From this list, we identified all patients who were seen at the 2 main referral centers that treat the vast majority (>95%) of AML patients in the province, the University of Alberta Hospital (UAH) in Edmonton and the Tom Baker Cancer Centre (TBCC) in Calgary. Through a comprehensive chart review at these 2 centers, we identified all patients within this cohort with RR disease.
Definitions
Relapse was defined as having achieved a documented CR regardless of primary treatment, followed by subsequent disease recurrence. For patients receiving IT, refractory disease was defined as failure to achieve a CR after 2 induction attempts with different intensive regimens; patients not considered candidates for intensive re-induction after 1 induction regimen were also included. For patients receiving NIT upfront, refractory disease was defined as failure to achieve an objective complete or partial response to either 6 cycles of an HMA (generally azacitidine, as decitabine was not available in Canada), or 4 cycles of LDAC; patients with clear evidence of disease progression after 2-3 LDAC cycles were also included. Patients with RR-AML who received either an HMA or LDAC combined with another agent (usually as part of a clinical trial), or non-intensive investigational agents, were included. Date of diagnosis of RR-AML was defined as the date of documented RR-AML status, usually by bone marrow aspirate/biopsy.
Further chart reviews were conducted on the patients with RR-AML to collect information regarding baseline clinical and laboratory characteristics, subsequent therapy and outcomes. Based on the treatment regimen received following the diagnosis of RR-AML, patients were grouped as (1) IT: patients who received ≥1 line of intensive re-induction therapy at any time after RR-AML diagnosis; (2) NIT: patients who received ≥1 line of non-intensive cytotoxic therapy (HMA or LDAC ± other agent, or an alternative agent) but no IT; and (3) BSC: patients who did not receive any cytotoxic or other disease-modifying therapy except hydroxyurea.
Responses were defined by standard Cheson criteria [23]. Overall survival (OS) was defined as the time from diagnosis of RR-AML to date of death or last follow-up. For prognostic evaluation, the European LeukemiaNet (ELN) 2010 classification was used [24], mainly because this was the system in use at the time, and only limited molecular profiling was available (FLT3, NPM1, and CEBPA). For most patients, cytogenetics and molecular data were not available at the time of RR disease; therefore, the initial diagnostic cytogenetic and molecular data were used. As this was a retrospective analysis, data with respect to performance status were also not available. Ethics approval was obtained from the provincial Cancer Research Ethics Board prior to the initiation of this retrospective study.
Statistical evaluation
Data from the study were merged and analyzed on a secure computing platform with whole-disc 128-bit AES encryption with a 256-bit key as well as biometric and 2-factor authentication. To analyze the data, we used custom software written in Perl in conjunction with several additional software packages for data management and statistical analysis, including The R Project for Statistical Computing (Version 3.6.2) with the “survival” package, Microsoft Excel (Version 16.33), Sequel Pro (Version 1.1.2), MySQL (Version 8.0.18) and MAMP (Version 5.5). Descriptive statistics were used to describe the study population, baseline characteristics and treatment patterns. We used the Fisher exact test to determine the P values of our descriptive statistics. Survival analyses, which included survival curves, median survival, survival rates and confidence intervals (CIs) of survival metrices, were done using the Kaplan-Meier method. The Mantel-Cox test was used to detect significance in the survival analysis.
Cox proportional hazards regressions were used to test several patient characteristics for independent associations with mortality. Hazard ratios for mortality were estimated for age group (age ≥60 vs age <60), treatment intensity post-RR-AML (NIT vs BSC; IT vs BSC), best response pre-RR-AML (CR ≥12 months vs NR; CR <12 months vs NR) and ELN risk classification (intermediate vs favorable; adverse vs favorable). P values <0.05 were considered statistically significant.
Results
A total of 572 patients were diagnosed with AML within the specified time period. Of these, 199 were identified at the 2 centers as meeting the predefined criteria for RR-AML and were included in the analysis. The baseline characteristics of the entire group, and according to treatment administered following RR-AML diagnosis, are summarized in Table 1.
Table 1.
Baseline characteristics of patients with RR-AML
| Characteristic | All patients (n=199) | IT (n=46) | NIT (n=65) | BSC (n=88) | P value |
|---|---|---|---|---|---|
| Age, mean (range), years | 63.2 (20.4-92.8) | 52.1 (20.4-74.6) | 67.7 (23-90.6) | 65.7 (21-92.8) | |
| Male, n (%) | 124 (62) | 28 (61) | 41 (63) | 55 (63) | NS |
| ELN 2010 risk classification at diagnosis, n (%)a | |||||
| Favorable | 35 (18) | 19 (41) | 6 (9) | 10 (11) | <0.001 |
| Intermediate-1 | 100 (50) | 18 (39) | 42 (65) | 40 (45) | |
| Intermediate-2 | 5 (3) | 1 (2) | 3 (5) | 1 (1) | |
| Adverse | 59 (30) | 8 (17) | 14 (22) | 37 (42) | |
| Prior hematologic malignancy, n (%) | 25 (13) | 6 (13) | 8 (12) | 11 (13) | NS |
| Lines of therapy pre-RR-AML, n (%) | |||||
| 1 | 172 (86) | 41 (89) | 57 (88) | 74 (84) | NS |
| 2 | 19 (10) | 5 (11) | 5 (8) | 9 (10) | |
| Treatment intensity pre-RR-AML, n (%) | |||||
| IT | 124 (62) | 46 (100) | 32 (49) | 46 (52) | NS |
| NIT | 75 (38) | 0 (0) | 33 (51) | 42 (48) | |
| Best response to pre-RR-AML therapy, n (%) | |||||
| CR ≥12 months | 38 (19) | 18 (39) | 11 (17) | 9 (10) | <0.001 |
| CR <12 months | 85 (43) | 22 (48) | 30 (46) | 33 (38) | |
| NCR | 76 (38) | 6 (13) | 24 (37) | 46 (52) | |
| alloSCT pre-RR-AML, n (%) | 25 (13) | 2 (4) | 8 (12) | 15 (17) | NS |
alloSCT: allogeneic stem cell transplantation; BSC: best supportive care; CR: complete response; ELN: European LeukemiaNet; IT: intensive therapy; NIT: non-intensive therapy; NCR: no complete response; NS: not significant; RR-AML: relapsed/refractory acute myeloid leukemia.
Rounding may cause total ≠ 100%.
Treatments administered
As shown in Table 1, 23% of patients received IT following RR diagnosis, 33% received NIT and the remaining 44% received BSC. Patients receiving IT after RR diagnosis tended to be younger and were more likely to have a favorable ELN risk score. In contrast, the NIT and BSC groups did not differ by age, and were more likely to be ELN intermediate risk, while those receiving BSC were more likely than treated patients to be ELN adverse risk. Patients whose first CR duration was ≥12 months were more likely to receive IT at relapse, as compared with those with CR <12 months or primary refractory disease. Patients relapsing after alloSCT were more likely to receive either NIT or BSC.
In terms of prior IT, 118 patients had received 3+7 induction chemotherapy initially, while 23 received either FLAG or FLAG-IDA. Of these, 27 primary induction failures received re-induction with either NOVE (mitoxantrone and etoposide) ± HiDAC (high-dose cytarabine) [25], FLAG-IDA or another regimen. For those achieving CR, consolidation therapy generally consisted of up to 4 cycles cytarabine 3 g/m2 × 6 doses (1 g/m2 for patient age >60 years); 25 had received alloSCT in first CR. In terms of prior NIT, azacitidine ± other agent was used in 61 patients and LDAC ± other agent in 17; 6 patients received another non-intensive agent in the setting of a clinical trial. No patients had received venetoclax prior to RR.
The first IT post-RR-AML consisted of FLAG-IDA in 29 cases (63%) and FLAG in 5 cases (11%); 2 patients each received NOVE-HiDAC and NOVE, while 1 patient each received a 3+7 regimen, high-dose cyclophosphamide-etoposide, and high-dose etoposide alone. Two patients (4%) proceeded to receive alloSCT directly. The first NIT consisted of azacitidine ± other agent in 49 cases (75%) and LDAC ± other agent in 12 cases (19%); 3 patients received selinexor in a clinical trial, while 1 patient each received gilteritinib, guadecitabine, and venetoclax plus idasanutlin, all in clinical trials. The number of patients (n=67) who received NIT first post-RR-AML exceeded the number of patients in the non-intensive cohort (n=65) because 2 patients who received first NIT post-RR-AML went on to receive IT in either the second or third line; these patients were included in the IT group. Seven patients received azacitidine as the second line post-RR, while 4 received LDAC.
Response data
The response data are shown in Table 2. Of the 30 patients achieving CR in the IT group, 6 were classified as achieving complete response with incomplete count recovery (CRi), while 4 in the NIT group were classified as CRi. As shown, ELN favorable risk patients were more likely to achieve a CR with IT post-RR; however, this difference did not reach statistical significance. In contrast, patients with a first CR duration ≥12 months were significantly more likely to achieve CR with IT post-RR. Responses to NIT were similar regardless of ELN risk group or CR1 duration. There were no CRs in the BSC group.
Table 2.
CR rates and OS according to treatment intensity after RR-AML
| Characteristic | CR + CRi/n (%) | Median OS (95% CI), months from diagnosis of RR-AML | |||
|---|---|---|---|---|---|
|
|
|
||||
| IT | NIT | IT | NIT | BSC | |
| All patients | 30/46 (65) | 9/65 (14) | 13.6 (9.6-NR) | 9.4 (5.8-12.8) | 1.9 (1.5-3.4) |
| Initial ELN 2010 risk group | |||||
| Favorable | 16/19 (84) | 1/6 (17) | NR (13.6-NR) | 6.9 (3.8-NR) | 1.3 (1.1-NR) |
| Intermediate | 10/19 (53) | 5/45 (11) | 11.0 (4.7-NR) | 10.4 (5.3-18.4) | 2.1 (1.5-3.8) |
| Adverse | 4/8 (50) | 3/14 (21) | 9.1 (4.3-NR) | 8.6 (5.4-NR) | 2.3 (1.7-4.1) |
| P value | 0.075 | NS | 0.05 | NS | NS |
| Best response pre-RR-AML | |||||
| CR ≥12 months | 16/18 (89) | 0/11 (0) | NR (12.4-NR) | 18.5 (9.3-NR) | 3.4 (2.0-NR) |
| CR <12 months | 13/22 (59) | 5/30 (17) | 10.3 (4.7-NR) | 7.6 (3.8-NR) | 1.8 (1.5-4.2) |
| NCR | 1/6 (17) | 4/24 (17) | 9.2 (5.3-NR) | 6.5 (4.9-11.5) | 1.8 (0.9-3.6) |
| P value | 0.004 | NS | NS | 0.08 | NS |
| Age at diagnosis of AML | |||||
| <60 years | 21/36 (58) | 2/13 (15) | 15.8 (9.2-NR) | 9.4 (5.8-NR) | 3.4 (1.8-4.0) |
| ≥60 years | 9/10 (90) | 7/52 (14) | 12.1 (4.3-NR) | 8.9 (5.3-11.5) | 1.8 (1.4-3.5) |
| P value | NS | NS | NS | NS | NS |
| Response post-RR-AML | |||||
| CR | N/A | N/A | NR (13.8-NR) | 44.8 (18.4-NR) | N/A |
| CRi | 7.6 (5.4-NR) | 11.3 (9.4-NR) | |||
| NCR | 5.4 (4.5-NR) | 9.3 (5.3-23.8) | |||
| P value | <0.001 | 0.01 | |||
BSC: best supportive care; CI: confidence interval; CR: complete response; CRi: complete response with incomplete count recovery; ELN: European LeukemiaNet; IT: intensive therapy; N/A: not applicable; NCR: no complete response; NIT: non-intensive therapy; NR: not reached; NS: not significant (only p values <0.1 are shown); OS: overall survival; RR-AML: relapsed/refractory acute myeloid leukemia.
Survival data
The OS of the entire group is shown in Figure 1A; the unadjusted median OS for the overall study cohort was 5.3 months from the time of diagnosis of RR-AML, with a 1-year OS rate of 29.6% (95% CI 29.0-30.3) and a 5-year OS rate of 12.6% (95% CI 7.5-21.1). The OS according to treatment intensity administered after RR-AML is shown in Figure 1B; the median OS outcomes were 13.6, 9.4, and 2.0 months for the IT, NIT, and BSC groups, respectively (P<0.001). The OS according to age at diagnosis is shown in Figure 2A; the OS for patients aged <60 years was 6.0 months, versus 4.5 months for those aged ≥60 years (P=0.009). The OS according to ELN risk group is shown in Figure 2B; the median OS for patients with ELN favorable, intermediate (high and low), and adverse risk profiles were 9.9, 4.7, and 4.0 months, respectively (P=0.003). The respective median OS outcomes in different subgroups according to treatment intensity are shown in Table 2. Patients with a first CR ≥12 months tended to have a longer OS than other patients, regardless of whether they received IT or NIT; however, these differences were not significant (P=0.2 and 0.08 for IT and NIT patients, respectively). In contrast, achievement of CR post-RR, regardless of whether patients received IT or NIT, was significantly associated with superior OS.
Figure 1.
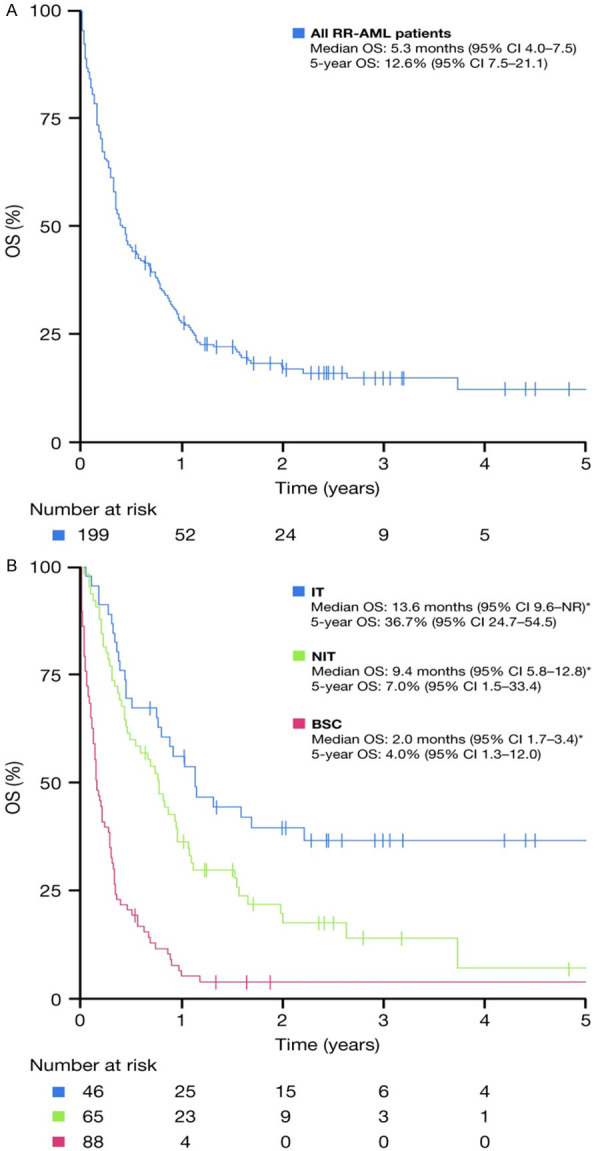
OS (A) of the entire cohort and (B) according to treatment intensity post-RR-AML. BSC: best supportive care; CI: confidence interval; IT: intensive therapy; NIT: non-intensive therapy; NR: not reached; OS: overall survival; RR-AML: relapsed/refractory acute myeloid leukemia. *P<0.001 between IT, NIT, and BSC groups.
Figure 2.
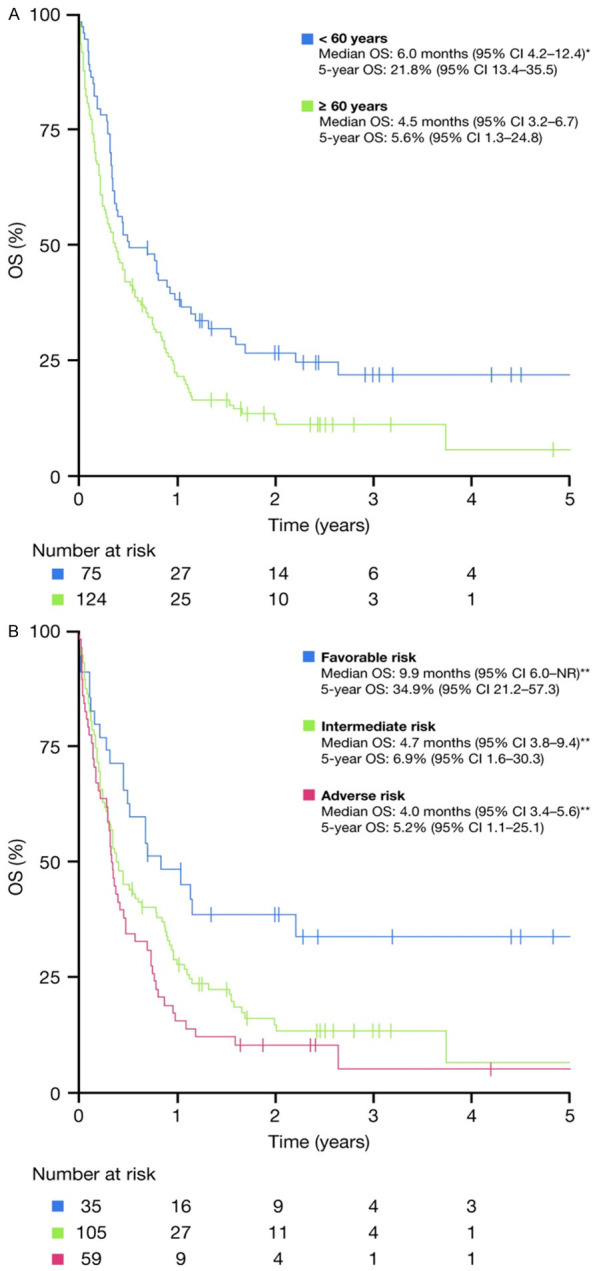
OS (A) according to age at diagnosis and (B) according to ELN risk group. CI: confidence interval; ELN: European LeukemiaNet; NR: not reached; OS: overall survival. *P=0.009 compared with ≥60 years, **P=0.003 between ELN risk groups.
A total of 16 patients underwent alloSCT post-RR, 12 of whom had received IT at RR and were in CR at the time of transplantation. The OS curves for the patients who underwent alloSCT in CR, and for those who did not undergo alloSCT after achieving CR with IT, are shown in Figure 3. The median OS for all patients who received alloSCT following diagnosis of RR-AML was 20.2 months.
Figure 3.
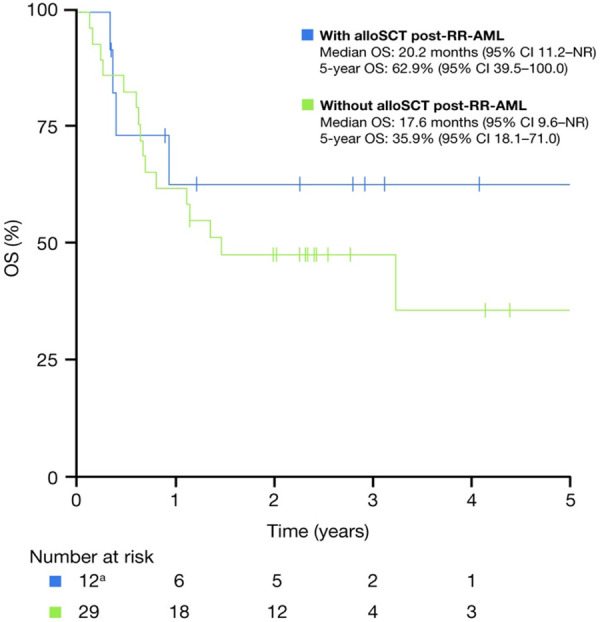
OS for the patients achieving CR post-RR-AML according to whether the patients underwent subsequent alloSCT. alloSCT: allogeneic stem cell transplantation; CI: confidence interval; CR: complete response; NR: not reached; OS: overall survival; RR-AML: relapsed/refractory acute myeloid leukemia. aFour patients who had alloSCT post-RR-AML were excluded because they did not attain CR status, the status was incorrectly entered, or the status was missing.
A multivariable Cox regression analysis was performed, incorporating age, ELN risk profile, treatment intensity before diagnosis of RR-AML, number of treatment lines before diagnosis of RR-AML, best response before diagnosis of RR-AML, treatment intensity after diagnosis of RR-AML, and alloSCT after diagnosis of RR-AML. In this analysis, any treatment (either IT or NIT) given post-RR was significantly associated with better OS versus BSC (Table 3). CR1 duration ≥12 months was of borderline significance for better OS, while other factors were not significantly associated with OS.
Table 3.
Multivariate analysis for OS after RR-AML
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age (<60 vs ≥60 years) | 1.20 (0.82-1.76) | 0.35 |
| Treatment intensity post-RR-AML | ||
| IT vs BSC | 0.26 (0.16-0.43) | <0.001 |
| NIT vs BSC | 0.32 (0.23-0.48) | <0.001 |
| IT vs NIT | 0.80 (0.38-1.7) | 0.56 |
| Best response pre-RR-AML | ||
| CR <12 months vs NCR | 0.87 (0.6-1.25) | 0.44 |
| CR ≥12 months vs NCR | 0.55 (0.33-0.92) | 0.03 |
| CR ≥12 months vs <12 months | 0.64 (0.38-1.05) | 0.08 |
| ELN risk score | ||
| Favorable vs intermediate | 1.42 (0.8-2.5) | 0.23 |
| Favorable vs adverse | 1.21 (0.64-2.3) | 0.56 |
| Intermediate vs adverse | 1.17 (0.79-1.74) | 0.44 |
BSC: best supportive care; CI: confidence interval; CR: complete response; ELN: European LeukemiaNet; HR: hazard ratio; IT: intensive therapy; NIT: non-intensive therapy; NCR: no complete response; OS: overall survival; RR-AML: relapsed/refractory acute myeloid leukemia.
Discussion
Considering the poor prognosis of patients with RR-AML, a number of investigational agents have been, and are being evaluated, in clinical trials. However, many of these are single-arm studies and therefore lack control groups, while randomized controlled trials generally have fairly stringent eligibility criteria. These studies do not indicate how many potential patients declined enrollment or were excluded. It is therefore difficult to ascertain how these results compare with those from real-world settings. Our study evaluated all patients treated at the two primary leukemia centers in Alberta at the time of diagnosis, and therefore would have captured all patients at the RR stage, including those who subsequently received palliation at their local hospitals without being referred back to tertiary care centers.
The most striking finding was that 44% of patients received only BSC following RR. The reasons for this are difficult to ascertain in a retrospective study but may include a lack of available effective treatment options or clinical trials, poor performance status, a moribund state with imminent death, or patient preference. These are often not independent variables; a patient’s decision to opt for BSC alone may be influenced by a lack of effective treatments as well as frail state, which may dissuade the patient from returning to the leukemia center. In particular, patients failing HMA therapy generally did not have effective treatment options available at the time of the analysis outside of clinical trials; this may have strongly influenced their decision.
Our study found that patients who receive any treatment, including palliative NIT, have a better survival than those who receive only BSC. Although intensive re-induction was associated with a superior OS on univariate analysis, there was no significant difference in OS between IT and NIT patients on multivariate analysis, suggesting that outcomes are also influenced by covariates such as prior therapy, first CR duration, age, performance status, and disease biology. In particular, patients relapsing after alloSCT, older patients, those with very short CR duration or no CR, and those with adverse-risk ELN scores were much less likely to receive IT, as shown in Table 1. Conversely, those who received IT at relapse tended to have more favorable biologic and clinical factors. As with previous studies, there were few long-term survivors following RR with the exception of those who underwent alloSCT.
For those who received antileukemic treatment post-RR, achievement of a true CR appears to be the most important predictor of survival regardless of treatment intensity, as shown in Table 2. Therefore, this remains an important goal of treatment. While ELN risk score was significantly associated with OS (Figure 2B), the lack of significance on multivariate analysis suggests that this was mainly due to the fact that adverse-risk patients were more likely to receive only BSC post-RR (63%), and that favorable-risk patients were more likely (54%) to receive IT post-RR than other groups (Table 1).
The main strength of this study is its population-based analysis, which eliminates the selection bias present in most clinical trials and therefore provides a more accurate reflection of real-world outcomes. Major limitations include the inability to ascertain the influence of certain factors such as performance status, which were not accurately obtainable retrospectively, and the inability to accurately determine the rationale behind decision-making with respect to treatment choice in many cases.
Since this analysis was performed, a number of targeted agents have been reported to produce favorable results in RR patients. These include the FLT3 inhibitors quizartinib and gilteritinib, each of which demonstrated a survival benefit compared with standard treatments in randomized clinical trials [11,12]; the OS in the gilteritinib arm of the ADMIRAL phase 3 study was 9.3 months [12]. The IDH2 inhibitor enasidenib showed an overall response rate of approximately 40%, with a median OS of 9.3 months in a phase 2 study of patients with AML harboring an IDH2 mutation [15], while the IDH1 inhibitor ivosidenib showed a comparable response rate in AML patients harboring an IDH1 mutation [16]. While these OS results do not appear superior to the NIT group in our study, many patients in those studies had already failed other treatments and would otherwise have been relegated to BSC alone. Several of these new agents have now been approved by regulatory agencies and are in clinical use. While these emerging therapies are likely to have a favorable impact on OS in these subsets of patients and allow some RR patients to receive antileukemic therapy, the majority of patients with AML do not harbor these mutations. Furthermore, these targeted agents are generally not curative when used as single agents in the RR setting, and should therefore be evaluated as part of frontline combination regimens where they may be more effective. This study emphasizes the need for newer therapies that will favorably impact a greater majority of patients with RR-AML, particularly those who have failed all available conventional therapies.
Acknowledgements
This study was funded by Bristol-Myers Squibb. The authors received editorial support in the preparation of this manuscript from Saba Choudhary, PhD, of Excerpta Medica, funded by Bristol-Myers Squibb. The authors are fully responsible for all content and editorial decisions.
Disclosure of conflict of interest
JMB has received honoraria from Bristol Myers Squibb, Novartis, Otsuka, Pfizer, Jazz Pharmaceuticals and Teva. MNG has received honoraria from Bristol Myers Squibb, Novartis, Otsuka, Pfizer, Jazz Pharmaceuticals. DY has received honoraria from Bristol Myers Squibb to conduct this study. FFL is an employee of Bristol Myers Squibb. CW is an employee of Celgene Inc., a Bristol Myers Squibb company. WYC has received research funding and consulting fees from Bristol Myers Squibb. The remaining authors have no conflicts of interest to disclose.
References
- 1.O’Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE, Damon LE, Goorha S, Lancet J, Maness LJ, Marcucci G, Millenson MM, Moore JO, Ravandi F, Shami PJ, Smith BD, Stone RM, Strickland SA, Tallman MS, Wang ES, Naganuma M, Gregory KM. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984–1021. doi: 10.6004/jnccn.2012.0103. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities amongst 5876 younger adult patients treated in the UK Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, Wheatley K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 4.Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Récher C, Sandhu I, Bernal del Castillo T, Al-Ali HK, Martinelli G, Falantes J, Noppeney R, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Döhner H. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysák D, Minden M, Arthur C. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastore D, Specchia G, Carluccio P, Liso A, Mestice A, Rizzi R, Greco G, Buquicchio C, Liso V. FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single center experience. Ann Hematol. 2003;82:231–235. doi: 10.1007/s00277-003-0624-2. [DOI] [PubMed] [Google Scholar]
- 7.Amadori S, Arcese W, Isacchi G, Meloni G, Petti MC, Monarca B, Testi AM, Mandelli F. Mitoxantrone, etoposide, and intermediate-dose cytarabine: an effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J. Clin. Oncol. 1991;9:1210–1214. doi: 10.1200/JCO.1991.9.7.1210. [DOI] [PubMed] [Google Scholar]
- 8.Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, Sakura T, Kanamori H, Usuki K, Yamashita T, Okoshi Y, Shibayama H, Nakamae H, Mawatari M, Hatanaka K, Sunami K, Shimoyama M, Fujishima N, Maeda Y, Miura I, Takaue Y, Fukuda T. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;95:1857–1864. doi: 10.3324/haematol.2010.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashidi A, Weisdorf DJ, Bajanyan N. Treatment of relapsed/refractory acute myeloid leukaemia in adults. Br J Haematol. 2018;181:27–37. doi: 10.1111/bjh.15077. [DOI] [PubMed] [Google Scholar]
- 10.Ganzel C, Sun Z, Cripe LD, Fernandez HF, Douer D, Rowe JM, Paietta EM, Ketterling R, O’Connell MJ, Wiernik PH, Bennett JM, Litzow MR, Luger SM, Lazarus HM, Tallman MS. Very poor long-term survival in past and more recent studies for relapsed AML patients: the ECOG-ACRIN experience. Am J Hematol. 2018;93:1074–1081. doi: 10.1002/ajh.25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes JE, Tallman MS, Schiller GJ, Trone D, Gammon G, Goldberg SL, Perl AE, Marie JP, Martinelli G, Kantarjian HM, Levis MJ. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory AML. Blood. 2018;132:598–607. doi: 10.1182/blood-2018-01-821629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl A, Martinelli G, Cortes J, Neubauer A, Berman E, Paolini S, Montesinos P, Baer M, Larson R, Ustun C, Fabbiano F, Di Stasi A, Stuart R, Olin R, Kasner M, Ciceri F, Chou WC, Podoltsev N, Recher C, Yokoyama H, Hosono N, Yoon SS, Lee JH, Pardee T, Fathi A, Liu C, Liu X, Bahceci E, Levis M. Gilteritinib significantly prolongs overall survival in patients with FLT3-mutated relapsed/refractory acute myeloid leukemia: results from the ADMIRAL trial. EHA Annual Meeting. 2019;267459:S876. [Google Scholar]
- 13.Ohanian M, Kantarjian HM, Borthakur G, Kadia TM, Konopleva M, Garcia-Manero G, Estrov Z, Ferrajoli A, Takahashi K, Jabbour EJ, Daver N, Kornblau SM, Wierda WG, Burger JA, Naqvi K, Benton CB, Bose P, Eckardt JR, Ravandi F, Cortes JE. Efficacy of a type I FLT3 inhibitor, crenolanib, with idarubicin and high-dose ara-C in multiply relapsed/refractory FLT3+ AML. Blood. 2016;128:2744. [Google Scholar]
- 14.Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, Faderl S, Verstovsek S, Mathews S, Andreeff M, Cortes JE. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96:62–68. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, Stone RM, DeAngelo DJ, Levine RL, Flinn IW, Kantarjian HM, Collins R, Patel MR, Frankel AE, Stein A, Sekeres MA, Swords RT, Medeiros BC, Willekens C, Vyas P, Tosolini A, Xu Q, Knight RD, Yen KE, Agresta S, de Botton S, Tallman MS. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiNardo CD, de Botton S, Stein EM, Roboz GJ, Mims AS, Pollyea DA, Swords RT, Altman JK, Collins RH, Mannis GN, Uy GL, Donnellan W, Pigneux A, Fathi AT, Stein AS, Erba HP, Prince GT, Foran JM, Traer E, Stuart RK, Arellano ML, Slack JL, Sekeres MA, Yen K, Kapsalis SM, Liu H, Goldwasser M, Agresta S, Attar EC, Tallman MS, Stone RM, Kantarjian HM. Ivosidenib (AG-120) in mutant IDH1 AML and advanced hematologic malignancies: results of a phase 1 dose escalation and expansion study. Blood. 2017;130(Suppl 1):725. [Google Scholar]
- 17.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A. Efficacy and biological correlates of response in a phase 2 study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6:1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinelli G, Pappayannidis C, Yee K, Vey N, Drummond M, Kelly K, Dickinson M, Lee JH, Seiter K, Yoon SS, Assouline SS, Kasner MM, Nichols G, Middleton S, Blotner S, Zhi J, Pierceall W, Chen LC. Phase 1b results of idasanutlin + cytarabine (Ara-C) in acute myeloid leukemia (AML) patients (pts) Haematologica. 2016;101:S504. [Google Scholar]
- 19.Sievers EL, Larson RA, Stadmauer EA, Estey E, Löwenberg B, Dombret H, Karanes C, Theobald M, Bennett JM, Sherman ML, Berger MS, Eten CB, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 20.Ravandi F, Ritchie EK, Sayar H, Lancet JE, Craig MD, Vey N, Strickland SA, Schiller GJ, Jabbour E, Erba HP, Pigneux A, Horst HA, Recher C, Klimek VM, Cortes J, Roboz GJ, Odenike O, Thomas X, Havelange V, Maertens J, Derigs HG, Heuser M, Damon L, Powell BL, Gaidano G, Carella AM, Wei A, Hogge D, Craig AR, Fox JA, Ward R, Smith JA, Acton G, Mehta C, Stuart RK, Kantarjian HM. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukemia (VALOR): a randomized controlled double-blind international phase 3 study. Lancet Oncol. 2015;16:1025–1036. doi: 10.1016/S1470-2045(15)00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juliusson G, Lazarevic V, Hörstedt AS, Hagberg O, Höglund M. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119:3890–3899. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B, Bloomfield CD. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 25.Brandwein JM, Gupta V, Schuh AC, Schimmer AD, Yee K, Xu W, Messner HA, Lipton JH, Minden MD. Predictors of response to reinduction chemotherapy for patients with acute myeloid leukemia who do not achieve complete remission with frontline induction chemotherapy. Am J Hematol. 2007;83:54–58. doi: 10.1002/ajh.21034. [DOI] [PubMed] [Google Scholar]


