Abstract
Background/purpose
Dental enamel defects are related to celiac disease and the dentists are in a perfect situation to identify and report suspected cases. The aim was to evaluate the symmetry of enamel defects in a pediatric Portuguese population with celiac disease and compare it with healthy controls.
Materials and methods
a case-control study was performed in 80 patients with celiac disease and 80 healthy individuals aged 6–18 years old as controls. Data was collected by a questionnaire and clinical observation. Colour, type, and site of enamel defects were recorded and classified according to Aine criteria. Data analysis was performed, and any p-value <0.05 was considered significant.
Results
Enamel defects were found in 55% of patients with celiac disease and 27.5% in the control individuals (p < 0.001). Grade I of Aine's classification was the most found in both groups, but it was higher in the celiac disease group, not only in the permanent dentition, but also in both dentitions with statistically significant difference (p = 0.002 and p = 0.001 respectively). Grade II was found only in the celiac disease group. It was observed that enamel defects in celiac disease were symmetric and the most affected teeth were the first permanent molars (p = 0.003) and the permanent incisors (p = 0.001).
Conclusion
Symmetric dental enamel defects in population with celiac disease are more predominant than in general population. Therefore, individuals with enamel defects, especially those with symmetric lesions, should be well evaluated and the possibility of having celiac disease in the clinical history must be taken into account.
Keywords: Celiac disease, Dental enamel defects, Enamel hypoplasia, Oral manifestations
Introduction
Celiac disease (CD) is an immune-mediated enteropathy that affects genetically susceptible people following exposure to dietary gluten.1, 2, 3, 4, 5, 6, 7 CD individuals may have gastrointestinal symptoms with a typical enteropathy, characterized by a malabsorption syndrome, extraintestinal symptoms or none of these.6,8,9
A greater prevalence of some oral manifestations in patients with celiac disease is described by other authors and it may be considered a diagnostic clue in silent-atypical forms of CD.3,4,10 Oral manifestations related to CD are recurrent aphthous stomatitis, delayed tooth eruption, caries, geographic tongue, angular cheilitis, atrophic glossitis, burning tongue, dry mouth and dental enamel defects.3,8,11,12,14, 15, 16 Impairment of dental crowns mineralization may occur in numerous systemic diseases but the defects found in celiac patients are highly specific and often in a symmetrical way.4,13 Dental enamel defects are mainly characterized by pitting, grooving and sometimes a complete loss of the enamel.4,15 These specific enamel defects have to be symmetrically and chronologically detectable in all four sections of the dentition.4,13 Other enamel defects (discolorations, hypoplasia or opacities) that are neither symmetrical nor chronological and are not present in the same teeth of both hemi-arches are considered unspecific. Dental enamel structural defects may be diverse and may contain hypoplasia (functional disturbances) as well as hypomineralization (qualitative disturbances).4
There are no articles about this topic in Portuguese population so, these considerations led us to perform the rst study in Portuguese children with CD aimed at evaluating the percentage of enamel defects among diagnosed patients with CD and compare the results with those of healthy controls, as well as the difference in symmetry in both groups.
Materials and methods
The sample included 80 children with celiac disease and 80 controls, aged between 6 and 18 years old in both groups. Two subgroups were created for each group: [6, 12] and [13, 18]. For the purpose of calculating age, the last birthday was considered, that is, if the patient was 12 years old and eleven months old, he was considered 12 years old and was placed in the group from 6 to 12 years old. Celiac patients, treated regularly in the Gastroenterology and Nutrition department/Pediatric Hospital in São João Hospital Center, in Oporto city, Portugal, were invited to participate in the study. The control group included 80 children and adolescents followed in the pediatric dentistry class of the Dentistry Department of Oporto University, in Oporto city, Portugal. This choice was intentional considering that it was an exclusion factor to present other systemic diseases, local causes associated with enamel defects, which could have triggered tooth pigmentation. If the control group consisted of non-celiac patients who were followed at the hospital, they could have presented problems of nutritional or systemic etiological nature and thus, alter the results of the study.
The inclusion criteria implemented for CD group were: previous diagnosis of CD, age between 6 and 18 and signed informed consent forms by children's legal representatives. Inclusion criteria for the control group were the same except for having CD. The exclusion criteria for the 2 groups were: patients who didn't allow a complete examination; the presence of fixed orthodontic appliance; the presence of dental fluorosis or other local/systemic diseases or local causes associated with enamel defects; previous use of drugs that may have caused dental pigmentation, such as tetracycline. Any child from the control group who reported any gastrointestinal disease and/or having a family history of CD was excluded.
The clinical examination of all participants was carried out by a certified dentist. In order to observe the patients clinically an Asa dental mirror, an Asa probe, and a sterile gauzes or cotton were used. Observation was made using artificial and natural light and its registry was carried out tooth by tooth. Aine et al.17,18 classified the specific enamel defects in grades I–IV according to the severity of their clinical aspect (Table 1) so that he could assess the enamel defects. Colour, type, and site of defects were recorded. The symmetrical dental defects distribution was evaluated by the involvement of the homologous teeth in the upper and lower hemi-arches and all hemi-arches. This study was approved by the ethical committee of the Faculty of Dentistry of Oporto University and by the ethical committee of São João Hospital (Oporto, Portugal). Informed consent was obtained from the legal representatives of all individual participants included in the study, according to the Declaration of Helsinki of 2002.
Table 1.
Classification of dental enamel defects according to Aine, adapted by Shteyer E.17
| Grade 0 | No defects. |
| Grade I | Defect in enamel colour. Single or multiple cream, yellow or brown opacities (marks) with clear or hazed boundary, part of the dental enamel may lack transparency. |
| Grade II | Slight structural enamel defects, rough surface with horizontal groves or pits, distortion of enamel colour and transparency. |
| Grade III | Evident structural defects. A part or the entire surface of enamel rough and filled with deep horizontal grooves that vary in width or have large vertical pits; large opacities of different colours or strong discolorations may appear in combination. |
| Grade IV | Severe structural defects. The shape of the tooth changed. The tips of cusps are sharp-pointed and/or the incisal edges are unevenly thinned and rough. The thinning of the enamel material is easily detectable, and the lesion may be strongly discoloured. |
All intraoral observations were performed by a single examiner; however, it was necessary to verify the intra-observer reliability degree over time and the reproducibility of the examination. For this purpose, 16 double observations were performed, which corresponded to approximately 10% of the total intraoral observations performed. All pairwise observations were made in the control group. Cohen's Kappa coefficient for dichotomous views and weighted Cohen's Kappa coefficient for ordinal observations were used to calculate intra-observer agreement, with a value of 0.875 and 0.915, considered as "near-perfect".19 For the assessment of the examiner and confirmation of the calibration of the observer, observations were made by another trained dentist. The double observations were performed whenever possible on different days with a distance that never exceeded 30 days, so that there were no significant changes in oral health status. The agreement between both was considered very good/near perfect based on a Kappa of 0.830, with a standard error of 0.040.19
For a descriptive analysis of the results, appropriate summary statistics were applied. Sample percentage of enamel defects and respective 95% confidence levels for population percentage of enamel defect were calculated using the adjusted Wald method as this provides the best coverage for the specified interval when sample sizes are less than about 150. A p-value lower than 0.05 was statistically significant. The analysis was performed using the statistical analysis software IBM® SPSS® v.24.0 (IBM Corporation, USA).
Results
A homogeneous sample of 80 celiac patients and 80 control patients aged 6–18 years, of both genders, with no significant difference in age (p = 0.172)/age range (p = 0.080) or gender (p = 0.631) between groups (Table 2).
Table 2.
Characteristics of the study groups.
| Variable | Characteristic | CD (n = 80) n (%) |
Control (n = 80) n (%) |
p-value |
|---|---|---|---|---|
| Age (years) | average (SD) | 13.3 (3.5) | 12.5 (3.7) | 0.172a |
| 95%CI mean | 12.5–14 | 11.7–13.3 | ||
| range | 6.5–18.7 | 7–18.9 | ||
| Age range | 6–12 years old | 30 (37.5%) | 41 (51.3%) | 0.080b |
| 13–18 years old | 50 (62.5%) | 39 (48.8%) | ||
| Gender | Female | 48 (60%) | 45 (56.3%) | 0.631b |
| Male | 32 (40%) | 35 (43.8%) |
CD – celiac disease; SD−standard deviation.
t test.
Chi-square test.
The presence of at least one enamel defect was more frequently found in the CD group in the permanent dentition (p = 0.001) as well as when both dentitions were counted (p < 0.001). Dental enamel defects in both dentitions were found in 55% celiac disease and in 27.5% control subjects (p < 0.001). In the deciduous tooth dentition, the number of enamel defects was very small, therefore, the inference for the population is irrelevant, the reason why no more results were included for this condition (Table 3). When performing a subgroup age analysis in both groups, CD group continued to have a higher percentage of enamel defects, at least in the 13–18 years old subgroup (p < 0.001). The results concerning this analysis can be found in Table 4.
Table 3.
Comparison of the presence of enamel defects in the CD and control group.
| Variable | Characteristic | CD (n = 80) n (%) |
Control (n = 80) n (%) |
p-value |
|---|---|---|---|---|
| ≥1 enamel defect Permanent dentition |
yes | 42 a (53.2) | 22 b (27.5) | 0.001 |
| no | 37 b (46.8) | 58 a (72.5) | ||
| ≥1 enamel defect Deciduous tooth dentition |
yes | 3 (10.3) | 2 (5.7) | 0.492 |
| no | 26 (89.7) | 33 (94.3) | ||
| ≥1 enamel defect Both dentitions |
yes | 44a (55) | 22b (27.5) | <0.001 |
| no | 36b (45) | 58a (72.5) |
CD – celiac disease; a,b-different letters show significant differences regarding counts, according to the chi-square test.
Table 4.
Comparison of the presence of enamel defects in the different age subgroups in CD and control group.
| Variable | 6–12 years old |
p-value | 13–18 years old |
p-value | |||
|---|---|---|---|---|---|---|---|
| CD n (%) |
Control n (%) |
CD n (%) |
Control n (%) |
||||
| ≥1 enamel defect Permanent dentition |
yes | 14 (48.3) | 14 (34.1) | 0.235 | 28a (56) | 8b (20.5) | 0.001 |
| no | 15 (51.7) | 27 (65.9) | 22b (44) | 31a (79.5) | |||
| ≥1 enamel defect Both dentitions |
yes | 16 (53.3) | 14 (34.1) | 0.106 | 28a (56) | 8b (20.5) | 0.001 |
| no | 14 (46.7) | 27 (65.9) | 22b (44) | 31a (79.5) | |||
CD – celiac disease; a,b-different letters show significant differences regarding counts, according to the chi-square test.
Dental enamel defects were classified using grades 0 to IV according to Aine criteria.17,18 In this study, only patients with grade I or II of enamel defects were found. Grade I was the most found in both groups (Fig. 1), but the percentage was significantly higher in the celiac disease group, not only in the permanent dentition, but also in both dentitions (p = 0.002 and p = 0.001 respectively). Moreover, grade II was found only in the CD group (Table 5 and Fig. 2).
Figure 1.
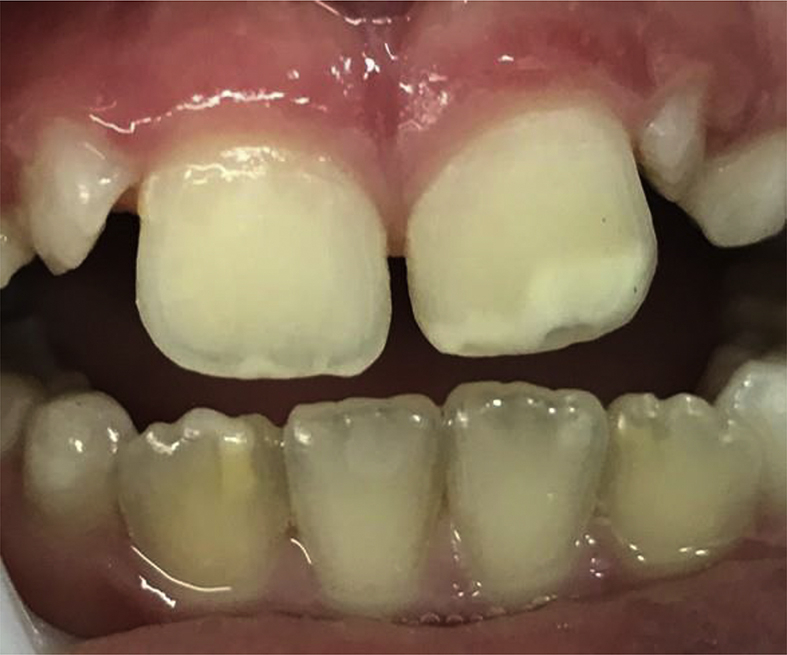
Presence of symmetrical enamel defects in the homologous teeth, Grade I Aine classification, in the permanent dentition in the celiac disease group.
Table 5.
Study of the distribution of different degrees of enamel defects (according to the Aine classification) in the celiac disease versus control group.
| Variable | Characteristic | CD n (%) n = 80 |
Control n (%) n = 80 |
p-value |
|---|---|---|---|---|
| ≥1 enamel defect Permanent dentition |
Grade I | 41 (51.3%) | 22 (27.5%) | 0.002a |
| Grade II | 9 (11.3%) | 0 (0%) | 0.003b | |
| ≥1 enamel defect Both dentitions |
Grade I | 43 (53.8%) | 22 (27.5%) | 0.001a |
| Grade II | 10 (12.5%) | 0 (0%) | 0.001a |
CD – celiac disease.
The chi-square test.
Fisher exact test.
Figure 2.
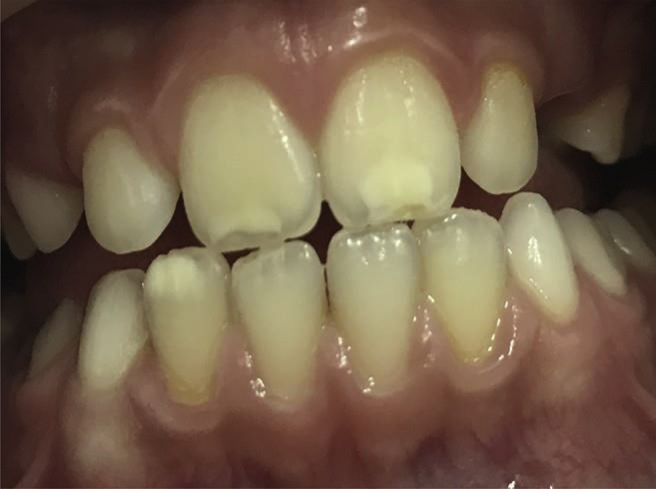
Presence of symmetrical enamel defects in the homologous teeth, Grade II Aine classification, in the permanent dentition in the celiac disease group.
This study found a significant higher number of symmetric enamel defects in the first upper molars (p = 0.003) and first lower molars (p = 0.003) and at the same time found higher number of symmetric enamel defects in all first molars (p = 0.028). Besides, a higher number of symmetric enamel defects in the lateral upper incisors (p = 0.001) and central upper incisors (p = 0.001) was found (Table 6).
Table 6.
Study of symmetry of enamel defects in the homologous teeth in the permanent dentition in celiac disease group versus control group.
| Permanent Dentition | ||||||
|---|---|---|---|---|---|---|
| Teeth | Number of symmetries in 2 homologous teeth CD | Number of symmetries in 2 homologous teeth Control |
p-value | Number of symmetries in 4 homologous teeth CD |
Number of symmetries in 4 homologous teeth Control |
p-value |
| 17/27 | 0 | 1 | 1.000a | 0 | 1 | 1.000a |
| 37/47 | 0 | 1 | 1.000a | |||
| 16/26 | 9 | 0 | 0.003a | 6 | 0 | 0.028a |
| 36/46 | 13 | 2 | 0.003b | |||
| 15/25 | 1 | 1 | 1.000a | 1 | 1 | 1.000a |
| 35/45 | 1 | 1 | 1.000a | |||
| 14/24 | 2 | 1 | 1.000a | 1 | 1 | 1.000a |
| 34/44 | 2 | 1 | 1.000a | |||
| 13/23 | 1 | 0 | 1.000a | 1 | 0 | 1.000a |
| 33/43 | 2 | 0 | 0.497a | |||
| 12/22 | 10 | 0 | 0.001b | 0 | 0 | n.a |
| 32/42 | 2 | 0 | 0.497a | |||
| 11/21 | 27 | 9 | 0.001b | 4 | 0 | 0.120a |
| 31/41 | 5 | 0 | 0.059a | |||
CD – celiac disease; n.a – not applicable.
Chi-square test.
Fisher test.
Discussion
In the CD Portuguese group, the percentage of at least one tooth with enamel defect was significantly higher than that of the control group. These results were similar to the literature found in celiac patients from other countries.8,11,20, 21, 22, 23, 24, 25 Enamel defects could be a major sign of CD.11,21,26 The overall prevalence of this oral manifestation ranges from 38% to 83%.5,11,14,20,21 Souto-Souza et al. investigated the relationship between enamel defects and CD and in their meta-analysis, observed that CD had a significantly higher prevalence of enamel defects; and they observed that only enamel defects diagnosed using Aine method were strictly related to CD.5 Nieri et al. performed a systematic review and a meta-analysis on the presence of enamel defects in CD versus healthy individuals and verified that CD had a significantly higher prevalence of enamel defects.27
It is important to note that defects in the enamel structure may develop in the prenatal, neonatal, and postnatal periods, and their extension will depend on the duration, intensity, and the period in which the etiological factor was present during the formation of the dental crown.28 The presence of enamel defects found in deciduous tooth dentition supports the hypothesis that immunogenic factors are probably more related to the development of enamel defects in CD than environmental factors.14 In our study, there were no differences between the two groups regarding the enamel defects in the deciduous tooth dentition. As reported in the literature, enamel defects are more frequent in the permanent dentition and normally there are not so many defects in the deciduous tooth dentition. It should be noted that the sample of individuals in deciduous tooth dentition in the present study was reduced, which makes it difficult to infer conclusions.
The distribution of the different degrees of enamel defects in the permanent dentition (according to Aine's classification) shows that grade I and II are the most common, which agrees with the results of the present study. In our study, CD group presented a higher occurrence of grade I enamel defects of Aine classification in the permanent dentition, being these results similar to those of some studies in the literature.11,15,18,19,29 Trotta et al. found dental enamel defects in most celiac patients. The defects are systematic, showing a symmetrical and chronological distribution in the four hemi arches. The most commonly affected teeth were the incisors, followed by molars, premolars and canines. Moreover, in the study by Trotta et al., grade I and II of Aine classification were the most found.12 Campisi et al. evaluated the severity of enamel defects according to Aine criteria and found 87% of grade I, 11% grade II and 2% grade IV.8 Mina et al. found 86% of grade I and 14% of grade II in CD patients.30 It is unclear what factors affect the degree of severity of enamel defects in CD.
Mineralization impairment of dental crowns can occur in numerous systemic diseases, but the enamel defects found in CD normally are symmetrically and chronologically detectable in all four sections of the dentition.13,31,32 The present study found a significant higher number of symmetric enamel defects and the most affected teeth were the first permanent molars and the permanent incisors. These defects were more frequently found in the permanent dentition, symmetrically (on both sides of the dental arch) and occurring in both the maxilla and the mandible, results similar to the literature.4,13,31,33 Celiac disease may predispose patients to enamel defects in the incisors and first molars as a result of the time course of odontogenesis and its relation to the active phases of the disease.15 Consequently, an early diagnosis is important and a gluten-free diet could prevent or reduce these enamel alterations.15
The incisors and first molars are the most affected teeth as a result of odontogenesis and its relationship with the active phases of the disease. The higher percentage in the permanent dentition can be explained by the fact that the development of the crown in this dentition occurs between the first months of life and the 7–8 years, whereas the formation of the deciduous tooth dentition occurs predominantly in utero. This correlation with the chronology of the development of the permanent dentition is also justified by the fact that the incisors and the first molars are the first teeth to calcify.14 Age at CD diagnosis plays an important role in the pathogenesis of enamel defects.15 Therefore, it is believed that when a systemic disease occurs in the period of mineralization of the temporary or permanent teeth, it can result in anomalies in the enamel.
Enamel defects are more prevalent and symmetrical in patients with celiac disease when compared to a non-celiac pediatric population and this difference is highly significant. The identification and the knowledge of this particularity (symmetric enamel defects) in this population may have an important role for the dentist to suspect of CD and subsequently to refer the patient to the physician, in order to perform specific tests to confirm the diagnosis.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
None.
References
- 1.Bai J.C., Fried M., Corazza G.R. World Gastroenterology Organisation global guidelines on celiac disease. J Clin Gastroenterol. 2013;47:121–126. doi: 10.1097/MCG.0b013e31827a6f83. [DOI] [PubMed] [Google Scholar]
- 2.Husby S., Koletzko S., Korponay-Szabo I.R. European society for pediatric Gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 3.Kneepkens C.M., von Blomberg B.M. Clinical practice: coeliac disease. Eur J Pediatr. 2012;171:1011–1021. doi: 10.1007/s00431-012-1714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macho V.M.P., Coelho A.S., Veloso E.S.D.M., de Andrade D.J.C. Oral manifestations in pediatric patients with coeliac disease - a review article. Open Dent J. 2017;11:539–545. doi: 10.2174/1874210601711010539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souto-Souza D., da Consolação Soares M.E., Rezende V.S., de Lacerda Dantas P.C., Galvão E.L., Falci S.G.M. Association between developmental defects of enamel and celiac disease: a meta-analysis. Arch Oral Biol. 2017;87:180–190. doi: 10.1016/j.archoralbio.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Turner J.M. Diagnosis of celiac disease: taking a bite out of the controversy. Dig Dis Sci. 2018;63:1384–1391. doi: 10.1007/s10620-018-5050-3. [DOI] [PubMed] [Google Scholar]
- 7.Lodhi M.U., Stammann T., Kuzel A.R., Syed I.A., Ishtiaq R., Rahim M. Celiac disease and concomitant conditions: a case-based review. Cureus. 2018;10:e2143. doi: 10.7759/cureus.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campisi G., Di Liberto C., Carroccio A. Coeliac disease: oral ulcer prevalence, assessment of risk and association with gluten-free diet in children. Dig Liver Dis. 2008;40:104–107. doi: 10.1016/j.dld.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Torres M.I., Lopez Casado M.A., Rios A. New aspects in celiac disease. World J Gastroenterol. 2007;13:1156–1161. doi: 10.3748/wjg.v13.i8.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris L.A., Park J.Y., Voltaggio L., Lam-Himlin D. Celiac disease: clinical, endoscopic, and histopathologic review. Gastrointest Endosc. 2012;76:625–640. doi: 10.1016/j.gie.2012.04.473. [DOI] [PubMed] [Google Scholar]
- 11.Avsar A., Kalayci A.G. The presence and distribution of dental enamel defects and caries in children with celiac disease. Turk J Pediatr. 2008;50:45–50. [PubMed] [Google Scholar]
- 12.Trotta L., Biagi F., Bianchi P.I. Dental enamel defects in adult coeliac disease: prevalence and correlation with symptoms and age at diagnosis. Eur J Intern Med. 2013;24:832–834. doi: 10.1016/j.ejim.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 13.de Carvalho F.K., de Queiroz A.M., Bezerra da Silva R.A. Oral aspects in celiac disease children: clinical and dental enamel chemical evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:636–643. doi: 10.1016/j.oooo.2015.02.483. [DOI] [PubMed] [Google Scholar]
- 14.Pastore L., Carroccio A., Compilato D., Panzarella V., Serpico R., Lo Muzio L. Oral manifestations of celiac disease. J Clin Gastroenterol. 2008;42:224–232. doi: 10.1097/MCG.0b013e318074dd98. [DOI] [PubMed] [Google Scholar]
- 15.Bucci P., Carile F., Sangianantoni A., D'Angio F., Santarelli A., Lo Muzio L. Oral aphthous ulcers and dental enamel defects in children with coeliac disease. Acta Paediatr. 2006;95:203–207. doi: 10.1080/08035250500355022. [DOI] [PubMed] [Google Scholar]
- 16.Lahteenoja H., Toivanen A., Viander M. Oral mucosal changes in coeliac patients on a gluten-free diet. Eur J Oral Sci. 1998;106:899–906. doi: 10.1046/j.0909-8836.1998.eos106501.x. [DOI] [PubMed] [Google Scholar]
- 17.Shteyer E., Berson T., Lachmanovitz O. Oral health status and salivary properties in relation to gluten-free diet in children with celiac disease. J Pediatr Gastroenterol Nutr. 2013;57:49–52. doi: 10.1097/MPG.0b013e31828b3705. [DOI] [PubMed] [Google Scholar]
- 18.Aine L. Dental enamel defects and dental maturity in children and adolescents with celiac disease. Proc Finn Dent Soc. 1986;82:227–229. [PubMed] [Google Scholar]
- 19.W.H.O. 4nd ed. WHO.; Geneva: 1997. Oral health surveys – basic methods. [Google Scholar]
- 20.Wierink C.D., van Diermen D.E., Aartman I.H., Heymans H.S. Dental enamel defects in children with coeliac disease. Int J Paediatr Dent. 2007;17:163–168. doi: 10.1111/j.1365-263X.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 21.Cantekin K., Arslan D., Delikan E. Presence and distribution of dental enamel defects, recurrent aphthous lesions and dental caries in children with celiac disease. Pak J Med Sci. 2015;31:606–609. doi: 10.12669/pjms.313.6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bramanti E., Cicciu M., Matacena G., Costa S., Magazzu G. Clinical evaluation of specific oral manifestations in pediatric patients with ascertained versus potential coeliac disease: a cross-sectional study. Gastroent Res Pract. 2014;2014:934159. doi: 10.1155/2014/934159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costacurta M., Maturo P., Bartolino M., Docimo R. Oral manifestations of coeliac disease.: a clinical-statistic study. Oral Implantol. 2010;3:12–19. [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J., Malahias T., Brar P., Minaya M.T., Green P.H. The association between celiac disease, dental enamel defects, and aphthous ulcers in a United States cohort. J Clin Gastroenterol. 2010;44:191–194. doi: 10.1097/MCG.0b013e3181ac9942. [DOI] [PubMed] [Google Scholar]
- 25.Franco K.M.D., Line S.R.P., de Moura-Ribeiro M.V.L. Prenatal and neonatal variables associated with enamel hypoplasia in deciduous teeth in low birth weight preterm infants. J Appl Oral Sci. 2007;15:518–523. doi: 10.1590/S1678-77572007000600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Procaccini M., Campisi G., Bufo P. Lack of association between celiac disease and dental enamel hypoplasia in a case-control study from an Italian central region. Head Face Med. 2007;3:25. doi: 10.1186/1746-160X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieri M., Tofani E., Defraia E., Giuntini V., Franchi L. Enamel defects and aphthous stomatitis in celiac and healthy subjects: systematic review and meta-analysis of controlled studies. J Dent. 2017;65:1–10. doi: 10.1016/j.jdent.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Vello M.A., Martinez-Costa C., Catala M., Fons J., Brines J., Guijarro-Martinez R. Prenatal and neonatal risk factors for the development of enamel defects in low birth weight children. Oral Dis. 2010;16:257–262. doi: 10.1111/j.1601-0825.2009.01629.x. [DOI] [PubMed] [Google Scholar]
- 29.Acar S., Yetkiner A.A., Ersin N., Oncag O., Aydogdu S., Arikan C. Oral findings and salivary parameters in children with celiac disease: a preliminary study. Med Princ Pract. 2012;21:129–133. doi: 10.1159/000331794. [DOI] [PubMed] [Google Scholar]
- 30.Mina S., Riga C., Azcurra A.I., Brunotto M. Oral ecosystem alterations in celiac children: a follow-up study. Arch Oral Biol. 2012;57:154–160. doi: 10.1016/j.archoralbio.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Bossu M., Bartoli A., Orsini G., Luppino E., Polimeni A. Enamel hypoplasia in coeliac children: a potential clinical marker of early diagnosis. Eur J Paediatr Dent. 2007;8:31–37. [PubMed] [Google Scholar]
- 32.Krzywicka B., Herman K., Kowalczyk-Zajac M., Pytrus T. Celiac disease and its impact on the oral health status - review of the literature. Adv Clin Exp Med. 2014;23:675–681. doi: 10.17219/acem/37212. [DOI] [PubMed] [Google Scholar]
- 33.Priovolou C.H., Vanderas A.P., Papagiannoulis L. A comparative study on the prevalence of enamel defects and dental caries in children and adolescents with and without coeliac disease. Eur J Paediatr Dent. 2004;5:102–106. [PubMed] [Google Scholar]


