Abstract
Non-alcoholic fatty liver disease (NAFLD) and cardiovascular diseases (CVD) share similar risk factors. Recent studies have focused on obesity and insulin resistance, but the link between NAFLD and CVD persists regardless of traditional risk factors. Despite the increased incidence and prevalence of NAFLD worldwide, there has been no thorough investigation of gender disparities nor a closer look taken into investigating the role gender may play in increased cardiovascular (CV) mortality in people with NAFLD. We assessed the incidence and prevalence of CV events and mortality based on gender in people with NAFLD, at any stage of fibrosis. A meta-regression was conducted to further analyze the impact of age on both genders. An aggregate analysis was performed on ten studies with NAFLD people. A random-effects model was used to pool the overall incidence and prevalence rates of CV events and mortality as well as all-cause mortality to examine any gender disparity. We also performed a meta-regression analysis to evaluate the effect of age on mortality for men versus women with NAFLD and CV events and mortality. Summary odds ratios (OR) and 95% confidence intervals (CI) were estimated using a random-effects model. In 108,711 people with NAFLD, of which 44% were females and 56% were males, all-cause mortality was 1.5x higher in women compared to men (OR 1.65, 95% CI 1.12-2.43, P<0.012). CV events and mortality were also 2x higher in women compared to men (OR 2.12 95% CI 1.65-2.73, P<0.001)). On meta-regression, females had higher mortality with advancing age starting at age 42 (coefficient =0.0518, P=0.00001). For people with NAFLD, women had a markedly higher incidence and prevalence of CV events, CV mortality, and all-cause mortality when compared to men. As the incidence and prevalence of NAFLD and concomitant CV events increase worldwide, we urge the medical community to increase surveillance and perform rigorous cardiovascular risk assessments for women, especially beginning at age 42. Additionally, we recommend heterogeneous surveys of gender disparities, increased focus on gender as a decisive factor for downstream CV events, the relationship between NAFLD severity and gender-based mortality differences, and larger studies representing equivalent male and female populations.
Keywords: NAFLD, CVD, CAD, gender, women’s health, cardiovascular mortality
Introduction
Every year, ninety million people are diagnosed with nonalcoholic fatty liver disease (NAFLD) and it has become one of the top etiologies for chronic liver disease [1-3]. NAFLD encompasses a spectrum of diseases that histologically varies from mild steatosis that can advance to fibrosis, cirrhosis, and eventually hepatocellular carcinoma [1-5]. NAFLD results from causes not attributed to alcohol consumption, such as viruses, drugs, toxins, and autoimmune diseases [1,2].
At first, cirrhosis was believed to be protective against coronary artery disease (CAD), but recent studies have demonstrated a strong correlation between cardiovascular disease (CVD) and the development of NAFLD [1,6,5-10]. NAFLD and CVD share common risk factors such as diabetes, smoking, unhealthy diets, dyslipidemia, obesity, presence of certain shared post-transcriptional regulators in microRNAs, hypertension, increased expression of the GCKR gene, and high levels of visceral fat [6,8-14]. As a result, a common pathophysiological pathway also exists via insulin resistance, inflammation and oxidative stress pathways [12,15]. People with NAFLD and CVD have increased Framingham risk scores and decreased life expectancy [16]. Cardiovascular (CV) complications in people with NAFLD are the following: CAD, subclinical atherosclerosis, cardiac arrhythmias, and heart failure [6].
CVD was established as the top cause of mortality for women throughout the world, accounting for 43% female deaths in the US since 1997. Despite such a strong association between NAFLD and CVD, gender differences have rarely been studied [17]. Women are underrepresented in most clinical trials and there is little information outlining the differences in care between the genders [18]. In this paper we discuss the association of NAFLD with CVD in men and women, the mechanisms underlying this association, and the differences between the genders with regards to age and incidence.
Methods
Search methods and study selection
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement was used to perform a meta-analysis of the research articles. Two co-authors (YSK and NRD) independently searched published studies indexed in OVID, Cochrane Central Register of Controlled Trials (CENTRAL) via the Wiley Interface, Web of Science Core Collection, MEDLINE, EMBASE, and Google Scholar from the beginning of the project on October 1, 2019 to January 30, 2020. We searched the terms “cardiovascular diseases” and “cardiovascular mortality” in all fields with each of the following words: “non-alcoholic fatty liver disease”, “non-alcoholic steatohepatitis”, “non-alcoholic fatty liver disease”, “nonalcoholic fatty liver disease,” “fatty liver”, “liver fat”, “steatosis”, “NAFLD”, “NASH”, “women,” “gender,” “all-cause mortality”. Terms used to define the outcomes included “prevalence” and “incidence”. All detected references were saved electronically in the Zotero reference management program after removal of duplicates. The systematic search was supplemented by manual review of all references in the retrieved eligible studies.
Randomized, prospective, retrospective, cross-sectional, and nonrandomized controlled studies were considered for inclusion if they reported the incidence of CVD and mortality in people with NAFLD. When the needed data was not directly found in the published articles, we obtained the necessary data from the authors through electronic communication or via reviewing their supplemental reports. We excluded all studies that did not meet these criteria or that reported insufficient data. We used the checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modeling Studies (CHARMS) for study quality assessment. The search methods, study selection, and eligibility criteria are highlighted in Figure 1 [19].
Figure 1.
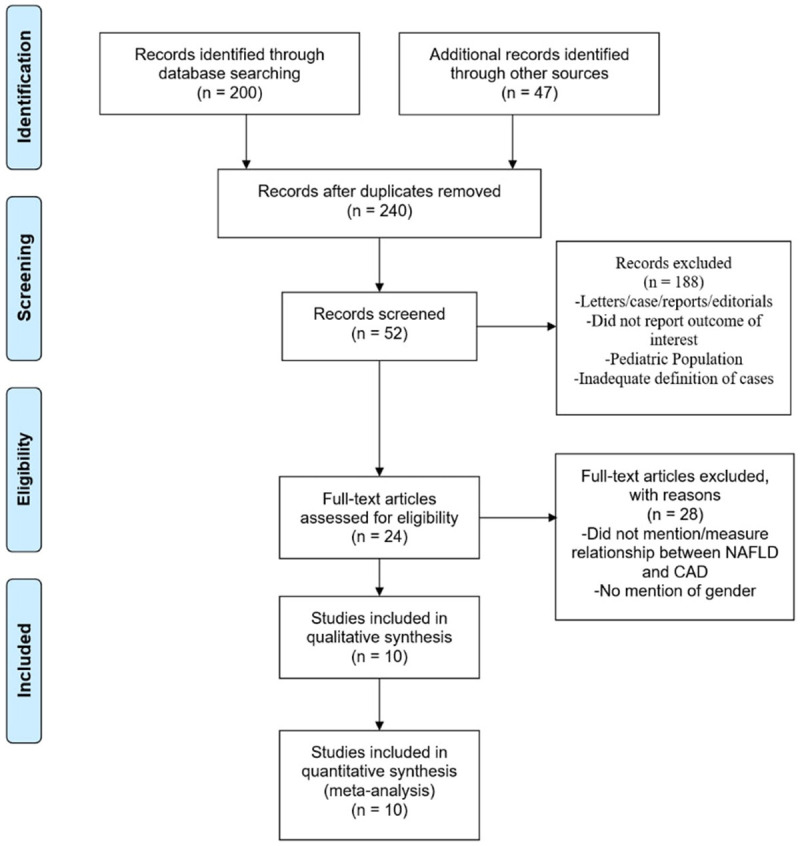
PRISMA flow chart of studies screened and included in meta-analysis.
Data extraction
A standardized data collection form was used to extract the following information: last name of the first author, title of the article, study design, year of study, country of origin, year of publication, sample size, characteristics of participants; evaluation of NAFLD through liver ultrasound, liver function tests (AST, ALT, GGT) or liver biopsy; evaluation of subclinical atherosclerosis (pathological carotid intima media thickness or presence of carotid plaques); and evaluation of CAD. The table was completed by the first author and verified by a study team member.
Meta-analysis
All statistical tests were two-tailed and the type I error rate was set at 5%. Confidence intervals (CIs) of individual studies were calculated. The Der Simonian & Laird method was utilized to complete the statistical data analysis using MedCalc software with: (1) a summary of data from individual studies; (2) an evaluation of heterogeneity, graphically and statistically, for all included studies; (3) graphical illustration via Forest Plots. A secondary analysis was performed using the Hartung-Knapp-Sidik-Jonkman (HKSJ) method through Comprehensive Meta-Analysis software. Heterogeneity was determined utilizing the Chi-square test and I2 statistic with each of the following values: low heterogeneity (0-25%), moderate heterogeneity (25-75%), and high heterogeneity (>75%). Meta-regression was analyzed using a generalization of Littenberg and Moses Linear model. The Newcastle-Ottawa scale (0 to 9 points) was utilized to quantify study quality (Tables 1 and 2). Publication bias was assessed using funnel plots and the Egger and Begg test using I2 and Q statistics.
Table 1.
Newcastle-ottawa scale assessment for studies
| Publication | Year | Newcastle-Ottawa Scale | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Selection | Comparability | Outcome | ||||||||
|
|
|
|||||||||
| Representatives of exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome demonstration at start | Assessment of outcome | Follow-up long enough for outcome to occur | Adequacy of follow-up | ||||
| El-Azeem | 2013 | * | * | * | * | ** | * | * | * | 9 |
| Hamaguchi | 2007 | * | * | * | * | ** | * | * | * | 9 |
| Haring | 2009 | * | * | * | * | ** | * | * | * | 9 |
| Hwang | 2008 | * | * | * | * | ** | * | * | * | 9 |
| Lee | 2006 | * | * | * | * | ** | * | * | * | 9 |
| Lu | 2009 | * | * | * | * | ** | * | * | * | 9 |
| Ruttman | 2005 | * | * | * | * | ** | * | * | * | 9 |
| Stepanova | 2012 | * | * | * | * | ** | * | * | * | 9 |
| Yu | 2008 | * | * | * | * | ** | * | * | * | 9 |
| Zheng | 2018 | * | * | * | * | ** | * | * | * | 9 |
Table 2.
Quality data for studies
| Publication | Year | Objective Defined | Objective Described | Characteristics Described | Confounders Described | Main Findings Outlined | Heterogeneous Population | Individuals Generating Data Blind to Outcomes | Reproducibility Assessed | Recruiting all subjects over same time period |
|---|---|---|---|---|---|---|---|---|---|---|
| El-Azeem | 2013 | Yes | Yes | Yes | Yes | Yes | No | NS | Yes | Yes |
| Hamaguchi | 2007 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Haring | 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Hwang | 2008 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Lee | 2006 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| Lu | 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Ruttman | 2005 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Stepanova | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Yu | 2008 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Zheng | 2018 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
Results
Baseline patient and study characteristics
Figure 1 illustrates how the studies were picked. At first, reviewing the literature revealed 247 potential studies that could possibly be analyzed. After removing retrospective studies, abstracts, case report studies with insufficient data, review articles, and articles with overlapping study populations and redundant data, a total of 10 studies were included in the final analysis (Table 3). All studies were conducted in the USA, Europe, Japan, Saudia Arabia, South Korea, and China. 8 studies reported incidence, 4 of which were retrospective and 4 were prospective. 2 studies were cross-sectional studies that reported prevalence. The number of people in each study varied with over 80,000 people in the largest study and 268 people in the smallest study. Among 108,711 people with NAFLD, 44% were females and 56% were males, with a weighted mean age of 50 years.
Table 3.
Baseline characteristics of included studies in the meta-analysis
| Author/Publication Year/Type of Study | Country | Duration (Years) | Sample (#) | Male (%) | Female (%) | Mean Age (Years) | Diagnosis of CVD | Diagnosis of NAFLD | CV Outcome, Incidence, and Prevalence | Study Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|
| El Azeem, 2013, Prospective Study | Saudi Arabia | 3.0 | 268 | 50.0 | 50.0 | 51.4±11.3 | Hospital Records, EKG | Liver Ultrasound | CV Mortality, 50.7% | No Adjustments |
| Hamaguchi, 2007, Prospective Study | Japan | 5.0 | 1221 | 61.0 | 39.0 | 49.1±8.7 | Self-Reporting Questionnaire, Metabolic Syndrome based on Waist Circumference | Liver Ultrasound | CV Events, 1.8% | No Adjustments |
| Haring, 2009, Retrospective Study | Germany | 7.3 | 4160 | 49.0 | 51.0 | 49.0±1.0 | ICD 10 Codes | GGT > ULN; Liver Ultrasound | CV mortality, 7.5% | Age (Decades), WC, Alcohol Consumption, Physical Activity, Education, Civil Status, Equalized Income, Functional Comorbidity Index |
| Hwang 2018, Retrospective Study | South Korea | 5.7 | 82899 | 75.6 | 24.4 | 41.6±10.7 | Death Certificate, ICD 10 Codes | Liver Ultrasound | CV mortality, 11.0% | No Adjustments |
| Lee 2006, Prospective Study | Finland | 12.0 | 2862 | 47.9 | 52.1 | 48.0±1.0 | Fatal and Non-Fatal events (ICD 8, 9, 10 codes) | GGT >64 U/L (Male); GGT >32 U/L (Female) | CVD Events, 4.0%; CV Mortality, 3.4% | Age, BMI, Cigarette Smoking, Alcohol Consumption, Physical Activity, SBP, Total Cholesterol, HDL-Cholesterol |
| Lu 2009 Cross-Sectional Survey | China | 7.0 | 421 | 62.7 | 37.3 | 56.4±6.6 | Patient History, Symptoms, EKG Findings, LHC | Liver Ultrasound; ALT >40 IU/L | CV events, 38.7%* | BMI, WC, SBP, DBP, FBG, Triglycerides, Total Cholesterol, HDL-Cholesterol, LDL-Cholesterol; ALT, AST, GGT, FINS, MetS |
| Ruttman, 2005 Prospective Study | Austria | 17.0 | 8795 | 56.5 | 23.5 | 42±15.0 | ICD 9 Codes | GGT >56 U/L | CV Mortality 2.1% Male and 4.3% Females | Age, BMI, SBP, Total Cholesterol, Triglycerides, Glucose, Smoking, Work Status, Year of Examination |
| Stepanova 2012, Retrospective Study | USA | 6.0 | 2492 | 52.8 | 47.2 | 54.0±1.0 | Self-Reported History | Liver Ultrasound | CV Mortality 3.76% | No Adjustments |
| Yun 2008, Retrospective Study | South Korea | 5.0 | 4022 | 80.5 | 19.5 | 50.0±8.0 | ICD 10 Codes | ALT >40 IU/L | CV Mortality, 22.0% | Age, Sex, Past Medical History, Smoking, Alcohol Consumption, Exercise, Education, Income, BMI, SBP, DBP, FPG, Total Cholesterol, HDL-Cholesterol, Triglycerides, Calculated GFR |
| Zheng 2018, Retrospective Study | China | 1.0 | 1571 | 64.4 | 35.6 | 56.2±11.2 | CIMT, ba-PWV | Liver Biopsy | CIMT, 43.8%* | Age, Gender, BMI, Exercise, Smoking, WC, LDL-Cholesterol Triglycerides, Diabetes Mellitus, Hypertension |
ICD = International Statistical Classification of Diseases and Related Health Problems, CV = Cardiovascular, EKG = Electrocardiogram, LHC = Left Heart Catheterization, CIMT = Carotid Intima Media Thickness, ba-PWV = Brachial-Ankle Pulse Wave Velocity, GGT = Gamma-Glutamyltransferase, ULN = Upper Limit of Normal (For that test), ALT = Alanine Transaminase, BMI = Body Mass Index, SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, FBG = Fasting Blood Glucose, HDL = High-Density Lipoproteins, LDL = Low-Density Lipoproteins, AST = Aspartate Aminotransferase, WC = Waist Circumference, MetS = Metabolic Syndrome, GFR = Glomerular Filtration Rate. Data are presented as mean § SD, median [interquartile range].
Defining outcomes
Asymptomatic atherosclerosis and CAD were defined as: carotid intima media thickness on carotid ultrasound, coronary angiography with >50% plaque in coronary vessels, aortic plaque on abdominal ultrasound, coronary computed tomography angiography (CCTA) showing >50% plaque in coronary vessels, exercise or nuclear stress testing results (positive results only), carotid plaques (measured by carotid ultrasound), and peripheral vascular arterial disease on arterial ultrasound. Cardiovascular events were defined as the one of the categories listed above. CVD was defined as symptomatic cardiovascular events resulting in hospitalization and morbidity as the end-point. Cardiovascular mortality was defined as death from one of the following causes: stroke, acute coronary syndrome (including unstable angina), and heart failure requiring hospitalization.
In our studies, NAFLD was diagnosed by various different imaging techniques: liver ultrasound, liver function tests, and liver biopsy (when possible).
Incidence and prevalence of cardiovascular events and mortality
For women, cardiovascular events and mortality were found to be 2-times higher than men with known NAFLD (OR 2.12 95% CI 1.65-2.73, P<0.001) (Figure 2; Tables 4, 5). All-cause mortality was also 1.5-times higher in women compared to men with NAFLD (OR 1.65, 95% CI 1.12-2.43, P<0.012) (Figure 3; Tables 6, 7). Similar results were noted both in the fixed and random effect models.
Figure 2.
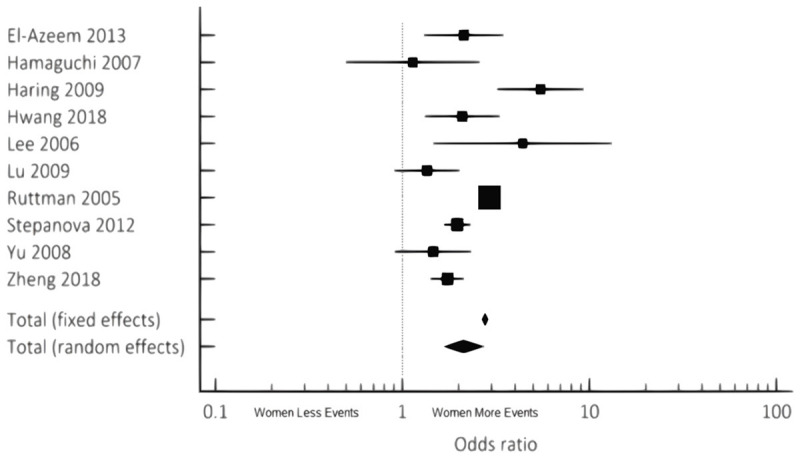
Cumulative incidence and prevalence of CV Events/Mortality noted in NAFLD females compared to males via combined fixed and random effects.
Table 4.
Odds Ratio, Confidence Interval, and Weight of Studies for CV Events/Mortality noted in NAFLD females compared to males via combined fixed and random effects
| Study | Odds Ratio | 95% CI | Z-score | P-value | Weight (%) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Fixed | Random | |||||
| El-Azeem 2013 | 2.129 | 1.308 to 3.467 | 0.65 | 9.45 | ||
| Hamaguchi 2007 | 1.136 | 0.499 to 2.584 | 0.23 | 5.68 | ||
| Haring 2009 | 5.479 | 3.217 to 9.329 | 0.55 | 8.84 | ||
| Hwang 2018 | 2.091 | 1.321 to 3.312 | 0.74 | 9.84 | ||
| Lee 2006 | 4.396 | 1.466 to 13.181 | 0.13 | 3.83 | ||
| Lu 2009 | 1.354 | 0.907 to 2.021 | 0.97 | 10.70 | ||
| Ruttman 2005 | 2.918 | 2.797 to 3.044 | 86.72 | 14.70 | ||
| Stepanova 2012 | 1.968 | 1.667 to 2.322 | 5.68 | 13.86 | ||
| Yu 2008 | 1.459 | 0.912 to 2.334 | 0.71 | 9.70 | ||
| Zheng 2018 | 1.738 | 1.413 to 2.138 | 3.63 | 13.40 | ||
| Total (Fixed Effects) | 2.765 | 2.658 to 2.876 | 50.599 | <0.001 | 100.00 | 100.00 |
| Total (Random Effects) | 2.123 | 1.653 to 2.725 | 5.904 | <0.001 | 100.00 | 100.00 |
Table 5.
Test for Heterogeneity of Studies for CV Events/Mortality noted in NAFLD females compared to males via combined fixed and random effects
| Test for Heterogeneity | |
|---|---|
| Q | 75.0879 |
| DF | 9 |
| Significance Level | P<0.0001 |
| I2 (Inconsistency) | 88.01% |
| 95% CI for I2 | 80.01 to 92.81 |
Figure 3.
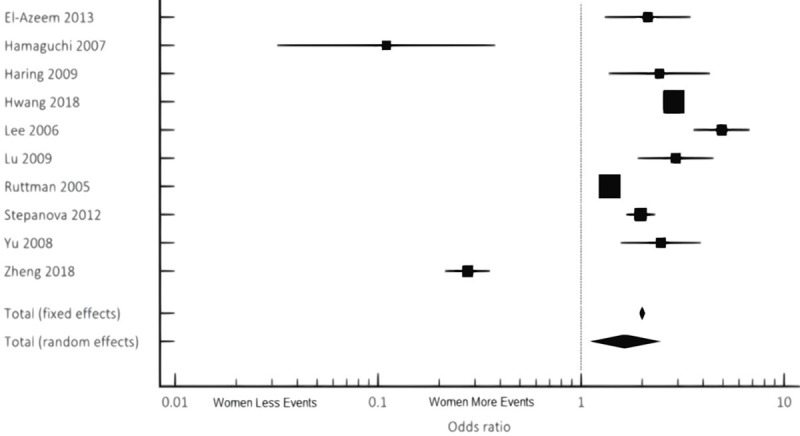
Cumulative incidence and prevalence of all-cause mortality noted in NAFLD females compared to males via combined fixed and random effects.
Table 6.
Odds Ratio, Confidence Interval, and Weight of Studies for all-cause mortality noted in NAFLD females compared to males via combined fixed and random effects
| Study | Odds Ratio | 95% CI | z-Score | P-Value | Weight (%) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Fixed | Random | |||||
| El-Azeem 2013 | 2.129 | 1.308 to 3.467 | 0.46 | 9.73 | ||
| Hamaguchi 2007 | 0.110 | 0.0319 to 0.378 | 0.071 | 5.33 | ||
| Haring 2009 | 2.433 | 1.370 to 4.322 | 0.33 | 9.19 | ||
| Hwang 2018 | 2.866 | 2.727 to 3.012 | 44.13 | 11.46 | ||
| Lee 2006 | 4.930 | 3.582 to 6.785 | 1.07 | 10.66 | ||
| Lu 2009 | 2.925 | 1.898 to 4.508 | 0.58 | 10.06 | ||
| Ruttman 2005 | 1.382 | 1.318 to 1.450 | 47.21 | 11.46 | ||
| Stepanova 2012 | 1.968 | 1.667 to 2.322 | 3.97 | 11.25 | ||
| Yu 2008 | 2.475 | 1.568 to 3.906 | 0.52 | 9.92 | ||
| Zheng 2018 | 0.276 | 0.214 to 0.357 | 1.66 | 10.94 | ||
| Total (Fixed Effects) | 1.999 | 1.936 to 2.064 | 42.281 | <0.001 | 100.00 | 100.00 |
| Total (Random Effects) | 1.646 | 1.115 to 2.431 | 2.507 | 0.012 | 100.00 | 100.00 |
Table 7.
Test for Heterogeneity of Studies for All-Cause Mortality noted in NAFLD females compared to males via combined fixed and random effects
| Test for Heterogeneity | |
|---|---|
| Q | 714.3491 |
| DF | 9 |
| Significance Level | P<0.0001 |
| I2 (Inconsistency) | 98.74% |
| 95% CI for I2 | 98.37 to 99.02 |
On univariate meta-regression (Figure 4), when plotting log odds ratio of cardiovascular events and mortality among males versus females (y-axis) against age (x-axis), women had higher mortality with advancing age starting at age of 42 based on our analysis (regression coefficient =0.0518, P=0.00001). Q and I2 statistics were analyzed to assess heterogeneity; the results of which can be found in Tables 5 and 7. Heterogeneity was higher than 50% meaning there were significant differences between the studies we included (88% for cardiovascular events and mortality and 98% for all-cause mortality). However, our analysis was done using both random and fixed effects models which showed the same results. The fixed effect model can be done when significant heterogeneity exists and it showed the same result as the random effects model.
Figure 4.
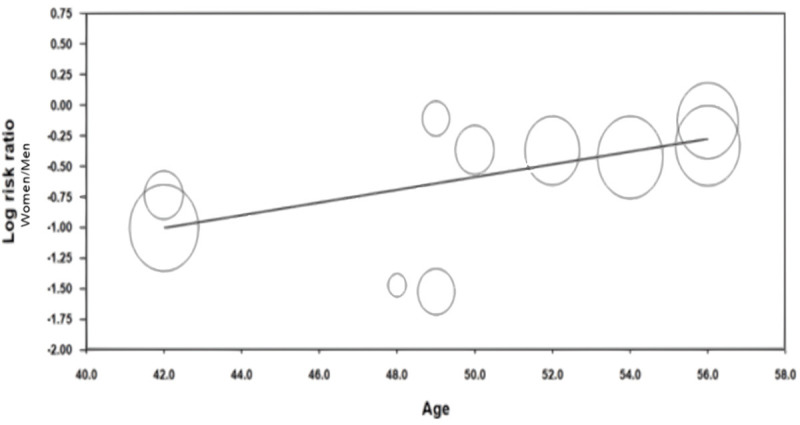
Meta-regression to assess incidence and prevalence of CV events/mortality of NAFLD female people with age.
Funnel plot analysis (Appendices A, B, C, D) did not reveal asymmetry around the axis for the treatment effect by Egger’s test. There was minimal publication bias.
Discussion
In this meta-analysis and meta-regression, the impact of gender on both the incidence and prevalence of CVD and mortality for adults with NAFLD was evaluated. The main findings of our study are the following: i) females were found to have greater incidence and prevalence of cardiovascular events and mortality; ii) all-cause mortality incidence and prevalence was higher for females; iii) men and women had a higher incidence and prevalence of mortality with advancing age. This is the first systematic review and meta-analysis assessing the role of gender in CVD and mortality for a cohort with NAFLD. These findings illustrate the need for future investigations and the development of new screening guidelines for women as they are an understudied patient population.
NAFLD is a multifactorial disease and the complex interplay of lifestyle and genetics shapes the development of the disease [5,6,20,21]. NAFLD and CVD both have significant similarities in terms of risk factors, genetic predispositions, and pathophysiology. NAFLD has also been linked to reduced inflammation, coronary artery blood flow, and insulin resistance, which are all major contributors to CVD [8,22]. This meta-analysis revealed a 2-fold increased incidence of CV events and mortality in females when compared to males with NAFLD which further increased with aging (beginning at age 42) (Figures 2 and 4).
Our study identifies that the female gender has a strong yet independent impact on cardiovascular events and mortality. For females, robust screening measures should be implemented earlier as increased incidence of carotid artery disease has been observed prior to cardiovascular events [23]. Previous studies on people with both NAFLD and CVD do not analyze the effects of gender on the incidence of cardiovascular events and mortality. Late presentation of these diseases results in prolonged ischemia and thus increases the incidence of complications that may affect people with both NAFLD and CVD [24,25]. Women are underrepresented in most drug trials, but most publications and trials claim equal efficacy for both sexes. In our study, females only represented 44% of the patient population [26] and as a result, we focused on gender to elucidate the disparities between the two populations. We strongly suggest researchers to focus on gender disparity analysis in future trials to further elucidate the observed and clinically relevant differences that arise from the statistical analysis.
Based on current literature, there appears to be a very definitive relationship between NAFLD and a higher risk of CVD that can be seen with imaging [2,3,7]. In this analysis of 10 studies, the initial definition of CVD varied by author. Zheng et al. defined CV disease as carotid intima media thickening >0.8 mm as a marker for clinical cardiovascular disease [20]. Yun et al., Harring et al., and Hwang et al. defined cardiovascular mortality via ICD 10 codes. Ruttman et al. similarly defined CV disease via ICD 9 codes. Stepanova et al. utilized patients’ self-reporting of their history of CV disease. Lu et al., however, used history, symptoms, EKG, and coronary angiography findings. Lee et al. utilized ICD 8, 9, and 10 codes of fatal and non-fatal CV events. Hamaguchi et al. used survey answers and defined metabolic syndrome using waist circumference. El-Azeem et al. used hospital records and EKG findings. Although carotid intima media thickness, symptoms, EKG, and coronary angiography findings are markers that may not necessarily correlate to clinical relevance and significance, they could be an early indicator of cardiovascular disease and should be monitored closely by clinicians. Multiple studies have demonstrated that NAFLD leads to a higher incidence and prevalence of CAD, even in asymptomatic patients [27]. Despite being asymptomatic, these people may develop clinically significant CVD thus highlighting the importance of monitoring our markers of CVD in NAFLD people and potentially preventing future CV events.
With regards to the diagnosis of NAFLD, the majority of studies analyzed did not use liver biopsy to diagnose NAFLD. Liver ultrasound findings were considered adequate evidence of NAFLD. Some studies only used elevated liver biomarkers, such as ALT and GGT greater than two-thirds the upper limit of normal to diagnose NAFLD, when all other causes of liver disease were eliminated (Table 3). Ideally, the studies should have diagnosed NAFLD using liver biopsies - the gold standard - to achieve uniformity. However, biopsies are invasive procedures with their own sets of complications and are avoided, as a result, for diagnosing NAFLD or considered unnecessary when NAFLD is identified through ultrasounds or biomarkers. Thus, we recommend earlier preliminary screening of people who have metabolic syndrome or other risk factors for CV disease via the use of liver indexes Fibrosis-4 score, FibroScan or NAFLD fibrosis score [28].
Our study further revealed an increased incidence and prevalence of all-cause mortality in the female population of NAFLD (Figure 3). In a meta-analysis of 40 studies by Muso et al., it was reported mortality was ten times higher if a patient was diagnosed with NAFLD [29]. This extensive increase in mortality may have been attributable to liver fibrosis as the underlying etiology. Ekstedt et al. postulated that the most accurate prognosticator of all-cause mortality in those with NAFLD was the extent of hepatic injury [29]. Having additional data in terms of the degree of liver fibrosis and disease stage could have helped us further understand why NAFLD increases all-cause mortality for this patient population. We would then be able to analyze the impact of the degree of liver fibrosis and disease severity on outcomes. Based on the most recent data from 2017, most patients that require a liver transplant have underlying NAFLD [30]. Our study reinforces the need for further investigation of NAFLD related all-cause mortality in terms of gender differences.
Our meta-regression analysis revealed that advancing age (beginning at age 42) was associated with higher cardiovascular mortality in both men and women, but a higher incidence and prevalence of mortality was noted for women with advancing age when compared to men. This gender disparity is very critical to note as women tend to present later in the course of NAFLD and CVD possibly due to the presence of atypical symptoms which can delay diagnosis and treatment [31,32]. Increasing age has been persistently identified as a risk factor for fibrotic progression to cirrhosis throughout multiple studies and there have been several theories about why females with NAFLD have increased mortality with advancing age [33]. One theory postulates that older postmenopausal females with estrogen deficiency tend to have more severe liver damage with NAFLD [33]. Per Ludwig et al., women older than the age of forty have increased comorbidities, NAFLD included, and therefore higher mortality [34]. Interestingly, per earlier studies, men tended to be diagnosed with NAFLD at a higher rate until the age of fifty and then this rate starts to drop, whereas the opposite is true for women. Women tend to have lower prevalence of NAFLD until the fifth decade of life, and peak during their sixties [35,36]. Our meta-regression substantiates these findings from earlier studies, and this may contribute to the increased all-cause and cardiovascular mortality for females seen in our study.
Study limitations and strengths
Our study had numerous strengths. First, a variety of distinct reference databases were utilized. We also analyzed a large patient population.
Our meta-analysis also has several limitations. Although the quality assessment and bias assessments methods used in our studies were considered high quality and with minimal bias, ultimately there was a high degree of heterogeneity between the studies. Differences in the inclusion criteria and human error associated with recording data could have added confounders to our analysis. Finally, this review is limited by the classification and diversity of CVD and CAD definitions. Our study results must be interpreted cautiously with respect to these myriad definitions. More research on the relationship between CVD and NAFLD in the form of randomized clinical trials where both NAFLD and CVD pathology is defined and evaluated clearly is necessary to solidify our current findings. Additionally, because our findings reveal that both age and gender have precedence in terms of disease outcomes and mortality, the need for research specifically focused on the link between the two disease states and these parameters only increases as the incidence and prevalence rates of both diseases continues to rise.
Conclusions
In the near future, NAFLD will be the main cause of end-stage liver disease when people have need of a liver transplant to prolong survival (7). Both increased cardiovascular events mortality in female people with both NAFLD and CVD cannot be overlooked and require thorough investigations performed by large, multi-center trials. This can be further investigated in future studies in terms of the pathophysiology and genetics of NAFLD and CVD [31,32]. Women had a markedly higher incidence and prevalence of CV event-related mortality and all-cause mortality when compared to men for this population. Advancing age increased mortality among female people with NAFLD. Our study suggests the importance of monitoring silent markers as well as early preventative medicine in female populations with NAFLD, due to their increased risk of developing CVD. We believe this can be achieved by encouraging this subset of people to reduce certain risk factors that overlap in both disease processes, such as weight and sedentary behavior, to help improve overall metabolic health and decrease disease burden. Additionally, changes to unhealthy dietary habits in favor of a healthier diet (i.e. Mediterranean Diet) would help to further reduce the risk factors for this subpopulation.
Appendix A.
Meta Regression Analysis Statistics
| Covariate | Coefficient | Standard Error | 95% Lower | 95% Upper | z-Value | 2-sided P-value |
|---|---|---|---|---|---|---|
| Intercept | -3.1739 | 0.6451 | -4.4382 | -1.9096 | -4.92 | 0.0000 |
| Age | 0.0518 | 0.0127 | 0.0270 | 0.0766 | 4.10 | 0.0000 |
Appendix B.
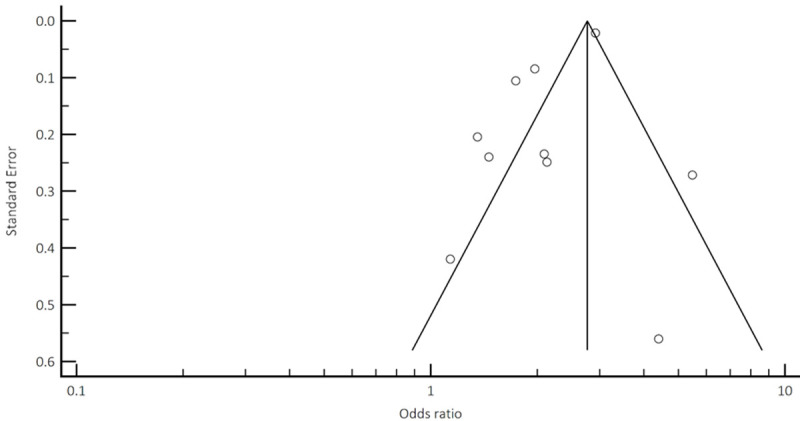
Funnel plot analysis to assess potential publication bias and/or presence of heterogeneity for CV mortality and events.
Appendix C.
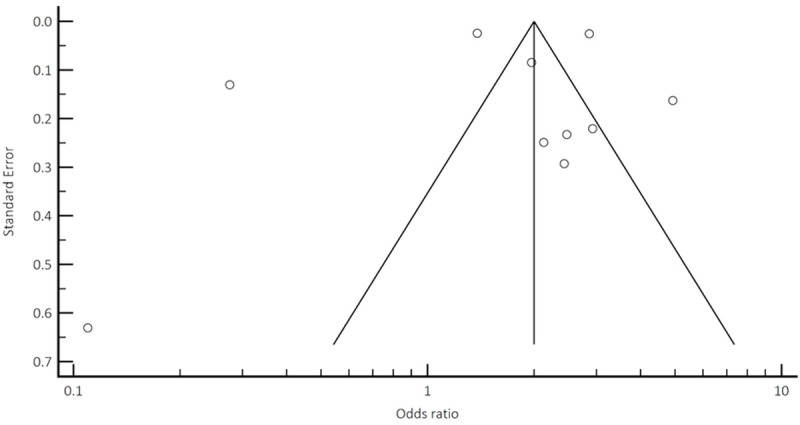
Funnel plot analysis to assess potential publication bias and/or presence of heterogeneity for all-cause mortality.
Appendix D.
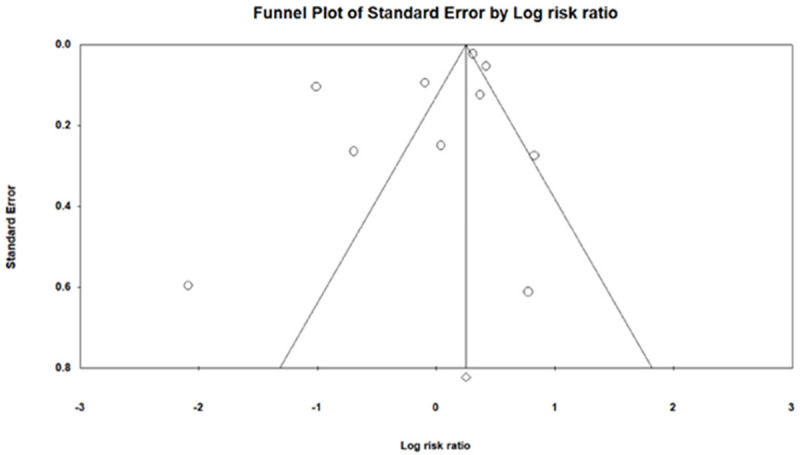
Funnel plot analysis to assess potential publication bias and/or presence of heterogeneity for meta regression.
Disclosure of conflict of interest
None.
References
- 1.VanWagner LB. New insights into the link between NAFLD and atherosclerotic coronary heart disease? J Hepatol. 2018;68:890–892. doi: 10.1016/j.jhep.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon TG, Trejo MEP, McClelland R, Bradley R, Blaha MJ, Zeb I, Corey KE, Budoff MJ, Chung RT. Circulating interleukin-6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: results from the multi-ethnic study of atherosclerosis. Int J Cardiol. 2018;259:198–204. doi: 10.1016/j.ijcard.2018.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuenza LR, Razon TLJ, Dayrit JC. Correlation between severity of ultrasonographic nonalcoholic fatty liver disease and cardiometabolic risk among filipino wellness people. J Cardiovasc Thorac Res. 2017;9:85–89. doi: 10.15171/jcvtr.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y, Ryu S, Sung KC, Cho YK, Sung E, Kim HN, Jung HS, Yun KE, Ahn J, Shin H, Wild SH, Byrne CD. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the kangbuk samsung health study. Gut. 2019;68:1667–1675. doi: 10.1136/gutjnl-2018-317666. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Park HY, Lee HS, Jung KS, Lee MH, Jhee JH, Kim TH, Lee JE, Kim HJ, Kim BS, Park HC, Lee BK, Choi HY. Association between non-alcoholic fatty liver disease and coronary calcification depending on sex and obesity. Sci Rep. 2020;10:1–8. doi: 10.1038/s41598-020-57894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ismaiel A, Dumitrascu DL. Cardiovascular risk in fatty liver disease: the liver-heart axis-literature review. Front Med. 2019;6:202. doi: 10.3389/fmed.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadayifci A, Tan V, Ursell PC, Merriman RB, Bass NM. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: a comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol. 2008;49:595–599. doi: 10.1016/j.jhep.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mahonen M, Ngu Blackett K, Lisheng L Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction. World health organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40:139–146. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- 9.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 10.Harrington RA. Targeting inflammation in coronary artery disease. N Engl J Med. 2017;377:1197–1198. doi: 10.1056/NEJMe1709904. [DOI] [PubMed] [Google Scholar]
- 11.Madika AL, Mounier-Vehier C. La maladie coronaire de la femme: de vraies spécificités à bien connaître pour améliorer les prises en charge. La Presse Médicale. 2016;45:577–587. doi: 10.1016/j.lpm.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Braza-Boïls A, Marí-Alexandre J, Molina P, Arnau MA, Barceló-Molina M, Domingo D, Girbes J, Giner J, Martínez-Dolz L, Zorio E. Deregulated hepatic microRNAs underlie the association between non-alcoholic fatty liver disease and coronary artery disease. Liver Int. 2016;36:1221–1229. doi: 10.1111/liv.13097. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Wang YC, Lu CQ, Zeng C, Chang D, Ju S. Correlations between the abdominal fat-related parameters and severity of coronary artery disease assessed by computed tomography. Quant Imaging Med Surg. 2018;8:579–587. doi: 10.21037/qims.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Li F, Guo J, Wang C, Xu D, Li C. Effects of liver function, insulin resistance and inflammatory factors on vascular endothelial dilation function and prognosis of coronary heart disease people complicated with NAFLD. Exp Ther Med. 2019;17:1306–1311. doi: 10.3892/etm.2018.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vita T, Murphy DJ, Osborne MT, Bajaj NS, Keraliya A, Jacob S, Diaz-Martinez AJ, Nodoushani A, Bravo P, Hainer J, Bibbo CF, Steigner ML, Taqueti VR, Skali H, Blankstein R, DeCarli MF, Dorbala S. Association between nonalcoholic fatty liver disease at CT and coronary microvascular dysfunction at myocardial perfusion PET/CT. Radiology. 2019;291:330–337. doi: 10.1148/radiol.2019181793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerut SE, Balart JT, Kerut EK, McMullan MR. Diagnosis of fatty liver by computed tomography coronary artery calcium score. Echocardiography. 2017;34:937–938. doi: 10.1111/echo.13545. [DOI] [PubMed] [Google Scholar]
- 17.Nabel EG. coronary heart disease in women-an ounce of prevention. N Engl J Med. 2000;343:572–4. doi: 10.1056/NEJM200008243430809. [DOI] [PubMed] [Google Scholar]
- 18.Prata J, Ramos S, Martins AQ, Rocha-Gonçalves F, Coelho R. Women with coronary artery disease: do psychosocial factors contribute to a higher cardiovascular risk? Cardiol Rev. 2014;22:25–29. doi: 10.1097/CRD.0b013e31829e852b. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niikura T, Imajo K, Ozaki A, Kobayashi T, Iwaki M, Honda Y, Kessoku T, Ogawa Y, Yoneda M, Kirikoshi H, Saito S, Nakajima A. Coronary artery disease is more severe in people with non-alcoholic steatohepatitis than fatty liver. Diagnostics. 2020;10:129. doi: 10.3390/diagnostics10030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Li D, Song X, Liu F, Wang X, Ma Q, Zhang X, Li X. Joint associations of serum uric acid and ALT with NAFLD in elderly men and women: a Chinese cross-sectional study. J Transl Med. 2018;16:285. doi: 10.1186/s12967-018-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sao R, Aronow WS. Association of non-alcoholic fatty liver disease with cardiovascular disease and subclinical atherosclerosis. Arch Med Sci. 2018;14:1233–1244. doi: 10.5114/aoms.2017.68821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petta S, Argano C, Colomba D, Cammà C, Di Marco V, Cabibi D, Tuttolomondo A, Marchesini G, Pinto A, Licata G, Craxì A. Epicardial fat, cardiac geometry and cardiac function in people with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62:928–933. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM, D’Onofrio G. Presentation, clinical profile, and prognosis of young people with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc. 2018;7:e009174. doi: 10.1161/JAHA.118.009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bugiardini R, Bairey Merz CN. Angina with normal coronary arteries: a changing philosophy. JAMA. 2005;293:477–84. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 26.Liu KA, Dipietro Mager NA. Women’s involvement in clinical trials: historical perspective and future implications. Pharm Pract (Granada) 2016;14:708. doi: 10.18549/PharmPract.2016.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, Jung SW, Jeong ID, Bang SJ, Shin JW, Park NH, Yang DH, Kang JW, Lim TH, Kim HK, Choe J, Lee HC. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J Hepatol. 2018;68:1018–1024. doi: 10.1016/j.jhep.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Nalbantoglu IL, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:9026–9037. doi: 10.3748/wjg.v20.i27.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 30.Calzadilla-Bertot L, Jeffrey GP, Jacques B, McCaughan G, Crawford M, Angus P, Jones R, Gane E, Munn S, Macdonald G, Fawcett J, Wigg A, Chen J, Fink M, Adams LA. Increasing incidence of nonalcoholic steatohepatitis as an indication for liver transplantation in Australia and New Zealand. Liver Transpl. 2019;25:25–34. doi: 10.1002/lt.25361. [DOI] [PubMed] [Google Scholar]
- 31.Perdoncin E, Duvernoy C. Treatment of coronary artery disease in women. Methodist Debakey Cardiovasc J. 2017;13:201–208. doi: 10.14797/mdcj-13-4-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX Jr, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LK CRUSADE Investigators. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 33.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34:1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig J, McGILL DB, Lindor KD. REVIEW: nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 1997;12:398–403. doi: 10.1111/j.1440-1746.1997.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 35.Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 36.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]


