Significance
The chromatin remodeler CHD8 is one of the most frequently mutated genes in autism spectrum disorder (ASD), but the mechanistic basis remains unclear. Here, we identify dosage-sensitive roles for CHD8 in the regulation of transcription and define CHD8’s role in regulating genome-wide accessibility. Importantly, we present new results that help to define the molecular function of CHD8 both in the context of pluripotency and in neural differentiation with implications for its role in ASD. By determining the execution point at which mutations in Chd8 might contribute to the disease, we hope to discover the potential for therapeutic approaches.
Keywords: autism spectrum disorder (ASD), chromatin remodeling, pluripotency, neural progenitors, chromodomain helicase DNA-binding protein 8 (CHD8)
Abstract
The chromatin remodeler CHD8 is among the most frequently mutated genes in autism spectrum disorder (ASD). CHD8 has a dosage-sensitive role in ASD, but when and how it becomes critical to human social function is unclear. Here, we conducted genomic analyses of heterozygous and homozygous Chd8 mouse embryonic stem cells and differentiated neural progenitors. We identify dosage-sensitive CHD8 transcriptional targets, sites of regulated accessibility, and an unexpected cooperation with SOX transcription factors. Collectively, our findings reveal that CHD8 negatively regulates expression of neuronal genes to maintain pluripotency and also during differentiation. Thus, CHD8 is essential for both the maintenance of pluripotency and neural differentiation, providing mechanistic insight into its function with potential implications for ASD.
Dynamic regulation of chromatin structure is required to ensure rapid transcriptional responses during development and to maintain these programs in order to safeguard cellular identity. Central to these regulatory processes are chromatin-remodeling enzymes, which hydrolyze adenosine 5′-triphosphate (ATP) to modify chromatin structure and DNA accessibility by repositioning, editing, or evicting nucleosomes (1). There are four major subfamilies of ATP-dependent nucleosome remodelers classified by phylogenetic relationships and distinct functional activities (2). Of these, the chromodomain helicase DNA-binding (CHD) class of remodelers is of particular interest because it is implicated in human diseases. In mammals, there are nine CHD family members, each containing two tandemly arranged chromodomains upstream of their catalytic SNF2 helicase domain that are involved in nucleosome spacing and histone variant H3.3 incorporation (3, 4). Individual CHD family members are frequently inactivated in specific human diseases, including Hodgkin’s lymphoma (CHD3), neuroblastoma (CHD5), CHARGE syndrome (CHD7), and autism spectrum disorder (ASD) (CHD8) (5). CHD8 is of particular interest because it is one of the most commonly mutated genes in ASD (6), but the mechanistic basis remains unclear.
CHD8 regulates important developmental pathways and is essential during embryogenesis. For example, CHD8 inhibits β-catenin and Wnt-signaling pathways as well as p53-dependent transactivation, preventing widespread apoptosis (7–9). Consistent with a specific role in neurodevelopmental disorders, CHD8 targets pathways associated with ASD and intellectual disability (10–13). Additionally, Chd8+/− and CHD8 knockdown mouse models recapitulate some ASD-like behavioral phenotypes, suggesting a causal role in promoting ASD etiology (14–17). While these studies have demonstrated that CHD8 is a critical transcriptional regulator during neurogenesis, there are discrepancies regarding CHD8’s role in cell cycle exit (18); moreover, it remains unclear how CHD8 is targeted to its chromatin sites, the downstream effect of its recruitment on accessibility, and how these biochemical activities regulate transcription.
Here, we utilized mouse embryonic stem cells (ESCs) and differentiation into neural progenitor cells (NPCs) as a model system to study the role of CHD8 in early embryonic gene regulation and control of genome-wide accessibility. We created both homozygous and heterozygous Chd8 deletions, enabling us to characterize the effect of gene dosage on these important processes. We demonstrate that CHD8 has a dosage-sensitive effect on transcriptional regulation in ESCs and upon differentiation into NPCs that is consistent with premature neuronal differentiation. Surprisingly, we find that CHD8 directly associates with SOX2 to maintain accessibility in support of the pluripotency gene network. Collectively, we demonstrate an essential role for CHD8 in regulating the accessible chromatin landscape and gene expression, providing insight into the function of CHD8 with potential implications for ASD.
Results
Dosage-Sensitive Effects of CHD8 Deletion on Transcription in ESCs.
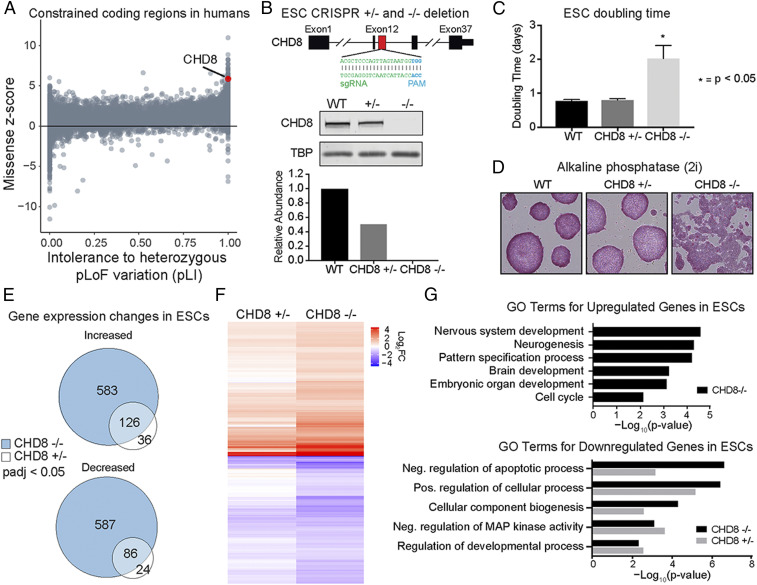
We were particularly motivated to study CHD8 gene dosage in pluripotency and in neurogenesis because it is one of the most highly constrained genes in the human genome (19) and is haploinsufficient for human neurodevelopment and human social behavior, as indicated by its role in ASD. Consistent with these observations, CHD8 has a very high probability of being intolerant to the loss of function of a single allele in humans (Fig. 1A). Previous work has shown that CHD8 expression at embryonic day 12.5 (E12.5) is ∼10-fold higher compared to the adult, and Chd8 knockout mice arrest at E7.5, implying an essential role for CHD8 during the neural progenitor stage (20). Thus, we generated single or double allelic Chd8 deletions in mouse ESCs, a well-studied and context-relevant model system. Using a single guide RNA (sgRNA), we targeted CRISPR-Cas9 to exon 12, which includes the ATPase/helicase domain (Fig. 1B) (21). Single or double deletion clones were validated by Sanger sequencing and Western blotting, which confirmed that CHD8 protein level was reduced to ∼50% in the Chd8+/− line and was undetectable in the Chd8−/− cell line, enabling investigation into CHD8 dosage.
Fig. 1.
Dosage-sensitive effect of CHD8 deletion on transcription in ESCs. (A) The gnomAD human gene constraint scores (missense and probability of intolerance to heterozygous pLoF variation [pLI] for all genes with CHD8 labeled). (B) Schematic showing the CHD8 CRISPR-mediated deletion approach and Western blot of CHD8 levels in Chd8+/− and Chd8−/− ESC lines. (C) Doubling time of WT, Chd8+/−, and Chd8−/− cells. Asterisks denote P < 0.05 (*). Values are expressed as the mean ± SE, n = 3. (D) Alkaline phosphatase staining of WT, Chd8+/−, and Chd8−/− ESCs in 2i media. Magnification: 200×. (E) Venn diagrams depicting overlap of DEGs in Chd8+/− and Chd8−/− ESCs. Genes with increased expression are on top, and genes with decreased expression are on the bottom (FDR < 0.05). (F) Heatmap showing dosage-sensitive transcriptional response for genes that are differentially expressed in Chd8−/−. (G) GO terms for DEGs (padj < 0.05).
Consistent with previous results (22), CHD8 knockout cells proliferated at a significantly slower rate, with a doubling time that was twice as long as wild-type (WT) and Chd8+/− cells (Fig. 1C; n = 3, P < 0.05). The decreased proliferation rate was not due to cell cycle arrest (SI Appendix, Fig. S1A). To assay the effect of CHD8 deletion on pluripotency, we stained cells for alkaline phosphatase (Fig. 1D). While all genotypes stained with similar intensity, the Chd8−/− cells did not form colonies like the WT and Chd8+/− cells (2i growth conditions), indicative of compromised pluripotency. To explore this result, we used RNA sequencing (RNA-seq) to characterize the effect of CHD8 deletion on gene expression changes over the approximately five passages required to generate the mutant ESC lines (SI Appendix, Fig. S1B). As expected, there were significantly more differentially expressed genes (DEGs) upon homozygous CHD8 deletion (709 up-regulated and 673 down-regulated) compared to heterozygous deletion (162 up-regulated and 110 down-regulated genes) (Fig. 1E). Interestingly, the Chd8+/− DEGs showed substantial overlap with Chd8−/− for both increased (77.8% overlap, 126 of 162) and decreased (78.2% overlap, 86 of 110) genes, confirming that CHD8 activity is dosage sensitive (haploinsufficient) in ESCs. Consistent with this finding, many DEGs in the Chd8−/− ESCs exhibited moderate dosage-sensitive expression changes when a single allele of CHD8 was deleted (Fig. 1F).
To identify the key regulatory pathways controlled by CHD8, we conducted gene ontology (GO) term analysis of biological processes on DEGs (Fig. 1G). Genes that were up-regulated in Chd8−/− were enriched for processes associated with nervous system development, neurogenesis, and cell cycle (P < 0.01 for all terms), whereas Chd8+/− did not show significant GO enrichment. In contrast, the Chd8+/− and Chd8−/− ESCs showed reduced expression of genes enriched for suppression of apoptotic process, cellular component biogenesis, and regulation of developmental process (P < 0.01 for all terms). Gene set enrichment analysis (GSEA) showed significant enrichment for pathways indicative of derepressed transforming growth factor beta (TGFβ) signaling (SI Appendix, Fig. S1D; FDR-adjusted P = 0.007). TGFβ plays a central role in tissue morphogenesis, and stimulation with TGFβ causes neural stem cells to lose multipotency and differentiate along the neuronal lineage (23, 24). On the other hand, CHD8 knockout showed enrichment of genes up-regulated upon inactivation of lysine-specific histone demethylase 1 (LSD1/KDM1A) (FDR-adjusted P = 0.003), which demethylates monomethylated and dimethylated histone H3 lysine 4 (H3K4me1 and H3K4me2) and is critical for mouse embryonic development beyond E6.5 (25). Collectively, our results reveal that CHD8 is required to repress neural differentiation but not for maintenance of the core pluripotency network OSKM (Oct4, Sox2, Klf4, and c-Myc) and has a modest dosage-sensitive role in transcription.
CHD8 Knockout Results in Up-Regulation of Neuronal Genes and Sox TFs upon Differentiation into NPCs.
Having characterized the role of CHD8 in ESCs, we differentiated Chd8+/− and Chd8−/− ESCs into self-renewing NPCs to define the role of CHD8 in a disease-relevant context (Fig. 2A). Additionally, neural induction is the default differentiation pathway in early vertebrate embryos (26) and can be faithfully driven in vitro with autocrine signaling pathways (27). To define the genes regulated by CHD8 upon differentiation, we conducted RNA-seq in the Chd8+/− and Chd8−/− NPCs (SI Appendix, Fig. S1C). We found that, upon differentiation, there were many more DEGs compared to ESCs (∼1.5-fold), but, similar to ESCs, there was substantial overlap between Chd8+/− and Chd8−/− NPCs for both increased (76.5% overlap, 297 of 388) and decreased (76.1% overlap, 305 of 401) DEGs (Fig. 2B) with a similar dosage-sensitive response to ESCs (Fig. 2C). GO term analysis of genes with increased expression in CHD8 mutants revealed significant enrichment for terms associated with brain development, such as neuron differentiation, axonogenesis, and corpus callosum morphogenesis (Fig. 2D; P < 0.003 for all terms). In human ASD patients with disruptive mutations in CHD8, corpus callosum hypogenesis is a prevalent phenotype (28). In contrast, genes with decreased expression in both Chd8+/− and Chd8−/− showed significant enrichment for embryogenesis terms, such as epithelial tube and embryonic placenta morphogenesis (P < 0.0001 for all terms). Genes showing reduced expression in Chd8−/− NPCs were associated with GO terms for cell cycle and suppression of apoptotic process, similar to ESCs. GSEA showed a significant enrichment of p53 target genes in neuroepithelium (FDR-adjusted P < 0.0001), an important regulator of cell cycle and apoptosis (SI Appendix, Fig. S1D), consistent with previous results (8, 20). Many of the most significantly up-regulated genes in Chd8+/− and Chd8−/− NPCs are involved in later stages of neuronal development, including Ascl1 [a central driver of neural reprogramming (29)], Dcx, Map2, Nefm, Neurod4, and Neurog1 (Fig. 2 E and F). Additionally, we found that Sox3 is derepressed in both Chd8+/− and Chd8−/− NPCs, and several other Sox TF members (Sox2, Sox7, and Sox11) became derepressed in the Chd8−/− cells (SI Appendix, Fig. S5C). SOX proteins act in a sequential manner during neurogenesis to select neural genes in ESCs for later activation in NPCs or neurons (30).
Fig. 2.
CHD8 deletion results in up-regulation of neuronal genes and Sox TFs during differentiation into NPCs. (A) Schematic showing the differentiation of WT, Chd8+/−, and Chd8−/− ESCs into NPCs. (B) Venn diagrams depicting overlap of DEGs in Chd8+/− and Chd8−/− NPCs. Genes with increased gene expression are on top, and genes with decreased expression are on bottom (FDR < 0.05). (C) Heatmap showing dosage sensitive transcriptional response for genes that are differentially expressed in Chd8−/−. (D) GO terms for DEGs. (E and F) Volcano plots of RNA-seq data for CHD8+/− and CHD8−/−. DEGs are highlighted in red (FDR < 0.05), and genes involved with mature neuronal development and Sox TFs are labeled. Genes labeled with yellow background were stained by IF in G. (G) Representative IF staining of day 8 NPCs, with antibodies listed on left side and respective genotypes on top (n = 2 biological replicates with similar results). Magnification: 200×.
We confirmed these transcriptional changes by immunofluorescence (IF) staining of the NPC cultures (Fig. 2G). WT and both mutant Chd8 lines stained positive for Nestin, a neural stem cell marker; however, Chd8+/− and Chd8−/− NPCs showed stronger staining for immature (DCX, NEUROD1, and TuJ1) and mature (MAP2A) neurons, as well radial glia (Pax6). Interestingly, DCX, NEUROD1, MAP2a, PAX6, and TUJ1 staining seemed to show a dose-dependent negative correlation with CHD8 levels (Fig. 2G), confirming that the observed transcription changes result in changes to protein levels. Collectively, these results reveal that CHD8 is required at full dosage to inhibit transcription of late differentiation genes and transcription factors, such that its inactivation causes cells to differentiate beyond NPCs toward a more mature neuronal cell type.
CHD8 Regulates Accessibility at Key Regions in ESCs and during Differentiation to NPCs.
Chromatin remodelers are important regulators of genomic accessibility. To investigate the role of CHD8 in regulating the accessible chromatin landscape in mutant ESCs and NPCs, we performed Assay for Transposase-Accessible Chromatin with high-throughput sequencing (ATAC-seq) (31). In contrast to transcriptional changes, we found that Chd8+/− had a modest effect on accessibility in both ESCs (two increased and three decreased sites) and NPCs (86 increased and 40 decreased sites), considering the number of called peaks (n = 93,093 and 43,190, respectively) (SI Appendix, Figs. S2A and S3A). This was somewhat surprising, since Chd8+/− mutation resulted in many more DEGs, yet other studies have noted a less than deterministic relationship between accessibility (ATAC-seq) and transcription (32). Nonetheless, we found that a fraction of DEGs had differential accessibility at their promoter (ESC = 14.7% and NPC = 10.2%) (SI Appendix, Fig. S3 D and E). This lack of a strong haploinsufficient effect on accessibility is further reflected in the heat map clustering and principal component analysis, which showed that the accessibility states of WT and Chd8+/− cells are more similar to each other than to Chd8−/− cells (SI Appendix, Fig. S2 B and C). Similarly, mSWI/SNF or BAF complexes are also haploinsufficient in human disease and generate accessibility, yet heterozygous deletion in ESCs produced subtle changes in accessibility measured by ATAC-seq (33).
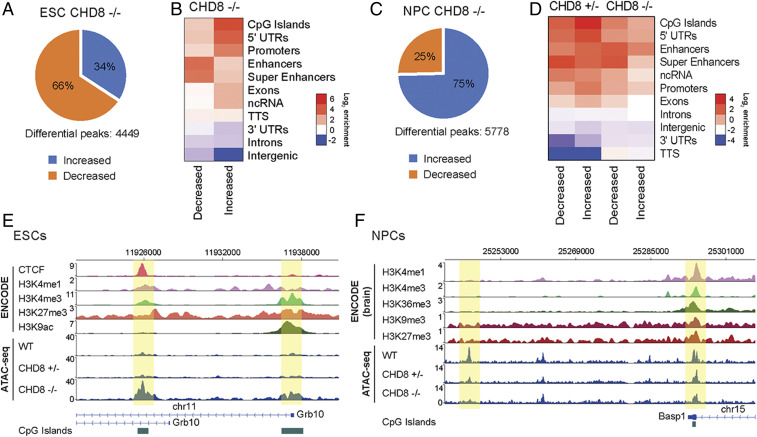
Chd8−/− ESCs and NPCs had considerably more accessibility changes (ESCs: 1,522 increased and 2,927 decreased sites; NPCs: 4,313 increased and 1,465 decreased sites). Surprisingly, the overall effect of Chd8−/− on the direction of accessibility changes was opposite in ESCs compared to NPCs, such that the majority of sites became less accessible in ESCs and more accessible in NPCs upon loss of CHD8 (Fig. 3 A and C). This suggests that CHD8 primarily functions to promote accessibility in the context of maintaining pluripotency but represses accessibility during differentiation, likely reflecting differentiation slightly beyond the NPC state. We next sought to determine whether CHD8 has a direct role in regulating accessibility at the changed sites. We reasoned that defining CHD8 localization in ESCs would be most appropriate, considering that differentiation following CHD8 deletion induced the up-regulation of many neurogenic transcription factors and resulted in a greater change to cellular identity, which likely resulted in accessibility changes that are independent of CHD8 remodeling activity. We conducted CHD8 chromatin immunoprecipitation sequencing (ChIP-seq) in ESCs and observed a striking enrichment at differential accessibility sites (SI Appendix, Fig. S3B). Thus, CHD8 deletion in ESCs and NPCs resulted in many reproducible accessibility changes that are consistent with being direct targets of CHD8.
Fig. 3.
CHD8 regulates accessibility at key regions in ESCs and during differentiation to NPCs. (A) Percentage of increased (blue) and decreased (orange) peaks for Chd8−/− ESCs. (B) Genomic annotation enrichment for increased and decreased sites for Chd8−/− ESC ATAC-seq datasets. (C) Percentage of increased (blue) and decreased (orange) peaks for Chd8−/− NPCs. (D) Genomic annotation enrichment for increased and decreased sites for Chd8+/−and Chd8−/− NPC ATAC-seq datasets. (E) Genome tracks showing gain of accessibility at Grb10 locus in ESCs. (F) Genome tracks showing loss of accessibility at Basp1 locus in NPCs.
We next analyzed the genomic annotations of differentially accessible sites in Chd8−/− ESCs. We found that the sites that lost accessibility were highly enriched for enhancers and superenhancers (Fig. 3B; Log2 enrichment = 3.64 and 3.41, respectively). In contrast, sites that gained accessibility were enriched at CpG islands, 5′ untranslated regions, and promoters (Log2 enrichment = 4.37, 3.93, and 3.22, respectively) but were depleted at intergenic regions (Log2 enrichment = −1.24). To determine whether specific genomic marks or factors were associated with the CHD8-induced accessibility changes, we leveraged the wealth of available ChIP-seq datasets in ESCs. We conducted Lasso multivariate regression (34, 35) for our ATAC-seq datasets and found the strongest positive association between increased accessibility sites and LSD1, H3K4me2, H3K4me3, and H3K27me3 ChIP signal (SI Appendix, Fig. S3C). This suggests that the chromodomain, which binds dimethylated histone H3K4 (22), likely targets CHD8 to render these sites less accessible. Bivalent genes that contain both H3K4me3 and H3K27me3 play an important role in cell differentiation (36, 37), and these general changes can be seen in a representative browser snapshot for Grb10 (Fig. 3E). In NPCs, we found that both decreased and increased sites were enriched at CpG islands, promoters, and enhancers but were depleted at intergenic regions and transcription termination sites (Fig. 3D). The accessibility changes in NPCs share genomic features similar to ESCs. These changes can be observed in representative browser tracks at the Basp1 gene, which, despite very subtle genome-wide changes, appears to exhibit a dosage-sensitive effect on accessibility during NPC differentiation (Fig. 3F). We next analyzed the pathway and ontology of genes nearest to differentially accessible peaks (SI Appendix, Fig. S4). Consistent with the examples shown, we find strong enrichment for pathways like Wnt signaling and GO terms, such as development and neurogenesis. Thus, CHD8 regulates chromatin accessibility at the promoters of important regulators of embryonic development and cell differentiation.
CHD8 Cooperates with Sox TFs to Regulate Accessibility and Transcription.
Chromatin-remodeling enzymes lack sequence-specific DNA-binding domains, so we next sought to investigate a potential cooperation between CHD8 and transcription factors. Using chromVAR (38), we defined the sequence motifs with the highest deviation in accessibility across genotypes. This analysis revealed that accessibility increased at sequence motifs for p53 and Ctcf when CHD8 is deleted in ESCs (Fig. 4A). CHD8 has previously been shown to antagonize p53-mediated apoptosis and to support CTCF insulator function through a direct interaction (7, 20). Our data suggest that CHD8 executes these roles by directly repressing genomic accessibility, perhaps through histone H1 recruitment or nucleosome movement. In contrast, accessibility was most reduced at pluripotency factor sequence motifs including Oct4 (Pou5f1), Sox2, and Esrrb when CHD8 was deleted in ESCs. This result is somewhat surprising considering that these pluripotency factors extensively autoregulate each other (39) and were not differentially expressed in Chd8−/− ESCs (SI Appendix, Fig. S5B). Yet, CHD8 ChIP localization was strongly biased toward promoters and enriched for binding OCT4−Sox2−TCF−Nanog and Ctcf motifs, supporting a direct role in regulating genomic accessibility (SI Appendix, Fig. S5 E and F). CHD8 and Sox2 regulate a significant number of overlapping genes (40) (SI Appendix, Fig. S5G; P value = 0.0045), yet none of the characteristic trophectoderm differentiation markers seen in the SOX2 knockdown cells were also up-regulated in the Chd8−/− ESCs, suggesting the overall effect of these accessibility changes does not phenocopy SOX2 loss. To further determine whether transcription factors are physically bound to these differentially accessible sites, we leveraged existing ChIP-seq datasets of known developmental regulatory and pluripotency factors. Consistent with a direct role for CHD8 in cooperating with these factors to generate accessibility, we observed that the decreased accessibility sites were strongly enriched for these factors in Chd8−/− ESCs, including SOX2, NANOG, and OCT4 (SI Appendix, Fig. S5A). In contrast, increased accessibility sites in Chd8−/− ESCs were strongly depleted where these factors bind.
Fig. 4.
CHD8 cooperates with Sox TFs to regulate accessibility and transcription. (A) Heatmap representation of ATAC-seq chromVAR bias-corrected deviations in the 50 most variable TF motifs across ESC WT and Chd8−/− ATAC-seq replicates. (B) Heatmap representation of ATAC-seq chromVAR bias-corrected deviations in the 50 most variable TF motifs across NPC WT and Chd8−/− ATAC-seq replicates. (C) Western blot showing co-IP of CHD8 with SOX2 in ESCs (antibodies used are the following: SOX2 A: sc-365823; SOX2 B: AB5603). (D) Heat map displaying CHD8 ChIP-seq at SOX2 peaks (41) (Left) and SOX2 ChIP-seq at CHD8 peaks (Right).
To characterize the types of accessibility changes at these motifs, we calculated changes in flanking accessibility (FA) and footprint depth (FPD) upon Chd8 deletion for a set of 579 transcription factors. We modified the BaGplot approach (42) to visualize changes in FA and FPD (SI Appendix, Fig. S6A). The outliers in the bagplot represent transcription factors with the greatest changes in FA and FPD as compared to the average. We found that the Oct4:Sox2 binding motif is one of the strongest outliers and that the Ctcf motif appeared at the fence border, which confirm results from the chromVAR analysis. In addition, among outliers, we found other transcription factors important for maintenance of the ESC pluripotent state, such as Gbx2 (43), Mybl2 (44), Zscan4 (45), Foxd3 (46), and Smad3 (47). We found that these transcription factors either directly interact with each other or have correlated expression, which suggests their functional relationship (SI Appendix, Fig. S6B). Thus, CHD8 facilitates pluripotency by cooperatively generating accessibility at sites that are bound by pluripotency transcription factors.
We anticipated that NPCs differentiated from Chd8−/− ESCs would show enrichment of sequence motifs for the many neurogenic TFs that were strongly derepressed by Chd8−/−(e.g., Ascl1). Instead, the most strongly enriched motifs were nearly exclusive to the Sox TF family (Fig. 4B). We considered that CHD8 might have a dual role in mediating accessibility for SOX2 in ESCs and repressing the Sox family of TFs in NPCs. However, it is more likely that the increased accessibility we observed is due to strong up-regulation of a few Sox TF genes (Sox2, Sox3, Sox7, and Sox11) in Chd8−/− NPCs (SI Appendix, Fig. S5C), which exhibit pioneer activity and are able to outcompete nucleosomes to generate accessibility independent of CHD8 remodeling activity. We confirmed accessibility changes in Sox family motifs using bagplot (SI Appendix, Fig. S6C), where Sox TFs appear as a single cluster near the fence border. Interestingly, the Oct4:Sox2 motif, which is one of the outliers in ESCs, does not come up in the Sox cluster in NPCs. Remarkably, outlier TFs in bagplot also include the family of Retinoid X Receptors (Rxra, Rxrb, Rxrg), known regulators of neural cell fate specification (48), and the Gata TF family. Gata TFs play a crucial role in cell fate determination (49); in particular, Gata2 determines the choice between GABAergic and glutamatergic interneuron specification (50). GATA2 and RXRA are functionally related (SI Appendix, Fig. S6D) and have been shown to interact in hematopoietic progenitors (51).
Since CHD8 deletion did not significantly affect Sox2 expression in ESCs but did result in the loss of accessibility at Sox2 binding motifs, we sought to determine whether the reliance on CHD8 for accessibility at these sites was mediated by a direct protein−protein interaction. We immunopurified SOX2 from ESCs using two different antibodies, and, in both cases, we observed clear CHD8 co-immunoprecipitation (co-IP) that was not seen with IgG control antibodies (Fig. 4C). We also tested SOX3 from NPCs and detected a weaker co-IP signal, but considering that Sox3 is up-regulated in the Chd8−/− NPCs, we cannot determine whether this interaction facilitates repression (SI Appendix, Fig. S4D). We reasoned that, if CHD8 is being physically targeted to SOX2 binding sites to facilitate accessibility, then there should be substantial overlap between them. To test this hypothesis, we plotted CHD8 occupancy at SOX2 binding sites (41) and plotted SOX2 at CHD8 binding sites. Consistent with direct targeting by SOX2, we found extensive colocalization (Fig. 4D). Thus, CHD8 both positively and negatively regulates accessibility through cooperation with transcription factors and, indirectly, by maintaining their repression.
Discussion
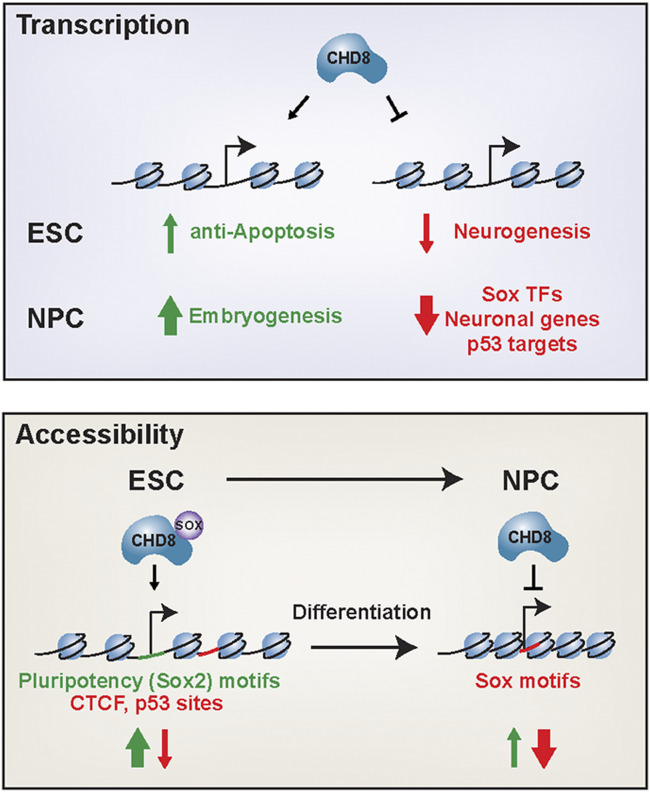
Here, we investigated the role of CHD8 in the maintenance of pluripotency and during differentiation into NPCs (Fig. 5). Utilizing heterozygous and homozygous genetic deletions, we showed that CHD8 primarily functions to repress cell differentiation and nervous system development pathways in addition to previously implicated pathways, such as p53 and the cell cycle. We demonstrated that CHD8 is a dosage-sensitive repressor of neuronal pathways that results in premature differentiation when inactivated. Finally, we defined the role of CHD8 in regulating genome-wide accessibility. In ESCs, CHD8 represses accessibility at p53/Ctcf motifs, while maintaining accessibility at pluripotency motifs, at least in part, through a new, direct protein−protein interaction with SOX2, whereas, in NPCs, CHD8 represses accessibility of Sox motifs, which could be mediated through a direct interaction or as a downstream consequence of derepressing the transcription factors that bind these motifs. Interestingly, these effects were largely dosage insensitive, suggesting that loss of a single allele of CHD8 compromises transcription to a greater degree than accessibility. However, we did find a small but consistent set of differentially accessible peaks that were dosage sensitive during early neural differentiation, so it is possible that CHD8 exerts a stronger haploinsufficient effect during complete neurogenesis.
Fig. 5.
Model summarizing findings.
How, then, does CHD8 regulate transcription and accessibility at specific genomic loci? We found that CHD8 repressed accessibility in ESCs at sites enriched for H3K4me2/3 and promoted accessibility at loci bound by pluripotency factors, suggesting two dominant modes of recruitment: 1) binding to posttranslational modifications on chromatin and 2) interaction with transcription factors, like SOX2. A well-established pioneer factor, SOX2 is able to bind nucleosomal DNA without the requirement for remodeling (52, 53). Despite this, our data reveal that maintenance of accessibility at these motifs depends, at least in part, on the activity of CHD8. It is intriguing that CHD8 has a dual role in maintaining accessibility at pluripotency motifs while repressing accessibility at Ctcf and p53 motifs. Previously, CHD8 has been shown to load H1 as a mechanism of repression (20), and our findings suggest that this could potentially be one mechanism by which CHD8 represses accessibility. It is likely, then, that local cooperative interactions determine whether CHD8 promotes or represses genomic accessibility. For example, CHD8 likely mediates accessibility through cooperative action of OCT4, SOX2, ESRRB, histone modifications, and perhaps other transcriptional activators to facilitate transcription initiation. In contrast, CHD8 likely represses accessibility at p53 motifs and Ctcf binding sites through sampling-based mechanisms, direct recruitment, and perhaps loading of histone H1 (7). Future studies are needed to distinguish the precise mechanisms that tip the balance between promoting accessibility and repression.
Of the nine members of the CHD family, CHD7 and CHD8 have been shown to play vital roles in neurodevelopment (54). Heterozygous mutations in CHD7 and CHD8 result in neurodevelopmental disorders, such as CHARGE syndrome and ASD, respectively. Previously, CHD7 has been shown to physically interact with SOX2 and regulate neurogenesis in NSCs (55). Additionally, in oligodendroglia, CHD7 and CHD8 appear to cooperate with SOX10 to activate stage-specific genes (56). Our finding that CHD8 also physically associates with SOX2/3 suggests that there might be conserved functional interplay between other CHD family members, and perhaps other SOX TFs in neural cell types as a general principle.
One of the significant questions relating to the pathogenesis of ASD has been the execution point at which mutated genes contribute to the disease. The significance of this question derives from the potential for therapeutic approaches after ASD is diagnosed. Our studies support work indicating that the execution point for CHD8 might be quite early and dosage dependent. Furthermore, we find that CHD8 is bound to many other ASD risk genes, consistent with other studies (10). Given our unique findings demonstrating the importance of CHD8 in regulating genome-wide accessibility and transcription of critical genes in pluripotency and neuronal differentiation, studies are needed in Chd8−/− mouse models and in vitro to further examine the mechanisms described here.
Methods
Culturing of Mouse Embryonic Stem Cells.
TC1 ESCs were cultured on 0.1% gelatin-coated plates at 37 °C with 5% CO2 in 2i media: Dulbecco’s modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12), Neurobasal Medium, 3% fetal bovine serum (FBS), N2 and B27 supplements, 100× penicillin/streptomycin, 2 mM GlutaMAX, 0.1 mM minimal essential media non-essential amino acids, Hepes, Sodium Pyruvate, 0.1 mM β-mercaptoethanol, and 1,000 U/mL leukemia inhibitory factor. The complete 2i media was made with 1 µM PD0325901 and 3 µM CHIR99021. ES cells were passaged every 2 d.
Generation of CRISPR Cell Lines.
Low-passage TC1(129) mouse ESCs were thawed onto mouse embryonic fibroblast (MEF)-coated dishes and passaged once on gelatin-coated dishes before transfection. Then 2 M cells were nucleofected (Lonza #VVPH-1001, A-013 program) with 8 µg PX459V2.0 (Addgene #62988) containing sgRNA targeting CHD8 exon 12 that was published previously (5′-ACGCTCCCAGTTAGTAATGG-3′) (21). Following transfection, 0.5 M cells were seeded onto 6-cm dishes coated with DR4 MEFs and cultured for 24 h before puromycin selection (1.0 µg/mL, 1.25 µg/mL, or 1.5 µg/mL) for 48 h. Single colonies were manually picked, dissociated with trypsin, and expanded on MEFs. Colonies containing heterozygous and homozygous insertions were confirmed by PCR and Western blotting.
Neural Differentiation Protocol.
Neural differentiation of ESCs was performed as previously described by Abranches et al. (27). In brief, ESCs were maintained using standard conditions in media containing DMEM, 15% ES-sure FBS, 100× penicillin/streptomycin, 2 mM GlutaMAX, 0.1 mM MEM-NEAA, Hepes, Sodium Pyruvate, 0.1 mM β-mercaptoethanol, and 1,000 U/mL LIF. To start the differentiation protocol, ESCs were plated at 1 × 104/cm2 on 0.1% gelatin-coated plates in N2B27 media: DMEM/F12, Neurobasal Medium, N2 and B27 supplements, 100× penicillin/streptomycin, 2 mM GlutaMAX, 0.1 mM MEM-NEAA, Hepes, Sodium Pyruvate, and 0.1 mM β-mercaptoethanol. The N2B27 media was changed every other day. On day 4, cells were dissociated with Accutase and plated at 2 × 104/cm2 on laminin-coated plates in N2B27 media supplemented with 5 ng/mL of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF).
Western Blot.
Cells were lysed for 30 min at 4 °C in radioimmunoprecipitation assay buffer (RIPA) buffer (50 mM Tris⋅HCl pH 8.0, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1% Nonidet P-40) supplemented with 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM protease inhibitor mixture (Roche), and 1:200 benzonase (Sigma-Aldrich). Lysates were centrifuged at maximum speed (14,000 × g), and total protein concentration was measured by Bradford assay (Bio-Rad). Extracts were mixed with Laemmli sample buffer containing 50 mM DTT and loaded onto a 4 to 12% Bis-Tris SDS/polyacrylamide gel electrophoresis (Thermo Fisher Scientific). Protein was transferred to Protran 0.1-mm nitrocellulose membrane (Thermo Fisher Scientific) and blocked in 5% milk/Tris-buffered saline, 0.1% Tween-20 Detergent (TBST). Blots were then probed with primary antibodies followed by staining with fluorescence-conjugated secondary antibodies (Li-Cor), and bands were detected using an Odyssey CLX imaging system (Li-Cor).
Preparation of Nuclear Extracts for Immunoprecipitation.
ESCs were dissociated with trypsin, and NPCs were dissociated with Accutase and washed with PBS. Cells were lysed on ice in Buffer A (25 mM Hepes [pH 7.6], 5 mM MgCl2, 25 mM KCl, 0.05 mM EDTA, 10% glycerol, 0.1% Nonidet P-40), supplemented with 1 mM DTT, 0.5 mM PMSF, 1 mM protease inhibitor mixture (Roche). Nuclei were centrifuged (500 × g) and resuspended in Buffer C (10 mM Hepes [pH 7.6], 3 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, 10% glycerol), supplemented with 1 mM DTT, 0.5 mM PMSF, 1 mM protease inhibitor mixture (Roche). Nuclei were lysed with the addition of ammonium sulfate to a final concentration of 0.3 M, and ultracentrifugation (100,000 × g) was used to separate the soluble nuclear proteins from the insoluble chromatin fraction. Soluble nuclear proteins were precipitated with the addition of 0.3 mg/mL ammonium sulfate for 20 min on ice, and proteins were isolated by ultracentrifugation (100,000 × g). Nuclear extract was stored at −80 °C for later use in IP assays.
Immunoprecipitation Assays.
Nuclear extracts obtained from ESCs or NPCs were resuspended in IP Buffer (150 mM NaCl, 50 mM Tris [pH 7.5], 1 mM EDTA, 10% glycerol, 0.5% Triton X-100), supplemented with 1 mM DTT, 0.5 mM PMSF, 1 mM protease inhibitor mixture (Roche). Bradford assays were used to determine protein concentration, which was adjusted to 300 µg for each IP. Samples were incubated with 5 µg of antibody and Protein A Dynabeads (Thermo Fisher) rotating overnight at 4 °C. The following day, the magnetic beads were washed three times with 1 mL per wash of IP Buffer at room temperature and then resuspended in 20 µL of 2× loading buffer (LDS, Thermo Fisher) with 10 mM DTT for subsequent immunoblot analysis.
ATAC Library Preparation.
ATAC libraries were independently prepared from separately cultured samples in duplicate as described previously (31). Briefly, we dissociated cells with Accutase (Life Technologies) and obtained nuclei from 50,000 viable cells by resuspending cells in 0.5 mL of lysis buffer (0.1% Tween-20 in RSB buffer [10 mM Tris⋅HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2]) and incubated them for 10 min on ice. Nuclei were pelleted by centrifugation for 10 min at 500 × g, resuspended in 50 μL of transposition mix (1× Tagmentation DNA buffer, 2.5 μL Tagment DNA enzyme [Illumina]), and incubated for 30 min at 37 °C. DNA was purified with a MinElute PCR purification kit (Qiagen), and libraries were amplified by PCR with barcoded Nextera primers (Illumina). For sequencing, libraries were size-selected with Agencourt AMPure XP (Beckman Coulter) for fragments between ∼50 bp and 1,000 bp in length according to the manufacturer’s instructions. Libraries were sequenced using paired-end reads on the Illumina HiSeq2000 sequencer.
Processing of ATAC-seq Data.
The following techniques were applied uniformly to all datasets as previously described. Processing of paired-end reads was performed by mapping to the mm9 reference mouse genome using Bowtie 2.1.0 (57). Only high-quality unique reads were kept, while duplicate fragments and reads with mapping quality of <10 were discarded. Two pseudofragments were generated for each fragment and occupied 200 bp centered at the true fragment ends. The density of pseudofragments was used to perform peak calling by MACS 2.1.1 (58). All peaks from control and mutant datasets within ± 1 kb were merged for differential analysis, and peaks were discarded if they were below a threshold of 10 RPM in at least one dataset to remove low-quality peak calls. The mean read density in the 100-bp window 8 kb away from each peak was used to obtain the background density of ATAC-seq signal. This background density was subtracted from the overall read density within each peak in each dataset. Differential peak calling was performed by comparing the total number of background-adjusted reads overlapping each of the resulting peaks for each dataset. Using the summed number of background-adjusted reads at the top 5% of highest read-density sites as size factors, differential peak calls were made using DESeq2 (59), which accounts for individual site variances across all replicates to make differential peak calls. The default use of maximum a posteriori estimation using a zero-mean normal prior (Tikhonov–Ridge regularization) was used to calculate log2-fold changes. The Benjamini–Hochberg procedure was used to calculate FDR-corrected P values, and differential calls were made by requiring fold changes of >1.5-fold in either direction and FDR-corrected P < 0.10. The mean values across both replicates from each condition are the RPM values in the genome tracks. Bwtool (60) was used to compute mean density profiles by calculating the mean base pair coverage across all replicates for a given condition. Bedtools (61) was used to determine overlap of peaks.
ChIP-seq Library Preparation.
As previously described, ChIP libraries were independently prepared from separately cultured samples in duplicate (33, 62–64). Briefly, 10 million to 30 million cells were fixed in 1% formaldehyde for 10 min to 12 min at room temperature. Cross-linking was stopped with the addition of glycine to 125 mM to quench excess formaldehyde. Cells were washed and centrifuged, and pellet was flash frozen in liquid nitrogen. Thawed pellets were then resuspended in NP Rinse 1 buffer (50 mM Hepes-KOH, pH 8.0, 140 mM NaCl, 1 mM EDTA, pH 8.0, 10% glycerol, 0.5% Nonidet P-40, 0.25% Triton X-100) and incubated on ice for 10 min. Isolated nuclei were washed once with NP Rinse 2 buffer (10 mM Tris⋅HCl, pH 8.0, 1 mM EDTA, pH 8.0, 0.5 mM EGTA, pH 8.0, 200 mM NaCl) and twice with Covaris Shearing buffer (0.1% SDS, 1 mM EDTA, pH 8.0, 10 mM Tris⋅HCl, pH 8.0) to remove salt. Pellets were resuspended in 0.9 mL of Covaris Shearing buffer with 1 mM protease inhibitor mixture (Roche) and sonicated for 10 min to 12 min in a Covaris E220 sonicator. This generated chromatin fragments between 200 bp and 1,000 bp in length which were then immunoprecipitated with FLAG M2 antibody (endogenous C terminus of CHD8 was tagged) bound to Protein G Dynabeads (Life Technologies) in ChIP buffer (50 mM Hepes-KOH, pH 7.5, 300 mM NaCl, 1 mM EDTA, pH 8.0, 1% Triton X-100, 0.1% DOC, 0.1% SDS) overnight at 4 °C. The next day, the beads were washed four times with ChIP buffer, once with DOC buffer (10 mM Tris⋅HCl, pH 8.0, 250 mM LiCl, 0.5% Nonidet P-40, 0.5% DOC, 1 mM EDTA, pH 8.0), and once with Tris-EDTA (TE) buffer. Libraries were constructed on beads by ChIPmentation (65). Sequencing was performed single-end on the Illumina HiSeq2000 sequencer.
Processing of ChIP-seq Data.
The following techniques were applied uniformly to all datasets as previously described (33). Processing of single-end ChIP-seq reads was performed by mapping to the mm9 reference mouse genome using Bowtie 2.1.0 (57). Reads that contained more than a single mismatch and duplicates were discarded, leaving only unique reads. By comparing to input samples for each cell type, peak calling was performed by MACS 2.1.1 (58) for all analyses. The mean values across both replicates from each condition are the RPM values in the genome tracks. Bwtool (60) was used to compute mean genome track densities.
RNA-seq Library Preparation and Data Analysis.
Cells were dissociated with Accutase, washed with PBS, and immediately resuspended in Trisure (Bioline # BIO-38033). Total RNA was isolated following manufacturer’s guidelines and digested with DNaseI (Thermo Fisher #18068015), and digestion reaction was cleaned up with acid-phenol:chloroform. RNA sequencing libraries were made from 1 µg of RNA (RIN > 9) using the SMARTer kit (Takara Bio # 634874) which produces stranded libraries from ribosomal RNA-depleted total RNA. Libraries were amplified with 12 PCR cycles and quantified with Qubit, and size distribution was determined by Bioanalyzer. Libraries were sequenced on an Illumina Nextseq, and the first three bases were trimmed with cutadapt (66) before quantification. Transcript abundances were quantified by Kallisto (67) using the University of California Santa Cruz Genome Browser transcript tables for the mm9 genome assembly. Transcript-level abundance estimates from Kallisto and gene-level count matrices were created using Tximport (68). Differential expression analysis was conducted with DESeq2 version 1.22.2 (59) using default parameters, after prefiltering genes with low counts (rowSums > 10). Differential calls were made by requiring FDR-corrected P < 0.05. GO and pathway analysis was conducted using the PANTHER classification system (69).
GSEA.
As described previously, a list of Refseq transcripts was prepared based on the output of DESeq2 from RNA-seq data, which was used to perform GSEA (33). RefSeq genes, ranked by log2-fold change, were imported into the GSEA application created by the Broad Institute. Enrichment plots and FDR-corrected P values were obtained directly from the modified Kolmogorov–Smirnov test performed by the GSEA application.
Genome Annotation Enrichment.
Enrichment for overlap with genomic annotations was performed as previously described by comparing the frequency that each peak fell into basic genomic annotations (33). This was separately determined for each group of differential peak call described above. HOMER was used to calculate enriched GO terms and log enrichment of genomic annotation overlap for each group of sites (70).
Lasso Multivariate Regression.
Lasso multivariate regression (35) was performed as previously described (33). Briefly, the fold change in ATAC-seq read density was related to a linear combination of 111 individual chromatin features,
The number of reads within ±3 kb from the center of each ATAC peak was summed for each of the 111 chromatin features from previously published datasets. At each site, the summed read count was log10 transformed and scaled to unit variance across all sites (xi). The R package “glmnet” (71) was used to perform the Lasso regression with the default mixing penalty parameter α = 1. Values for the restricted parameter (λ=∑iβi) were obtained for the Chd8+/− and Chd8−/− ESCs and NPCs by 10-fold cross-validation with the minimal value selected that provided the lowest mean cross-validation error.
chromVAR.
The chromVAR was performed similarly to that previously described (38). Briefly, normalized peaks were filtered to only include peaks containing one fragment across all samples. Peaks were matched to mouse_pwms_v1 motifs, and the top 50 most variable motifs across the samples were calculated and plotted.
FPD and FA Calculation.
We used vertebrate subset of JASPAR CORE motif database accounting for 579 motifs in total (72). TF FPD and FA were defined as FPD = log2 and FA = log2, where , , and are average number of Tn5 inserts calculated in [−5 bp, +5 bp], [−55 bp, −6 bp] U [6 bp, 55 bp] and [−200 bp, −250 bp] U [200 bp, 250 bp] intervals around motif center, respectively. Only motifs found within ATAC-seq peaks were used in calculations. The positions of the Tn5 inserts were defined as the first base pair in the sequencing read offset by 4 bp (by −5 bp) for reads aligned to + (to −) strand (31). We accommodated the visualization technique from the BagFoot package (42) to present changes in FPD and FA across conditions as a bagplot plot. In brief, the bagplot is defined by its median point, the bag and the fence. The bag is constructed around median point and contains about 50% of all points. The fence is obtained by inflating the bag by factor 3. STRING (73) was used to evaluate transcription factor mutual associations such as direct interaction or coexpression.
Public Datasets Analyzed in This Study.
Read densities were obtained from publicly available National Center for Biotechnology Information Gene Expression Omnibus datasets. For each of these chromatin feature datasets, read fragments were extended to 200 bp from the 3′ end, and base pair coverage was determined using Bedtools (61).
Antibodies Used in This Study.
Antibodies used in this study are mouse a-FLAG M2 (1:1,000, Sigma-Aldrich), rabbit a-CHD8 (1:2,500, Novus Biologicals #NB100-60417; 1:2,000, Novus Biologicals #NB100-60418), mouse a-TBP (1:2,000, Abcam #ab818), rabbit a-DCX (1:200, CST #4604S), mouse a-Nestin (1:200, #MAB353), mouse a-Tuj1 (1:200, BioLegend #801201), rabbit a-Pax6 (1:200, BioLegend #901301), rabbit a-NeuroD1 (1:200, Proteintech #12081-1-AP), and mouse a-Map2a (1:200, M9942).
Alkaline Phosphatase.
ESCs were cultured in 6-cm plates for 4 d and fixed with 4% paraformaldehyde. Reagents were prepared according to the protocol included in the Alkaline Phosphatase Detection Kit (Sigma-Aldrich, #SCR004). Briefly, dishes were incubated in stain solution at room temperature in the dark for 15 min. The AP-expressing cells were stained red.
IF.
NPCs were fixed in 4% paraformaldehyde for 15 min, blocked, and permeabilized with 3% normal donkey serum and 0.25% Triton in TBS. They were then incubated with primary antibodies ON at 4 °C followed by secondary goat anti-rabbit or anti-mouse antibodies (Invitrogen) and DAPI, washed with TBS, and imaged on a Keyence BZ-X710 fluorescent microscope.
Supplementary Material
Acknowledgments
We thank the Genome Aggregation Database (gnomAD) and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at https://gnomad.broadinstitute.org/about. We are grateful to Thomas Edlund from Umeå University in Sweden for kindly sharing the Sox3 antibody and to Wendy Wenderski for critical comments on the manuscript. We thank V. Natu, D. Wagh, and J. Coller at the Stanford Functional Genomics Facility for their help with sequencing. S.S. is supported by NSF Graduate Research Fellowship 2013169249 and the National Institute of Mental Health (Program F31-MH111157). G.R.C. is supported by Howard Hughes Medical Institute, NIH Grant R37 NS046789 and the Simons Foundation Autism Research Initiative Grant 306063. C.M.W. is supported by the Walter V. and Idun Berry & GSK Sir James Black Fellowship. H.C.H. is supported by NIH Grant R00CA187565.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921963117/-/DCSupplemental.
Data Availability.
All sequencing data have been deposited in Gene Expression Omnibus (accession number GSE155218).
References
- 1.Narlikar G. J., Sundaramoorthy R., Owen-Hughes T., Mechanisms and functions of ATP-dependent chromatin-remodeling enzymes. Cell 154, 490–503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clapier C. R., Iwasa J., Cairns B. R., Peterson C. L., Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 18, 407–422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konev A. Y. et al., CHD1 motor protein is required for deposition of histone variant H3.3 into chromatin in vivo. Science 317, 1087–1090 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micucci J. A., Sperry E. D., Martin D. M., Chromodomain helicase DNA-binding proteins in stem cells and human developmental diseases. Stem Cells Dev. 24, 917–926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marfella C. G. A., Imbalzano A. N., The Chd family of chromatin remodelers. Mutat. Res. 618, 30–40 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee-Basu S., Packer A., SFARI Gene: an evolving database for the autism research community. Dis. Model Mech. 3, 133–135, 10.1242/dmm.005439 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Ishihara K., Oshimura M., Nakao M., CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol. Cell 23, 733–742 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama M. et al., Early embryonic death in mice lacking the beta-catenin-binding protein Duplin. Mol. Cell. Biol. 24, 8386–8394 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson B. A., Tremblay V., Lin G., Bochar D. A., CHD8 is an ATP-dependent chromatin remodeling factor that regulates beta-catenin target genes. Mol. Cell. Biol. 28, 3894–3904 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotney J. et al., The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 6, 6404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugathan A. et al., CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc. Natl. Acad. Sci. U.S.A. 111, E4468–E4477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson B. et al., The autism-associated gene chromodomain helicase DNA-binding protein 8 (CHD8) regulates noncoding RNAs and autism-related genes. Transl. Psychiatry 5, e568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahir F. et al., Novel deletions of 14q11.2 associated with developmental delay, cognitive impairment and similar minor anomalies in three children. J. Med. Genet. 44, 556–561 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gompers A. L. et al., Germline Chd8 haploinsufficiency alters brain development in mouse. Nat. Neurosci. 20, 1062–1073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama Y. et al., CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 537, 675–679 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Platt R. J. et al., Chd8 mutation leads to autistic-like behaviors and impaired striatal circuits. Cell Rep. 19, 335–350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suetterlin P. et al., Altered neocortical gene expression, brain overgrowth and functional over-connectivity in chd8 haploinsufficient mice. Cereb. Cortex 28, 2192–2206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breuss M. W., Gleeson J. G., When size matters: CHD8 in autism. Nat. Neurosci. 19, 1430–1432 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Karczewski K. J., et al. , The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama M. et al., CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis. Nat. Cell Biol. 11, 172–182 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen C. et al., NSD3-Short is an adaptor protein that couples BRD4 to the CHD8 chromatin remodeler. Mol. Cell 60, 847–859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez-Paredes M., Ceballos-Chávez M., Esteller M., García-Domínguez M., Reyes J. C., The chromatin remodeling factor CHD8 interacts with elongating RNA polymerase II and controls expression of the cyclin E2 gene. Nucleic Acids Res. 37, 2449–2460 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fueyo R. et al., Lineage specific transcription factors and epigenetic regulators mediate TGFβ-dependent enhancer activation. Nucleic Acids Res. 46, 3351–3365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plasari G. et al., Nuclear factor I-C links platelet-derived growth factor and transforming growth factor beta1 signaling to skin wound healing progression. Mol. Cell. Biol. 29, 6006–6017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster C. T. et al., Lysine-specific demethylase 1 regulates the embryonic transcriptome and CoREST stability. Mol. Cell. Biol. 30, 4851–4863 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz-Sanjuán I., Brivanlou A. H., Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3, 271–280 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Abranches E. et al., Neural differentiation of embryonic stem cells in vitro: A road map to neurogenesis in the embryo. PLoS One 4, e6286 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao C. et al., Dual requirement of CHD8 for chromatin landscape establishment and histone methyltransferase recruitment to promote CNS myelination and repair. Dev. Cell 45, 753–768.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wapinski O. L. et al., Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 155, 621–635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergsland M. et al., Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 25, 2453–2464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buenrostro J. D., Giresi P. G., Zaba L. C., Chang H. Y., Greenleaf W. J., Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klemm S. L., Shipony Z., Greenleaf W. J., Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 20, 207–220 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Hodges H. C. et al., Dominant-negative SMARCA4 mutants alter the accessibility landscape of tissue-unrestricted enhancers. Nat. Struct. Mol. Biol. 25, 61–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanton B. Z. et al., Smarca4 ATPase mutations disrupt direct eviction of PRC1 from chromatin. Nat. Genet. 49, 282–288 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibshirani R., Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. B 58, 267–288 (1996). [Google Scholar]
- 36.Bernstein B. E. et al., A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Meissner A. et al., Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454, 766–770 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schep A. N., Wu B., Buenrostro J. D., Greenleaf W. J., chromVAR: Inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young R. A., Control of the embryonic stem cell state. Cell 144, 940–954 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masui S. et al., Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 9, 625–635 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Whyte W. A. et al., Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baek S., Goldstein I., Hager G. L., Bivariate genomic footprinting detects changes in transcription factor Activity. Cell Rep. 19, 1710–1722 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M., Tang L., Liu D., Ying Q. L., Ye S., The transcription factor Gbx2 induces expression of Kruppel-like factor 4 to maintain and induce naïve pluripotency of embryonic stem cells. J. Biol. Chem. 292, 17121–17128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musa J., Aynaud M. M., Mirabeau O., Delattre O., Grünewald T. G., MYBL2 (B-myb): A central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis. 8, e2895 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amano T. et al., Zscan4 restores the developmental potency of embryonic stem cells. Nat. Commun. 4, 1966 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plank J. L., Suflita M. T., Galindo C. L., Labosky P. A., Transcriptional targets of Foxd3 in murine ES cells. Stem Cell Res. (Amst.) 12, 233–240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi X. et al., TGF-β/Smad3 stimulates stem cell/developmental gene expression and vascular smooth muscle cell de-differentiation. PLoS One 9, e93995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simandi Z. et al., RXR heterodimers orchestrate transcriptional control of neurogenesis and cell fate specification. Mol. Cell. Endocrinol. 471, 51–62 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Tremblay M., Sanchez-Ferras O., Bouchard M., GATA transcription factors in development and disease. Development 145, dev164384 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Joshi K., Lee S., Lee B., Lee J. W., Lee S. K., LMO4 controls the balance between excitatory and inhibitory spinal V2 interneurons. Neuron 61, 839–851 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuzuki S. et al., Cross talk between retinoic acid signaling and transcription factor GATA-2. Mol. Cell. Biol. 24, 6824–6836 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soufi A., Donahue G., Zaret K. S., Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell 151, 994–1004 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soufi A. et al., Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 161, 555–568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodman J. V., Bonni A., Regulation of neuronal connectivity in the mammalian brain by chromatin remodeling. Curr. Opin. Neurobiol. 59, 59–68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelen E. et al., Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat. Genet. 43, 607–611 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Marie C. et al., Oligodendrocyte precursor survival and differentiation requires chromatin remodeling by Chd7 and Chd8. Proc. Natl. Acad. Sci. U.S.A. 115, E8246–E8255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langmead B., Trapnell C., Pop M., Salzberg S. L., Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y. et al., Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pohl A., Beato M., bwtool: A tool for bigWig files. Bioinformatics 30, 1618–1619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinlan A. R., Hall I. M., BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barski A. et al., High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Jin W. et al., Genome-wide detection of DNase I hypersensitive sites in single cells and FFPE tissue samples. Nature 528, 142–146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kidder B. L., Zhao K., Efficient library preparation for next-generation sequencing analysis of genome-wide epigenetic and transcriptional landscapes in embryonic stem cells. Methods Mol. Biol. 1150, 3–20 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Schmidl C., Rendeiro A. F., Sheffield N. C., Bock C., ChIPmentation: Fast, robust, low-input ChIP-seq for histones and transcription factors. Nat. Methods 12, 963–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin M., Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 17, 3 (2011). [Google Scholar]
- 67.Bray N. L., Pimentel H., Melsted P., Pachter L., Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Soneson C., Love M. I., Robinson M. D., Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000 Res. 4, 1521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mi H. et al., Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 14, 703–721 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heinz S. et al., Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedman J. H., Hastie T., Tibshirani R., Regularization paths for generalized linear models via coordinate descent. J. Stat. Software 33, 22 (2010). [PMC free article] [PubMed] [Google Scholar]
- 72.Khan A. et al., JASPAR 2018: Update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46, D260–D266 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Mering C. et al., STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 33, D433–D437 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data have been deposited in Gene Expression Omnibus (accession number GSE155218).