Significance
Experiencing poverty early in life is associated with continued risk for mental and physical health problems in childhood and adulthood. As such, it is important to understand the mechanisms by which poverty contributes to lasting risk for poor developmental outcomes. Here we use longitudinal prospectively acquired data starting in preschool to demonstrate that early poverty is associated with disruptions in developmental testosterone trajectories and hippocampal volume growth across school age and adolescent development, as well as with greater emotion dysregulation and depression in adolescence. Further, we provide evidence that such disruptions in hormonal and brain development are part of the pathway linking poverty to subsequent problems with emotion regulation and depression.
Keywords: poverty, hippocampus, testosterone, development, emotion regulation
Abstract
There is robust evidence that early poverty is associated with poor developmental outcomes, including impaired emotion regulation and depression. However, the specific mechanisms that mediate this risk are less clear. Here we test the hypothesis that one pathway involves hormone mechanisms (testosterone and DHEA) that contribute to disruption of hippocampal brain development, which in turn contributes to perturbed emotion regulation and subsequent risk for depression. To do so, we used data from 167 children participating in the Preschool Depression Study, a longitudinal study that followed children from preschool (ages 3 to 5 y) to late adolescence, and which includes prospective assessments of poverty in preschool, measures of testosterone, DHEA, and hippocampal volume across school age and adolescence, and measures of emotion regulation and depression in adolescence. Using multilevel modeling and linear regression, we found that early poverty predicted shallower increases of testosterone, but not DHEA, across development, which in turn predicted shallower trajectories of hippocampal development. Further, we found that early poverty predicted both impaired emotion regulation and depression. The relationship between early poverty and self-reported depression in adolescence was explained by serial mediation through testosterone to hippocampus to emotion dysregulation. There were no significant interactions with sex. These results provide evidence about a hormonal pathway by which early poverty may contribute to disrupted brain development and risk for mental health problems later in life. Identification of such pathways provide evidence for potential points of intervention that might help mitigate the impact of early adversity on brain development.
There is robust evidence that early experiences of poverty and other forms of early adversity experienced prenatally (1–3) and/or postnatally (4–12) contribute to an increased risk for poor developmental outcomes, including mental health problems in childhood and adulthood. The overwhelming evidence of such associations has encouraged efforts aimed at early intervention and prevention (11, 13). However, the success of such efforts is contingent upon a better understanding of the mechanisms linking early poverty to poor developmental outcomes. There is growing evidence that early adversity contributes to difficulties with emotion dysregulation, potentially related to disruptions in the development of brain regions such as the hippocampus. Importantly, there is also evidence that early adversity disrupts the hypothalamic–pituitary–adrenal (HPA) axis, which impacts the regulation of hormones such as DHEA (product of HPA axis) (14) as well as testosterone through modulation of the hypothalamic–pituitary–gonadal (HPG) axis. There, HPG function has shown to modulate HPA function as well (15). As anabolic steroids, these hormones have neuroprotective effects on hippocampus in rodent models (16–27), and thus dysregulation of DHEA or testosterone could have negative impacts on hippocampal development. Here we test the hypotheses that modulation of trajectories of testosterone and DHEA across development is one pathway linking poverty to disruptions in development of the hippocampus and emotion dysregulation and increased risk for depression.
Many forms of early adversity have been found to relate to hippocampal structure and function, including reductions in hippocampal volume associated with poverty (28–37), reduced maternal support (38, 39), and abuse/adverse childhood experiences (ACEs) (40–42). There is also evidence for associations between early poverty and altered hippocampal connectivity (43) and function (40, 41), though this evidence is less robust than for hippocampal volume. Interestingly, such relationships may be most apparent in children and adolescents, with less consistent evidence for an association in adults (44, 45). These findings in humans are consistent with the animal literature showing opposite effects of stress and environmental enrichment on hippocampal cell proliferation and dendritic length and branching (46–49).
There is evidence that hippocampus is important for appropriate stress reactivity, disruptions of which increase risk for emotion dysregulation and the development of mental health disorders, including depression (50). In fact, Palacios-Barrios and colleagues have argued that emotion dysregulation, and impairments in self-regulation more broadly, may be a final common pathway linking early adversity, alterations in hippocampal development and other brain regions, and poor physical and mental health outcomes (37). Most of this research has focused on a pathway that involves dysregulation of the HPA axis (51), with the argument that chronic stress leads to heightened response to stress and glucocorticoid secretion, which disrupt hippocampal development (52–54), which may in turn contribute to emotion dysregulation and negative emotion lability. However, substantially less attention has been paid to other endocrine axes, such as the HPG axis biomarkers of DHEA and testosterone, which are also stress responsive and impact the hippocampus, and thus could also contribute to emotion dysregulation.
Specifically, the relationships of early adversity, including poverty, to the development of HPG axis biomarkers has been less examined. Both acute and chronic stress can impact DHEA, as well as testosterone through HPG modulation. Using puberty as a proxy for HPG development, some data suggest that early adversity, stress, and poverty can accelerate pubertal development and maturation/aging (55–58). While the rise in HPG axis biomarkers are responsible for pubertal maturation, HPG hormonal biomarkers and pubertal status are not interchangeable and exert unique development effects (59–61). Intriguingly, there is evidence from the animal literature that chronic stress can reduce the responsiveness of the HPG axis, which is responsible for stimulating the release of hormones such as testosterone and estrogen from the ovaries and the testes (62, 63). Further, there is evidence that both DHEA and testosterone are neuroprotective and can support neurogenesis and cell survival in the hippocampus in adult rodents (16–27), though less is known about developmental effects (64). Thus, it is possible that early poverty and its associated chronic stress might alter the timing or level of DHEA and/or testosterone, and in turn have impacts on hippocampal development via the role of these hormones as anabolic steroids that can have neuroprotective effects on hippocampus in rodent models (16–27).
The literature reviewed above indicates that (i) poverty and stress are associated with reduced hippocampal volume, (ii) increased stress can be associated with altered testosterone and/or DHEA, and (iii) testosterone and DHEA support hippocampal neurogenesis and have neuroprotective effects. Together, these findings suggest that there could be relationships between early adversity, including poverty, and DHEA or testosterone, and this in turn could contribute to disruptions in hippocampal volume in humans and subsequent emotion dysregulation, negative emotion lability, and poor mental health outcomes, including increased risk for depression. A few studies have begun to examine the relationship between hormones such as DHEA and testosterone and brain development, including the hippocampus, with mixed results. Several studies have found no relationships between testosterone and hippocampal volume across development (65, 66) or in adolescence (67), and one small study found a negative relationship (68). However, other work has found a positive relationship between hippocampal volume and DHEA in school-age male and female children and a positive relationship to testosterone in girls (69). Importantly, a recent study of 600+ scans from participants of ages 8 to 29 y found strong evidence for a positive relationship between testosterone level and hippocampal volume in both males and females even when controlling for age. However, the majority of these studies have focused on between-person differences in hormone levels rather than within-person change over pubertal development. Developmental data are critical to understand this process during the transition from school age to adolescence given the key role of hormones on neurodevelopment during this period. To date, there have been few data to inform the relationships between trajectories of testosterone or DHEA levels and hippocampal volume change over this developmental period, or whether this is associated with early poverty and/or emotion dysregulation or depression risk.
The goal of the current study was to use longitudinal data from the Preschool Depression Study to explore potential hormonal mechanisms for the relationship between adversity and brain development. We examined relationships of early poverty (preschool) to trajectories of testosterone and DHEA levels and the relationships of these hormones to patterns of hippocampal volume development. We also sought to investigate subsequent relationships of adversity and hormones and hippocampal development to later emotion dysregulation/negative lability and risk for depression. More specifically, we wished to test the hypotheses that (i) greater poverty early in life would be associated with lower overall levels and/or shallower slopes of testosterone and DHEA levels across development, (ii) lower levels and shallower slopes of testosterone and/or DHEA would be associated with small hippocampal volumes, and (iii) hormonal levels and hippocampal volumes would mediate, at least in part, a relationship between early poverty and emotion regulation and depression in adolescence/young adulthood. Further, to test the specificity of such effects, we conducted parallel analyses in several comparison brain regions, including amygdala, caudate, dorsal anterior cingulate, and dorsolateral prefrontal cortex.
Results
Participant Characteristics.
Participants were a subsample of youth enrolled in an ongoing, longitudinal study focused on examining the trajectory of preschool-onset depression and brain development (SI Appendix, Fig. S1 shows a flowchart of the study). These children have had between one and nine assessment waves and between one and four scan waves (SI Appendix, Fig. S1). Given the goals of the study, we focused our analysis on youth who had data on poverty from T1 (thus not including the 42 children added at MRI1) who also had both hormone and hippocampal volume data (N = 167).
The demographic characteristics and means on all of the variables of interest for these children included are shown in Table 1. These children did not differ from those not analyzed (i.e., who did not have T1 income-to-needs or hormone/hippocampal data) on any hormone, hippocampal, emotion dysregulation, or depression variable analyzed, or on age at T9 or sex (all P > 0.37).
Table 1.
Participant characteristics
| Characteristic | Mean | SD | Min | Max |
| Age, y | 15.83 | 1.11 | 13 | 19 |
| T1 income-to-needs | 1.99 | 1.11 | 0 | 3.93 |
| MRI1 natural log testosterone | 3.72 | 0.35 | 3.50 | 4.12 |
| MRI2 natural log testosterone | 4.07 | 0.49 | 2.20 | 5.08 |
| MRI3 natural log testosterone | 4.09 | 0.48 | 2.78 | 5.56 |
| T9/MRI4 natural log testosterone | 4.47 | 0.74 | 2.88 | 7.18 |
| Log testosterone slope across MRI1–T9/MRI4 | 0.11 | 0.12 | -0.19 | 0.27 |
| Log testosterone intercept across MRI1–T9/MRI4 | 4.12 | 0.18 | 3.57 | 4.66 |
| MRI1 natural log DHEA | 3.78 | 0.38 | 3.34 | 4.01 |
| MRI2 natural log DHEA | 4.35 | 0.75 | 1.77 | 5.60 |
| MRI3 natural log DHEA | 4.46 | 0.75 | 1.63 | 5.97 |
| T9/MRI4 natural log DHEA | 5.14 | 0.65 | 3.37 | 6.90 |
| Log DHEA slope across MRI1–T9/MRI4 | 0.19 | 0.08 | −0.078 | 0.51 |
| Log DHEA intercept across MRI1–T9/MRI4 | 4.50 | 0.46 | 3.01 | 5.58 |
| MRI1 hippocampal volume, cm3 | 4.069 | 0.38 | 3.05 | 5.44 |
| MRI2 hippocampal volume, cm3 | 4.074 | 0.40 | 2.92 | 5.48 |
| MRI3 hippocampal volume, cm3 | 4.095 | 0.41 | 3.04 | 5.56 |
| Hippocampal slope across MRI1–MRI3, cm3 | 0.018 | 0.0057 | 0.0011 | 0.037 |
| Hippocampal intercept across MRI1–MRI3, cm3 | 4.19 | 0.39 | 3.05 | 5.45 |
| T9/MRI4 emotion dysregulation | 25.76 | 7.28 | 15 | 48 |
| T9/MRI4 Child Depression Inventory | 48.03 | 8.03 | 40 | 90 |
Participant breakdown by sex: 48.5% female; by race, 38.9% Black.
Does Early Poverty Predict Hormones, Hippocampal Volume, Emotion Dysregulation, or Depression?
We started by using hierarchical linear regressions to determine whether early poverty (T1 income-to-needs) predicted the variables of interest. As shown in Table 2, greater early poverty (i.e., lower T1 income-to-needs) predicted shallower testosterone increase over adolescence (i.e., flatter slope), but did not significantly predict testosterone intercepts. Of note, consistent with a lack of results for testosterone intercepts, T1 income-to needs did not predict testosterone individually at MRI 1, 2, or 3 (all P > 0.14), though it did at MRI4/T9 (B = 0.11, t = 2.28, P = 0.024). Early poverty did not predict DHEA intercepts or slopes (Table 2). In addition, greater early poverty predicted a shallower slope of hippocampal growth across development. Further, greater early poverty predicted both greater emotion dysregulation and greater depression at T9 (average age 15.8 y). All of these significant effects survived FDR correction. Notably, poverty did not significantly interact with sex to predict any additional variance (Table 2). Further, all significant predictions from T1 income-to-needs remained significant if T1 depression scores were also included as an additional predictor, including prediction of adolescent (T9/MRI4; SI Appendix, Fig. S1) youth-reported depression (SI Appendix, Table S1), as well as when controlling for pubertal status at T9/MRI4 (SI Appendix, Table S2). T1 income-to-needs did not relate to any of the comparison brain regions (i.e., amygdala, caudate, dACC, DLPFC; SI Appendix, Table S3).
Table 2.
Early poverty predicting hormones, hippocampal volume, emotion dysregulation, and depression
| Outcome variable | B | Lower 95% CI | Upper 95% CI | t | P | R2 adj. step 1 |
| Prediction from T1 income-to-needs in step 1 | ||||||
| Testosterone slope | 0.069 | 0.006 | 0.132 | 2.18 | 0.031* | 0.838 |
| Testosterone intercept | 0.032 | −0.098 | 0.163 | 0.49 | 0.627 | 0.281 |
| DHEA slope | 0.067 | −0.078 | 0.212 | 0.91 | 0.365 | 0.059 |
| DHEA intercept | 0.012 | −0.124 | 0.148 | 0.18 | 0.859 | 0.128 |
| Hippocampal slope | 0.251 | 0.097 | 0.405 | 3.22 | 0.002** | 0.048 |
| T9 emotion dysregulation | −0.340 | −0.500 | −0.180 | −4.19 | 0.001*** | 0.096 |
| T9 Child Depression Inventory | −0.226 | −0.393 | −0.059 | −2.68 | 0.008* | 0.060 |
| Prediction from interaction between T1 income-to-needs and sex in step 2 | ||||||
| Testosterone slope | 0.044 | −0.018 | 0.107 | 1.39 | 0.166 | 0.839 |
| Testosterone intercept | 0.114 | −0.016 | 0.244 | 1.74 | 0.084 | 0.290 |
| DHEA slope | 0.007 | −0.139 | 0.153 | 0.10 | 0.922 | 0.059 |
| DHEA intercept | 0.087 | −0.049 | 0.223 | 1.26 | 0.208 | 0.131 |
| Hippocampal slope | 0.033 | −0.121 | 0.188 | 0.43 | 0.671 | 0.043 |
| T9 emotion dysregulation | −0.080 | −0.240 | 0.080 | −0.99 | 0.326 | 0.096 |
| T9 Child Depression Inventory | −0.130 | −0.296 | 0.037 | −1.54 | 0.125 | 0.070 |
FDR-adjusted P < 0.05*; <0.01**, <0.005***. Significant values are presented in boldface type.
Are Testosterone Slopes and Hippocampal Volume Slopes or Intercepts Related?
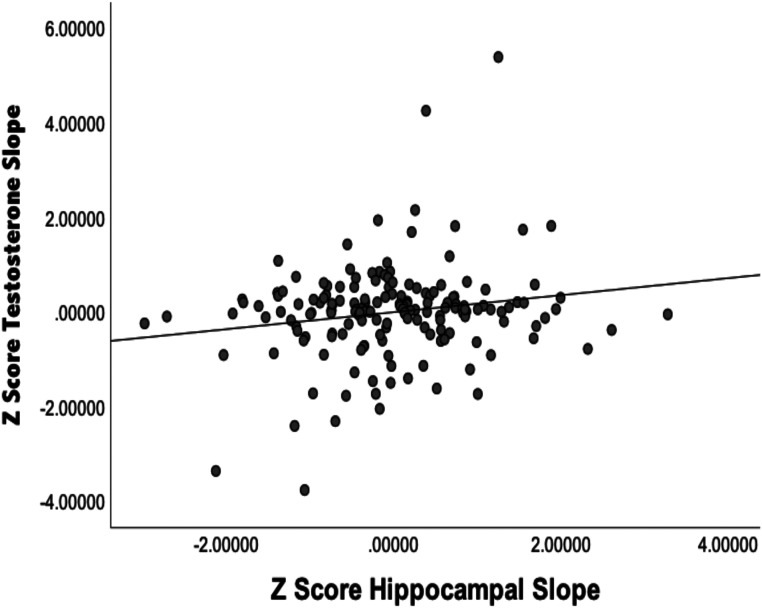
Next, we used hierarchical linear regression to examine the relationships between testosterone slopes and hippocampal volume slopes, as both were related to early poverty. As shown in Fig. 1, a greater increase in testosterone over development (larger slope) was associated with a greater increase in hippocampal growth over adolescence (R2Adj = 0.02, B = 0.447, bootstrapped CI 95%+/− = 0.068 to 826, t = 2.33, P = 0.021). There were again no significant interactions with sex (P > 0.64). Testosterone slopes did not relate to any of the comparison brain regions (SI Appendix, Table S4), indicating some level of specificity.
Fig. 1.
Graph illustrating the relationships between testosterone slopes and hippocampal slopes.
Do Testosterone Slopes or Hippocampal Volume Slopes Relate to Emotion Dysregulation or Depression?
As shown in Table 3, hierarchical linear regressions indicated that greater testosterone increase across development (larger slope) related to both lower emotion dysregulation and lower child-reported depression at T9 (mean age 15.8 y). Further, greater hippocampal volume growth across adolescence (larger slope) also related to lower emotion dysregulation, though not lower depression (Table 3). There were again no significant interactions with sex (Table 3), and all results remained the same when T9 pubertal status was added as a covariate. None of the comparison regions significantly related to either emotion dysregulation (P = 0.079 to 0.718) or depression P = 0.395 to 0.859).
Table 3.
Hormones and hippocampal volume predicting emotion dysregulation and depression In Table 2, is the explanation of boldface values correct as added?
| Variable | B | Lower 95% CI | Upper 95% CI | t | P | R2 adj. step 1 |
| Main effect of testosterone slope in step 1 | ||||||
| T9 emotion dysregulation | −0.406 | −0.804 | −0.009 | −2.02 | 0.045 | 0.011 |
| T9 Child Depression Inventory | −0.463 | −0.884 | −0.043 | −2.18 | 0.031 | 0.043 |
| Interaction between testosterone slope and sex in step 2 | ||||||
| T9 emotion dysregulation | 0.875 | −0.671 | 2.42 | 1.12 | 0.265 | 0.013 |
| T9 Child Depression Inventory | 0.199 | −1.52 | 1.92 | −0.23 | 0.819 | 0.036 |
| Main effect of hippocampal slope in step 1 | ||||||
| T9 emotion dysregulation | −0.298 | −0.454 | −0.141 | −3.76 | 0.001 | 0.076 |
| T9 child depression inventory | −0.014 | −0.181 | 0.152 | −0.170 | 0.865 | 0.031 |
| Interaction between hippocampal slope and sex in step 2 | ||||||
| T9 emotion dysregulation | 0.040 | −0.281 | 0.361 | −0.247 | 0.805 | 0.069 |
| T9 child depression inventory | −0.277 | −0.617 | 0.063 | −1.61 | 0.109 | 0.050 |
Significant values are presented in boldface type.
Mediation.
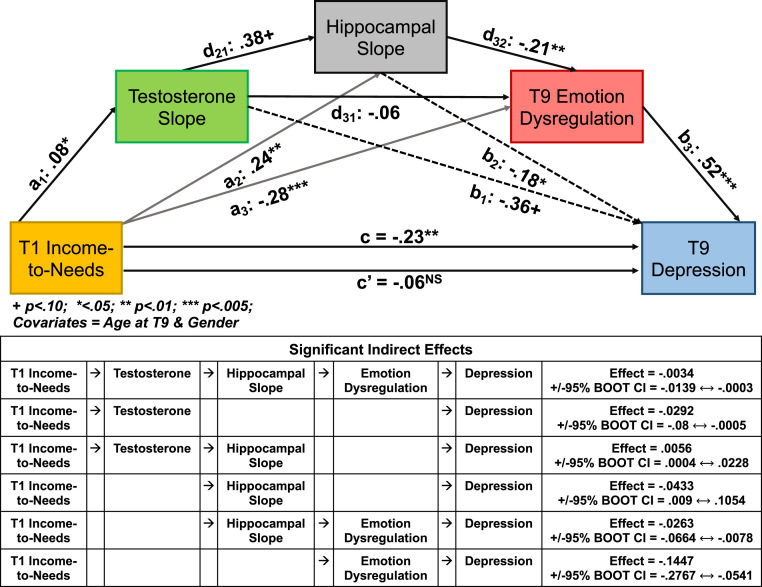
We next tested the hypotheses that early poverty related to emotion dysregulation based on parent report and risk for depression (child report on the CDI-I) through modulation of testosterone and hippocampal volume growth across adolescence. This serial mediation model is depicted in Fig. 2, using the notation suggested by Hayes (70). As shown in Fig. 2, this model indicated significant mediation, such that the direct effect of T1 income-to-needs on depression in adolescence (T9) was no longer significant with testosterone slope, hippocampal volume slope, and emotion dysregulation in the model. Importantly, as shown in Fig. 2, the full indirect effect pathway linking T1 income-to-needs to depression was significant via the path from testosterone slope across adolescence to hippocampal slope across adolescence to emotion dysregulation, as well as some additional indirect pathways. In addition, this model is robust to the inclusion of depression symptoms at T1 as an additional covariate, becoming, if anything, stronger, with the full indirect serial mediation path remaining significant (SI Appendix, Fig. S2), with a similar result when including T9 pubertal status as a covariate (SI Appendix, Fig. S3). Further, as shown in SI Appendix, Fig. S4, with inclusion of the same assessment of emotion dysregulation at MRI3 (one wave earlier than the depression assessment), this same serial mediation model is significant, with the full indirect path continuing to be significant.
Fig. 2.
Results of serial mediation model between early poverty and adolescent depression. (Top) Illustration of the components of the serial mediation model using the Hayes notation. (Bottom) Chart illustrating all of the individually significant indirect effects.
In addition, this full indirect serial mediation path remains significant if you control for lifetime history of medication use (effect = −0.0046, bootstrap SE = 0.0038, +/− 95% bootstrap CI = −0.0176 to −0.0004) or lifetime history of externalizing disorders such as attention deficit hyperactivity disorder or conduct disorder (effect = −0.0023, bootstrap SE = 0.0024, +/− 95% bootstrap CI = −0.0121 to −0.0001). Notably, examination of the comparable mediation of early poverty to externalizing problems is not significant (effect = −0.0104, bootstrap SE = 0.0114, +/− 95% bootstrap CI = −0.0507 to 0.0001), providing some evidence of specificity. Importantly, if we switched the order of effects to put hippocampal slope before testosterone, the full indirect path is no longer significant (effect = −0.0005, bootstrap SE = 0.0017, +/− 95% bootstrap CI = −0.0058 to 0.0017). Further, if we test an alternative model asking whether depression and emotion regulation at MRI1 mediate the relationship between T1 income-to-needs and testosterone and hippocampal slopes, there is no significant mediation (effect = 0.0506, bootstrap SE = 0.0481, +/− 95% bootstrap CI = −0.0375 to 0.1638). There was also no significant mediation by T1 depression (effect = −0.003, bootstrap SE = 0.006, +/− 95% bootstrap CI = −0.0217 to 0.0046).
Discussion
The results of the current study provide evidence consistent with the hypothesis that one pathway mediating the relationship between early childhood poverty and later risk for emotion dysregulation and depression is disruption in the systems regulating testosterone and related hippocampal volume development. More specifically, we found that early poverty, even when controlling for early depression, related to shallower testosterone slopes across the transition from school age to adolescence, as well as smaller intercepts and shallower slopes of hippocampal volume. In turn, steeper increases in testosterone across development related to less emotion dysregulation and depression in late adolescence, with steeper slopes of hippocampal volume also predicting less depression. Further, mediation models indicated that the relationship between early poverty and adolescent depression was fully mediated by a pathway through testosterone to hippocampal volume to emotion dysregulation, even when controlling for early levels of depression and lifetime history of externalizing psychopathology. These findings provide evidence about a key previously underexplored pathway in brain development and emotion dysregulation that may be disrupted by early poverty and which may contribute to lifetime risk for depression, providing a potentially novel intervention target. These results also point to a potential mechanism for the established effects of poverty on hippocampal development through the effects of androgenic hormones, which have been shown in a rodent model to facilitate neurogenesis in the hippocampus by promoting cell survival (20–22).
Our findings extend the literature suggesting that early poverty is contributing to disruptions in HPG axis function, which is responsible for stimulating the release of hormones such as testosterone (62, 63). As described in the Introduction, the animal literature suggests that chronic stress can reduce the responsiveness of the HPG axis, but studies which find that psychosocial stress advances pubertal onset, timing, and tempo suggest that stress would increase the responsiveness of the HPG axis and testosterone (14, 56). Our findings are consistent with the animal literature insofar as poverty was associated with lower testosterone and a shallow developmental trajectory. It was also notable that that testosterone was not interchangeable with puberty, as those statistical associations were independent of puberty and the largest links between poverty and low testosterone were, on average, age 15 y, and the bulk of pubertal maturation would be completed. Like others (60, 71), our findings suggest that testosterone is distinct from puberty, and we extend this to an understudied measure of testosterone’s developmental trajectory.
Our results are also consistent with prior work suggesting that testosterone may have beneficial effects on hippocampal development, including evidence for a positive relationship to neurogenesis and cell proliferation in rodents (16–22). Further, our data showing a positive relationship between testosterone slope and hippocampal slope across development are consistent with findings from the majority of the previous studies examining the relationship between androgen levels and brain development, which have also found similar positive relationships (61, 69). Importantly, previous research has primarily been cross-sectional, and our results extend this work by showing that steeper increases in testosterone within an individual are associated with steeper increases in hippocampal volume. Further, we did not find similar effects for any of our comparison regions, including amygdala, caudate, dorsal anterior cingulate, or dorsolateral prefrontal cortex. This suggests some level of specificity to hippocampus. However, while there was a significant relationship between testosterone and hippocampal volume, and the serial mediation model was significant, there was clearly variation in the relationship between early poverty and hippocampal volume that was not accounted for by testosterone slopes. There are likely a number of factors associated with poverty that could also be influencing hippocampal development, including stress/cortisol, nutrition, and exposure to environmental toxins that also need to be examined in future research.
A key question is how our findings fit into the context of prior work on pubertal timing and risk for depression. The literature is mixed in terms of such relationships. Some studies suggest that earlier pubertal timing (72, 73) and higher levels of testosterone (72) are associated with greater risk of depression in adolescence, at least among girls. In contrast, there is also literature to suggest that testosterone can be protective against anxiety and depression (74) and evidence that higher testosterone levels are associated with lower depression (69, 75), or even both depending on the absolute level of testosterone (76). Our findings are more consistent with the later studies on the protective effects of testosterone. Interestingly, we did not find any evidence of sex differences in these relationships, which is consistent with the evidence that, although testosterone levels reach overall higher levels in males, they are important for development in both males and females. For example, Wierenga et al. found that testosterone level better predicted hippocampal volume than chronological age for both boys and girls (61). Further, we did not find evidence for any similar relationships to externalizing psychopathology outcomes, suggesting at least some specificity to depression.
The strengths of the current study center on its prospective longitudinal assessments of multiple constructs relevant to testing hypotheses about the mechanisms by which early poverty might relate to risk for depression through neurodevelopmental processes. However, there are also a number of limitations to the current study. First, we did not have measures of hippocampal volume in preschool, as imaging only started when the children entered school age. As such, we do not know how early relationships of hippocampal volume to poverty and testosterone levels emerge. Second, we had measures of only testosterone and DHEA in the current study, and not estradiol and progesterone. It is intriguing that we did not also see similar findings for DHEA. The release of DHEA as part of adrenarche developmentally occurs earlier than HPG development, typically occurring between the ages of 6 and 9 y (60). Our first wave of hormone assessment (MRI1) included children ages 6 to 12 y, but only 43% were age 9 y or younger at that first assessment, and only 7% were aged 9 y at MRI2. Thus, we may have missed adrenarche for many of the children, meaning that it is possible that we may have seen stronger relationships of poverty to DHEA had we started assessing children even younger. Third, the methods for hormone assessment changed at the fourth wave for all youth, limiting inferences about absolute changes at the average level, something we did not attempt to do (our focus was on individual differences). Fourth, we did not have measures of cortisol, which will be important to examine to more fully test hypotheses as to whether the relationships between early poverty and HPG function were mediated by a relationship of early poverty to HPA function. Fifth, there was little SES mobility in our sample, such that children who experienced early poverty continued to experience poverty throughout development, meaning that we could not isolate the specific effects of early poverty from chronic poverty. Sixth, the results of the mediation model should be taken as evidence consistent with our hypotheses, but not all of the measures meet the temporal precedence necessary for a more definitive test of a causal model, something that will need to be addressed in future work where assessments of all constructs are acquired at every time point through development.
The pathways by which poverty contributes to negative developmental outcomes in children are multifaceted and include factors such as limitations on educational opportunities, family stress, and adverse environmental exposures, such as lead, cigarette smoke, poor nutrition, and air pollution (6, 9, 77). Here we provide evidence consistent with the idea that part of this pathway includes disruptions in hormonal function that may in turn contribute to disruptions in hippocampal development and subsequent risk for emotion dysregulation and depression. These results seem to be relatively specific to testosterone (as compared to DHEA) and to hippocampus (as compared to amygdala, caudate, dACC, and DLPFC). Such findings provide a perspective on this risk pathway, providing evidence for a potential avenue for early intervention that may help promote healthy brain development even in the face of adversity. Direct modulation of hormone levels as an intervention strategy would require a very high level of evidence to justify. However, factors that may modulate the relationship between poverty and hormones (e.g., stress reactivity interventions, environmental supports) are safer and more feasible targets, and hormone levels may be important indicators of the effectiveness of such interventions that could help guide timing and dose.
Methods
Participants.
At baseline, 306 children aged 3.0 to 5.92 y and their primary caregivers were recruited from the St. Louis, MO, area, using a checklist to oversample preschoolers with elevated symptoms of depression (78) and then followed longitudinally. At school age (7–12 y), healthy children and those with a history of depression from this sample were invited for participation in brain imaging, along with recruitment of an additional 42 healthy children (n = 210 completed the first wave of imaging). Exclusion criteria included (i) head injury with loss of consciousness >5 min, (ii) neurological illness, (iii) diagnosis of an autism spectrum disorder, (iv) treatment for lead poisoning, or (v) contraindications for MRI scanning (added starting at first scan wave). All study methods were reviewed and approved by the institutional review board at the Washington University School of Medicine (IRB no. 201502094; PDS-III Imaging). Written informed consent and assent was obtained from all study participants.
Poverty.
Poverty was operationalized as the income-to-needs ratio, which was defined as the total family income divided by the federal poverty level based on family size (79). The value was calculated based on T1 (SI Appendix, Fig. S1) data of caregiver-reported total family income and total number of people living in the household.
Emotion Regulation.
In order to assess youths’ emotional dysregulation, the caregivers completed the Emotion Regulation Checklist (ERC) (80) at each of the last four assessment waves (SI Appendix, Fig. S1, MRI1, MRI2, MRI3, T9/MRI4). The ERC targets affective lability, intensity, valence, and flexibility and includes both positively and negatively weighted items rated on a four-point Likert scale. It has two subscales: emotional regulation (higher scores indicate more positive/effective emotion regulation) and negative lability (higher scores indicate more emotional lability and less effective emotion regulation). Here we focus on the negative lability score at T9/MRI4 (with supplemental analysis from MRI3) as capturing emotion dysregulation. A higher score indicates greater emotion dysregulation.
Parent-Reported Depression at Preschool Age.
Trained staff from the Early Emotional Development Program conducted up to nine in-person assessment sessions with participants and their primary caregivers over the course of the study (SI Appendix, Fig. S1). The children were between the ages of 3.0 and 5.11 y at the time of their first interview (T1) and between the ages of 13.3 and 19.4 at the most recent assessment wave (T9/MRI4). The first three interviews (T1 to T3) used the Preschool-Age Psychiatric Assessment (PAPA) (81, 82) as a diagnostic assessment. The PAPA is designed for diagnostic use with children ages 2.0 to 6.0 y (but has been used up to age 8.0 y), has acceptable reliability (83), and consists of a series of developmentally appropriate questions answered by the primary caregiver, which cover the DSM-IV criteria for all Axis I disorders, including MDD, ADHD, and anxiety disorders. We created a dimensional T1 depression score by computing the number of core depression items from the PAPA endorsed by the parents at T1 (SI Appendix, Fig. S1) to give us an estimate of depression levels in children at the first wave.
Child-Reported Depression.
Youths completed the Child Depression Inventory (CDI) (83) at each of the four MRI assessments waves, on the day of scanning (SI Appendix, Fig. S1, MRI1, MRI2, MRI3, T9/MRI4), with the CDI-I at MRI1 to MRI3 and the CDI-II at T9/MRI4. This measure assesses a range of depression symptoms and has good reliability and validity (84). A higher score indicates greater depression. Our primary outcome variable was the CDI score at T9/MRI4.
Testosterone and DHEA Assessment and Analysis.
Puberty hormones were assessed across four waves through saliva samples starting at MRI1 through T9/MRI4 (SI Appendix, Fig. S1), along with pubertal status using a validated self-report measure, the Pubertal Development Scale (85). In the first three waves of hormone assessment (MRI1 to 3), saliva was collected at the laboratory on the morning of the scan, typically between 2 and 4 h post waking. Children were instructed to not eat for at least 60 min prior to arrival, to avoid dairy products for 20 min prior to sample collection, and to not have sugary, acidic, or caffeinated products immediately prior to arrival (confirmed by questionnaire at time of sample collection). Each child provided three samples (10, 30, and 75 min after arrival) through passive drool cryovials that were immediately refrigerated. On completion of the session, the research assistants pooled 500 μL from each cryovial into a fresh tube that was then frozen at −80 °C. For T9/MRI4, sample collection switched to at home within 30 min of rising (before eating or brushing their teeth). Youth were sent home from their behavioral assessment session at T9/MRI4 with a passive drool cryovial kit and an ice pack. Youth were instructed to immediately place the saliva sample in the freezer and to bring it to the MR session in the ice pack kit. For males as well as females who had not yet had a menstrual cycle, data collection occurred on the day of the scan. For females who had started menstruating but were not taking birth control, saliva collection occurred in the first day of their menstrual cycle. For females taking oral birth control, saliva collection occurred when they took the last pill in their birth control pack. Youth were called and reminded of data collection procedures the night before. Saliva samples were transferred to the −80 °C freezer as soon as youth arrived for the MR scan.
The saliva samples were assayed for testosterone and DHEA by the Washington University Core Lab facilities using ELISA kits from Salimetrics that employed competitive immunoassay validated to measure testosterone and DHEA. For DHEA, the standard curve ranged from 10.2 to 1,000 pg/mL, with samples repeated if duplicates differed by more than 15% (two samples). The between-assay CVs, based on the controls in each run, were 6.3% at 56.4 pg/mL and 6.5% at 538.9 pg/mL. For testosterone, the standard curve ranged from 6.1 to 600 pg/mL. Samples above 600 pg/mL were repeated on dilution. The between-assay CVs were 12.1% at 15.1 pg/mL and 2.7% at 165.4 pg/mL
As is typical, testosterone and DHEA values were log-transformed. To generate individual intercepts and slopes of testosterone and DHEA at S1 to S4 for each youth, we used multilevel linear models (MLMs) that included both random intercept and random slope components (with an unstructured covariance matrix between the two). Time was coded as age at scan and was centered at median age 13 y. Separate models were run for males and females. In partial correlations controlling for sex, the intercepts and slopes of testosterone were only modestly related (r = 0.25), so we examined both in relationship to early poverty.
Imaging Acquisition.
The focus of imaging data in the current manuscript is on how the trajectory of hippocampal volume development relates to poverty and testosterone and DHEA levels. As such, we focus on the hippocampal data from MRI scans 1 to 3, which have already been processed through the FreeSurfer Longitudinal pipeline (see below). Structural images were also acquired at T9/MRI scan 4. However, MRI scan 4 switched to using a 3.0-T Siemens Prisma whole-body scanner with a 32-channel head coil using Human Connectome Project-style acquisitions (86). We are determining the best way to integrate the first three scan waves of Trio scanner data with the newer Prisma data. Thus, the current analyses focus on scan waves 1 through 3, which were performed using a 3.0-T Siemens Tim Trio whole-body scanner with a 12-channel head coil. Quality-assurance measures included having subjects practice in an MRI simulator, evaluating head motion during structural scans, and recollection of data if necessary. Structural data were obtained using two 3D T1-weighted scans (TR 2,300 ms, TE 3.16 ms, TI 1,200 ms, flip angle 8°, 160 slices, 256 × 256 matrix, field of view 256 mm, 1.0-mm3 voxels, 6:18 min per scan) in the sagittal plane using a magnetization-prepared rapid gradient echo (MPRAGE) sequence. Two resting-state fMRI (rsfMRI) scans were obtained during the same session with T2*-weighted gradient-echo echoplanar sequence; neither of these modalities is of focus here.
Structural Imaging Processing.
Hippocampal volumes were generated using the same longitudinal FreeSurfer processing stream as in Luby et al. (2016) (87). Specifically, for each scan session, the two MPRAGE scans were assessed visually, and the best in terms of quality and contrast selected by blind raters. Processing of structural data was accomplished using the FreeSurfer longitudinal pipeline v5.3 (http://surfer.nmr.mgh.harvard.edu) (88). When necessary, visual inspection of the white and pial surfaces for errors and regeneration with manual intervention to correct for errors was completed by an experienced rater blinded to diagnostic category. Processing steps included skull stripping, atlas registration, spherical surface registration, and parcellation. Importantly, the longitudinal “stream” included initialization from an unbiased within-subject template (created across the longitudinal scans), which reduces the bias that would otherwise be present in selecting a single scan as “baseline.” Using an unbiased longitudinal template significantly increases reliability and statistical power (89). For ∼10% of sessions, poor scan quality (in both MPRAGEs) required excluding those sessions from the longitudinal analysis (n = 29, n = 22, and n = 18 at the three waves, respectively). In those cases, FreeSurfer’s longitudinal stream was run using the remaining available sessions for that participant.
Volume of the left and right hippocampus in the subject’s “native space” were obtained using FreeSurfer’s “aseg.stats” report. We did not have a priori hypotheses about left or right hippocampus, and thus we averaged the two together even though the patterns were the same for left and right hippocampus. To generate individual intercepts and slopes of hippocampal volume at S1 to S3 for each youth, we used an MLM that included both random intercept and random slope components (with an unstructured covariance matrix between the two). Time was coded as age at scan and was centered at median age 12 y. The model included sex (0 = male, 1 = female) as covariate. This was the same type of model used in our prior work to examine the relationship between maternal support and hippocampal developmental trajectories (87). Of the 167 youth included in these analyses, there were 105 with 3 scans, 41 with 2 scans, and 21 with 1 scan. In partial correlations controlling for sex, hippocampal slopes and intercepts were highly correlated (r = 0.90), and thus we just focus on hippocampal slope, though the results were essentially identical with the intercepts. In addition, to assess the specificity of any results to the hippocampus, we generated the same measures for the amygdala and caudate (from “aseg.stats”) and for the dorsal anterior cingulate (G_and_S_cingul_Mid_Ant_volume) and dorsolateral prefrontal cortex (G_front_middle_volume + S_front_middle_volume) from the Destrieux Atlas (90) as comparison regions, since disruptions in all have been associated with poverty and/or depression, but the animal literature has focused on hippocampus as a key locus of the potential neuroprotective effects of HPG hormones.
Statistical Analysis.
All variables were examined for distributions and outliers. There was one outlier in the hormone slopes, and that value was Winsorized to the 99th percentile of the data. We started by examining the relationships of early poverty to each of the outcomes of interest (testosterone and DHEA slopes and intercepts across MRI1 to T9/MRI4, hippocampal slopes and intercepts across MRI1 to 3, T9/MRI4 emotion dysregulation, T9/MRI4 depression). Using hierarchical regressions, T1 income-to-needs was entered as a predictor in step 1, along with sex as a covariate, and then the interaction between T1 income-to-needs and sex was entered as well to determine if it accounted for an increase in variance, which would indicate a different relationship for males and females. Regressions predicting the hormones and hippocampal volume did not include age as a predictor because the multilevel models used to generate individual slopes and intercepts used age. The regressions predicting emotion dysregulation and depression included age at T9 as an additional covariate as well as sex in step 1. FDR was used to correct for multiple comparisons across all eight of these initial regressions. For any significant regression, we then asked if T1 income-to-needs continued to predict if we also included the T1 parent-reported depression score. Next, we examined whether any of the hormone or hippocampal measures that were related to poverty were related to each other or to either emotion dysregulation or CDI-I depression at T9/MRI4, again using hierarchal regression with sex as a main effect in step 1 and the interaction with sex in step 2. Last, we conducted serial mediation analyses using the PROCESS procedure (model 6) in SPSS (91, 92) with age at T9/MRI4 and sex as covariates. We did not include moderation by sex in the regressions since none of the regressions found any significant interactions with sex.
Data Sharing.
The study data were collected prior to the common use of consent forms that allow broad data sharing and the depositing of data in data repositories. However, anonymized data and code can be requested from the first author with a signed data use agreement.
Supplementary Material
Acknowledgments
This study was supported by grants 2R01 MH064769-06 and R01 MH098454.
Footnotes
Competing interest statement: J.L.L. receives royalties from Guilford Press.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004363117/-/DCSupplemental.
References
- 1.Dufford A. J., Kim P., Evans G. W., The impact of childhood poverty on brain health: Emerging evidence from neuroimaging across the lifespan. Int. Rev. Neurobiol. 150, 77–105 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Reynolds R. M., Labad J., Buss C., Ghaemmaghami P., Räikkönen K., Transmitting biological effects of stress in utero: Implications for mother and offspring. Psychoneuroendocrinology 38, 1843–1849 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Schwabe L., Bohbot V. D., Wolf O. T., Prenatal stress changes learning strategies in adulthood. Hippocampus 22, 2136–2143 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Carneiro P. M., Heckman J. J., Eds., Human Capital Policy, (MIT Press, Cambridge, MA, 2003). [Google Scholar]
- 5.Brooks-Gunn J., Duncan G. J., The effects of poverty on children. Future Child. 7, 55–71 (1997). [PubMed] [Google Scholar]
- 6.Freedman D., Woods G. W., Neighborhood effects, mental illness and criminal behavior: A review. J. Politics Law 6, 1–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung J. T., Shek D. T., Poverty and adolescent developmental outcomes: A critical review. Int. J. Adolesc. Med. Health 23, 109–114 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Perkins S. C., Finegood E. D., Swain J. E., Poverty and language development: Roles of parenting and stress. Innov. Clin. Neurosci. 10, 10–19 (2013). [PMC free article] [PubMed] [Google Scholar]
- 9.Rauch S. A., Lanphear B. P., Prevention of disability in children: Elevating the role of environment. Future Child. 22, 193–217 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Raver C. C., Low-income children’s self-regulation in the classroom: Scientific inquiry for social change. Am. Psychol. 67, 681–689 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair C., Raver C. C., Poverty, stress, and brain development: New directions for prevention and intervention. Acad. Pediatr. 16 (3, suppl.), S30–S36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa H., Aber J. L., Beardslee W. R., The effects of poverty on the mental, emotional, and behavioral health of children and youth: Implications for prevention. Am. Psychol. 67, 272–284 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Hanson J. L. et al., A family focused intervention influences hippocampal-prefrontal connectivity through gains in self-regulation. Child Dev. 90, 1389–1401 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belsky J., Ruttle P. L., Boyce W. T., Armstrong J. M., Essex M. J., Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Dev. Psychol. 51, 816–822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakreski E. et al., “Developmental trajectories of HPA-HPG dual axes coupling: Implications for social neuroendocrinology” in Routledge International Handbook of Social Neuroendocrinology, Schultheisss O. C., Mehta P. H., Eds. (Routledge, Abingdon-on-Thames, United Kingdom, 2018). [Google Scholar]
- 16.Duarte-Guterman P. et al., Androgens enhance adult hippocampal neurogenesis in males but not females in an age-dependent manner. Endocrinology 160, 2128–2136 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farinetti A. et al., Testosterone and estradiol differentially affect cell proliferation in the subventricular zone of young adult gonadectomized male and female rats. Neuroscience 286, 162–170 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Galea L. A., Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res. Brain Res. Rev. 57, 332–341 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Galea L. A., Spritzer M. D., Barker J. M., Pawluski J. L., Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus 16, 225–232 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Gheorghe A., Qiu W., Galea L. A. M., Hormonal regulation of hippocampal neurogenesis: Implications for depression and exercise. Curr. Top. Behav. Neurosci. 43, 379–421 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Heberden C., Sex steroids and neurogenesis. Biochem. Pharmacol. 141, 56–62 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Spritzer M. D., Galea L. A., Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev. Neurobiol. 67, 1321–1333 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Vieira-Marques C. et al., Dehydroepiandrosterone protects male and female hippocampal neurons and neuroblastoma cells from glucose deprivation. Brain Res. 1644, 176–182 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Karishma K. K., Herbert J., Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur. J. Neurosci. 16, 445–453 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Cardounel A., Regelson W., Kalimi M., Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: Mechanism of action. Proc. Soc. Exp. Biol. Med. 222, 145–149 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Bastianetto S., Ramassamy C., Poirier J., Quirion R., Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Brain Res. Mol. Brain Res. 66, 35–41 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Kimonides V. G., Khatibi N. H., Svendsen C. N., Sofroniew M. V., Herbert J., Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 95, 1852–1857 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barch D. et al., Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am J Psychiatry 173, appiajp201515081014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luby J. et al., The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatr. 167, 1135–1142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson J. L. et al., Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol. Psychiatry 77, 314–323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jednoróg K. et al., The influence of socioeconomic status on children’s brain structure. PLoS One 7, e42486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson J. L., Chandra A., Wolfe B. L., Pollak S. D., Association between income and the hippocampus. PLoS One 6, e18712 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble K. G. et al., Hippocampal volume varies with educational attainment across the life-span. Front. Hum. Neurosci. 6, 307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butterworth P., Cherbuin N., Sachdev P., Anstey K. J., The association between financial hardship and amygdala and hippocampal volumes: Results from the PATH through life project. Soc. Cogn. Affect. Neurosci. 7, 548–556 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piras F., Cherubini A., Caltagirone C., Spalletta G., Education mediates microstructural changes in bilateral hippocampus. Hum. Brain Mapp. 32, 282–289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staff R. T. et al., Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann. Neurol. 71, 653–660 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Palacios-Barrios E. E., Hanson J. L., Poverty and self-regulation: Connecting psychosocial processes, neurobiology, and the risk for psychopathology. Compr. Psychiatry 90, 52–64 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Luby J. L. et al., Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. U.S.A. 109, 2854–2859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luby J. L., Belden A., Harms M. P., Tillman R., Barch D. M., Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc. Natl. Acad. Sci. U.S.A. 113, 5742–5747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bick J., Nelson C. A., Early adverse experiences and the developing brain. Neuropsychopharmacology 41, 177–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin K. A., Weissman D., Bitran D., Childhood adversity and neural development: A systematic review. Annu. Rev. Dev. Psychol. 1, 277–312 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herzog J. I., Schmahl C., Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front. Psychiatry 9, 420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barch D. et al., Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am. J. Psychiatry 173, 625–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calem M., Bromis K., McGuire P., Morgan C., Kempton M. J., Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 14, 471–479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farah M. J., Socioeconomic status and the brain: Prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 19, 428–438 (2018). [DOI] [PubMed] [Google Scholar]
- 46.van Praag H., Kempermann G., Gage F. H., Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Hirase H., Shinohara Y., Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 280, 282–298 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Okuda H. et al., Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 87, 3546–3553 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Eiland L., Ramroop J., Hill M. N., Manley J., McEwen B. S., Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology 37, 39–47 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Bodegom M., Homberg J. R., Henckens M. J. A. G., Modulation of the hypothalamic-pituitary-adrenal Axis by early life stress exposure. Front. Cell. Neurosci. 11, 87 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson S. B., Riis J. L., Noble K. G., State of the art review: Poverty and the developing brain. Pediatrics 137, e20153075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berens A. E., Jensen S. K. G., Nelson C. A. 3rd, Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Med. 15, 135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheline Y. I., Depression and the hippocampus: Cause or effect? Biol. Psychiatry 70, 308–309 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheline Y. I., Hippocampal atrophy in major depression: A result of depression-induced neurotoxicity? Mol. Psychiatry 1, 298–299 (1996). [PubMed] [Google Scholar]
- 55.Ellis B. J., Essex M. J., Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Dev. 78, 1799–1817 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Ellis B. J., Timing of pubertal maturation in girls: An integrated life history approach. Psychol. Bull. 130, 920–958 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Drury S. S. et al., Growing up or growing old? Cellular aging linked with testosterone reactivity to stress in youth. Am. J. Med. Sci. 348, 92–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drury S. S. et al., Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Mol. Psychiatry 17, 719–727 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ge X., Natsuaki M. N., In search of explanations for early pubertal timing effects on developmental psychopathology. Curr. Dir. Psychol. Sci. 18, 327–331 (2009). [Google Scholar]
- 60.Vijayakumar N., Op de Macks Z., Shirtcliff E. A., Pfeifer J. H., Puberty and the human brain: Insights into adolescent development. Neurosci. Biobehav. Rev. 92, 417–436 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wierenga L. M. et al., Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology 91, 105–114 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Tilbrook A. J., Turner A. I., Clarke I. J., Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev. Reprod. 5, 105–113 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Tilbrook A. J., Turner A. I., Clarke I. J., Stress and reproduction: Central mechanisms and sex differences in non-rodent species. Stress 5, 83–100 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Allen K. M., Fung S. J., Rothmond D. A., Noble P. L., Weickert C. S., Gonadectomy increases neurogenesis in the male adolescent rhesus macaque hippocampus. Hippocampus 24, 225–238 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Koolschijn P. C., Peper J. S., Crone E. A., The influence of sex steroids on structural brain maturation in adolescence. PLoS One 9, e83929 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atwi S., McMahon D., Scharfman H., MacLusky N. J., Androgen modulation of hippocampal structure and function. Neuroscientist 22, 46–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herting M. M. et al., The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Hum. Brain Mapp. 35, 5633–5645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neufang S. et al., Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex 19, 464–473 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Ellis R. et al., Relationships between adrenarcheal hormones, hippocampal volumes and depressive symptoms in children. Psychoneuroendocrinology 104, 55–63 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Hayes A. F., Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression-Based Approach, (The Guilford Press, New York, ed. 2, 2018). [Google Scholar]
- 71.Grotzinger A. D. et al., Genetic and environmental influences on pubertal hormones in human hair across development. Psychoneuroendocrinology 90, 76–84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Copeland W. E., Worthman C., Shanahan L., Costello E. J., Angold A., Early pubertal timing and testosterone associated with higher levels of adolescent depression in girls. J. Am. Acad. Child Adolesc. Psychiatry 58, 1197–1206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Copeland W. et al., Outcomes of early pubertal timing in young women: A prospective population-based study. Am. J. Psychiatry 167, 1218–1225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McHenry J., Carrier N., Hull E., Kabbaj M., Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocrinol. 35, 42–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Booth A., Johnson D. R., Granger D. A., Crouter A. C., McHale S., Testosterone and child and adolescent adjustment: The moderating role of parent-child relationships. Dev. Psychol. 39, 85–98 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Booth A., Johnson D. R., Granger D. A., Testosterone and men’s depression: The role of social behavior. J. Health Soc. Behav. 40, 130–140 (1999). [PubMed] [Google Scholar]
- 77.Roosa M. W., Jones S., Tein J. Y., Cree W., Prevention science and neighborhood influences on low-income children’s development: Theoretical and methodological issues. Am. J. Community Psychol. 31, 55–72 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Luby J. L., Heffelfinger A., Koenig-McNaught A. L., Brown K., Spitznagel E., ThePreschool feelings checklist: A brief and sensitive screening measure for depression in young children. J. Am. Acad. Child Adolesc. Psychiatry 43, 708–717 (2004). [DOI] [PubMed] [Google Scholar]
- 79.McLoyd V. C., Socioeconomic disadvantage and child development. Am. Psychol. 53, 185–204 (1998). [DOI] [PubMed] [Google Scholar]
- 80.Shields A., Cicchetti D., Emotion regulation among school-age children: The development and validation of a new criterion Q-sort scale. Dev. Psychol. 33, 906–916 (1997). [DOI] [PubMed] [Google Scholar]
- 81.Egger H. L., Psychiatric assessment of young children. Child Adolesc. Psychiatr. Clin. N. Am. 18, 559–580 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Egger H. L. et al., Test-retest reliability of the preschool age psychiatric assessment (PAPA). J. Am. Acad. Child Adolesc. Psychiatry 45, 538–549 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Kovacs M., Children’s Depression Inventory (CDI2): Technical Manual, (Multi-Health Systems, Inc, North Tonawanda, NY, ed. 2, 2011). [Google Scholar]
- 84.Carnevale T., An integrative review of adolescent depression screening instruments: Applicability for use by school nurses. J. Child Adolesc. Psychiatr. Nurs. 24, 51–57 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Petersen A. C., Crockett L., Richards M., Boxer A., A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc. 17, 117–133 (1988). [DOI] [PubMed] [Google Scholar]
- 86.Glasser M. F. et al., The Human Connectome Project’s neuroimaging approach. Nat. Neurosci. 19, 1175–1187 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luby J. L. et al., Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry 73, 31–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reuter M., Rosas H. D., Fischl B., Highly accurate inverse consistent registration: A robust approach. Neuroimage 53, 1181–1196 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reuter M., Schmansky N. J., Rosas H. D., Fischl B., Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Destrieux C., Fischl B., Dale A., Halgren E., Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Preacher K. J., Hayes A. F., Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40, 879–891 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Hayes A. F., Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, (The Guildford Press, New York, NY, 2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.