Abstract
Rituximab (RTX) for immune-mediated inflammatory disease (IMID) with interstitial pneumonitis (IP) results in non-response in about a third of patients for reasons not well understood. Complete peripheral B-cell depletion in IMID-IP does not seem to correlate with successful treatment outcome. A hypothesis is that splenic B cells might play a role in B-cell recovery and attraction of naïve B cells in non-responsive patients. The aim of this post hoc analysis of clinical trial data is to search for indicators in [89Zr]Zr-rituximab PET/CT data from the spleen that might explain non-responsiveness. PET/CT data of 20 patients with IMID-IP, who were enrolled in a phase II trial and treated with RTX were analyzed. Clinical outcome was categorized into responders (RSP) and non-responders (NR) after 6 months of initial RTX by two independent pulmonologists. Patients were examined separately to search for associations between clinical outcome, splenic activity on PET/CT, lymphocyte counts and other biomarkers. Treatment failure was found in 6/20 patients (30%) while all patients exhibited B-cell depletion from the circulation. NR patients demonstrated significantly higher splenic activity than RSP patients (non-preload protocol: SUV 4.9±1.96 and SUV 2.3±1.08 respectively, P=0.025). No correlations between treatment outcome and serum lymphocyte subsets were found. Our findings suggest a potential splenic mechanism in IMID-IP patients non-responding to RTX and warrant further consideration and investigation.
Keywords: Spleen, clinical research, interstitial lung disease, rituximab, [89Zr]Zr-rituximab, PET/CT, CD20 cells, immunology
Introduction
Treatment failure following anti-CD20 therapy in immune-mediated inflammatory disease in interstitial pneumonitis (IMID-IP) using rituximab (RTX) occurs in approximately 30% of patients after initial treatment, and the mechanisms of treatment failure in non-responders are still incompletely understood [1]. Progression of IMID-IP can have serious and fatal consequences; therefore it is essential to understand the mechanisms underlying failure of RTX therapy and relapse following RTX. Complete B-cell depletion in IMID has not been shown to correspond with expected successful treatment outcome after 3-6 months from baseline, while the earliest cases of re-population of B-cell to normal values have been demonstrated to occur well beyond 6 months of initial RTX therapy [2]. In light of these observations, alternative diagnostic tools are required to understand the treatment failure in RTX therapy, with a focus on the role of the spleen, as it is involved in a broad array of immune system processes. Histological studies demonstrated that RTX efficiently depleted splenic and circulating B cells one month after initial therapy; nevertheless, this did not correlate with successful treatment response [3]. In an autoimmune encephalomyelitis model, it was demonstrated that B cells repopulated spleen substantially earlier than the blood compartment [4]. This could be indicative of why autoimmune encephalomyelitis patients relapse sooner than in rheumatoid arthritis patients and also why the recommended effective dose of RTX given during the course of the disease, is more than twice higher in autoimmune encephalomyelitis [5,6]. The spleen contains distinct B cell lineages, and it is not clear how these lineages relate to treatment outcome, especially in IMID-ILD [7]. RTX antibody availability has also been considered a factor in treatment response, while closely related to the B cell depletion rate, it is not related to clinical outcome in patients with rheumatoid arthritis [8].
Understanding the role of the spleen and RTX might guide management for re-treatment or alternative immunologic dosing regimen in RTX refractory patients. Recently, the role of the spleen and RTX treatment has come under attention through a novel imaging technique using [89Zr]Zr-rituximab PET/CT [9]. This post hoc analysis study explores the targeting of CD20 cells in the spleen using this technique and its correlation to treatment response.
Methods
Inclusion criteria
A Dutch cohort of patients with IMID-IP, part of a phase II prospective trial, were referred to the department of pulmonology of St. Antonius hospital Nieuwegein, a tertiary center of pulmonology for treatment of rare lung diseases between May 2015-April 2017. All patients gave written informed consent prior to participation. Patients with pulmonary function decline were included according to strict selection criteria, published in full detail at ClinicalTrials.gov identifier: NCT02251964 (https://clinicaltrials.gov/ct2/show/NCT02251964). Patients younger than 18 and older than 70 were excluded. All were therapy refractory to first-line (corticosteroids) and second-line therapy (cyclophosphamide or azathioprine). Exclusion criteria were residual lung volume >120% predicted at screening, DLCO <25% of the predicted value at screening measured at rest. Patients having any signs of infection were not eligible.
Intervention
A dose of 1 g rituximab was administered intravenously on day 1 and day 14 preceded by premedication: acetaminophen, dexamethasone and antihistamine according to protocol. Adverse events and serious adverse events were documented and reported to the medical research ethics committee united (MEC-U) under NL49534.100.14.
Biomarker measurements
Blood samples in EDTA were taken before infusion of RTX to determine baseline values of CA 15.3, IL-18, procalcitonin (PCT) in plasma, and CD19+ B cells, CD56+ NK cells, and CD3+/CD4+CD8+ T cells in whole blood. Blood samples were stained with monoclonal antibodies against CD3, CD8, CD45, CD4, CD16, CD56 and CD19 (Becton Dickinson, New Jersey, USA), and flow cytometric analysis was performed using the FACS Canto II (Becton Dickinson, New Jersey, USA). The following lymphocyte subpopulations were quantitatively measured: T-lymphocytes (CD3+), B-lymphocytes (CD19+), Natural Killer (NK) cells (CD3-/CD16+56+), T-helper cells (CD4+) and cytotoxic/suppressor T-cells (CD8+). Two weeks, three months and six months after RTX lymphocyte measurements were repeated and CD4+/CD8+ ratio calculated. Frequencies of B-cell compartments were only determined at baseline due to a low B-cell count during the remaining months. The following serological markers were measured using a Human Magnetic Luminex Assay (R&D Systems, Minnesota, USA): CA 15.3, IL-18, B-Cell Activating Factor (BAFF) and PCT.
Criteria of response assessment
RTX treatment success or failure after 6 months was determined by two independent pulmonologists specialized in ILD according to specific criteria until consensus reached. The criteria were as follows: 1) the overall clinical state judged on vital signs, 2) indicators, pulmonary function parameters (forced vital capacity and diffusing capacity for carbon monoxide) and 3) changes in imaging by way of chest x-ray, high-resolution computer tomography (HRCT). Patients with a worsening clinical state were defined as non-responders (NR), patients with disease stabilization or with a clinical improvement as responders (RSP).
[89Zr]Zr-rituximab PET/CT
Using 10 mg of RTX, [89Zr]Zr-N-suc-DFO-rituximab, abbreviated as [89Zr]Zr-rituximab was synthesized using 18 MBq Zr89 (half-life 78.4 hours) according to previously published protocol [10,11]. Detailed description of [89Zr]Zr-rituximab production and QC procedures can be found in the supplementary methods in (Table S2). Previous studies with [89Zr]Zr-rituximab were mainly performed in lymphoma patients with considerable CD20-expressing tumor load, and a substantially higher likelihood of ‘left-over’ CD20 targets for [89Zr]Zr-rituximab after preloading with RTX. By contrast, IMIDs are fundamentally different from lymphomas and large monoclonal B-cell populations are non-existent by definition. It was hypothesized that after preloading with therapeutic RTX, all the CD20 targets in the lungs might be already fully saturated, preventing [89Zr]Zr-rituximab from binding to CD20 targets. The saturation effect of RTX in the lungs must be excluded, therefore after the first ten patients we have amended the study protocol to allow a PET preparation protocol without a preload. The doses of RTX and [89Zr]Zr-rituximab were similar in both protocols [9]. In the preload protocol (n=10) subjects received therapeutic RTX within 4 hours prior to infusion with 18 MBq [89Zr]Zr-rituximab (containing 10 mg RTX), and in the non-preload protocol (n=10) patients received RTX within 4 hours subsequent to the infusion. Patients were scanned using an analogue Philips Gemini TF PET/CT (Philips Medical Systems, Best, the Netherlands), in accordance with Zr-harmonization protocol [12,13]. PET images were taken 15 minutes per bed position from the lower neck to splenic region, 3 days after injection of [89Zr]Zr-rituximab. Standardized uptake values (SUV) were measured in the liver and spleen using a 4-14 cm3 volume (2 cm radius) of a predefined volume of interest (VOIs).
Statistics
For the descriptive statistics on continuous data the independent and paired-sample T-tests were performed, and a P-value <0.05 was regarded as statistically significant. A Bonferroni correction (for multiple testing) was applied where relevant. Additional correlations between subsets and outcome measures were performed using bivariate correlation using the Pearson correlation coefficient and two-tailed test of significance. Due to the low sample size a ROC curve was not of added value. The statistical evaluation was performed using SPSS version 22 (IBM, Armonk, New York, USA).
Results
Clinical characteristics
Twenty patients completed the study protocol including RTX therapy, blood tests and [89Zr]Zr-rituximab PET/CT (male [n=9], female [n=11], mean age 60.2±10.4 years). A supplement is available further detailing all patient characteristics (Table S1). Four patients (20%) had RA-associated interstitial lung disease (ILD), six patients (30%) antisynthetase syndrome (ASS) related IP, seven (30%) patients chronic extrinsic allergic alveolitis (cEAA) and four patients (20%) other types of connective tissue disease-related IP (CTD-ILD). At baseline, all patients had abnormal median values of the following: CA 15.3 was 116.0±233.9 (reference 7-30 U/ml), IL-18 was 267.2±264.9 (reference 0-70 pg/ml) and PCT was 27.2±19.7 (reference <100 ng/ml). After 6 months, all 20 patients were categorized into two groups: 70% is RSP or treatment success (n=14) and 30% NR or treatment failure (n=6) by consensus of two independent pulmonologists.
Correlation of clinical outcome and lymphocytes after RTX
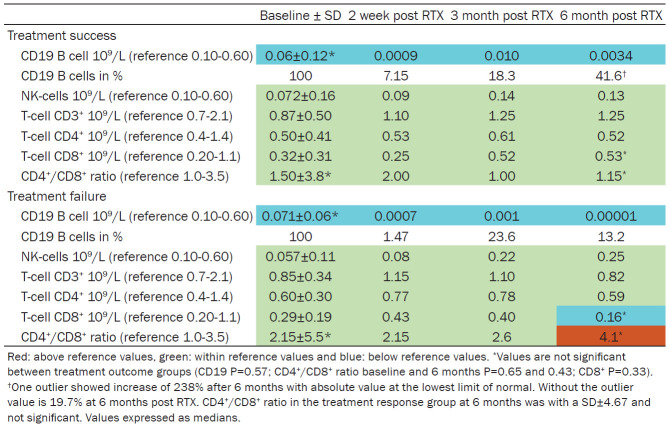
Baseline absolute counts of CD19+ B cells decreased significantly in all patients to levels less than 5% of initial values, and absolute values remained below normal during the follow-up period (Table 1 and Figure 1). NK-cells, CD3+, CD4+, CD8+ were within normal range and without significant differences between groups. In the NR group, a higher average CD4+/CD8+ ratio was observed in comparison with the RSP group, although this was not statistically significant. At 6 months the CD8+ count was lower in the NR group, but did not statistically differ from the RSP group. Mean BAFF measurements showed higher absolute concentration of 1874.5±1138.6 ng/ml in NR patients [n=5] versus 1333.2±744.9 ng/ml for RSP [n=14] (reference <2 ng/ml) however not significantly different.
Table 1.
Biomarkers before and after rituximab treatment (N=20)
Figure 1.
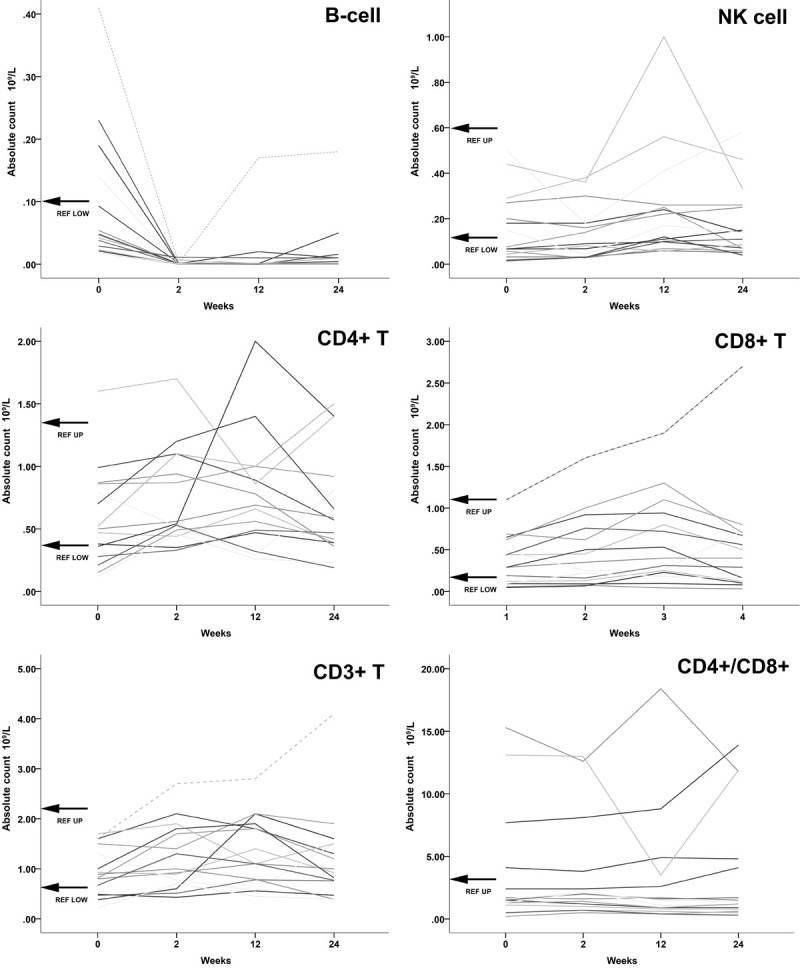
All absolute values displayed of cell counts in 109/L and weeks after initial RTX therapy at baseline (0 weeks) and second infusion at week 2. Top left: B-cells. Absolute B-cell counts were far below lower normal reference values within 2 weeks, therefore upper limit is not visible in this scale. One patient showed B cell recovery after 3 months to lower range reference values. Top right: NK cells. Most values did not appear to change significantly. Middle left: CD4+ T-cell. Values fluctuated mostly within normal reference values with no clear indication of changes. Middle right: CD8+ T-cell. Values mostly within normal limits with no apparent change after RTX. Bottom left: CD3+ T-cell. No apparent changes after RTX therapy with values well within normal limits. Bottom right: CD4+/CD8+ ratio. Few patients with higher ratios but remained without significant changes after 3-months.
Correlation of clinical outcome and [89Zr]Zr-rituximab PET
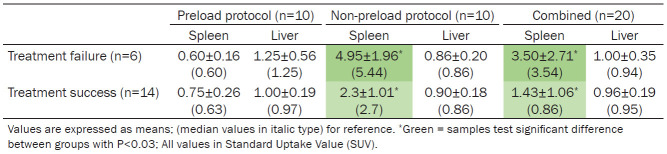
A subgroup analysis was performed between NR and RSP patients and injection protocol. Visually the non-preload protocol demonstrated higher splenic activity and sometimes bone marrow activity while preload patients demonstrated lower splenic activity with lower bone marrow activity, consistent with previous studies. Quantitative measurement using SUV mean values in the spleen were expectedly different between groups using the preload and non-preload protocol (Table 2). Subgroup analysis demonstrated no significant differences in absolute [89Zr]Zr-rituximab liver activity between preload/non-preload protocol within NR and RSP. [89Zr]Zr-rituximab with non-preload protocol showed a higher splenic SUV for NR (n=4) patients than RSP (n=6) patients: SUV 4.95±1.96 and SUV 2.3±1.08, respectively, also demonstrating a negative correlation with clinical outcome, a higher splenic activity correlated with worse outcome (Pearson K=0.7 and P=0.03) (Figure 2). In addition, post hoc test confirmed splenic activity significance (P=0.022) in non-preload group. When reviewing the splenic activity in the non-preload protocol, in both NR and RSP patients the spleen appears to be more active than the liver, however a higher measurable difference was demonstrated in the spleen of the NR patients (Figure 3).
Table 2.
[89Zr]Zr-rituximab activity in spleen and liver using two different protocols
Figure 2.
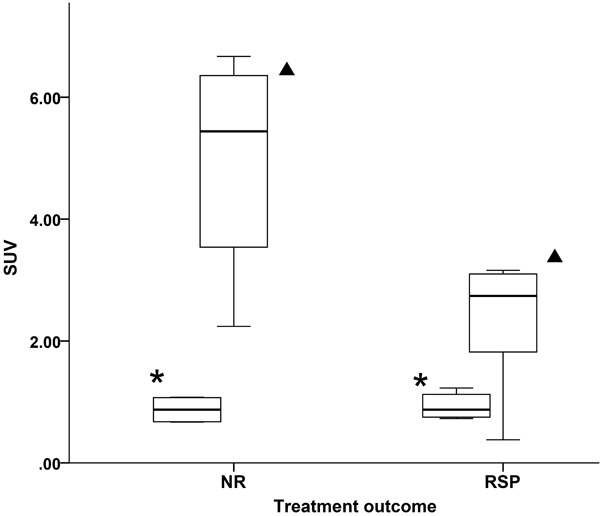
[89Zr]Zr-rituximab activity measured in SUV in the spleen (triangle) and liver (asterisk) reference using the non-preload protocol, between treatment outcomes: non-responders (NR, n=4) and responders (RSP, n=6). There is significantly higher splenic activity measured in the NR group.
Figure 3.
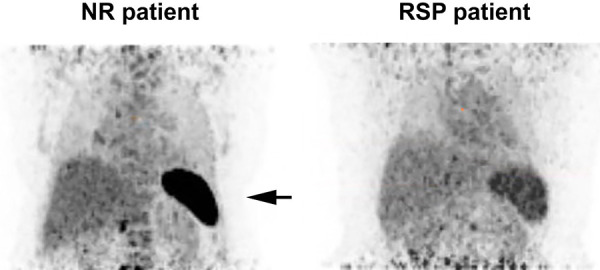
[89Zr]Zr-rituximab activity in the spleen using the non-preload protocol, between treatment outcomes: non-responder (NR) on the left and responder (RSP) on the right. There is significantly higher measurable splenic activity in the NR patient (arrow) and a slight bone marrow uptake. No particular adjustment is made on the scale bar to reflect the difference.
Discussion
[89Zr]Zr-rituximab is a novel imaging method of the pharmacokinetics of rituximab in vivo. Utilizing this technique, higher splenic activity was found in NR patients in this study using the [89Zr]Zr-rituximab non-preload protocol, while absolute lymphocyte counts were not statistically different compared to the RSP group. Prior to inferring normative conclusions from these findings, it is important to establish (1) if the [89Zr]Zr-rituximab PET/CT is a reliable method for measuring splenic activity, (2) the validity of the methods used, and (3) the premise and other evidence supporting these findings. Regarding the former, our experience with [89Zr]Zr-rituximab in IMID-IP patients has demonstrated it to be a highly specific tracer to visualize CD20 cells in vivo [11,14,15]. Although histologic evidence is considered the gold standard, splenic biopsies were outside the scope of our initial study in IMID-IP patients as this is an explorative post hoc study. Also given the high-risk nature of this procedure, a biopsy proposition must be based on substantial evidence beforehand. [89Zr]Zr-rituximab seems to be a highly specific imaging technique to establish a CD20 cell count/estimate in various body parts, in vivo. However splenic biopsies may have to be performed in future studies.
Several caveats and limitations in this study should be considered, including the PET protocol, technical PET image quality, therapeutic rituximab dosing and statistics. As this was the first study performed in IMIDs with interstitial pneumonitis using [89Zr]Zr-rituximab, an established PET preloading protocol was used according to previous immuno-PET studies performed on patients with lymphoma. In hematological patients, where large volumes of circulating clonal cells are characteristic, therapeutic RTX was initially given within 4 hours before [89Zr]Zr-rituximab, which has been demonstrated to improve image B-cell targeting and PET image quality [16,17]. When a fixed treatment dose of 1 g rituximab is given prior to intravenous administration of 10 mg of labelled [89Zr]Zr-rituximab, the amount of [89Zr]Zr-rituximab trapped in the spleen and bone marrow is expected to decrease, and more [89Zr]Zr-rituximab would become available for targeting CD20 cells. However variation of splenic activity was still measured in patients despite preloading with RTX, the amount of activity varied from mGy/MBq 2.8 to 51.0, indicating high variability [18]. Unfortunately, there was no clinical follow-up of this particular study to asses correlation of splenic activity and treatment effect. As there are major differences in CD20 target volumes between IMID-IP patients and hematological patients suffering from lymphomas, the preload protocol in IMIDs was debated. We proposed in our study that there was a higher likelihood of blocking all CD20 targets in IMID patients in the lungs with preloading, therefore reducing [89Zr]Zr-rituximab available CD20 targets in the lungs. Higher absolute SUV values were found in the lungs when preloading was not used, suggesting that dosing protocols and subsequently biodistribution of the tracer had to be adapted and optimized in patients with IMID [11]. Current data show that a non-preload protocol with [89Zr]Zr-rituximab injection prior to therapeutic RTX result in higher SUV values in different target organs, such as the lungs, spleen and blood pool, while the activity in the liver remained relatively constant.
While the [89Zr]Zr-rituximab has been thoroughly tested and validated using mouse studies, the [89Zr]Zr-rituximab PET/CT has been validated with physics studies, including the development of a [89Zr] harmonization protocol and human studies through various multicenter validation studies with excellent reproducibility [12,19,20]. To minimize signal-to-noise effects, the scanning time was 15 minutes per bed position, considerably longer than a normal routine PET scan. In our study only three bed positions were performed per patient (from the chest and part of the liver/spleen area), and this took approximately 45 minutes scanning time while the patient was inside the PET scanner. It was well tolerated since oxygen was available and no movement artefacts occurred. Even though we utilized a long scanning time to acquire sufficient signals, the end image quality result was still quite less than routine PET with a higher dose, a limitation also addressed in our previous publication [9]. This limitation in image quality was also an important reason not to include bone marrow PET activity, as the measurements were less reliable, and we have taken several immunological markers related to bone marrow development of immune cells from the blood instead. Using state-of-the-art digital PET scanners with higher sensitivity, however, several image quality issues can be overcome in the future. Another limitation in our scans was that the spleen was not always completely visible depending on the scanner range. As a result, total organ volume assessment was not always feasible. To overcome this limitation, the VOI was measured instead and proved quite reliable as the spleen demonstrated a diffuse [89Zr]Zr-rituximab uptake. Using a VOI or ROI technique delivers the same SUVmean values on the liver [21]. Measurements showed a difference in the standard deviation, but not the SUVmean measurement itself. We have only used absolute SUVmean values for statistical analysis per patients and avoided measuring on the scanning borders of the scanned area. As these are particularly noisy areas, smaller VOIs were applied. There have been studies that measured the spleen and liver using 2D ROI, which correlated well with white blood counts in studies using fluorodeoxyglucose [18F]-FDG [22]. Although SUVmean worked satisfactorily, for future studies it is recommended to perform a total volume measurement of the spleen.
There are a few aspects of [89Zr]Zr-rituximab and RTX therapy that are noteworthy. First and foremost, there is no evidence in the literature that a change in injection protocol of [89Zr]Zr-rituximab 4 h prior or after therapeutic RTX, has any effect on treatment outcome since [89Zr]Zr-rituximab dose is 200 times lower than the therapeutic range, therefore it is considered negligible. In both NR as RSP patients, a significant reduction in CD19 B cells was found, thus validating the effectiveness of rituximab in these patients. In addition, dose effectiveness trials have shown that treatment with a fixed dose of rituximab using 2× 1000 mg dose is as effective as 2× 500 mg dose in patients with rheumatoid arthritis, and there are even indications to using lower doses [23,24]. As rheumatoid arthritis is considered an IMID, our IMID-IP patient population fall within the group of autoimmune disorders that include rheumatoid arthritis, we have no indication that NR patients have been undertreated. Furthermore, no major differences in body weight, neither were other confounding factors noted, including age. Since extreme bodyweight also may potentially lead to attenuation artefacts, and lower quality PET images we have not selected patients above 100 kg.
A major limitation of this study is the small sample size, with n=6 and 14 in the NR and RSP group, respectively. Dividing these patients further into subgroups resulted in 2 NR patients in the preload group and 4 NR patients in the non-preload group. Within the NR subgroup, 2 NR patients received the preload. Considering the small group sizes, it was realistic to keep statistics mostly descriptive. Larger patient groups must be included in future studies, perhaps in pooled collaborative efforts, to conduct inferential statistics.
Possible splenic immune-mediated mechanisms
In this post hoc analysis in a small subset of patients, we did demonstrate a higher activity in the spleen in NR patients. Even though this finding requires validation by larger studies and using more sensitive scanners, it has significant implications if verified. The spleen is the largest lymphoid organ containing specific subsets of lymphocytes and myeloid cells, which are known to be involved in the progression of multiple diseases. There are several B cell lineages in the spleen which may or may not have a causal role in treatment failure even after effective CD20 therapy (measured in the peripheral blood). As immature B cells do not have CD20+ receptors, they will not be depleted by RTX. So 4-6 weeks after the last RTX dose, these cells migrate from the bone marrow and start repopulation. Repopulation is a slow process and immature cells will migrate to BAFF producing locations, such as lymph nodes and spleen. We found a noteworthy trend of higher levels of BAFF in the NR patients compared to RSP. Steadily, more publications are focusing on splenic mechanisms in immune-mediated conditions, including work on sympathetic nerve hyperactivity in the spleen as a causal effect in IMIDs, or anti-inflammatory pathways via the spleen using vagal nerve stimulation [25,26]. Considering rituximab is very specific for CD20, and CD20 is exclusively expressed on B cells, our data infers a possible association between recovery of splenic B cell numbers and disease non-responsiveness in IMID-IP. As B cells are still absent in the blood, it appears newly generated B cells rapidly migrate from the bone marrow to the spleen in these patients, while they may distribute more broadly in RSP patients. It is not likely that these B cells in the spleen of NR patients are directly responsible for disease activity, it is more likely the ongoing disease activity in the spleen (most likely mediated by T and myeloid cells) attracts and activates naïve B cells. As different immune system mechanisms can be implicated in NR patients when using anti-CD20 therapy, the current imaging findings have rendered a research agenda for a possible splenic process being involved.
Conclusion
Differences in splenic CD20 activity between IMID patients with a response or non-response to treatment have been demonstrated in this explorative study in a small subset of patients, without preloading. While RTX is highly effective in reducing CD19 B cells, treatment outcome was not associated with differences in peripheral blood lymphocyte counts in between IMID patients. A direct causal effect is not proven, but these preliminary data could suggest that ongoing disease activity in the spleen might attract naïve B cells thus leading to treatment failure, even after effective CD19 B cell reduction. Current findings warrant further consideration and a need to further substantiate a possible link between splenic involvement in cases with RTX treatment failure.
Acknowledgements
This investigator-initiated study was financially supported by ZonMw research grant for Goed Gebruik Geneesmiddelen (GGG) which is the Netherlands Organization for Health Research and Development commissioned by the Ministry of Health, Welfare and Sport of the Netherlands. There is no conflict of interest. This study was funded by ZonMW (grant number 836021009).
Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, Nicholson AG, Hansell DM, Wells AU, Renzoni EA. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–9. doi: 10.1111/resp.12214. [DOI] [PubMed] [Google Scholar]
- 2.Thiel J, Rizzi M, Engesser M, Dufner AK, Troilo A, Lorenzetti R, Voll RE, Venhoff N. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther. 2017;19:101. doi: 10.1186/s13075-017-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audia S, Samson M, Guy J, Janikashvili N, Fraszczak J, Trad M, Ciudad M, Leguy V, Berthier S, Petrella T, Aho-Glele S, Martin L, Maynadie M, Lorcerie B, Rat P, Cheynel N, Katsanis E, Larmonier N, Bonnotte B. Immunologic effects of rituximab on the human spleen in immune thrombocytopenia. Blood. 2011;118:4394–400. doi: 10.1182/blood-2011-03-344051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausler D, Hausser-Kinzel S, Feldmann L, Torke S, Lepennetier G, Bernard CCA, Zamvil SS, Bruck W, Lehmann-Horn K, Weber MS. Functional characterization of reappearing B cells after anti-CD20 treatment of CNS autoimmune disease. Proc Natl Acad Sci U S A. 2018;115:9773–9778. doi: 10.1073/pnas.1810470115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin YW, Lee ST, Park KI, Jung KH, Jung KY, Lee SK, Chu K. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. 2017;11:1756285617722347. doi: 10.1177/1756285617722347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredemeier M, Campos GG, de Oliveira FK. Updated systematic review and meta-analysis of randomized controlled trials comparing low- versus high-dose rituximab for rheumatoid arthritis. Clin Rheumatol. 2015;34:1801–5. doi: 10.1007/s10067-015-2977-z. [DOI] [PubMed] [Google Scholar]
- 7.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–18. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Torne C, Ortiz MA, Sarmiento M, Diaz-Lopez C, Corominas H, Casademont J, Vidal S. Rituximab levels are associated with the B cell homeostasis but not with the clinical response in patients with rheumatoid arthritis. Eur J Rheumatol. 2019;6:81–4. doi: 10.5152/eurjrheum.2019.18109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams H, van de Garde EM, van Moorsel CH, Vugts DJ, van Dongen GA, Grutters JC, Keijsers RG. [(89)Zr]Zr-rituximab PET/CT activity in patients with therapy refractory interstitial pneumonitis: a feasibility study. Am J Nucl Med Mol Imaging. 2019;9:296–308. [PMC free article] [PubMed] [Google Scholar]
- 10.Verel I, Visser GW, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GA. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med. 2003;44:1271–81. [PubMed] [Google Scholar]
- 11.Adams H, van de Garde EM, van Moorsel CH, Vugts DJ, van Dongen GA, Grutters JC, Keijsers RG. [89Zr]Zr-rituximab PET/CT activity in patients with IMID-IP: a feasibility study. Am J Nucl Med Mol Imaging. 2019;9:296–308. [PMC free article] [PubMed] [Google Scholar]
- 12.Makris NE, Boellaard R, Visser EP, de Jong JR, Vanderlinden B, Wierts R, van der Veen BJ, Greuter HJ, Vugts DJ, van Dongen GA, Lammertsma AA, Huisman MC. Multicenter harmonization of 89Zr PET/CT performance. J Nucl Med. 2014;55:264–7. doi: 10.2967/jnumed.113.130112. [DOI] [PubMed] [Google Scholar]
- 13.Makris NE, van Velden FH, Huisman MC, Menke CW, Lammertsma AA, Boellaard R. Validation of simplified dosimetry approaches in (8)(9)Zr-PET/CT: the use of manual versus semi-automatic delineation methods to estimate organ absorbed doses. Med Phys. 2014;41:102503. doi: 10.1118/1.4895973. [DOI] [PubMed] [Google Scholar]
- 14.Jauw YWS, O’Donoghue JA, Zijlstra JM, Hoekstra OS, Menke-van der Houven van Oordt CW, Morschhauser F, Carrasquillo JA, Zweegman S, Pandit-Taskar N, Lammertsma AA, van Dongen GAMS, Boellaard R, Weber WA, Huisman MC. (89)Zr-Immuno-PET: toward a noninvasive clinical tool to measure target engagement of therapeutic antibodies in vivo. J Nucl Med. 2019;60:1825–32. doi: 10.2967/jnumed.118.224568. [DOI] [PubMed] [Google Scholar]
- 15.Van Dongen GA, Huisman MC, Boellaard R, Harry Hendrikse N, Windhorst AD, Visser GW, Molthoff CF, Vugts DJ. 89Zr-immuno-PET for imaging of long circulating drugs and disease targets: why, how and when to be applied? Q J Nucl Med Mol Imaging. 2015;59:18–38. [PubMed] [Google Scholar]
- 16.Bruijnen S, Tsang-A-Sjoe M, Raterman H, Ramwadhdoebe T, Vugts D, van Dongen G, Huisman M, Hoekstra O, Tak PP, Voskuyl A, van der Laken C. B-cell imaging with zirconium-89 labelled rituximab PET-CT at baseline is associated with therapeutic response 24 weeks after initiation of rituximab treatment in rheumatoid arthritis patients. Arthritis Res Ther. 2016;18:266. doi: 10.1186/s13075-016-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jauw YW, Zijlstra JM, de Jong D, Vugts DJ, Zweegman S, Hoekstra OS, van Dongen GA, Huisman MC. Performance of 89Zr-labeled-Rituximab-PET as an imaging biomarker to assess CD20 targeting: a pilot study in patients with relapsed/refractory diffuse large B cell lymphoma. PLoS One. 2017;12:e0169828. doi: 10.1371/journal.pone.0169828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muylle K, Flamen P, Vugts DJ, Guiot T, Ghanem G, Meuleman N, Bourgeois P, Vanderlinden B, van Dongen GA, Everaert H, Vaes M, Bron D. Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. Eur J Nucl Med Mol Imaging. 2015;42:1304–14. doi: 10.1007/s00259-015-3025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natarajan A, Habte F, Liu H, Sathirachinda A, Hu X, Cheng Z, Nagamine CM, Gambhir SS. Evaluation of 89Zr-rituximab tracer by Cerenkov luminescence imaging and correlation with PET in a humanized transgenic mouse model to image NHL. Mol Imaging Biol. 2013;15:468–75. doi: 10.1007/s11307-013-0624-0. [DOI] [PubMed] [Google Scholar]
- 20.Jauw YWS, Bensch F, Brouwers AH, Hoekstra OS, Zijlstra JM, Pieplenbosch S, Schröder CP, Zweegman S, van Dongen GAMS, Menke-van der Houven van Oordt CW, de Vries EGE, de Vet HCW, Boellaard R, Huisman MC. Interobserver reproducibility of tumor uptake quantification with (89)Zr-immuno-PET: a multicenter analysis. Eur J Nucl Med Mol Imaging. 2019;46:1840–9. doi: 10.1007/s00259-019-04377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amakusa S, Matsuoka K, Kawano M, Hasegawa K, Ouchida M, Date A, Yoshida T, Sasaki M. Influence of region-of-interest determination on measurement of signal-to-noise ratio in liver on PET images. Ann Nucl Med. 2018;32:1–6. doi: 10.1007/s12149-017-1215-y. [DOI] [PubMed] [Google Scholar]
- 22.Nunez R, Rini JN, Tronco GG, Tomas MB, Nichols K, Palestro CJ. Correlation of hematologic parameters with bone marrow and spleen uptake in FDG PET. Rev Esp Med Nucl. 2005;24:107–12. doi: 10.1157/13071686. [DOI] [PubMed] [Google Scholar]
- 23.Bredemeier M, Campos GG, de Oliveira FK. Updated systematic review and meta-analysis of randomized controlled trials comparing low- versus high-dose rituximab for rheumatoid arthritis. Clin Rheumatol. 2015;34:1801–5. doi: 10.1007/s10067-015-2977-z. [DOI] [PubMed] [Google Scholar]
- 24.den Broeder AA, Verhoef LM, Fransen J, Thurlings R, van den Bemt BJF, Teerenstra S, Boers N, den Broeder N, van den Hoogen FHJ. Ultra-low dose of rituximab in rheumatoid arthritis: study protocol for a randomised controlled trial. Trials. 2017;18:403. doi: 10.1186/s13063-017-2134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellinger DL, Lorton D. Sympathetic nerve hyperactivity in the spleen: causal for nonpathogenic-driven chronic Immune-Mediated Inflammatory Diseases (IMIDs)? Int J Mol Sci. 2018;19:1188. doi: 10.3390/ijms19041188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannon EC, Sun J, Wilson K, Brands M, Martinez-Quinones P, Baban B, O’Connor PM. A basic solution to activate the cholinergic anti-inflammatory pathway via the mesothelium? Pharmacol Res. 2019;141:236–48. doi: 10.1016/j.phrs.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.