Abstract
N6-methyladenosine (m6A) is the most common RNA modification and has an important role in normal development and tumorigenesis. The abnormal expression of m6A regulators can lead to an imbalance in m6A levels in cancer cells, leading to the dysregulated expression of oncogenes and tumor suppressor genes that may contribute to cancer development, patient response to chemoradiotherapy, and clinical prognosis. Recent studies demonstrate that non-coding RNAs are involved in epigenetic modification of both DNA and RNA in tumor cells, and may also affect the development and progression of cancer by targeting m6A regulators. In this review, we describe the functional crosstalk between m6A and non-coding RNAs, particularly microRNA, long non-coding RNA, and circular RNA, and illustrate their roles in tumor regulation. Finally, we discuss the significance of non-coding RNA and m6A modification in the diagnosis, treatment, and prognosis of cancer patients, as well as potential future research directions.
Keywords: cancer, N6-methyladenosine, non-coding RNA, microRNA, long non-coding RNA, circular RNA
Graphical Abstract
As a pivotal member of RNA modification, m6A plays an important role in the initiation and progression of cancer. However, the mechanism between m6A regulators and non-coding RNAs is largely unknown. Gao and colleagues summarize the crosstalk between m6A and non-coding RNAs in cancers and provide potential novel research directions.
Main Text
According to recent global cancer statistics, cancer remains an important factor threatening human health.1 N6-methyladenosine (m6A) is the most common RNA modification and has attracted significant attention from researchers in the fields of tumorigenesis and development.2,3 Since the discovery of m6A in the early 1970s, studies have shown that it accumulates predominantly near the stop codons and 3′ untranslated regions (3′ UTRs) of mRNA.4, 5, 6, 7, 8 The abnormal expression of m6A regulators can lead to an imbalance in m6A levels in cancer cells, leading to the dysregulated expression of oncogenes and tumor suppressor genes that may contribute to cancer development, patient response to chemoradiotherapy, and clinical prognosis.2,7,9,10
Previous studies confirm that dysregulation of m6A regulators may be detected in precancerous lesions, highlighting their potential as molecular markers for the early diagnosis of cancer.11 Fat mass and obesity-associated protein (FTO) has been identified as an m6A demethylase that can selectively remove the m6A modification from target RNAs.12 A recent study showed that combination of FTO inhibitor and nilotinib can restrain the growth of leukemia and increase the sensitivity of leukemia cells to tyrosine kinase inhibitors, highlighting the potential therapeutic value of targeting m6A regulators in drug-resistant cancers.13 Although the FTO inhibitor, entacapone, has been approved by The Food and Drug Administration (FDA) for the treatment of cancer and other related diseases,14 specific inhibitors have not yet been identified for other m6A regulatory proteins.
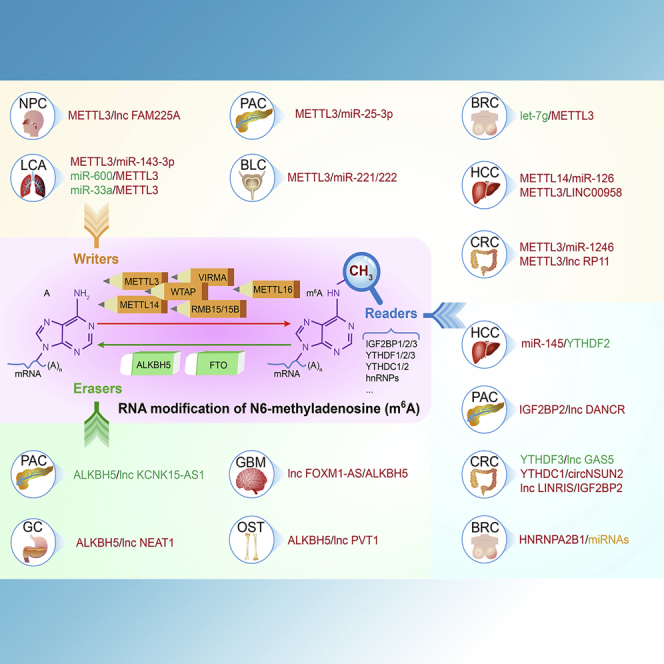
Although the function of m6A modification in cancer is becoming increasingly clear, its effect on protein translation and the molecular mechanisms underlying the effect of this mark on cancer progression remain unclear. Following the development of MeRIP-seq (methylated RNA immunoprecipitation sequencing) and miCLIP (m6A individual-nucleotide-resolution cross-linking and immunoprecipitation) technologies, researchers have found that non-coding RNAs, including long non-coding RNA (lncRNA), microRNA (miRNA), circular RNA (circRNA), transfer RNA, ribosomal RNA, and small nuclear RNA, are also capable of modifying DNA and RNA bases in cancer cells.15,16 Furthermore, non-coding RNA also participates in the regulation of m6A modification, thus affecting cancer progression (Figure 1).3,17
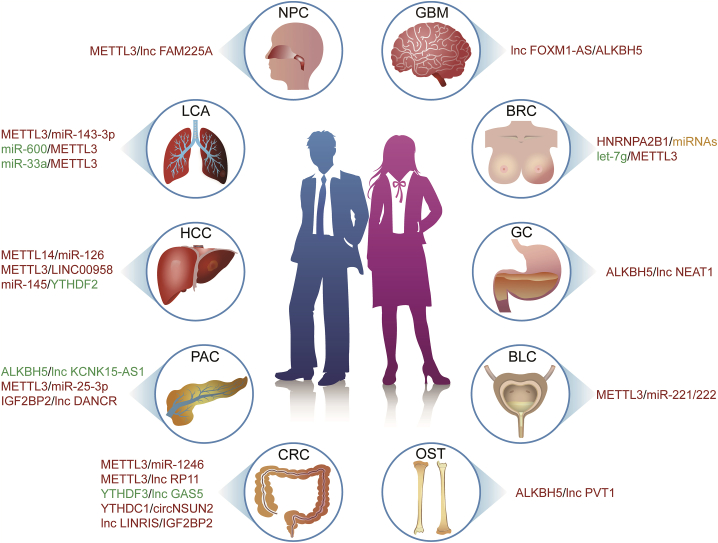
Figure 1.
Non-coding RNAs and N6-methyladenosine (m6A) Modifications in Cancer
Red font indicates an oncogenic role, green font indicates a tumor suppressor role, and yellow font indicates involvement of both oncogenic and tumor suppressor activities. BLC, bladder cancer; BRC, breast cancer; CRC, colorectal cancer; GBM, glioblastoma; GC, gastric cancer; HCC, hepatocellular carcinoma; LCA, lung cancer; NPC, nasopharyngeal carcinoma; OST, osteosarcoma; PAC, pancreatic cancer.
In this review, we describe the functional crosstalk between m6A and non-coding RNA, particularly miRNA, lncRNA, and circRNA, and illustrate how deregulation of these networks plays a role in tumors. Finally, we discuss the significance of non-coding RNA and m6A modifications in the diagnosis, treatment, and prognosis of cancer patients and possible future research directions.
Overview of m6A Writers, Erasers, and Readers
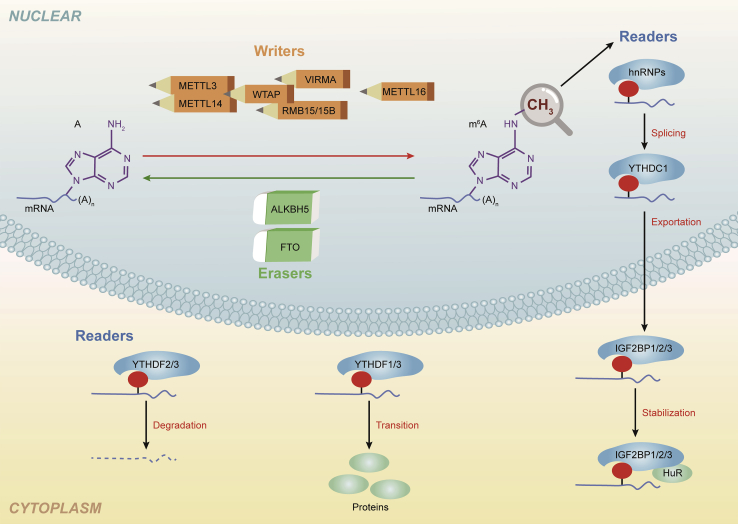
m6A modification is a dynamic and reversible process that has a critical role in regulating RNA stability, splicing, and translation. This modification is controlled by regulatory proteins referred to as “writers,” “erasers,” and “readers” (Figure 2). Epigenetic writers included methyltransferase-like 3/14/16 (METTL3/14/16), wt1-associated protein (WTAP), RNA binding motif protein 15/15B (RBM15/15B), and vir-like m6A methyltransferase-associated protein (VIRMA, also known as KIAA1429). METTL3/14 can form complexes to cause m6A methylation to be written into mRNA,18 and WTAP aids METTL3/14 to locate nuclear spots and maintain the catalytic activity of m6A methyltransferase in vivo.19 Meanwhile, METTL3 expression is essential for WTAP protein homeostasis.20 Moreover, RBM15, RBM15B, and VIRMA have roles in the regulation of METTL3 and METTL14 activity.21
Figure 2.
Summary of m6A Modification and Its Effects on mRNA Function
m6A modification is a dynamic and reversible process. RNA can be methylated by “writers,” demethylated by “erasers,” and recognized by “readers.”
Erasers include FTO and AlkB homolog 5 (ALKBH5). These proteins can selectively remove the m6A mark targeting mRNA through a series of complex intermediate reactions, thereby affecting tumor-specific biological processes.22 In 2001, researchers from the laboratory of Chuan He confirmed that FTO is an important DNA and RNA demethylase, particularly for m6A demethylation.12 The oncogenic role of FTO has since been confirmed in numerous cancers, including cervical cancer, breast cancer (BRC), and gastric cancer (GC).23, 24, 25
To date, several hypotheses have inferred that m6A modifications function by altering RNA structure or recruiting m6A readers. The most common readers include m6A RNA binding protein 1/2/3 (YTHDF1/2/3), YTH domain-containing 1/2 (YTHDC1/2), insulin-like growth factor 2 mRNA binding proteins 1/2/3 (IGF2BP1/2/3), heterogeneous nuclear ribonucleoproteins (HNRNPs), and zinc-finger CCCH domain-containing protein 13 (ZC3H13).26 Reader proteins function by binding to m6A sites on the target RNA and mediating its modification, thereby controlling RNA fate.26,27
In addition to revealing the functional roles and mechanisms of m6A RNA methylation in various cancers, recent studies highlighted the impact of m6A RNA methylation regulators on the diagnosis and prognosis of cancer patients (Table 1). Du et al.10 performed univariate Cox regression analysis to evaluate the clinical prognostic values of m6A RNA methylation regulators in glioblastoma (GBM), and revealed that HNRNPC, ALKBH5, and ZC3H13 are favorable prognostic markers, whereas FTO is an unfavorable prognostic marker for GBM. Furthermore, METTL3, YTHDC2, and YTHDF2 were identified as independent predictors of overall survival in liver cancer (LC).28 Moreover, Zhuang et al.29 built a 10-gene risk score model in lung adenocarcinoma (LUAD) through combined analysis of expression levels of m6A RNA regulators and clinicopathological characters. They found that the expression patterns of ALKBH5, FTO, HNRNPC, YTHDF2, YTHDF1, YTHDC2, RBM15, KIAA1429, WTAP, and METTL3 were correlated with TNM stage, lymph node stage, and sex, as well as the living status of patients with LUAD. A two-gene signature consisting of METTL3 and METTL14 was identified as an independent prognostic indicator for distinguishing clear cell renal cell carcinoma (ccRCC) patients.30 These studies suggested that m6A regulators are potential diagnostic and prognostic markers for various cancers.
Table 1.
Impact of m6A Modification Regulator on Diagnosis and Prognosis of Cancer Patients
| Cancer Type | m6A Regulator | Diagnosis/Prognosis | References |
|---|---|---|---|
| GBM | HNRNPC, ZC3H13, ALKBH5 | unfavorable prognostic marker | 10 |
| FTO | favorable prognostic marker | ||
| LC | METTL3, YTHDC2, YTHDF2 | unfavorable prognostic marker | 28 |
| LUAD | ALKBH5, HNRNPC, YTHDF2, YTHDF1, YTHDC2, RBM15, KIAA1429, WTAP, METTL3, FTO | diagnostic marker, prognostic marker | 29 |
| ccRCC | METTL3 | unfavorable prognostic marker | 30 |
| METTL14 | favorable prognostic marker |
m6A Modification of Non-coding RNA
Non-coding RNAs comprise a large class of RNA transcripts without protein-coding potential that regulate gene expression and are important regulators of cancer cell proliferation, apoptosis, migration, immune response, and autophagy.31 m6A modification of non-coding RNA regulates important processes controlling RNA function, including processing, stability, and transport (Figure 3).32
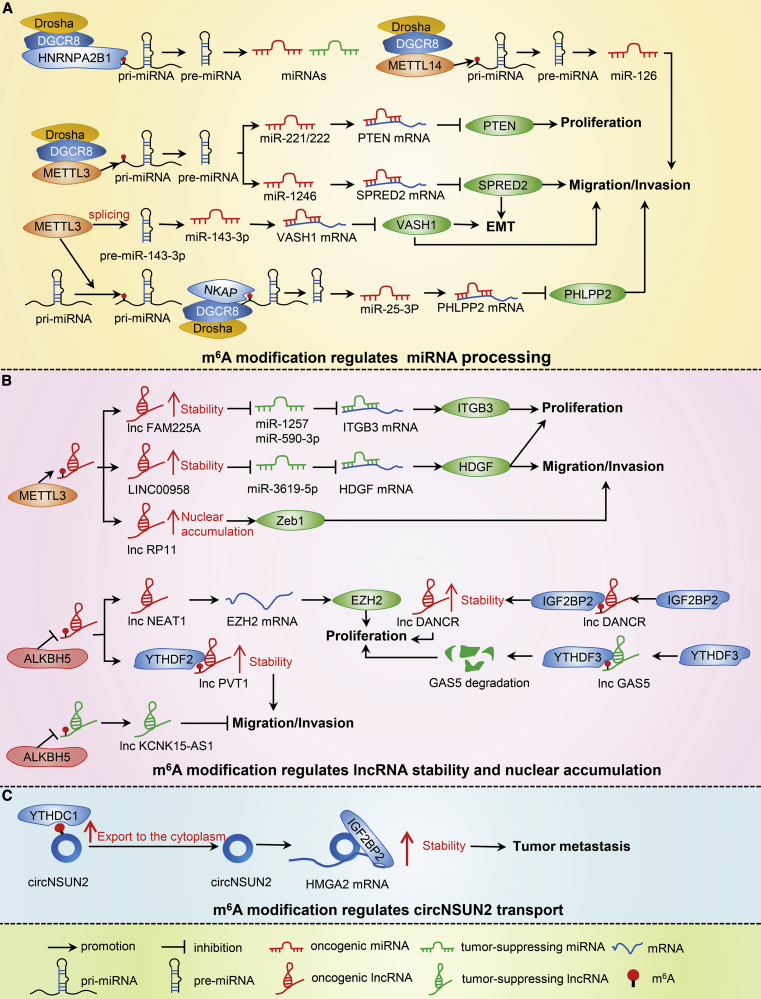
Figure 3.
m6A Modifications in Non-coding RNA
(A) m6A modification regulates miRNA processing. (B) m6A modification regulates lncRNA stability and nuclear accumulation. (C) m6A modification regulates circNSUN2 transport.
m6A Modification of miRNA
On the basis of our current understanding, miRNA biogenesis can be divided into three steps.33 In the nucleus, RNA polymerase II or III transcribes miRNA-related genes into primary miRNA (pri-miRNA). pri-miRNA is transformed into precursor miRNA (pre-miRNA) by the microprocessor complex, DGCR8-Drosha, and is subsequently transported out of the nucleus by the exportin-5-Ran-GTP complex. Finally, the microprocessor component, Dicer, cleaves the pre-miRNA into mature mRNA in the cytoplasm.34,35 miRNAs have important roles in the regulation of gene expression, mainly through their association with AGO2 as part of the RNA-induced silencing complex (RISC), via binding to mRNA 3′ UTR, leading to degradation and inhibition of translation.36,37 In 2002, the Croce team first identified the role of miRNAs in cancer, demonstrating low expression of miR-15 and miR-16 in chronic lymphocytic leukemia patients.38 Since then, numerous studies have indicated that abnormal expression of miRNA underlies many pathological processes related to tumorigenesis.39 Notably, there is a strong association between m6A and miRNA binding sites in mammals.4
The synthesis and function of miRNAs may be affected by m6A modification at multiple levels (Table 2). Studies by Alarcón et al.40 indicate that heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) can read m6A marks and enhance DGCR8 binding to pri-miRNA transcripts, affecting miRNA processing. Similarly, the m6A writer METTL14 can interact with DGCR8 and promote miR-126 processing in an m6A-dependent manner in hepatocellular carcinoma (HCC).41 In bladder cancer, METTL3 is overexpressed and regulates the processing of miR-221/miR-222 in an m6A-dependent manner via recruitment of DCGR8.42 METTL3 also promotes pri-miR-1246 maturation via a similar mechanism and positively modulates tumor cell metastasis.43 Studies by Zhang et al.44 emphasize the importance of the m6A writer METTL3 on miR-25-3p maturation and identified NKAP as an m6A reader for pri-miR-25 processing in pancreatic cancer (PAC). METTL3 can accelerate the brain metastasis of cancer cells and promote the splicing of pre-miR-143-3p to produce mature miRNA, and may be associated with Dicer in lung cancer (LCA).45 As previously discussed, m6A promotes miRNA maturation by regulating its processing, thereby enhancing the degradation and translational inhibition of downstream target mRNAs.
Table 2.
m6A Modification Regulates miRNA Processing
| m6A Regulator | Associated Cancer | Function | References |
|---|---|---|---|
| Reader | |||
| HNRNPA2B1 | BRC | promotes miRNAs processing | 40 |
| Writer | |||
| METTL14 | HCC | promotes the processing of miR-126 via DGCR8 | 41 |
| METTL3 | BLC | promotes the processing of miR-221/222 via DGCR8 | 42 |
| METTL3 | CRC | promotes the processing of miR-1246 by DGCR8 | 43 |
| METTL3 | PAC | promotes miR-25-3p maturation | 44 |
| METTL3 | LCA | promotes splicing of pre-miR-143-3p | 45 |
Interestingly, m6A modifications can also protect mRNA degradation mediated by miRNA. Müller et al.46 demonstrated that IGF2BP1 affects miRNA-directed decay of SRF mRNA, increasing SRF expression in an m6A-dependent manner. In colorectal cancer (CRC), IGF2BP2 maintains RAF1 mRNA stability by blocking miRNA-mediated degradation, thereby increasing cancer cell proliferation.47 Furthermore, m6A modification of AGO2 mRNA has also been reported to affect miRNA levels.48 In conclusion, m6A modification plays an important role in miRNA biogenesis, and the effects of m6A-mediated miRNA level variation require further investigation.
m6A Modification of lncRNA
The role of lncRNA in tumor development is complex and diverse.49 lncRNAs may regulate gene transcription via binding to gene promoters,50 affect the variable splicing of RNA and maintain the normal function of intracellular organelles,51 act as miRNA sponges, relieving inhibition of miRNA target genes,52 and affect the stability and translation of mRNA via RNA interactions.53,54 They can also affect protein function by acting as a scaffold for protein-protein interactions, modulating their localization to chromatin, and regulating protein post-translational modifications and stability.55 lncRNAs are located in different subcellular structures, including the cell membrane, cytoplasm, nucleus, and paraspeckles, and their functions and regulatory mechanisms are closely related to their localization in cancer cells.56
m6A modification of lncRNA regulates numerous processes affecting cancer cell activity (Table 3). In HCC, METTL3-mediated m6A leads to upregulation of LINC00958 by enhancing its stability, thereby promoting cancer progression.57 METTL3 has also been shown to increase the stability of FAM225A, a lncRNA overexpressed in nasopharyngeal carcinoma (NPC), to promote tumorigenesis.58 Moreover, studies have shown that METTL3 can upregulate expression of RP11 lncRNA by increasing its nuclear accumulation in CRC.59 The m6A eraser ALKBH5 has been shown to act as both a tumor suppressor and promoter, and has the ability to demethylate m6A on single-stranded RNA and DNA.60,61,65 As a tumor suppressor, ALKBH5 is significantly downregulated in PAC, and its expression is related to patient survival, as well as being an independent marker of prognosis. ALKBH5 can also regulate the expression of KCNK15-AS1 via demethylation, leading to inhibition of PAC cell migration and invasion.60 ALKBH5 also plays a role in tumor progression, promoting osteosarcoma (OST) cell proliferation via upregulation of lncRNA PVT1.61 Additionally, ALKBH5 is upregulated in GC cells and increases invasion and metastasis via inhibition of NEAT1 methylation.62 The m6A readers IGF2BP2 and YTHDF3 are also involved in the regulation of lncRNA. IGF2BP2 is highly expressed in PAC and interacts with lncRNA DANCR, leading to an increase in its stability and promoting cancer cell proliferation.63 In a similar manner, YTHDF3 can negatively regulate GAS5 lncRNA and promote progression of CRC.64
Table 3.
m6A Modification Regulates lncRNA
| m6A Regulator | Associated Cancer | Function | References |
|---|---|---|---|
| Writer | |||
| METTL3 | HCC | enhances LINC00958 stability | 57 |
| METTL3 | NPC | increases lnc FAM225A stability | 58 |
| METTL3 | CRC | increases lnc RP11 nuclear accumulation | 59 |
| Eraser | |||
| ALKBH5 | PAC | inhibits lnc KCNK15-AS1 methylation | 60 |
| ALKBH5 | OST | decreases the m6A modification of lnc PVT1 | 61 |
| ALKBH5 | GC | decreases methylation of the lnc NEAT1 | 62 |
| Reader | |||
| IGF2BP2 | PAC | increases lnc DANCR stability | 63 |
| YTHDF3 | CRC | promotes decay of lnc GAS5 | 64 |
In the nucleus, lncRNAs may recruit regulatory proteins and interact with mRNAs or act as competing endogenous RNAs (ceRNAs), regulating the translation and stability of mRNA.66 Therefore, we infer that m6A modifications may affect similar regulatory functions of cytoplasmic lncRNAs. However, our understanding of m6A modifications of lncRNA is still limited.
m6A Modification of circRNA
circRNA was first identified in eukaryotes nearly 40 years ago and was subsequently discovered in humans infected with hepatitis delta virus (HDV).67,68 Studies demonstrated that circRNA can specifically adsorb and bind miRNA, releasing the inhibition of miRNA on downstream target genes and directly binding proteins to modulate their function.69,70 In human cancer, circRNA regulates critical cellular processes, including proliferation, metastasis, differentiation, autophagy, and drug resistance.71, 72, 73, 74
Recent studies revealed that circRNA has the potential to be translated. Yang et al.75 demonstrated that m6A levels in circRNA can promote efficient initiation of protein translation from human cells. Other studies have shown that YTHDF1 and YTHDF2 can interact with circRNAs, and that METTL3 also affects circRNA m6A levels, suggesting that m6A is modified by the same machinery in both circRNAs and mRNAs.76 However, enrichment of m6A in circRNAs is mainly at the translation start site of their corresponding mRNAs, differing from mRNA.76 The m6A reader YTHDC1 has been shown to increase circNSUN2 export to the cytoplasm (Table 4), leading to the formation of a circNSUN2-IGF2BP2-HMGA2 RNA-protein ternary complex that can stabilize HMGA2 mRNA and enhance colorectal liver metastasis.77 Another study also reports that METTL3 can impact circZNF609 m6A modification, and YTHDC1 regulates back-splicing of circZNF609, which highlights the critical role of m6A modification in circZNF609 biogenesis and translation in HeLa cells.78 These studies provide a new perspective on m6A modification of circRNA.
Table 4.
m6A Modification Regulates circRNA
| m6A Regulator | Associated Cancer | Function | Reference |
|---|---|---|---|
| YTHDC1 | CRC | increases circNSUN2 export to the cytoplasm | 77 |
Regulation of m6A Modification by Non-coding RNAs
miRNA Affects m6A Modification
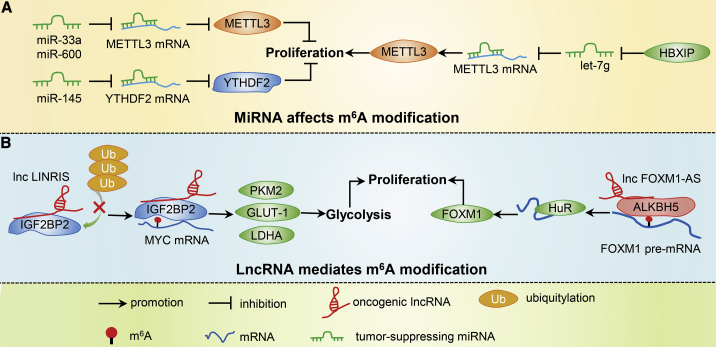
Studies have shown that m6A modifications can be controlled by miRNA levels (Table 5). In LCA, miR-600 decreases the expression of METTL3 and reverses the effect of METTL3 on cancer cell progression.79 In keeping with these findings, miR-33a targeting of the METTL3 3′ UTR leads to the downregulation of METTL3 expression and suppression of non-small cell lung cancer (NSCLC) proliferation.80 Let-7g miRNA can also inhibit METTL3 expression by targeting its 3′ UTR; moreover, HBXIP increases METTL3 expression by restraining let-7g in BRC.81 Yang et al.82 reveal that overexpression of miR-145 strongly increases m6A levels via targeting of the 3′ UTR of YTHDF2 in HCC (Figure 4A). Together, these studies indicate that miRNAs may affect m6A modification by controlling the levels of m6A regulators.
Table 5.
miRNA Affects m6A Modification
| miRNA | Associated Cancer | Function | m6A Regulator | References |
|---|---|---|---|---|
| miR-600 | LCA | downregulates the expression of METTL3 | writer | 79 |
| miR-33a | NSCLC | decreases METTL3 through targeting its 3′ UTR | writer | 80 |
| let-7g | BRC | restrains METTL3 expression by targeting its 3′ UTR | writer | 81 |
| miR-145 | HCC | inhibits YTHDF2 by targeting its 3′ UTR | reader | 82 |
Figure 4.
Overview of Non-coding RNA-Mediated Regulation of m6A Modifications in Cancer
(A) miRNA affects m6A modification. (B) lncRNA mediates m6A modification.
lncRNAs Regulate m6A Modification
As discussed above, m6A modification participates in lncRNA biogenesis and can affect functional activity. Conversely, lncRNAs can also affect m6A regulators and influence their function in cancer cells (Table 6). For example, the lncRNA LINRIS is upregulated in CRC cells and maintains IGF2BP2 protein stability via blocking the ubiquitination-proteasome pathway.83 ALKBH5 is highly expressed in primary GBM cell lines and promotes cancer cell proliferation. The lncRNA FOXM1-AS promotes the interaction between ALKBH5 and FOXM1, leading to demethylation of FOXM1 mRNA and overexpression of FOXM1 (Figure 4B).84 These studies suggest that regulation of m6A modifications by antisense lncRNAs may be a common mechanism. As a regulatory subunit of IGF2BP1, the peptide RBRP encoded by LINC0266-1 can recognize m6A modification via binding to IGF2BP1 and recruit stable RNA molecules to maintain the stability of MYC mRNA, thus promoting the occurrence and development of tumors.85 This study enriches our understanding of the effect of lncRNA on m6A modification.
Table 6.
lncRNA Regulates m6A Modification
Conclusions
m6A regulators can modulate non-coding RNAs via multiple mechanisms, including regulation of pri-miRNA processing, affecting m6A-dependent ceRNA networks, promoting the nucleation of circRNA, and even by regulating the interaction between lncRNAs and proteins. These studies typically use poly(A)+ RNA for m6A mapping, excluding many regulatory ncRNA species that do not contain poly(A) tails. In addition, miRNA and lncRNA can also regulate m6A levels in cancer cells. miRNAs can target the corresponding mRNA of m6A regulators to silence their expression, thus altering m6A levels in cancer cells. In the nucleus, lncRNAs may act as scaffolds, providing a platform for other effector molecules to interact with m6A. Additionally, lncRNAs may be involved in maintaining the stability of m6A-related proteins. Notably, Huang et al.86 reported that circSTAG1 can bind ALKBH5 and inhibit its nucleation, thus changing the total RNA m6A modification and increasing the m6A modification level of RNAs, including FAAH mRNA in the chronic unpredictable stress mouse hippocampus. This study provides the foundation for analyzing the relationship between circRNA and m6A modification. circRNA can not only be modified by m6A, but can also regulate the process of m6A modification by binding to m6A-modified proteins. However, the regulation of m6A modification by circRNA has not yet been reported in human cancer.
The crosstalk between non-coding RNAs and m6A modifications provides a new perspective for us to study normal development and tumorigenesis and to understand its complex regulatory network. Dynamic m6A modification of non-coding RNA represents a novel mechanism to regulate genetic information in cancer cells and adds to our understanding of how m6A modifications regulate RNA and downstream biological processes. The finding that m6A levels can in turn be regulated by non-coding RNAs enriches our understanding of non-coding RNA molecular networks in cancer progression. Together, these findings provide new directions to study the mechanism of non-coding RNA in tumorigenesis and development.
Future Prospects
According to the central dogma, generation of the entire proteome from the genome requires regulation at four main stages: RNA production (including epigenetic regulation and transcriptional regulation), RNA degradation, protein production (translation regulation), and protein degradation. Of these, regulation of translation constitutes the most important mode of regulation in the cell, accounting for more than half of all regulatory events.87 To date, there have been few studies in this field because of the limitations of the current research methods and other factors.
At present, RNA methylation and translation-omics are new directions in epigenetic research and will provide important insights into the novel mechanisms governing normal physiological and abnormal cellular processes. Research on the functional interaction of non-coding RNA and m6A modification deserves particular attention. It is worth noting that studies investigating the crosstalk between non-coding RNA and m6A regulators typically involve members transcribed from different parental genes. Whether non-coding RNA and m6A regulators transcribed from the same gene can interact to regulate downstream target genes through positive or negative feedback loops will be of interest in the future. Studying the cross regulation of non-coding RNA and m6A modification will facilitate the discovery of critical targets for the diagnosis and treatment of cancer patients, which is the ultimate goal of personalized medicine. Given the involvement of these regulatory processes in normal development and other diseases, these findings are likely to have widespread applications, although the specific mechanisms in these cell types require further exploration.48,88
Author Contributions
W.G., W.X., and Y.W. conceived this manuscript. F.D., Y.L., C.A., and L.Z. collected and prepared the related references. F.D., Y.W., and Y.L. drafted the manuscript. Y.G. and F.D. drew the figures. Y.L., L.D., and L.Z. performed data analysis and tabulation. W.G., Y.W., H.L., and W.X. supervised and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 81602394); Postdoctoral Research Foundation of China (grant 2017M610174); The Excellent Talent Science and Technology Innovation Project of Shanxi Province (grant 201705D211018); Outstanding Youth Development Foundation of The First Hospital Affiliated with Shanxi Medical University (YR1601); and The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant 2019-RC-HL-004).
Contributor Information
Yongyan Wu, Email: wuyongyan@sxent.org.
Wei Xu, Email: xuwhns@126.com.
Wei Gao, Email: gaoweisxent@sxent.org.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Lan Q., Liu P.Y., Haase J., Bell J.L., Hüttelmaier S., Liu T. The Critical Role of RNA m6A Methylation in Cancer. Cancer Res. 2019;79:1285–1292. doi: 10.1158/0008-5472.CAN-18-2965. [DOI] [PubMed] [Google Scholar]
- 3.Dai D., Wang H., Zhu L., Jin H., Wang X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 2018;9:124. doi: 10.1038/s41419-017-0129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alarcón C.R., Lee H., Goodarzi H., Halberg N., Tavazoie S.F. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M., Kim B., Kim V.N. Emerging roles of RNA modification: m(6)A and U-tail. Cell. 2014;158:980–987. doi: 10.1016/j.cell.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng X., Su R., Weng H., Huang H., Li Z., Chen J. RNA N6-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J., Hou K., Mi S., Ji H., Ma S., Ba Y., Hu S., Xie R., Chen L. Malignant Evaluation and Clinical Prognostic Values of m6A RNA Methylation Regulators in Glioblastoma. Front. Oncol. 2020;10:208. doi: 10.3389/fonc.2020.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W., Qi C.B., Lv S.W., Xie M., Feng Y.Q., Huang W.H., Yuan B.F. Determination of DNA and RNA Methylation in Circulating Tumor Cells by Mass Spectrometry. Anal. Chem. 2016;88:1378–1384. doi: 10.1021/acs.analchem.5b03962. [DOI] [PubMed] [Google Scholar]
- 12.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.G., He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan F., Al-Kali A., Zhang Z., Liu J., Pang J., Zhao N., He C., Litzow M.R., Liu S. A dynamic N6-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res. 2018;28:1062–1076. doi: 10.1038/s41422-018-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Major J.M., Dong D., Cunningham F., By K., Hur K., Shih D.C., Jiang R., Podskalny G.D., Wei X., Pinheiro S. Entacapone and prostate cancer in Parkinson’s disease patients: A large Veterans Affairs healthcare system study. Parkinsonism Relat. Disord. 2018;53:46–52. doi: 10.1016/j.parkreldis.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Pendleton K.E., Chen B., Liu K., Hunter O.V., Xie Y., Tu B.P., Conrad N.K. The U6 snRNA m6A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell. 2017;169:824–835.e14. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y., Hou G., Zhang H., Dou J., He J., Guo Y., Li L., Chen R., Wang Y., Deng R. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46:5195–5208. doi: 10.1093/nar/gky156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fazi F., Fatica A. Interplay Between N6-Methyladenosine (m6A) and Non-coding RNAs in Cell Development and Cancer. Front. Cell Dev. Biol. 2019;7:116. doi: 10.3389/fcell.2019.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Q., Shi H., Ye P., Li L., Qu Q., Sun G., Sun G., Lu Z., Huang Y., Yang C.G. m6A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ping X.L., Sun B.F., Wang L., Xiao W., Yang X., Wang W.J., Adhikari S., Shi Y., Lv Y., Chen Y.S. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorci M., Ianniello Z., Cruciani S., Larivera S., Ginistrelli L.C., Capuano E., Marchioni M., Fazi F., Fatica A. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018;9:796. doi: 10.1038/s41419-018-0843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ianniello Z., Fatica A. N6-Methyladenosine Role in Acute Myeloid Leukaemia. Int. J. Mol. Sci. 2018;19:2345. doi: 10.3390/ijms19082345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y., Yan J., Li Q., Li J., Gong S., Zhou H., Gan J., Jiang H., Jia G.F., Luo C., Yang C.G. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43:373–384. doi: 10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou D., Dong L., Li C., Yin Z., Rao S., Zhou Q. The m6A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019;19:321. doi: 10.1186/s12935-019-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu Y., Lin Z., Wan A., Chen H., Liang H., Sun L., Wang Y., Li X., Xiong X.F., Wei B. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol. Cancer. 2019;18:46. doi: 10.1186/s12943-019-1004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge L., Zhang N., Chen Z., Song J., Wu Y., Li Z., Chen F., Wu J., Li D., Li J. Level of N6-Methyladenosine in Peripheral Blood RNA: A Novel Predictive Biomarker for Gastric Cancer. Clin. Chem. 2020;66:342–351. doi: 10.1093/clinchem/hvz004. [DOI] [PubMed] [Google Scholar]
- 26.Meyer K.D., Jaffrey S.R. Rethinking m6A Readers, Writers, and Erasers. Annu. Rev. Cell Dev. Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M., Wong C.M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol. Cancer. 2020;19:44. doi: 10.1186/s12943-020-01172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W., Sun B., Xia Y., Sun S., He C. RNA N6-Methyladenosine-Related Gene Contribute to Clinical Prognostic Impact on Patients With Liver Cancer. Front. Genet. 2020;11:306. doi: 10.3389/fgene.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang Z., Chen L., Mao Y., Zheng Q., Li H., Huang Y., Hu Z., Jin Y. Diagnostic, progressive and prognostic performance of m6A methylation RNA regulators in lung adenocarcinoma. Int. J. Biol. Sci. 2020;16:1785–1797. doi: 10.7150/ijbs.39046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Yu K., Zhong G., Shen W. Identification of a m6A RNA methylation regulators-based signature for predicting the prognosis of clear cell renal carcinoma. Cancer Cell Int. 2020;20:157. doi: 10.1186/s12935-020-01238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beermann J., Piccoli M.T., Viereck J., Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 32.Huang H., Weng H., Chen J. m6A Modification in Coding and Non-coding RNAs: Roles and Therapeutic Implications in Cancer. Cancer Cell. 2020;37:270–288. doi: 10.1016/j.ccell.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 34.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 35.Mirihana Arachchilage G., Dassanayake A.C., Basu S. A potassium ion-dependent RNA structural switch regulates human pre-miRNA 92b maturation. Chem. Biol. 2015;22:262–272. doi: 10.1016/j.chembiol.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 38.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 40.Alarcón C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J.Z., Yang F., Zhou C.C., Liu F., Yuan J.H., Wang F., Wang T.T., Xu Q.G., Zhou W.P., Sun S.H. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6 -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 42.Han J., Wang J.Z., Yang X., Yu H., Zhou R., Lu H.C., Yuan W.B., Lu J.C., Zhou Z.J., Lu Q. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol. Cancer. 2019;18:110. doi: 10.1186/s12943-019-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng W., Li J., Chen R., Gu Q., Yang P., Qian W., Ji D., Wang Q., Zhang Z., Tang J., Sun Y. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:393. doi: 10.1186/s13046-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J., Bai R., Li M., Ye H., Wu C., Wang C., Li S., Tan L., Mai D., Li G. Excessive miR-25-3p maturation via N6-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat. Commun. 2019;10:1858. doi: 10.1038/s41467-019-09712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H., Deng Q., Lv Z., Ling Y., Hou X., Chen Z., Dinglin X., Ma S., Li D., Wu Y. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol. Cancer. 2019;18:181. doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Müller S., Glaß M., Singh A.K., Haase J., Bley N., Fuchs T., Lederer M., Dahl A., Huang H., Chen J. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47:375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye S., Song W., Xu X., Zhao X., Yang L. IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF-1 degradation by miR-195. FEBS Lett. 2016;590:1641–1650. doi: 10.1002/1873-3468.12205. [DOI] [PubMed] [Google Scholar]
- 48.Min K.W., Zealy R.W., Davila S., Fomin M., Cummings J.C., Makowsky D., Mcdowell C.H., Thigpen H., Hafner M., Kwon S.H. Profiling of m6A RNA modifications identified an age-associated regulation of AGO2 mRNA stability. Aging Cell. 2018;17:e12753. doi: 10.1111/acel.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Zhang X., Chen W., Hu X., Li J., Liu C. Regulatory roles of long noncoding RNAs implicated in cancer hallmarks. Int. J. Cancer. 2020;146:906–916. doi: 10.1002/ijc.32277. [DOI] [PubMed] [Google Scholar]
- 50.Wang X., Sun W., Shen W., Xia M., Chen C., Xiang D., Ning B., Cui X., Li H., Li X. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J. Hepatol. 2016;64:1283–1294. doi: 10.1016/j.jhep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 51.Fang Z., Zhang S., Wang Y., Shen S., Wang F., Hao Y., Li Y., Zhang B., Zhou Y., Yang H. Long non-coding RNA MALAT-1 modulates metastatic potential of tongue squamous cell carcinomas partially through the regulation of small proline rich proteins. BMC Cancer. 2016;16:706. doi: 10.1186/s12885-016-2735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao G., Yao J., Kong D., Ye C., Chen R., Li L., Zeng T., Wang L., Zhang W., Shi X. The Long Noncoding RNA TTTY15, Which Is Located on the Y Chromosome, Promotes Prostate Cancer Progression by Sponging let-7. Eur. Urol. 2019;76:315–326. doi: 10.1016/j.eururo.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Zhuo W., Liu Y., Li S., Guo D., Sun Q., Jin J., Rao X., Li M., Sun M., Jiang M. Long Noncoding RNA GMAN, Up-regulated in Gastric Cancer Tissues, Is Associated With Metastasis in Patients and Promotes Translation of Ephrin A1 by Competitively Binding GMAN-AS. Gastroenterology. 2019;156:676–691.e11. doi: 10.1053/j.gastro.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 54.Deng S.J., Chen H.Y., Zeng Z., Deng S., Zhu S., Ye Z., He C., Liu M.L., Huang K., Zhong J.X. Nutrient Stress-Dysregulated Antisense lncRNA GLS-AS Impairs GLS-Mediated Metabolism and Represses Pancreatic Cancer Progression. Cancer Res. 2019;79:1398–1412. doi: 10.1158/0008-5472.CAN-18-0419. [DOI] [PubMed] [Google Scholar]
- 55.Liu B., Sun L., Liu Q., Gong C., Yao Y., Lv X., Lin L., Yao H., Su F., Li D. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Kopp F., Mendell J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuo X., Chen Z., Gao W., Zhang Y., Wang J., Wang J., Cao M., Cai J., Wu J., Wang X. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J. Hematol. Oncol. 2020;13:5. doi: 10.1186/s13045-019-0839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Z.Q., Li Z.X., Zhou G.Q., Lin L., Zhang L.L., Lv J.W., Huang X.D., Liu R.Q., Chen F., He X.J. Long Noncoding RNA FAM225A Promotes Nasopharyngeal Carcinoma Tumorigenesis and Metastasis by Acting as ceRNA to Sponge miR-590-3p/miR-1275 and Upregulate ITGB3. Cancer Res. 2019;79:4612–4626. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y., Yang X., Chen Z., Tian L., Jiang G., Chen F., Li J., An P., Lu L., Luo N. m6A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol. Cancer. 2019;18:87. doi: 10.1186/s12943-019-1014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He Y., Hu H., Wang Y., Yuan H., Lu Z., Wu P., Liu D., Tian L., Yin J., Jiang K., Miao Y. ALKBH5 Inhibits Pancreatic Cancer Motility by Decreasing Long Non-Coding RNA KCNK15-AS1 Methylation. Cell. Physiol. Biochem. 2018;48:838–846. doi: 10.1159/000491915. [DOI] [PubMed] [Google Scholar]
- 61.Chen S., Zhou L., Wang Y. ALKBH5-mediated m6A demethylation of lncRNA PVT1 plays an oncogenic role in osteosarcoma. Cancer Cell Int. 2020;20:34. doi: 10.1186/s12935-020-1105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J., Guo S., Piao H.Y., Wang Y., Wu Y., Meng X.Y., Yang D., Zheng Z.C., Zhao Y. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J. Physiol. Biochem. 2019;75:379–389. doi: 10.1007/s13105-019-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu X., Peng W.-X., Zhou H., Jiang J., Zhou X., Huang D., Mo Y.-Y., Yang L. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 2020;27:1782–1794. doi: 10.1038/s41418-019-0461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ni W., Yao S., Zhou Y., Liu Y., Huang P., Zhou A., Liu J., Che L., Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol. Cancer. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J., Vågbø C.B., Shi Y., Wang W.L., Song S.H. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 67.Kos A., Dijkema R., Arnberg A.C., van der Meide P.H., Schellekens H. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 1986;323:558–560. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- 68.Hsu M.T., Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 69.Li Q., Wang Y., Wu S., Zhou Z., Ding X., Shi R., Thorne R.F., Zhang X.D., Hu W., Wu M. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell Metab. 2019;30:157–173.e7. doi: 10.1016/j.cmet.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Wang R., Zhang S., Chen X., Li N., Li J., Jia R., Pan Y., Liang H. CircNT5E Acts as a Sponge of miR-422a to Promote Glioblastoma Tumorigenesis. Cancer Res. 2018;78:4812–4825. doi: 10.1158/0008-5472.CAN-18-0532. [DOI] [PubMed] [Google Scholar]
- 71.Su M., Xiao Y., Ma J., Tang Y., Tian B., Zhang Y., Li X., Wu Z., Yang D., Zhou Y. Circular RNAs in Cancer: emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer. 2019;18:90. doi: 10.1186/s12943-019-1002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J., Qi X., Liu L., Hu X., Liu J., Yang J., Yang J., Lu L., Zhang Z., Ma S. Emerging Epigenetic Regulation of Circular RNAs in Human Cancer. Mol. Ther. Nucleic Acids. 2019;16:589–596. doi: 10.1016/j.omtn.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng S., Zhou H., Feng Z., Xu Z., Tang Y., Li P., Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol. Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Y., Zhang Y., Zheng X., Dai F., Lu Y., Dai L., Niu M., Guo H., Li W., Xue X. Circular RNA circCORO1C promotes laryngeal squamous cell carcinoma progression by modulating the let-7c-5p/PBX3 axis. Mol. Cancer. 2020;19:99. doi: 10.1186/s12943-020-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y., Fan X., Mao M., Song X., Wu P., Zhang Y., Jin Y., Yang Y., Chen L.L., Wang Y. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou C., Molinie B., Daneshvar K., Pondick J.V., Wang J., Van Wittenberghe N., Xing Y., Giallourakis C.C., Mullen A.C. Genome-Wide Maps of m6A circRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns that Are Distinct from mRNAs. Cell Rep. 2017;20:2262–2276. doi: 10.1016/j.celrep.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen R.X., Chen X., Xia L.P., Zhang J.X., Pan Z.Z., Ma X.D., Han K., Chen J.W., Judde J.G., Deas O. N6-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat. Commun. 2019;10:4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Timoteo G., Dattilo D., Centrón-Broco A., Colantoni A., Guarnacci M., Rossi F., Incarnato D., Oliviero S., Fatica A., Morlando M., Bozzoni I. Modulation of circRNA Metabolism by m6A Modification. Cell Rep. 2020;31:107641. doi: 10.1016/j.celrep.2020.107641. [DOI] [PubMed] [Google Scholar]
- 79.Wei W., Huo B., Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag. Res. 2019;11:1177–1187. doi: 10.2147/CMAR.S181058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du M., Zhang Y., Mao Y., Mou J., Zhao J., Xue Q., Wang D., Huang J., Gao S., Gao Y. MiR-33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem. Biophys. Res. Commun. 2017;482:582–589. doi: 10.1016/j.bbrc.2016.11.077. [DOI] [PubMed] [Google Scholar]
- 81.Cai X., Wang X., Cao C., Gao Y., Zhang S., Yang Z., Liu Y., Zhang X., Zhang W., Ye L. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–19. doi: 10.1016/j.canlet.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 82.Yang Z., Li J., Feng G., Gao S., Wang Y., Zhang S., Liu Y., Ye L., Li Y., Zhang X. MicroRNA-145 Modulates N6-Methyladenosine Levels by Targeting the 3′-Untranslated mRNA Region of the N6-Methyladenosine Binding YTH Domain Family 2 Protein. J. Biol. Chem. 2017;292:3614–3623. doi: 10.1074/jbc.M116.749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Lu J.H., Wu Q.N., Jin Y., Wang D.S., Chen Y.X., Liu J., Luo X.J., Meng Q., Pu H.Y. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer. 2019;18:174. doi: 10.1186/s12943-019-1105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang S., Zhao B.S., Zhou A., Lin K., Zheng S., Lu Z., Chen Y., Sulman E.P., Xie K., Bögler O. m6A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell. 2017;31:591–606.e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu S., Wang J.Z., Chen D., He Y.T., Meng N., Chen M., Lu R.X., Chen X.H., Zhang X.L., Yan G.R. An oncopeptide regulates m6A recognition by the m6A reader IGF2BP1 and tumorigenesis. Nat. Commun. 2020;11:1685. doi: 10.1038/s41467-020-15403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang R., Zhang Y., Bai Y., Han B., Ju M., Chen B., Yang L., Wang Y., Zhang H., Zhang H. N6-methyladenosine modification of fatty acid amide hydrolase messenger RNA in circular RNA STAG1-regulated astrocyte dysfunction and depressive-like behaviors. Biol Psychiatry. 2020;88:392–404. doi: 10.1016/j.biopsych.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 87.Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 88.Yang D., Qiao J., Wang G., Lan Y., Li G., Guo X., Xi J., Ye D., Zhu S., Chen W. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46:3906–3920. doi: 10.1093/nar/gky130. [DOI] [PMC free article] [PubMed] [Google Scholar]