Abstract
Purpose of review
To review acute findings commonly encountered during routine clinical FDG PET/CT studies and present key imaging features to differentiate them from malignant counterparts.
Recent findings
FDG PET/CT is extensively used in routine clinical practice for oncology patients. Incidental acute findings in patients undergoing FDG PET/CT are increasingly common, and awareness of these findings and their mimics are important in delivering a clinically relevant and accurate radiological report for directing further management.
Summary
This article will review examples of common acute findings encountered during routine FDG PET/CT scans, compare them against examples of FDG-avid malignancy that can mimic these findings and emphasize key imaging findings to differentiate acute findings from their malignant mimics.
Keywords: FDG, PET/CT, Infection, Inflammation, Mimics, Malignancy
Introduction
18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is a routinely performed imaging study in oncology patients. However, FDG uptake is not limited to neoplastic processes, as infections and inflammation can also result in focal FDG uptake, which may lead to false-positive results. Interpreting physicians must be aware of these potential pitfalls in order to avoid misdiagnosis. Patient history, concurrent CT finding, and knowledge of typical patterns of metastatic spread can guide interpretation of the abnormal FDG uptake.
18F-FDG, a glucose analogue, has been used for the detection and evaluation of a wide range of solid and hematological malignancies due to the increased rate of glucose utilization in cancer cells. However, increased glucose uptake and utilization is not specific for malignant cells. Inflammatory cells such as neutrophils, activated macrophages, and lymphocytes also demonstrate increased 18F- FDG accumulation through a shared mechanism [1, 2]. Therefore, infectious and inflammatory processes have the potential to be misinterpreted as metastatic disease. Metser et al. reviewed greater than one thousand 18F- FDG PET/CTs and found one-quarter of these examinations contained incidental foci of benign FDG uptake [3]. The majority of these were related to infection and inflammation. Pneumonia, upper respiratory tract infections, and wound infections were among the most common etiologies identified incidentally on 18F-FDG PET/CT done for oncologic indications [4]. In addition, numerous additional common and uncommon benign causes of FDG uptake have been described [5].
In this review, we will present examples of common infectious and inflammatory processes encountered in oncologic 18F-FDG PET/CT, compare them with potential mimics of FDG-avid malignancy of these findings, and emphasize key imaging features to differentiate acute findings from their malignant mimics.
Thorax
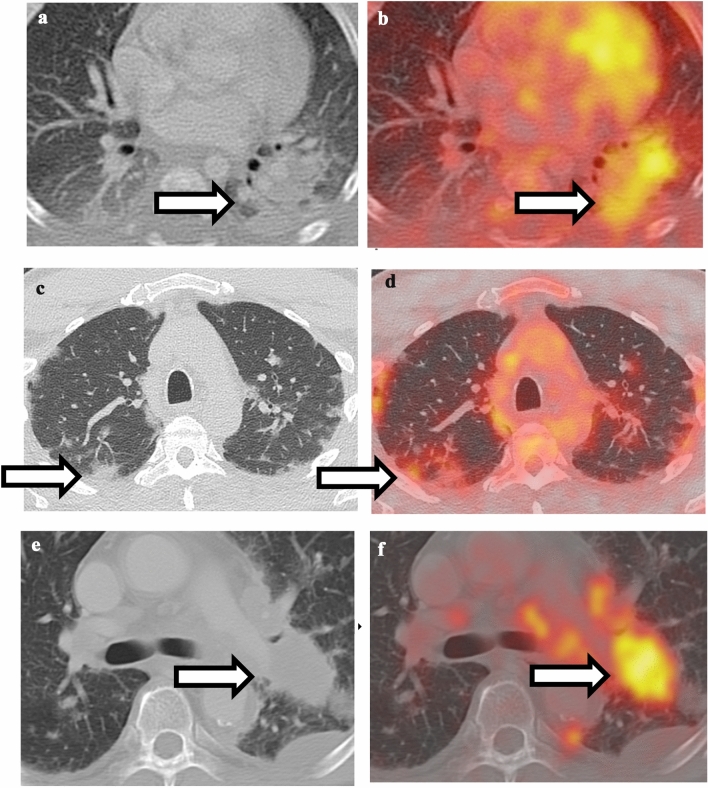
A wide variety of pulmonary infections can be 18F-FDG-avid, including typical and atypical organisms such as bacterial, fungal, TB, nocardia, and pneumocystis [6•, 7–9]. Bacterial pneumonia is one of the most commonly encountered scenarios during routine 18F-FDG PET/CT scan. Key differentiating considerations are CT appearance and clinical symptoms. If the FDG uptake is diffuse and corresponding to ill-defined consolidation and patient has clinical symptom of infection, bacterial pneumonia is likely the underlying cause. On the other hand, if the focal FDG uptake corresponds to a solid nodule/mass on CT and is round or oblong in appearance, then malignancy should be highly considered. In the current COVID-19 pandemic, there have been case reports of PET-CT findings in COVID patients [10, 11]. The characteristic CT appearance of peripheral ground-glass opacities in combination with high FDG uptake is highly suggestive of COVID-19 infection (Fig. 1). Occasionally, pulmonary edema can be confused with lymphangitic spread of tumor since both conditions can show interlobular septal thickening and ground-glass opacities [12]. Key differentiating features are that in pulmonary edema, the septal thickening is symmetric and smooth, and without associated hypermetabolism, while in lymphangitic spread of tumor, the septal thickening tends to be irregular and nodular, and is often associated with increased FDG uptake (Fig. 2).
Fig. 1.
Comparison between pulmonary infection and malignancy. a Non-contrast CT of the chest shows ill-defined consolidation in the left lower lobe. b Fusion of PET with CT shows hypermetabolism associated with left lower lobe consolidation, compatible with bacterial pneumonia. c Non-contrast CT of the chest shows bilateral peripheral ground-glass opacities. d Fusion of PET and CT shows hypermetabolism associated with ground-glass opacities, highly suggestive of COVID-19 infection. e Non-contrast CT of the chest shows a well-defined, round mass in the left lower lobe. f Fusion of PET with CT shows hypermetabolism associated with left lower lobe mass, compatible with lung malignancy
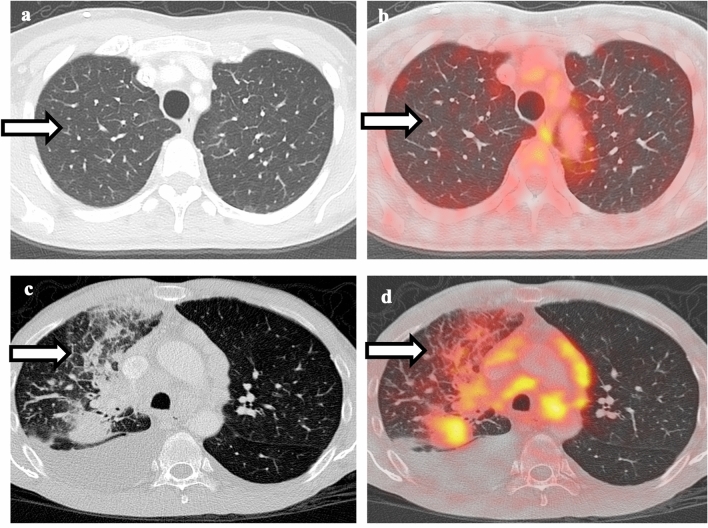
Fig. 2.
Comparison between pulmonary edema and lymphangitic spread of tumor. a Non-contrast CT of the chest shows smooth interlobular septal thickening in bilateral upper lobes. b Fusion of PET with CT shows no associated hypermetabolism, compatible with pulmonary edema. c Non-contrast CT of the chest shows right lower lobe mass, irregular and nodular thickening of interlobular septa, and small right pleural effusion. d Fusion of PET with CT shows hypermetabolism associated with the right lower lobe mass as well as the interlobular septa, compatible with lymphangitic spread of tumor
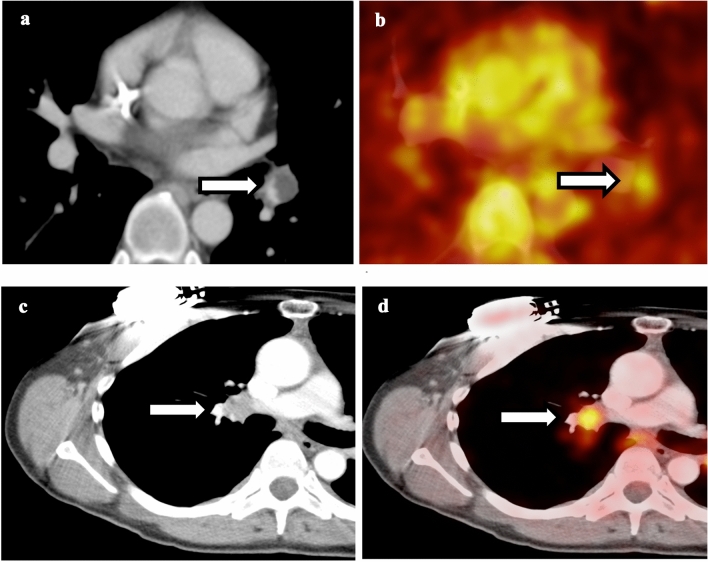
Hypercoagulability in cancer patients predispose them to develop thrombosis. Incidental pulmonary embolism can sometimes be seen in FDG PET/CT scan and some of the emboli are associated with increased FDG uptake [13]. CT appearance and location of the FDG uptake are key. If the FDG uptake is low level, along a vessel, has a curvilinear configuration, and corresponding to filling defect in pulmonary artery on post-contrast CTs, then PE is likely the culprit of FDG uptake. The most common scenario this can be confused with is hypermetabolic perihilar lymph nodes given both of them are central in location and close attention should be paid to differentiate them (Fig. 3). In addition, PE-associated hypermetabolism can also mimic pulmonary artery sarcoma. SUVmax value can distinguish these two entities as pulmonary artery sarcoma demonstrates much higher SUVmax compared to that of pulmonary embolism [14].
Fig. 3.
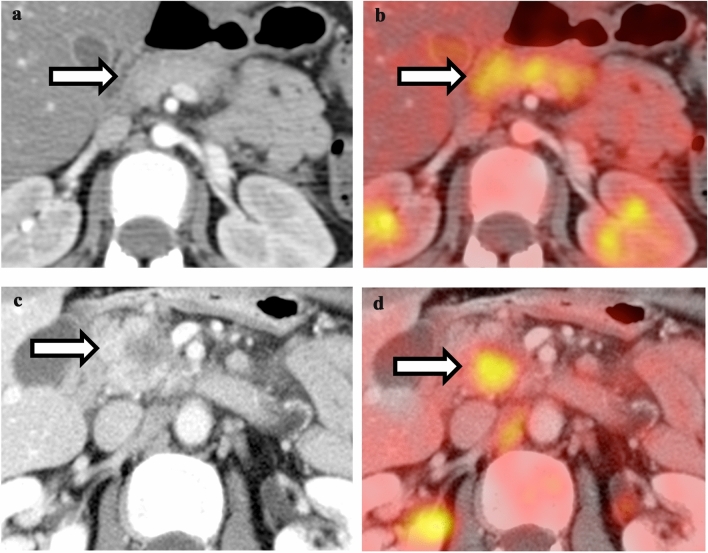
Comparison between pulmonary embolism and perihilar lymph node. a Contrast-enhanced CT of the chest shows curvilinear filling defect in the left lower lobe pulmonary artery. b Fusion of PET with CT shows low-level hypermetabolism associated with the pulmonary artery embolism. c Contrast-enhanced CT of the chest shows an enlarged right hilar lymph node. d Fusion of PET with CT shows hypermetabolism associated with the right hilar lymph node, compatible with hilar lymphadenopathy
Some inflammatory conditions including organizing pneumonia, sarcoidosis, Wegener granulomatosis, immunotherapy-induced pneumonitis, as well as post-radiation changes can also demonstrate 18F-FDG avidity [6, 15–22]. Radiation therapy-induced inflammation in lung parenchyma is often geographical and diffuse or linear configuration, distinguishing it from more focal uptake from malignancy. Additionally, FDG uptake related to radiation-induced inflammation should slowly resolve over time and nearly completely resolve 12 weeks after radiation. Conversely, talc pleurodesis performed for management of malignant and benign pleural effusions is associated with intense inflammatory foreign body response and also demonstrates high FDG uptake, which can be present for many years after the procedure [23, 24]. Thus, clinical history of prior talc pleurodesis should be correlated so that it is not confused with pleural spread of malignancy.
Extra-pulmonic infectious and inflammatory foci are also routinely encountered. Esophagitis can be various patterns of FDG uptake. For example, low-level liner FDG uptake at gastroesophageal junction can be seen in the setting of reflux esophagitis [25], as well as prior radiation, recent nasogastric tube, or other causes of esophagitis. However, intense, focal esophageal uptake, especially in the mid-thoracic esophagus, is worrisome for malignancy and further evaluation with endoscopy should be considered. Breast uptake on FDG PET/CT can be secondary to variety of processes including malignancy, inflammation, as well as benign breast masses, including silicone granuloma, fat necrosis, fibroadenoma, and postsurgical changes [26, 27]. Symmetric ill-defined low-level uptake in a premenopausal woman is usually physiological. Conversely, rounded, focal uptake should be followed up with a mammogram as it is challenging to differentiate benign versus malignant masses on PET/CT [28]. Cardiac uptake is relatively common on oncologic PET/CTs, most of which are related to increased circulating glucose or insulin level. However, if there is clinical concern for myocardial inflammation, specific tailored exam such as sarcoid PET/CT should be performed. Moreover, pericarditis, myocarditis, as well as uptake in the adjacent great vessels due to vasculitis can demonstrate increased FDG uptake [29•, 30]. These should be differentiated from FDG-avid pericardiophrenic lymph node by correlation with CT.
Abdomen and Pelvis
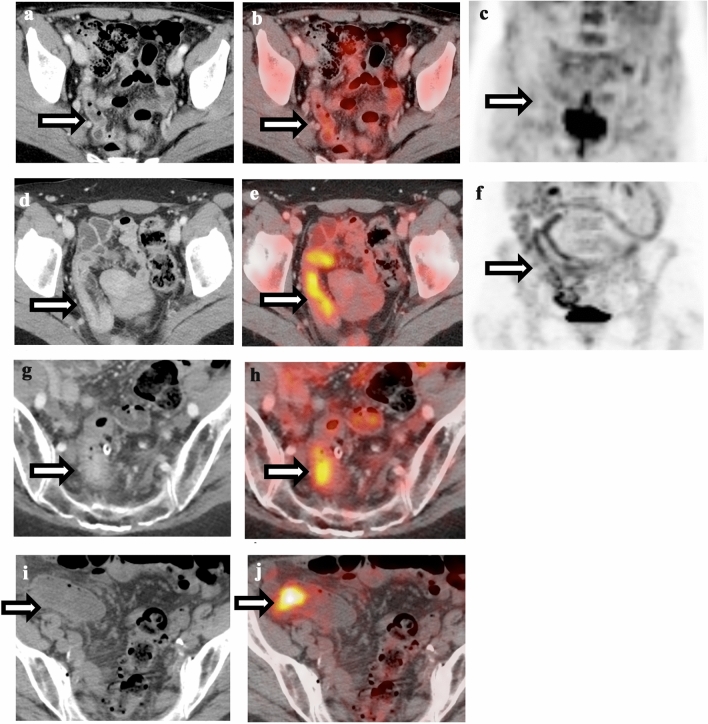
Many acute intra-abdominal infectious or inflammatory process demonstrate increased FDG uptake and can mimic malignancy. Bowel uptake of FDG is relatively common and can be physiologic, infectious/inflammatory, or neoplastic in origin. Physiologic uptake is usually low level, linear and the CT appearance of the bowel is normal (Fig. 4). Focal FDG uptake in bowel can be related to abscesses, appendicitis, diverticulitis, infectious and inflammatory colitis, as well as immunotherapy-related colitis [31–37]. Moreover, it is well known that metformin can lead to intense FDG uptake in the colon [38]. Figure 4 demonstrates examples of physiologic uptake, immunotherapy-induced colitis, diverticulitis, and colon cancer. If there is diffuse FDG uptake corresponding to inflammatory changes around diverticula, diverticulitis is more likely. Immunotherapy-induced colitis demonstrates diffuse bowel wall thickening, surrounding fat stranding, and diffuse FDG uptake. Conversely, focal, rounded uptake in the colon regardless of CT correlate should raise concerns for colon cancer, especially if it persists over multiple exams, and colonoscopy should be considered.
Fig. 4.
Comparison of physiological bowel uptake, immunotherapy-induced colitis, diverticulitis, and colon cancer. a Contrast-enhanced CT of the abdomen shows normal appearance of the bowel. b Fusion of PET and CT shows diffuse low-level metabolism throughout the bowel, compatible with physiological uptake. c Maximum intensity projection (MIP) of the same patient as (a) and (b) shows diffuse low-level metabolism throughout the bowel, compatible with physiological uptake. d Contrast-enhanced CT of a patient on immunotherapy shows long segment bowel wall thickening of sigmoid colon. e Fusion of PET and CT shows increased hypermetabolism in the segment of thick-walled sigmoid colon, compatible with immunotherapy-induced colitis. f MIP of the same patient as (d) and (e) shows increased hypermetabolism in the segment of thick-walled sigmoid colon, compatible with immunotherapy-induced colitis. g Contrast-enhanced CT of the abdomen shows focal bowel wall thickening around an inflamed diverticulum with surrounding fat stranding. h Fusion of PET and CT shows hypermetabolism associated with inflamed diverticulum, compatible with diverticulitis. i Non-contrast CT of the abdomen shows subtle mass in the sigmoid colon without surrounding inflammatory changes. j Fusion of PET and CT shows intense FDG uptake associated with the colonic mass, compatible with colon cancer
Visceral organs can also demonstrate increased FDG uptake. For example, acute pancreatitis shows increased FDG uptake [39–41]. CT appearance, FDG uptake pattern, and clinical symptoms are key. If patient has acute onset of epigastric pain, CT shows diffuse pancreatic enlargement, and peripancreatic fat stranding with associated lab shows increased lipase, then pancreatitis is more likely. On the other hand, pancreatic cancer will show soft tissue mass with focal FDG uptake resulting in distal main pancreatic ductal dilatation (Fig. 5). Any focal pancreatic abnormality, whether on the PET or CT, should be handled with care. Follow-up dedicated pancreatic imaging should be seriously considered. Like acute pancreatitis, acute cholecystitis will show intense FDG uptake [42, 43]. CT appearance, FDG uptake pattern, and clinical symptoms are key. If patient has acute onset of right upper quadrant pain and CT shows circumferential gallbladder wall thickening with diffuse FDG uptake, acute cholecystitis is highly likely. Conversely, gallbladder cancer will show soft tissue mass in the gallbladder wall with possible direct invasion into the adjacent liver parenchyma (Fig. 6). The patient can be asymptomatic or has persistent, long-term right upper quadrant pain. Tumefactive sludge can be a mimic of gallbladder cancer on CT; however, this will demonstrate no FDG uptake.
Fig. 5.
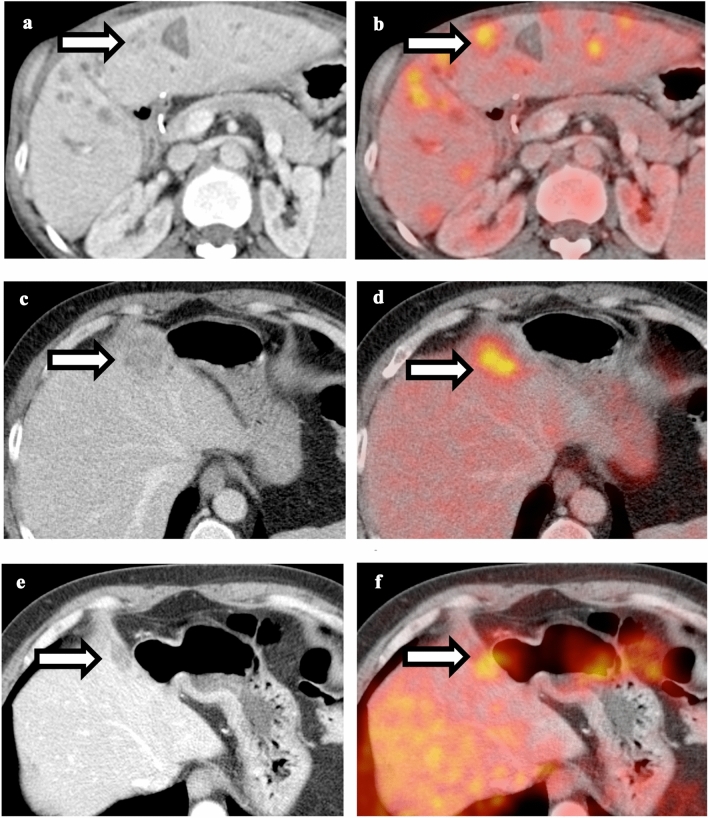
Comparison between pancreatitis and pancreatic cancer. a Contrast-enhanced CT of the abdomen shows diffuse pancreatic edema and peripancreatic stranding. b Fusion of PET and CT shows diffuse hypermetabolism throughout the pancreas, compatible with acute pancreatitis. c Contrast-enhanced CT of the abdomen shows ill-defined hypo-enhancing mass in the pancreatic head. d Fusion of PET and CT shows intense focal FDG uptake in the pancreatic head mass, compatible with pancreatic cancer
Fig. 6.
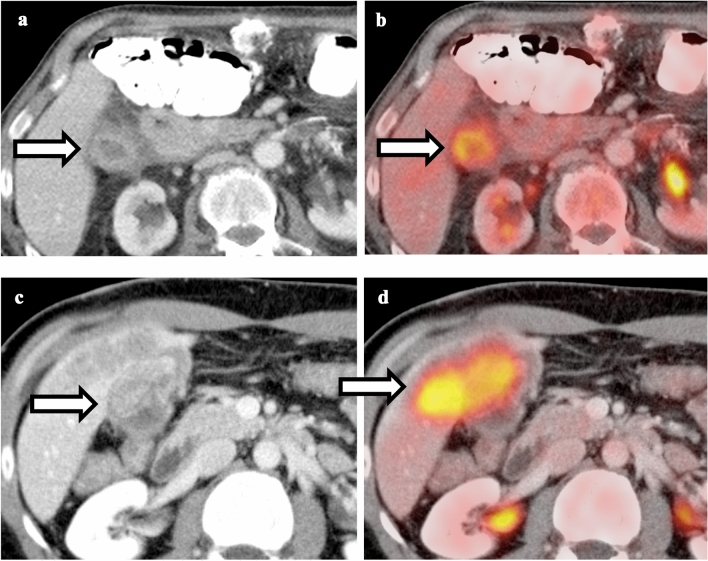
Comparison between acute cholecystitis and gallbladder cancer. a Contrast-enhanced CT of the abdomen shows circumferential gallbladder wall thickening and pericholecystic stranding. b Fusion of PET and CT shows hypermetabolism associated with thickened gallbladder wall without focal mass, compatible with acute cholecystitis. c Contrast-enhanced CT of the abdomen shows focal mass arising from the gallbladder wall with invasion into the adjacent liver parenchyma. d Fusion of PET and CT shows intense FDG uptake in the gallbladder mass and liver parenchyma, compatible with gallbladder cancer
Both hepatic metastases and infection/abscess can demonstrate intense FDG uptake [44, 45]. CT appearance, clinical history, and time course are key to differentiate them from metastatic lesions (Fig. 7). If patient has clinical symptoms and lab evidence of infection, infectious etiology should be high on differential. Also, if the patient has other signs of infection, especially intra-abdominal infection, or recent interventions such as ERCP or percutaneous biopsy, then abscesses should be considered. On imaging, larger abscesses (greater than 1 cm) can be centrally photopenic on FDG PET. Sometimes, systemic antibiotics or chemotherapy treatment and short-term follow-up are necessary to differentiate abscess versus metastases. Similarly, focal renal infections like renal tuberculosis, acute pyelonephritis, xanthogranulomatous pyelonephritis, and renal abscesses have all been encountered on 18F-FDG PET/CT and may be mistaken for a neoplastic process [46–49]. Gonadal infection such as tubo-ovarian abscesses and orchitis has been mistaken for neoplasm on 18F-FDG PET/CT [50–52].
Fig. 7.
Comparison between liver abscess and metastatic lesion. a Contrast-enhanced CT of the abdomen shows multiple ill-defined hypoattenuating lesions throughout the liver. b Fusion of PET and CT shows hypermetabolism associated with the lesions. Patient was treated with intravenous antibiotics and follow-up PET-CT shows resolution of these lesions, compatible with liver abscess. c Contrast-enhanced CT of a patient with history of colon cancer shows ill-defined hypoattenuating lesion in the right hepatic lobe. d Fusion of PET and CT shows hypermetabolism associated with the lesion, suspicious for metastatic lesion. e and f Follow-up PET-CT after chemotherapy shows decreased size and metabolism of the lesion, compatible with treated metastatic disease. Liver abscess and metastasis can have similar CT and PET appearance, and thus clinical correlation and follow-up are necessary to differentiate these two conditions
In addition to the above-mentioned infectious or inflammatory process in the abdominal and pelvic organs, hernias can also demonstrate increased FDG uptake due to incarceration of fat and/or bowel, which can mimic tumor [53]. Figure 8 shows a case of FDG-avid inguinal hernia and a companion case of local tumor recurrence. CT is key. If the FDG uptake corresponds to bowel-containing inguinal hernia without adjacent soft tissue mass, do not mistake it for local recurrence. An additional potential mimic of tumor is inguinal hernia repair with prolene mesh, which can show increased FDG uptake [54]. CT can help differentiate local recurrence from prolene mesh as mesh will have lower Hounsfield units compared to local recurrence, which usually demonstrates attenuation similar to that of muscle. In equivocal cases, surgical history will be helpful. Additionally, FDG uptake can be seen along the tract of a surgical intervention due to granulation tissue. However, these regions should not be dismissed because these are common sites of recurrence after surgical resections. Any focal or new soft tissue masses should be considered suspicious for tumor and ultrasound-guided biopsy should be considered. Some other miscellaneous conditions such as acute portal venous thrombosis, peritonitis, idiopathic mesenteric necrotizing granuloma, and sclerosing mesenteritis have also been reported to show increased FDG uptake [55–57], which can potentially mimic peritoneal carcinomatosis. The location, clinical history, and CT findings can be helpful to differentiate inflammatory from malignant changes; however, often times, it is evolution of time and changes in tumor markers that differentiate them.
Fig. 8.
Comparison between inguinal hernia and intramuscular malignancy. a Contrast-enhanced CT of the pelvis shows right bowel-containing inguinal hernia. b Fusion of PET and CT shows mild hypermetabolism associated with the right inguinal hernia, due to physiological bowel uptake. c Contrast-enhanced CT of the pelvis shows mildly hyper-attenuating lesion in the left adductor musculature. d Fusion of PET and CT shows intense FDG uptake associated with the intramuscular lesion, compatible with recurrent malignancy
Musculoskeletal
Low-level physiologic FDG uptake can be seen in the axial and proximal appendicular skeleton. Maximum intensity projection (MIP) images can delineate the diffuse physiologic skeletal uptake nicely. Meanwhile, a variety of musculoskeletal processes can lead to focal uptake on FDG PET/CT. Incidental inflammatory uptake in joints from degenerative osteoarthritis is almost ubiquitously visualized. Acute processes such as herniated discs, discitis, osteomyelitis, and septic arthritis can also demonstrate focal FDG uptake and may be mistaken for osseous metastases [58, 59]. Focal FDG uptake in the muscles can be related to tendinous insertion, myositis, or intramuscular infection and mistaken for sites of malignancy [60–62]. Most times, the pattern of FDG uptake can distinguish the two scenarios as metastasis are usually focally rounded or oblong in appearance, while infectious/inflammatory uptake is usually more diffuse and linear (Fig. 9). MRI may be used for ambiguous cases. In addition, osseous remodeling after fracture will show increased FDG uptake and should be differentiated from osseous metastasis [63]. CT and FDG patterns are key to differentiate these two etiologies. If the FDG uptake is less intense and extends linearly along the fracture line, then insufficiency fracture is likely. Conversely, if the FDG uptake is more intense with a corresponding sclerotic or lytic osseous lesion on CT, then osseous metastasis should be considered (Figs. 10, 11). In cases of no CT correlate, the pattern of FDG uptake can be helpful. Multiple areas of FDG uptake make metastasis more likely. However, an isolated FDG-avid lesion can be due to both benign and malignant etiologies. For example, case report that pigmented villonodular synovitis and solid aneurysmal bone cyst can demonstrate increased FDG uptake and mimic malignancy [64, 65]. In these situations, MRI would be helpful to differentiate.
Fig. 9.
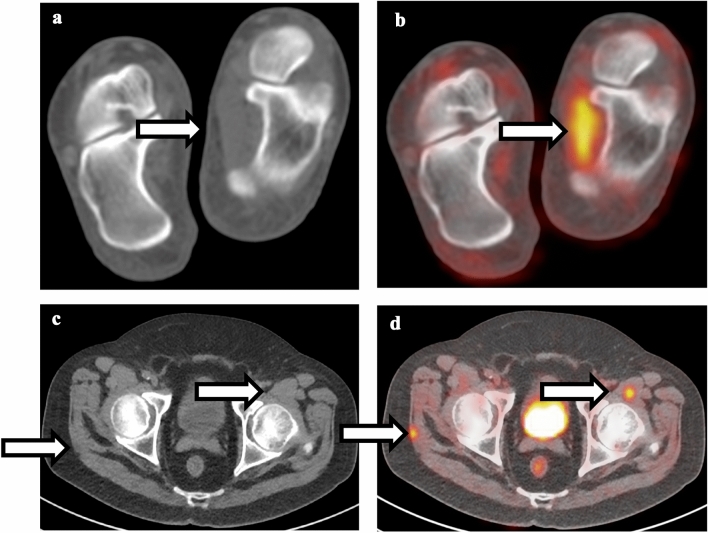
Comparison between muscle strain and intramuscular metastatic disease. a Non-contrast CT of the foot shows normal appearance of the left abductor hallucis muscle. b Fusion of PET and CT shows linear hypermetabolism associated with the left abductor hallucis muscle, compatible with muscle strain. c Non-contrast CT of the pelvis shows subtle lesions in the right gluteus maximum and left iliopsoas muscle. d Fusion of PET and CT shows focal intense FDG uptake associated with these lesions, compatible with intramuscular metastatic disease
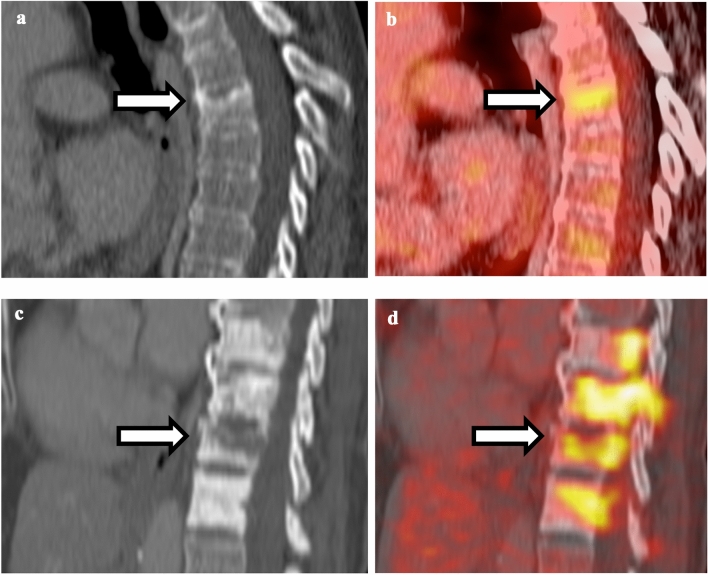
Fig. 10.
Comparison between vertebral body insufficiency and pathological fracture. a Non-contrast CT of the thoracic spine shows compression fracture of superior endplate of mid-thoracic vertebral body without sclerotic changes. b Fusion of PET and CT shows mild hypermetabolism associated with the superior endplate fracture, compatible with insufficiency fracture. c Non-contrast CT of the thoracic spine shows multiple sclerotic vertebral bodies with lucent fracture line in one of the vertebral bodies. d Fusion of PET and CT shows intense FDG uptake associated with fracture line as well as adjacent sclerotic vertebral bodies, compatible with pathological fracture
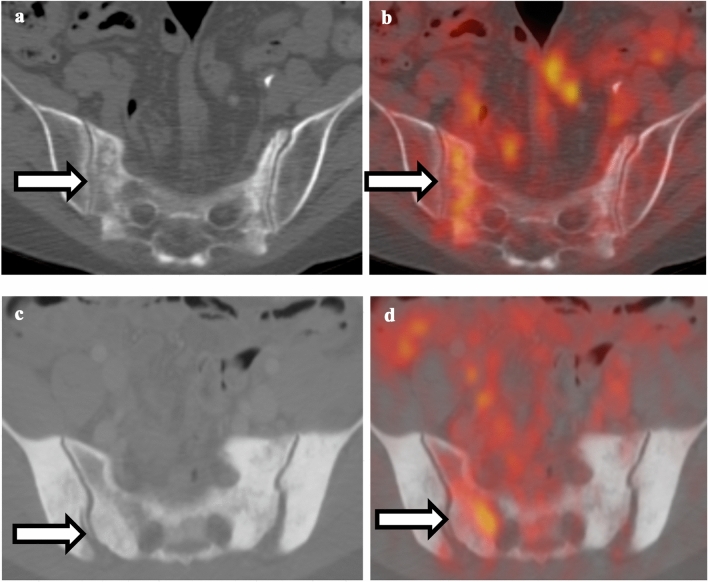
Fig. 11.
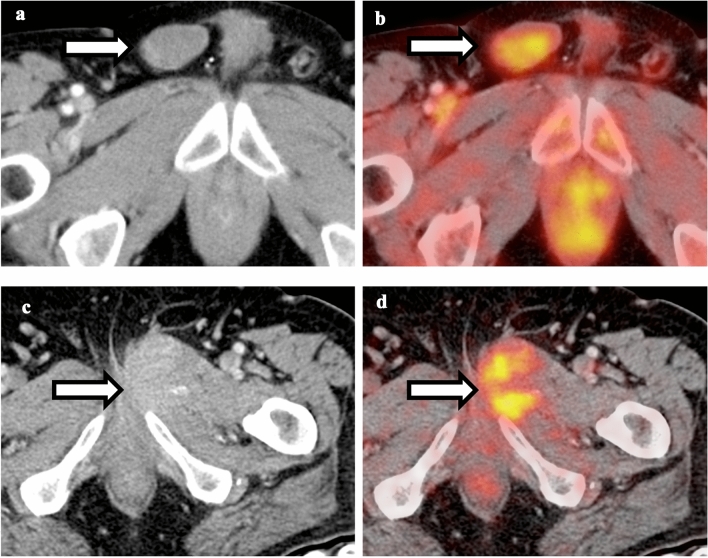
Comparison between sacral insufficiency and pathological fracture. a Non-contrast CT of the pelvis shows right sacral fracture. b Fusion of PET and CT shows hypermetabolism associated with the right sacral fracture. Given the relatively normal appearance of the rest of the sacrum, this is compatible with insufficiency fracture. c Non-contrast CT of the pelvis shows diffuse sclerosis of the sacrum and bilateral iliac bones. d Fusion of PET and CT shows hypermetabolism in the right sacrum; given diffuse sclerosis, this is compatible with pathological fracture
Many cutaneous and subcutaneous lesions can demonstrate increased 18F-FDG uptake, including common processes such as sebaceous cysts and acne vulgaris to less common entities such as hidradenitis suppurativa [66•,67]. Fortunately, direct inspection of the skin is easily done clinically and can assist to determining the etiology of abnormal uptake; thus, these scenarios rarely cause diagnostic conundrum.
Conclusion
18F-FDG PET/CT is a routinely performed imaging modality for cancer staging and evaluation of treatment response. A variety of conditions other than malignancy can cause increased FDG uptake and should be always be considered when reviewing these complex patients. Ways to differentiate acute findings from cancer including time course of change, co-existing findings on CT, patient’s symptom and lab values, primary tumor type, and intensity of hypermetabolism. The interpreting physician need be aware and familiar with the acute causes of focal uptake to avoid diagnostic error and route the patient to the appropriate treatment.
Compliance with Ethical Guideline
Conflict of interest
SC.B.—(1) Cancer Targeted Technology (CTT)—Research Grant (2) Advanced Accelerator Applications (AAA)—Advisory Board. Y.Li—No conflict of interest.
Footnotes
This article is part of the Topical collection on Nuclear Medicine & PET/CT Imaging.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Recently published papers of particular interest have been highlighted as: • Of importance
- 1.Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation–current and emerging clinical applications. Clin Radiol. 2015;70(7):787–800. doi: 10.1016/j.crad.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25(5):1357–1368. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 3.Metser U, Miller E, Lerman H, Even-Sapir E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence. AJR Am J Roentgenol. 2007;189(5):1203–1210. doi: 10.2214/AJR.07.2083. [DOI] [PubMed] [Google Scholar]
- 4.Wong PS, Lau WF, Worth LJ, Thursky KA, Drummond E, Slavin MA, Hicks RJ. Clinically important detection of infection as an’ incidental’ finding during cancer staging using FDG-PET/CT. Intern. Med. J. 2012;42(2):176–183. doi: 10.1111/j.1445-5994.2011.02450.x. [DOI] [PubMed] [Google Scholar]
- 5.Bakheet SM, Powe J. Benign causes of 18-FDG uptake on whole body imaging. Semin Nucl Med. 1998;28(4):352–358. doi: 10.1016/s0001-2998(98)80038-x. [DOI] [PubMed] [Google Scholar]
- 6.•Matthew, VR et al. Lung masses of unusual histologies mimicking malignancy: flurodeoxyglucose positron emission tomography-computed tomography appearance. Indian J Nucl Med. 2019;34(4):295–301, Enlisted variety of benign neoplasms and inflammatory lesions that can mimic pulmonary malignancy, discussed their metabolic and morphologic characteristics and provided a pathophysiological basis for their FDG uptake. [DOI] [PMC free article] [PubMed]
- 7.Mascarenhas NB, Lam D, Lynch GR, Fisher RE. PET imaging of cerebral and pulmonary Nocardia infection. Clin Nucl Med. 2006;31(3):131–133. doi: 10.1097/01.rlu.0000200597.42832.39. [DOI] [PubMed] [Google Scholar]
- 8.Kapucu LO, Meltzer CC, Townsend DW, Keenan RJ, Luketich JD. Fluorine-18- fluorodeoxyglucose uptake in pneumonia. J Nucl Med. 1998;39(7):1267–1269. [PubMed] [Google Scholar]
- 9.Kono M, Yamashita H, Kubota K, Kano T, Mimori A. FDG PET imaging in pneumocystis pneumonia. Clin Nucl Med. 2015;40(8):679–681. doi: 10.1097/RLU.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 10.Qin C, Liu F, Lan X. 18 F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47(5):1281–1286. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Y, Lei L, Zhang W. The potential added value of FDG PET/CT for COVID-19 pneumonia. Eur J Nucl Med Mol Imaging. 2020;21:1–2. doi: 10.1007/s00259-020-04767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash P, Kalra MK, Digumarthy SR. FDG PET/CT in assessment of pulmonary lymphangitic carcinomatosis. Am J Roentgenol. 2010;194:231–236. doi: 10.2214/AJR.09.3059. [DOI] [PubMed] [Google Scholar]
- 13.Flavell RR, Behr SC, Pampaloni MH. The incidence of pulmonary embolism and associated FDG-PET Findings in IV contrast-enhanced PET/CT. Acad Radiol. 2014;21(6):718–725. doi: 10.1016/j.acra.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Kubota K, Matsuda H. Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med. 2009;23:671–676. doi: 10.1007/s12149-009-0292-y. [DOI] [PubMed] [Google Scholar]
- 15.Widmann G, Nguyen VA, Plaickner J, Jaschke W. Imaging features of toxicities by immune checkpoint inhibitors in cancer therapy. Curr. Radiol. Rep. 2016;5(11):59. doi: 10.1007/s40134-017-0256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raad RA, Kannan R, Madden K, Pavlick A. Ipilimumab-induced organizing pneumonia on 18F-FDG PET/CT in a patient with malignant melanoma. Clin Nucl Med. 2017;42(7):e345–e346. doi: 10.1097/RLU.0000000000001673. [DOI] [PubMed] [Google Scholar]
- 17.Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics. 2015;35(2):424–437. doi: 10.1148/rg.352140121. [DOI] [PubMed] [Google Scholar]
- 18.Baha A, Yildirim F, Kokturk N, Akdemir UO, Demircan S, Turktas H. 18F-FDG uptake in focal organising pneumonia mimicking bronchial carcinoma. Clin. Respir. J. 2016;10(6):740–745. doi: 10.1111/crj.12280. [DOI] [PubMed] [Google Scholar]
- 19.Lewis PJ, Salama A. Uptake of fluorine-18-fluorodeoxyglucose in sarcoidosis. J Nucl Med. 1994;35(10):1647–1649. [PubMed] [Google Scholar]
- 20.Zivin S, David O, Lu Y. Sarcoidosis mimicking metastatic breast cancer on FDG PET/CT. Intern Med. 2014;53(21):2555–2556. doi: 10.2169/internalmedicine.53.3333. [DOI] [PubMed] [Google Scholar]
- 21.Prabhakar HB, Rabinowitz CB, Gibbons FK, O’Donnell WJ, Shepard JA, Aquino SL. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am. J. Roentgenol. 2008;190(3):S1–S6. doi: 10.2214/AJR.07.7001. [DOI] [PubMed] [Google Scholar]
- 22.Makis W, Palayew M, Rush C, Probst S. Disseminated multi-system sarcoidosis mimicking metastases on (18)F-FDG PET/CT. Mol Imaging Radionucl Ther. 2018;27(2):91–95. doi: 10.4274/mirt.29200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss N, Solomon SB. Talc pleurodesis mimics pleural metastases differentiation with positron emission tomography/computed tomography. Clin Nucl Med. 2003;28(10):811–814. doi: 10.1097/01.rlu.0000089522.86184.49. [DOI] [PubMed] [Google Scholar]
- 24.Vandemoortele T, Laroumagne S, Astoul P, et al. Positive FDG-PET/CT of the pleura twenty years after talc pleurodesis: three cases of benign talcoma. Respiration. 2014;87(3):243–248. doi: 10.1159/000356752. [DOI] [PubMed] [Google Scholar]
- 25.Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. Radiographics. 2007;27(1):145–159. doi: 10.1148/rg.271065080. [DOI] [PubMed] [Google Scholar]
- 26.Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol. 2012;198(3):W304–W314. doi: 10.2214/AJR.11.7130. [DOI] [PubMed] [Google Scholar]
- 27.Akkas BE, Ucmak Vural G. Fat necrosis may mimic local recurrence of breast cancer in FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2013;32(2):105–106. doi: 10.1016/j.remn.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Shin KM, Kim HJ, Park JY. Incidental breast lesions identified by 18F-FDG PET/CT: which clinical variables differentiate between benign and malignant breast lesions? J Breast Cancer. 2015;18(1):73–79. doi: 10.4048/jbc.2015.18.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.•BetancourtCuellar SL, Palacio D, Benveniste MF, Carter BW, Gladish G. Pitfalls and misinterpretations of cardiac findings on PET/CT imaging: a careful look at the heart in oncology patients. Curr Probl Diagn Radiol. 2018. Reviewed the variations of normal cardiac 18F-FDG uptake observed in oncology patients and the appearances of other patterns of pathologic metabolic activity, such as post-therapeutic effects after radiation therapy, systemic diseases, or cardiomyopathy that may lead to false-negative and false-positive results.
- 30.Lobert P, Brown RK, Dvorak RA, Corbett JR, Kazerooni EA, Wong KK. Spectrum of physiological and pathological cardiac and pericardial uptake of FDG in oncology PET-CT. Clin Radiol. 2013;68(1):e59–e71. doi: 10.1016/j.crad.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Bachmeyer C, Kerrou K, Chosidow O, Frances C, Montravers F. 18-F fluorodeoxyglucose positron emission tomography indicating unsuspected infections in two patients with dermatomyositis. Clin Exp Dermatol. 2009;34(8):e769–e771. doi: 10.1111/j.1365-2230.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 32.Hannah A, Scott AM, Akhurst T, Berlangieri S, Bishop J, McKay WJ. Abnormal colonic accumulation of fluorine-18-FDG in pseudomembranous colitis. J Nucl Med. 1996;37(10):1683–1685. [PubMed] [Google Scholar]
- 33.Kim BW, Kim HW, Won KS, Kang YN, Choi BW. Colonic parasitic infection mimicking peritoneal seeding on 18F-FDG PET/CT. Clin Nucl Med. 2017;42(8):e365–e366. doi: 10.1097/RLU.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 34.Koff SG, Sterbis JR, Davison JM, Montilla-Soler JL. A unique presentation of appendicitis: f-18 FDG PET/CT. Clin Nucl Med. 2006;31(11):704–706. doi: 10.1097/01.rlu.0000242723.61455.a8. [DOI] [PubMed] [Google Scholar]
- 35.Rayamajhi SJ, Gorla AKR, Basher RK, Sood A, Mittal BR. Unsuspected active ulcerative colitis in a patient with dermatomyositis: a rare association detected on (18)F-FDG PET/CT during the search for an occult malignancy. Indian J Nucl Med. 2017;32(2):130–132. doi: 10.4103/0972-3919.202238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaz SC, Oliveira C, Castanheira JC, Silva AF, Costa DC. 18F-FDG uptake in ischemic colitis during follow-up of a patient with lung cancer. Clin Nucl Med. 2017;42(8):e367–e370. doi: 10.1097/RLU.0000000000001723. [DOI] [PubMed] [Google Scholar]
- 37.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197(6):W992–W1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 38.Steenkemp DW, McDonnell M, Meibom S. Metformin may be associated with false-negative cancer detection in the gastrointestinal tract on PET/CT. Endocr Pract. 2014;20(10):1079–1083. doi: 10.4158/EP14127.RA. [DOI] [PubMed] [Google Scholar]
- 39.Hsu WL, Chang SM, Wu PY, Chang CC. Localized autoimmune pancreatitis mimicking pancreatic cancer: case report and literature review. J Int Med Res. 2018;46(4):1657–1665. doi: 10.1177/0300060517742303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng L, Xing H, Li F, Huo L. Focal autoimmune pancreatitis mimicking pancreatic cancer on FDG PET/CT imaging. Clin Nucl Med. 2018;43(1):57–59. doi: 10.1097/RLU.0000000000001901. [DOI] [PubMed] [Google Scholar]
- 41.Dong A, Dong H, Zhang L, Zuo C. Hypermetabolic lesions of the pancreas on FDG PET/CT. Clin Nucl Med. 2013;38(9):e354–e366. doi: 10.1097/RLU.0b013e3182708503. [DOI] [PubMed] [Google Scholar]
- 42.Kitazono MT, Colletti PM. FDG PET imaging of acute cholecystitis. Clin Nucl Med. 2006;31(1):23–24. doi: 10.1097/01.rlu.0000191567.16067.83. [DOI] [PubMed] [Google Scholar]
- 43.Yu JQ, Kung JW, Potenta S, Xiu Y, Alavi A, Zhuang H. Chronic cholecystitis detected by FDG-PET. Clin Nucl Med. 2004;29(8):496–497. doi: 10.1097/01.rlu.0000132952.14515.9c. [DOI] [PubMed] [Google Scholar]
- 44.Albano D, Bosio G, Bertoli M, Petrilli G, Bertagna F. Hepatosplenic Candidiasis Detected by (18)F-FDG-PET/CT, Asia Ocean. J. Nucl. Med. Biol. 2016;4(2):106–108. doi: 10.7508/aojnmb.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavailloles FA, Mure A, Nasser H, Lecapitaine AL, Granier F. Multiple liver amoebic abscesses detected on FDG PET/CT. Clin Nucl Med. 2014;39(1):79–80. doi: 10.1097/RLU.0b013e31828e98ad. [DOI] [PubMed] [Google Scholar]
- 46.Cheng G, Torigian DA, Alavi A. FDG PET/CT and MRI findings in a patient with focal xanthogranulomatous pyelonephritis mimicking cystic renal malignancy. Clin Nephrol. 2011;76(6):484–486. doi: 10.5414/cn106762. [DOI] [PubMed] [Google Scholar]
- 47.Jung JS, Lee SM, Kim HJ, Jang SH, Lee JW. A case of septic pulmonary embolism associated with renal abscess mimicking pulmonary metastases of renal malignancy. Ann Nucl Med. 2014;28(4):381–385. doi: 10.1007/s12149-014-0811-3. [DOI] [PubMed] [Google Scholar]
- 48.McCammack KC, Hawkes NC, Silverman ED, Paz DA. PET/CT appearance of acute pyelonephritis. Clin Nucl Med. 2013;38(7):e299–e301. doi: 10.1097/RLU.0b013e31827a218d. [DOI] [PubMed] [Google Scholar]
- 49.Subramanyam P, Palaniswamy SS. Dual time point (18)F-FDG PET/CT imaging identifies bilateral renal tuberculosis in an immunocompromised patient with an unknown primary malignancy. Infect Chemother. 2015;47(2):117–119. doi: 10.3947/ic.2015.47.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YI, Kim SK, Lee JW, Lee SM, Kim TS. Ovarian mass mimicking malignancy: a case report. Nucl Med Mol Imaging. 2010;44(4):290–293. doi: 10.1007/s13139-010-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansberg R, Ho B, Bui C. False Positive FDG PET/CT of recurrent testicular tumor due to orchitis. Mol Imaging Radionucl Ther. 2014;23(1):28–30. doi: 10.4274/Mirt.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakheja R, Makis W, Hickeson M. Bilateral tubo-ovarian abscess mimics ovarian cancer on MRI and (18)F-FDG PET/CT. Nucl Med Mol Imaging. 2011;45(3):223–228. doi: 10.1007/s13139-011-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhargava P, Zhuang H, Alavi A. Hiatal hernia mimics centrally necrotic cancer in the lung on FDG positron emission tomographic imaging. Clin Nucl Med. 2003;28(4):347–349. doi: 10.1097/01.RLU.0000057621.02252.42. [DOI] [PubMed] [Google Scholar]
- 54.Cronin CG, Harsinghani MG, Blake MA. Multitechnique imaging findings of prolene plug hernia repair. Am J Roentgenol. 2010;195:701–706. doi: 10.2214/AJR.09.4142. [DOI] [PubMed] [Google Scholar]
- 55.GW Cowell, LK Thomson, M. Digby, FW Poon. Acute portal vein thrombosis: an important mimic of malignancy on FDG PET/CT. BMJ Case Rep. 10.1136/bcr.07.2011.4482. [DOI] [PMC free article] [PubMed]
- 56.Liu YY. Large idiopathic mesenteric necrotizing granuloma mimicking metastatic disease on FDG PET/CT. Clin Nucl Med. 2020 doi: 10.1097/rlu.0000000000002958. [DOI] [PubMed] [Google Scholar]
- 57.Makis W. Progressing sclerosing mesenteritis (Mesenteric Panniculitis) mimics progression of malignancy after neoadjuvant chemotherapy for gastri adenocarcinoma on serial 18F-FDG PET/CT. Clin Nucl Med. 2016;41:313–316. doi: 10.1097/RLU.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 58.SaadAldin E, Sekar P, SaadEddin Z, Keller J, Pollard J. Incidental diagnosis of sternoclavicular septic arthritis with Moraxella nonliquefaciens. IDCases. 2018;12:44–46. doi: 10.1016/j.idcr.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaha H, Onomura M, Nishikuramori Y. Pyogenic vertebral osteomyelitis in a breast cancer patient: report of a case. Surg Today. 2012;42(10):1022–1025. doi: 10.1007/s00595-012-0158-0. [DOI] [PubMed] [Google Scholar]
- 60.Chen SJ, Wang XY, Hua FC, Guan YH. Detection of multiple muscle involvement in eosinophilic myositis with (1)(8)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40(8):1297. doi: 10.1007/s00259-013-2412-0. [DOI] [PubMed] [Google Scholar]
- 61.Bennett O, Ravi Kumar AS, Agnew J. Focal inflammatory myositis on 18F-FDG PET/CT. Clin. Nucl. Med. 2016;41(6):469–471. doi: 10.1097/RLU.0000000000001218. [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Zheng X, Lin J, Tang K. A case of intramuscular cryptococcosis mimicking a malignant tumor on FDG PET/CT. Clin Nucl Med. 2019;44(7):12569–12574. doi: 10.1097/RLU.0000000000002488. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Wang Q, Qiu L. MS 18F-FDG PET/CT in a patient with pregnancy and lactation-associated osteoporosis. Clin Nucl Med. 2018;43:742–743. doi: 10.1097/RLU.0000000000002182. [DOI] [PubMed] [Google Scholar]
- 64.Elumogo CO, Kochenderfer JN, Civelek AC, Bluemke DA. Pigmented villonodular synovitis mimics metastases on fluorine 18 fluorodeoxyglucose position emission tomography-computed tomography. Quant Imaging Med Surg. 2016;6(2):218–223. doi: 10.21037/qims.2016.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matcuk GR, Jr, Chopra S, Menendez LR. Solid aneurysmal bone cyst of the humerus mimics metastasis or brown tumor. Clin Imaging. 2018;52:117–122. doi: 10.1016/j.clinimag.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 66.•Metser U, Tau N. Benign cutaneous and subcutaneous lesions on FDG-PET/CT. Semin Nucl Med. 2017;47(4):352–361. Reviewed various benign cutaneous and subcutaneous conditions encountered in routine clinical practice and discussed morphologic features of the lesion on CT to help differentiate them from malignancy. [DOI] [PubMed]
- 67.Asamoah P, Wale DJ, Viglianti BL, Wong KK, Gross M. Multiple hypermetabolic subcutaneous lesions from hidradenitis suppurativa mimicking metastases on 18F-FDG PET/CT. Clin Nucl Med. 2018;43(1):73–74. doi: 10.1097/RLU.0000000000001911. [DOI] [PubMed] [Google Scholar]