Abstract
Background/purpose
Alveolar bone deficiency is sometimes found in the patients who need dental implantation. This study used autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation for the patients with alveolar bone deficiency but requiring dental implantation.
Materials and methods
In this study, autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation was performed in four patients with different situations of alveolar bone deficiency. The titanium mesh was used as the barrier membrane and provided support to the compartment which was filled with calcium sulfate materials. Autogenous bone fragments harvested from adjacent implant osteotomy or from cortical bone of the recipient site were spread on the external surface of titanium mesh as the resources of osteoblasts for new bone formation.
Results
Four months after above-mentioned procedures, cone-beam computed tomography showed adequate alveolar bone formation. The titanium mesh was removed and dental implant was placed in the augmented alveolar ridge at the same time. We found that secondary bone graft combined with autogenous bone and inorganic bovine bone were covered by the pseudo-periosteum and suitable for dental implantation in our four patients. The implants were submerged for 3–4 months till uncovering, and then the prostheses were delivered one month afterwards with successful clinical outcomes.
Conclusion
The clinical outcomes of our four patients indicate that the vital autogenous bone grafts and the titanium mesh possess the ability to induce and guide new bone formation in four months and can be successful used for alveolar ridge augmentation and subsequent dental implantation.
Keywords: Alveolar ridge augmentation, Autograft, New bone formation, Dental implantation, Guided tissue regeneration
Introduction
Alveolar bone deficiency is a common problem encountered in implant dentistry. Some studies argued the necessity of bone graft materials in guided bone formation. Xenograft or allograft material actually does not have a better ability to induce alveolar bone formation than an empty control in the histological level.1 Less bone volume, more fibrous tissue, and foreign body reaction are the key microscopic findings around xenograft or allograft materials compared to an empty control, even though the clinical gross examination shows dense new hard tissue formation.2 Previous studies also showed no need of bone graft materials for new bone regeneration when a well-contained environment surrounded by a rigid membrane or in sinus lifting.3,4 Therefore, the necessity of bone graft materials should be reconsidered.
When alveolar ridge augmentation is indicated, autogenous bone graft is still considered to be the gold standard to maintain the space and to provide growth factors for stimulating new bone formation.5,6 The autogenous bone grafts provide not only the osteoinductive ability due to the production of growth factors but also the osteoconductive ability acting as the space fillers.
Beside osteoconduction and osteoinduction, the autogenous bone grafts contain osteoblast-like cells with the ability to proliferate and express bone cell markers. The osteoblast-like cells within the bone chips are well utilized in in-vitro study as the cell source for new bone formation and bone tissue engineering but few studies describe the usage of these osteoblastic-like cells directly in the clinical cases.
This case series described a new technique to apply the concepts of bone tissue engineering on the recipient site, to culture the osteoblast-like cells in the body, and to tailor the titanium mesh as the scaffold. The new bone formation could achieve adequate volume and density within four months of in-vivo culturing. Our results indicate that the autogenous bone grafts and titanium mesh-guided new bone formation can be successful used for alveolar ridge augmentation and subsequent dental implantation.
Materials and methods
Inclusion criteria and general requirement for the patients
All the procedures were done in a private clinic by the same dentist (M.D. Jeng) who finished the autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation, dental implantation, and final prosthesis delivery. The patients were included in this study when they had alveolar bone deficiency and required alveolar ridge augmentation before dental implantation, when they could sign informed consent for the bone grafting procedure and dental implantation therapy, and when their systemic condition was suitable for undergoing alveolar ridge augmentation and dental implantation surgeries in a dental clinic.
Clinical protocols for autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation
All patients received cone-beam computed tomography (CBCT) examinations prior to the bone grafting procedure. The treatment plans were conducted after CBCT evaluation and thorough discussion with the patients. When the alveolar ridge augmentation procedure was indicated, amoxicillin 500 mg, acetaminophen 500 mg, and celecoxib 200 mg were given to the patient one hour before the bone grafting procedure. Supra-crestal incision with intra-sulcular extension was made with vertical releasing incisions at the adjacent teeth. After a full-thickness flap elevated, autogenous bone grafts were collected from the apical cortical bone of the recipient sites or from the osteotomy of implant placement at the same time at a low speed drilling sequence that was less than 200 rpm. Calcium sulfate powder mixed with normal saline was packed on the bony defect which was further covered with titanium mesh fixed by titanium fixation screws. The autogenous bone chips were applied on the titanium mesh surface, mainly to seal the holes on the titanium mesh. Periosteal releasing was made and primary closure layer by layer was achieved with vertical mattress and interrupted sutures. Amoxicillin 500 mg and acetaminophen 500 mg four times per day plus celecoxib 200 mg QS were prescribed to the patient for 5 days.
After four months of autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation, the patients were scheduled for a second CBCT examination to re-evaluate the bone quantity and quality. Then, dental implant with appropriate dimensions (IDEOSS, Taipei, Taiwan) were chosen and placed in the augmented alveolar ridge at the second surgery. In cases when secondary bone grafting was needed, autogenous bone chips obtained from the dental implant osteotomy combined with inorganic bovine bone at 1:1 ratio were placed on the dental implant top and the wound was closed by suturing layer by layer including pseudo-periosteal and alveolar mucosal layers (Figure 1, Figure 2). After another three to four months of undisturbed healing, the implants were all osteointegrated, implants were uncovered, and fixed prosthesis was delivered.
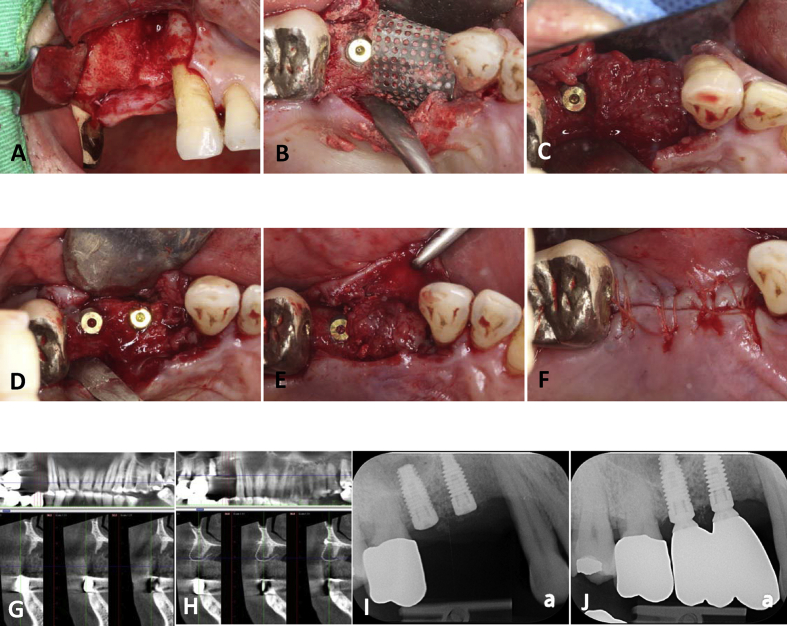
Figure 1.
The clinical and radiographic photographs of patient 1. (A) Buccal view of tooth 14 bone defect. (B) The bone defect was filled with calcium sulfate and then covered by the titanium mesh. A dental implant was inserted at tooth 15 position and the bone chips from the osteotomy were collected and packed on the surface of the titanium mesh. (C) The pseudo-periosteal membrane was found under the titanium mesh after four months of grafting. (D) Placement of tooth 14 implant. (E) Autogenous bone and bovine bone were mixed as secondary bone graft and the pseudo-periosteal layer was sutured back with absorbable stitches. (F) Primary closure of the alveolar mucosa without flap releasing. (G) Buccal bony defect of tooth 14 on pre-operative CT image. (H) CT image four months after grafting showing adequate bone volume for implant even though the bone density is not dense enough. (I) Periapical radiograph after tooth 14 implant was inserted. (J) Periapical radiograph taken six months after delivery of the prosthesis.
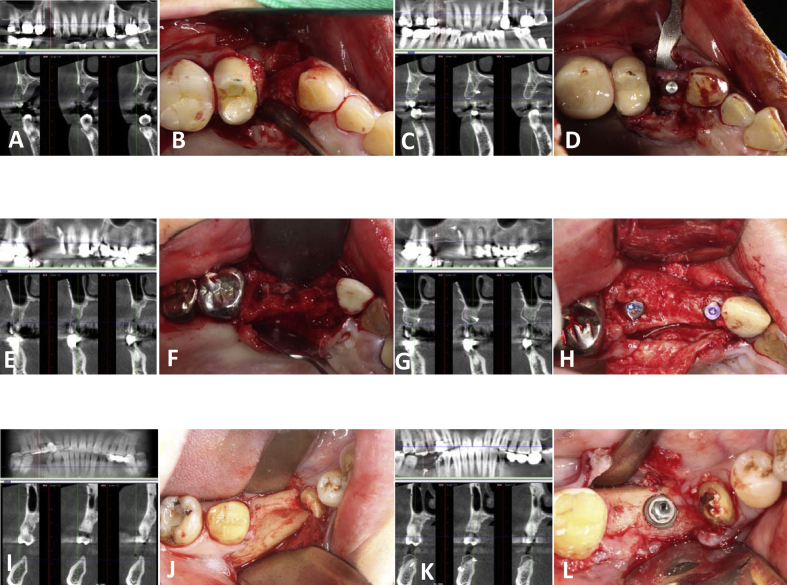
Figure 2.
Radiographic and clinical photographs of our three patients. (A, B, C and D) The bony defect and grafting result of tooth 14 implant in patient 2. (E, F, G and H) The bony defect and grafting results of teeth 13 and 15 implants in patient 3. (I, J, K and L) The bony defect and grafting result of tooth 46 implant in patient 4. Left column: pre-operative CT images. Middle left column: occlusal view of the bone defects. Middle right column: CT images after four-month bone healing. Right column: photographs of implant surgery.
To quantify the bone formation, the CBCT sections at the planned implant sites were further analyzed. The CBCT images before and after bone grafting were superimposed in different colors. The vertical bone gain measurement started from the most occlusal point of recipient bone to the most occlusal point of new bone formation. Horizontal bone gain was measured at the widest part of the new bone regeneration (Fig. 3). These measurements were based on the orientation of implant position and occlusal plane. All the image merging and measuring were done using the software “Image J”.
Figure 3.
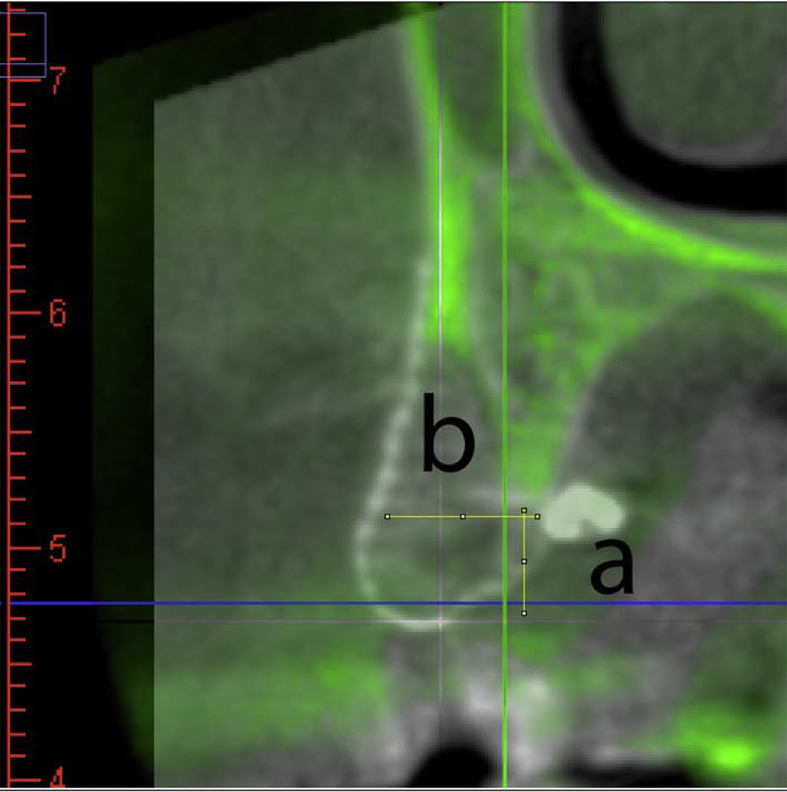
Superimposed images of pre-operative and post-operative CT. The pre-operative CT image was transformed into green shade and matched with the basal bone of the post-operative CT image. The measurement was set as the implant orientation and perpendicular to the occlusal plane. The vertical bone gain (a) was measured from the most coronal part of the original bone contour to the most coronal part of the new bone, and the horizontal bone gain (b) was measured at the widest part of the new bone formation. The horizontal and vertical bone gain results were recorded in Table 1.
Results
Patient 1
A 59-year-old male patient came for construction of fixed prostheses for the right maxillary first and second premolars (Table 1). Buccal alveolar bone deficiency of tooth 14 was noted in the CBCT examination taken three months after tooth extraction. As the alveolar ridge augmentation procedure was indicated and the patient was medicated, supra-crestal incision with intra-sulcular extension was made with a vertical releasing incision at the mesial side of tooth 13. After a full-thickness flap elevated, buccal dehiscence was found as CBCT predicted. Calcium sulfate powder mixed with normal saline was packed on the buccal bony defect which was further covered with titanium mesh fixed by two screws. Autogenous bone grafts collected from tooth 15 implant site were applied on the titanium mesh surface for sealing the holes on the titanium mesh. The wound was sutured as described. The treatment procedures are illustrated in Fig. 1.
Table 1.
Surgical sites treated with autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation for subsequent implant placement in our 4 patients.
| Patient number (Surgical site number) | Gender of patient | Age of patient | Tooth number | Horizontal bone gain | Vertical bone gain |
|---|---|---|---|---|---|
| 1 (1) | Male | 59 | 14 | 6.03 mm | −0.20 mma |
| 2 (2) | Female | 63 | 14 | 6.36 mm | 4.37 mm |
| 3 (3) | Male | 48 | 13 | 1.15 mm | 1.11 mm |
| 3 (4) | Male | 48 | 15 | 7.11 mm | −1.8 mma |
| 4 (5) | Male | 42 | 46 | 1.65 mm | −0.62 mma |
Negative values inferred bone remodeling.
After four months of uneventful healing, CBCT scan showed new bone formation underneath the titanium mesh. The interface between new bone and residual bone could be identified by the radiography due to the different densities of the bones. Although the bone density was not strong enough to look like the cortical bone, sufficient alveolar ridge bone volume was achieved for dental implantation. A second surgical intervention was made to remove the titanium mesh. The pseudo-periosteum under titanium mesh was elevated to the buccal side, a ø4 × 11 mm dental implant (IDEOSS) was placed using the lower speed drill (<200 rpm) to harvest autogenous bone particles. In spite of the low density in the CBCT evaluation, type II bone quantity7 was experienced in the clinical procedures during drilling sequence. Secondary bone grafts yielded from autogenous bone and inorganic bovine bone were packed on the top of tooth 14 implant. The pseudo-periosteal layer was sutured backed with absorbable chromic gut and the alveolar mucosa layer was closed with polyglycolic acid (PGA) stitches. After another four months of undisturbed healing, the implants were all osteointegrated, implants were uncovered and teeth 14 and 15 splinted fixed prosthesis was delivered. Radiographic image showed stable bone level after follow-up for six months (Fig. 1).
Patient 2
This 63-year-old female patient had insufficient alveolar bone found by CBCT three months after extraction of tooth 14 owing to the root fracture. The patient needed alveolar ridge augmentation for the alveolar defect of tooth 14. In brief, the mucosal flap was elevated after two vertical releasing incisions were performed at the distal ling angle of tooth 13 and mesial ling angle of tooth 15. Autogenous bone chips, harvested from apical cortical bone of tooth 15 area by a bone scrapping tool at a drilling speed of 200 rpm, were packed on the surface of the titanium mesh. With relief of the buccal flap, primary closure was achieved as described previously.
Four months after the autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation, CBCT re-evaluation showed bone gain at both width and height and thus a ø4 × 13 mm implant was placed after removal of the titanium mesh. The implant was uncovered after four months of implant placement and the implant supported prosthesis was cemented one month later. Periapical radiographies displayed steady bone level around tooth 14 implant up to 17 months after the prosthesis delivery. The clinical procedures and CT images are demonstrated in Fig. 2A, B, C and D.
Patient 3
This 48-year-old male patient visited our clinic for treatment of a loose fixed bridge from tooth 12 to tooth 15. The tooth 13 was missing and the teeth 14 and 15 were hopeless and thus were extracted subsequently. The tooth sockets were carefully curetted to allow the wound healing for six weeks. CBCT evaluation after oral mucosa and socket healing showed inadequate bone width at teeth 13 and 15 sites where were planned for dental implantation. The autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation was performed at tooth 13 to tooth 15 region as previously described.
Four months after the autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation, abundant bone formation and nice bone contour were unveiled after removal of the titanium mesh. Two implants at teeth 13 and 15 were inserted with stage approach (Fig. 2E, F, G and H). The second stage of implant uncovering was performed three months later. The final prosthesis of tooth 12 crown and teeth 13 to 15 fixed implant-supported prosthesis were delivered within ten months after teeth 14 and 15 were extracted. The patient completed the 12-month recall without any untoward complications.
Patient 4
This 42-year-old male patient suffered from tooth 45 pain, which was an abutment of a bridge from tooth 45 to tooth 47. The tooth 45 was indicated for root canal therapy. Subsequent to the removal of the fixed prosthesis, the endodontic treatment of tooth 45 was performed. After a thorough discussion, the patient preferred to have an individual prosthesis on tooth 45, 46, or 47 rather than a splinted fixed prosthesis for the three teeth. However, inadequate alveolar ridge bone volume for tooth 46 implant was perceived in CBCT. Thus, the patient was treated by the procedures of autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation as those described for the patients 1, 2 and 3. Unfortunately, early titanium mesh membrane exposure confronted the oral environment on the third week post-surgery. No attempt was made to cover the exposed mesh, nor special care was given to the patient except that oral hygiene maintenance was re-enforced.
Although the patient had the disaster of early titanium mesh membrane exposure and no special care was given to the patient, no active infection was found at the exposure site. Thus, even under the worse circumstance of early wound opening, there was still some new bone formation just enough for a ø5 × 9.5 mm implant placement shown on CBCT four months later (Fig. 2I, J, K and L). The prostheses on tooth 46 implant and 45 and 47 natural teeth were finished three months after the implant surgery. Stable bone around tooth 46 implant was revealed by periapical radiography at the 9-month follow-up.
Quantification of autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation
The grafted sites all showed perfect results. The horizontal bone gain ranged from 1.15 mm to 7.11 mm and the bone height could be increased up to 4.73 mm. Some clinical insignificant marginal bone remodeling was observed without interfering implant placement (Table 1). All the patients displayed stable bone level by periapical radiography throughout the follow-up periods.
Discussion
This new autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation technique could be considered as bone tissue engineering in situ. It required osteoblast-like cells derived from the collected bone chips and residual bone structures for new bone formation, in which the titanium mesh acted as the scaffold, the calcium sulfate acted as the culture dish, and blood supply acted as the culture medium for the osteoblast-like cells.
According to studies by Robey and Termine7 and by Sodek and Berkman,8 the bone chips collected from the cortical bone, eliminated any soft tissue parts, stored in an isotonic solution to maintain the vitality of the cells, and cultured in a dish for several days still possessed the ability to differentiate into osteoblast-like cells which could express bone cell markers such as alkaline phosphate and osteocalcin. These cells were commonly cultured for in-vitro studies and could be utilized in researches on bone tissue engineering.
The vitality of the osteoblast-like cells was largely influenced by the methods to collect them. Studies showed bone chips acquired by hand instrument or by low speed drilling (bellow 200 rpm without irrigation) contained higher cell vitality and activity than those collected by drill sludge at a standard implant drilling procedure (>800 rpm with irrigation) or by piezosurgry.8,9 The cells in the former bone chips also expressed higher amount of growth factors including bone morphogenesis protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) and better mineralization after differentiating medium induction.10 Therefore, a bone scraper or low speed drilling sequence was essential for bone harvesting in the new autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation technique for osteoblast-forced new bone formation.
In the present technique, primary closure was critical to keep the graft bone chips in a vital condition. Rigid titanium mesh membrane and solid fixation were the two important keys for the new bone formation. The undesired cells should be separated away from the compartment. Osteoblasts or osteoprogenitor cells can only be drafted from cortical bone to eliminate possible influence by other kinds of cells such as those derived from adipose or connective tissues. For obtaining the proper blood supply, decortication is indicated in the mandibular alveolar ridge with dense cortical plate receiving alveolar ridge augmentation. However, maxillary alveolar ridge is porous to provide blood supply and decortication sometimes is not required.
The firm structure of titanium-mesh as the scaffold and barrier membrane were well documented in the literature and screw fixation was mandatory to gain stability of the structure.11, 12, 13, 14 According to the study by Degidi et al., titanium mesh is helpful in maintaining space with a large defect,15 and the titanium mesh has generally been regarded to be highly biocompatible. The holes on the titanium mesh allowed for the blood supply from the flaps to the surgical site, in contrary to other types of membrane that may block the blood circulation to the surgical site.
In conclusion, the new autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation technique only requires small amount of autogenous bone grafts that can be easily harvested from adjacent bone. Admittedly, this skill results in fast bone ingrowth and creates an augmented solid alveolar bone ridge allowing for implant placement within four months of healing.
Declaration of Competing Interest
Ming-Dih Jeng is the shareholder of IDEOSS Biotech Inc., Taipei, Taiwan.
References
- 1.Stavropoulos A., Kostopoulos L., Nyengaard J.R., Karring T. Deproteinized bovine bone (Bio-Oss) and bioactive glass (Biogran) arrest bone formation when used as an adjunct to guided tissue regeneration (GTR): an experimental study in the rat. J Clin Periodontol. 2003;30:636–643. doi: 10.1034/j.1600-051x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 2.Stavropoulos A., Chiantella G., Costa D., Steigmann M., Windisch P., Sculean A. Clinical and histologic evaluation of a granular bovine bone biomaterial used as an adjunct to GTR with a bioresorbable bovine pericardium collagen membrane in the treatment of intrabony defects. J Periodontol. 2011;82:462–470. doi: 10.1902/jop.2010.100331. [DOI] [PubMed] [Google Scholar]
- 3.Chen T.W., Chang H.S., Leung K.W., Lai Y.L., Kao S.Y. Implant placement immediately after the lateral approach of the trap door window procedure to create a maxillary sinus lift without bone grafting: a 2-year retrospective evaluation of 47 implants in 33 patients. J Oral Maxillofac Surg. 2007;65:2324–2328. doi: 10.1016/j.joms.2007.06.649. [DOI] [PubMed] [Google Scholar]
- 4.Linde A., Thoren C., Dahlin C., Sandberg E. Creation of new bone by an osteopromotive membrane technique: an experimental study in rats. J Oral Maxillofac Surg. 1993;51:892–897. doi: 10.1016/s0278-2391(10)80111-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Tabassum A., Wu G., Deng L., Wismeijer D., Liu Y. Bone regeneration in critical-sized bone defect enhanced by introducing osteoinductivity to biphasic calcium phosphate granules. Clin Oral Implants Res. 2017;28:251–260. doi: 10.1111/clr.12791. [DOI] [PubMed] [Google Scholar]
- 6.Leong D.J., Oh T.J., Benavides E., Al-Hezaimi K., Misch C.E., Wang H.L. Comparison between sandwich bone augmentation and allogenic block graft for vertical ridge augmentation in the posterior mandible. Implant Dent. 2015;24:4–12. doi: 10.1097/ID.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 7.Lekholm U., Zarb G.A., Albrektsson T. Quintessence; Chicago: 1985. Tissue-integrated prostheses: osseointegration in clinical dentistry. [Google Scholar]
- 8.Tabassum A., Wismeijer D., Hogervorst J., Tahmaseb A. Comparison of proliferation and differentiation of human osteoblast-like cells harvested during implant osteotomy preparation using two different drilling protocols. Int J Oral Maxillofac Implants. 2020;35:141–149. doi: 10.11607/jomi.7648. [DOI] [PubMed] [Google Scholar]
- 9.Springer I.N., Terheyden H., Geiss S., Harle F., Hedderich J., Acil Y. Particulated bone grafts-effectiveness of bone cell supply. Clin Oral Implants Res. 2004;15:205–212. doi: 10.1111/j.1600-0501.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 10.Miron R.J., Gruber R., Hedbom E. Impact of bone harvesting techniques on cell viability and the release of growth factors of autografts. Clin Implant Dent Relat Res. 2013;15:481–489. doi: 10.1111/j.1708-8208.2012.00440.x. [DOI] [PubMed] [Google Scholar]
- 11.von Arx T., Kurt B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh: a prospective clinical study with 20 implants. Clin Oral Implants Res. 1999;10:24–33. doi: 10.1034/j.1600-0501.1999.100104.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan H.L., Benavides E., Tsai C.Y., Wang H.L. A Titanium mesh and particulate allograft for vertical ridge augmentation in the posterior mandible: a pilot study. Int J Periodontics Restor Dent. 2015;35:515–522. doi: 10.11607/prd.1980. [DOI] [PubMed] [Google Scholar]
- 13.Yamada H., Nakaoka K., Horiuchi T. Mandibular reconstruction using custom-made titanium mesh tray and particulate cancellous bone and marrow harvested from bilateral posterior ilia. J Plastic Surg hand Surg. 2014;48:183–190. doi: 10.3109/2000656X.2013.848809. [DOI] [PubMed] [Google Scholar]
- 14.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 15.Degidi M., Scarano A., Piattelli A. Regeneration of the alveolar crest using titanium micromesh with autologous bone and a resorbable membrane. J Oral Implantol. 2003;29:86–90. doi: 10.1563/1548-1336(2003)029<0086:ROTACU>2.3.CO;2. [DOI] [PubMed] [Google Scholar]