Highlights
-
•
High birthweight significantly increases risk of urinary incontinence after delivery.
-
•
Increasing birthweight (>3500 and >4000 g) leads to increasing risk of incontinence.
-
•
The association is also found in separate analyses on vaginal delivery, primiparous, and stress UI.
Abbreviations: UI, urinary incontinence; OR, odds ratio; CI, confidence interval
Keywords: birthweight, childbirth, postpartum, puerperium, urinary incontinence
Abstract
Birthweight and urinary incontinence after childbirth: a systematic review and meta-analysis
Stian Langeland WESNES, Elin SEIM MD
Urinary incontinence (UI) is common after childbirth. Many cohort and cross-sectional studies have reported data on birthweight, but results have not been pooled. It is unclear how birthweight affects UI after childbirth. The objective is to review the effect of birthweight on UI after childbirth through meta-analyses.
Searches were performed in Medline, Embase, Svemed+, ClinicalTrials.gov, Cochrane, and Cinahl in August 2016. Additional reference checking was performed. Included articles evaluated birthweight as a possible risk factor for maternal UI. We included articles that were presented in Norwegian, Danish, Swedish, or English. Two independent reviewers extracted the data and analysed it using Review Manager 5.3 software. Available data from included studies on birthweight (≥4000 g and ≥3500 g, respectively) and UI were combined in meta-analyses. PRISMA and MOOSE guidelines were used.
Eighteen studies (N = 30 070) reported data on birthweight >4000 g vs <4000 g. Birthweight>4000 g compared to weight <4000 g was associated with a significantly increased OR of any UI (OR 1.49, 95% CI 1.24 – 1.80). Five studies (N = 15 066) reported data on birthweight >3500 g vs <3500 g. Birthweight>3500 g was also associated with a significantly increased OR of UI (OR 1.26, 95% CI 1.15 – 1.37).
High birthweight appears to increase OR of UI after childbirth. Preventative strategies should be targeted towards women at particular risk.
1. Introduction
Urinary incontinence (UI) is a common problem after childbirth. Prevalence estimates vary from 14 – 45% [1]. A systematic review reported pooled prevalence of any UI to be 32-36% three months postpartum [2]. Reviews on epidural [3], episiotomy [4], cesarean section [2] and instrumental childbirth [5] have clarified their association with UI postpartum. There is inconsistency in the literature regarding remaining birth parameters. By identifying significant risk factors for UI among women after childbirth, future research can identify and validate preventive measures that can be targeted towards these women. Many cohort and cross-sectional studies have reported data on birthweight, but results have not been pooled. In studies using electromyography heavier babies have been associated with evidence of pudendal nerve damage in the pelvic floor after vaginal birth [6], with uncertain clinical significance. The objective of this study was to review the literature to identify studies reporting on the association of high birthweight on urinary incontinence after all modes of childbirth, and to perform meta-analyses on the association of high birthweight on UI after childbirth. If birthweight can be isolated as a risk factor for postpartum urinary incontinence, patients at particular risk can be identified.
2. Material and methods
Literature searches were done in Medline, Embase, Svemed+, and Cinahl. Additional search was done in ClinicalTrials.gov and Cochrane Database of Systematic Reviews. A librarian from the University in Bergen assisted in the search in May 09. 2014 and August 24. 2016. Additional search was done May 03. – 14.2017. The search included the following MESH terms and free text; urinary incontinence, leak, urine, bladder, delivery, obstetric, postpartum, postpartum period, puerperium, birthweight, infant, new-born, large, small, SGA, LGA. Abstracts and articles in Norwegian, Danish, Swedish, or English were considered. Both conference abstracts and full publications were included. Additional literature was added based on authors’ knowledge and after reading references in identified literature. Grey literature was not identified.
In the four-part PICO question for this systematic review, we compared women who gave birth with birthweight >4,000 g or >3,500 g, to women giving birth with birthweight <4,000 g or <3,500 g. The outcome was any UI, and stress UI after childbirth.
Search was done in headings and abstracts. Birthweight was sometimes one of several risk factors included in sub-analysis in papers, often not presented in the abstract, and thereby not found by the search strategy. Additional articles were added based on the authors’ knowledge of relevant literature, and after reading references. Identified literature was reviewed separately by both authors. Articles evaluating obstetric risk factors for maternal urinary incontinence in title or abstract were reviewed in full by both authors. When discrepancies between the two authors occurred (Seim, Wesnes), the article was discussed with a third researcher (Rortveit, see Acknowledgement). Criteria for inclusion were that the article or conference abstract evaluated birthweight as a possible risk factor for maternal urinary incontinence, with results presented in Norwegian, Danish, Swedish, or English The process for selecting studies is presented in Table 1. Information about origin, study design, response rate, number of participants, method of data collection, adjusted results, time of UI, mode of childbirth, BMI, weight of new-born, age, parity, and main findings for all included studies were extracted.
Table 1.
Flowchart of included and excluded studies.
 |
No reviews of this topic were identified. For obvious reasons, no randomized controlled studies (RCT) on birthweight and UI have been conducted. A considerable number of cohort studies and cross-sectional studies of high quality have been performed. Even though RCT’s provide the highest level of evidence, a summary of results from cohort studies and cross-sectional studies will be essential in order to evaluate a possible causal association between birthweight and UI.
2.1. Birth weight
Mean birth weight in Europe and USA [7] is approximately 3500 g. Weight cut-off at 3500 g and 4000 g gives information on the association between UI and birthweight beyond mean, as well as extreme birthweight, respectively. Weight cut-off on 3500 g and 4000 g were most common in identified studies, and were therefore chosen for this review.
Birthweight in one study was originally analysed according to the 50th and 90th percentile for birthweight (3,541 g and 4,180 g, respectively). These data have been re-categorized into 3500 g and 4000 g, and data has thereafter been reanalysed and stratified for mode of childbirth. Data were adjusted for BMI and weight loss after childbirth [8]. Birthweight in one study was categorized according to birthweight quartiles [9]; birthweight >3925 g from this study was included in analyses on the association between birthweight >4000 g and UI. Boyles et al used pounds [10]; birthweight of >8 lb. (3639 g) from this study was included in analyses on the association between birthweight >3500 g and UI.
2.2. Urinary incontinence
Information on UI after childbirth was categorized as questionnaires, objective testing, structured interviews, phone interviews, or reviews of existing medical records. No minimum cut-off for frequency, amount or severity of UI was set to be included in this article. Stress UI is more common after childbirth than urgency UI and mixed UI. The prevalence of pure stress UI is reported to be 2 – 8 times higher than the prevalence of pure urgency UI in pregnancy [1]. The stress/urgency ratio is reduced postpartum as prevalence of stress UI decline. Several studies focus solely on stress UI [[11], [12], [13], [14]], but few studies have data on urgency UI. Data on any UI was used in this meta-analysis. Separate analyses on available data on stress UI were also performed.
2.3. Assessment of quality and bias
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Initiative has published recommendations on how to report data in cohort and cross-sectional studies [15]. STROBE was used to assess methodological quality. There was a high threshold to exclude studies. Studies with insufficient methodological information on study design, setting, statistical methods, and study participants were excluded from the systematic review and meta-analysis. Selection bias can affect the meta-analytic estimate. Selection bias was therefore considered in studies used in the meta-analyses, based on setting, study population and response rate. Risk of selection bias was considered as low, medium, high, and unclear. Information on studies included in meta-analyses regarding selection bias, adjustment of effect estimates, and reporting of all data (effect estimate, N in exposed and unexposed group, N with UI and continence) were collected. Funnel plot asymmetry based on standard error (log [OR]) was used to explore possible reporting biases.
2.4. Data synthesis
Birthweight was categorized as a dichotomous variable with two categories; <4000 g vs >4000 g, and <3500 g vs >3500 g, respectively. Available original data from included studies on birthweight (≥4000 g and ≥3500 g, respectively) and UI were extracted. Adjusted effect estimates were extracted when available. Unadjusted effect estimates were used when adjusted estimates were not presented; raw data and absolute numbers were then converted to unadjusted OR. Relative risk in one study was treated as OR [16]. To enable comparison across studies, Log [OR] with SE were calculated for each included study. Results were pooled and combined in meta-analyses. Estimates were inserted into Review Manager 5.3 for meta-analyses.
To reduce diversity in study characteristics, separate sub-group meta-analyses were performed according to type of UI, mode of delivery, primiparous women, and UI 3 – 18 months postpartum. Mode of delivery was categorized as any vaginal delivery or any CS. Main findings on the association between birthweight and UI are presented as odds ratio, and in Forest plot figures. Both adjusted data and unadjusted data are presented in Forest plot figures.
Heterogeneity among studies was assessed by I2. An I2 of 0% to 40% represents minimal heterogeneity, while 75% to 100% represents considerable heterogeneity. Adjusted data and unadjusted data had in general moderate to substantial heterogeneity in effect estimates. Random effect estimates were therefore used.
The review was registered in PROSPERO (73021); NHS’ International prospective register of systematic reviews. The review adheres to the PRISMA guidelines and MOOSE guidelines for meta-analyses and systematic reviews of observational studies.
3. Results
A total of 477 articles were identified. Fifteen external articles were added based on the authors’ knowledge of relevant literature, and after reading references. A total of 385 articles remained after removing duplicates (Table 1). Fifty-seven articles (N = 164,600) were included in this systematic review. Descriptive data are presented in Table 2. Twenty-two articles had data that could be included in the meta-analyses. Descriptive data are presented in Table 3.
Table 2.
Descriptive data on studies included in the systematic review.
| Origin | Design | Asso- ciation | N | Data gathering | Respons-rate | Adjust. | Time point of UI | Birth-weight | Parity | Age | BMI | Delivery | Main finding | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altaweel [21] * | Saudi Arabia | Cross-sectional | ++ | 2,180 | Quest.: UDI-6, IIQ-7 | 30 % | Yes | > 4 kg | All | 30 ± 10 | All | SVD and CS | Birthweight of baby >4 kg OR 1.7 (1.4–2). Not data on type or severity by birthweight. | |
| Arya [41] | USA | Cohort | - | 315 | Telephone interview and questionnaire. IIQ-7 | Yes | 2 weeks, 3 months, 1 year after delivery | Primiparous | 21-23 | SVD 48% | On univariate analysis, there was no association between the presences of urinary incontinence at any follow-up period after delivery infant birthweight. Hazard Ratio: 1,0 (95% CI 0,9-1,1) | |||
| Forceps 29% Vacuum 24% | ||||||||||||||
| Baracho [42] | Brazil | Cross-sectional | ++ | 192 | Delivery charts. Interview, ICIQ-SF, physical exam | Yes | 5-7 mth pp | >2.988 kg | Primiparous | 23,2 | BMI>25: 39/192 | SVD | Newborn weight (g), mean (SD) among women with UI: 3,206.4 (364.8). Among women without UI: 3,128.3 (372.8) 0.14*. Significant finding. Sign. association between SUI and weight >2.988 g | |
| Boyles [10] * | USA | Cross- sectional | ++ | 5,599 | Quest. | 39 % | Yes | 3-6 mth pp | > 8 lb. | Primiparous, continent before pregn. | 27 | 24 | CS 27%. Forceps/vacuum 13% | Increasing UI with birthweight >8 lb., but only among women delivering by vaginal delivery: adjusted OR 1.22, (1.03–1.45). OR 0.84 (0.53–1.35) among women delivering by CS. |
| Brown [22] * | Australia | Cross- sectional | ++ | 1,336 | Statewide postal survey. | 62 % | Yes | 6-7 mth pp | >4 kg | All | SVD 69%. Forceps 11%. Emergency CS 9%. Elective CS 9%. | Infants weighing ≥ 4000 g associated with higher rates of urinary incontinence (37/196 [18.9%] versus 101/1097 [9.2%], OR 2.29 [95% CI 1.5-3.5]). Associations of assisted vaginal births controlling for duration of labor, birthweight of infant and perineal trauma. Infant birthweight: <3000 g, 3000-3999 g, >4000 g: Adjusted OR for UI: 1.90 [1.2-3.1] | ||
| Burgio [43] | USA | Cohort | - | 523 | Interview day 2. and 3, week 6 and 3, 6, and 12 mth pp. | Yes | 6 weeks – 12 mth pp. | Mixed, parity 1,9 | 28,6 | Heaviest previous birthweight OR 0.999, 95% CI 0.990- 1.008. p = 793 | ||||
| Cardo [44] | Spain | Cohort | - | 272 | Interviewed at term and 4 months pp. ICQ-SF and KHQ | 4 mth pp | Mixed | 31,8 | SVD 62%. Forceps 4%. Vacuum 21%. CS 21%. |
When only vaginal delivery is analyzed, no statistical association with newborn's weight was found. | ||||
| Caseym [23] * | USA | Cohort | + | 3,887 | Interview | 37 % | Yes | 5-7 mth pp | > 4 kg | Primiparous | 22,5 | BMI 30 | Univariate analyses: Birthweight >4000 g in 279 women (7%). Among these Urge UI 14 (9%, OR 1.3 (0.7- 2.3)). Stress UI 10 (7%, OR 1.0 (0.5- 1.9)). Adjusted analyses: association between stress UI and weight >4000 g: OR 1.2: 0.6 – 2.3. | |
| Castillo [45] | Spain | Cohort | - | 243 | Quest; ICIQ-SF | 6 mth pp. | Mixed | 29,9 | BMI 26,2 | VD 66%. | No statistically significant differences were found between a worsening on quality of life and birthweight | |||
| Chaliha [46] | UK | Cohort | + | 549 | Interview, examination | 100 % | 3 mth pp | Mean 3.37 kg ± 0.49 | Primiparous | 29 | SVD 53%. CS 24% |
Fetal weight ass with urge UI: OR 11.3 95% CI 0.4- 352.8. Stress UI: OR 2.5 95% CI 1.1- 6.1 | ||
| Chou [47] | Taiwan | Cross-sectional | - | 378 | Interview by telephone | Yes | 1 year pp | Mean 3.116 kg | Primiparous | 28,1 | BMI 27,0 | Vaginal 48%. CS 52% | Vaginal delivery: Birth body weight OR 0.999 (95% CI 0.997- 1.002, p = 0.543) for incident stress UI. When CS: Birth body weight OR 0.997 (95% CI 0.997- 1.002, p = 0.543) for incident stress UI (identical to vaginal delivery) | |
| Connolly [48] | USA | Cross-sectional | - | 3,205 | Interview. Sandvik SI score >3. | 36 % | Yes | > 4 kg | Mixed | 49,2 | Vaginal | There was an overall difference in the odds of moderate/ severe UI between the <4,000 g group, the ≥4,000 g group. | ||
| Diez-Itza [24] * | Spain | Cohort | - | 376 | Interview | Yes | 6 weeks pp | > 4 kg | Mixed | 32,4 | Vaginal | Urgency only. Birthweight > 4 kg were not associated with UUI 6 weeks postpartum (OR 0.6, 95% CI 0.05 - 3.10) | ||
| Diez-Itza [17] | Spain | Cohort | - | 272 | Interview | Yes | 2 years pp | Primiparous, continent before pregn. | 31,2 | BMI 23,4 | Vaginal 86%. CS 14%. |
Incident stress UI. No stress UI 2 years postpartum: Mean birthweight 3306. Stress UI 2 years postpartum: Mean birthweight 3281. P = 0.74. | ||
| Dimpfl [31] * | Germany | Cohort | - | 350 | Interview | No | 6 and 12 weeks pp. | 3.5 kg | Continent before pregn. | Vaginal 83%. CS 17% | Mothers, who gave birth to infants with a birthweight above 3500 g (permanent SUI: 4.7%) had no significantly higher incidence of pp UI than mothers with infants under 3500 g (7.0%). Chi’ = 0.22-n.s. | |||
| Dolan [32] * | UK | Cross- sectional | -/+ | 1,861 | Sheffield Pelvic Floor Questionnaires. | 62 % | Yes/No | 20 years after delivery | Mean 3.285 kg | Parity 1,6 | 45,7 | 24,8 | Vaginal 86%, CS 13,9 | Adjusted OR for UI 12 years after delivery in primiparous < 3000 g OR 1.32 (0.73-2.39). 3000 – 3500 g Ref. > 3500 g OR 1.05 (0.59-1.86). Adjusted OR for UI 12 years after delivery in parous: < 3000 g OR 1.25 (0.96-1.62). 3000 – 3500 g Ref. > 3500 g OR 1.21 (0.97- 1.52). |
| Eason [25] * | Canada | Cohort | - | 949 | Quest. Info collected during a RCT of perineal massage during the 3. trimester. | 79 % | Yes | 3 mth pp | 4 kg | Mixed | 28,6 | CS 18% | Baby's weight (g): <4000 N: 837 Risk: 31% OR 1.00. Baby's weight (g): ≥4000 N 112 Risk: 30% Crude OR 0.96 95% CI 0.73-1.48 | |
| Eftekhar [49] | Iran | Cohort | ++ with frequen-cy | 702 | Quest. at prenatal visit week 28-29. | 70 % | 4 mth pp | 3 kg | Primiparous continent before pregn. | Vaginal 51%. CS 49% | Stress UI. A birthweight greater than 3000 g appeared to be associated with increased frequency of SUI P = 0.000; x2 = 22.5. | |||
| Emanuela [50] | Italy | Cohort | - | 93 | Clinical examinations before delivery and at 3 and 6 months pp. | 100 % | 3 and 6 mth pp | 32,6 | Newborn weight did not show statistical differences in continent and incontinent patients | |||||
| Farrell [37] | Canada | Cohort | - | 484 | Questionnaire and hospital charts. | 83,50 % | No | 6 weeks and 6 mth pp | Mean 3.489 kg | Primiparous | 28 | CS 25%. SVD 56%. Instrumental 19%. |
Birthweight (kg) continent women: 3458 g, incontinent women: 3425 g, not significant difference. | |
| Frias [51] | Spain | Cohort | + | 89 | Sandvik questionnaire, ICIQ-SF, PISQ-12 | 2 mth | 53,7% primiparous | 31,3 | 28,3 | Eutocic 68%. Forceps 4% CS 28%. | More women with UI had babies >3000 g than women without…. 84% of women with UI had a newborn weight >3000 g compared with a rate of 60% of women without UI. No statistical differences. | |||
| Fritel [52] | France | Cohort | - | 307 | Questionnaire | 46% | No | 4 years | 4 kg | Primiparous | 29,3 | 21,3 | CS 21% Forceps 36% |
Univariate comparisons between women with current SUI and those with no SUI found no significant association between current birth weight |
| Gartland [26] * | Australia | Cohort | + | 1,283 | hospital records, quest and telephone interviews | 28–31% | No | 3, 6, 9, 12 and 18 mth pp | 4 kg | Primiparous, continent before pregnancy | 31 | SVD 31%. CS 21%. Instr 32%. |
Persistent UI 4 – 18 months postpartum. Birthweight were not significantly associated with persistent UI. Birthweight (g) <2500 OR 1.41Birthweight (g) 2500–3999 OR 1.0 (ref). Birthweight (g) ≥ 4000 OR 1.32. | |
| Glazener [38] | Aberdeen; Scotland, Birmingham; England, Dunedin, NZ | Cross-sectional | ++ for persistent UI starting in pregn. | 3,405 | Questionnaire survey in Maternity units and obstetric case note data | 70-84% | Yes | 3 mth pp | Mean 3.296 kg. Used quartiles. | Primiparous | 26,7 | SVD 58%, CS 17%, Instr 25% |
Incontinence first occurring during pregnancy and still present at 3 months was associated with heavier babies (birthweight in top quartile, OR 1.56, 95% CI 1.12-2.19). Incident UI after delivery: < 3 kg Ref. 3.00–3.35 kg OR 1.26. 3.36–3.69 kg OR 1.42. ≥ 3.70 kg OR 1.33. Persistent UI starting during pregnancy: < 3 kg Ref. 3.00–3.35 kg OR 1.33. 3.36–3.69 kg OR 1.45. ≥ 3.70 OR 1.56. |
|
| Grodstein [53] | USA | Cohort(?) | - | 83,168 | Nurses’ health study | Yes | Late in life | All | 60,4 | 20 | For birthweight of the heaviest child, little association with UI. Somewhat lower risks with infant of >10.5 pounds at birth compared with <8.5 pounds. Risk for UI: <3.86 kg OR 1.00. 3.86-4.3 kg OR 1.03. 4.35-4.76 kg OR 1.05. > 4.76 kg OR 0.97. | |||
| Groutz [11] * | Israel | Cross-sectional | ++ | 300 | Interview | 100%? | 3 days p | 3.5/4 kg | 100 nulliparous. 100 primiparous. 100 ≥ para 5 | 20 - 43 | VD only | No correlation between birthweight of the first newborn and prevalence of persistent, non-pregnancy-related stress urinary incontinence. Prevalence of persistent, stress UI among grand multiparous women delivering at least one baby >4000 g was 29.4%. Prevalence of persistent stress UI among grand multiparous whose newborns did not weigh more than 4,000 g was significantly lower (16.7%, P < 0.05). | ||
| Groutz [54] | Israel | Cross-sectional | - | 363 | Interview, hospital charts | 1 year pp | mean | Primiparous continent before preg. | 28-32 | 60-63 kg | SVD and CS | Birthweight among continent women: 3240 ± 408. Birthweight among incontinent women: 3330 ± 330. No significant difference. | ||
| Gyhagen [35] | Sweden | Cross-sectional | + | 5,236 | Questionnaire and birth registry | 65 % | Yes | 22 years pp | 4.5 kg | Primiparous | 50-53 | 26 | VD 76% CS 24%. |
Weight > 4 500 g compared to < 4 500 g among CS. OR 0.66 (95% CI 0.33–1.29). Weight > 4 500 g compared to < 4 500 g among VD 1.23 (95% CI 0.87–1.76). The risk of UI after VD vs CS increased with increasing birthweight. |
| Hatem [18] * | Canada | Cross-sectional | - | 1,291 | Questionnaire | 52 % | 6 months pp | 4 kg | Primiparous | 27,20 | 25,2 | Mix | No association between birthweight > 4000 g and UI: OR 0.63 (0.30–1.31) | |
| Hvidman [19] * | Denmark | Cross-sectional | - | 376 | Questionnaire | 1 % | Yes | Few days pp and 3 mth pp | Mixed | 29 | CS 9%. Instr. 7% |
Risk of UI first days PP and 6 mth pp OR 1.0 pr 500 g in adjusted analyses. Adjusted OR for UI first days PP 1.0. OR for UI > 4 weeks PP 1.2 (not sign). Adjusted OR for UI ≥ 12 weeks pp 1.1 (not sign). | ||
| Iwanowicz [12] * | Poland | Cross-sectional | ++ | 313 | Women treated for stress UI; medical history and urodynamic test. | 4 kg | Mixed | 50-53 | The probability of the occurrence of SUI is statistically higher after vaginal delivery of a baby with birthweight of 4000 g or more. 45% of women with UI and 34% of women without UI had babies >4000 g (sign finding). | |||||
| Kashanian [55] | Iran | Cohort | - | 1,400 | Questionnaire | 1 year pp | VD 400. ECS 600. Acute CS 400 | There was no significant difference between the women with SUI and without according to neonatal weight | ||||||
| Koveleva [9] | Russian Federation | Cohort | ++ for mixed UI | 518 | Interview | 4 mth pp | Mean | All | 30,1 | VD and CS | Mean weight of the newborn in group of patients with mixed UI was 3544 + 519 g, in the control group and 3173 + 740 g, (p < 0.01). A relative risk of occurrence mixed UI in group of women with weight of the newborn above 3544 + 519 g was higher (RR = 1,38; 95% CI - 1,02 to 1,85; p < 0,05). | |||
| Krue [13] * | Denmark | Cross-sectional | + | 119 | Questionnaire | 89 % | No | 6-30 mth pp | 4 kg | Mix | >30 | VD | Birthweight >4000 g vs <4000 g; stress UI 34% vs 31%, urge UI 6% vs 4%, mixed UI 15% vs 11%: p > 0.10. In the group whose infant birthweight was 4000 g or more the prevalence of stress incontinence 6–30 months postpartum was higher than in the <4000 g group (34.0% vs. 30.6%) (p > 0.10) | |
| Mallah [20] * | Iran | Cohort | ++ | 441 | Examination, medical records | Yes | 3 mth pp. | 4 kg | Primiparous | 28,1 | 31,5 | Mix | The incidence of UI was higher in cases of vaginal delivery and birthweight greater than 4 kg. OR 4,8 (95% CI 3,0 - 7,7) | |
|
Marsh [56] |
UK | Cross-sectional | - | 324 | Questionnaire | 3 mth pp. | Mean 3.586 kg | 82% primiparous | VD | Birthweight was not associated with increased risk of developing stress urinary incontinence | ||||
| McKinnie [57] | USA | Cross-sectional | ++ | 978 | Questionnaire | Yes | 42,7 | VD | For each additional 16 ounces of infant weight delivered vaginally, the OR for UI increased by 1.13 (1.06–1.20). | |||||
| Obioah [27] * | Nigeria | Cohort | ++ | 230 | Questionnaire interview | Yes | 6 weeks, 3 mth pp | 80% multipara, continent before pregn. | 31,4 | SVD 90% | Birthweight > 4 kg significantly associated with UI 3 months postpartum OR 5.60 (1.21–25.92) | |||
| Rørtveit [14] * | Norway | Cross-sectional | ++ | 11,397 | Questionnaire and birth registry | 80 % | Yes | 4 kg | VD | Significant associations between any UI and birthweight ≥ 4000 g (OR 1.1, 95% CI 1.0-1.2); moderate or severe incontinence OR 1.0 (0.9-1.2). ≥ 4000 g also associated with stress UI. | ||||
| Samuelsson [28] * | Sweden | Cross-sectional | + | 487 | Questionnaire, gyno.examin. | 76 % | Yes | Quar-tiles | Mixed | 39 | 65 kg | Mixed | There were no significant correlations with birthweights >3925 g. | |
| Schytt [16] * | Sweden | Cohort | ++ | 2,390 | Questionnaire Swedish Birth Register | 53 % | Yes/no | 1 year pp | 3.5-4 kg | 44% primiparous. Strati-fied on primiparous. | 30,5 | VD 79%. CS 13%. Instr 13%. |
Birthweight >3500 g was associated with stress UI in multiparas (RR 1.4, CI 95% 1.1–1.7). Within the vaginal group: infant birthweight >3500 g (RR 1.3; CI 95% 1.1–1.6). There was no association in multivariate analyses. Some results are adjusted. | |
| Seshan [58] | India | Cross-sectional | + | 598 | Questionnaire | Yes | Mixed | 20-60 | The weight of the largest baby delivered had the strongest impact on predicting UI symptom severity (UISS) | |||||
| Solans-Domenech [29] * | Spain | Cohort | + | 1,128 | Questionnaire | No | 7 weeks pp | > 4 kg | Continent, nulliparous women | CS 20%. VD 80%. |
UI among 12/56 women with baby >4000 g, UI among 140/832 among women with baby <4000 g. Adjusted HR for incident UI postpartum among women with baby >4000 g: 2.8 (0.9–8.4) | |||
| Thom [30] * | USA | Retro-spective cohort | ++ | 1,521 | Questionnaire, interview, abstraction of labor and delivery records. | Yes | > 4 kg | 56 | VD | Weekly UI significantly associated with weighing 4,000 g or more (OR 1.47, 95% CI 1.16–1.86). When analyzed as a continuous variable, greatest birthweight showed evidence of a threshold effect with an increase in the risk of UI associated with increasing birthweight above about 3,200 g. | ||||
| Torkestani [34] | Iran | Case-control | - | 250 | Questionnaire gyno.exam. | Yes | Mix | 33-40 | Mix | OR 0.928. 95% CI 0.43-2.00 for association with birthweight. | ||||
| Van Brummen [59] | The Netherlands | Cohort | - - | 344 | Questionnaire | 723 % | Yes | 3 and 12 mth pp | 3,418 vs 3,549 | Nulliparous | 30-31 | 21-26 | VD 83%. CS 17%. |
Infant birthweight 3,418 vs 3,549 as risk factor for urgency 1 year pp among women delivering by VD: adjusted OR 0.9 (0.98–0.99). No association was found for stress UI or urge UI. |
| Viktrup [60] | Denmark | Cohort | + | 305 | Questionnaire | 12 mth pp | Mix | VD 82%. CS 18%. |
Birthweight was increased in infants of mothers who developed stress UI after deliver, but not significantly: p = 0,07 | |||||
| Volloyhaug [61] | Norway | Cross-sectional | + | 1,641 | Questionnaire | Yes | Mean 20 years | Parous, mean 2,3 | 47 | 25,8 | VD 42% OD 42% CD 14% | Parity and the largest infant’s birthweight were additional independent risk factors for UI but did not remain significant in a multivariable logistic regression analyzes. | ||
| Wesnes [62] * | Norway | Cohort | ++ | 5,219 | Questionnaire, birth registry | 45 % | Yes | 6 mth pp | 50/ 90 percentile. Re-analyzed on 3,5/4 kg | Primiparous continent before- and during pregn. | 27 | 23,6 | SVD only | Baby's birthweight between the 50th - 90th percentile (3541 - 4180 g) and > 90th percentile (> 4,180 g) were statistically significant risk factors for incident UI 6 months postpartum (OR 1.4; 95% CI 1.2 - 1.6 and OR 1.6; 95% CI 1.2 - 2.0, respectively) as compared to birthweight below the 50th percentile. Data reanalyzed for 3500 g and 4000 g. |
| Williams [63] | UK | Retro-spective, cross-sectional | ++(stress) -- (urge) | 482 | Questionnaire | 23 % | 12 mth pp. | Birthweight was associated with incident stress UI (spearman r coefficient r = 0,04) and protective on incident urge UI (r coefficient r= - 0,04) | ||||||
| Wu [64] | China | Cross-sectional | + + | 2,500 | Interview | 43,5 | Mix | Fetal weight was associated with stress UI only OR 1,64 (95% CI 1.27–2.13), p < 0,001 for macrocosmic infant compared with normal birthweight | ||||||
| Yang [65] | China | Cohort | - | 1,889 | Telephone interview | Yes | 6 mth pp | Primiparous | 30,6 | 72,9 kg | VD 45%. CS 55%. |
No association between neonate birthweight and SUI, UUI or MUI. | ||
| Yohay [66] | Israel | Cohort | - | 37 | Questionnaire medical records, telephone interview | 32 % | 3 mth pp | 3.344 kg | Multiparous mean 2,7 | 30,8 | SVD 73% CS 23% OD 4% |
Other obstetrical parameters including episiotomy and birthweight were not found to be significantly associated with any of the PFD items. | ||
| Yip [67] | Hong Kong | Cohort | - | 148 | Telephone interview | 4 years pp. | 3.2 kg | Nulliparous, continent before pregn. | 27-28 | VD 100% | The logistic regression analysis showed that birthweight was not significantly associated SUI 4 years after the index pregnancy- | |||
| Zanelli [68] | Italy | Cohort | ++ | 452 | Questionnaire | 3 and 12 mth pp | Statistical correlation with incontinence 3 months postpartum was found for high fetal weight | |||||||
| Zhang [69] | China | Cross-sectional | ++ | 4,684 | Questionnaire | 72 % | Yes | 1,1 | 40 | 21,9 | VD 80% CS 20%. |
A multiple logistic regression analysis showed fetal birthweight was common potential risk factors for LUTS (OR 1.40, 1.07–1.85), voiding (OR 1.42, 1.08–1.87) and storage symptoms (OR 1.63, 1.16–2.28). | ||
| Zhu [70] | China | Cross-sectional | - | 5,221 | Interview | ? | Yes | Birthweight was not identified as potential risk factors of female SUI. |
Preg = pregnancy. PP = postpartum. Quest = questionnaire. SVD = spontaneous vaginal delivery. CS = cesarean section. VD = vaginal delivery. Instr = instrumental delivery. OD = operative delivery. Adj. = adjusted analyses. OR = odds ratio. RR = relative risk. Mth = months. UI = urinary incontinence. SUI = stress urinary incontinence. UUI = urgency urinary incontinence. MUI = mixed urinary incontinence. OR = odds ratio. ++ = significant positive association between birthweight and UI. + = non-significant positive association between birthweight and UI. - = non-significant negative association between birthweight and UI. -- = significant negative association between birthweight and UI. * = studies used in meta-analysis.
Table 3.
Descriptive data on studies included in meta-analyses.
| Study | Data on 4000 g | Data on 3500 g | Data on stress UI | Data 3-18 months postpartum | Data on vaginal delivery | Data on primi-parous | Risk of selection bias | Adjusted effect estimates |
|---|---|---|---|---|---|---|---|---|
| Altaweel [21] | X | Low | X | |||||
| Boyles [10] | X (8 lb) | X | X | X | Low | X | ||
| Brown [22] | X | X | Low | X | ||||
| Casey [23] | X | X | X | X | Low | For stress UI | ||
| Diez-Itza [24] | X | X | High | X | ||||
| Dimpfl [31] | X | X | X | Low | ||||
| Dolan [32] | X | X | Low | Partly | ||||
| Eason [25] | X | X | Low | |||||
| Gartland [26] | X | X | X | Low | ||||
| Groutz [11] | X | X | X | Low | ||||
| Hatem [18] | X | X | X | Moderate | ||||
| Hvidman [19] | X | X | Moderate | X | ||||
| Iwanowicz [12] | X | X | X | Unclear | ||||
| Krue [13] | X | X | X | High | ||||
| Mallah [20] | X | X | X | Unclear | X | |||
| Obioah [27] | X | X | Low | X | ||||
| Rørtveit [14] | X | X | X | Low | X | |||
| Samuelsson [28] | 3925 | Low | ||||||
| Schytt [16] | X | X | X | X | X | Low | ||
| Solans-Domenech [29] | X | X | Low | |||||
| Thom [30] | X | X | Low | X | ||||
| Wesnes [62] | X | X | X | X | X | Low | X |
UI = urinary incontinence.
Selection bias was considered in studies used in the meta-analyses. Risk of selection bias was considered as high in 2/22 studies [13,17], moderate in 2/22 studies [18,19], unclear in 2/22 studies [12,20], and low in 16/22 studies (Table 3). Unadjusted association between birthweight and UI was reported in 9/22 studies. Funnel plot did not reveal publication bias, as it spread evenly on both sides of the average, creating a roughly funnel-shaped distribution.
There was a significant positive association between high birthweight and UI after childbirth in 35% (20/57) of the studies. There was a non-significant positive association in 19% (11/57) of the studies. There was no association in 46% (26/57) of the studies. A significant protective association between high birthweight and urgency UI was also found in one of the above studies.
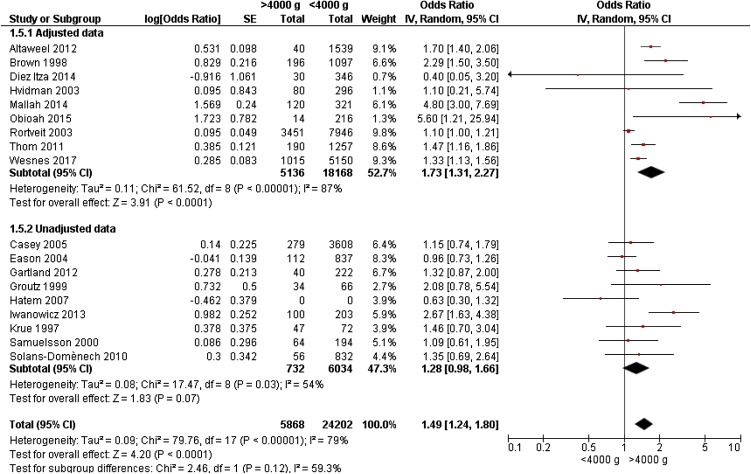
3.1. Birthweight >4000g
Eighteen studies [8,[11], [12], [13], [14]] [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]], reported data on 30,070 women for review on birthweight >4000 g and UI. Birthweight >4000 g compared to weight <4000 g was associated with a significantly increased risk of any UI in meta-analyses (OR 1.49, 95% CI 1.24 – 1.80) (Fig. 1). Meta-analyses revealed higher OR of UI in adjusted data than unadjusted data (OR 1.73 and OR 1.28, respectively). Funnel plot did not reveal publication bias, as it spread evenly on both sides of the average, creating a roughly funnel-shaped distribution.
Fig. 1.
Forest plot of the association between urinary incontinence and birthweight >4000 g vs <4000 g, stratified for adjusted and unadjusted data.
3.1.1. Birthweight >4000 g and stress UI
Data from four European [[11], [12], [13], [14]] and one American [23] study were available for meta-analyses. Time of recording UI varied from 3 days postpartum [11] to several decades after childbirth 14] Weight >4000 g was associated with a significant increased risk of stress UI (OR 1.52, 95% CI 1.03 – 2.25) when analysing available data from these five studies with a total of 15,806 women.
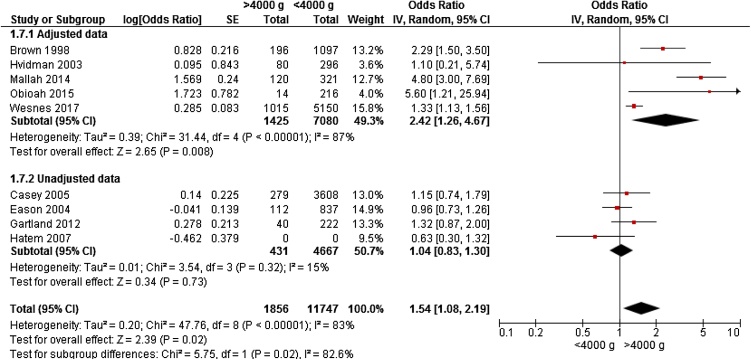
3.1.2. Birthweight >4000 g and UI 3 – 18 months postpartum
Nine studies; six cohort studies [8,20,23,[25], [26], [27]] and three cross-sectional studies [18,19,22] gave data on 13,603 women for meta-analyses. The studies were conducted in Europe [8,19], Africa 27], Asia20], Australia22,26], and America[18,23,25]. Age was 22 – 31 years in the cohorts; two cross-sectional studies reported age 27 – 29 years. Five studies included primiparous only [8,[18,20,23,26]. Birthweight >4000 g lead to a significantly increased OR 1.54 (95% CI 1.08 – 2.19) for UI 3 – 18 months postpartum compared to women delivering infants with birthweight <4000 g (Fig. 2).
Fig. 2.
Forest plot of the association between urinary incontinence 3 – 18 months postpartum, and birthweight >4000 g vs <4000 g, stratified for adjusted and unadjusted data.
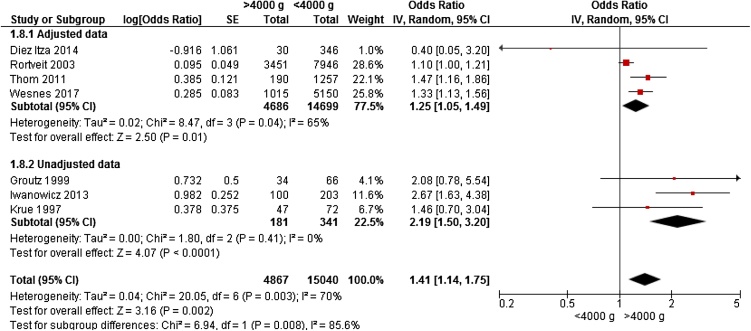
3.1.3. Birthweight >4000 g and UI after vaginal birth
Seven studies gave data for meta-analyses on 19,907 women on the association between UI and birth weight >4000 g among women delivering by any vaginal birth; three cohort studies [8,24,30] and four cross-sectional studies [[11], [12], [13], [14]] were identified. Three large studies with adjusted data were included; Rortveit et al.14] enrolled 11,397 women; Wesnes et al. [8] enrolled 5,219 women, and Thom et al. enrolled 1,521 women. However, mean age, parity and time of UI varied in these studies. Weight >4000 g was associated with a significantly increased risk of UI after vaginal birth (OR 1.41, 95% CI 1.14 – 1.75) (Fig. 3).
Fig. 3.
Forest plot of the association between urinary incontinence after any vaginal delivery, and birthweight >4000 g vs <4000 g, stratified for adjusted and unadjusted data.
Only one study had additional data on birthweight >4000 g and birth by CS [8]. OR for UI after birth by any CS of child >4000 g compared to <4000 g was 1.38 (95% CI 0.84 – 2.28).
3.1.4. Birthweight >4000 g and UI among primiparous women
Five cohort studies [8,20,23,26,29] and one cross-sectional study [18] had data on 11,643 women for meta-analyses on birthweight >4000 g among primiparous women. All studies had data on UI 2 – 18 months postpartum. Three studies included only women who were continent before pregnancy [8,26,29]. Weight >4000 g was associated with a non-significantly increased risk of UI among primiparous women (OR 1.46, 95% CI 0.95 – 2.26). Only two studies gave adjusted effect estimates [8,20], leading to an OR of 2.48 (95% CI 0.70 – 8.71) for UI among primiparous women delivering babies >4000 g compared to <4000 g. However, due to heterogeneity in effect estimates, I2 was 96%. Unadjusted analyses gave OR of 1.15 (95% CI 0.88 – 1.50).
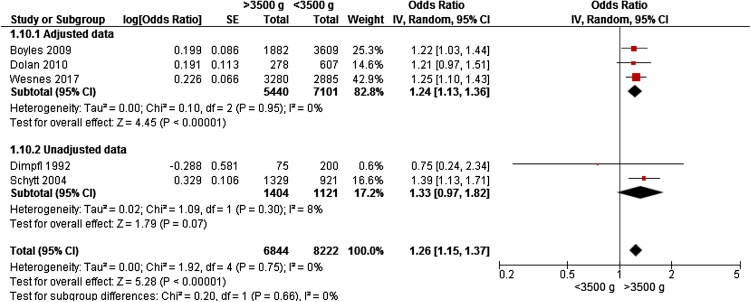
3.2. Birthweight >3500 g
Only four studies from Europe [8,16,31,32] and one from America [10] gave data on birthweight >3500 g and risk of any UI, including 15,066 women for meta-analyses. Two studies reported data on primiparous women[8, [10]; three studies included women who were continent before pregnancy [8,[10,31]. Weight >3500 g was associated with a significantly increased risk of UI (OR 1.26, 95% CI 1.15 – 1.37) (Fig. 4).
Fig. 4.
Forest plot of the association between urinary incontinence and birthweight >3500 g vs <3500 g, stratified for adjusted and unadjusted data.
3.2.1. Birthweight >3500 g stress UI
Two studies had unadjusted data on the association between birthweight >3500 g and stress UI [16,31]. Data was collected 6 weeks – 1 year after childbirth. None of these studies reached statistical significance on the association between birthweight >3500 g and stress UI. Birthweight >3500 g was associated with a non-significantly increased risk of stress UI in meta-analyses of 2525 women (OR 1.33, 95% CI 0.97 – 1.82).
3.2.2. Birthweight >3500 g and UI 3 – 12 months postpartum
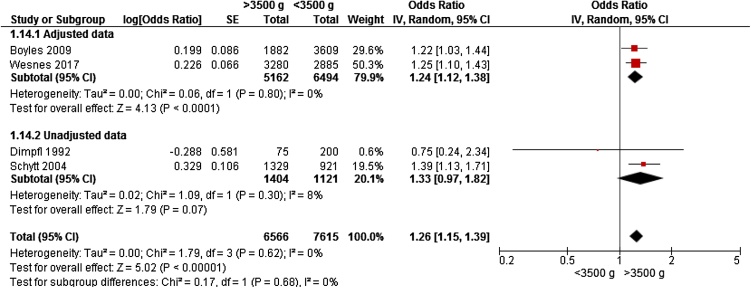
Four studies reported data on birth weight >3500 g and UI 3 – 12 months after childbirth [8,10,16,31]. Included studies were rather similar regarding study population; three studies reported data on primiparous [8,10,16], and three studies reported data on women who were continent before pregnancy [8,10,31]. Meta-analyses on 14,181 women found a significantly increased risk of UI 3 – 12 months postpartum (OR 1.26, 95% CI 1.15 – 1.39) (Fig. 5).
Fig. 5.
Forest plot of the association between urinary incontinence 3 – 12 months postpartum and birthweight >3500 g vs <3500 g, stratified for adjusted and unadjusted data.
3.2.3. Birthweight >3500 g and UI after vaginal childbirth
Three large studies with 5599 [10], 2390 [16], and 5219 [8] participants had data on the association between birthweight >3500 g and UI after vaginal childbirth. All studies presented adjusted data on UI 3 – 12 months postpartum with OR 1.22, 1.30 and 1.25, respectively. In meta-analyses, weight >3500 g was associated with a significantly increased risk of UI after vaginal childbirth (OR 1.26, 95% CI 1.15 – 1.37). I2 was 0%.
Boyles [10] and Wesnes [8] reported stratified data for CS: there was no association between birthweight >3500 g and UI after CS (OR 1.04, 95% CI 0.67 – 1.63).
3.2.4. Birthweight >3500 g and UI among primiparous women
Four large questionnaire-based studies investigated the association of birthweight >3,500 g on UI [8,10,16,32] in women 3 – 12 months after childbirth. Three studies reported adjusted results. Two studies found a significant positive association [8,10] between birthweight >3,500 g and UI among primiparous women, two studies found a non-significant positive association 16,32]. Meta-analyses from these studies gave an OR of 1.23 (95% CI 1.11 – 1.35) for UI among primiparous women delivering infants with birthweight >3500 g compared to <3500 g.
Schytt et al [16] reported stratified data on primiparous and multiparous women. Results indicated that birthweight >3500 g lead to higher RR for UI postpartum among multiparous compared to primiparous women (RR 1.5 (95% CI 1.2–1.9) and RR 1.1 (95% CI 0.8–1.4), respectively).
4. Discussion
This is the first systematic review on the association between birthweight and UI after childbirth. UI postpartum is a common condition, affecting 32 – 36% of women [2]. Many risk factors have been brought forth in studies, most are not well documented. Reviews on epidural [3], episiotomy [4], cesarean section [2] and instrumental childbirth [5] have made their association with UI postpartum clear. Some birth variables are more commonly extracted from birth registries (birthweight, head circumference, rupture) and often included in analyses. There has been need to summarize knowledge on these potential risk factors.
4.1. Main findings
Birthweight >4000 g and >3500 g were associated with significantly increased risk of UI (OR 1.49 and 1.26, respectively). Separate analyses on stress UI, UI 3 – 18 months postpartum, UI after vaginal birth and UI among primiparous women also revealed significantly increased risk of UI.
4.2. Strengths and limitations
Prevalence of UI after childbirth varies with time of information gathering, type of UI, mode of childbirth, and characteristics of the study population [1]. Diversity in studies included in meta-analyses needs to be addressed. To control for some of these parameters, separate meta-analyses were performed for time of UI, stress UI, vaginal birth, and parity. Subgroup meta-analyses on the reported variables showed significantly increased OR in the range 1.41 – 1.54 for UI and birth weight >4000 g. Corresponding analyses on UI and birth weight >3500 g gave significantly increased OR in the range 1.23 – 1.33. This indicates that the overall risk estimates are robust. Studies have shown that selection bias affects prevalence estimates, but data are still valid for risk estimates [33]. High birthweight is also associated with high BMI in the mother, prolonged birth, CS, rupture, episiotomy, birth by forceps and vacuum. We cannot rule out confounding.
A total of 33/57 studies report on the association between birthweight and UI as secondary finding, without authors reporting values, percentages or risks. Information from these articles was applicable for this review, but not for the meta-analysis.
The literature search also needs to be addressed. Birth weight and UI was the main objective in few articles. Literature search retrieved information from headings and abstracts, therefore it did not retrieve articles where relevant information was solely in the main text. 476 articles were identified by literature search. Author’s knowledges of literature, and full reference reading identified 15 external articles (Table 1). A total of 27 articles were not evaluated for inclusion due to foreign language or lack of access. We must therefore accept that the sensitivity of the search was high, but not complete. Publication bias may be a problem, as significant data are more likely to be published, and presented in abstracts.
Categorizing of weight groups and reference groups can affect the results. Birthweight >3500 g and > 4000 g led to OR 1.26 and 1.49, respectively. The reference groups were birthweight <3500 g and < 4000 g, respectively. One study with 5,219 women used lower reference value [8]. OR of UI was 1.6 (95% CI 1.2 – 2.0) after birthweight >4,180 g (90 percentile) compared to birthweight 3,540 g (50 percentile) [8]. Another study compared birthweight >3,925 to <3,199, finding OR of 1.4 [28]. Risk estimates in studies might have been higher if reference groups consisted of lower birthweights.
Few studies use weight groups >4500 g [16,34,35]. Few study participants in the exposure group makes it difficult to do meta-analyses. One study reported adjusted OR 1.23 (95% CI 0.87 – 1.76) for UI among women delivering babies with birthweight >4,500 g compared to < 4,500 g 35].
Vaginal birth is associated with higher risk of UI than CS in most studies [2]. Weight >4000 g was associated with UI after vaginal birth and CS (OR 1.41 95% CI 1.14 – 1.75 and OR 1.38, 95% CI 0.84 – 2.28, respectively). Weight >3500 g was also associated with UI after vaginal birth and CS (OR 1.26, 95% CI 1.15 – 1.37, and OR 1.04 95% CI 0.67 – 1.63, respectively). These meta-analyses on birthweight and UI are in line with what is previous shown regarding birth mode and UI [2]; lower risk of UI after CS, than after vaginal birth. Studies including women delivering by vaginal birth and CS indicate that birthweight is a significant risk factor for UI only in association with vaginal birth [8,35]. Mode of birth is a confounder that is likely to affect the association between birthweight and UI in the remaining sub-analysis. There were, however, no data available for further stratified sub-analysis on CS and vaginal birth.
It is unclear how high birthweight lead to UI. Trauma to the pelvic floor when delivering large babies is a plausible contributing factor. Heavier babies have been associated with EMG evidence of pudendal nerve damage in the pelvic floor after vaginal birth [6,36].
The majority of studies report grams, others report pounds [10]. Several studies analysed on mean birthweight [37], some use percentiles [8] or quartiles [38]. Birthweight was often categorized arbitrary without authors reporting reason for cut-off, most studies uses 500-gram groups (3500 g, 4000 g, and 4500 g). Varying reporting and different weight categorizing makes it difficult to summarize published results. Weight cut-off on 3500 g and 4000 g were most common, and were therefore chosen for this review. As 3500 g generally represents mean birth weight, these weight cut-offs gives important information on the association between UI and birthweight beyond mean, as well as extreme birthweight >4000 g.
4.3. Interpretation
Birthweight appears to be a risk factor for UI after childbirth. Data in this meta-analysis are gathered from epidemiological cohort and cross-sectional studies. When planning future epidemiological studies, data on birthweight ought to be gathered when possible. Birthweight is likely to effect risk estimates on UI after deliver, and adjustment should be considered in future research.
The Hill Criteria are useful when considering a possible causal relationship between exposure and outcome. Birthweight satisfies several of the Hill Criteria for causation; it is biologically plausible, exposure precedes outcome, and there is consistency in the majority of studies. There is a dose-response association, as birthweight >4000 g gave higher OR for UI than >3500 g.
To predict future risk of UI after childbirth, the UR-CHOICE risk calculator intend to include eight variables, of which birthweight is the only fetal factor [39]. Birthweight is included in the risk calculator due to the high likelihood of causation.
Even though results appear biological plausible, results in the meta-analysis are based on epidemiological research and can thereby not automatically be applied into a clinical setting. As high birthweight is associated with UI postpartum, clinical preventive strategies ought to be identified. Strategies might be targeted on identifying mothers at risk of having babies with high birthweight (for instance by identifying high maternal BMI, identifying previous deliveries of babies with high birthweight), avoiding high birthweight (for instance by avoiding high maternal weight gain during pregnancy, detecting gestational diabetes), detecting high birthweight (for instance by growth charts, ultrasound, symphysis-fundus height measurements), reducing risk of incident UI postpartum (for instance by aiming at normal weight before pregnancy, and at regaining pre-pregnancy weight postpartum, considering CS, performing PFMT), or by treating UI postpartum, for instance by PFMT. Future research will need to find ways to identify which women are likely to give birth to babies >3500 g, and look into the best preventive strategies.
Pelvic floor muscle training is generally recommended in pregnancy and postpartum. It has been unclear which women benefit the most from this training. Women delivering babies with birthweight > 3500 g are women at risk of developing UI. No preventive strategy is validated in this study, but pelvic floor muscle training has documented effect on preventing UI among women delivering heavy babies [40].
5. Conclusion
We conclude that birthweight appears to be a risk factor for UI after childbirth. A causal relationship between birthweight and UI is biologically plausible. Strategies towards preventing UI postpartum should be targeted towards women at higher risk, like women giving birth to babies with high birthweight.
Author contribution to the manuscript
Wesnes: Project development, data collection, manuscript writing, statistical analysis.
Seim: Data collection, manuscript writing.
Funding, financial disclaimers
None.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We acknowledge Guri Rortveit’s contribution to this paper. Rortveit’s affiliation: Research Group for General Practice, Department of Global Public Health and Primary Care, University of Bergen, Norway, and Research Unit for General Practice, Uni Research Health, Bergen, Norway. Rortveit has not received any funding in association with this article.
Biographies
Stian Langeland Wesnes: M.D., working as specialist in general medicine. Ph.D in 2011 with the title “Urinary incontinence in pregnancy and postpartum: incidence, prevalence and risk factors”.
Elin Seim: M.D. since 2014, specializing in neurology at Haukeland University Hospital.
References
- 1.Wesnes S.L., Hunskaar S., Rortveit G. Epidemiology of Urinary Incontinence in Pregnancy and Postpartum. In: Alhasso A., editor. Urinary Incontinence. InTech; Rijeka: 2012. pp. 21–40. [Google Scholar]
- 2.Thom D.H., Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89(12):1511–1522. doi: 10.3109/00016349.2010.526188. [DOI] [PubMed] [Google Scholar]
- 3.Leighton B.L., Halpern S.H. Epidural analgesia: effects on labor progress and maternal and neonatal outcome. Semin Perinatol. 2002;26(2):122–135. doi: 10.1053/sper.2002.32201. [DOI] [PubMed] [Google Scholar]
- 4.Carroli G., Mignini L. Episiotomy for vaginal birth. Cochrane database of systematic reviews. 2009;(1) doi: 10.1002/14651858.CD000081.pub2. CD000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towner D.R., Ciotti M.C. Operative vaginal delivery: a cause of birth injury or is it? Clinical obstetrics and gynecology. 2007;50(3):563–581. doi: 10.1097/GRF.0b013e31811eaa39. [DOI] [PubMed] [Google Scholar]
- 6.Allen R.E., Hosker G.L., Smith A.R., Warrell D.W. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97(9):770–779. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 7.Donahue S.M., Kleinman K.P., Gillman M.W., Oken E. Trends in birth weight and gestational length among singleton term births in the United States: 1990-2005. Obstet Gynecol. 2010;115(2 Pt 1):357–364. doi: 10.1097/AOG.0b013e3181cbd5f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wesnes S.L., Hannestad Y., Rortveit G. Delivery parameters, neonatal parameters and incidence of urinary incontinence six months postpartum: a cohort study. Acta Obstet Gynecol Scand. 2017;96(10):1214–1222. doi: 10.1111/aogs.13183. [DOI] [PubMed] [Google Scholar]
- 9.Kovaleva L. Assessment of risk factors of urinary incontinence in pregnancy, effect of pelvic floor muscle training. International Urogynecology Journal and Pelvic Floor Dysfunction. 2011;22:S1473. [Google Scholar]
- 10.Boyles S.H., Li H., Mori T., Osterweil P., Guise J.M. Effect of mode of delivery on the incidence of urinary incontinence in primiparous women. Obstet Gynecol. 2009;113(1):134–141. doi: 10.1097/AOG.0b013e318191bb37. [DOI] [PubMed] [Google Scholar]
- 11.Groutz A., Gordon D., Keidar R. Stress urinary incontinence: prevalence among nulliparous compared with primiparous and grand multiparous premenopausal women. Neurourol Urodyn. 1999;18(5):419–425. doi: 10.1002/(sici)1520-6777(1999)18:5<419::aid-nau2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Iwanowicz-Palus G.J., Stadnicka G., Wloszczak-Szubzda A. Medical and psychosocial factors conditioning development of stress urinary incontinence (SUI) Annals of Agricultural and Environmental Medicine. 2013;20(1):135–139. [PubMed] [Google Scholar]
- 13.Krue S., Jensen H., Agger A.O., Rasmussen K.L. The influence of infant birth weight on post partum stress incontinence in obese women. Arch Gynecol Obstet. 1997;259(3):143–145. doi: 10.1007/BF02505323. [DOI] [PubMed] [Google Scholar]
- 14.Rortveit G., Daltveit A.K., Hannestad Y.S., Hunskaar S. Vaginal delivery parameters and urinary incontinence: the Norwegian EPINCONT study. Am J Obstet Gynecol. 2003;189(5):1268–1274. doi: 10.1067/s0002-9378(03)00588-x. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Schytt E., Lindmark G., Waldenstrom U. Symptoms of stress incontinence 1 year after childbirth: prevalence and predictors in a national Swedish sample. Acta Obstet Gynecol Scand. 2004;83(10):928–936. doi: 10.1111/j.0001-6349.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 17.Diez Itza I., Arrue M., Ibanez L. Factors involved in stress urinary incontinence 2 years after first delivery. Neurourology and Urodynamics. 2010;29(6):1136–1137. doi: 10.1002/nau.24442. [DOI] [PubMed] [Google Scholar]
- 18.Hatem M., Pasquier J.C., Fraser W., Lepire E. Factors Associated With Postpartum Urinary/Anal Incontinence in Primiparous Women in Quebec. Journal of Obstetrics and Gynaecology Canada. 2007;29(3):232–239. doi: 10.1016/S1701-2163(16)32402-1. [DOI] [PubMed] [Google Scholar]
- 19.Hvidman L., Foldspang A., Mommsen S., Nielsen J.B. Postpartum urinary incontinence. Acta Obstet Gynecol Scand. 2003;82(6):556–563. doi: 10.1034/j.1600-0412.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 20.Mallah F., Tasbihi P., Navali N., Azadi A. Urinary incontinence during pregnancy and postpartum incidence, Severity and risk factors in alzahra and taleqani hospitals in Tabriz, Iran, 2011-2012. International Journal of Women’s Health and Reproduction Sciences. 2014;2(3):178–185. [Google Scholar]
- 21.Altaweel W., Alharbi M. Urinary incontinence: Prevalence, risk factors, and impact on health related quality of life in Saudi women. Neurourology and Urodynamics. 2012;31(5):642–645. doi: 10.1002/nau.22201. [DOI] [PubMed] [Google Scholar]
- 22.Brown S., Lumley J. Maternal health after childbirth: results of an Australian population based survey. Br J Obstet Gynaecol. 1998;105(2):156–161. doi: 10.1111/j.1471-0528.1998.tb10045.x. [DOI] [PubMed] [Google Scholar]
- 23.Casey B.M., Schaffer J.I., Bloom S.L., Heartwell S.F., McIntire D.D., Leveno K.J. Obstetric antecedents for postpartum pelvic floor dysfunction. Am J Obstet Gynecol. 2005;192(5):1655–1662. doi: 10.1016/j.ajog.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Diez Itza I., Goyeneche L., Uranga S., Salgueiro D., Goiri C., Lekuona A. Assessment of overactive bladder and urgency urinary incontinence six weeks after vaginal delivery. Neurourology and Urodynamics. 2014;33(6):767–768. [Google Scholar]
- 25.Eason E., Labrecque M., Marcoux S., Mondor M. Effects of carrying a pregnancy and of method of delivery on urinary incontinence: A prospective cohort study. BMC Pregnancy and Childbirth. 2004;4(4) doi: 10.1186/1471-2393-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gartland D., Donath S., MacArthur C., Brown S.J. The onset, recurrence and associated obstetric risk factors for urinary incontinence in the first 18 months after a first birth: An Australian nulliparous cohort study. BJOG: An International Journal of Obstetrics and Gynaecology. 2012;119(11):1361–1369. doi: 10.1111/j.1471-0528.2012.03437.x. [DOI] [PubMed] [Google Scholar]
- 27.Obioha K.C., Ugwu E.O., Obi S.N., Dim C.C., Oguanuo T.C. Prevalence and predictors of urinary/anal incontinence after vaginal delivery: prospective study of Nigerian women. Int Urogynecol J Pelvic Floor Dysfunct. 2015;26(9):1347–1354. doi: 10.1007/s00192-015-2690-0. [DOI] [PubMed] [Google Scholar]
- 28.Samuelsson E., Victor A., Svardsudd K. Determinants of urinary incontinence in a population of young and middle-aged women. Acta Obstet Gynecol Scand. 2000;79(3):208–215. [PubMed] [Google Scholar]
- 29.Solans-Domenech M., Sanchez E., Espuna-Pons M. Pelvic Floor Research G. Urinary and anal incontinence during pregnancy and postpartum: incidence, severity, and risk factors. Obstet Gynecol. 2010;115(3):618–628. doi: 10.1097/AOG.0b013e3181d04dff. [DOI] [PubMed] [Google Scholar]
- 30.Thom D.H., Brown J.S., Schembri M., Ragins A.I., Creasman J.M., Van Den Eeden S.K. Parturition events and risk of urinary incontinence in later life. Neurourol Urodyn. 2011;30(8):1456–1461. doi: 10.1002/nau.21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimpfl T., Hesse U., Schussler B. Incidence and cause of postpartum urinary stress incontinence. Eur J Obstet Gynecol Reprod Biol. 1992;43(1):29–33. doi: 10.1016/0028-2243(92)90239-u. [DOI] [PubMed] [Google Scholar]
- 32.Dolan L.M., Hilton P. Obstetric risk factors and pelvic floor dysfunction 20 years after first delivery. Int Urogynecol J. 2010;21(5):535–544. doi: 10.1007/s00192-009-1074-8. [DOI] [PubMed] [Google Scholar]
- 33.Nilsen R.M., Vollset S.E., Gjessing H.K. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 34.Torkestani F., Zafarghandi N., Davati A., Hadavand S.H., Garshasbi M. Case-controlled study of the relationship between delivery method and incidence of post-partum urinary incontinence. J Int Med Res. 2009;37(1):214–219. doi: 10.1177/147323000903700126. [DOI] [PubMed] [Google Scholar]
- 35.Gyhagen M., Bullarbo M., Nielsen T.F., Milsom I. The prevalence of urinary incontinence 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. Bjog. 2013;120(2):144–151. doi: 10.1111/j.1471-0528.2012.03301.x. [DOI] [PubMed] [Google Scholar]
- 36.Snooks S.J., Swash M., Henry M.M., Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. Int J Colorectal Dis. 1986;1(1):20–24. doi: 10.1007/BF01648831. [DOI] [PubMed] [Google Scholar]
- 37.Farrell S.A., Allen V.M., Baskett T.F. Parturition and urinary incontinence in primiparas. Obstet Gynecol. 2001;97(3):350–356. doi: 10.1016/s0029-7844(00)01164-9. [DOI] [PubMed] [Google Scholar]
- 38.Glazener C.M., Herbison G.P., MacArthur C. New postnatal urinary incontinence: obstetric and other risk factors in primiparae. Bjog. 2006;113(2):208–217. doi: 10.1111/j.1471-0528.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 39.Wilson D., Dornan J., Milsom I., Freeman R. UR-CHOICE: can we provide mothers-to-be with information about the risk of future pelvic floor dysfunction? International Urogynecology Journal and Pelvic Floor Dysfunction. 2014;25(11):1449–1452. doi: 10.1007/s00192-014-2376-z. [DOI] [PubMed] [Google Scholar]
- 40.Chiarelli P., Cockburn J. Promoting urinary continence in women after delivery: Randomised controlled trial. Br Med J. 2002;324(7348):1241–1244. doi: 10.1136/bmj.324.7348.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arya L.A., Jackson N.D., Myers D.L., Verma A. Risk of new-onset urinary incontinence after forceps and vacuum delivery in primiparous women. American Journal of Obstetrics and Gynecology. 2001;185(6):1318–1324. doi: 10.1067/mob.2001.120365. [DOI] [PubMed] [Google Scholar]
- 42.Baracho S.M., Barbosa da Silva L., Baracho E., Lopes da Silva Filho A., Sampaio R.F., Mello de Figueiredo E. Pelvic floor muscle strength predicts stress urinary incontinence in primiparous women after vaginal delivery. Int Urogynecol J Pelvic Floor Dysfunct. 2012;23(7):899–906. doi: 10.1007/s00192-012-1681-7. [DOI] [PubMed] [Google Scholar]
- 43.Burgio K.L., Zyczynski H., Locher J.L., Richter H.E., Redden D.T., Wright K.C. Urinary incontinence in the 12-month postpartum period. Obstet Gynecol. 2003;102(6):1291–1298. doi: 10.1016/j.obstetgynecol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Cardo Maza A., Alamar Provecho J., Moreno Selva R. Urinary incontinence during pregnancy and for months after delivery. Risk factors and impact on quality of life. International Urogynecology Journal and Pelvic Floor Dysfunction. 2011;22:S1883–S1884. [Google Scholar]
- 45.Castillo Vico M.T., Agramunt S., Banos N. Obstetrical risk factors and urinary incontinence: A prospective cohort approach. International Urogynecology Journal and Pelvic Floor Dysfunction. 2011;22:S1486. [Google Scholar]
- 46.Chaliha C., Kalia V., Stanton S.L., Monga A., Sultan A.H. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol. 1999;94(5 Pt 1):689–694. doi: 10.1016/s0029-7844(99)00364-6. [DOI] [PubMed] [Google Scholar]
- 47.Chou P.L., Chen F.P., Teng L.F. Factors associated with urinary stress incontinence in primiparas. Taiwanese Journal of Obstetrics and Gynecology. 2005;44(1):42–47. [Google Scholar]
- 48.Connolly T.J., Litman H.J., Tennstedt S.L., Link C.L., McKinlay J.B. The effect of mode of delivery, parity, and birth weight on risk of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(9):1033–1042. doi: 10.1007/s00192-006-0286-4. [DOI] [PubMed] [Google Scholar]
- 49.Eftekhar T., Hajibaratali B., Ramezanzadeh F., Shariat M. Postpartum evaluation of stress urinary incontinence among primiparas. Int J Gynaecol Obstet. 2006;94(2):114–118. doi: 10.1016/j.ijgo.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 50.Emanuela F., Amelia P., Raffaela B. Post-partum urinary incontinence and childbirth-related risk factors. Neurourology and Urodynamics. 2012;31:S3. [Google Scholar]
- 51.Frias Aldeguer L., Crispin Milart P.H., Martinez Moron V. How does pregnancyand delivery affect urinary continence and sexual function? International Urogynecology Journal and Pelvic Floor Dysfunction. 2011;22:S1904–S1905. [Google Scholar]
- 52.Fritel X., Fauconnier A., Levet C., Benifla J.L. Stress urinary incontinence 4 years after the first delivery: a retrospective cohort survey. Acta Obstet Gynecol Scand. 2004;83(10):941–945. doi: 10.1111/j.0001-6349.2004.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grodstein F., Fretts R., Lifford K., Resnick N., Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434. doi: 10.1067/s0002-9378(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 54.Groutz A., Rimon E., Peled S. Cesarean section: does it really prevent the development of postpartum stress urinary incontinence? A prospective study of 363 women one year after their first delivery. Neurourol Urodyn. 2004;23(1):2–6. doi: 10.1002/nau.10166. [DOI] [PubMed] [Google Scholar]
- 55.Kashanian M., Parashi S. Evaluation of the relationship between the mode of delivery and stress urinary incontinence 1 year after first delivery. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20(3 SUPPL):S445. doi: 10.1007/s00192-008-0767-8. [DOI] [PubMed] [Google Scholar]
- 56.Marsh F., Johnson A., Rogerson L., Landon C., Wright A. Factors associated with prolonged faecal and urinary symptoms following obstetric anal sphincter injury (OASIS) from 2004-2008. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20(3 SUPPL):S271–S272. [Google Scholar]
- 57.McKinnie V., Swift S.E., Wang W. The effect of pregnancy and mode of delivery on the prevalence of urinary and fecal incontinence… includes discussion. Am J Obstet Gynecol. 2005;193(2):512–518. doi: 10.1016/j.ajog.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 58.Seshan V., Muliira J.K. Self-reported urinary incontinence and factors associated with symptom severity in community dwelling adult women: Implications for women’s health promotion. BMC Women’s Health. 2013;13(1) doi: 10.1186/1472-6874-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Brummen H.J., Bruinse H.W., van de Pol G., Heintz A.P., van der Vaart C.H. The effect of vaginal and cesarean delivery on lower urinary tract symptoms: what makes the difference? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(2):133–139. doi: 10.1007/s00192-006-0119-5. [DOI] [PubMed] [Google Scholar]
- 60.Viktrup L., Lose G., Rolff M., Barfoed K. The symptom of stress incontinence caused by pregnancy or delivery in primiparas. Obstet Gynecol. 1992;79(6):945–949. [PubMed] [Google Scholar]
- 61.Volloyhaug I., Morkved S., Salvesen O., Salvesen K.A. Pelvic organ prolapse and incontinence 15-23 years after first delivery: A cross-sectional study. BJOG: An International Journal of Obstetrics and Gynaecology. 2015;122(7):964–971. doi: 10.1111/1471-0528.13322. [DOI] [PubMed] [Google Scholar]
- 62.Wesnes S.L., Lose G. Preventing urinary incontinence during pregnancy and postpartum: a review. Int Urogynecol J. 2013;24(6):889–899. doi: 10.1007/s00192-012-2017-3. [DOI] [PubMed] [Google Scholar]
- 63.Williams A., Herron-Marx S., Knibb R. The prevalence of enduring postnatal perineal morbidity and its relationship to type of birth and birth risk factors. J Clin Nurs. 2007;16(3):549–561. doi: 10.1111/j.1365-2702.2006.01593.x. [DOI] [PubMed] [Google Scholar]
- 64.Wu X.H., Liu X.X., Xie K.H., Wang R.M., Wu Y.X., Liu Y.G. Prevalence and related factors of urinary incontinence among Hebei women of China. Gynecologic and Obstetric Investigation. 2011;71(4):262–267. doi: 10.1159/000320333. [DOI] [PubMed] [Google Scholar]
- 65.Yang X., Zhang H.X., Yu HY Gao XL, Yang H.X., Dong Y. The prevalence of fecal incontinence and urinary incontinence in primiparous postpartum Chinese women. Eur J Obstet Gynecol Reprod Biol. 2010;152(2):214–217. doi: 10.1016/j.ejogrb.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 66.Yohay D., Weintraub A.Y., Mauer-Perry N. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35–39. doi: 10.1016/j.ejogrb.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 67.Yip S.K., Sahota D., Chang A., Chung T. Effect of one interval vaginal delivery on the prevalence of stress urinary incontinence: a prospective cohort study. Neurourol Urodyn. 2003;22(6):558–562. doi: 10.1002/nau.10113. [DOI] [PubMed] [Google Scholar]
- 68.Zanelli S., Minini G.F., Cesana B.M. Obstetric risk factors for pelvic floor damage leading to urinary incontinence: A prospective study on 452 women after first delivery. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20(3 SUPPL):S308–S309. [Google Scholar]
- 69.Zhang W., Song Y., He X. Prevalence and risk factors of lower urinary tract symptoms in Fuzhou Chinese women. European Urology. 2005;48(2):309–313. doi: 10.1016/j.eururo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Zhu L., Lang J., Wang H., Han S., Huang J. The prevalence of and potential risk factors for female urinary incontinence in Beijing, China. Menopause. 2008;15(3):566–569. doi: 10.1097/gme.0b013e31816054ac. [DOI] [PubMed] [Google Scholar]