Abstract
Over the past decade, nanoparticle-based therapeutic modalities have become promising strategies in cancer therapy. Selective delivery of anticancer drugs to the lesion sites is critical for elimination of the tumor and an improved prognosis. Innovative design and advanced biointerface engineering have promoted the development of various nanocarriers for optimized drug delivery. Keeping in mind the biological framework of the tumor microenvironment, biomembrane-camouflaged nanoplatforms have been a research focus, reflecting their superiority in cancer targeting. In this review, we summarize the development of various biomimetic cell membrane-camouflaged nanoplatforms for cancer-targeted drug delivery, which are classified according to the membranes from different cells. The challenges and opportunities of the advanced biointerface engineering drug delivery nanosystems in cancer therapy are discussed.
Keywords: Cell membrane-camouflaged nanoplatform, Biofunctionalization, Tumor microenvironment, Controlled drug delivery, Targeted cancer therapy
Graphical abstract
In this review article, we summarize the development and mechanisms of various biointerface engineering nanoplatforms for cancer-targeted drug delivery, which are prepared by coating different cell membranes onto the nanoparticles. In addition, the challenges and opportunities of the smart nanocarriers for selective drug delivery are predicted.
1. Introduction
Cancer morbidity and mortality have risen globally in recent decades, with approximately 18.1 million new cancer diagnoses and 9.6 million cancer-related deaths occurring in 2018 [1]. Surgery remains the most effective method for cancer therapy. However, in cases of terminal cancer, surgery is not always an option for many patients. Meanwhile, conventional treatments, such as chemotherapy, radiotherapy, and molecule targeted therapy, are insufficient. These conventional treatments also generate severe side effects that include hepatotoxicity, renal toxicity, myelosuppression, radiation-induced enteritis, and multidrug resistance, and so forth. Despite the many studies done, these therapeutic formulations remain mostly unchanged, and their drawbacks remain. Several alternative approaches for cancer therapy have emerged in recent decades. The most successful example of these is immunotherapy, which has been recommended in many current cancer treatment guidelines [2]. However, with its widespread clinical application, some problems have emerged. They include immunotherapy-associated side effects and relatively lower treatment efficiency [3], [4], [5]. Besides, a perplexing issue is the susceptibility of some, but not all, patients to immunotherapy. Another emerging therapeutic approach is phototherapy, which has become very popular in anticancer research and has been proved efficient in cancer suppression [6,7]. However, there are also limitations to phototherapy. For example, only superficial tumors can feasibly be treated using phototherapy. Moreover, the low efficiency of photothermal transition necessitates the further modification of conventional photosensitizers to allow phototherapy to be used more broadly.
With the progress in nanotechnology and increasing research efforts in nanomedicine, nanoparticle-based anti-neoplastic solutions are being considered a superior treatment option [8,9]. To improve anticancer efficiency, a variety of nanocarriers have been invented and rationally designed. These include micelles, liposomes, nanogels, nanocapsules, nanoemulsions, nanocomplexes, and other designs [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Some of these nanoformulations have been employed in clinical anticancer treatment [23], [24], [25], [26]. Typically, various nanocarriers are administered as drug vehicles that function to deliver their payload to the tumor with various anticancer agents loaded in the nanocarriers or chemically conjugated to the surface [27], [28], [29], [30]. Certain types of nanomedicines are also capable of inhibiting tumors due to their physical and chemical characteristics [27,31].
The primary therapeutic capability of the various types of nanomedicines relies on their local accumulation at the tumor site, where target specificity remains a challenge. The nonspecific distribution of nanocarriers in other major organs and healthy cells always blunts the therapeutic outcomes and causes severe systematic side effects. The goal of many ongoing types of research is to develop strategies that enhance the intratumor accumulation of various nanomedicines, either passively or actively.
Due to the abnormal leakage of tumor vessels and cacoethic lymphatic drainage, the enhanced permeability and retention (EPR) effect passively facilitates the local accumulation of nanoparticles at the targeted areas [32], [33], [34]. However, because most nanoparticle-based drug delivery systems are artificial, the recipient biological organisms enable the accurate and efficient identification of the “non-self” nanoparticles. These nanomaterials, therefore, are rapidly removed through the reticuloendothelial system [35,36]. Thus, proper modifications that increase biocompatibility and extend the circulation halftime enhance the EPR effect, thereby improving the passive accumulation of various nanoparticles. The use of poly(ethylene glycol) (PEG) to modify the biointerfaces of nanoparticles was once considered an effective means to increase the biocompatibility and circulation halftime of the nanoparticles. However, repeated administration of PEG-modified nanocarriers was demonstrated to stimulate the secretion of anti-PEG antibodies and induce immune responses against PEGylated nanomedicines, in turn accelerating the premature clearance of anti-neoplastic agents from the body [37,38].
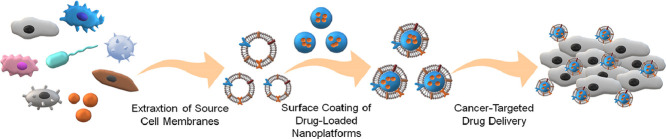
Another strategy that is being explored is the targeting strategy. Apart from the means of embellishing nanocarriers with biological ligands [39], [40], [41], [42], [43], or endowing them with internal/external stimuli-responsive properties [44], [45], [46], [47], nanomedicine researchers have developed the cell membrane-engineered nanoplatforms. Different cells have various and specific functions within biological microenvironments based on their intrinsic membrane proteins and saccharides. Thus, by coating nanoparticles with various cell membranes, the biomimetic strategy aims at disguising nanoparticles as different types of living cells. Accordingly, the membrane-enshrouded nanoparticles carry out the defined functions of the source cells to some extent, as depicted in Fig. 1. In living organisms, specific cells enable the immune escape and have a long circulation half-life while others communicate with tumor cells or biological microenvironment. The communication involves cell recognition, adhesion, and cytokine-induced recruitment, which is facilitated by the activities of membrane adhesion molecules or secreted cytokines [48,49]. Various aspects are summarized in Table 1. Based on the natural affinity between the source cells and tumor cells, the cell-mimetic nanocarriers tend to actively recognize and adhere to the tumor cells in a ligand-receptor manner. Some types of the cell-engineered nanoplatforms are also recruited by the chemical signals of cytokines in the tumor microenvironment (TME), as the biological behavior of their source cells. Modifying the nanoparticles with such selective cell membranes drives the targeting.
Fig. 1.
Schematic illustration of cell membrane-camouflaged nanoplatforms for cancer-targeted drug delivery.
Table 1.
Synopsis of various cell membrane derivations and their active targeting mechanism.
| Cell derivation | Tumor cell targeting | Chemokine recruitment | Targeted region | Cell adhesion molecule for active targeting | Targeted ligand or attractant | Ref. |
|---|---|---|---|---|---|---|
| Cancer cell | + | − | Cancer cell | EpCAM, galectin-3, N-cadherin | EpCAM, galectin-3, N-cadherin | [61], [62], [63], [64], [65], [66] |
| WBC | + | + | Cancer cell/TME | CXCR2, CCL18, α4 integrin, endothelin-B receptor, CSF-1 receptor, EGF, VEGFR, TCR | CXCL1/2/3/4/5/7/8, VCAM-1, GM-CSF ET-2, CSF-1, EGFR, VEGF, Melan-A/MART-1, tyrosinase, gp100, MAGEs, so-called cancer/testis antigens, tumor-restricted antigens | [75], [76], [77], [78], [79], [80], [81], [82],90] |
| Platelet | + | − | Cancer cell | P-selectin | CD44 receptor | [105,106] |
| Mesenchymal stem cell | + | + | Cancer cell | CXCR4, EGF, integrins, extracellular matrix molecules | SDF-1, EGFR (HER2) | [108], [109], [110], [111] |
| Bacteria | + | − | Cancer cell | HA, engineered anti-HER2 affibody | CD44 receptor, HER2 | [119,120] |
| Exosome | + | − | Cancer cell | Lysosome-associated membrane glycoprotein 2b, GE-11 peptide, lymphocyte function-associated antigen-1, hEGF | Acetylcholine receptor, HER2, CAMs, magnetic field | [121,[123], [124], [125], [126],129] |
+: available; −: not available.
Nowadays, the development of biomimetic membrane-camouflaged nanoplatforms has become a hot area of research. Moreover, several research groups have been focusing on the membrane-camouflaged nanoplatforms in cancer theranostics [50], [51], [52]. Based on the multiple research, we comprehensively summarized various biomimetic membrane-camouflaged nanoplatforms that have been applied for cancer-targeted drug delivery. Apart from erythrocyte membrane-camouflaged nanoplatform, which is a passively targeted example, all the other researches in this review are actively targeted nanoformulations. The biological targeting mechanism of each type of cell membrane derivation is briefly described and discussed in each section. Relevant research examples we discuss are summarized in Table 2, and the diverse membrane extraction technologies and membrane coating procedures are attached in Table 3.
Table 2.
Features of nanoparticles used for cancer-targeted drug delivery.
| Membrane derivation | Excipient of nanoparticle | Model drug | Targeted cell line | Ref. |
|---|---|---|---|---|
| RBC | Methoxy poly(ethylene glycol)-block-poly(D,L-lactide) | PTX, TPC | HeLa | [60] |
| Cancer cell | PLGA | DOX, Hb | MCF-7 | [70] |
| PLGA | R837 | RM-1 | [68] | |
| WBC | PLGA | DOX | MCF-7 | [84] |
| Bismuth selenide | QE | 4T1 | [90] | |
| Dual-end PEGylation of poly(β-amino ester) | PTX | MDA-MB-231 | [91] | |
| PLGA | PTX | MKN-45 | [93] | |
| Lipids 1,2-dioleoyl-sn‑glycero-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt) (DOTAP), cholesterol | DOX | MCF-7 | [96] | |
| PLGA | CFZ | 4T1 | [103] | |
| Platelet | Acrylamide, N-(3-aminopropyl) methacrylamide, glycerol dimethacrylate | TRAIL, DOX | MDA-MB-231 | [107] |
| Mesenchymal stem cell | PLGA | DOX | MHCC97H | [112] |
| Bacteria | Selenium-PEI | siRNA | HepG2 | [119] |
| − | siRNA | HCC-1954 | [120] | |
| Exosome | Transferrin | DOX | H22 | [129] |
| − | DOX, ICG | BT474, MDA-MB-468 | [126] | |
| WBC/Cancer cell | − | PTX | HN12, B16 | [131] |
| RBC/Cancer cell | CuS | DOX | B16F10 | [132] |
Table 3.
Typical example of extraction technologies of each membrane and membrane coating process of cell-derived nanoplatforms.
| Membrane derivation | Extraction technologies of different membranes | Membrane coating procedure | Ref. | |
|---|---|---|---|---|
| RBC | The whole blood was centrifuged to collect the RBC, followed by washing with cold PBS. The obtained RBC was put into a cold hypotonic buffer for hemolysis. The free hemoglobin was eliminated by centrifugation at 4 °C. The pink pellet was obtained by washing with cold hypotonic buffer. Afterward, the RBC vesicle was collected by extruding the empty RBC membrane through 450 nm polycarbonate membrane. | The RBC membrane-coated nanoplatforms were prepared by co-extruding RBC vesicles and nanoparticles through 450 nm porous membrane. Free RBC vesicle was eliminated by centrifugation at 4 °C. | [60] | |
| Cancer cells | The source membranes were obtained by hypotonic lysis of cancer cells, mechanical membrane disruption, and the different speed of centrifuge. Then the intracellular contents of cancer cells were removed, and the cancer cell membrane was collected. Finally, the membrane was extruded through the 220 nm polycarbonate membrane with DSPE-PEG2000. | The cancer cell-coated nanoplatforms were harvested by extruding the nanoparticles and source membranes with a 220 nm polycarbonate membrane. | [70] | |
| WBCs | Monocytes | Cell membranes of U937 monocytes were obtained by hypotonic lysis, homogenization, and subsequent isolation of the membrane fraction by serial ultracentrifugation. | The obtained cell membranes were coated onto nanoparticles through serial extrusion using polycarbonate membrane with a size of 400 and 200 nm. | [84] |
| Macrophages | The macrophage ghost was obtained by freezing and thawing in liquid nitrogen of macrophages suspension in 50 nM phenylmethanesulfonyl fluoride (PMSF) added 0.25 × PBS. The lysate was washed with the buffer and centrifuged to obtain the macrophage ghost. | The resulting fresh macrophage ghost was sonicated with nanoparticle solution, followed by extruding through 400 and 100 nm polycarbonate porous membrane with an Avanti mini extruder. | [91] | |
| hCTLs | Lymphocytes were washed with PBS and lysed in hypotonic lysis buffer. The cells were disrupted on ice by the homogenizer. The homogenized cells were centrifuged at 4 °C. The supernatant was saved, then the pellet was resuspended in hypotonic lysis buffer and made another 20 passes and centrifuged again. The process was repeated until no intact cells remain. All the supernatants were subjected to sucrose density gradient ultracentrifugation at 4 °C. The collected membranes were retained, lyophilized, weighed, resuspended in 0.9% saline solution, and stored at 4 °C. | The isolated cell membranes were sonicated using the bath sonicator. The collected membrane vesicles were then coated onto nanoparticles through coextrusion them using an Avanti mini extruder. | [93] | |
| NK cells | Source cells were washed with PBS and centrifuged. The purified pellet was suspended in homogenization buffer and homogenized on ice. The homogenized mixture was collected and subjected to sucrose density gradient ultracentrifugation at 4 °C. The collected gradients were ultra-centrifuged 4 °C. Then, the obtained membrane fractions were diluted with saline and ultracentrifuged for purification. The isolated membranes were lyophilized, weighed, and stored at 4 °C. | The liposome was extruded together with the isolated NK cell membranes by polycarbonate membrane filter with the size 200 nm to get the resulting NKsomes. | [96] | |
| Neutrophils | Neutrophils were suspended in the ice-cold isolation buffer, and were homogenized using a homogenizer. The homogenate was then centrifuged at 4 °C. The supernatant was then collected and centrifuged at 4 °C to remove the mitochondria. The collected supernatant was then centrifuged at 4 °C. The cell membranes containing pellets were washed. After freeze-drying and weighting, the obtained membranes were stored at −80 °C for further use. | The membrane vesicles were suspended in water and sonicated on ice. Then, the vesicles were mixed with nanoparticles, and the mixture was sonicated again on ice. The collected solution was centrifuged at 4 °C to remove the excess neutrophil membranes. | [103] | |
| Platelets | Platelets were isolated by gradient centrifugation from whole blood. After that, the collected platelets were added into a lysis buffer for dissociation. Then, the solution was centrifuged to acquire the purified platelets membrane. | The nanoparticles and platelet membranes were sonicated together. Then, the mixture was stirred and maintained overnight to coat the platelet membranes onto nanoparticles. | [107] | |
| MSCs | The MSC membranes were washed with ice-cold PBS and put into lysis buffer. Afterward, the suspension was centrifuged, and the cell pellet was then collected, homogenized in solution, which contains mannitol, sucrose, bovine serum albumin, EDTA, Tris, and phosphatase and protease inhibitor cocktail. The collected solution was centrifuged at 4 °C. The supernatant was then ultracentrifuged at 4 °C. The resulting pellet was obtained for further use. | The obtained MSC membrane pellet solution was mixed with the prepared nanoparticles and ultrasonicated together. The mixture was centrifuged, and the MSC membrane-coated nanoparticles were obtained. | [112] | |
| Bacteria | The OMVs were purified by multiple centrifugation and filtration steps to ensure complete elimination of parent bacterial debris and free endotoxins. The crude OMVs collected were further separated by sequential density sucrose gradient ultracentrifugation. After that, the removal of free endotoxin was completed using endotoxin-removing columns. Then, the obtained OMVs were resuspended in 15% glycerol, filtered by 0.20 µm cellulose acetate filter, and stored at − 80 °C. | − | [120] | |
| Exosomes | The cell surface was induced to bud and generate giant plasma membrane vesicles (GPMVs) with the function of sodium deoxycholate. Then, GPMVs were purified under the low power of ultrasonic vibration to generate nanosized exosomes. | − | [126] | |
| RBC/Cancer cells | Apart from the manufacture of RBC membrane vesicles, as shown above, the cancer cells were washed with PBS at 4 °C and suspended in membrane protein extraction reagent A containing PMSF. The mixture was incubated in an ice bath and then centrifuged. The collected supernatant was further centrifuged to obtain the membrane. Moreover, the membrane was lyophilized and stored at −80 °C for further use. After that, RBC membrane vesicle was added to the cancer cell membrane. Then, they were sonicated at 37 °C to complete the fusion of different membranes. | The nanoparticles solution was added to the hybrid membrane solution. Then the mixture was sonicated to achieve the membrane-coated nanoplatforms. The resulting solution was centrifuged to get rid of the excess membrane. At last, the nanoplatforms were resuspended in deionized water for future use. | [132] | |
2. Types of cell-derived nanoplatforms fabricated for cancer-targeted drug delivery
2.1. Erythrocyte membrane-camouflaged nanoplatforms
Red blood cells (RBCs), also termed erythrocytes, are the most abundant cells in the blood circulation system. They are responsible for the gas exchange that occurs between the blood and all tissues or organs, including oxygen uptake and carbon dioxide dislodgement [53]. Erythrocytes can be functionally active for up to 120d, because of the presence of a protein called a cluster of differentiation 47 (CD47). CD47 is a transmembrane protein that is exposed on the membrane surface. It is an autologous identification marker that prevents cells from being engulfed and degraded by macrophages [54,55]. Erythrocytes are numerous in blood and easy to collect. Hence, the erythrocyte membrane was the first type of cell membrane used in the manufacture of biomimetic nanoplatforms. Coating erythrocyte membrane to the various nanocarriers was done to prolong residence in the blood and encourage their passive accumulation at the targeted tumor site. To date, erythrocyte membrane-camouflaged nanoplatforms have been the most exploited nanoformulation and have been widely employed in cancer chemotherapy, radiotherapy, molecular targeted therapy, photothermal therapy (PTT), and photodynamic therapy (PDT) [41,[56], [57], [58], [59].
In one study, Pei and the coworkers designed RBC membrane-camouflaged nanoparticles (RBC(M(TPC-PTX))), loaded with paclitaxel (PTX) dimeric prodrug (PTX2-TK) and photosensitizer 5,10,15,20-tetraphenylchlorin (TPC) for synergistic chemotherapy and PDT (Fig. 2A) [60]. As expected, nanoparticles loaded with PTX or TPC, and those loaded with both drugs had a homogeneous spherical structure. By co-extruding the RBC membrane-derived vesicles with PTX2-TK and TCP encapsulated nanoparticles (M(TPC-PTX)) through a 450 nm pore size polycarbonate membrane, the harvested RBC(M(TPC-PTX)) displayed a spherical core–shell structure. Under the camouflage provided by the RBC membranes, the concentration of the anticancer agent at the tumor site was improved, which illustrated that a prolonged circulation was achieved (Fig. 2B). To further investigate the in vitro cytotoxicity, HeLa cells of each group were identified using the established live–dead cell assay. In the control group, which used phosphate-buffered saline (PBS), the cells remained alive. In other groups, markedly more dead cells were observed in RBC(M(TPC-PTX)) (L+) group than those in the M(TPC-PTX) and M(TPC-PTX) (L+) groups. The outcome illustrated the synergetic inhibition evoked by chemotherapy and PDT. The synergetic anticancer efficacy was also investigated in vivo. Animals treated with RBC(M(TPC-PTX)) (L+) displayed more significant tumor ablation compared with other groups (Fig. 2C). The results indicate the potential of RBC(M(TPC-PTX)) nanoformulations in synergistic cancer therapy. As the most popular nanoplatform, the erythrocyte membrane-camouflaged formulations have demonstrated the practicability of biomimetic nanoplatforms in cancer-targeted drug delivery.
Fig. 2.
Manufacture of RBC(M(TPC-PTX)), brief mechanism in cancer therapy, pharmacokinetics performance, and inhibition of tumor growth. (A) Fabrication of RBC membrane-camouflaged nanoparticles for synergistic photodynamic/chemotherapy. (B) Pharmacokinetics data of PTX2-TK in RBC(M(TCP-PTX)) and M(TCP-PTX) groups. (C) Tumor suppression curve of subcutaneous xenografts in different groups. (A: PBS B: PBS(L+) C: M(PTX) D: M(PTX) (L+) E: M(TPC-PTX) F: M(TPC-PTX) (L+) G: RBC(M(TPC-PTX)) H: RBC(M(TPC-PTX)) (L+)). Reproduced with permission from [60]. Copyright 2018 American Chemical Society.
2.2. Cancer cell membrane-cloaked nanoplatforms
Cancer cells are one of the most frequently used source cells that have been investigated for biomimetic nanoparticles engineering. Nanoparticles coated with cancer cell membranes reportedly displayed identical surface adhesion molecules, such as epithelial cell adhesion molecule (EpCAM), galectin-3, and N-cadherin, as the source cancer cells. As such, they show a source cell-specific targeting phenomenon [61], which reflected the homologous binding mechanism of multicellular aggregation formation repeatedly noted in cancers [62], [63], [64], [65], [66]. Nowadays, various cancer cell membranes have been used to cloak the nanoparticles to direct their targeted drug delivery in cancer therapy [67], [68], [69], [70], [71].
In a study by Tian et al., a type of doxorubicin (DOX)/hemoglobin (Hb)-loaded and cancer cell membrane-cloaked nanoparticle (DHCNP) was fabricated (Fig. 3A) [70]. DHCNP was designed to selectively deliver the oxygen-loaded Hb to influence oxygen supply in hypoxia TME and further to overcome hypoxia-induced multidrug resistance. The results confirmed the preservation of the galectin-3, EpCAM, and N-cadherin proteins from the source MCF-7 cell line. To demonstrate the homologous targeting ability of the functionalized nanoplatforms, DHCNPs were incubated with various cell lines, and cell internalization was visualized through the fluorescence intensity detection of DOX. The fluorescence intensity in MCF-7 cells was much stronger than those in other groups, which verified the source cell-specific targeting behavior (Fig. 3B). In vivo anticancer efficacy data were also provided. As shown in Fig. 3C, compared with other groups, the DHCNP group displayed the lowest relative tumor volume, which demonstrated the promise of oxygen interference strategy in overcoming hypoxia-induced multidrug resistance. The results indicated the broad prospect of cancer cell membrane-derived nanoplatforms in cancer-targeted drug delivery.
Fig. 3.
Preparation of Oxy-DHCNP, characterization of homologous targeting property, and inhibition of tumor growth. (A) Fabrication process of DHCNP and therapeutic mechanism in overcoming hypoxia-induced multidrug resistance. (B) Homologous targeting behavior of DHCNP toward source MCF-7 cells, verified by fluorescence intensity. (C) Relative tumor volumes of different groups treated, respectively, with DHCNP, DCNP, DNP, free DOX, and PBS. Reproduced with permission from [70]. Copyright 2017 John Wiley & Sons.
In another example, Li et al. fabricated the multiantigenic nanoparticles (MANPs) using the cancer cell membrane (Fig. 4A) [72]. Through the coating of the cancer cell membrane, the resulting nanoparticles exhibited multiple tumor-associated antigens and were administered as the anticancer vaccines. The R837-loaded MANPs with different particle diameters were prepared, and their capability of immune stimulation was investigated. In vivo prophylactic effects on cancer revealed that, after prophylactic vaccination with different nanovaccine formulations, MANP/R837 with the size of 83 nm (MANP83/R837) displayed the most efficient antigen presentation through activating mature of dendritic cells (DCs), and showed the best efficacy of tumor prevention (Fig. 4B). At the same time, the median survival of MANP83/R837 group was the most extended compared with those of the other groups (Fig. 4C). Subsequent studies on cancer cell membrane-engineered nanoplatforms have been reported, although considerable progress is still required before these will be ready for clinical applications.
Fig. 4.
Brief mechanism in tumor suppression, in vivo prophylactic effects on tumor growth, and median survival data. (A) Process of immune stimulation by MANPs and mechanism in cancer prevention and treatment. (B) Tumor volumes of different groups vaccinated by MANP83/R837, MANP103/R837, MANP122/R837, MANP83, NP60/R837, NP60, or PBS. (C) Median survival of different treatment groups. ID, intradermal injection. Reproduced with permission from [72]. Copyright 2019 John Wiley & Sons.
2.3. White blood cell membrane-coated nanoplatforms
White blood cells (WBCs) constitute essential cells in the blood, with diameters ranging from 7 to 20 µm [73]. According to their different functions, WBCs are divided into numerous subtypes, including monocytes, macrophages, lymphocytes, neutrophils, eosinophils, basophilic granulocytes, and mastocytes. WBCs are crucial in the immune system, and protect the body against infection and foreign aggression [74]. When the body is infected or invaded, WBCs are recruited from the blood circulation to the injured site, at which point inflammation occurs together with the secretion of various cytokines [75]. Considering that the chronic inflammatory response is a primary characteristic of tumor tissue [76], and based on the inflammatory chemotactic properties of WBCs, many subtypes of WBCs are susceptible to the tumor-derived chemoattractants. These chemoattractants include chemokine (C−X−C motif) ligand (CXCL)1/2/3/4/5/7/8, vascular cell adhesion molecule-1 (VCAM-1), granulocyte-macrophage colony-stimulating factor (GM-CSF), endothelin-2 (ET-2), colony-stimulating factor-1 (CSF-1), and so forth [77], [78], [79], [80], [81]. Besides, through activation of the T cell receptors, T cells recognize and attack tumor cells [82]. Various WBCs have already been utilized in nanoparticle engineering for drug delivery. Notably, such WBC membrane-coated nanoparticles exhibit superior active targeting toward neoplastic cells with the minimal nonspecific distribution of the loaded drugs.
2.3.1. Monocyte membrane-wrapped nanoplatforms
Monocytes originate from hematopoietic stem cells of the bone marrow and are the precursors of macrophages. As the biggest WBCs, monocytes serve as a significant component of the body's defense system. They are capable of engulfing damaged and aging cells and the cell fragments with apparent deformational movements. They participate in the immune response by presenting the engulfed antigenic determinants to lymphocytes for the induction of specific immune responses. Under pathological conditions, the secretion of chemokine (C—C motif) ligand 2 (CCL2) by the tumor or stroma cells serves to recruit inflammatory monocytes, which express the chemokine (C—C motif) receptor 2 (CCR2), resulting in their accumulation at the tumor sites. Moreover, the presence of tumor-specific monocytes is often related to tumor metastasis and progression, along with poor clinical prognosis [83].
To exploit the binding mechanism between monocytes and tumor cells, Krishnamurthy et al. synthesized the DOX-loaded biodegradable nanoparticle, which had a poly(lactic-co-glycolic acid) (PLGA) core wrapped by monocyte membrane [84]. In this nanoplatform, the membrane protein pattern was consistent between monocyte (U937) membrane and the shell of the prepared nanoparticle, verifying the preservation of cell membrane conjugating proteins. In addition, monocyte membrane-coated nanoparticle avidly targeted MCF-7 cells, better than bare PLGA NP, further demonstrating the cell receptor-specific binding phenomenon. In another example, Li et al. developed a nanoparticle-based drug delivery system against an astrocytoma cell line incorporating a bone marrow-derived monocyte membrane coating as a shell [85]. The monocyte membrane shell protected the nanoparticle from being eliminated by the mononuclear phagocyte system, with the chemokine receptor CXCR4 on monocyte membrane able to promote the active tumor-tropism of the nanoparticle-based drug delivery system. The outcomes from these two studies revealed that the monocyte membrane-wrapped nanoparticles have great potential for cancer-targeted drug delivery. Further studies will explore this potential.
2.3.2. Macrophage membrane-biomimetic nanoplatforms
Macrophages are categorized as a type of functionalized immune cells that are derived from monocytes [86]. They are widely distributed in various tissues and organs of the body and are capable of recognizing and engulfing foreign invaders or other non-self-materials. The engulfed antigens can then be presented to DCs or T cells after phagocytosis, and the step is essential in antigen-specific immune responses. Similar to monocyte, macrophage membranes have been extracted and used in the manufacture of nanoparticle-based drug delivery systems, and have proven to be crucial in cancer therapy owing to their inherent cell membrane proteins [87], [88], [89], [90]. For example, Zhao et al. reported the development of macrophage membrane-coated quercetin (QE)-loaded bismuth selenide nanoparticles (M@BS-QE NPs) for the inhibition of breast cancer lung metastasis [90]. The recruitment of M@BS-QE NPs was through the way of CCL2/CCR2 chemokine interaction. Besides, tumor recognition of M@BS-QE NPs was realized via α4/VCAM-1 interaction. QE encapsulated in nanoparticles acted as a synergistic PTT-sensitive agent by inhibiting the expression of heat shock protein 70 and preventing tumor progression through the reduction of p-Akt/matrix metalloproteinase-9 pathway signaling. The therapeutic effect against lung metastasis of breast cancer was shown in a BALB/c mouse model, which further indicated the synergistic inhibition efficacy associated with M@BS-QE NPs.
As reported in most recent studies, different types of cell membrane-engineered nanoparticles are integrally endocytosed by the targeted cells, including both the internal core and the outer cell membranes. However, a newly published study reported that prior to the endocytosis, the outer envelope membrane surface was removed, which was termed the membrane escape phenomenon [91]. The phenomenon, also termed the “proton sponge” effect [92], is based on the ability of cationic polymer nanoplatforms to cause an influx of extra electrolytes and water into the acidic late endosome, which obtains an internal balance of ionic strength and electrical neutrality. This process caused the surface membrane rupturing with the overflow of the internal core. Owing to the membrane escape phenomenon, the macrophage membrane-coated nanoparticles actively targeted the TME and then displayed a step-by-step release of anticancer agents following the removal of the outer membrane when the pH value changed in the extracellular and intracellular TME [91]. This work inspired the rational design of membrane-biomimetic nanoplatforms in cancer-targeted drug delivery, although additional verification and exploration are required for further biomedical application.
Furthermore, because of the fluid character of lipid bilayers in living cells, we hypothesize that during the endocytosis of nanoparticles, the outer shell of the nanoparticle is likely to interact with the targeted cell membranes through the partial exchange of lipid bilayers. Thus, this process might be utilized for tumor cell membrane-specific drug delivery and therapy, similar to that afforded by the small molecule programmed cell death-ligand 1 inhibitors. However, there is as yet no relevant research evidence in support of this hypothesis. Such studies are warranted.
2.3.3. Lymphocyte membrane-covered nanoplatforms
Lymphocytes are essential components in the human body's immune response, although they are the smallest WBCs. Depending on their occurrence, surface molecules, and individual functions, lymphocytes are categorized as T cells, B cells, and natural killer (NK) cells. Both T cells and B cells are antigen-specific lymphocytes, as they become mature and functional after encountering an antigen trigger, thereby producing a cytotoxic effect or secretion of the corresponding antibodies. NK cells have cytotoxic effects without relying on antigen stimulation and have wide-ranging effects on targets.
Taking advantage of the capability of lymphocyte antigen recognition, several lymphocyte membrane-covered nanoplatforms have been developed and applied for cancer-targeted drug delivery. In the work of Zhang et al., membrane vesicles were extracted from human cytotoxic T lymphocytes (hCTLs) [93]. The vesicles used to cover PTX-loaded PLGA NP to form an hCTL-inspired nanoformulation. Low-dose irradiation (LDI) promotes local infiltration of intratumoral CD8+ T cells [94], resulting from the upregulation of membrane adhesion molecule expression on tumor vasculature and the enhanced release of chemoattractants [95]. As such, LDI was administered in the study to facilitate targeting of the hCTL-inspired nanoformulations toward the tumor site. In addition, through the synergistic chemotherapeutic inhibition mediated by the encapsulated PTX, therapeutic efficacy against a subcutaneous gastric cancer xenograft model was also amplified.
NK cells, the crucial effector cells in innate immunity, have also been utilized for nanoparticle-based research. The NK cell membranes were employed to coat DOX-loaded nanoparticle for cancer-targeted treatment [96]. Notably, the lipid bilayer of the membrane-coated nanoparticle and targeted tumor cells fused prior to the discharge of the DOX from the nanoparticle inside the tumor cells, with the membrane-covered nanoparticle not being integrally engulfed. B cells have not yet been used in the design of biomimetic nanoformulations, although additional anticancer studies are expected to be conducted based on other types of lymphocytes.
2.3.4. Neutrophil membrane-enveloped nanoplatforms
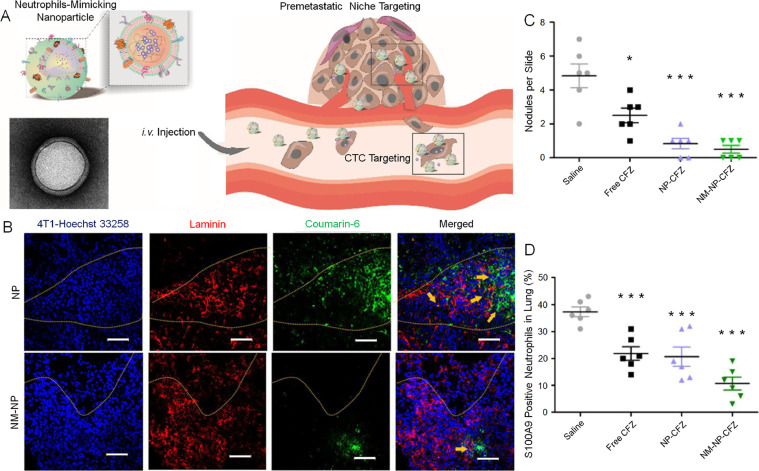
Typically, neutrophils serve as a crucial barrier against foreign intruders. Early studies described that neutrophils were cytotoxic to malignant cells [97,98]. However, other studies showed that tumor-associated neutrophils isolated from both tumor-bearing mice and patients with gastric cancer were not cytotoxic to the tumor cells [99,100]. Instead, these neutrophils contributed to tumor progression and metastasis, with adhesion molecules exposed on the neutrophil membranes implicating in the adherence to cancer cells [101,102]. Kang et al. designed a neutrophil membrane-enveloped nanoparticle (NM-NP) that was capable of actively targeting circulating tumor cells and inhibiting the pre-metastatic niche (Fig. 5A) [103]. In an animal model of tumor pre-metastasis, different mice were intravenously injected with coumarin-6-labeled nanoparticles or NM-NPs. As shown in the outcome, the neutrophil membrane coating resulted in better targeting to the pre-metastatic region in the lung (Fig. 5B). Moreover, after loading with carfilzomib (CFZ), the nanoplatforms demonstrated the enhanced therapeutic capability to reduce the metastatic foci. Moreover, the quantification of metastasis nodules in lung tissue slides revealed the fewest metastasis nodules in the CFZ-loaded NM-NP group (Fig. 5C). S100A9, expressed by neutrophils, is a metastatic niche-promoting molecule. The recruitment of S100A9-expressing neutrophils to metastatic sites was also investigated [103]. Dramatically reduced expression of S100A9 in the lung was noted after administration of CFZ-loaded NM-NPs compared to those in the other groups, and lung sections from the CFZ-loaded NM-NP group revealed the lowest level of S100A9-positive neutrophils (Fig. 5D). These results demonstrated the marked potential for treating metastatic tumors and regulating the TME through the bioengineering of various nanoparticles with neutrophil membranes.
Fig. 5.
Fabrication of neutrophil-mimicking nanoparticle (NM-NP), assessment of pre-metastatic niche targeting, and application in lung metastasis therapy. (A) NM-NP for targeting CTCs and pre-metastatic niche. (B) Targeting property of NM-NP compared with naked nanoparticle in the pre-metastatic region as displayed by confocal microscopy. Scale bar: 50 µm. (C) Quantification of metastasis nodules in lung tissue slides of different groups. (D) S100A9 positive neutrophil level in different treating groups. Reproduced with permission from [103]. Copyright 2017 American Chemical Society.
2.4. Platelet membrane-masquerading nanoplatforms
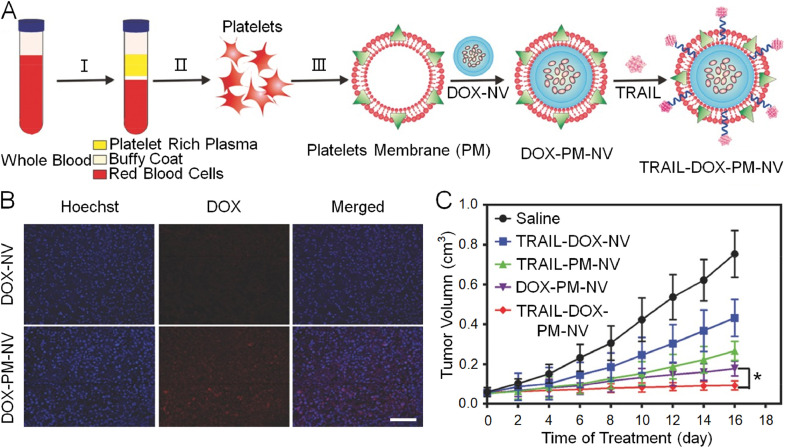
Platelets are generically derived from mature megakaryocytes. Their primary physiological function is participation in hemostasis. When blood vessels are injured and leaking, platelets are quickly recruited around the damaged vascular endothelial cells and aggregate into clusters to form a thrombus, thereby blocking the leakage and benefiting the recovery of the body. However, a recent study revealed their contribution to tumor progression and metastasis [104]. Typically, the membrane protein P-selectin on platelets combines with the CD44 receptor, which is overexpressed on tumor cell membranes. The combination facilitates interaction and adherence of platelets around the circulating tumor cells and promotes the formation of distant metastatic lesions [105,106]. Based on this distinct adherence mechanism between platelets and cancer cells, Hu et al. fabricated a platelet-mimicking nanovehicle, which was further modified by decoration with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and loading with DOX (Fig. 6A) [107]. Masked by the platelet membranes, the synthetic platelets membrane-mimicking nanovehicles exhibited a favorable performance with respect to hemodynamics, and active targeting capability was observed (Fig. 6B). The platelet-mimicking nanovehicles also displayed enhanced anticancer efficacy due to TRAIL decoration and DOX loading, as shown in Fig. 6C. Other studies have identified the key determinant of the platelet membrane in targeted drug accumulation. Further studies will be needed before this potential translates to clinical applications.
Fig. 6.
Fabrication process of TRAIL-DOX-PM-NV, active targeting property, and inhibition of tumor growth. (A) Schematic illustration of preparation of TRAIL-DOX-PM-NV. (B) Fluorescence distribution of tumors treated with TRAIL-DOX-NV and TRAIL-DOX-PM-NV. Scale bar: 100 µm. (C) Tumor volume following administration of saline, TRAIL-DOX-NV, TRAIL-PM-NV, DOX-PM-NV, and TRAIL-DOX-PM-NV. Reproduced with permission from [107]. Copyright 2015 John Wiley & Sons.
2.5. Mesenchymal stem cell membrane-shrouded nanoplatforms
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into a variety of cell types. Additionally, they exhibit immunomodulatory properties in pathological situations, including tumor occurrence, and often show a high affinity toward neoplastic cells [108]. Consequently, they are widely applied in cancer and immune-related research. Considering that MSCs express specific cell adhesion molecules, such as CXCR4, epidermal growth factor (EGF), integrins, and extracellular matrix, they are easily attracted by the tumoral chemoattractants that include stromal cell-derived factor 1 or directly interact with the targeted ligands like human epidermal growth factor receptor-2 (HER2) [108], [109], [110], [111]. Moreover, taking advantage of the property of their affinities with neoplastic cells, MSCs have been utilized to decorate nanoparticles for cancer-targeted drug delivery.
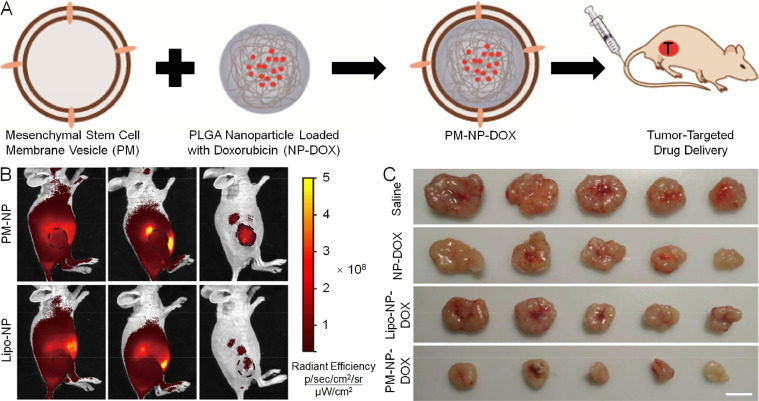
In a recent study, Yang et al. functionalized synthetic PLGA NPs with the biological properties of MSCs [112]. The PLGA NPs harboring DOX were coated with MSC membranes and administered intravenously for active tumor-specific drug delivery (Fig. 7A). A tumor-specific tropism assay was conducted in vivo between the liposome-coated nanoparticles and MSC membrane-coated nanoparticle groups. Twenty hours after administration, distinct fluorescence distribution was noted between the two groups, which illustrated that tumor-specific tropism was inherited from the source MSCs (Fig. 7B). The tumor inhibition assay was also carried out. After four administrations over 15 days, tumor growth was inhibited best in mice who received MSC membrane-coated nanoparticles containing DOX (Fig. 7C). In two other studies, MSC membrane-shrouded nanoparticles also displayed active tumor targeting and efficacious treatment of cancer-based on PDT and chemotherapy in vitro and in vivo [113,114].
Fig. 7.
Preparation of PM-NP-DOX, evaluation of tumor-specific targeting ability, and inhibition of tumor growth. (A) MSC membrane-shrouded polymeric PLGA nanoplatform for tumor-specific DOX delivery. (B) Tumor-specific targeting capability between lipo-NP and PM-NP groups. (C) Tumor inhibition efficacy after different treatments. PM: cell plasma membrane. Reproduced with permission from [112]. Copyright 2018 American Chemical Society.
2.6. Bacteria-based nanoplatforms
The human body hosts many microbes that are essential to metabolism and other biological activities. These microbes include bacteria, fungi, rickettsia, and viruses. Bacteria are unicellular organisms that lack both organelles and a membrane-bound nucleus [115]. While some bacteria are pathogenic, others can be very beneficial for preventing or treating diseases, and there are many ongoing studies on the potential use of bacteria in nanoparticle engineering for cancer-targeted drug delivery.
Typically, bacteria-based nanoparticles are constructed to replicate a particular bacterial cell component, such as the S-layer, bacterial ghosts, outer membrane vesicles (OMVs), endospores, and magnetosomes. Each bacterium has unique properties and different biologically active components, which may be useful for active tumor targeting. For example, the Streptococcus bacteria contains a bacterial polymer called hyaluronic acid (HA) targeting the cell membrane protein CD44 receptor and then produces HA-based nanoparticles that are effective cargo carriers for drug delivery [116], [117], [118]. Xie et al. reported that siRNA-loaded nanoparticles ornamented with HA displayed enhanced inhibition of hepatocellular carcinoma. Due to this property of HA, it has been widely employed to decorate nanoparticles in order to improve drug delivery [119]. In another example, bacterial OMVs have also been utilized for cancer-targeted drug delivery (Fig. 8A) [120]. OMV-based nanoparticles are cytotoxic to tumor cells in vitro, and they are able to actively target HER2-overexpressed tumor cells and induce inhibition of tumor growth in vivo after being modified with an engineered anti-HER2 antibody (Fig. 8B and C). Despite the numerous benefits of using bacteria-based drug delivery vectors, it is essential to recognize the risk for potential infections or antibiotic infections in clinical trials.
Fig. 8.
Fabrication of Escherichia coli (E. coli) generating OMV and assessment of in vitro and in vivo efficacy. (A) E. coli generating OMV displaying HER2-specific affibody and loaded siRNA for cancer therapy. (B) In vitro cell viability evaluation among different groups. (C) Excised tumor tissues of different groups after therapy. Reproduced with permission from [120]. Copyright 2014 American Chemical Society.
2.7. Exosome-mimicking nanoplatforms
The exosomes are multifunctional vehicles that transfer biological information between cells upon stimulation. They are the smallest types of extracellular vehicles with particle sizes ranging from 40 to 100 nm [121]. Although once regarded as the transporters of waste materials from cells, exosomes are currently thought to be multifunctional vehicles that transfer biological information between diverse cells [122]. After being externally secreted, exosomes usually move toward the binding molecules, such as acetylcholine receptor, HER2, and endothelial cell adhesion molecules on the surface of the targeted cells [121,[123], [124], [125], [126]. They are internalized by these targeted recipient cells, with the loaded cargos. These cargos include mRNAs or miRNAs, which cause the targeted gene silencing or the production of certain encoded proteins [127,128].
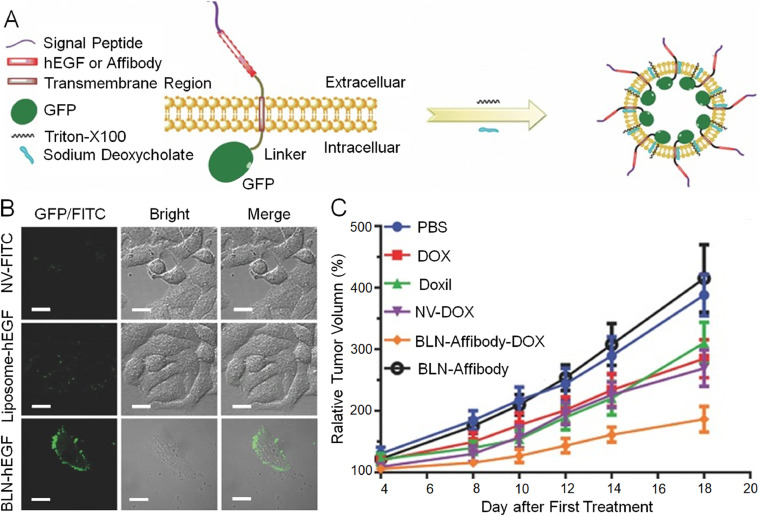
Due to this active targeting property, exosomes have been studied for their potential applications in cancer therapeutics. Qi et al. isolated transferrin receptor-expressing exosomes from blood plasma and loaded them with DOX. These nanosized exosomes were then modified with engineered transferrin, giving them a super-paramagnetic property. Under an external magnetic field, the super-paramagnetic nanoparticles actively targeted and inhibited tumor growth [129]. In another study, Zhang et al. genetically engineered biofunctionalized liposome-like nanovesicles (BLNs) to express human EGF on their biointerface as targeting moieties and loaded them with DOX (Fig. 9A) [126]. These BLNs displayed better-targeting capabilities than artificial liposome nanovesicles covalently combined with hEGF and marked with fluorescein isothiocyanate (Fig. 9B). Moreover, they even exhibited better anticancer efficacy than clinically approved liposomal doxorubicin (DoxilⓇ) when administered in HER2-overexpressing tumor xenograft models (Fig. 9C). These studies demonstrated that with excellent targeting property and smart engineering strategies, exosome-based nanoparticles have considerable potential for future clinical applications.
Fig. 9.
Illustration of forming process of BLN, characterization of targeting performance, and inhibition of tumor growth. (A) By induction of surfactants, BLN budding from cell surface was completed. (B) Superior targeting performance of BLN-hEGF. (C) Relative tumor volume of different groups. Reproduced with permission from [126]. Copyright 2017 John Wiley & Sons.
2.8. Hybrid membrane-veiled nanoplatforms
Various cell membrane sources have been utilized in nanoparticle engineering since the original use of RBC membranes. The shell of the nanoparticles displayed unitary cell-derived formulations in most cases. In order to harness the distinct characteristics from different cells, Dehaini et al. fabricated an erythrocyte-platelet hybrid membrane-veiled nanoparticle as a proof-of-concept [130]. Notably, the researchers demonstrated that the dual-membrane formulation was realized with the inheritance of both source cell properties. Subsequently, hybrid membrane-veiled nanoparticles were further developed for cancer-targeted drug delivery. He et al. reported a composite cell membrane modality wherein leukocytes, and cancer cells were employed as the source cells [131]. Because of the dual-membrane extraction, both the preeminent homing toward the tumor site and prolonged circulation were realized compared with the single source cell formulation. Moreover, the encapsulation of PTX improved the anticancer efficacy without inducing severe side effects. In another example, a similar method was adopted based on RBCs and B16F10 cancer cells as the membrane sources [132]. The hollow copper sulfide nanoparticles were prepared and encapsulated with DOX (DCuS NPs), followed by hybrid membrane coating (Fig. 10A). Because of the membrane camouflage, DCuS NPs exhibited active tumor-specific tropism together with prolonged blood retention. The composite membrane-veiled nanoparticles (DCuS@[RBC-B16] NPs) also showed near-total inhibition of tumor growth with synergistic PTT and chemotherapy (Fig. 10B). Systematic toxicity was also investigated. No obvious body weight loss was observed in the DCuS@[RBC-B16] NPs-treated animals (Fig. 10C).
Fig. 10.
Manufacture of DCuS@[RBC-B16] NP, inhibition of tumor growth, and systematic safety assessment. (A) Schematic presentation of DCuS@[RBC-B16] NP synthesis. (B) Relative tumor volume following administration of each groups (1: NS, 2: CuS@[RBC-B16], 3: DOX, 4: NIR laser (1064 nm, 1.0 W/cm2), 5: DCuS@[RBC-B16], 6: CuS@[RBC-B16] with NIR laser (1064 nm, 1.0 W/cm2), 7: DCuS@[RBC-B16] with NIR laser (1064 nm, 1.0 W cm−2)). (C) Follow-up investigation of body weight in each group. Reproduced with permission from [132]. Copyright 2018 American Chemical Society.
3. Discussion and outlook
We have summarized different paradigms of membrane-camouflaged nanoplatforms that have been extensively developed for cancer-targeted drug delivery. This new biointerface engineering strategy leverages the interaction between the modified biomimetic nanomaterials and the organism itself, the targeted neoplastic cells, or the TME. Numerous cell types have been explored for the engineering of nanoparticle-based drug delivery systems, including RBCs, tumor cells, WBCs, platelets, and stem cells. As well, material and medical science have been advancing encouragingly. This progress has synergistically advanced cancer therapy and improved the therapeutic outcomes of these biofunctionalized nanoplatforms.
Nevertheless, additional efforts are necessary, and many issues deserve further exploration. For example, most studies have established that in the metabolism of these biomimetic nanocarriers, the outer biofilms were endocytosed by the targeted cancer cells together with the inner cores. However, considering that certain functions of membrane proteins are preserved after membrane coating, it is possible that the properties of the phospholipid bilayer itself, such as membrane fluidity, may be retained after being extracted and coated onto the nanoparticles, along with the occurrence of membrane exchange between the nanoparticles and targeted cells during endocytosis activity. This process, if confirmed by additional studies, might be used to targeted deliver anticancer drugs from the shell of the nanoparticles to tumor cell membranes and fulfill cancer cell membrane-specific drug delivery. In addition, as indicated in one study, the outer membranes of nanoparticles were removed in the acidic TME before endocytosis [91]. However, relevant data are scant, and more studies are required. If confirmed, anticancer drugs loaded in outer membranes and inner nanoparticles can be respectively delivered to different target sites.
Clinical transformations and applications are the goals of cancer-associated research, including cell membrane-engineered nanomedicines. Several issues remain to be solved. For example, cancer cell membrane-coated nanoparticles are popular in targeted anticancer research on animals. However, patients may not accept the use of the tumor-derived components that need to be administered back into their bodies for therapy. Clinical trials in this area will be challenging in terms of approval and execution. The use of standardized cell lines in cancer research is usually done. Nevertheless, heterogeneity is a principal attribute of cancer, which often results in treatment failure. As a result, bench-to-bedside individualized treatment based on cancer heterogeneity is the most critical challenge to be solved.
In conclusion, there are still many opportunities for additional studies, especially on the mechanisms of tumorigenesis and progression. Moreover, additional opportunities ought to exist for the application of nanotechnology in targeted oncological research and clinical translation. The myriad innovative advances in the future will promote the development of accurate and efficient nanomedicine and will hopefully solve the crucial problems of cancer-targeted drug delivery to benefit human health.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Grant Nos. 51973216, 51873207, 51803006, 51673190, 51603204, 51673187, and 51520105004), the Science and Technology Development Program of Jilin Province (Grant Nos. 20190201068JC, 20170101102JC, and 20160414047GH), the Medical and Health Program of Jilin Province (Grant No. 20190304047YY), the Youth Talents Promotion Project of Jilin Province (Grant No. 181909), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (Grant No. 2019005).
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Haanen J.B.A.G., Carbonnel F., Robert C., Kerr K.M., Peters S., Larkin J. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:119–142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 3.Cukier P., Santini F.C., Scaranti M., Hoff A.O. Endocrine side effects of cancer immunotherapy. Endocr-Relat Cancer. 2017;24(12):T331–T347. doi: 10.1530/ERC-17-0358. [DOI] [PubMed] [Google Scholar]
- 4.Hescot S., Haissaguerre M., Pautier P., Kuhn E., Schlumberger M., Berdelou A. Immunotherapy-induced Addison's disease: a rare, persistent and potentially lethal side-effect. Eur J Cancer. 2018;97:57–58. doi: 10.1016/j.ejca.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Pfoeher C., Eichler H., Burgard B., Krecke N., Mueller C.S.L., Vogt T. A case of immune thrombocytopenia as a rare side effect of an immunotherapy with PD1-blocking agents for metastatic melanoma. Transfus Med Hemother. 2017;44(6):426–428. doi: 10.1159/000479237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Wang G.D., Chuang Y.J., Zhen Z., Chen X., Biddinger P. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 2015;15(4):2249–2256. doi: 10.1021/nl504044p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L., Liu Y., Chang R., Xing R., Yan X. Supramolecular photothermal nanomaterials as an emerging paradigm toward precision cancer therapy. Adv Funct Mater. 2019;29(4) [Google Scholar]
- 8.Li S., Feng X., Wang J., He L., Wang C., Ding J. Polymer nanoparticles as adjuvants in cancer immunotherapy. Nano Res. 2018;11(11):5769–5786. [Google Scholar]
- 9.Wang Q., Zhang P., Li Z., Feng X., Lv C., Zhang H. Evaluation of polymer nanoformulations in hepatoma therapy by established rodent models. Theranostics. 2019;9(5):1426. doi: 10.7150/thno.31683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H., Dai F., Ma G., Zhang X. Theranostic gold nanomicelles made from biocompatible comb-like polymers for thermochemotherapy and multifunctional imaging with rapid clearance. Adv Mater. 2015;27(24):3645–3653. doi: 10.1002/adma.201501420. [DOI] [PubMed] [Google Scholar]
- 11.Feng L., Cheng L., Dong Z., Tao D., Barnhart T.E., Cai W. Theranostic liposomes with hypoxiaactivated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano. 2017;11(1):927–937. doi: 10.1021/acsnano.6b07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianella A., Jarzyna P.A., Mani V., Ramachandran S., Calcagno C., Tang J. Multifunctional nanoemulsion platform for imaging guided therapy evaluated in experimental cancer. ACS Nano. 2011;5(6):4422–4433. doi: 10.1021/nn103336a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M., Li Z.H., Xu F.J., Lai L.H., Wang Q.Q., Tang G.P. An oligopeptide ligand-mediated therapeutic gene nanocomplex for liver cancer-targeted therapy. Biomaterials. 2012;33(7):2240–2250. doi: 10.1016/j.biomaterials.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 14.Lu X., Wang Q.Q., Xu F.J., Tang G.P., Yang W.T. A cationic prodrug/therapeutic gene nanocomplex for the synergistic treatment of tumors. Biomaterials. 2011;32(21):4849–4856. doi: 10.1016/j.biomaterials.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Ragelle H., Crauste-Manciet S., Seguin J., Brossard D., Scherman D., Arnaud P. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int J Pharm. 2012;427(2):452–459. doi: 10.1016/j.ijpharm.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Xiao H., Song H., Yang Q., Cai H., Qi R., Yan L. A prodrug strategy to deliver cisplatin(IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials. 2012;33(27):6507–6519. doi: 10.1016/j.biomaterials.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X., Luo M., Liu G., Wang X., Tao W., Lin Y. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv Sci. 2018;5(10) doi: 10.1002/advs.201800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R., Song X., Liang C., Yi X., Song G., Chao Y. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials. 2017;138:13–21. doi: 10.1016/j.biomaterials.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M., Hu B., Gu Z., Joo K.I., Wang P., Tang Y. Degradable polymeric nanocapsule for efficient intracellular delivery of a high molecular weight tumor-selective protein complex. Nano Today. 2013;8(1):11–20. [Google Scholar]
- 20.Feng X., Xu W., Li Z., Song W., Ding J., Chen X. Immunomodulatory nanosystems. Adv Sci. 2019;6(17) doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng X.R., Ding J.X., Gref R., Chen X.S. Poly(β-cyclodextrin)-mediated polylactide-cholesterol stereocomplex micelles for controlled drug delivery. Chin J Polym Sci. 2017;35(6):693–699. [Google Scholar]
- 22.Jiang Z., Liu Y., Feng X., Ding J. Functional polypeptide nanogels. J Funct Polym. 2019;32(1):13–27. [Google Scholar]
- 23.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 24.Ji X., Wang C., Tang M., Guo D., Peng F., Zhong Y. Biocompatible protamine sulfate@silicon nanoparticle-based gene nanocarriers featuring strong and stable fluorescence. Nanoscale. 2018;10(30):14455–14463. doi: 10.1039/c8nr03107j. [DOI] [PubMed] [Google Scholar]
- 25.Wang A.Z., Langer R., Farokhzad O.C. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M., Hagan C.T., Min Y., Foley H., Tian X., Yang F. Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials. 2018;169:1–10. doi: 10.1016/j.biomaterials.2018.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conde J., Oliva N., Zhang Y., Artzi N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat Mater. 2016;15(10):1128–1138. doi: 10.1038/nmat4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta B., Ramasamy T., Poudel B.K., Pathak S., Regmi S., Choi J.Y. Development of bioactive pegylated nanostructured platforms for sequential delivery of doxorubicin and imatinib to overcome drug resistance in metastatic tumors. ACS Appl Mater Interfaces. 2017;9(11):9280–9290. doi: 10.1021/acsami.6b09163. [DOI] [PubMed] [Google Scholar]
- 29.Rodell C.B., Arlauckas S.P., Cuccarese M.F., Garris C.S., Ahmed R.L.M.S., Kohler R.H. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2(8):578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao D., Li M., Wang Z., Zheng X., Lao Y.H., Chang Z. Bioinspired diselenide-bridged mesoporous silica nanoparticles for dual-responsive protein delivery. Adv Mater. 2018;30(29) doi: 10.1002/adma.201801198. [DOI] [PubMed] [Google Scholar]
- 31.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshantri A.K., Moreira A.V., Ecker V., Mandhane S.N., Schiffelers R.M., Buchner M. Nanomedicines for the treatment of hematological malignancies. J Controlled Release. 2018;287:194–215. doi: 10.1016/j.jconrel.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 33.Liu R., Xiao W., Hu C., Xie R., Gao H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Controlled Release. 2018;278:127–139. doi: 10.1016/j.jconrel.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z.B., Long J., Zhao Y.Y., Yang J.B., Jiang W., Liu Q.Z. Adaptive immune cells are necessary for the enhanced therapeutic effect of sorafenib-loaded nanoparticles. Biomater Sci. 2018;6(4):893–900. doi: 10.1039/c8bm00106e. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Li H., Chen Y., Jin Q., Ren K., Ji J. Mixed-charge nanoparticles for long circulation, low reticuloendothelial system clearance, and high tumor accumulation. Adv Healthcare Mater. 2014;3(9):1439–1447. doi: 10.1002/adhm.201300617. [DOI] [PubMed] [Google Scholar]
- 36.Rao L., Xu J.H., Cai B., Liu H., Li M., Jia Y. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology. 2016;27(8) doi: 10.1088/0957-4484/27/8/085106. [DOI] [PubMed] [Google Scholar]
- 37.Ishihara T., Takeda M., Sakamoto H., Kimoto A., Kobayashi C., Takasaki N. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res. 2009;26(10):2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 38.Yang Q., Lai S.K. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2015;7(5):655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi K.Y., Yoon H.Y., Kim J.H., Bae S.M., Park R.W., Kang Y.M. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. ACS Nano. 2011;5(11):8591–8599. doi: 10.1021/nn202070n. [DOI] [PubMed] [Google Scholar]
- 40.Duo Y., Yang M., Du Z., Feng C., Xing C., Wu Y. Cx-5461-loaded nucleolus-targeting nanoplatform for cancer therapy through induction of pro-death autophagy. Acta Biomater. 2018;79:317–330. doi: 10.1016/j.actbio.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Fu Q., Lv P., Chen Z., Ni D., Zhang L., Yue H. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale. 2015;7(9):4020–4030. doi: 10.1039/c4nr07027e. [DOI] [PubMed] [Google Scholar]
- 42.Lv Y., Cao Y., Li P., Liu J., Chen H., Hu W. Ultrasound-triggered destruction of folate-functionalized mesoporous silica nanoparticle-loaded microbubble for targeted tumor therapy. Adv Healthcare Mater. 2017;6(18) doi: 10.1002/adhm.201700354. [DOI] [PubMed] [Google Scholar]
- 43.Roncato F., Rruga F., Porcu E., Casarin E., Ronca R., Maccarinelli F. Improvement and extension of anti-EGFR targeting in breast cancer therapy by integration with the avidin-nucleic-acid-nano-assemblies. Nat Commun. 2018;9(1):4070. doi: 10.1038/s41467-018-06602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.H., Jang J.T., Choi J.S., Moon S.H., Noh S.H., Kim J.W. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nanotechnol. 2011;6(7):418–422. doi: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]
- 45.Park D., Cho Y., Goh S.H., Choi Y. Hyaluronic acid-polypyrrole nanoparticles as pH-responsive theranostics. Chem Commun. 2014;50(95):15014–15017. doi: 10.1039/c4cc06349j. [DOI] [PubMed] [Google Scholar]
- 46.Stephen Z.R., Kievit F.M., Veiseh O., Chiarelli P.A., Fang C., Wang K. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O-6-benzylguanine to brain tumors. ACS Nano. 2014;8(10):10383–10395. doi: 10.1021/nn503735w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X., Liu X., Liu Z., Pu F., Ren J., Qu X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv Mater. 2012;24(21):2890–2895. doi: 10.1002/adma.201104797. [DOI] [PubMed] [Google Scholar]
- 48.Gong Y.K., Winnik F.M. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale. 2012;4(2):360–368. doi: 10.1039/c1nr11297j. [DOI] [PubMed] [Google Scholar]
- 49.Tuosto L., Xu C. Editorial: membrane lipids in T cell functions. Front Immunol. 2018;9:01608. doi: 10.3389/fimmu.2018.01608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vijayan V., Uthaman S., Park I.K. Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers (Basel) 2018;10(9):983. doi: 10.3390/polym10090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bose R.J.C., Paulmurugan R., Moon J., Lee S.H., Park H. Cell membrane-coated nanocarriers: the emerging targeted delivery system for cancer theranostics. Drug Discov Today. 2018;23(4):891–899. doi: 10.1016/j.drudis.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Wu M., Le W., Mei T., Wang Y., Chen B., Liu Z. Cell membrane camouflaged nanoparticles: a new biomimetic platform for cancer photothermal therapy. Int J Nanomed. 2019;14:4431–4448. doi: 10.2147/IJN.S200284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugie J., Intaglietta M., Sung L.A. Water transport and homeostasis as a major function of erythrocyte. Am J Physiol: Heart Circ Physiol. 2018;314(5):H1098–H1107. doi: 10.1152/ajpheart.00263.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 55.Sosale N.G., Spinier K.R., Alvey C., Discher D.E. Macrophage engulfment of a cell or nanoparticle is regulated by unavoidable opsonization, a species-specific 'Marker of self' CD47, and target physical properties. Curr Opin Immunol. 2015;35:107–112. doi: 10.1016/j.coi.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aryal S., Hu C.M.J., Fang R.H., Dehaini D., Carpenter C., Zhang D.E. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine. 2013;8(8):1271–1280. doi: 10.2217/nnm.12.153. [DOI] [PubMed] [Google Scholar]
- 57.Liu T., Shi C., Duan L., Zhang Z., Luo L., Goel S. A highly hemocompatible erythrocyte membrane-coated ultrasmall selenium nanosystem for simultaneous cancer radiosensitization and precise antiangiogenesis. J Mater Chem B. 2018;6(29):4756–4764. doi: 10.1039/C8TB01398E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piao J.G., Wang L., Gao F., You Y.Z., Xiong Y., Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8(10):10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 59.Ren X., Zheng R., Fang X., Wang X., Zhang X., Yang W. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials. 2016;92:13–24. doi: 10.1016/j.biomaterials.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 60.Pei Q., Hu X., Zheng X., Liu S., Li Y., Jing X. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano. 2018;12(2):1630–1641. doi: 10.1021/acsnano.7b08219. [DOI] [PubMed] [Google Scholar]
- 61.Fang R.H., Hu C.M.J., Luk B.T., Gao W., Copp J.A., Tai Y. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glinsky V.V., Glinsky G.V., Glinskii O.V., Huxley V.H., Turk J.R., Mossine V.V. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63(13):3805–3811. [PubMed] [Google Scholar]
- 63.Iurisci I., Cumashi A., Sherman A.A., Tsvetkov Y.E., Tinari N., Piccolo E. Synthetic inhibitors of galectin-1 and -3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res. 2009;29(1):403–410. [PubMed] [Google Scholar]
- 64.Khaldoyanidi S.K., Glinsky V.V., Sikora L., Glinskii A.B., Mossine V.V., Quinn T.P. MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by thomsen-friedenreich antigen-galectin-3 interactions. J Biol Chem. 2003;278(6):4127–4134. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- 65.Osta W.A., Chen Y., Mikhitarian K., Mitas M., Salem M., Hannun Y.A. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64(16):5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 66.Yue X.S., Murakami Y., Tamai T., Nagaoka M., Cho C.S., Ito Y. A fusion protein N-cadherin-Fc as an artificial extracellular matrix surface for maintenance of stem cell features. Biomaterials. 2010;31(20):5287–5296. doi: 10.1016/j.biomaterials.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z., Zhao P., Luo Z., Zheng M., Tian H., Gong P. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 68.Li J., Wang X., Zheng D., Lin X., Wei Z., Zhang D. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater Sci. 2018;6(7):1834–1845. doi: 10.1039/c8bm00343b. [DOI] [PubMed] [Google Scholar]
- 69.Li S.Y., Cheng H., Qiu W.X., Zhang L., Wan S.S., Zeng J.Y. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials. 2017;142:149–161. doi: 10.1016/j.biomaterials.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 70.Tian H., Luo Z., Liu L., Zheng M., Chen Z., Ma A. Cancer cell membrane-biomimetic oxygen nanocarrier for breaking hypoxia-induced chemoresistance. Adv Funct Mater. 2017;27(38) [Google Scholar]
- 71.Zhang N., Li M., Sun X., Jia H., Liu W. NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials. 2018;159:25–36. doi: 10.1016/j.biomaterials.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Li S., Feng X., Wang J., Xu W., Islam M.A., Sun T. Multiantigenic nanoformulations activate anticancer immunity depending on size. Adv Funct Mater. 2019 [Google Scholar]
- 73.Li R., He Y., Zhang S., Qin J., Wang J. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B. 2018;8(1):14–22. doi: 10.1016/j.apsb.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedl P., Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 75.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 76.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Grimshaw M.J., Wilson J.L., Balkwill F.R. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. Eur J Immunol. 2002;32(9):2393–2400. doi: 10.1002/1521-4141(200209)32:9<2393::AID-IMMU2393>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 79.Labelle M., Begum S., Hynes R.O. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111(30):E3053–E3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis C.E., Pollard J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 81.Su S., Liu Q., Chen J., Chen J., Chen F., He C. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Rivoltini L., Carrabba M., Huber V., Castelli C., Novellino L., Dalerba P. Immunity to cancer: attack and escape in t lymphocyte-tumor cell interaction. Immunol Rev. 2002;188:97–113. doi: 10.1034/j.1600-065x.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 83.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krishnamurthy S., Gnanasammandhan M.K., Xie C., Huang K., Cui M.Y., Chan J.M. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale. 2016;8(13):6981–6985. doi: 10.1039/c5nr07588b. [DOI] [PubMed] [Google Scholar]
- 85.Li Y.N., Chang C.W., Chiang C.S. SDF-1/CXCR4 axis-mediated tumor-tropism of monocyte membrane-coated nanoparticles. Cancer Res. 2017;77(13):2196. [Google Scholar]
- 86.Wan S.W., Wu-Hsieh B.A., Lin Y.S., Chen W.Y., Huang Y., Anderson R. The monocyte-macrophage-mast cell axis in dengue pathogenesis. J Biomed Sci. 2018;25:77. doi: 10.1186/s12929-018-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao H., Dan Z., He X., Zhang Z., Yu H., Yin Q. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–7748. doi: 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- 88.Rao L., He Z., Meng Q.F., Zhou Z., Bu L.L., Guo S.S. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J Biomed Mater Res, Part A. 2017;105(2):521–530. doi: 10.1002/jbm.a.35927. [DOI] [PubMed] [Google Scholar]
- 89.Xuan M., Shao J., Dai L., Li J., He Q. Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 90.Zhao H., Li L., Zhang J., Zheng C., Ding K., Xiao H. C−C chemokine ligand 2 (CCL2) recruits macrophage-membrane-camouflaged hollow bismuth selenide nanoparticles to facilitate photothermal sensitivity and inhibit lung metastasis of breast cancer. ACS Appl Mater Interfaces. 2018;10(37):31124–31135. doi: 10.1021/acsami.8b11645. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Gai K., Li C., Guo Q., Chen Q., He X. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18(3):1908–1915. doi: 10.1021/acs.nanolett.7b05263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neuberg P., Kichler A. Recent developments in nucleic acid delivery with polyethylenimines. Adv Genet. 2014;88:263–288. doi: 10.1016/B978-0-12-800148-6.00009-2. [DOI] [PubMed] [Google Scholar]
- 93.Zhang L., Li R., Chen H., Wei J., Qian H., Su S. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomed. 2017;12:2129–2142. doi: 10.2147/IJN.S126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Draghiciu O., Walczak M., Hoogeboom B.N., Franken K.L.M.C., Melief K.J.M., Nijman H.W. Therapeutic immunization and local low-dose tumor irradiation, a reinforcing combination. Int J Cancer. 2014;134(4):859–872. doi: 10.1002/ijc.28418. [DOI] [PubMed] [Google Scholar]
- 95.Lugade A.A., Sorensen E.W., Gerber S.A., Moran J.P., Frelinger J.G., Lord E.M. Radiation-induced IFN-γ production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 96.Pitchaimani A., Tuyen Duong Thanh N., Aryal S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials. 2018;160:124–137. doi: 10.1016/j.biomaterials.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 97.Chee D.O., Townsend C.M., Galbraith M.A., Eilber F.R., Morton D.L. Selective reduction of human tumor cell populations by human granulocytes in vitro. Cancer Res. 1978;38(12):4534–4539. [PubMed] [Google Scholar]
- 98.Dvorak A.M., Connell A.B., Proppe K., Dvorak H.F. Immunological rejection of mammary adenocarcinoma (TA3-St) in C57BL-6 mice: participation of neutrophils and activated macrophages with fibrin formation. J Immunol. 1978;120(4):1240–1248. [PubMed] [Google Scholar]
- 99.Aeed P.A., Welch D.R. Sensitivity of locally recurrent rat mammary tumor cell lines to syngeneic polymorphonuclear cell, macrophage and natural killer cell cytolysis. Br J Cancer. 1988;58(6):746–752. doi: 10.1038/bjc.1988.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dallegri F., Ballestrero A., Ottonello L., Patrone F. Defective antibody-dependent tumor cell lysis by neutrophils from cancer patients. Clin Exp Immunol. 1989;77(1):58–61. [PMC free article] [PubMed] [Google Scholar]
- 101.Aeed P.A., Nakajima M., Welch D.R. The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer. 1988;42(5):748–759. doi: 10.1002/ijc.2910420521. [DOI] [PubMed] [Google Scholar]
- 102.Wislez M., Rabbe N., Marchal J., Milleron B., Crestani B., Mayaud C. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63(6):1405–1412. [PubMed] [Google Scholar]
- 103.Kang T., Zhu Q., Wei D., Feng J., Yao J., Jiang T. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11(2):1397–1411. doi: 10.1021/acsnano.6b06477. [DOI] [PubMed] [Google Scholar]
- 104.Labelle M., Begum S., Hynes R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Borsig L., Wong R., Feramisco J., Nadeau D.R., Varki N.M., Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98(6):3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 107.Hu Q., Sun W., Qian C., Wang C., Bomba H.N., Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27(44):7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 109.Furman N.E.T., Lupu-Haber Y., Bronshtein T., Kaneti L., Letko N., Weinstein E. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett. 2013;13(7):3248–3255. doi: 10.1021/nl401376w. [DOI] [PubMed] [Google Scholar]
- 110.Menon L.G., Picinich S., Koneru R., Gao H., Lin S.Y., Koneru M. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25(2):520–528. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- 111.Roorda B.D., ter Elst A., Meeuwsen-de Boer T.G.J., Kamps W.A., de Bont E.S.J.M. Mesenchymal stem cells contribute to tumor cell proliferation by direct cell−cell contact interactions. Cancer Invest. 2010;28(5):526–534. doi: 10.3109/07357900903179625. [DOI] [PubMed] [Google Scholar]
- 112.Yang N., Ding Y., Zhang Y., Wang B., Zhao X., Cheng K. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl Mater Interfaces. 2018;10(27):22963–22973. doi: 10.1021/acsami.8b05363. [DOI] [PubMed] [Google Scholar]
- 113.Gao C., Lin Z., Jurado-Sanchez B., Lin X., Wu Z., He Q. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small. 2016;12(30):4056–4062. doi: 10.1002/smll.201600624. [DOI] [PubMed] [Google Scholar]
- 114.Gao C., Lin Z., Wu Z., Lin X., He Q. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl Mater Interfaces. 2016;8(50):34252–34260. doi: 10.1021/acsami.6b12865. [DOI] [PubMed] [Google Scholar]
- 115.Farjadian F., Moghoofei M., Mirkiani S., Ghasemi A., Rabiee N., Hadifar S. Bacterial components as naturally inspired nanocarriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol Adv. 2018;36(4):968–985. doi: 10.1016/j.biotechadv.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanbrunt J. More to hyaluronic acid than meets the eye. Bio-Technology. 1986;4(9):780–782. [Google Scholar]
- 117.Wang H., Agarwal P., Zhao S., Yu J., Lu X., He X. Combined cancer therapy with hyaluronan-decorated fullerene-silica multifunctional nanoparticles to target cancer stem-like cells. Biomaterials. 2016;97:62–73. doi: 10.1016/j.biomaterials.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang L., Jia E. Ovarian cancer targeted hyaluronic acid-based nanoparticle system for paclitaxel delivery to overcome drug resistance. Drug Deliv. 2016;23(5):1810–1817. doi: 10.3109/10717544.2015.1101792. [DOI] [PubMed] [Google Scholar]
- 119.Xia Y., Guo M., Xu T., Li Y., Wang C., Lin Z. siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. Int J Nanomed. 2018;13:1539–1552. doi: 10.2147/IJN.S157519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gujrati V., Kim S., Kim S.H., Min J.J., Choy H.E., Kim S.C. Bioengineered bacterial outer membrane vesicles as cell-specific drug delivery vehicles for cancer therapy. ACS Nano. 2014;8(2):1525–1537. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- 121.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Delivery Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 122.Koppers-Lalic D., Hackenberg M., Bijnsdorp I.V., van Eijndhoven M.A.J., Sadek P., Sie D. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 123.Jang S.C., Kim O.Y., Yoon C.M., Choi D.S., Roh T.Y., Park J. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 124.Ohno S.I., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 126.Zhang P., Zhang L., Qin Z., Hua S., Guo Z., Chu C. Genetically engineered liposome-like nanovesicles as active targeted transport platform. Adv Mater. 2018;30(7) doi: 10.1002/adma.201705350. [DOI] [PubMed] [Google Scholar]
- 127.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A.J., Hopmans E.S., Lindenberg J.L. Functional delivery of viral miRNA via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 129.Qi H., Liu C., Long L., Ren Y., Zhang S., Chang X. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10(3):3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- 130.Dehaini D., Wei X., Fang R.H., Masson S., Angsantikul P., Luk B.T. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29(16) doi: 10.1002/adma.201606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He H., Guo C., Wang J., Korzun W.J., Wang X.Y., Ghosh S. Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano Lett. 2018;18(10):6164–6174. doi: 10.1021/acs.nanolett.8b01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang D., Dong H., Li M., Cao Y., Yang F., Zhang K. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–5252. doi: 10.1021/acsnano.7b08355. [DOI] [PubMed] [Google Scholar]