Abstract
Background/purpose
Maxillary sinus lift without grafting is an alternative procedure that is used to lower the risk of infection and facilitate the surgical procedure. The objective of this study was to evaluate the tenting effect of the dental implant by measuring the amount and morphology of bone formation around it.
Material and methods
49 implants were placed in 26 patients by maxillary sinus lift without grafting. Radiographic images were taken preoperatively and at 6 months postoperatively and used to evaluate the height of the residual bone, the width of the maxillary sinus, the amount of bone formation, and the adjacent tooth.
Results
The most common type of bone formed around the implant, as seen in 23 cases, was the same height as the apex of the implant; in 11 cases, it was 0–2 mm above the apex of the implant, and in 7 cases, 2 mm or more. Meanwhile, 5 cases showed defects. The tent type of bone formation, which showed more bone formation at the implant apex than in the surrounding bone, was overwhelmingly the most common. (80.4%) The amount of bone formation increased in proportion to the difference between the residual bone height and the implant length. (P < .001).
Conclusion
The amount of bone formation in the sinus lift without grafting increased in proportion to the length of the implants in the maxillary sinus due to the tenting effect of the implant in the maxillary sinus membrane.
Keywords: Dental implant, Bone formation, Maxillary sinus augmentation, Sinus lift
Introduction
Pneumatization of the maxillary sinus and alveolar bone resorption after extraction of maxillary posterior teeth result in horizontal and vertical bone resorption. The atrophic posterior maxilla is a challenging site for placement of dental implants. A variety of surgical techniques have been developed to reconstruct the posterior maxilla when bone volume is insufficient. Sinus lift with bone graft has been considered to be the best option, the conventional procedure nowadays being a lateral approach with bone graft along with immediate or delayed implantation.1, 2, 3, 4, 5, 6
Autogenous bone is the most well established material used to fill the area of the lifted maxillary sinus. Indeed, with its osteogenic, osteoinductive and osteoconductive characteristics, it is the gold standard in alveolar bone reconstruction.7, 8, 9 However, in view of some of the disadvantages and systemic limitations, such as the need for a second surgical site and post-operative morbidity, diverse bone substitutes have been developed, among which are materials of homogeneous, heterogeneous and alloplastic origin.9, 10, 11 These materials have the limitations of having only osteoconductive properties and of possibly transmitting diseases and contamination.12 Improvements in graft materials and technically demanding procedures are necessary.
The possibility of new bone formation with only maxillary sinus membrane elevation has been reported in human and animal studies.13, 14, 15 In 2003, Lundgren et al. reported spontaneous bone formation in the maxillary sinus three months after extirpating an intrasinusal cyst, having had to raise the sinus membrane to stitch.16 In 2006, Palma et al. found, after carrying out experimental studies on goats, that the amount of bony tissue increase post-elevation of the maxillary sinus, either with or without autogenous grafting, was similar after 6 months of healing.14 In 2007, Thor et al. placed implants in the sinus without grafting, arguing that the implants’ titanium surface provided sufficient thrombogenicity in activating the coagulation system and platelets and stimulating cell and bone growth thereby.15 Some studies have indicated that implant placement in to the sinus without graft materials can stimulate new bone formation in the sinus cavity.16 Specifically, blood cells induce the new bone formation by stimulating the bone precursor cells to evolve to osteoclasts, the activated osteoclasts activating, in turn, other, bone-forming osteoclasts that begin producing bone.17
With the sinus lift procedure, immediate implantation without grafting is possible, provided that primary stabilization in the residual ridge is first achieved. The implant affords a vertical limit to the upper position of the elevated maxillary sinus membrane, while the space is maintained by the formation of a blood clot.18 Then, spontaneous bone formation occurs in this space. Moreover, because bone grafts are not used, complications such as infection can be reduced.
On the other hand, when bone graft material is not used, the space-maintenance ability by blood-clot formation alone might be diminished. Thus, when bone graft is not used, it is necessary to study the amount and morphology of bone formation, especially as they relate to implant length; and it is also necessary to evaluate the morphology of the maxillary sinus.
The aim of the present study was to investigate the clinical and radiographic results of new bone formation after membrane elevation in the maxillary sinus and simultaneous placement of dental implants without additional bone graft materials. Cone-beam computerized tomography (CBCT) was obtained at post-operative 6 months for analysis of linear bone height measurements and bone morphology.
Materials and methods
Patients and pre-surgical evaluation
We retrospectively evaluated 26 patients (11 women and 15 men, with a mean age of 52) who had been treated at the Department of Oral and Maxillofacial Surgery at Dong-A University Hospital for implant rehabilitation and maxillary sinus floor grafting between February 2012 and February 2017. A total of 49 dental implants were placed. The present study's protocol was reviewed and approved by the Institutional Review Board of Dong-A University Hospital (IRB No. 19–032).
Preoperative panoramic radiographs and CBCT were taken to evaluate residual bone height and sinus pathosis. None of the patients had significant sinus pathosis. The residual bone height of the edentulous site for implant placement ranged from 1.3 to 9.2 mm (mean, 6.1 mm).
Inclusion criteria
The following inclusion criteria were applied
(a) Patients who were more than 18 years old., (b) Patients who had a lateral approach of maxillary sinus lift without grafting with simultaneous implant placement., (c) Patients with implant placement between 4 and 5 mm in diameter and between 10 and 13 mm in length., (d) Patients who were understand and sign the informed consent form and compliant with supportive maintenance therapy after surgical procedures.
Exclusion criteria
The following exclusion criteria were applied
(a) Patients with active infection or disease affecting bone and wound healing., (b) Patients who had maxillary sinusitis or pathosis., (c) Patients without the use of other bone augmentation techniques. (e.g., guided bone regeneration), (d) Patients who were administered prescription medications that could affect bone metabolism, such as steroids, bisphosphonates, and agents for rheumatism. (e.g., immunosuppressive agents), (e) Patients with a history of head or neck radiation therapy., (f) Patients who were pregnant.
Surgical procedure
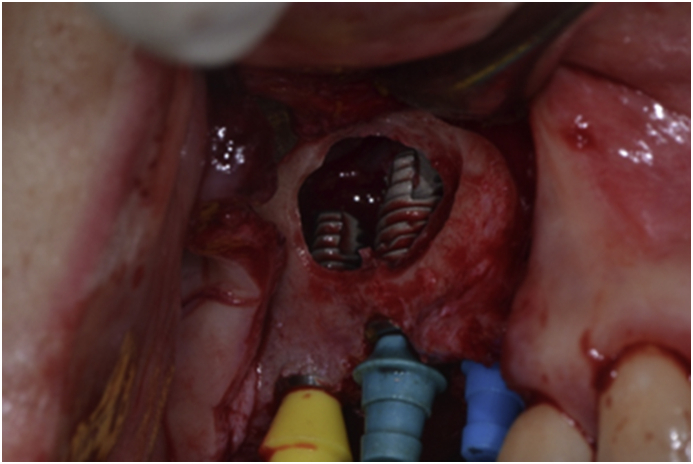
The operation was carried out with the patient under local anesthesia (2% LidoHCl with 1:100,000 epinephrine). The perioral areas were aseptically prepared. A crestal incision was made on the midline of the gingiva attached to the edentulous ridge. The flap was elevated carefully and extended labially to expose the bone. A mesial and distal vertical releasing incision was made as needed. The mucosal flap was denuded subperiosteally to fully expose the sharp and thin alveolar ridge and the lateral wall of the maxillary sinus. Extreme care was taken to radically elevate the sinus membrane from the lateral access window opened by using an electric-motor drill with appropriate water cooling. The floor, lateral wall, medial and posterior wall of the sinus membrane were meticulously detached and pushed upward to allow for the placement of dental implants into the bone chamber. The implant fixture was positioned from the crestal bone and extended into the space, with primary stabilization provided by the retained alveolar bone (Fig. 1). Two submerged implant systems (Zimmer; Zimmer Dental Inc., Carsbad, CA, USA, and Dentis; Dentis Dental Inc., Daegu, South Korea) were used in patients with focal edentulous areas. Instead of placing autogenous bone or allogenic bone substitute into the sinus space as fillers, we tented the elevated sinus membrane by using the fixture to maintain the elevated sinus space. The incision line was sutured with 4–0 black silk. After surgery, the patient received cephalosporins antibiotics, non-steroidal anti-inflammatory drugs, and .1% chlorhexidine for 5 days. The sutures were removed 7 days after surgery. The surgery was performed according to a two-stage procedure. Six-to-eight months after the initial surgery, the second-stage operation was carried out to expose the implanted fixtures. A labially positioned palatal flap was used to ensure sufficient keratinized gingiva at the buccal side of the fixture. A minimum of 1 month was required for healing of the flap and peri-implant tissues. The prosthetic procedures were started at 7–9 months and with initial force loading at about 10 months after sinus-lifting implant surgery.
Figure 1.
Clinical aspects of maxillary sinus lift and implant placement procedure.
Post-surgical evaluation
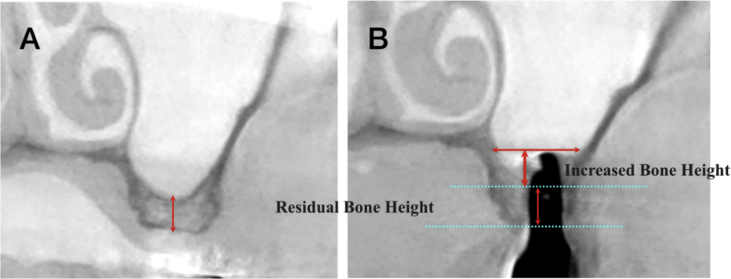
At the second stage of surgery, post-surgical CBCT was used to assess the lifted bone height, and the morphology was evaluated at the same time (Fig. 2). The outcome of the dental implantation was defined as “survival” when the prosthesis had been delivered and followed without infection or pain or more than 1.5 mm of peri-implant bone loss over the course of 12 months post-loading. The preoperative and post-operative CBCT cross-sections of the implant position were measured, as was the regenerated bone gained from the sinus elevation procedure between the primary cortical floor and the lifted sinus wall. In order to compare the degree of bone formation according to the morphology of the maxillary sinus, the mesial-to-lateral diameters of the maxillary sinus at the implant apex were measured (Fig. 3). Additionally, the anteroposterior shapes of the maxillary sinus were identified with reference to the adjacent teeth; also, the bone height of the implant apex and the bone morphology around the implant were investigated by CBCT (Fig. 4).
Figure 2.
Coronal images on CBCT scans. (A) Preoperative radiograph, (B) Radiograph 6 months after surgery.
Figure 3.
Schematic representation of investigated parameters. A: residual bone height, B: width of maxillary sinus, C: length of implant protrusion, D: bone height relative to implant apex, E: range of increase of bone height. Among these parameters, A and B were analyzed on preoperative CBCT.
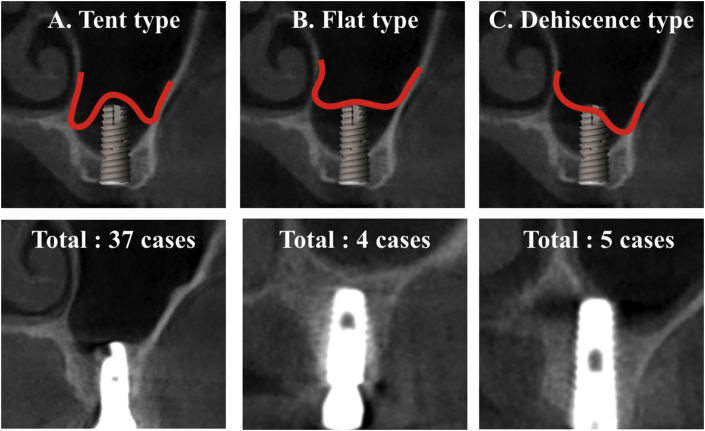
Figure 4.
Morphology of bone formation relative to implant apex. (A) Tent type, bone formation being higher at implant apex than in surrounding bone, (B) Flat type, bone formation being same or lower at implant apex than in surrounding bone, (C) Dehiscence type, presence of bone defect.
Statistical analysis
All data were analyzed using SPSS Win 22.0 (SPSS Inc., Chicago, IL). Descriptive statistics such as mean, standard deviation and range (minimum, maximum) were provided for all groups and outcome parameters. The correlations between height of bone formation relative to implant apex and type of morphology of bone formation relative to implant apex and parameters were assessed using the Pearson's Correlation Test. The correlations between each parameter (increase of bone height, residual bone height, width of maxillary sinus, increase rate, adjacent teeth) was assessed using the Pearson's Correlation Test. In order to evaluate the amount of bone formation according to the residual bone height, divided into two groups based on 4 mm, and the bone formation according to gap between the inserted implant length and the residual bone height was assessed using the Pearson's Correlation Test. The level of statistically significant differences was set at P < 0.05 for the analysis.
Results
A total of 49 implants ranging from 4.1 to 4.8 mm in diameter (mean, 4.68 mm) and 10–13 mm in length (mean, 11.26 mm) were placed in the first premolar (1), second premolar (7), first molar (23), and second molar (18) areas. All of the implants were placed according to the 2-stage system. No patients developed sinusitis or infection. However, three implants were removed due to failed osseointegration. The initial torque values in these cases were 5, 11, 13 N/cm. The remaining 46 implants healed well; no infection or implant mobility was observed on initiation of loading force from the prosthetic components. The implant survival rate was 93.5% (Table 1).
Table 1.
Clinical features of 46 implants in 26 patients.
| Average age (yr) | 51 |
| Gender | |
| Men | 15 |
| Women | 11 |
| Classification of implant location | |
| First premolar | 1 |
| Second premolar | 7 |
| First molar | 23 |
| Second molar | 18 |
| Length of fixture (mm) | |
| 10 | 17 |
| 11.5 | 18 |
| 12 | 13 |
| 13 | 1 |
| Diameter of fixture (mm) | |
| 4.1 | 1 |
| 4.3 | 6 |
| 4.7 | 18 |
| 4.8 | 24 |
| Residual bone height on pre-surgical CBCT (mm) | 1.3–9.2 |
| Range of increase of bone height (mm) | 2.2–11.7 |
| Average increase of bone level in post-surgical CBCT (mm) | 6 |
| Survival rate of implants (%) | 93.5 |
The height of the primary edentulous ridge below the sinus floor ranged from 1.3 to 9.2 mm (mean, 5.9 mm). The increases in lifted sinus bone height ranged from 2.2 to 11.7 mm with an average of 6 mm. The bone height above the implant apex ranged from 0 to 6 mm (mean, .96 mm). Among the implants, 23 were at the same level of the implant apex and lifted sinus bone apex. The bone height above the implant apex was 0–2 mm in 11 implants, and more than 2 mm in 7 implants. Five (5) implants showed bone dehiscence in the palatal area (4) or buccal area (1) ranging from 1.3 to 4 mm (mean, 2.66 mm) (Table 2). The morphologies of bone formation based on the implant apex were as follows: 37 cases of the tent type (bone formation is higher at implant apex than in surrounding bone), 4 cases of the flat type (bone formation is same or lower at implant apex than in surrounding bone), and 5 cases of the dehiscence type (presence of bone defect) (Table 3).
Table 2.
Height of bone formation relative to implant apex.
| Bone height (mm) | Number of implants |
|---|---|
| 0 | 23 |
| 0–2 | 11 |
| >2 | 7 |
| <0 | 5 |
Table 3.
Morphology of bone formation relative to implant apex.
| Type | Number of implants |
|---|---|
| Tent type | 37 |
| Flat type | 4 |
| Dehiscence type | 5 |
The correlations between the parameters were investigated. The smaller the amount of residual bone height, the higher the increase of bone height and the higher the increase rate, with statistical significance. The mesial-to-lateral diameters of the maxillary sinus at the implant apex ranged from 10.1 to 23.5 mm (mean, 15.1 mm). In 11 cases, the adjacent teeth were anterior and posterior to the implant; in 16 cases, only anterior; in 19 cases, free-end. On the other hand, neither sinus width nor adjacent teeth are important considerations in sinus lift, and in any case, the data were not statistically significant (Table 4).
Table 4.
Correlations between investigated parameters. IBH: increase of bone height, RBH: residual bone height, WMS: width of maxillary sinus, IR: increase rate, AT(M): adjacent teeth (mesial), AT(D): adjacent teeth (distal).
| IBH | RBH | WMS | IR | AT(M) | AT(D) | |
|---|---|---|---|---|---|---|
| IBH | 1 | |||||
| RBH | -.790∗∗ | 1 | ||||
| WMS | .173 | -.372∗ | 1 | |||
| IR | .816∗∗ | -.672∗∗ | .029 | 1 | ||
| AT(M) | -.106 | .215 | .290 | -.183 | 1 | |
| AT(D) | -.013 | .039 | .056 | -.014 | .363∗∗ | 1 |
Pearson's Correlation Test, ∗P < .005, ∗∗P < .001.
We divided the 46 implants into 2 groups according to residual bone height. Group 1 (< 4 mm) gained more regenerated bone than did group 2 (> 4 mm). This difference was statistically significant (Table 5).
Table 5.
Relationship between residual bone height and gained bone height.
| Residual bone height (mm) | Number of implants | Av. bone height (mm) | Gained bone height (mm) |
|---|---|---|---|
| Group 1 (<4) | 8 | 2.9 | 9.4 |
| Group 2 (>4) | 38 | 5.9 | 5.9 |
Pearson's Correlation Test, P < .001.
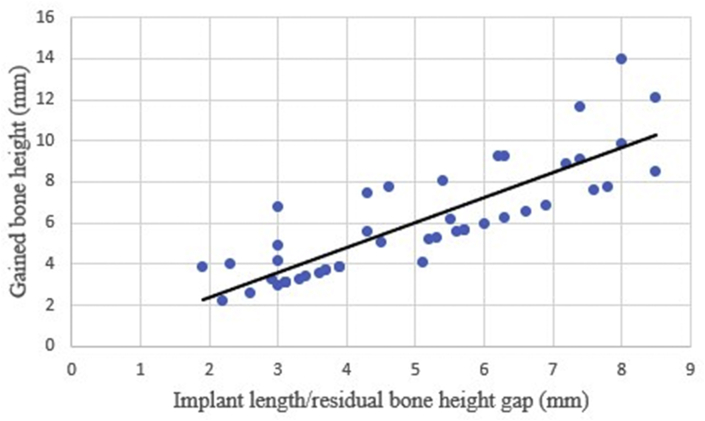
We also found that the gap between the inserted implant length and the residual bone height was related to the gained bone height. The higher the gap between the inserted implant length and the residual bone height was, the more bone height was gained (Fig. 5). Especially, when the implant length was more than twice the residual bone height (implant length-to-residual bone height ratio: over 200%), the gained bone height was significantly high. This also was statistically significant (Table 6).
Figure 5.
Relationship of implant length/residual bone height gap to gained bone height.
Table 6.
Relationship between Implant length-to-residual bone height ratio and gained bone height. IL: implant length, BH: bone height.
| IL /residual BH ∗ 100 (%) | Number of implants | IL /residual BH ∗ 100 (%) | Gained BH (mm) |
|---|---|---|---|
| <200 | 28 | 152 | 4.3 |
| >200 | 18 | 312 | 8.5 |
Pearson's Correlation Test, P = .020.
Discussion
Maxillary sinus lift by a lateral approach has been the most widely used technique in cases where an increase in bone height in the maxillary posterior region is required for placement of implants.1, 2, 3, 4,6 Currently there is general agreement on the efficacy of either autogenous bone grafts or allogeneic bone substitutes in maxillary sinus lift to enable increased height of bone extending into the maxillary sinus.
Various theories have been put forth to explain the bone formation that occurs without the use of bone graft material. The findings of Lindhe et al. are consistent with bone regeneration after creation and maintenance of an isolated space between the periosteum and the calvarial cortex.19 It is conceivable that formation of new bone in the maxillary sinus does not require the presence of various grafts as scaffolds. An alternative mechanism is as follows: maintenance of space for blood clot formation, followed by resorption and deposition of bone cells derived from the sinus periosteum or peripheral cancellous marrow in the maxilla. Srouji et al. showed that cells derived from the sinus membrane can grow in culture expressing osteoprogenitor cell markers and that osteogenic differentiation can be induced along with new bone formation in the transplant area.20 This is evidence of the presence of osteoprogenitor cells within the maxillary sinus membrane. Important factors in this process are the elevation of the membrane and the exposure of the medial sinus wall, because mesenchymal cells migrate from the exposed sinus wall. Since bone formation requires the recruitment, migration and differentiation of pluripotent mesenchymal cells into osteoblasts, the periosteum of the sinus membrane is another possible source of bone-forming cells.21
In this study, results similar to those of the above-noted studies were obtained. The present study of 49 consecutive sinus augmentations with only membrane elevation and simultaneous implant placement showed new bone formation in the maxillary sinus on radiographic evaluation. Follow-up CBCT after 6 months showed a newly developed sinus floor. This was the result of spontaneous osteogenesis occurring via osteogenic factors including the elevated sinus membrane and surrounding sinus bone. Even in the three cases of implant removal due to failure of osseointegration consequent upon failure to obtain primary stabilization, bone formation in the maxillary sinus could be confirmed.
Insertion of implants at the same time as elevation and sinus grafting is a widely used technique that is well documented clinically. Blomquist et al. pointed out the advantage of this technique in minimizing both costs and surgery time, as well as the fact that the loading can be carried out beforehand, thus allowing for maintenance of the graft.22 Many studies have indicated that there are no clinical or histological differences between immediate placement of the implant after maxillary sinus elevation and delayed insertion.23, 24, 25 However, primary stabilization with no implant motion in the residual bone should be obtained. Implant placement requires a minimum bone height of 3 mm to ensure the primary stability of the implant, which in turn guarantees the success of the treatment.26 In this study, the heights of residual bone in the three cases of implantation failure were 3.2, 1.3, and 6.2 mm, and the initial torque values were 13, 11, and 5 N/cm, respectively. The height of residual bone in the cases of the remaining, successful implants was more than 3 mm, except for one case in which the height was 1.5 mm.
Maxillary sinus lift without grafting offers a series of advantages over the conventional grafting technique: it needs no graft material; a second surgical donor site is not necessary (in the case of autologous bone); there is less morbidity (again in the case of autologous bone); a lower infection risk; no risk of failure of the graft material; it is simpler, and, not least, there is greater patient cooperation and acceptance when no filler is inserted. None of the patients in this study showed any infection such as sinusitis or infection. Infection is the main cause of failure of sinus lift, causing bone graft loss when bone graft material is used.
Maintaining the integrity of the maxillary sinus membrane, making sure it stays raised for there to be enough space to build bone, and forming a blood clot, are the prerequisites for bone formation in cases of maxillary sinus lift without grafting. The space is maintained thanks to the primary stability of the implant that is sustained in turn by the membrane, thereby creating a limit and enabling subsequent formation of a blood clot. Palma et al. reported that the amount of augmented bone in the maxillary sinus after sinus membrane elevation with or without adjunctive autogenous bone grafts did not differ after 6 months of healing, and that new bone was frequently deposited in contact with the maxillary sinus membrane at coagulum-alone sites, thus indicating the osteoinductive potential of the membrane.14 In the case of bone grafting into the sinus, bone graft materials play the role of filler in providing space within the sinus; however, in the present study, the dental implant and a blood clot under the sinus membrane acted as space maintainers for new bone formation in the maxillary sinus. Significant new bone formation around the implant placed in the sinus was found in all cases, as was a new sinus floor. According to the results of this study, though, the amount of bone formation was determined according to the length of the implant in the maxillary sinus, which fact resulted in only 14 of 46 implants showing bone formation above the apex. And of those 14 cases, only 7 showed bone formation more than 2 mm above the apex of the implant. The tent type, which showed more bone formation at the implant apex than in the surrounding bone, was overwhelmingly high (37/46, 80.4%). The longer the length of the implants into the maxillary sinus, the more bone formation was observed. These results indicate that initially, space maintenance is performed by the implant and the blood clot, but that as the blood clot is absorbed, the space is finally retained by the implant. Therefore, primary stabilization of implants by residual bone is essential. The three implantation failures in this study was due to the failure to acquire initial stability in the residual bone, though bone increase was nonetheless achieved.
Haas et al. conducted an interesting evaluation of the visual behavior of implants placed in the maxillary sinus without grafting and with autogenous, heterogeneous and homogeneous bone graft material.27,28 Total membrane collapse onto the implant was observed at the sites without grafting, whereas no such collapse was noted when the autogenous graft had been placed at the almost apical level of the implant and the other biomaterials were 2–4 mm over the apical limit of the implant. In terms of new bone formation in the four groups, the highest level was observed at the sites with the autogenous bone graft, followed by the graftless sites. The latter exceeded more than 10% of the bone formation of the sites grafted with biomaterial, which fact demonstrates that maintenance with a blood clot alone would aid in the formation of more bone tissue than would the use of some intrasinus fillings. Among the mechanical load measurements, there were no significant differences. Thus, in summary, there is no problem in terms of bone formation or bone quality when bone graft material is not used in the maxillary sinus, but space maintenance problems can occur. In the present study, bone defects were found around 5 implants (5/46, 10.9%), 4 of which were in the palatal region and 1 in the buccal region. The cause of this phenomenon was probably that air pressure in the maxillary sinus caused the maxillary sinus membrane to fall onto the implant, thereby inhibiting the stability of the blood clot in the created space in the maxillary sinus. The reason that bone defect occurs mainly in the palatal region (present study: 4/5, 80%) is the lack of dissection of the medial wall membrane of the sinus. However, among the current results, there were no bone defects on both sides (only on one side), and the defects were not severe enough to cause functional problems of the implant (mean, 2.66 mm).
The role of the walls of the adjacent bone in the intrasinus cavity has been poorly analyzed in previous studies; when the residual alveolar bone for implant placement is at least 3 mm height and there are adjacent teeth, there must be well defined superior and inferior cortical and cancellous bone to permit adequate blood flow in the area. Thor et al. immediately placed implants and performed maxillary sinus lift without grafting in areas with these characteristics, with the result that the cavity-type defect encountered behaved similarly to a three-wall defect.15,29 The size of the sinus cavity likely is another important factor. De Moraes et al. established the need for cavity reconstruction of up to 2 cm3 in cases of highly pneumatized maxillary sinuses, and determined that large sinus cavities are considered critical defects; indeed, in such cases, the blood clot is incapable of contributing to bone regeneration.30,31 An extensive volume of the maxillary sinus (high pneumatization) shows more cortical bone (less vascularization) than cancellous bone on the buccal side; the periosteum and cancellous bone that remain in the maxillary sinus contain smaller numbers of osteoprogenitor cells, and thus lessen the capacity for new bone formation.32 In the present study, the presence of adjacent teeth was not statistically significant, though it was expected that the amount of bone formation would increase due to the improved blood supply. To determine the degree of bone formation according to the volume of the maxillary sinus, the mesial-to-lateral diameter of the maxillary sinus was measured, but was not statistically relevant. These results indicate that implant placement during the same surgery can ensure that the size of the defect is smaller and that the stabilized blood clot contributes to bone-tissue formation. It appears that for this reason, the influence of the adjacent teeth and the volume of the maxillary sinus are reduced.
In this study, the amount of bone formation in sinus lift without grafting increased in proportion to the length of the implants in the maxillary sinus. Therefore, longer-length implants are needed to obtain more bone formation. Half of the implants (23/46, 50%) obtained bone formation as long as the implant length, and only 7 implants (7/46, 15.2%) obtained bone formation of more than 2 mm above the implant. There were 5 implants (5/46, 10.9%) with bone defect around the implant. There were no statistically significant differences in the degree of bone formation among the initial residual bone height, the presence or absence of adjacent teeth, and the size of the maxillary sinus. Bone formation was obtained with no problem incurred to implant function, and there were complications such as infection. Maxillary sinus lift without bone graft reduces post-surgical infections and simplifies the procedure, which facts are beneficial to both the surgeon and the patient; moreover, it allows for sufficient bone formation due to the tenting effect of the implant in the maxillary sinus membrane. However, during the long follow-up period, bone changes after implant function as well as bone-quality analysis are needed. For more definitive evaluation of the bone formation process and support of our radiographic results, improved histologic and histomorphometric analyses with larger sample sizes are required. Furthermore, studies assessing new techniques and materials that can maintain the membrane's elevation in a superior position are called for.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study received no specific grant from any funding agency.
References
- 1.Esposito M., Grusovin M.G., Worthington H.V., Coulthard P. Interventions for replacing missing teeth: bone augmentation techniques for dental implants treatment. Cochrane Database Syst Rev. 2006;1:CD003607. doi: 10.1002/14651858.CD003607.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Garg A.K. Augmentation grafting of the maxillary sinus for placement of dental implants: anatomy, physiology, and procedures. Implant Dent. 1999;8:36–46. doi: 10.1097/00008505-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Chanavaz M. Maxillary sinus: anatomy, physiology, surgery, and bone grafting related to implantology - eleven years of surgical experience. J Oral Implantol. 1990;16:199–209. [PubMed] [Google Scholar]
- 4.Boyne P.J., James R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38:613–616. [PubMed] [Google Scholar]
- 5.Schwartz-Arad D., Herzberg R., Dolev E. The prevalence of surgical complications of the sinus graft procedure and their impact on implant survival. J Periodontol. 2004;75:511–516. doi: 10.1902/jop.2004.75.4.511. [DOI] [PubMed] [Google Scholar]
- 6.Tong D.C., Rioux K., Drangsholt M., Beirne O.R. A review of survival rates for implants placed in grafted maxillary sinuses using meta-analysis. Int J Oral Maxillofac Implants. 1998;13:175–182. [PubMed] [Google Scholar]
- 7.Liu Y., Möller B., Wiltfang J., Warnke P.H., Terheyden H. Tissue engineering of a vascularized bone graft of critical size with an osteogenic and angiogenic factor-based in vivo bioreactor. Tissue Eng. 2014;20:3189–3197. doi: 10.1089/ten.TEA.2013.0653. [DOI] [PubMed] [Google Scholar]
- 8.Kim J.W., Cho M.H., Kim S.J., Kim M.R. Alveolar distraction osteogenesis versus autogenous onlay bone graft for vertical augmentation of severely atrophied alveolar ridges after 12 years of long-term follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:540–549. doi: 10.1016/j.oooo.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Rickert D., Slater J.J., Meijer H.J., Vissink A., Raghoebar G.M. Maxillary sinus lift with solely autogenous bone compared to a combination of autogenous bone and growth factors or (solely) bone substitutes. a systematic review. Int J Oral Maxillofac Surg. 2012;41:160–167. doi: 10.1016/j.ijom.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Almasri M., Altalibi M. Efficacy of reconstruction of alveolar bone using an alloplastic hydroxyapatite tricalcium phosphate graft under biodegradable chambers. Br J Oral Maxillofac Surg. 2011;49:469–473. doi: 10.1016/j.bjoms.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Polo-Corrales L., Latorre-Esteves M., Ramirez-Vick J.E. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14:15–56. doi: 10.1166/jnn.2014.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiarello E., Cadossi M., Tedesco G. Allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin Exp Res. 2013;25:101–103. doi: 10.1007/s40520-013-0088-8. [DOI] [PubMed] [Google Scholar]
- 13.Lundgren S., Andersson S., Sennerby L. Spontaneous bone formation in the maxillary sinus after removal of a cyst: coincidence or consequence. Clin Implant Dent Relat Res. 2003;5:78–81. doi: 10.1111/j.1708-8208.2003.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 14.Palma V.C., Magro-Filho O., de Oliveira J.A., Lundgren S., Salata L.A., Sennerby L. Bone reformation and implant integration following maxillary sinus membrane elevation: an experimental study in primates. Clin Implant Dent Relat Res. 2006;8:11–24. doi: 10.2310/j.6480.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- 15.Thor A., Sennerby L., Hirsch J.M., Rasmusson L. Bone formation at the maxillary sinus floor following simultaneous elevation of the mucosal lining and implant installation without graft material: an evaluation of 20 patients treated with 44 Astra Tech implants. J Oral Maxillofac Surg. 2007;65:64–72. doi: 10.1016/j.joms.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 16.Lundgren S., Andersson S., Gualini F., Sennerby L. Bone reformation with sinus membrane elevation: a new surgical technique for maxillary sinus floor augmentation. Clin Implant Dent Relat Res. 2004;6:165–173. [PubMed] [Google Scholar]
- 17.Borges F.L., Dias R.O., Piattelli A. Simultaneous sinus membrane elevation and dental implant placement without bone graft: a 6-month follow-up study. J Periodontol. 2011;82:403–412. doi: 10.1902/jop.2010.100343. [DOI] [PubMed] [Google Scholar]
- 18.Chen T., Chang H., Leung K.W., Lai Y.L., Kao S. Implant placement immediately after the lateral approach of the trap door window procedure to create a maxillary sinus lift without bone grafting: a 2-year retrospective evaluation of 47 implants in 33 patients. J Oral Maxillofac Surg. 2007;65:2324–2328. doi: 10.1016/j.joms.2007.06.649. [DOI] [PubMed] [Google Scholar]
- 19.Linde A., Thoren C., Dahlin C., Sandberg E. Creation of new bone by an osteopromotive membrane technique: an experimental study in rats. J Oral Maxillofac Surg. 1993;51:892–897. doi: 10.1016/s0278-2391(10)80111-9. [DOI] [PubMed] [Google Scholar]
- 20.Srouji S., Kizhner T., Ben David D., Riminucci M., Bianco P., Livne E. The Schneiderian membrane contains osteoprogenitor cells: in vivo and in vitro study. Calcif Tissue Int. 2009;84:138–145. doi: 10.1007/s00223-008-9202-x. [DOI] [PubMed] [Google Scholar]
- 21.Sohn D.S., Lee J.S., Ahn M.R., Shin H.I. New bone formation in the maxillary sinus without bone grafts. Implant Dent. 2008;17:321–331. doi: 10.1097/ID.0b013e318182f01b. [DOI] [PubMed] [Google Scholar]
- 22.Blomqvist J.E., Alberius P., Isaksson S. Retrospective analysis of one-stage maxillary sinus augmentation with endosseous implants. Int J Oral Maxillofac Implants. 1996;11:512–521. [PubMed] [Google Scholar]
- 23.Jensen O.T., Shulman L.B., Block M.S., Iacono V.J. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants. 1998;13:11–45. [PubMed] [Google Scholar]
- 24.Wallace S., Froum S. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003;8:328–343. doi: 10.1902/annals.2003.8.1.328. [DOI] [PubMed] [Google Scholar]
- 25.Del Fabbro M., Rosano G., Taschieni S. Implants survival rates after maxillary sinus augmentation. Eur J Oral Sci. 2008;116:497–506. doi: 10.1111/j.1600-0722.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 26.Esposito M., Grusovin M.G., Rees J. Effectiveness of sinus lift procedures for dental implant rehabilitation: a Cochrane systematic review. Eur J Oral Implant. 2010;3:7–26. [PubMed] [Google Scholar]
- 27.Haas R., Haidvogl D., Donath K., Watzek G. Freeze-dried homogeneous and heterogeneous bone for sinus augmentation in sheep. Part I: histological findings. Clin Oral Implants Res. 2002;13:396–404. doi: 10.1034/j.1600-0501.2002.130408.x. [DOI] [PubMed] [Google Scholar]
- 28.Haas R., Haidvogl D., Dörtbudak O., Mailath G. Freeze-dried bone for maxillary sinus augmentation in sheep. Part II: biomechanical findings. Clin Oral Implants Res. 2002;13:581–586. doi: 10.1034/j.1600-0501.2002.130602.x. [DOI] [PubMed] [Google Scholar]
- 29.Choi J.Y., Jung U.W., Lee I.S., Kim C.S., Lee Y.K., Choi S.H. Resolution of surgically created three-wall intrabony defects in implants using three different biomaterials: an in vivo study. Clin Oral Implants Res. 2011;22:343–348. doi: 10.1111/j.1600-0501.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- 30.De Moraes P.H., Costa V.O.C., Olate S., Caria P.H.F., Barbosa J.R.A. Morphometric study of maxillary sinus by computed tomography. assessment of sinus floor bone reconstruction. Int J Morphol. 2012;30:592–598. [Google Scholar]
- 31.Callan D.P., Rohrer M.D. Use of bovine-derived hydroxyapatite in the treatment of edentulous ridge defects: a human clinical and histologic case report. J Periodontol. 1993;64:575–582. doi: 10.1902/jop.1993.64.6.575. [DOI] [PubMed] [Google Scholar]
- 32.Bruder S.P., Fink D.J., Caplan A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]