Abstract
The present treatise chronicles one decade of experience pertaining to clinical PRRT services in a large-volume tertiary cancer care centre in India delivering over 4,000 therapies, an exemplar of successful PRRT programme employing indigenous 177Lutetium production and resources. For the purpose of systematic discussion, we have sub-divided the communication into 3 specific parts: (a) Radiopharmaceutical aspects that describes 177Lutetium production through ‘Direct’ Neutron Activation Route and the subsequent radiolabeling procedures, (b) The specific clinical nuances and finer learning points (apart from the routine standard procedure) based upon clinical experience and how it has undergone practice evolution in our setting and (c) Dosimetry results with this indigenous product and radiation safety/health physics aspects involved in PRRT services. Initiated in 2010 at our centre, the PRRT programme is a perfect example of affordable quality health care delivery, with indigenous production of the radionuclide (177Lu) in the reactor and subsequent radiolabeling of the radiopharmaceutical ([177Lu]Lu-DOTATATE) at the hospital radiopharmacy unit of the centre, which enabled catering to the needs of a large number of patients of progressive, metastatic and advanced Neuroendocrine Neoplasms (NENs) and related malignancies.
Keywords: Neuroendocrine neoplasms (NENs), neuroendocrine tumor, peptide receptor radionuclide (PRRT), somatostatin receptor (SSTR), [177Lu]Lu-DOTATATE, [90Y]Y-DOTATATE, Direct’ neutron activation route, 177Lutetium, chemo-PRRT, duo-PRRT
Introduction
The somatostatin receptor (SSTR)-targeted theranostics integrates [99mTc]Tc/[111In]In/[68Ga]Ga-labelled diagnostic SPECT or PET imaging and [177Lu]Lu/[90Y]Y-labelled therapeutic radiopharmaceuticals for ‘Peptide Receptor Radionuclide Therapy’ (PRRT), that has seen rapidly increasing applications for metastatic or advanced and progressive neuroendocrine neoplasms (NENs), and gratifying cumulative clinical experience for disease stabilization in majority and partial response in a sizeable fraction of patients. The somatostatin receptor subtype 2 (SSTR2), a member of the G-protein coupled somatostatin receptor family (SSTR 1-5) is the principal target for PRRT, in view of its predominance in the NENs. The therapy is accomplished through intravenously administered unsealed radiopharmaceutical, the most common across the world being [177Lu]Lu-DOTATATE (DOTATATE = DOTA0-(Tyr3)-octreotate) [177Lu: Eβ(max) = 0.497 MeV; maximum tissue range ~2.5 mm], while [90Y]Y-DOTATATE or DOTATOC is the other beta emitting therapeutic alternative, in which the harder beta energy [90Y: Eβ(max) = 2.28 MeV] and longer range of 90Y (maximum tissue range ~11 mm) makes it more suitable for larger and heterogeneous tumors (albeit also accounting for its more nephrotoxicity), while [177Lu]Lu-DOTATATE is more efficacious for smaller tumors.
Radiopharmaceutical aspects: the 177Lutetium revolution
Production of 177Lu suitable for formulation of [177Lu]Lu-DOTA0-Tyr3-octreotate ([177Lu]Lu-DOTATATE) for PRRT
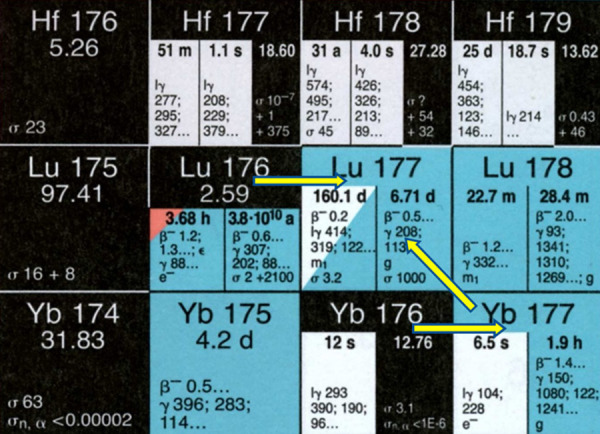
Two alternative routes are available for production of 177Lu with adequate specific activity required for its utility in PRRT, as shown in Figure 1. The first is the “direct” route which involves the thermal neutron activation of highly enriched (in 176Lu) lutetium target [176Lu(n, γ)177Lu] in research reactors with medium to high thermal neutron flux [1-7]. The second route or the “indirect” route is based on neutron irradiation of highly enriched (in 176Yb) ytterbium targets leading to the formation of no-carrier-added (NCA) 177Lu from the β- decay of the short-lived activation product 177Yb (T1/2 = 1.9 h) [3,8-10]. The post-irradition radiochemical processing in the former case involves simple dissolution of the irradiated target, whereas the latter route involves elaborate procedure to separate 177Lu from ytterbium targets as well as its radionuclides. The neutron activation products of natural lutetium and ytterbium targets along with the nuclear decay characteristics of the product radionuclides are given in Table 1.
Figure 1.
Alternate routes for production of 177Lu in Nuclear Reactor.
Table 1.
Neutron activation products of natural lutetium and ytterbium targets along with the nuclear decay characteristics of the product radionuclides
| Target element | Target isotope | % Abundance | σ (barns) | Activation product | Mode of decay | T1/2 | Decay product |
|---|---|---|---|---|---|---|---|
| Lu | 175Lu | 97.41 | 16.7 | 176mLu | β-, γ | 3.66 h | 176Hf |
| 6.6 | 176Lu | β-, γ | 4×1010 y | 176Hf | |||
| 176Lu | 2.59 | 2.8 | 177mLu | β-, γ & IT | 160.4 d | 177Hf (78.6%) | |
| 177Lu (21.4%) | |||||||
| 2090 | 177Lu | β-, γ | 6.65 d | 177Hf | |||
| Yb | 168Yb | 0.13 | 2300 | 169Yb | EC | 32.02 d | 169Tm |
| 170Yb | 3.04 | 9.9 | 171Yb | Stable | |||
| 171Yb | 14.28 | 58.3 | 172Yb | Stable | |||
| 172Yb | 21.83 | 1.3 | 173Yb | Stable | |||
| 173Yb | 16.13 | 15.5 | 174Yb | Stable | |||
| 174Yb | 31.83 | 63 | 175Yb | β-, γ | 4.18 d | 175Lu | |
| 176Yb | 12.76 | 2.85 | 177Yb | β-, γ | 1.9 h | 177Lu |
The indirect route of production offers two distinct advantages over the direct route, (i) it provides NCA 177Lu (theoretical specific activity 4.1 TBq/mg, 110.9 Ci/mg), which in turn leads to higher specific activity of the radiolabeled peptide (ii) 177Lu produced from indirect route is practically free from any radionuclide contaminant, provided a robust radiochemical separation strategy is adapted to separate 177Lu from radionuclides of ytterbium. Different approaches have been successfully utilized in the separation of clinical grade NCA 177Lu from bulk quantity of neutron irradiated ytterbium target [8-11]. Although, NCA 177Lu is available from commercial sources, the absolute need of a complex radiochemical separation procedure to isolate 177Lu of requisite purity is challenging and expensive. It can be shown by theoretical calculation that irradiation of 1 mg of 99% enriched (in 176Yb) Yb2O3 target at a thermal neutron flux of 5×1014 n/cm2.s will produce only ~3.2 GBq (~87 mCi) of 177Lu.
In contrast to any other medically useful radionuclides, direct (n, γ) route can be utilized for large-scale production of 177Lu in nuclear reactors, having medium to high thermal neutron flux (1.0×1014 n.cm-2.s-1 or higher), using enriched lutetium target (80% or more in 176Lu), with specific activity adequate for preparing receptor-specific therapeutic radiopharmaceuticals. This is possible due to the following two reasons, (i) 176Lu has very high thermal neutron capture cross section (σ = 2090 b, I0 = 1087 b) for formation of 177Lu and also that (ii) the neutron capture cross section of 176Lu does not follow 1/v law and there is a strong resonance very close to the thermal region [12]. Consequently, it is possible to produce 177Lu of specific activity of more than 20 Ci/mg (740 GBq/mg) in a medium flux reactor. The most significant advantage of direct route over the indirect route is the simplicity of post-irradiation chemical treatment procedure, which makes it much less technologically demanding as well as cost-effective compared to the other route. Moreover, there is absolutely no impediment towards scaling up the production as per its clinical requirement. On the contrary, the major concern in the large-scale clinical utilization of 177Lu produced via the direct route, is the presence of the co-produced 177mLu, with a long T1/2 of 160.4 days (Table 1), which creates problem in the disposal of radioactive wastes arising from the treatment of large number of patients beside the additional radiation dose burden to the patients. A careful optimization of the duration of irradiation depending on the available thermal neutron flux of the reactor is essential in the direct route to obtain 177Lu in the highest achievable specific activity while keeping the contamination from 177mLu to a minimum [1,3,5,8,13].
In India, the clinical grade 177Lu used in PRRT is indigenously produced following the direct route, by irradiation of enriched lutetium oxide target (~82% enriched in 176Lu) at a thermal neutron flux of 1.6×1014 n.cm-2.s-1 at Dhruva research reactor, Bhabha Atomic Research Centre (BARC), Mumbai. The irradiated target is dissolved in 0.01 M ultrapure HCl solution in an aseptic environment to obtain sterile, pyrogen free, [177Lu]LuCl3 solution as the radiopharmaceutical precursor.
The theoretical yield of 177Lu ‘A’ (in Bq) produced via 176Lu(n, γ)177Lu at the end of irradiation is calculated using the formula (Equation 1):
 |
Where, where, N0 = number of 176Lu atoms used as target (at t = 0), λ = decay constant of 177Lu (in s-1), σ = thermal neutron capture cross section of 176Lu at the neutron velocity of 2200 m.s-1 (2090 b), σ’ = thermal neutron capture cross section of 177Lu at the neutron velocity of 2200 m.s-1 (1000 b), φ = thermal neutron flux of the reactor (in n.cm-2.s-1), t = time of irradiation and ‘k’ is so called k-factor, the value of ‘k’ is reported to be between 1.5-2.5 [7]. This expression of the yield of 177Lu is based on the assumption that the neutron flux is highly thermalized and there is practically no contribution of epithermal neutrons towards 177Lu formation. Based on Equation 1, the variation of theoretical yield of 177Lu as a function of irradiation time at the irradiation position in Dhruva (where k = 1.75) is shown in Figure 2. It is evident for the figure that the yield is maximum (~21 Ci/mg of Lu) when irradiation is carried out for 13-14 days. Hence, Lu targets are irradiated for 2 weeks for production of 177Lu in India. Also, it is very interesting to note that, in case of 177Lu, there is a mismatch between the theoretically calculated specific activity and that actually obtained l from its yield per unit mass of the target irradiated. This is due to the significant burn up of target during the irradiation for which the actual mass of lutetium present in the system, post irradiation is significantly reduced compared to the initial mass of the target irradiated. Using the burn-up correction, the actual specific activity S (Bq/mol) of 177Lu can be expressed as (Equation 2):
Figure 2.

Theoretical yield 177Lu as a function of irradiation time at the irradiation position in Dhruva research reactor in India (ф = ~1.6×1014 n.cm-2.s-1, k = 1.75).
 |
where, n0 is the number of moles of the target isotope 176Lu at the start of irradiation and n is the number of moles of other lutetium isotopes which do not lead to the formation of 177Lu by (n, γ) process. Based on Equation 2, the specific activity of 177Lu has been found to be 1.25 times higher compared to the yield of 177Lu, when irradiations of Lu targets (~82% enriched 176Lu) were carried out in Dhruva at a thermal neutron flux of 1.6×1014 n.cm-2.s-1 for 14 d. This enhancement of specific activity is an added advantage for 177Lu towards its utilization in receptor specific therapeutic radiopharmaceuticals. Practically, the specific activity of 177Lu has been found to be 900 ± 78 GBq/mg (24.3 ± 2.1 Ci/mg) (averaged over 300 batches).
The impurity burden of 177mLu in 177Lu produced in our case was found be ~0.015% of 177Lu activity produce at end of irradiation (EOI) and ~0.02% of 177Lu activity produced at 48 h post-EOI, when 177Lu is generally used in the clinic. A typical gamma ray spectrum of [177Lu]LuCl3 used for formulation of [177Lu]LuDOTATATE is shown in Figure 3, which confirms very high radionuclidic purity of the radiopharmaceutical precursor. This implies that for each patient administered with 7.4 GBq dose of 177Lu-labeled-S-analogue peptide, the administered dose of 177mLu is ~1.48 MBq (40 μCi). The presence of 177mLu at this level is not a matter of serious concern from the point of view of radiation dose burden to the patients. However, the use of each GBq of 177Lu will lead to the accumulation of ~200 kBq of radioactive waste of 177mLu to be disposed by following delay and decay approach.
Figure 3.

Typical gamma-ray spectrum of [177Lu]LuCl3 used for the formulation of [177Lu]Lu-DOTATATE.
Formulation of [177Lu]Lu-DOTATATE for clinical use
The therapy doses of [177Lu]Lu-DOTATATE radiopharmaceutical are formulated mainly in hospital radiopharmacy prior to clinical use following a protocol optimized after extensive radiochemical studies. Moderate specific activity [177Lu]LuCl3 produced (20-24 Ci/mg) indigenously by direct neutron activation route is used for the formulation. The general protocol followed for the synthesis of the radiopharmaceutical is presented schematically in Figure 4. During the last decade more than 400 batches of the [177Lu]Lu-DOTATATE were synthesized (10-15 therapeutic doses in each batch) with >95% radiochemical purity, as confirmed by radio-HPLC (Figure 5). The specific activity of the formulation varied from 1.0 mCi/µg (53.1 GBq/µmol) to 1.2 mCi/µg (63.8 GBq/µmol) of DOTATATE. The specifications of the radiopharmaceutical formulation are summarized in Table 2.
Figure 4.

Schema of General protocol for the synthesis of [177Lu]Lu-DOTATATE.
Figure 5.

Typical HPLC radio-chromatogram of [177Lu]Lu-DOTATATE formulation. HPLC was carried out using a dual pump HPLC unit with a C-18 reversed phase HiQ-Sil (5 μM, 25 cm×0.46 cm) column. The elution was monitored both by detecting UV signals at 270 nm as well as by radioactivity signal using NaI(Tl) detector. Gradient elution with Water (A) and acetonitrile (B) mixtures with 0.1% trifluoroacetic acid were used as the mobile phase (0-5 min 90% A, 5-15 min 90% A to 10% A, 15-20 min 10% A, 20-25 min 10% A to 90% A, 25-30 min 90% A). Flow rate was maintained at 1 mL.min-1.
Table 2.
Specifications of [177Lu]Lu-DOTATATE therapy dose (~7.4 GBq, 200 mCi) formulation
| Parameter | Specification |
|---|---|
| Appearance | Clear pale yellow solution without any suspended particulate |
| pH | ~4.5 |
| Volume | ~1.0 mL |
| Radionuclidic purity | >99.95% |
| Radiochemical purity | >95.0% |
| Sterility | Sterile formulation |
| Apyrogenicity | Pyrogen content <25 IU/mL |
PRRT: clinical services
The PRRT clinical services was initiated at the Radiation Medicine Centre (RMC) -Tata Memorial Hospital (TMH) premises in 2010, by the joint efforts of Radiation Medicine Centre (RMC), BARC (Bhabha Atomic Research Centre) and GI services of the Tata Memorial Hospital (TMH). More than 4,000 therapies have been successfully administered with [177Lu]Lu-DOTATATE in around 1000 patients, and has involved patients of (i) Gastroenteropancreatic NENs (GEP-NENs), (ii) Metastatic NEN with unknown primary (CUP-NEN) (iii) Bronchopulmonary, Mediastinal and Thymic NENs, (iv) Medullary thyroid carcinoma (MTC), (v) non-[131I]I-MIBG concentrating metastatic Paraganglioma & Pheochromocytomas, and (vi) other miscellaneous tumors (non-iodine concentrating metastasis of differentiated thyroid carcinoma or TENIS, metastatic Merkel Cell carcinoma, Meningioma, recurrent/inoperable Phosphaturic Mesenchymal Tumor and sinonasal neuroendocrine carcinoma) (Table 3; Figure 6). The PRRT with [90Y]Y-DOTATATE is relatively new addition that has started from September 2019 as a part of duo-PRRT protocol, which is at its optimization phase at present (detailed later). The important milestones in our clinical PRRT programme has been depicted in Figure 7. Under this sections, we have elaborated upon the learning points and have shared our perspectives on these themes or issues.
Table 3.
Spectrum of Malignancies treated by PRRT with 177Lu-DOTATATE and their relative distribution
| Patients characteristics | Number of Patients |
|---|---|
| 177Lu-DOTATATE PRRT | 1000 |
| 90Y-DOTATATE PRRT | 11 |
| Site of primary disease for 1000 pts | |
| Gastroenteropancreatic NENs (GEP-NENs) | 781 |
| Metastatic NEN with unknown primary (CUP-NEN) | 87 |
| Lung NENs | 30 |
| Mediastinal/thymus NENs | 27 |
| Medullary Thyroid carcinoma (MTC) | 50 |
| Pheochromocytomas and Paraganglioma (PCC/PGL) | 15 |
| Miscellaneous | 10 |
Figure 6.

Pie Chart of Spectrum of Malignancies treated by PRRT with [177Lu]Lu-DOTATATE and their relative distribution.
Figure 7.

The important milestones in the Clinical PRRT Services and Practice Evolution in our set-up.
The clinical efficacy of 177Lu obtained through “Direct” route as a therapeutic radiopharmaceutical: results in major malignancies
As mentioned before, two alternative strategies are available for production of 177Lu with adequate specific activity required for its utility in PRRT. The first is the so called “direct” route which results in the production of carrier-added 177Lu. The second route or the “indirect” route is based on neutron irradiation of highly enriched (in 176Yb) ytterbium targets leading to the formation of no-carrier-added (NCA) 177Lu from the β- decay of the short-lived activation product 177Yb (T1/2 = 1.9 h).
In India, more than 90% of the PRRT procedures (nearly all government centres and majority of the private centres) are undertaken using 177Lu obtained via the first pathway indigenously in the research reactor. There has been some debate on the efficacy of 177Lu produced through the direct route. At our Centre, we have obtained gratifying response with the indigenous product in large number of patients with clinical results similar to that is reported with the product from the ‘indirect’ route (two clinical examples depicted in Figures 8, 9, 10 and 11) in the literature [14-18]. There was excellent concordance of the ranges in tumor absorbed doses (estimated in metastatic NET patients during different PRRT cycles) with indigenous [177Lu]Lu-DOTATATE and that by the 177Lu produced through ‘indirect’ route (a comparative table of the tumor absorbed doses with the reported studies and our own experience has been illustrated in Figure 11; Table 4). Herein we present the comparative data with contemporary literature in the 3 most common malignancies where the [177Lu]Lu-DOTATATE PRRT has been employed in our services viz. (i) Metastatic Advanced and Progressive Gastroenteropancreatic Neuroendocrine Tumors, (ii) Metastatic Advanced Pulmonary and Mediastinal Neuroendocrine Tumors, and (iii) Metastatic Medullary Carcinoma of thyroid.
Figure 8.

Post-PRRT complete response (CR) in a known patient of grade I (Ki-67 index = 1%) pancreatic NET with multiple hepatic metastases. The baseline [68Ga]Ga-DOTATATE scan (A) showed intensely SSTR avid multiple liver lesions and pancreatic tail lesion. Patient received 3 cycles of [177Lu]Lu-DOTATATE PRRT (cumulative dose of 550 mCi/20.35 GBq). The [177Lu]Lu-DOTATATE post-therapy scan (B) showed good concentration of tracer in liver lesions and pancreatic lesion. Following 3 cycles of PRRT, follow-up [68Ga]Ga-DOTATATE scan (C) showed complete resolution of liver lesions and pancreatic lesion suggestive of CR.
Figure 9.

Post-PRRT partial response (PR) in a case of grade II pancreatic NET (Ki-67 index = 5%) with liver metastases. Pre-PRRT [68Ga]Ga-DOTATATE PET-CT (A) showed intensely tracer avid pancreatic lesion measuring 8×7 cm and few metastatic liver lesions. The patient received 5 cycles of PRRT with [177Lu]Lu-DOTATATE (cumulative dose: 800 mCi/29.6 GBq). Post-PRRT, [68Ga]Ga-DOTATATE scan (B) showed reduction in size of pancreatic lesion (4×3.7 cm) and liver lesions suggestive of partial response to PRRT.
Figure 10.
(A) 53 year old female, diagnosed as a case of atypical carcinoid of lung (MiB1 index of 6-10%), MiB-1 index of 6-10%. Multiple SSTR positive lesions in liver, both lungs, multiple rib & right sided pelvis, received 3 cycles of chemotherapy with cisplatin and etoposide with progression of disease. Excellent partial response was observed in the [68Ga]Ga-DOTATATE PET-CT (A). The patient had a FDG negative disease at baseline (B). She reported a dramatic decrease in symptoms including decrease in abdominal pain and frequency of diarrhea. Also reported weight gain and overall improvement in general condition and health related quality of life.
Figure 11.
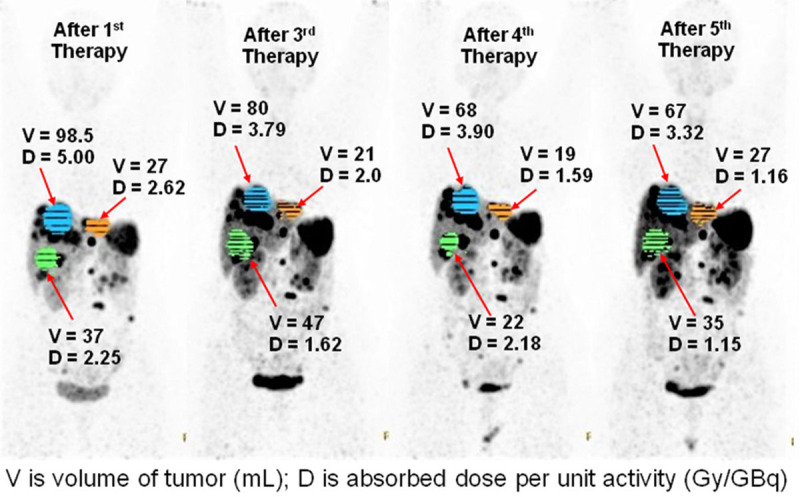
Illustration of comparison of tumor absorbed doses in different therapy cycle and a comparative table of the tumor absorbed doses with the reported studies and our own experience with indigenous [177Lu]Lu-DOTATATE, demonstrating excellent concordance (Table 4).
Table 4.
Comparisons of tumor absorbed doses with literature data
Metastatic advanced and progressive gastroenteropancreatic neuroendocrine tumors
An interim analysis during 2014-2015 at our centre looked into 50 patients with histopathologically confirmed metastatic/inoperable GEP-NETs (M:F 33:17, age: 26-71 years) who had undergone at least three cycles of PRRT with [177Lu]Lu-DOTATATE [19]. Symptomatic response was documented in more than 90% of patients in terms of improvement in health-related quality of life, the disease control rate (stable disease and partial response by scan) was observed in ~80% of cases. One point of note in this study was that (a) high pre-therapy FDG SUVmax values were associated with increased chances of treatment refractoriness (disease control rate 52.94% versus 96.97% in patients with absence of FDG uptake) and (b) symptomatic improvement was observed in most cases irrespective of tumor Ki-67 index (Table 5A and 5B). In a recent analysis of a total of 468 patients with proven GEP-NEN who underwent from 2 to 7 cycles (at the interval of 10-12 weeks) of intravenous [177Lu]Lu-DOTATATE PRRT at our centre, the overall clinical results are further depicted partial response by PERCIST and RECIST 1.1 of 25% and 27% and stable disease of 57% and 60% respectively. The PFS at 7 yrs was 71.1% and OS was 79.4%. A total of 39 (8%) patients died out of 468 patients at 7 years (unpublished work; personal communication). The PRRT procedures were well-tolerated with minimal nephrotoxicity (grade 1: 3.5%, grade 2, grade 3 and grade 4: <1% each) and hematotoxicity primarily of grade 1 (1.7%), grade 2 and grade 3, 0.2% each and no grade 4 hematotoxicity (Figures 8 and 9).
Table 5A.
PRRT response in total 50 GEP-NETs on Symptomatic and PET-CT imaging [19]
| Response | Symptomatic | 18F-FDG/68Ga-DOTATATE PET/CT imaging responders |
|---|---|---|
| Yes (PR+SD) | 96% | 82% |
| No (PD) | 4% | 18% |
Table 5B.
PRRT response in GEP-NETs with respect to SSTR-based and [18F]F-FDG PET/CT imaging findings [19]
| [18F]F-FDG and [68Ga]Ga-DOTATATE PET/CT finding → | Discordance (SSTR>FDG avid lesions, n = 33) | Concordance (SSTR≤FDG avid lesions, n = 17) |
|---|---|---|
| PRRT response ↓ | ||
| Yes (n = 41, 82%) | 32/33 (96.97%) | 9/17 (52.94%) |
| No (n = 9, 18%) | 1/33 (3.03%) | 8/17 (47.06%) |
In a short series of metastatic NETs (both GEP-NEN and pulmonary NENs) with extensive bone marrow involvement at diagnosis, the majority (four out of five patients, i.e. 80%) of the patients had excellent symptomatic response with at least stabilization of the disease at a follow-up period of 10-27 months [20]. The single patient who had a progressive disease also had a good symptomatic response in the initial 6 months from the first dose of PRRT. Despite the extensive bone marrow involvement, no hematological toxicity was observed (only one patient showed Grade I anaemia), suggesting that PRRT was well-tolerated by this particular subgroup. We have obtained gratifying response and improvement of health related quality of life in patients with extensive metastatic disease (Figure 12).
Figure 12.

A 35 year old female, diagnosed patient of duodenal NET (MiB1 labeling index 20%) with liver metastasis (involving left lobe) and history of radiofrequency ablation of the liver metastasis and external radiotherapy six months previously, was referred for exploring the feasibility of PRRT. Despite such extensive disease, the patient started working at 4 years. (A) [99mTc]Tc-HYNIC-TOC SSTR Imaging and (C) [177Lu]Lu-DOTATATE post therapy scan showing tracer uptake in residual primary tumor in duodenum (dotted arrow) and liver lesion (thin solid arrow) with fairly large area of photopenia suggesting necrosis. Diffuse uptake over axial and appendicular skeleton (with interspersed areas of focal uptake, particularly in left parietal bone) and intense bilateral uptake in breast nodules (thick solid arrows) indicate metastases from NET. (B) [18F]F-FDG PET/CT demonstrating relatively intense uptake in duodenal primary tumor (dotted arrow), intense uptake bilaterally in metastatic breast nodules (solid arrows), low-grade [18F]F-FDG uptake in liver lesion, and no uptake in bone marrow. (Reproduced with permission [21]).
A large body of literature and guidelines exist today demonstrating efficacy of [177Lu]Lu-DOTATATE in metastatic GEP-NENs [22-29]. Wang et al in a systematic review and meta-analysis of 22 studies (1758 patients) estimated an overall CR+PR of 35.0% and CR+PR+SD of 83% (on RECIST 1.1) [26]. Another systematic meta-analysis of 15 studies by Zhang et al reported a CR+PR of 27.58% on RECIST 1.1 and CR+PR+SD of 79.14% on RECIST 1.1 [27]. The PFS and OS were not estimated in both of these meta-analyses. Ezzidin et al [28] in their study of 74 patients with a median follow-up of 47 months, had documented a PFS and OS of 26 months and 55 months respectively, while that by Brabander et al in 443 patients, were 29 and 63 months respectively. Thus, the clinical results with the indigenously produced 177Lu has been in agreement with what has been reported in the literature (Table 6) [29].
Table 6.
Meta-analysis and literature reports of response and survival of PRRT in metastatic GEP-NETs
| Name/author of the study | No of studies/patients analyzed | Radionuclide used for PRRT | Follow up period after PRRT | Response on anatomical or molecular | PFS | OS |
|---|---|---|---|---|---|---|
| Wang LF et al [26] Systematic meta-analysis | 22 studies (1758 patients) | 177Lu and 90Y-DOTATATE | Review article | 35.0% = CR+PR on RECIST 1.1 | Not evaluated | Not evaluated |
| 83% = CR+PR+SD on RECIST 1.1 | ||||||
| Zhang J et al [27] Systematic meta-analysis | 15 studies | 177Lu and 90Y-DOTATATE | Review article | 27.58% = CR+PR on RECIST 1.1 | Not evaluated | Not evaluated |
| 79.14% = CR+PR+SD on RECIST 1.1 | ||||||
| Ezzidin et al [28] | 74 | 177Lu-DOTATATE | 47 months (median follow up) | 36.5% PR, 17.6% MR, 35.1% SD, and 10.8% PD | 26 mo | 55 mo |
| Brabander et al [29] | 443 | 177Lu-DOTATATE | Not mentioned | SD-36% | 29 mo | 63 mo |
Metastatic advanced pulmonary and mediastinal neuroendocrine tumors
Metastatic pulmonary and mediastinal neuroendocrine tumors form the next tumor group that is considered for the [177Lu]Lu-DOTATATE peptide receptor radionuclide therapy [PRRT] in our setting. In an initial analysis in metastatic/advanced pulmonary (NETs) in 19 patients, symptomatic response following PRRT was observed in 79%. A total of 63% were finally characterized as responders and 37% were overall non-responders to PRRT based upon predefined 3-scale response evaluation criteria. All 7 non-responders had moderate to intense FDG-avid primary lung lesion and 5 had FDG-avid metastatic liver disease (SUVmax >5) (Table 7). The median OS was 40 months [30]. The results obtained were well-commensurate with two studies reported in literature on 177Lu-octreotate in metastatic pulmonary neuroendocrine tumors, where the progressive disease reported were 32% and 30%, one in pure metastatic pulmonary NET scenario [31] and the other in a combined analysis of patients with gastroenteropancreatic and bronchial neuroendocrine tumors [29]. In the study by Sabet et al, the DCR was 67% (PR = 27%, SD = 41% and CR 0%) matches well with the responder group of 63% in our preliminary analysis [30,31]. Response evaluation to PRRT in 30 patients of metastatic pulmonary NETs over last 10 years shows a PD of 31% on RECIST 1.1 and 47% on PERCIST & biochemical parameters (unpublished work; personal communication). Thus, the DCR (SD+PR+CR) by RECIST 1.1 was 69% and by PERCIST & biochemical parameters was 53% (Table 8; Figures 10 and 13).
Table 7.
Response evaluation to PRRT in 19 pulmonary NET patients [30]
| Response | Symptomatic evaluation | Biochemical evaluation | PERCIST* | RECIST 1.1** |
|---|---|---|---|---|
| Complete response | 42% | 7% | 5% | 5% |
| Partial Response | 26% | 15% | 10% | 5% |
| Minor response | 20% | 20% | ||
| Stable disease | 12% | 31% | 18% | 37% |
| Progressive disease | 20% | 47% | 47% | 33% |
PERCIST=PET response criteria in solid tumors.
RECIST 1.1=Response Evaluation Criteria In Solid Tumors.
Table 8.
Response evaluation to PRRT in 30 patients of metastatic pulmonary NETs over last 10 years
| Response | Symptomatic | Biochemical | RECIST 1.1 | PERCIST |
|---|---|---|---|---|
| CR | 42% | 7% | 4% | 5% |
| PR | 26% | 15% | 8% | 12% |
| SD | 12% | 31% | 57% | 36% |
| PD | 20% | 47% | 31% | 47% |
Figure 13.

A. Patient of Pulmonary NET with hepatic metastases, presenting with profound anorexia and weight loss over previous 2 months. The baseline [68Ga]Ga-DOTATATE (a) and FDG PET-CT in May 2016 show tracer avid left lung mass and multiple liver lesions with minimal to absent FDG uptake in the lesions (b). The patient received 5 cycles of PRRT from June 2016 to Dec 2017 (750 mCi/27.75 GBq). B. In 2018, the patient was asymptomatic with decrease in serum CgA level and [68Ga]Ga-DOTATATE PET-CT showed no changes in size, number of lesions or tracer uptake in the primary left lung lesion and metastatic liver lesions, with increase in necrotic changes in liver and lung lesions-suggesting disease stabilization in this case.
With regard to mediastinal NETs, twenty-seven patients with histopathologically and radiologically proven metastatic or advanced mediastinal NET, who had undergone [177Lu]Lu-DOTATATE PRRT, metabolic PET-CT response evaluation demonstrated partial metabolic response in 33.3%, metabolically stable disease was seen in 18.5%, while 44.4% of the patients showed metabolic disease progression (Table 9; Figure 14). The median PFS and OS were 36 months and 66 months respectively. Significant level of association of PFS was observed with surgical intervention, higher cumulative PRRT dose, metabolic response, smaller sized primary lesion, and low lesional FDG uptake; with respect to the OS, higher cumulative PRRT dose, low FDG uptake and longer PFS showed significant association [32]. There is relatively sparse clinical study data in the literature till date on [177Lu]Lu-DOTATATE PRRT in metastatic mediastinal/thymic NETs, which are primarily reported as case reports.
Table 9.
Response evaluation results of Mediastinal NETs to [177Lu]Lu-DOTATATE PRRT [32]
| Response | Symptomatic | Metabolic | Anatomical |
|---|---|---|---|
| CR | 37% | 3.7% | 0% |
| PR | 25.9% | 33.3% | 22.2% |
| SD | 14.8% | 18.5% | 25.9% |
| PD | 22.2% | 44.4% | 51.9% |
Figure 14.

177Lu-DOTATATE PRRT in disease stabilization of Thymic NET. The patient was diagnosed case of MEN 1, with thymic NET (Mib-1 index = 15%), pituitary adenoma and parathyroid adenoma. He was operated for pituitary adenoma, parathyroid adenoma and inoperable for thymic NET. He was evaluated for PRRT in view of inoperable and metastatic disease. [68Ga]Ga-DOTATATE and FDG-PET/CT showed SSTR and FDG avid enlarged mediastinal lesions (A), patient received 2 cycles of PRRT (B), the follow-up [68Ga]Ga-DOTATATE and FDG PET/CT in Dec 2017 (C) demonstrates stable disease.
We have to mention here that for both metastatic pulmonary and mediastinal NENs, in addition to the aggressive biology of this tumor group, PRRT has been usually considered at an advanced state following chemotherapy failure which we believe could be partly responsible for its relatively inferior outcome compared to the GEP-NENs.
Metastatic medullary carcinoma of thyroid (MTC)
[68Ga]Ga-DOTATATE positive metastatic MTC forms the 3rd most common indication for PRRT in our set-up. In a total of 43 somatostatin receptor-positive metastatic MTC patients treated with [177Lu]Lu-DOTATATE PRRT [33], 61% were responders and 39% were nonresponders based upon PERCIST criteria, while with RECIST 1.1 criteria 62% were responders and 38% were non-responders (Figure 15). Significant association between responses to PRRT was observed with following prognostic variables: (a) size of lesions (<2 cm) and (b) FDG uptake in lesions (SUVmax of <5). PRRT was well tolerated in all patients without any major grade 3 or 4 toxicity. The median OS was 26 months and the median PFS 24 months. There are much smaller series that have been published in this domain and more results may be awaited for comparison with the indigenous [177Lu]Lu-DOTATATE (Table 10) [34,35].
Figure 15.

Metastatic MTC treated with [ 177 Lu]Lu-DOTATATE PRRT. Diagnosed case of MTC with past history of total thyroidectomy and bilateral selective neck dissection presents with follow-up serum calcitonin of 6271 pg/ml and CT abdomen showing metastatic liver lesions. Baseline [68Ga]Ga-DOTATATE PET-CT (A) showed intensely SSTR avid (Krenning score = 4) bilobar liver lesions (SUVmax 25) and bony metastasis in right acetabulum (SUVmax 23). Subsequently, the patient received 2 cycles of PRRT (300 mCi). 177Lu-DOTATATE post therapy scan (B) showed good concentration of tracer in liver and skeletal lesions. Post-PRRT [68Ga]Ga-DOTATATE PET-CT (C) showed reduction in SSTR uptake in liver lesions (SUVmax 16) and right acetabulum bony lesion (SUVmax 13) with significant reduction serum calcitonin level from 6271 to 2167 pg/ml, suggestive of favorable response to PRRT.
Table 10.
PRRT response evaluation in MTC patients in four categories [33]
| Response | Symptomatic | Biochemical | RECIST 1.1 | PERCIST |
|---|---|---|---|---|
| CR | 19% | 11% | 0% | 0% |
| PR | 28% | 30% | 4% | 10% |
| SD | 4% | 10% | 58% | 51% |
| PD | 49% | 49% | 38% | 39% |
Dual tracer PET-CT in individualizing treatment regimen in NENs: a valuable adjunct alongwith tumor Ki-67/MIB-1 index in assessing tumor biology
While the tumor Ki-67/MIB-1 labeling index forms an integral part of assessment of metastatic NENs, dual tracer PET-CT molecular imaging findings (somatostatin receptor based [68Ga]Ga-DOTATATE and tumor glucose metabolism with FDG) has developed into a valuable adjunct for personalizing the treatment regimen in this group of patients (Figure 15). Several studies in the early years including ours had observed that tumors with low or negligible FDG uptake respond well to somatostatin targeted therapy, while those with high FDG uptake demonstrates refractoriness to PRRT alone [19,37]. The relative uptake of [68Ga]Ga-DOTATATE and FDG enables assessment of the dynamic tumor biology of NENs on a continuous scale and is utilized for clinical decision making and personalizing the treatment strategies [38]; depending upon the dual tracer PET-CT findings, the patients are subdivided into 3 classical subgroups: (i) Tumors with low Ki-67 index, are usually positive on SSTR imaging with low/absent FDG uptake: they are candidates for SSA and PRRT, (ii) Tumors with high Ki-67 index, are usually negative on SSTR based imaging and shows high uptake on FDG-PET/CT: are candidates for chemotherapy (CAPTEM if the Ki-67<55% or platinum based chemotherapy if the Ki-67>55%) (Figure 16). (iii) Tumors demonstrating both high [68Ga]Ga-DOTATATE and 18F-FDG uptake on dual tracer PET/CT form the intermediate grey zone and intermediate Ki-67 index, wherein a combined approach of PRRT plus chemotherapy is employed (Figures 17, 18 and 19) [36,38,39].
Figure 16.

Dual tracer PET-CT as valuable adjunct in personalizing treatment regimen in metastatic NENs (Reproduced with permission from Basu et al [36]).
Figure 17.

[68Ga]Ga-DOTATATE and [18F]F-FDG PET-CT in a patient of CUP-NET with Ki-67 index = 2% showing matched histopathology and dual tracer molecular PET-CT imaging finding with minimal FDG and avid [68Ga]Ga-DOTATATE uptake.
Figure 18.

A known case of NEC with unknown primary site. Presenting with complaints of abdominal pain and cough, the biopsy from abdominal lesions showed metastatic carcinoma with neuroendocrine differentiation and positivity for CK7, synaptophysin and negativity for chromogranin and p40. Ki-67 index was 80-90%. The patient did not receive any treatment before the molecular imaging. [18F]F-FDG PET-CT scan (A) showed intensely FDG avid multiple liver lesions, lung nodules, skeletal lesions and mesenteric deposits with no uptake in above-mentioned lesions on [68Ga]Ga-DOTATATE PET-CT (B). The patient is NOT a suitable case for PRRT.
Figure 19.

Dual tracer PET finding matched with that predicted by HPR. Pancreatic NET with liver lesions, Ki-67 index = 15%, showing high uptake on both [68Ga]Ga-DOTATATE (A) and FDG (B) PET-CT. The patient is a typical case for consideration for combined chemo-PRRT.
The specific areas where this manoeuvre becomes particularly useful for clinical decision making are:
(i) The tumor biology in tumors with intermediate level of Ki-67 labeling index (e.g. 10-20%) and also those with Ki-67 LI between 20-30%
Wide variation in tumor behaviour at both of the above regions of Ki-67 index, where the relative uptake of [68Ga]Ga-DOTATATE and FDG becomes particularly useful for deciding on the therapy on an individualized basis. Having observed this early-on, we have adopted the practice routinely in our Institute.
The observation made over years have been recently reflected by the WHO 2017 NEN grading. Amongst the grade 3 NENs, there is now increasing recognition of a ‘well differentiated NEN composed of cells showing minimal to moderate atypia, lacking necrosis and expressing general markers of neuroendocrine differentiation (diffuse and intense synaptophysin and chromogranin A)’ which contrasts to the ‘poorly differentiated NEN is composed of highly atypical small or large cells expressing faint neuroendocrine differentiation markers’. Hence, two different subsets within the grade 3 NENs are now recognized by the recent WHO 2017 NEN classification, distinguishing well differentiated grade 3 neuroendocrine tumours (NET G3) from poorly differentiated grade 3 neuroendocrine carcinomas (NEC G3), which is an important step from management view-points.
Dual tracer PET-CT findings of the relative tracer accumulation in tumor lesions based upon somatostatin receptor expression (by [68Ga]Ga-DOTATATE/NOC PET-CT) and tumor glycolysis (by FDG-PET/CT), can potentially aid in deciphering the aforementioned tumor characteristics, and differentiating NET G3 from NEC G3. A high [68Ga]Ga-DOTATATE/NOC with low/absent FDG uptake would be more consistent with the diagnosis of G3-NEC.
(ii) Discordance between Ki-67/MIB-1 and the dual tracer PET-CT findings
Though the standard findings on dual tracer PET-CT is characteristic and remains in agreement with the reported Ki-67/MIB-1 labeling index, the outliers or exceptions are not uncommon in day-to-day practice. Herein two considerations need to be made: (a) Ki-67/MIB-1 labeling index from a single lesion and single-site biopsy could be fraught with its inability to assess the tumor biology as a whole on a continuous scale and (b) even in the same individual, interlesional heterogeneity can exist, thus one biopsy specimen may not be representative of all lesions, which could be better portrayed by the molecular PET-CT imaging (Images of case examples: Figures 20, 21). In our set-up the findings in the PET-CT imaging forms an important parameter and given equivalent important to the Ki-67 index for clinical decision-making of therapies in metastatic NENs, if not more.
Figure 20.

Discordance between Ki-67 and dual tracer PET-CT findings in a patient of metastatic NET of unknown primary site, liver biopsy reported as metastatic NET with Ki-67 index = 22%. [68Ga]Ga-DOTATATE PET-CT (A) showed intensely SSTR avid multiple liver and wide spreads skeletal lesions. [18F]F-FDG PET-CT (B) showed mild FDG uptake in liver lesions and faint FDG uptake in skeletal lesions.
Figure 21.

Discordance between Ki-67 and dual tracer PET-CT findings in a patient of pancreatic NET with pancreatic mass involving the body and tail with splenic and colonic infiltration. HPR:NET with Ki-67:1-2%. However, shows low grade SSTR based 68Ga-DOTATATE uptake (left panel) but high on FDG (right panel) PET-CT.
PRRT beyond classical indication of GEP-NENs: expanding the boundaries of SSTR based Theranostics
The classical standard indications for PRRT has been (i) metastatic and unresectable advanced grade I or II gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) that are (ii) progressive on Synthetic Somatostatin Analogues (SSAs) and demonstrate high grade uptake (Krenning score 3 or 4 on semi-quantitative scale) on SSTR based [68Ga]Ga-DOTATOC/TATE PET-CT or [99mTc]Tc-HYNIC-TOC SPECT-CT. (iii) Symptomatic functioning NENs, who are resistant to the long-acting SSAs (octreotide/lanreotide) form another group where PRRT is usually considered. Beyond the aforementioned indications, the clinical applications of PRRT has been widened substantially over the recent years, and can be subdivided into two groups: (A) extended indications within GEP-NENs and (B) applications in tumors beyond GEP-NENs.
Extended indications within GEP-NENs
The following two groups of GEP-NEN patients are now frequently considered for PRRT with 177Lu-DOTATATE and increasingly becoming clinical routine: (iv) In NENs with MIB-1 index between 20 and 30%, a sizeable fraction of patients demonstrate high uptake on [68Ga]Ga-DOTATATE PET-CT, where PRRT can be successfully employed [40]. Amongst this subgroup, a fraction of patients do show high uptake of FDG additionally, wherein we advocate combined chemo-PRRT (the regimen employed in our set-up is detailed later). The ESMO clinical practice guidelines for GEP-NENs advocates PRRT upto Ki-67 of 30%.
(v) While usually PRRT is considered in patients with disease progression on cold somatostatin analogues, there has been increasing adoption of this treatment modality upfront at diagnosis in patients with widely metastatic disease on whole body diagnostic SSTR based study, where the disease is already ‘progressed’ involving multiple sites.
Tumors beyond Gastroenteropancreatic NENs (GEP-NENs)
Over the years, we have now acquired fair amount of experience in various ‘beyond GEP-NENs’ indications, wherein 177Lu-DOTATATE PRRT provide good disease stabilization in substantial fraction of patients. These include: (a) metastatic/inoperable Bronchopulmonary and Mediastinal/Thymic NENs, [30,32]; (b) metastatic/inoperable Medullary thyroid carcinoma** [33]; (c) Non-131I-MIBG concentrating metastatic Paraganglioma & Pheochromocytoma (Figure 22); (d) Non-iodine concentrating metastasis of differentiated thyroid carcinoma (TENIS: only 15-20% of this patient subgroup demonstrates enough uptake to justify PRRT)**, [41]; (e) Other tumors with neuroendocrine tumor differentiation/characterization: metastatic Merkel Cell carcinoma, Meningioma and recurrent/inoperable Phosphaturic Mesenchymal Tumor, Recurrent Metastatic Sinonasal Neuroendocrine Carcinoma, Recurrent metastatic large cell neuroendocrine carcinoma (LCNEC) of larynx (Figure 23) [42-46].
Figure 22.

Diagnosed patient of invasive paraganglioma of carotid body, Mib-1 index 10%, the patient underwent excision of right carotid body tumor in 2013 and received post-operative EBRT in 2013. Presented with bony pain and headache in 2016. Patient evaluated for PRRT, the [68Ga]Ga-DOTATATE (B) and FDG PET/CT (A) showed multiple skeletal lesions and lung nodules, following which the patient received 2 cycles of PRRT (C). In 2017 both [68Ga]Ga-DOTATATE (D) and FDG PET/CT show SSTR and FDG avid lesions with no change as compared to baseline-suggesting stable disease (SD) in metastatic paraganglioma with PRRT.
Figure 23.

Observation of [68Ga]Ga-DOTATATE (A) avid metachronous Meningioma in a known patient of pancreatic NET (Ki-67 index = 4%) with metastatic liver lesions. Initially the patient underwent right hepatectomy and pancreaticoduodenectomy in 2015. In subsequent years, he complained of abdominal pain, weakness and headache. 68Ga-DOTATATE PET-CT showed SSTR avid brain lesion (B, blue arrows), liver lesion (C), skeletal lesion (D), abdominal lymph nodal and lung lesions (E). Meningioma was confirmed on MRI of brain region.
‘**’ represent clinical indications where PRRT has been considered even though there was a lesser degree of uptake (Krenning score 2) on SSTR based scanning, in view of the fact that alternative experimental therapies were either potentially toxic, only modest efficacious or expensive. The aforementioned list of PRRT indications is partly adapted with newer additions from Basu et al [46]).
The role of [99mTc]Tc-HYNIC-TOC SPECT/SPECT-CT as decision making scan in the absence of [68Ga]Ga-DOTATOC/NOC/TATE PET-CT
The diagnostic modality of choice for SSTR based imaging is [68Ga]Ga-DOTATOC/NOC/TATE PET-CT, which has been proven superior to the other conventional diagnostic modalities including the other whole body functional imaging (such as [111In]In-octreotide or [99mTc]Tc-HYNIC-TOC SPECT-CT), in view of its superior resolution and ability for quantification.
However, in the absence of PET-CT or 68Ge/68Ga generator, the mandatory requirements for [68Ga]Ga-DOTATOC/NOC/TATE PET-CT, a factor to be considered in several peripheral centres in developing nations, [99mTc]Tc-HYNIC-TOC planar and SPECT-CT gamma camera based imaging can do reasonably well for decision making of PRRT in patients with large volume disease. In India, [99mTc]Tc-HYNIC-TOC is available for kit formulation method which played important role in the initial years of PRRT development at our set-up (Figures 24, 25) [47].
Figure 24.

Pancreatic NET with no metastatic disease showing concordance between [99mTc]Tc-HYNIC-TOC (A) and [68Ga]Ga-DOTATATE PET/CT (B).
Figure 25.

Jejunal NET with metastatic disease in abdominal lymph nodes, liver and skeleton. [68Ga]Ga-DOTATATE PET-CT (B) has advantage in detecting small volume/tiny lesions as compared to [99mTc]Tc-HYNIC-TOC planar study (A). SPECT-CT enhances the lesion detectability.
Patient preparation in specific clinical scenarios
Large volume functioning hepatic metastases: minimizing the incidence of ‘carcinoid crisis’
Overall, PRRT is a very well-tolerated procedure with minimal adverse effects, however, post-PRRT acute carcinoid syndrome (“carcinoid crisis”) is a rare but life-threatening risk that requires emergency medical management. This is typically observed in patients with large volume functioning hepatic metastases. In our experience, adequate patient preparation is key to minimize the incidences in these cases, which would involve short acting octreotide injections (subcutaneous) till 1 day before of PRRT and initiate the same next day following PRRT continuing for 2 weeks. Priming this subgroup of patients with cyproheptadine on a regular basis 1 to 2 days before scheduled PRRT is also a helpful practice (it exerts its action as a potent antagonist of the 5-HT2 receptors).
Protein deficiency, poor nutrition status and generalized anasarca: enhancing the chances of undertaking PRRT
This is a challenge to the attending physicians, more relevant to the developing world, the protein deficiency being primarily due to poor nutritional status, gastrointestinal loss of protein and tryptophan and hepatic dysfunction due to large hepatic disease burden. Oral protein supplementation along with octreotide therapy enhances the general condition and enables to administer PRRT in the isolation ward.
For malnourished patients with anasarca, undergoing treatment, generally a high calorie & high protein diet is recommended. In patients with conditions like ascites and pedal edema, the energy requirement varies between 25-40 kcals/kg/day but if the patient is underweight: it would be 35-40 kcals/kg/day. The protein requirements is higher for these patients due to nutritional hypoalbunemia (1.2-2 g/kg/bw). Supplement amount depends on patient’s present condition and food intake. The greater the decrease in food intake the higher the quantity of prescribed supplement. If the patient is on oral diet, lesser quantity is prescribed like 100 gms a day or lesser but the quantity is more if he is on tube feeds.
Use of aprepitant as an additional antiemetic during PRRT
Nausea and vomiting is a common occurrence during PRRT primarily related to the metabolic acidosis due to the amino acid co-infusion used for nephroprotection, rather than the radiopharmaceutical. This could be Dexamethasone-ondansetron pre-treatment on a regular basis, however, there remains a small fraction of patients who show refractory vomiting during or soon after the therapy which could be well managed with oral Aprepitant, an NK1 antagonist in the central and peripheral nervous system, the typical adult dose is 125 mg, 80 mg and 80 mg on days 1, 2, and 3 respectively. In a patient who develops refractory vomiting during the first cycle of PRRT, he is considered for Aprepitant on a routine basis from 2nd cycle onwards.
Carcinoid heart disease: can PRRT be potentially helpful in enhancing the chances of corrective vulvular surgery?
Carcinoid Heart Disease is seen in the setting of high volume functioning disease, typically in patients with bulky hepatic involvement which is an important risk factor (Peak 5-HIAA forms a significant predictor of the disease progression) that leads secretion of vasoactive substances (5-hydroxytryptamine, tachykinins, and prostaglandins) reaching the right side of the heart through hepatic veins and consequent deposition of fibrous tissue on the endocardial surfaces of the heart resulting in pathologenesis of carcinoid heart disease. Though the usual reported incidence is upto 70% within the subgroup of patients with carcinoid syndrome, its incidence is now much reduced owing to widespread use of synthetic somatostatin analogues (SSA) and better control of the functioning disease. We have some limited experience of managing such patients with resistant disease, where [177Lu]Lu-DOTATATE helped in substantial reduction of 5-HIAA levels and resulted in symptomatic improvement and improved health related quality of life (from NYHA grade III at baseline to NYHA grade I after 4-6 cycles), finally enabling taking the patient for corrective valvular surgery [48].
One concern on advocating PRRT in patients of carcinoid heart disease has been the concern of volume overload in patients with features of cardiac insufficiency related to amino acid infusion and hence caution exerted in various guidelines [24]. Our Institutional protocol of a relatively prolonged mixed amino acid protocol of 7.5-8 hours adopted during PRRT is particularly helpful to address this concern, and can be recommended in this clinical setting of CaHD to undertake this treatment uneventfully. Furthermore, it needs to be mentioned that the renal absorbed dose has been reported to be further reduced by prolonging the infusion time of the amino acid solution over 10 hours by up to a factor of 39% [24].
PRRT in neoadjuvant setting
[177Lu]Lu-DOTATATE PRRT shows modest success in the neoadjuvant setting, where the primary intent is reduction of the size of an initially unresectable primary GEP-NENs to bring to the point when it becomes operable. We follow a slightly different regimen in this context, where a higher mean dose with shorter time interval is followed (i.e. 200 mCi at 8 weekly interval) compared to the metastatic setting, where the intent is primarily disease stabilization and palliative (mean of 150 mCi per cycle administered at 12 weekly intervals for 5 such cycles). We have observed excellent tolerability of this regimen in all patients without any major hematologic or renal toxicity.
In our initial experience, the inoperable disease became operable in around 26% of patients, assessed in a population of 57 (Figure 26). This is similar to the published results, the success for operability is around one-third of treated patients (~30%) [49,50] (unpublished work; personal communications). Another observation in a sizeable fraction of patients that could be made with neoadjuvant PRRT is substantial regression of liver disease, thereby facilitating surgical removal of primary and residual liver lesions. This has been also reported by investigators who have employed [90Y]Y-DOTATOC and [90Y]Y-DOTATATE in the neoadjuvant setting [51,52].
Figure 26.

Efficacy of PRRT in neoadjuvant setting. A 35 years old male, diagnosed case of pancreatic NET with Ki-67 of 1%. Baseline [68Ga]Ga-DOTATATE PET-CT scan (A) showed intensely SSTR avid (krenning score = 4) pancreatic head mass >180° contact with SMV (SUVmax 59, 5.6×4.0×7.2 cm) and SSTR avid peri-pancreatic lymph node (SUVmax 55, 3.4×2.6 cm). Subsequently, patient received 2 cycles of PRRT (400 mCi). 177Lu-DOTATATE post therapy scan (B) showed good concentration of tracer activity in pancreatic head mass and peri-pancreatic lymph node. Follow-up [68Ga]Ga-DOTATATE PET-CT scan (C) showed reduction in size and SSTR uptake of pancreatic head mass with <180° contact with SMV (SUVmax 34, 4.4×3.5×4.1 cm) and also reduction in SSTR uptake and size of peri-pancreatic lymph node (SUVmax 30, 2.3×1.7 cm), suggestive of favorable response to PRRT.
Sandwich Chemo-PRRT in patients with avid [68Ga]Ga-DOTATATE and [18F]F-FDG uptake on dual tracer PET-CT: its comparison with PRCRT
From our initial years of experience with PRRT, we had observed that these group of tumors usually demonstrate aggressive biology, treatment refractoriness to PRRT alone and increased incidence of progressive disease and mortality in due course. Since 2013, we had adopted a combination approach (oral chemotherapy with capecitabine-temozolamide and [177Lu]Lu-DOTATATE PRRT) for these group of patients. In chemo-PRRT protocol, 2 cycles of CAPTEM chemotherapy is sandwiched between 2 PRRT cycles of [177Lu]Lu-DOTATATE.
The standard CAPTEM regimen comprises of oral capecitabine (CAP), 750 mg/m2 twice daily for 14 days (D1-D14) and oral temozolomide (TEM) 200 mg/m2 once daily for 5 days (D10-D14) followed by two weeks rest period and another CAPTEM cycle given for total 28 days is followed by next cycle of PRRT at around 3 months (Figure 27). In a population of 38 patients with metastatic NENs with high uptake on both 68Ga-DOTATATE and 18F-FDG dual tracer PET/CT, who were treated with this Chemo-PRRT regimen, we found encouraging results with partial response in around 45%, stable disease in 39% and progressive disease in 16% on RECIST 1.1 with an overall disease control rate of 84% in this aggressive group of tumors (unpublished work; personal communications) (Figure 28).
Figure 27.

Protocol adopted for Sandwich Chemo-PRRT.
Figure 28.

Efficacy of ‘Sandwich Chemo-PRRT’. A diagnosed case of pancreatic NET (Ki-67 index = 8%) with hepatic and ovarian metastatic disease. The baseline [68Ga]Ga-DOTATATE (A) and [18F]F-FDG PET-CT scans (B) in 2015 showed both SSTR and FDG avid multiple liver lesions, abdominal lymph nodes, bilateral adnexal lesions. The patient received sandwich Chemo-PRRT from 2015 to 2018. Post chemo-PRRT follow up [68Ga]Ga-DOTATATE and [18F]F-FDG PET-CT scans in 2018 showed significant reduction of SSTR and FDG uptake in liver lesions, abdominal lymph nodes and bilateral adnexal lesions suggestive of PR after sandwich Chemo-PRRT treatment regimen.
Amongst the various combination regimen, PRCRT using 177Lu-octreotate with concomitant 5-FU radiosensitizing infusional chemotherapy has been employed at other centres [53,54] in patients with FDG-avid NET. The dosage of 5-FU in PRCRT in this regimen has been (200 mg/m2/24 h), starting approximately 4 days before the day of PRRT administration, and continued for 3 weeks in total and early discontinuation in the event of hand-foot syndrome or other acute toxicity. There was achievement of good partial response, with long progression-free survival of 48 months in a cohort of 52 patients [54]. The other investigators have used (i) First 14 days of capecitabine 1500 mg/m2 and last 5 days of temozolomide 200 mg/m2 commencing on the morning of PRRT [55], (ii) oral capecitabine (1250 mg/m2) for 15 consecutive days from the 1st day of PRRT [56], and (iii) oral capecitabine (500-1000 mg/m2) twice daily for 14 days (starting 1 day after the PRRT) and oral temozolomide (150-250 mg/m2) once per day for 5 days (days 10-14 after the PRRT) [57], with varying partial response (from 9% to 70%) being documented [55-57]. We believe that our aforementioned “sandwich chemo-PRRT” regimen could be more user friendly and easier to adopt in a busy clinical setting.
Duo-PRRT with [90Y]Y-DOTATATE in combination with [177Lu]Lu-DOTATATE in large volume lesions
The superior efficacy of [90Y]Y-DOTATATE for large sized lesions and heterogeneous lesions results from higher beta-particle energy of 2.28 MeV (mean energy of 0.94 MeV) of 90Y (maximum soft tissue range ~11 mm) compared to that of 177Lu [Eβ(max) = 0.497 MeV, maximum soft tissue range ~2.5 mm].
With the availability of indigenous [90Y]Y-DOTATATE, 11 NET patients with large volume lesions have received [90Y]Y-DOTATATE PRRT (Figure 29). Post PRRT stable disease has been observed in all 11 patients in the limited period of time, on imaging response evaluation with no major acute adverse effects. We are in the process of optimizing the duo-PRRT regimen at our set-up (with different regimen for the metastatic and neoadjuvant settings), including optimization of post-therapy Bremsstrahlung and PET-CT imaging following [90Y]Y-DOTATATE treatment (Figures 29 and 30) [58,59].
Figure 29.
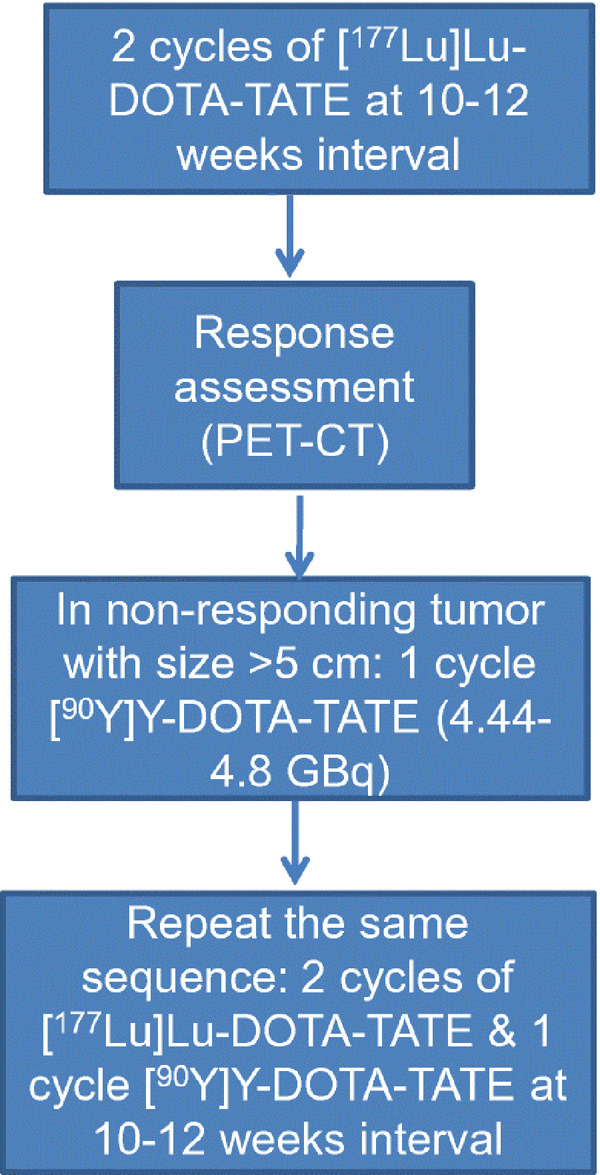
Treatment Protocol for “duo-PRRT” protocol for large volume disease (lesion size >5 cm) in metastatic and advanced Neuroendocrine Neoplasms (Reproduced with permission from Basu et al [42]).
Figure 30.

Post [90Y]Y-DOTATATE whole body planar bremsstralung image (A) and post [90Y]Y-DOTATATE regional PET-CT scan (B) in a known case of large sized pancreatic NET with liver metastatic disease (Ki-67 index = 1%), who had stable disease to [177Lu]Lu-DOTATATE PRRT, showing good tracer uptake in primary pancreatic and metastatic liver lesions.
Radiation safety aspects in [177Lu]Lu-DOTATATE PRRT
In our institute PRRT is administered as in-patient therapy procedure. World-over, there is some variation in this regard, while some countries consider PRRT as an outpatient procedure and some countries consider it as in-patient procedure. To minimise the radiation doses of staff, carers and general public each institute have their own radiation safety protocols. Our institute also have evolved elaborate radiation safety protocols, the salient points among them are mentioned in this review. Prior to administration of the [177Lu]Lu-DOTATATE, patients and their relatives were provided with radiation safety and hygiene practices instructions. Patients were explained about their stay in special (isolation) ward, because this physical preparation of patients helps in minimizing the radiation exposure and contamination of physicians, nursing staff, other staff members and visitors. To avoid the spread of contamination, entry to the isolation ward is restricted. Standard operating procedures are mandated to be followed by the physicians, radiation safety officers, radio-pharmacists and nursing staff, during synthesis of [177Lu]Lu-DOTATATE, preparation of patient doses, administration of [177Lu]Lu-DOTATATE and discharge of patients from isolation wards. Regular radiation surveys and contamination checks are performed to ensure the limits set by the regulatory body are adhered. In our institute, all the liquid waste generated in isolation wards, including urine and faeces of NET patients are collected in dual delay tank. Once activity levels decrease to discharge limits set by our national regulatory body, discharges are let to municipal sewerage system.
To establish the discharge criteria and ensure that public exposure is less than the limit set by national regulatory body radiation field from the patient’s body should be monitored. In literature [60] it reported that the average exposure at one meter immediately after treatment is 19 ± 5 µSv/h (for mean administered activity 7.09 GBq) and at time of discharge (4-5 h) is 9.8 ± 3.1 µSv/h. In our experience we found that the average exposure at one meter immediately after treatment is 20.22 ± 3.21 µSv/h (for mean administered activity 7.4 GBq) and at time of discharge (18-20 h) is 7.05 ± 2.94 µSv/h (Table 11). The predictive effective dose to the family members of NET patients is found to be less than 0.15 mSv (Occupancy factor 0.5) [61]. Before discharging the patients from the ward, once again all instruction of radiation safety and hygiene practices were provided in written as well as verbal, so as to minimise the dose to family members and general public.
Table 11.
A comparison of Radiation Dosimetry and related parameters obtained with [177Lu]Lu-DOTATATE through direct neutron activation route with that of reported literature
| Parameters | Types | Our Study | Literature Value | References |
|---|---|---|---|---|
| Dose Ratea (µSv/h) | Immediately after dosage administration | 3.92 ± 0.63 (2.69-5.61) | 2.68-4.47 | [60,61,72,73] |
| Per unit administered activity (µSv/h/GBq) | ||||
| At the time of Discharge | 7.05 ± 2.94b | 7.0 ± 3.0c | ||
| 9.8 ± 3.1d | ||||
| In-vivo Kinetics (Hours) | Fast component | 2.23 ± 0.95e (0.82-4.90) | 1.28-4.7 | [64,66,72] |
| Slow component | 36.20 ± 16.47 (12.15-71.94) | 49.5-87.2 | ||
| Urinary Excretionsf (%) | 0-2 Hours | 35 | [64,73] | |
| 0-6 Hours | 55 | 44-46 | ||
| 0-24 Hours | 70 | 58 | ||
| Dosimetry Estimates [Absorbed dosef (mGy/MBq)] | Kidneys | 0.72 ± 0.23 | 0.55-1.15 | [65,74-81] |
| Bone marrow | 0.041 ± 0.021 | 0.03-0.07 | ||
| Total Body | 0.071 ± 0.025 | 0.05-0.07 | ||
| Tumors | 8.83 ± 7.18 | 3.41-9.7 |
Dose rate at 1-meter distance from the surface of patient’s body.
Discharge time was 18-20 hours after dose administration.
Discharge time was 18 hours after dose administration.
Discharge time was 4-5 hours after dose administration.
During our study, we also found that the clearance of 177Lu follows bi-exponential decay in 83.33% NET patients and in 16.67% NET patients it follows mono-exponential decay.
Results from ongoing 177Lu-DOTATATE dosimetry work.
In vivo kinetics of lutetium and labelled radiopharmaceuticals
In vivo kinetics of lutetium
Till date there is no data available about the kinetics of lutetium in human body. However, data of lutetium kinetics in laboratory animals revealed that lutetium collects in tissue and organs (60% in bones, 2% in the liver and 0.5% in the kidneys). In addition, lutetium is reported to have biological half-life of 3500 days in bone and the liver and 10 days in the kidneys [62]. Hence most of the lutetium that enters the body accumulates in the bone over a period.
In-vivo kinetics of [177Lu]Lu-DOTATATE
After administration of [177Lu]Lu-DOTATATE intravenously to the NET patients, [177Lu]Lu-DOTATATE uptake is seen in the kidney, liver, spleen and metastatic sites within 2 hours. Most of the [177Lu]Lu-DOTATATE which in not absorbed in the body is quickly excreted out from the body in the urine. As per the literature reports [63], [177Lu]Lu-DOTATATE is primarily excreted in the urine, with a cumulative excretion of 44% with in 5 hours, 58% within 24 hours and 65% within 48 hours. In our experience of 24 hours urinary excretion studies, we found a cumulative excretion of 35% within 2 hours, 55% within 6 hours and 70% within 24 hours (results from ongoing 177Lu-DOTATATE dosimetry work). Sandstrom et al [64] studied in-vivo kinetics in patients administered with [177Lu]Lu-DOTATATE and reported that the clearance of [177Lu]Lu-DOTATATE is biphasic inside the NET patients. The effective half-life of fast component is 1.28 h (range 0.93-1.52 h) and slow component is 49.5 h (range 45.1-56.6 h). During our studies, we also found that the clearance of 177Lu is biphasic inside the NET patients with effective half-life of fast component is 2.23+/-0.95 hours and slow component is 36.20+/-16.47 hours (Table 11) [66].
ALARA principle for critical organs
Bone marrow and kidneys are the critical organs in PRRT. Proximal tubular reabsorption of the [177Lu]Lu-DOTATATE and subsequent retention results in high radiation exposure to the kidney. To reduce the high kidney retention of [177Lu]Lu-DOTATATE, positively charged amino acids are co-infused to completely inhibit reabsorption of the [177Lu]Lu-DOTATATE in the kidney. The co-administration of these amino acids reduces the kidney dose significantly in the range of 9% to 53% [67]. Renal absorbed dose is further reduced by up to 39% by extending the infusion time of the amino acids solution over 10 h, and up to 65% by extending the protection over 2 days after radiopeptide administration, thereby covering the renal excretion phase more efficiently [68,69].
Internal dosimetry
The absorbed dose estimation of normal organs and tumour/metastatic lesions provides the means for optimizing the dose administration of radio-peptide in PRRT, thereby full therapeutic window can be achieved with limited radiation-induced side effects to normal organs, particularly the kidney and bone marrow, which are considered as dose limiting organs in PRRT. The MIRD (Medical Internal Radiation Dose) scheme provides reference techniques for internal dosimetry. PRRT dose estimates in organs are generally calculated using the MIRD scheme, with the basic formula [70]:
Ď = Ã×S = A0 × τ × S
Where Ď is the Mean absorbed dose to target organ from a uniformly distributed activity in source organ, Ã is the Cumulative activity in the source organ, S is the ‘S’ factor, it is the radiation absorbed dose per unit cumulative activity and τ is the residence time/disintegrations corresponding to the total number of decays occurring in the organ divided by A0. For individualized patient dosimetry the values of S-factor rescaled according to the mass of the patient organ. Once the integral activities in the organs of interest are determined using mathematical models, absorbed doses are generally calculated using dedicated software programs such as OLINDA/EXM 1.0 or its newer version OLINDA 2.2.0. For obtaining the integral activities in the organs of interest input data include data from blood, urine, and whole-body scans at different time intervals sufficiently covering decay of fast and slow component of the radioisotope after PRRT. The planar images are useful to derive biokinetics over time, while SPECT and SPECT/CT fused images, although requiring more time to acquire, provide insight into organ-specific three-dimensional activity distribution. In our experience the radiation absorbed dose (mGy/MBq) to the kidney, spleen, liver, bone marrow, whole body and tumour are 0.69 ± 0.23, 0.93 ± 0.58, 0.13 ± 0.06, 0.04 ± 0.02, 0.06 ± 0.02 and 4.91 ± 3.73 respectively (Table 11, results from ongoing 177Lu-DOTATATE dosimetry work), which are comparable with that reported in the literature [68].
Conclusion
Thus in this treatise, the multifaceted aspects of PRRT were discussed (encompassing the radiopharmaceutical aspects, clinical PRRT services, radiation safety and dosimetry involving [177Lu]Lu-DOTATATE PRRT obtained from indigenous resources), with clinical practice points and lessons learnt over last 10 years from the experience of a large volume PRRT centre in India. The specific areas of clinical challenges such as combination regimen for FDG avid lesions and duo-PRRT for large sized tumors were emphasized. The role of alpha therapy with [225Ac]Ac-DOTATATE is evolving at this point and its place in the practice needs to be defined. The practical and unclear issues were discussed with examples and reference drawn from the published literature in each domain, to provide the readers an in-depth holistic overview of this subject.
Acknowledgements
The authors sincerely acknowledge the staff members of Reactor Group, Bhabha Atomic Research Centre for their valuable role in regular operation and maintenance of Dhruva research reactor, which was utilized for production of 177Lutetium throughout last decade.
One decade of ‘Bench-to-Bedside’ Peptide Receptor Radionuclide Therapy with indigenous [177Lu]Lu-DOTATATE obtained through ‘Direct’ Neutron Activation Route: Lessons learnt including Practice Evolution in an Indian Setting.
Disclosure of conflict of interest
None.
References
- 1.Pillai MR, Chakraborty S, Das T, Venkatesh M, Ramamoorthy N. Production logistics of 177Lu for radionuclide therapy. Appl Radiat Isot. 2003;59:109–118. doi: 10.1016/s0969-8043(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 2.Das T, Pillai MR. Options to meet the future global demand of radionuclides for radionuclide therapy. Nucl Med Biol. 2013;40:23–32. doi: 10.1016/j.nucmedbio.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Pillai MR, Knapp FF. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem Rev. 2015;115:2934–2974. doi: 10.1021/cr500171e. [DOI] [PubMed] [Google Scholar]
- 4.Cutler CS, Hennkens HM, Sisay N, Huclier-Markai S, Jurisson SS. Radiometals for combined imaging and therapy. Chem Rev. 2013;113:858–883. doi: 10.1021/cr3003104. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty S, Vimalnath KV, Lohar SP, Shetty PS, Dash A. On the practical aspects of large-scale production of 177Lu for peptide receptor radionuclide therapy using direct neutron activation of 176Lu in a medium flux research reactor: the Indian experience. J Radioanal Nucl Chem. 2014;302:233–242. [Google Scholar]
- 6.Mikolajczak R, Parus JL, Pawlak D, Zakrzewska E, Michalak W, Sasinowsk I. Reactor produced 177Lu of specific activity and purity suitable for medical applications. J Radioanal Nucl Chem. 2003;257:53–57. [Google Scholar]
- 7.Dvorakova Z, Henkelmann R, Lin X, Türler A, Gerstenberg H. Production of 177Lu at the new research reactor FRM-II: irradiation yield of 176Lu(n,gamma)177Lu. Appl Radiat Isotope. 2008;66:147–151. doi: 10.1016/j.apradiso.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarty R, Das T, Dash A, Venkatesh M. An electro-amalgamation approach to isolate no-carrier-added 177Lu from neutron irradiated Yb for biomedical applications. Nucl Med Biol. 2010;37:811–820. doi: 10.1016/j.nucmedbio.2010.04.082. [DOI] [PubMed] [Google Scholar]
- 9.Mirzadeh S, Du M, Beets AL, Knapp FF Jr, inventors. Method for preparing high specific activity 177Lu. 6716353. United States Patent. 2004
- 10.Lebedev NA, Novgorodov AF, Misiak R, Brockmann J, Roesch F. Radiochemical separation of no-carrier-added 177Lu as produced via the 176Yb(n, γ)177Yb→177Lu process. Appl Radiat Isotope. 2000;53:421–425. doi: 10.1016/s0969-8043(99)00284-5. [DOI] [PubMed] [Google Scholar]
- 11.Bilewicz A, Zuchowska K, Bartos B. Separation of Yb as YbSO4 from 176Yb target for production of 177Lu via the 176Yb(n, γ)177Yb→177Lu process. J Radioanal Nucl Chem. 2009;280:167–169. [Google Scholar]
- 12.Atlas of neutron capture cross sections (1997) IAEA Nuclear Data Section, Vienna [Google Scholar]
- 13.Zhernosekov KP, Perego RC, Dvorakova Z, Henkelmann R, Türler A. Target burn-up corrected specific activity of 177Lu produced via 176Lu(n, gamma) 177Lu nuclear reactions. Appl Radiat Isotope. 2008;66:1218–1220. doi: 10.1016/j.apradiso.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Parghane R, Ranade R, Thapa P, Ramaswamy A, Ostwal V, Sirohi B, Panda D, Shrikhande SV. Peptide receptor radionuclide therapy in the management of neuroendocrine tumors (Neoplasms)*: fundamentals and salient clinical practice points for medical oncologists. Indian J Med Paediatr Oncol. 2019;40:165–71. [Google Scholar]
- 15.Garske-Román U, Sandström M, Fröss Baron K, Lundin L, Hellman P, Welin S, Johansson S, Khan T, Lundqvist H, Eriksson B, Sundin A, Granberg D. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45:970–988. doi: 10.1007/s00259-018-3945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilan E, Sandström M, Wassberg C, Sundin A, Garske-Román U, Eriksson B, Granberg D, Lubberink M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med. 2015;56:177–82. doi: 10.2967/jnumed.114.148437. [DOI] [PubMed] [Google Scholar]
- 17.Wehrmann C, Senftleben S, Zachert C, Müller D, Baum RP. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm. 2007;22:406–416. doi: 10.1089/cbr.2006.325. [DOI] [PubMed] [Google Scholar]
- 18.Garkavij M, Nickel M, Sjögreen-Gleisner K, Ljungberg M, Ohlsson T, Wingårdh K, Strand SE, Tennvall J. 177Lu-[DOTA0,Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer. 2010;116(Suppl):1084–1092. doi: 10.1002/cncr.24796. [DOI] [PubMed] [Google Scholar]
- 19.Thapa P, Ranade R, Ostwal V, Shrikhande SV, Goel M, Basu S. Performance of 177Lu-DOTATATE-based peptide receptor radionuclide therapy in metastatic gastroenteropancreatic neuroendocrine tumor: a multiparametric response evaluation correlating with primary tumor site, tumor proliferation index, and dual tracer imaging characteristics. Nucl Med Commun. 2016;37:1030–7. doi: 10.1097/MNM.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 20.Basu S, Ranade R, Thapa P. Metastatic neuroendocrine tumor with extensive bone marrow involvement at diagnosis: evaluation of response and hematological toxicity profile of PRRT with (177)Lu-DOTATATE. World J Nucl Med. 2016;15:38–43. doi: 10.4103/1450-1147.165353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu S, Abhyankar A. The use of 99mTc-HYNIC-TOC and 18F-FDG PET/CT in the evaluation of duodenal neuroendocrine tumor with atypical and extensive metastasis responding dramatically to a single fraction of PRRT with 177Lu-DOTATATE. J Nucl Med Technol. 2014;42:296–298. doi: 10.2967/jnmt.114.139238. [DOI] [PubMed] [Google Scholar]
- 22.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, Lepensky C, Kwekkeboom DJ, Baum RP, Krenning EP, Modlin IM. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 23.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O’Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio MS, O’Dorisio TM, Howe JR, Cremonesi M, Kwekkeboom DJ, Zaknun JJ. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–16. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Öberg K, Knigge U, Kwekkeboom D, Perren A ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124–30. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 26.Wang LF, Lin L, Wang MJ, Li Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: a meta-analysis. Medicine (Baltimore) 2020;99:e19304. doi: 10.1097/MD.0000000000019304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Song Q, Cai L, Xie Y, Chen Y. The efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2020;146:1533–1543. doi: 10.1007/s00432-020-03181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, Willinek W, Biersack HJ, Sabet A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 29.Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23:4617–4624. doi: 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- 30.Parghane R, Talole S, Prabhash K, Basu S. Clinical response profile of metastatic/advanced pulmonary neuroendocrine tumors to peptide receptor radionuclide therapy with 177Lu-DOTATATE. Clin Nucl Med. 2017;42:428–435. doi: 10.1097/RLU.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 31.Sabet A, Haug AR, Eiden C, Auernhammer CJ, Simon B, Bartenstein P, Biersack HJ, Ezziddin S. Efficacy of peptide receptor radionuclide therapy with 177Lu-octreotate in metastatic pulmonary neuroendocrine tumors: a dual-centre analysis. Am J Nucl Med Mol Imaging. 2017;7:74–83. [PMC free article] [PubMed] [Google Scholar]
- 32.Adnan A, Kudachi S, Ramesh S, Prabhash K, Basu S. Metastatic or locally advanced mediastinal neuroendocrine tumours: outcome with 177Lu-DOTATATE-based peptide receptor radionuclide therapy and assessment of prognostic factors. Nucl Med Commun. 2019;40:947–957. doi: 10.1097/MNM.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 33.Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, Basu S. Clinical utility of 177Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck. 2020;42:401–416. doi: 10.1002/hed.26024. [DOI] [PubMed] [Google Scholar]
- 34.Beukhof CM, Brabander T, van Nederveen FH, van Velthuysen MF, de Rijke YB, Hofland LJ, Franssen GJH, Fröberg LAC, Kam BLR, Visser WE, de Herder WW, Peeters RP. Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: predictors and pitfalls. BMC Cancer. 2019;19:325. doi: 10.1186/s12885-019-5540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaisman F, Rosado de Castro PH, Lopes FP, Kendler DB, Pessoa CH, Bulzico DA, de Carvalho Leal D, Vilhena B, Vaisman M, Carneiro M, Corbo R. Is there a role for peptide receptor radionuclide therapy in medullary thyroid cancer? Clin Nucl Med. 2015;40:123–127. doi: 10.1097/RLU.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 36.Basu B, Basu S. Correlating and combining genomic and proteomic assessment with in vivo molecular functional imaging: will this be the future roadmap for personalized cancer management? Cancer Biother Radiopharm. 2016;31:75–84. doi: 10.1089/cbr.2015.1922. [DOI] [PubMed] [Google Scholar]
- 37.Adnan A, Sampathirao N, Basu S. Implications of fluorodeoxyglucose uptake in low-intermediate grade metastatic neuroendocrine tumors from peptide receptor radionuclide therapy outcome viewpoint: a semi-quantitative standardized uptake value-based analysis. World J Nucl Med. 2019;18:389–395. doi: 10.4103/wjnm.WJNM_62_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu S, Sirohi B, Shrikhande SV. Dual tracer imaging approach in assessing tumor biology and heterogeneity in neuroendocrine tumors: its correlation with tumor proliferation index and possible multifaceted implications for personalized clinical management decisions, with focus on PRRT. Eur J Nucl Med Mol Imaging. 2014;41:1492–1496. doi: 10.1007/s00259-014-2805-8. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Ranade R, Thapa P. Correlation and discordance of tumour proliferation index and molecular imaging characteristics and their implications for treatment decisions and outcome pertaining to peptide receptor radionuclide therapy in patients with advanced neuroendocrine tumour: developing a personalized model. Nucl Med Commun. 2015;36:766–774. doi: 10.1097/MNM.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 40.Basu S. Should grade of tracer uptake on somatostatin receptor-targeted imaging be the major determinant and break the barrier of histopathologic criteria for determining the suitability of Peptide receptor radionuclide therapy? J Nucl Med. 2013;54:2018–9. doi: 10.2967/jnumed.113.127829. [DOI] [PubMed] [Google Scholar]
- 41.Jois B, Asopa R, Basu S. Somatostatin receptor imaging in non-(131)I-avid metastatic differentiated thyroid carcinoma for determining the feasibility of peptide receptor radionuclide therapy with (177)Lu-DOTATATE: low fraction of patients suitable for peptide receptor radionuclide therapy and evidence of chromogranin a level-positive neuroendocrine differentiation. Clin Nucl Med. 2014;39:505–10. doi: 10.1097/RLU.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 42.Basu S, Ranade R. Favorable response of metastatic merkel cell carcinoma to targeted 177Lu-DOTATATE therapy: will PRRT evolve to become an important approach in receptor-positive cases? J Nucl Med Technol. 2016;44:85–7. doi: 10.2967/jnmt.115.163527. [DOI] [PubMed] [Google Scholar]
- 43.Parghane RV, Talole S, Basu S. Prevalence of hitherto unknown brain meningioma detected on 68Ga-DOTATATE positron-emission tomography/computed tomography in patients with metastatic neuroendocrine tumor and exploring potential of 177Lu-DOTATATE peptide receptor radionuclide therapy as single-shot treatment approach targeting both tumors. World J Nucl Med. 2019;18:160–170. doi: 10.4103/wjnm.WJNM_39_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu S, Fargose P. 177Lu-DOTATATE PRRT in recurrent skull-base phosphaturic mesenchymal tumor causing osteomalacia: a potential application of PRRT beyond neuroendocrine tumors. J Nucl Med Technol. 2016;44:248–250. doi: 10.2967/jnmt.116.177873. [DOI] [PubMed] [Google Scholar]
- 45.Adnan A, Basu S. Gratifying results with combined Chemo-PRRT (177Lu-DOTATATE and platinum-based chemotherapy) in recurrent metastatic sinonasal neuroendocrine carcinoma with high uptake on both 68Ga-DOTATATE and 18F-FDG PET-CT: a Promising Therapeutic Option. J Nucl Med Technol. 2020 doi: 10.2967/jnmt.119.237354. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Parghane RV, Ostwal V, Ramaswamy A, Bhandare M, Chaudhari V, Talole S, Shrikhande SV, Basu S. Long-term outcome of “Sandwich” chemo-PRRT: a novel treatment strategy for metastatic neuroendocrine tumors with both FDG- and SSTR-avid aggressive disease. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-05004-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Basu S, Kand P, Mallia M, Korde A, Shimpi H. Gratifying clinical experience with an indigenously formulated single-vial lyophilized HYNIC-TOC kit at the radiopharmaceutical division of BARC: a pivotal boost for building up a peptide receptor radionuclide therapy programme in an Indian setting. Eur J Nucl Med Mol Imaging. 2013;40:1622–4. doi: 10.1007/s00259-013-2501-0. [DOI] [PubMed] [Google Scholar]
- 48.Ramesh S, Kudachi S, Basu S. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in carcinoid heart disease: a contraindication or a promising treatment approach bettering chances for corrective surgery? J Nucl Med Technol. 2018;46:292–294. doi: 10.2967/jnmt.118.210179. [DOI] [PubMed] [Google Scholar]
- 49.van Vliet EI, van Eijck CH, de Krijger RR, Nieveen van Dijkum EJ, Teunissen JJ, Kam BL, de Herder WW, Feelders RA, Bonsing BA, Brabander T, Krenning EP, Kwekkeboom DJ. Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0,Tyr3]Octreotate. J Nucl Med. 2015;56:1647–1653. doi: 10.2967/jnumed.115.158899. [DOI] [PubMed] [Google Scholar]
- 50.Barber TW, Hofman MS, Thomson BN, Hicks RJ. The potential for induction peptide receptor chemoradionuclide therapy to render inoperable pancreatic and duodenal neuroendocrine tumours resectable. Eur J Surg Oncol. 2012;38:64–71. doi: 10.1016/j.ejso.2011.08.129. [DOI] [PubMed] [Google Scholar]
- 51.Stoeltzing O, Loss M, Huber E, Gross V, Eilles C, Mueller-Brand J, Schlitt HJ. Staged surgery with neoadjuvant 90Y-DOTATOC therapy for down-sizing synchronous bilobular hepatic metastases from a neuroendocrine pancreatic tumor. Langenbecks Arch Surg. 2010;395:185–192. doi: 10.1007/s00423-009-0520-x. [DOI] [PubMed] [Google Scholar]
- 52.Sowa-Staszczak A, Pach D, Chrzan R, Trofimiuk M, Stefańska A, Tomaszuk M, Kołodziej M, Mikołajczak R, Pawlak D, Hubalewska-Dydejczyk A. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inoperable neuroendocrine tumors (NETs) Eur J Nucl Med Mol Imaging. 2011;38:1669–1674. doi: 10.1007/s00259-011-1835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong G, Thompson M, Collins M, Herschtal A, Hofman MS, Johnston V, Eu P, Michael M, Hicks RJ. Assessment of predictors of response and long-termsurvival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT) Eur J Nucl Med Mol Imaging. 2014;41:1831–1844. doi: 10.1007/s00259-014-2788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kashyap R, Hofman MS, Michael M, Kong G, Akhurst T, Eu P, Zannino D, Hicks RJ. Favourable outcomes of 177Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2015;42:176–185. doi: 10.1007/s00259-014-2906-4. [DOI] [PubMed] [Google Scholar]
- 55.Claringbold PG, Turner JH. Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-octreotate capecitabine-temozolomide radiopeptide chemotherapy. Neuroendocrinology. 2016;103:432–439. doi: 10.1159/000434723. [DOI] [PubMed] [Google Scholar]
- 56.Ballal S, Yadav MP, Damle NA, Sahoo RK, Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in patients with advanced neuroendocrine tumors: a long-term-outcome, toxicity, survival, and quality-of-life study. Clin Nucl Med. 2017;42:e457–e466. doi: 10.1097/RLU.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 57.Yordanova A, Ahrens H, Feldmann G, Brossart P, Gaertner FC, Fottner C, Weber MM, Ahmadzadehfar H, Schreckenberger M, Miederer M, Essler M. Peptide receptor radionuclide therapy combined with chemotherapy in patients with neuroendocrine tumors. Clin Nucl Med. 2019;44:e329–e335. doi: 10.1097/RLU.0000000000002532. [DOI] [PubMed] [Google Scholar]
- 58.Basu S, Parghane RV, Banerjee S. Availability of both [177Lu]Lu-DOTA-TATE and [90Y]Y-DOTATATE as PRRT agents for neuroendocrine tumors: can we evolve a rational sequential duo-PRRT protocol for large volume resistant tumors? Eur J Nucl Med Mol Imaging. 2020;47:756–758. doi: 10.1007/s00259-019-04546-7. [DOI] [PubMed] [Google Scholar]
- 59.Parghane RV, Mitra A, Upadhye T, Rakshit S, Banerjee S, Basu S. Sequential duo-PRRT with indigenous 90Y-DOTATATE and 177Lu-DOTATATE in large volume neuroendocrine tumors: post-therapy bremsstrahlung and PET-CT imaging following 90Y-DOTATATE treatment. Clin Nucl Med. 2020;45:714–715. doi: 10.1097/RLU.0000000000003182. [DOI] [PubMed] [Google Scholar]
- 60.Basu S, Parghane RV, Kamaldeep , Chakrabarty S. Peptide receptor radionuclide therapy of neuroendocrine tumors. Semin Nucl Med. 2020;50:447–464. doi: 10.1053/j.semnuclmed.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Nelson KL, Sheetz MA. Radiation safety observation associated with 177Lu-Dotatate patients. Health Phys. 2019;117:680–687. doi: 10.1097/HP.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 62.Kamaldeep , Bhaktivinayagam A, Tervankar S, Kumar P, Kaisar S, et al. Estimate of effective dose to comfortors/family members of patients in peptide receptor radionuclide therapy with [lu-177]-DOTATATE. Proceedings of 31st National Conference on “Advances in Radiation Measurement Systems and Techniques. IARPNC- 2014; March 19 -21, 2014; Mumbai. p. 120. [Google Scholar]
- 63.Ann ICRP. 1981. ICRP Publication 30 (Part 3). Limits for intakes of radionuclides by workers; p. 6. [PubMed] [Google Scholar]
- 64.Lutathera Package Insert. 2018. Advanced Accelerator Applications; pp. 1–14. [Google Scholar]
- 65.Sandström M, Garske-Román U, Granberg D, Johansson S, Widström C, Eriksson B, Sundin A, Lundqvist H, Lubberink M. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33–41. doi: 10.2967/jnumed.112.107524. [DOI] [PubMed] [Google Scholar]
- 66.Kamaldeep , Wanage G, Sahoo S, Maletha P, Adnan A, Basu S, Das T, Banerjee S. Biokinetic studies of 177Lu-DOTATATE in patients with advanced/metastatic neuroendocrine tumor receiving peptide receptor radionuclide therapy. Indian Journal of Nuclear Medicine (Abstract Supplement Issue) 2017;32:S28. [Google Scholar]
- 67.De Jong M, Krenning EP. New advances in peptide receptor radionuclide therapy. J Nucl Med. 2002;43:617–20. [PubMed] [Google Scholar]
- 68.Jamar F, Barone R, Mathieu I, Walrand S, Labar D, Carlier P, de Camps J, Schran H, Chen T, Smith MC, Bouterfa H, Valkema R, Krenning EP, Kvols LK, Pauwels S. (86YDOTA0)-DPhe1-Tyr3-octreotide (SMT487) - a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging. 2003;30:510–8. doi: 10.1007/s00259-003-1117-1. [DOI] [PubMed] [Google Scholar]
- 69.Bodei L, Cremonesi M, Grana C, Rocca P, Bartolomei M, Chinol M, Paganelli G. Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3- octreotide (90Y-DOTATOC) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2004;31:1038–46. doi: 10.1007/s00259-004-1571-4. [DOI] [PubMed] [Google Scholar]
- 70.Loevinger R, Budinger TF, Watson EE. MIRD primer for absorbed dose calculations. New York: The Society of Nuclear Medicine; 1991. [Google Scholar]
- 71.Cremonesi M, Ferrari ME, Bodei L, Chiesa C, Sarnelli A, Garibaldi C, Pacilio M, Strigari L, Summers PE, Orecchia R, Grana CM, Botta F. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur J Nucl Med Mol Imaging. 2018;45:2426–2441. doi: 10.1007/s00259-018-4044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levart D, Kalogianni E, Carcoran B, Mulholl N, Vivian G. Radiation precautions for inpatient and outpatient 177Lu-DOTATATE peptide receptor radionuclide therapy of neuroendocrine tumours. EJNMMI Phys. 2019;6:7. doi: 10.1186/s40658-019-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calais PJ, Turner JH. Radiation safety of outpatient 177Lu-octreotate radiopeptide therapy of neuroendocrine tumors. Ann Nucl Med. 2014;28:531–539. doi: 10.1007/s12149-014-0843-8. [DOI] [PubMed] [Google Scholar]
- 74.Sandstrom M, Garske U, Granberg D, Sundin A, Lundqvist H. Individualized dosimetry in patients undergoing therapy with (177)Lu-DOTA-D-Phe (1)-Tyr (3)-octreotate. Eur J Nucl Med Mol Imaging. 2010;37:212–25. doi: 10.1007/s00259-009-1216-8. [DOI] [PubMed] [Google Scholar]
- 75.Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, Baio SM, Sansovini M, Paganelli G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–56. doi: 10.1007/s00259-008-0778-1. [DOI] [PubMed] [Google Scholar]
- 76.Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, Schmidt MA, Bugaj JL, de Jong M, Krenning EP. [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med. 2001;28:1319–25. doi: 10.1007/s002590100574. [DOI] [PubMed] [Google Scholar]
- 77.Del Prete M, Buteau FA, Beauregard JM. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study. Eur J Nucl Med Mol Imaging. 2017;44:1490–500. doi: 10.1007/s00259-017-3688-2. [DOI] [PubMed] [Google Scholar]
- 78.Wehrmann C, Senftleben S, Zachert C, Muller D, Baum RP. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm. 2007;22:406–16. doi: 10.1089/cbr.2006.325. [DOI] [PubMed] [Google Scholar]
- 79.Gupta SK, Singla S, Thakral P, Bal CS. Dosimetric analyses of kidneys, liver, spleen, pituitary gland, and neuroendocrine tumors of patients treated with 177Lu-DOTATATE. Clin Nucl Med. 2013;38:188–94. doi: 10.1097/RLU.0b013e3182814ac1. [DOI] [PubMed] [Google Scholar]
- 80.Garkavij M, Nickel M, Sjögreen-Gleisner K, Ljungberg M, Ohlsson T, Wingårdh K, Strand SE, Tennvall J. 177Lu-[DOTA0,Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer. 2010;116(Suppl 4):1084–92. doi: 10.1002/cncr.24796. [DOI] [PubMed] [Google Scholar]
- 81.Svensson J, Berg G, Wangberg B, Larsson M, Forssell-Aronsson E, Bernhardt P. Renal function affects absorbed dose to the kidneys and haematological toxicity during (1)(7)(7)Lu-DOTATATE treatment. Eur J Nucl Med Mol Imaging. 2015;42:947–55. doi: 10.1007/s00259-015-3001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


