Abstract
A simple method for the preparation of bromoferrocenes from stannylferrocenes is described: the preparation of 1,1′-dibromoferrocene is used as an example. Stannylferrocenes are reacted with bromine directly in dichloromethane to give bromoferrocenes in a self-indicating titration reaction. Also, an enhanced method of removal of the highly soluble organotin tin by-products formed in the preparation of 1,1′-diiodoferrocene from 1,1′-tri-n-butylstannylferrocene is reported allowing the preparation of large-scale quantities of 1,1′-diiodoferrocene, which is one of the most important starting materials in ferrocene chemistry.
Keywords: Inorganic chemistry, Ferrocene, Iodoferrocene, Synthesis, Purification, Bromoferrocene
Inorganic chemistry; Ferrocene; Iodoferrocene; Synthesis; Purification; Bromoferrocene.
1. Introduction
Halogenoferrocenes are particularly useful synthons in the preparation of a broad range of useful ferrocenes such as ferrocenylamines [1, 2], diisocyanoferrocenes [3], trifluorothioferrocenes [4], and of course in the metathesis reaction to lithioferrocenes [5, 6] (although lithioferrocenes to halogenoferrocenes is more common) which lead to hundreds of ferrocene-based compounds. Of the halogenoferrocenes arguably the most important are iodo- and bromo-ferrocenes because they are more reactive in standard organic coupling reactions, [7, 8]. There has been much debate on the synthesis of iodoferrocenes concerning the most useful and viable synthetic methodology some of which are summarised for just one representative compound, 1,1′-diiodoferrocene, Figure 1 [9, 10, 11, 12, 13, 14, 15, 16, 17].
Figure 1.
General synthetic routes to diiodoferrocene, A-E.
All the synthetic methods shown in Figure 1, use iodine as a synthon reacting with a ferrocene-metal or ferrocene-metalloid compound although clearly there are other quenching reagents such as iodine monobromide, iodine monochloride, diiodotetrafluoroethane (route A (ii), yields 90–95%)∗, and N-iodosuccinimide (35–40%)∗ which can replace iodine (60–65%)∗(∗yields quoted are those we have routinely obtained based on many synthetic attempts). The very first preparations of iodoferrocenes were made using the reactions of ferrocenyl mercuric chlorides (route E, Figure 1) which requires the preparation of the poorly soluble bis mercury salt which is difficult to crystallise and the reaction of lithioferrocenes with iodine (route A(i), Figure 1) which were reported by Rausch and co-workers, [9, 10]. Additionally, ferrocene based organometallic reagents such as Grignard reagents or ferrocenylboronic acids may be directly reacted with iodine (routes B and D, Figure 1). Both these routes require the formation of a precursor compound which is itself difficult to obtain pure without product loss. The synthetic route A (ii) using di-iodotetrafluoroethane was by far the best synthetic route as a cheap supply of this quenching reagent was available in the early 1990's but the industrial synthesis was halted. The reaction with iodine on large scale worked reasonably well although it was imperfect; nevertheless, we and others used the lithiation route routinely in the early 1980s although many by-products such as 1,1′′′′-di-iodobiferrocene, 7, Figure 2, which results from metathesis, were formed, [14]. Of course in some instances the metathesis reaction itself can be useful, for example, when [Fe(CO)2CpI] is used to quench 1,1′-dilithioferrocene to give the iron-substituted iodobiferrocene, 8, in good yields (20–25%) together with [Fe(CO)2Cp]2 and small quantities of other iodoferrocenes.
Figure 2.
Biferrocenes 7 and 8 obtained with iodine in coupling reaction of 1,1′-dilithioferrocenes.
The separation and purification of iodoferrocenes was carried out using column chromatography. This served well as an undergraduate teaching exercise to clean up the crude product but later a commercial temperature gradient sublimation apparatus was used to purify the iodoferrocenes. In response to the poor-quality synthesis we developed a new synthesis which used stannylferrocenes as intermediates, Figure 1, C [16]. This synthetic method is a cheap and high-yielding but it does suffer from one major disadvantage which is when the product is difficult to crystallise, i.e. it has a low melting point and is non-polar, so it is difficult to remove the tin by-products and trace ferrocene. Despite this difficulty the method has been well used, [18, 19, 20, 21]. In the original work the tin by-products were removed by adding fluoride, which turns them into a polymeric material which could be filtered off. However, the large-scale use of fluoride is not ideal because of its high toxicity. Two papers describe the clean-up methods [22, 23] for removal of trace ferrocene by selective oxidation which is a particularly elegant solution to the first problem. Given the inherent importance of iodoferrocenes it was decided to re-examine the second problem, i.e. the removal of the by-product, tri-n-butylstannyl iodide, on scales greater than 5g. Corey had proposed a method to remove similar alkyltins from organic reaction mixtures using mixed metal salts [24] but this turned out to be synthetically difficult because of the nature of the reagents and scales involved. We now report a solution to this problem which is simple, effective and the synthetic method has been developed into an alternative synthesis of bromoferrocenes too. It should be noted that (trimethylstannyl)ferrocenes also react with iodine to give iodoferrocenes and may be less problematic in the workup because of their reduced solubility, however we have focussed on the use of more readily available low cost n-butyltins to produce an efficient synthesis.
2. Results and discussion
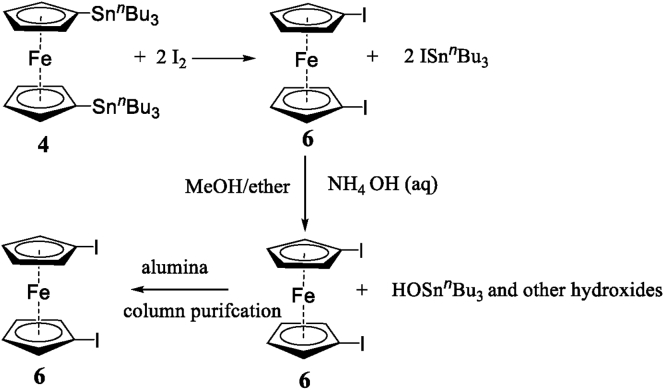
The preparation of tri (n-butylstannyl)ferrocenes from the reaction of lithioferrocenes with chloro-tri-n-butyltin is a routine procedure which can be carried out on very large scale in a broad range of dry solvents [25]. Following an exhaustive review of the literature where tin halides were unwanted by-products (and many trials) it was decided to attempt the use of aqueous sodium hydroxide or ammonia solutions to remove the tin containing compounds. (Supplementary Figures S1 and S2) This initially gave limited success but subsequently we developed the method using biphasic conditions to obtain pure iodoferrocenes. The method is summarised in Figure 3.
Figure 3.
Schematic of synthetic steps in 1,1′-diiododferrocene preparation and purification. The progress of iodination reaction, step 1, may be followed by NMR (Figure S3).
1,1′-Bis-tri-n-butylstannylferrocene, which may be prepared and used in situ, (1H NMR spectrum shown in Figure S4) was reacted with iodine in dichloromethane and the product was vigorously stirred overnight with aqueous ammonia with the addition of some methanol to enhance the reagent miscibility. A fine white precipitate removed. It is known that trimethyltin halides react with NaOH to form the corresponding tin hydroxides and also that trimethyl tin iodide reacts with trimethyl tin hydroxide to give a white crystalline solid, [(Me3SnOH)2SnMe3]+ I−. A similar chemistry is expected in this case where methyl substituents are replaced with n-Bu groups. The reason ammonium hydroxide is more efficient in the clean-up process than using aqueous sodium hydroxide is almost certainly a case of better miscibility of the reaction solvents. It is possible that bis-(tri-n-butyltin)oxides, such as (nBu3Sn)2O are also formed. Thus, the filtered solution was added to alumina and the solvent was removed to give a free-flowing sample. This solid was loaded onto a standard alumina column and the product was eluted with petrol as a yellow orange band to give pure 1,1′-di-iodoferrocene. (1H NMR, Figure S5). The remaining tin containing by-products adhere to the column and are apparent as a pale green and deep green bands allowing the elution of pure di-iodoferrocene with petrol (column photograph, Figure S6). The deep green band was eluted with copious amounts of diethyl ether however it turns yellow instantly on contact with air and after solvent removal it was clear that it contained only trace amounts of iodoferrocenes. We assume it is an oxidised ferrocene which accounts for the intense deep green colour. The reason for the adherence to the column is assumed to be that the remaining by-products are complex alkyltin hydroxides as described earlier. The reaction of 1,1′-bis-(tri-n-butylstannyl)ferrocenes with iodine monobromide likewise produces 1,1′-di-iodoferrocenes, again relatively cleanly with the formation of the by-product tri-n-butylstannylbromide. This was not too surprising – the only surprise was that the reaction was so clean with minimum halide scrambling. The reaction is faster than that of the corresponding reaction with iodine. The latter observation led us to examine the reaction of bromine with 1,1′-bis-(tri-n-butylstannyl)ferrocene, which proceeds even more rapidly and thus can be carried out as a titration reaction in dichloromethane: this effectively removes the possibility of oxidation of ferrocenes with excess bromine, Figure 4.
Figure 4.
Simple synthesis of 1,1′-dibromoferrocene from the 1,1′-tri-(n-butyl-stannyl)ferrocene24 using bromine.
When a bromine solution in dichloromethane is added to a dichloromethane solution of stannylferrocenes a deep green/black spot of oxidised ferrocene is instantaneously produced in the solution caused by local oxidation of the ferrocene but this quickly disappears within seconds or with rapid stirring. This means that the reaction may be carried out as a self-indicating titration. The product 1,1′-dibromoferrocene, 9, (1H NMR spectrum, Figure S7) does not suffer from the purification problems of 1,1′-di-iodoferrocene because it is so easy to crystallise from methanol or hexanes. As noted earlier the use of trimethyltin chloride as an alternative quenching reagent gives less soluble reaction products however the low cost of tri-n-butyltin chloride means that on large scale this is the reagent of choice and of course this clean-up method will also ameliorate the purification step with this reagent. Clearly the use of a surfactant as a phase transfer reagent could further enhance the removal of the tin containing by-products by increased solvent miscibility, but this was not found to be necessary in this case.
Supplementary Material Available: Figures (S1–S7) showing NMR spectra of the reaction compounds and a photograph of typical chromatographic column.
3. Summary
An improved method of producing pure 1,1′-di-iodoferrocene on a scale up to 100g has been developed. Adaption of this method can be used for the formation of bromoferrocenes. Although the clean-up method has focussed on diiodoferrocene it will have broad applicability towards other reactions containing alkyltin halide by-products.
4. Experimental section
All reagents used were of reagent grade. Note: Rubber gloves should be worn when working with alkyltin compounds because of their toxicity.
4.1. 1,1′-diiodoferrocene preparation
The general method given is suitable for scales between 5g and 50g ferrocene; the following is an example on 100mmol scale. All reactions are carried out under an inert atmosphere whereas workup methods are carried out under normal laboratory conditions. When using aqueous ammonium hydroxide it is particularly important to work in fume hood, neutralising any excess before disposal.
1,1′-Dilithioferrocene/TMEDA was prepared in hexanes as follows: a solution of n-butyllithium in hexanes (88 mL of a 2.5M solution) was added to a slurry of ferrocene (18.6g, 100mmol) and excess TMEDA (12.0g, 103 mmol) in hexanes (500ml). The mixture was stirred overnight, (min 12h. and it is important that stirring does not stop and a heavy-duty magnetic stirrer at slow speed is always used). The reaction flask is the immersed in an external cooling bath of acetone and the acetone is cooled to ca. -75 °C by addition of either dry ice or liquid nitrogen. Stirring is restarted and maintained throughout. A mixture of chloro-tri-n-butyltin (71.5g, 220 mmol) and diethyl ether (100mL) hexanes is then added dropwise over a period of 20 min to the reaction mixture while the cooling bath is maintained at -75 °C. The mixture was the allowed to warm to room temperature without removal of the cooling bath over a period of 2–3h. before being re-cooled to ca -30 °C. It was then hydrolysed slowly by the gradual addition of water (300mL) initially dropwise, increasing volumes as the hydrolysis proceeded. The organic layer was separated by addition of further diether ether (100mL) and the organic layer was separated. A further extraction was performed on the aqueous layer with diethyl ether 200mL and the organic layers were combined vacuum filtered through a loose plug of celite (in a 15cm Buchner funnel) and dried over stirred anhydrous magnesium sulfate. Volatiles and solvent were subsequently removed on a rotary evaporator to furnish a thick amber colored oil of the crude product 1,1′-bis-(tri-n-butylstannyl)ferrocene. This oil was dissolved in dichloromethane (250 ml) and this was treated incrementally with excess freshly ground iodine (2.2 equiv. I2). [slow addition is recommended as an exotherm occurs]. The slurry was stirred in the dark overnight under nitrogen in the dark, [16]. Following the addition of diethyl ether (250 ml) a saturated aqueous sodium thiosulfate solution was slowly added (again addition should be gradual as an exotherm occurs) to the rapidly stirred solution until the black color permanently discharged. The organic layer is then separated in a separatory funnel by addition of more diethyl ether and water if required to facilitate layer separation. The volatiles were then removed to leave a still wet oil of 1,1′-diiodoferrocene, iodo-tri-n-butyltin and other alkyltins. This amber oil was extracted with four times with methanol (4 × 100 mL) which leaves a thick oily residue of the tin compounds. The methanol fractions were separated from the oily residue by decantation, combined and ether (200ml) was added to these. Water was added (300 ml) followed by an aqueous solution of 50% ammonium hydroxide, 100ml. This forms 2 layers. The rapid mixing of this mixture was continued overnight. The key to this reaction is rapid agitation throughout to ensure the partially immiscible layers mix thoroughly. The organic layer is separated by addition of diethyl ether as required to facilitate layer separation and any residual fine white powder still present is removed by slow vacuum filtration through a magnesium sulfate plug (large Buchner funnel, ca 20cm diameter with 6 cm depth plug of magnesium sulfate, covered with a filter paper) which also dries the solution. Following complete removal of volatiles, column chromatography {column diameter 9cm, min 45 cm length with alumina (neutral Brockmann grade I) or silica gel for column chromatography (60Å particle size)}. [the diiodoferrocene is dissolved in dichloromethane 100ml in a round bottom flask suitable for use on a rotary evaporator and alumina 100g is added]. The solvent is then removed on a rotary evaporator which has loosely plugged cotton wool in the solvent reflux trap top to avoid bumping of the powder. The dry powder is added to the top of the column. The flow rate is that using gravity alone due to the weight of the solvent above the column. Hexanes, hexane or pet. ether (b.pt 60–80) are used as the eluent as even addition of 1% diethyl ether rapidly speeds up the elution of the trace by-products. The first yellow band is the product diiodoferrocene. (this may take 6–8hr to collect depending on flow rate as it is important not to apply pressure). The product may be collected as fractions and checked for purity by NMR spectroscopy. If there is trace residual ferrocene this may be removed by the method of Long and co-workers [23], but this is not usually required where diiodoferrocene is to be used as a reactant as trace ferrocene does not usually pose a problem in subsequent transformations. Final drying is performed on a high vacuum line. Product yields are generally 85–94%.
As discussed, the preceding general method is for reaction scales starting with greater than 5g of ferrocene as in small scale reactions trituration with methanol and column chromatography on alumina results in product isolation.
4.2. 1,1′-Dibromoferrocene
(General method): the solution of 1,1′-bis(tri-n-butylstannyl)ferrocene is dissolved in dichloromethane and the solution is treated with a solution of bromine (2.2 mol equiv.; 1.1 equiv, Br2) in dichloromethane (1:5 by vol) by slow addition. After each increment allow a few seconds for the color to go deep green/back to yellow/orange. When the first permanent brown color exists let stand and add a small quantity aqueous sodium thiosulfate solution and agitate well to ensure complete bromine removal. Separate the dichloromethane layer, remove the dichloromethane and re-dissolve the product in hexane or ether, filter through a plug of magnesium sulfate and alumina (layer of alumina on top of magnesium sulfate) to remove any oxidised by-products. Following complete solvent removal, the product can be crystallised from methanol or hexanes at low temperature; (dissolved at room temperature, filter if required and cool overnight). On large scales the oil which forms will crystallise at room temperature if left for 1 day. Yields are generally >80%.
It is noted that if bromine is added one drop at a time to the centre of an unstirred solution of bis-tri-n-butylstannylferrocene then the disappearance of the blue/black spot that forms in the centre is an excellent teaching demonstration.
Declarations
Author contribution statement
Ian R. Butler: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yassin T. H. Mehdar, Zahraa S. Al-Taie: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We gratefully acknowledge the provision of bench space provide by Dr P. Murphy and the help of Dr David Hughes with NMR experimentation.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Heliyon Supplmentary NEW
References
- 1.Shafir A., Power M.P., Whitener G.D., Arnold J. 1,1′-Diaminoferrocene. Organometallics. 2000;19:3978–3982. [Google Scholar]
- 2.Leonidova A., Joshi T., Nipkow D., Frei A., Penner J.E., Konatschnig S., Patra M., Gasser G. An environmentally benign and cost-effective synthesis of aminoferrocene and aminoruthenocene. Organometallics. 2013;32(6):2037–2040. [Google Scholar]
- 3.Siemeling U., Rothera D., Bruhna C. “Schizoid” reactivity of 1,1′-diisocyanoferrocene. Chem. Commun. 2007:4227–4229. doi: 10.1039/b709063c. http://www.rsc.org/suppdata/cc/b7/b709063c/b709063c.txt [DOI] [PubMed] [Google Scholar]
- 4.Hess J., Konatschnig S., Morard S., Pierroz V., Ferrari S., Spingler B., Gasser G. Novel, mercury-free synthetic pathway for trifluoromethylthio-substituted metallocenes. Inorg. Chem. 2014;53:3662–3667. doi: 10.1021/ic403169z. [DOI] [PubMed] [Google Scholar]
- 5.Butler I.R., Davies R.L. A rapid convenient synthesis of ferrocene-based triphos analogue ligands. Synthesis. 1996:1350–1354. [Google Scholar]
- 6.Lai L.-L., Dong T.-Y. A novel method to synthesize unsymmetrical disubstituted ferrocenes. J. Chem. Soc., Chem. Commun. 1994:2347–2348. [Google Scholar]
- 7.Imrie C., Loubser C., Engelbrecht P., McCleland C.W. The use of a modified Suzuki reaction for the synthesis of monoarylferrocenes. J. Chem. Soc., Perkin Trans. 1. 1999;17:2513–2523. [Google Scholar]
- 8.Neuse E.W., Loonat M.S. Synthesis of ferrocenylruthenocene. Transition Met. Chem. 1981;6:260–263. [Google Scholar]
- 9.Rausch M.D. Ferrocene and related organometallic π-complexes. IV. some ullmann reactions of haloferrocenes1. J. Org. Chem. 1961;26:1802–1805. [Google Scholar]
- 10.Roling P.V., Rausch M.D. Formation of 1,2-oligomeric ferrocenes from ullmann reactions of iodoferrocenes. J. Organomet. Chem. 1977;141:195–204. [Google Scholar]
- 11.Roling P.V., Rausch M.D. Formation and characterization of 1,2-diiodoferrocene and related derivatives. J. Org. Chem. 1974;39:1420–1424. [Google Scholar]
- 12.Shechter H., Helling J.F. Ferrocenyl and 1,1’-ferrocenylene grignard reagents1a. J. Org. Chem. 1961;26:1034–1037. [Google Scholar]
- 13.Fish R.W., Rosenblum M. A convenient synthesis of some haloferrocenes. J. Org. Chem. 1965;30:1253–1254. [Google Scholar]
- 14.Kamounah F.S., Christensen J.B. A new preparation of iodoferrocene. J. Chem. Res. (S) 1997;(4) 150-150. [Google Scholar]
- 15.Inkpen M.S., White A.J.P., Albrecht T., Long N.J. Rapid sonogashira cross-coupling of iodoferrocenes and the unexpected cyclo-oligomerization of 4-ethynylphenylthioacetate. Chem. Commun. 2013;49:5663–5665. doi: 10.1039/c3cc43116a. [DOI] [PubMed] [Google Scholar]
- 16.Butler I.R., Wilkes S.B., McDonald S.J., Hobson L.J., Taralp A., Wilde C.P. A convenient preparation of iodoferrocenes. Polyhedron. 1993;12:129–131. [Google Scholar]
- 17.Roemer M., Nijhuis C. Syntheses and purification of the versatile synthons iodoferrocene and 1,1′-diiodoferrocene. Dalton Trans. 2014;43:11815–11818. doi: 10.1039/c4dt01787k. [DOI] [PubMed] [Google Scholar]
- 18.Debroy P., Roy S. Recent advances in the synthesis and properties of ferrocenes having an unsaturated backbone. Coord. Chem. Rev. 2007;251:203–221. [Google Scholar]
- 19.Guillaneux D., Kagan H.B. High yield synthesis of monosubstituted ferrocenes. J. Org. Chem. 1995;60:2502–2505. [Google Scholar]
- 20.Park P., Lough A.J., Foucher D.A. Copper-mediated polycondensations of substituted diiodoferrocenes and bis(stannyl)ferrocenes: synthesis and properties of soluble polyferrocenylenes containing trimethylsilyl or methyl groups. Macromolecules. 2002;35:3810–3818. [Google Scholar]
- 21.Yuasa A., Sasamori T., Hosoi Y., Furukawa Y., Tokitoh N. Synthesis and properties of stable 1,2-bis(metallocenyl)disilenes: novel d–π conjugated systems with a Si=Si double bond. Bull. Chem. Soc. Jpn. 2009;82:793–805. [Google Scholar]
- 22.Goeltz J.C., Kubiak C.P. Facile purification of iodoferrocene. Organometallics. 2011;30:3908–3910. [Google Scholar]
- 23.Inkpen M.S., Du S., Driver M., Albrecht T., Long N.J. Oxidative purification of halogenated ferrocenes. Dalton Trans. 2013;42:2813–2816. doi: 10.1039/c2dt32779a. [DOI] [PubMed] [Google Scholar]
- 24.Edelson B.S., Stolz B.M., Corey E.J. A simple and effective procedure for removal of tri-n-butyltin halides from reaction mixtures. Tetrahedron Lett. 1999;40:6729–6730. [Google Scholar]
- 25.Wright M.E. 1,1’-Bis(tri-n-butylstannyl)ferrocene: selective transmetalation applied to the synthesis of new ferrocenyl ligands. Organometallics. 1990;9:853–856. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heliyon Supplmentary NEW