Abstract
Background
Spinal neurofibromas (SNFs) in neurofibromatosis type 1 (NF1) can cause progressive spinal cord compression and neurological dysfunction. The MEK inhibitor selumetinib shrinks the majority of plexiform neurofibromas (PNs) in patients with NF1. We assessed the effect of selumetinib on SNF.
Methods
Pediatric and adult patients with NF1 and inoperable PN participating in phase 2 studies of selumetinib for PN were included in this analysis if they had SNF and serial spine magnetic resonance imaging (MRI). Selumetinib was administered orally at the recommended dose of 25 mg/m2/dose twice daily (max 50 mg b.i.d.; 1 cycle = 28 days). We qualitatively assessed the effect of selumetinib on SNF-related spinal canal distortion, cerebrospinal fluid distribution, and spinal cord deformity on MRI.
Results
Twenty-four patients (18 male), median age 16.9 years (range, 6.2–60.3), had SNF, 22 of which were associated with the same nerves as the target PN assessed on the clinical trial. Twenty patients had spinal cord deformity. Twenty-three patients completed at least 12 treatment cycles to date. Eighteen patients showed subtle to a marked improvement in SNF burden, 5 remained stable, and no worsening was observed during treatment.
Conclusions
This is the first study describing the effect of selumetinib on SNF. Of 24 patients, 18 exhibited some improvement of SNF burden on imaging. These findings suggest that selumetinib may prevent the worsening of cord compression, potentially reducing the need for surgical interventions in select patients or benefitting patients who do not have a surgical option. Prospective evaluation of the clinical benefit of selumetinib for SNF is warranted.
Keywords: neurofibromatosis type 1, plexiform neurofibroma, selumetinib, spinal cord deformity, spinal neurofibroma
Key Points.
Selumetinib shrinks SNF in NF1.
Prospective trial of selumetinib for SNF is warranted.
Importance of the Study.
Spinal neurofibromas (SNFs) in patients with NF1 can lead to progressive neurological deficits and may require multiple surgeries to relieve spinal cord compression. MEK inhibitors have been recently shown to result in a sustained decrease of plexiform neurofibroma volumes in the majority of patients treated. In this study, we demonstrate that selumetinib also reduces SNF burden and its effect on the spinal cord. We propose that MEK inhibitors may have the potential to prevent cord compression and in select patients may obviate the need for surgery. Prospective standardized evaluations of selumetinib and its effect on SNF are needed to further define its role in the treatment of SNF.
Neurofibromatosis type 1 (NF1) is an autosomal dominant tumor predisposition syndrome caused by mutational inactivation of the NF1 gene on chromosome 17q11.2.1,2 Neurofibromas, the hallmark features of NF1, are histologically benign nerve sheath tumors. The initiating step in neurofibroma formation is the inactivation of the second NF1 allele in Schwann cells that leads to dysregulation of the RAS pathway and proliferation.3 Neurofibromas may affect small segments of a peripheral nerve and appear as discrete nodules, or they can form large conglomerate masses along multiple nerve trunks and/or branches, called plexiform neurofibromas (PNs).4 PN are documented in approximately 50% of NF1 patients who undergo whole-body magnetic resonance imaging (MRI), are frequently diagnosed early in life, can arise in many body locations, and can lead to multiple morbidities resulting from progressive growth.5–8 Neurofibromas that develop in the vicinity of critical organs are of particular concern. Spinal neurofibromas (SNFs) arise from the proximal portion of spinal nerves or the spinal nerve roots and can be discrete masses or part of PN. SNFs have the propensity to encroach on the central canal resulting in cord compression and severe neurologic compromise.9–12 Nguyen et al.10 evaluated a cohort of 97 NF1 patients with high PN burden and found that SNF prevalence increased with age; SNFs were present in 70% of patients younger than the age of 10 years, 80% in the 10–18 age group, and 89% of adults. Imaging signs of spinal cord compression were detected in 34% of patients. While spinal nerve thickening was observed throughout the spine, lesions intruding into the spinal canal originated mainly from cervical and lumbosacral nerves. In addition, paraspinal neurofibromas were associated with an increased incidence of vertebral abnormalities.
The clinical presentation of patients with SNF can be variable and symptoms may include pain, numbness, paresthesia, motor weakness, or gait abnormalities; however, tumor size may not be a direct indicator of symptom severity. Some patients with heavy SNF burden and advanced cord compression remain well compensated, functionally intact, and may never need interventions, while others with minor nerve enlargement suffer unrelenting pain.12
In most patients, symptoms can be managed conservatively. The primary indication for surgical intervention is symptomatic spinal cord compression.13 Decompression can be achieved by SNF resection and/or laminectomy; however, the surgery itself can cause significant morbidities.14 Surgical complications may develop as a result of injury to the spinal cord, the vertebral artery, or the parent nerve. The risks of postoperative neurologic deficits are greater with SNF that span multiple spinal levels as compared with the removal of an isolated SNF. Other postoperative surgical complications include cerebrospinal fluid (CSF) leak, bony deformity (scoliosis, kyphoscoliosis, and swan neck deformity), spinal instability, limited mobility, and postoperative pain. Spinal instability risks must be carefully assessed preoperatively and intraoperatively to determine the need for spinal fusion and instrumentation.13,15
There is an ongoing effort to find alternative treatment options to invasive surgical interventions. MEK inhibitors target the activated RAS pathway in PN and recent clinical trials of various MEK inhibitors in patients with NF1 and inoperable PNs have demonstrated at least 20% tumor volume shrinkage in the majority of patients (selumetinib, PD0325901, and trametinib).16–20 In addition, clinical benefit with improvement in PN-related symptoms such as pain and motor dysfunction was shown in children receiving selumetinib.16,17 Based on these results, in April 2020, selumetinib became the first medical therapy approved by the US Food and Drug Administration for the treatment of children with symptomatic inoperable NF1 PN.
Standard solid tumor response criteria using 1- or 2-dimensional line measurements have limited applicability to the assessment of PN. MRI with volumetric analysis is the most effective means to sensitively and reproducibly detect size change in PN and has been implemented for response assessment in most clinical trials directed at PN.21,22 However, volumetric analysis is not always feasible in SNF. Reliable volume measurements can only be performed on lesions above a certain size that have well-defined contours and many SNFs do not meet these criteria. More importantly, in patients with cord compression, minor volume reduction in the overall bulk of SNF may have no relevance unless the impact on the spinal cord is reduced as well, therefore the critical area of the spine deserves focused attention. Due to the lack of validated and sensitive measurement methods for SNF, we developed a qualitative method of assessing change during treatment with selumetinib. We characterized changes in SNF extension into the spinal canal, CSF distribution, and spinal cord deformity during therapy. In this retrospective study, we describe the effect of selumetinib on SNF in patients enrolled in our ongoing phase 2 trials of selumetinib for children and adults.
Patients and Methods
All subjects were enrolled in the ongoing pediatric (NCT01362803) or adult (NCT02407405) phase 2 trials of selumetinib for patients with NF1 and inoperable PN at the National Cancer Institute (NCI) between August 1, 2015 and October 22, 2018. Data for this analysis were collected until October 31, 2019. Both trials were approved by NCI’s institutional review board. Written informed consent was obtained from adult patients or from the guardians of minor patients. Child assent was obtained when appropriate. The primary objective of these studies is to determine the objective response rate, defined as the proportion of patients with at least a 20% volume decrease in the target PN, compared to baseline. The primary trial endpoint and clinical observations are reported separately17,20 and the focus of this analysis was to evaluate imaging response specifically in SNF occurring in these same patients.
Inclusion Criteria
All NF1 patients with PN enrolled at the NCI on either the adult or pediatric phase 2 study of selumetinib and who underwent serial spinal MRIs during treatment were included in this analysis.
Treatment Plan/Evaluation Schedule
Patients received selumetinib at the recommended adult (50 mg/dose) or pediatric (25 mg/m2/dose, maximum 50 mg/dose) dosage twice daily on a continuous dosing schedule (1 cycle = 28 days). MRI scans for PN response evaluation were obtained at baseline, then every 4 cycles up to cycle 24 and every 6 cycles thereafter until off therapy.
Image Acquisition
The region of the target PN was imaged by axial and coronal short T1 inversion recovery MRI sequences optimized for volumetric assessment.21 In patients with known or suspected SNF, MRIs of the cervical, thoracic, and lumbar spine were obtained, including sagittal T2-weighted (Repetition time (TR) = 3500 ms, echo time (TE) = 110 ms, field of view = 13 × 18 cm2, acquisition matrix = 256 × 358, slice thickness = 3 mm) and high-resolution axial balanced fast field echo sequences (TR = 5.47, TE = 2.36 ms, field of view = 12 × 12 cm2, acquisition matrix = 256 × 256, slice thickness = 1.5 mm). T1-weighted gadolinium-enhanced images were performed in select cases at the discretion of the neuroradiologist.
Image Analysis
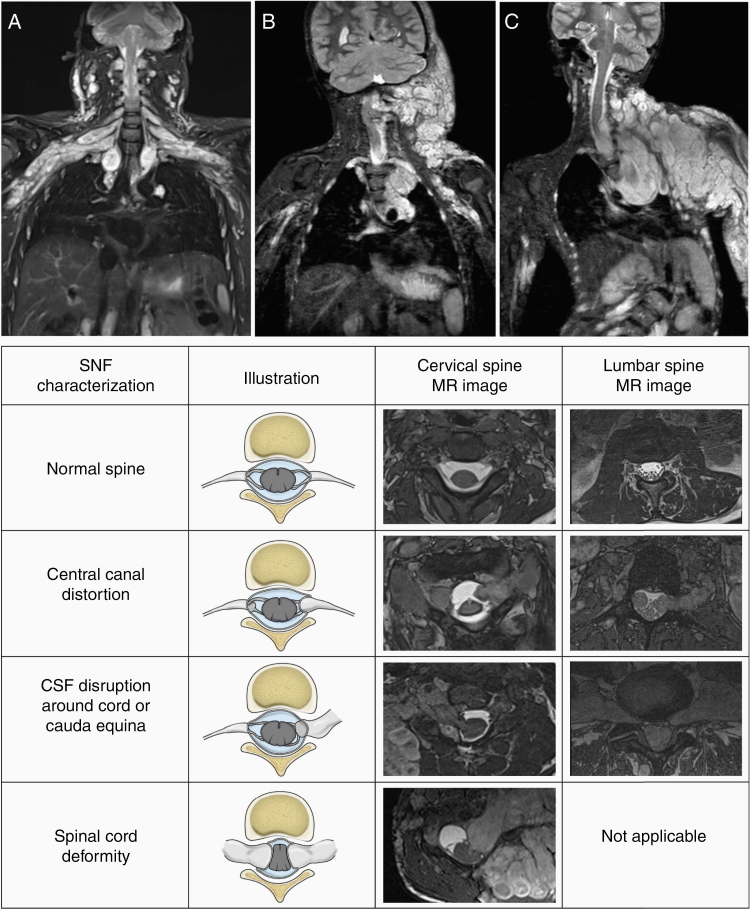
For the primary study endpoint, imaging response evaluation of the target PN was performed by volumetric MRI analysis at the National Institutes of Health.21,23 For this analysis, we evaluated the distribution and severity of SNF on baseline MRIs and qualitatively assessed changes during selumetinib therapy. SNF distribution was classified by the affected spinal region (cervical, thoracic, lumbosacral, or any combination of these), by the number of affected nerve roots (single nerve root, multilevel predominantly one-sided, or multilevel bilateral) and relationship to the primary target PN (same or different body region/nerve root). In patients with multilevel SNF, the most severely affected segment that could be consistently visualized was selected as the SNF region of interest. On baseline and follow-up MRIs, we independently rated the presence or absence of 3 SNF-related characteristics: (1) spinal canal deformity, (2) CSF disruption around the spinal cord or cauda equina, and (3) spinal cord deformity (Figure 1). Follow-up MRIs were also compared to baseline and rated as improved, unchanged, or worsened in each of these categories. The degree of overall change, taking into account the combination of the above visual assessments, was rated as subtle or marked. The subtle change implied that the layout of the region of interest changed slightly, for example, a minimal amount of CSF became detectable between the cord and SNF, or the shape of the spinal cord improved, but cord compression remained. Substantial structural changes, such as the complete resolution of cord deformity or indisputable size decrease in SNF, were rated as a marked improvement. Other relevant spinal findings, such as scoliosis, vertebral scalloping, spinal stenosis, kyphoscoliosis, vertebral erosion, swan neck deformity, postsurgical changes, and spinal instrumentation, were noted. Images acquired at baseline, after cycle 4, after cycle 12, and at the most recent evaluation were scored for the analysis.
Figure 1.
Spinal neurofibroma (SNF) burden characterization. Coronal short T1 inversion recovery MR images demonstrate the SNF distribution: multilevel symmetrical (A), multilevel predominantly one-sided (B), or single spinal nerve root (C). In addition to SNF, extensive plexiform neurofibroma (PN) is seen in the same body region in all 3 patients. The chart below illustrates our method of capturing the presence or absence of SNF-related spinal canal distortion, cerebrospinal fluid (CSF) disruption around the cord or cauda equina, and spinal cord deformity.
Data Analysis
This study was retrospective and descriptive with the aim of evaluating the effect of selumetinib on SNF.
Results
Fifty-eight patients (36 pediatric, 22 adults) enrolled on a pediatric phase 2 and an adult phase 2 study of selumetinib at the NCI between August 1, 2015 and October 22, 2018. Fifty-one patients could be evaluated for the presence of spinal tumors on MRIs acquired within 1 year prior to enrollment; in the remaining 7 pediatric patients with no clinical concern for cord compression, no MRI of the spinal region was obtained. In 14 patients (11 pediatric, 3 adults) whole-body MRI did not suggest spinal involvement, therefore detailed spinal imaging was not indicated. Dedicated spinal MRIs were performed in 37 patients (18 pediatric,19 adults) that identified 12 pediatric and 13 adult patients with SNFs invading the spinal canal. One adult with SNF was removed from the clinical trial without follow-up MRIs, therefore 24 patients (12 pediatric, 12 adults) with SNF and on-treatment data were included in this analysis.
Baseline characteristics are summarized in Table 1. The median age at trial entry was 16.9 years (range 6.2–60.3). The SNF region of interest was selected at the cervical spine (N = 18), lumbosacral spine (N = 3), cervicothoracic junction (N = 2), and thoracolumbar (N = 1); however, 14 patients had additional SNF in other locations. The distribution of SNF was multilevel symmetrical in 13, multilevel predominantly one-sided in 8, and a single nerve root in 3 patients. SNFs were disrupting the circumferential configuration of CSF in 20 patients by direct contact with the spinal cord (N = 19) or obstructing the spinal canal around the cauda equina (N = 1). Some degree of cord deformity by the SNF was observed in 20 patients (10 adults). Examples of SNF distribution and characterization are shown in Figure 1.
Table 1.
Baseline Patient, Spinal Neurofibroma (SNF) and Plexiform Neurofibroma (PN) Characteristics
| Baseline Characteristics (N = 24) | ||
|---|---|---|
| Age (years): median, range | 16.9, 6.2–60.3 | |
| Sex (male/female) | 18/6 | |
| SNF location | Cervical | 18 |
| Cervico-thoracic | 2 | |
| Thoraco-lumbar | 1 | |
| Lumbosacral | 3 | |
| SNF location in relation to target PN location | Same | 22 |
| Other | 2 | |
| SNF distribution | Multilevel symmetrical | 13 |
| Multilevel one-sided | 8 | |
| Single nerve root | 3 | |
| None | 7 | |
| Bony spine deformity | Kyphosis/scoliosis | 9 |
| Vertebral scalloping | 8 | |
| Spinal stenosis | 3 | |
| Vertebral erosion | 1 | |
| History of surgical decompression | 11 | |
| Spinal instrumentation | Fusion/stabilization | 5 |
| Scoliosis repair | 1 | |
| Target PN location | Cervical/brachial plexus distribution | 14 |
| Lumbosacral plexus distribution | 6 | |
| Whole body | 4 | |
| Baseline PN volume (mL): median, range | 890, 138–4444 | |
The majority of SNF were contiguous with extensive PN, and in 22 patients the most clinically relevant PN selected as target lesion for the clinical trial was in the same region as the target SNF for this analysis. Eleven patients (8 adults) had a history of spinal decompression surgery and 5 of them had undergone spinal fusion with instrumentation to stabilize the neck. One or more bony spinal abnormalities were observed in 16 patients. Spinal rods for scoliosis repair were present at baseline in 1 patient, and 2 other patients had the procedure performed after 15 and 27 cycles of treatment, respectively.
As of October 2019, the 24 study participants received a median of 36 treatment cycles (range 8–54) with 16 patients continuing on therapy. Representative MRI examples of treatment effect on SNF are shown in Figure 2. No worsening of any aspect of SNF-related imaging findings was detected during the first 12 treatment cycles in any patient. In 15 patients, the decreased impact of SNF on the central canal at the spinal region of interest could be observed after 4 cycles of treatment. At the same time, CSF regained circumferential distribution in 3 patients and the contact area between the cord and SNF decreased in 11 patients. Some improvement in cord deformity could be appreciated in 8 patients, including one case where the cord deformation completely resolved. By the end of cycle 12, 3 additional patients demonstrated decreased central canal distortion, circumferential CSF distribution could be detected in one more case, and improvement in cord compression in 2 additional patients. The summary of SNF evaluations at the end of cycle 12 is presented in Table 2. After 12 cycles of selumetinib treatment, the degree of overall imaging improvement was rated on a subjective scale as subtle in 10 (43%), marked in 8 (35%) patients, and no improvement was noted in 5 (22%) patients. SNF responses were maintained during further treatment cycles in all but 1 patient whose adherence to selumetinib treatment schedule declined between cycles 24 and 30, potentially contributing to tumor regrowth. In another patient, protocol mandated selumetinib discontinuation after 24 cycles due to the target PN not meeting response criteria resulted in clinical and imaging worsening of the SNF. Six months after stopping treatment this patient presented with new neurologic symptoms including clonus and gait disturbances, and the MRI findings confirmed worsening spinal cord compression. The patient underwent emergency decompression surgery and after recovery was eligible to restart selumetinib treatment per protocol. One year after restarting selumetinib the response in the remaining SNFs was similar to the SNF response achieved during the initial treatment phase.
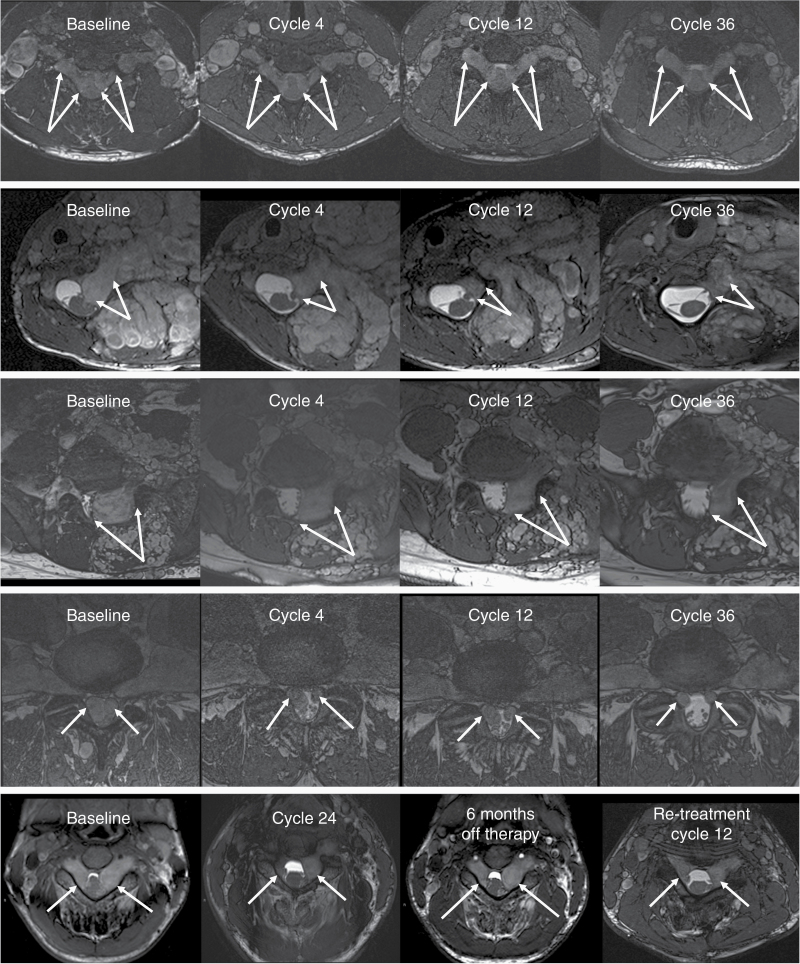
Figure 2.
MRI examples of subtle (top row) and marked improvement (rows 2–5) in patients with spinal neurofibroma (SNF) receiving selumetinib therapy. The area of the spinal canal is shown on axial balanced fast field echo sequences. Arrows indicate the SNFs of interest. First row: Bilateral dumbbell tumors compress the spinal cord at the cervical 5–6 level. There is a slight gradual improvement in the narrow wedge shape of the cord and an increase in cerebrospinal fluid (CSF) abundance. Also note the decreasing size of the adjacent brachial plexus plexiform neurofibroma (PN). Second row: SNF in the left cervical 6–7 neuroforamen extends into the central canal and deforms the spinal cord. The size of the mass is markedly reduced after 4 cycles of selumetinib therapy with further improvement through cycle 36. Third row: SNF below the level of the cord (L5-S1) displacing the thecal sac and nerve roots. SNF shrinkage is apparent by cycle 4 and the response is maintained through cycle 36. Fourth row: Lumbar 4–5 level bilateral SNFs completely fill the central canal at baseline. CSF becomes detectable around the nerve roots after 4 treatment cycles and the improvement continues through cycle 30. Fifth row: The spinal cord deformity at C3-4 level is reduced during 24 cycles of therapy. Interruption of selumetinib treatment for 6 months resulted in SNF regrowth; however, improvement is evident again upon retreatment with selumetinib.
Table 2.
Spinal Neurofibroma (SNF) Assessment After Completing 12 Cycles of Selumetinib Treatment
| Evaluation of the Effect of Seluemtinib on SNFs After 12 Cycles of Selumetinib | ||
|---|---|---|
| Evaluable Patients (N = 23) | Baseline | Cycle 12 |
| Spinal canal distortion (N) | Present: 23 | Resolved: 1 |
| Absent: 0 | Improved: 17 | |
| No change: 5 | ||
| Worsened: 0 | ||
| Disruption of circumferential CSF (N) | Present: 19 | Resolved: 4 |
| Absent: 4 | Improved: 13 | |
| No change: 2 | ||
| Worsened: 0 | ||
| Spinal cord deformity (N) | Present: 19 | Resolved: 1 |
| Absent: 1 | Improved: 9 | |
| Not applicable: 3 | No change: 9 | |
| Worsened: 0 | ||
Discussion
PNs arising from proximal spinal nerves often extend through the neural foramina into the spinal canal and have the propensity to compress the spinal cord, resulting in significant pain, disfigurement, and morbidity in patients with NF1.10,11 Recently, MEK inhibitors have been shown to shrink inoperable PN in the majority of NF1 patients.16–20 The MEK inhibitor selumetinib is now approved in the United States for the treatment of symptomatic, inoperable PN in children with NF1. The effect of MEK inhibitors on SNF burden in NF1 has not been previously evaluated, likely due to the inability to sensitively and reproducibly measure changes in SNF size. In the absence of standardized response criteria for SNF, we therefore qualitatively assessed the effect of selumetinib on imaging findings related to SNF in this study.
Among 51 patients participating in clinical trials of selumetinib directed at NF1 PN who underwent MRI of the spinal region, we found that 59% of adults (13 of 22) and 41% of children (12 of 29) had at least one SNF encroaching on the spinal canal. Spinal cord deformity was observed in 20 patients, despite 11 patients having undergone spinal decompression in the past. These findings highlight not only the high prevalence of SNF in NF1 patients with large PN burden, but also the substantial clinical impact and the difficulty of resolving the problem with surgical means.
Using qualitative analysis of serial spine MRIs in 24 clinical trial participants with SNF, we observed gradual improvement in SNF-related MRI findings in the majority of patients, and no one had worsening of SNF characteristics on selumetinib treatment. The most noticeable indicator of treatment response was increased abundance of CSF at the level of SNF on cross-sectional images, indicating less crowding of the central canal. Categorical change, such as the complete resolution of cord deformation (N = 1), restoration of circumferential CSF distribution (N = 4), or complete normalization of the spinal cord shape (N = 1), was achieved in few cases after approximately 1 year (12 cycles) of therapy. Qualitative improvement, including decreased cord deformity, was observed more frequently. At the time of the first follow-up, MRI evaluation after completing 4 cycles of therapy, 15 of 24 patients demonstrated some improvement in SNF burden. By the end of 12 treatment cycles, 18 of the 23 evaluable patients were found to have imaging improvement, with the degree of overall change rated as subtle in 10 and marked in 8 patients. Importantly, in patients with additional follow-up, the improvements continued or persisted for a median of 36 treatment cycles until data cutoff. Long-term administration of the therapeutic selumetinib dose may be necessary for disease control, as SNF regrowth was observed when treatment was interrupted in 2 patients. Notably, upon restarting selumetinib therapy in one of these patients, SNF response returned (Figure 2, bottom row).
The proportion of patients who demonstrated improvement in SNF burden in this study is similar to the previously reported response rate of PN to selumetinib in patients with NF1.16,17 The similarity is not surprising, as these tumors are intricately interconnected; however, on an individual level, one may find discrepancies. One such example is the above patient who had marked improvement in cord compression but failed to qualify for PN response by volumetric MRI criteria.
None of the patients with advanced cord compression reached complete normalization of the spinal cord, and the improvement was typically modest. However, potentially even a slight decrease in SNF size could be sufficient to reduce cord distress in severe cases or prevent further functional decline. In this analysis, we did not attempt to correlate imaging response with clinical outcomes, as our patients had substantial PN burden in the region of SNF, and distinguishing which clinical changes could be attributable to SNF versus the bulk of the PN would have been difficult. Further studies are needed to assess whether selumetinib treatment results in clinical benefit in NF1 patients with predominantly SNF burden.
However, designing clinical trials for SNF with response criteria based on clinical findings and symptoms alone would not be adequate. SNFs evolve slowly over years, and patients with severe cord compression can remain asymptomatic for prolonged time periods, which can be followed by rapid clinical deterioration in spite of stable or minimally worsened imaging appearance. An ideal window of opportunity for medical intervention in patients with worsening cord compression might therefore be the presymptomatic phase, with imaging response as a key outcome measure. The major barrier toward the development of clinical trials targeting SNF is the lack of appropriate objective response criteria for these tumors. Rating systems currently in use to describe the severity of acute cord compression and evaluate surgical success are not sensitive enough to capture small but potentially meaningful changes in this condition. In 2017, Mauda et al.24 crafted a novel scoring system to evaluate spinal and paraspinal neurofibromas. While their imaging observations provide a good means to qualify disease severity to correlate with clinical symptoms, they lack sensitivity to assess changes over time and/or with therapeutic intervention. The imaging working group of the Response Evaluation in Neurofibromatosis and Schwannomatosis international collaboration is in the process of designing an evaluation toolbox specifically for SNF that, if successful, could be used in future prospective studies.
Our retrospective evaluation of SNFs in the ongoing phase 2 trials with selumetinib has several limitations. Our analysis focused only on imaging findings. In the absence of established objective response criteria for SNF, we described qualitative changes during treatment. These qualitative assessments are subject to interpretation; while cases of marked improvement are unequivocal, some observers might rate more subtle findings differently. The evaluations were not done in a blinded fashion; therefore, investigator bias cannot be excluded.
In conclusion, we demonstrated that in patients with NF1-related PN selumetinib reduces SNF burden and its effect on the spinal canal, CSF distribution, and spinal cord shape. We believe that selumetinib treatment therefore could have a potential role in the management of patients who have symptomatic cord compression but are not surgical candidates, patients with asymptomatic cord compression who are at risk of becoming clinically symptomatic, and patients at high risk of developing cord compression. Prospective studies and the establishment of standardized response criteria for SNF will be necessary to more objectively evaluate the effect and clinical benefit of selumetinib and other upcoming medical therapies in patients with NF1 and SNF.
Acknowledgments
We are grateful for the time and support of our patients who participate in the clinical trials. We thank Dr. John Butman, a neuroradiologist at the NIH Clinical Center, Department of Radiology and Imaging Sciences for optimizing the spinal imaging protocol used in this study. We would also like to recognize the excellent team of dedicated radiology technologists who ensured the superior quality of the MRI exams, with special thanks to team supervisors Robert Evers and R. Reese Baehr.
Funding
This work was supported, in part, by the National Cancer Institute, Center for Cancer Research, Intramural Research Program.
Conflict of interest statement. None declared.
Authorship statement
B.C.W., P.W., E.D., A.M.G., and K.Y. contributed to experimental design. S.J., E.H.B., and E.D. analyzed the imaging of each subjected and interpreted findings. A.M.G., G.O., A.P.C., B.C.W., A.B., Je.D., C.T., Jo.D., and A.C. evaluated, recorded findings of, and cared for study participants. P.W., A.B., C.T., Je.D., Jo.D., A.C., A.M.G., G.O., A.P.C., and B.C.W. handled study participant enrollment, compliance with protocol participation, and coordination of subsequent study imaging. All authors have contributed to writing the manuscript at the draft and any revision stages and have read and approved the final version.
References
- 1. Korf BR. Neurofibromatosis. Handb Clin Neurol. 2013;111:333–340. [DOI] [PubMed] [Google Scholar]
- 2. Viskochil D, White R, Cawthon R. The neurofibromatosis type 1 gene. Annu Rev Neurosci. 1993;16:183–205. [DOI] [PubMed] [Google Scholar]
- 3. Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: schwann cell origin and role of tumor environment. Science. 2002;296(5569):920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89(1):31–37. [DOI] [PubMed] [Google Scholar]
- 5. Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [DOI] [PubMed] [Google Scholar]
- 6. Merker VL, Bredella MA, Cai W, et al. Relationship between whole-body tumor burden, clinical phenotype, and quality of life in patients with neurofibromatosis. Am J Med Genet A. 2014;164A(6):1431–1437. [DOI] [PubMed] [Google Scholar]
- 7. Gross AM, Singh G, Akshintala S, et al. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018;20(12):1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thakkar SD, Feigen U, Mautner VF. Spinal tumours in neurofibromatosis type 1: an MRI study of frequency, multiplicity and variety. Neuroradiology. 1999;41(9):625–629. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen R, Dombi E, Akshintala S, Baldwin A, Widemann BC. Characterization of spinal findings in children and adults with neurofibromatosis type 1 enrolled in a natural history study using magnetic resonance imaging. J Neurooncol. 2015;121(1):209–215. [DOI] [PubMed] [Google Scholar]
- 11. Leonard JR, Ferner RE, Thomas N, Gutmann DH. Cervical cord compression from plexiform neurofibromas in neurofibromatosis 1. J Neurol Neurosurg Psychiatry. 2007;78(12):1404–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruggieri M, Polizzi A, Spalice A, et al. The natural history of spinal neurofibromatosis: a critical review of clinical and genetic features. Clin Genet. 2015;87(5):401–410. [DOI] [PubMed] [Google Scholar]
- 13. Klekamp J, Samii M. Surgery of spinal nerve sheath tumors with special reference to neurofibromatosis. Neurosurgery. 1998;42(2):279–289; discussion 289–290. [DOI] [PubMed] [Google Scholar]
- 14. Taleb FS, Guha A, Arnold PM, Fehlings MG, Massicotte EM. Surgical management of cervical spine manifestations of neurofibromatosis type 1: long-term clinical and radiological follow-up in 22 cases. J Neurosurg Spine. 2011;14(3):356–366. [DOI] [PubMed] [Google Scholar]
- 15. Crawford AH, Schumaier AP, Mangano FT. Management of cervical instability as a complication of neurofibromatosis type 1 in children: a historical perspective with a 40-year experience. Spine Deform. 2018;6(6):719–729. [DOI] [PubMed] [Google Scholar]
- 16. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382(15):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiss B, Plotkin S, Widemann B, et al. NFM-06. NF106: phase 2 trial of the MEK inhibitor PD-0325901 in adolescents and adults with NF1-related plexiform neurofibromas: an NF clinical trials consortium study. Neuro Oncol. 2018;20(suppl 2):i143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCowage GB, Mueller S, Pratilas CA, et al. Trametinib in pediatric patients with neurofibromatosis type 1 (NF-1)–associated plexiform neurofibroma: a phase I/IIa study. J Clin Oncol. 2018;36(15):10504. [Google Scholar]
- 20. Coyne G, Gross A, Dombi E, et al. Phase II trial of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886 Hydrogen Sulfate) in adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PN). J Clin Oncol. 2020;38(15_suppl):3612. [Google Scholar]
- 21. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. ; REiNS International Collaboration Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21)(suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahlawat S, Fayad LM, Khan MS, et al. ; Whole Body MRI Committee for the REiNS International Collaboration ; REiNS International Collaboration Members 2016. Current whole-body MRI applications in the neurofibromatoses: NF1, NF2, and schwannomatosis. Neurology. 2016;87(7)(suppl 1):S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solomon J, Warren K, Dombi E, Patronas N, Widemann B. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28(5):257–265. [DOI] [PubMed] [Google Scholar]
- 24. Mauda-Havakuk M, Shofty B, Ben-Shachar S, Ben-Sira L, Constantini S, Bokstein F. Spinal and paraspinal plexiform neurofibromas in patients with neurofibromatosis type 1: a novel scoring system for radiological-clinical correlation. AJNR Am J Neuroradiol. 2017;38(10):1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]