Significance
Since fever is frequently a response to infection, immune responses likely occur commonly at fever temperatures. Are fever temperatures detected by immune cells, what molecular pathways are involved in this sensing, and what are the functional consequences? Our data show that CD4 T lymphocytes detect moderate fever temperature. A TRPV channel-mediated pathway involving Notch activation leads to a Th2 skewing of the effector T cell response. However, the antigen presenting dendritic cells that stimulate CD4 T cells, in turn, detect fever temperature and overproduce interleukin-12 to counter this Th2 skewing. The evolutionary genesis of these pathways and the functional consequences of this complex regulatory loop in specific infectious circumstances are, thus, likely to be of great interest.
Keywords: Fever, Th1/Th2, TRPV, Notch
Abstract
Fever is a conserved and prominent response to infection. Yet, the issue of how CD4 T cell responses are modulated if they occur at fever temperatures remains poorly addressed. We have examined the priming of naive CD4 T cells in vitro at fever temperatures, and we report notable fever-mediated modulation of their cytokine commitment. When naive CD4 T cells were primed by plate-bound anti-CD3 and anti-CD28 monoclonal antibodies at moderate fever temperature (39 °C), they enhanced commitment to IL4/5/13 (Th2) and away from IFNg (Th1). This was accompanied by up-regulation of the Th2-relevant transcription factor GATA3 and reduction in the Th1-relevant transcription factor Tbet. Fever sensing by CD4 T cells involved transient receptor potential vanilloid cation channels (TRPVs) since TRPV1/TRPV4 antagonism blocked the febrile Th2 switch, while TRPV1 agonists mediated a Th2 switch at 37 °C. The febrile Th2 switch was IL4 independent, but a γ-secretase inhibitor abrogated it, and it was not found in Notch1-null CD4 T cells, identifying the Notch pathway as a major mediator. However, when naive CD4 T cells were primed via antigen and dendritic cells (DCs) at fever temperatures, the Th2 switch was abrogated via increased production of IL12 from DCs at fever temperatures. Thus, immune cells directly sense fever temperatures with likely complex physiological consequences.
Fever is a hallmark response linking infection, immunity, and inflammation. Fever induction and maintenance in homeotherms involves coordinated interplay among microbial recognition by the innate immune system, signaling in the hypothalamus via microbial ligands and/or cytokines, and, subsequent, hypothalamic signals to the periphery regulating heat generation (1, 2). The fever response probably provides a survival advantage; even poikilotherms show induction of ‘"behavioral fever" (3), and antipyretic drug usage correlates with greater morbidity in infections (4).
Fever pleiotropically modulates infection outcomes. It reduces virus replication in cells (5) and increases bacterial susceptibility to complement-mediated lysis (6). However, uncontrolled fever is detrimental as in sepsis where lowering core temperature is beneficial (7). In innate immune cells, fever-range temperatures increase respiratory burst and bacteriolytic capacities in neutrophils and their recruitment (8, 9), the phagocytic abilities of DCs (10–12) and natural killer cell recruitment, and cytotoxicity (13). Hyperthermia causes HSP70-dependent enhancement of lipopolysaccharide (LPS)-mediated NF-κB activation (14). However, there are fewer studies of the effects of elevated temperatures on adaptive immunity and on innate-adaptive crosstalk. Fever-range temperatures augment lymphocyte trafficking through effects on binding activities of both l-selectin and α4β7-integrin (15–17) and enhance CD8 T cell effector differentiation (18) and Th17 differentiation (19).
Transient receptor potential channels, particularly, the TRPVs TRPVs1–4, are known to function as cellular thermosensors (20) and are expressed in many tissues (21). TRPV1 is a critical regulator of physiological body temperature and fever outside the central nervous system (22) and is expressed on cells of the immune system (21) with functional significance in CD4 T cells (23), macrophages, and DCs (24). However, the relationship between temperature and TRPVs in modulating immune cell functions is unclear.
On this background, we have examined the effects of fever temperatures on innate-adaptive immune crosstalk. We report that elevated temperature modulates antigen-presenting cell (APC)-free activation-induced naive CD4 T cell differentiation by enhancing expression of the transcription factor GATA3. This switch is dependent on TRPV1 and on enhanced Notch activation. However, we find that DCs show TRPV/Notch-independent enhanced IL12 production at fever temperatures. As a result, APC-mediated CD4 T cell activation at elevated temperatures does not lead to Th2 differentiation except under IL12 deficit. Thus, immune cells directly sense fever temperatures via TRPV1 with subsequent complex crosstalk.
Results
Fever-Range Temperatures Shift the Th1/Th2 Balance toward the Th2 Phenotype in Naive CD4 T Cells Differentiating In Vitro.
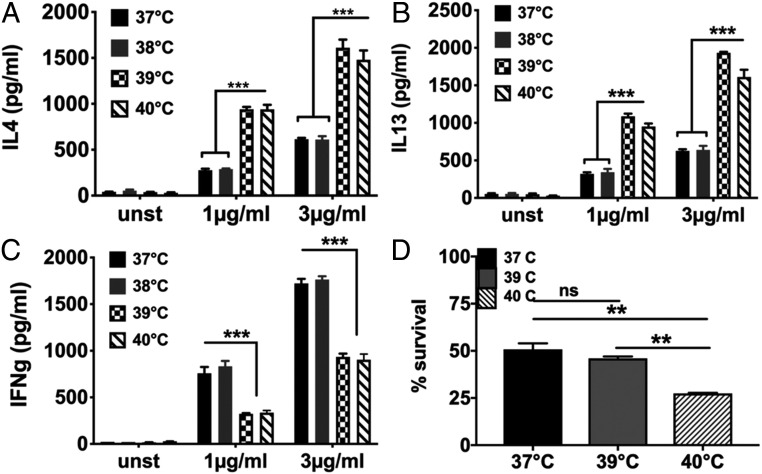
We examined the effect of mild (38 °C), moderate (39 °C), and severe (40 °C) febrile temperatures on differentiation of naive CD4 T cells stimulated in vitro. To ensure cell-intrinsic temperature detection, we used purified mouse naive CD4 T cells (CD4+CD44-CD25-CD62L+; NCD4T cells; SI Appendix, Fig. S1) responding in vitro to plate-coated anti-CD3+anti-CD28 monoclonal antibodies (mAbs) without any further differentiation-skewing modulation of the cultures. Cells primed for 72 h at various temperatures were rested for 24 h at 37 °C in IL2, restimulated with plate-coated anti-CD3+anti-CD28 at 37 °C, and cytokine levels in 24-h culture supernatants measured (SI Appendix, Fig. S2), ensuring that fever effects were limited to the priming phase. IFN-γ (IFNg) was measured as a Th1 cytokine, and interleukin-4 (IL4) and IL13 as Th2 cytokines. Priming at 38 °C did not alter cytokine commitment, but moderate (39 °C) and severe (40 °C) fever temperatures significantly enhanced Th2 commitment (Fig. 1 A and B) and reduction in Th1 commitment (Fig. 1C). Thus, responding NCD4T cells switched commitment to Th2 between 38 °C and 39 °C.
Fig. 1.
Effect of fever-range temperatures on effector cytokine programming of naive CD4 T cells (A–C). Levels of IL4 (A), IL13 (B), and IFNg (C) in culture supernatants of primed and restimulated NCD4T cells at indicated recall concentrations of anti-CD3. NCD4 cells were primed with anti-CD3+anti-CD28 at indicated temperatures for 3 d, rested at 37 °C for one day, and restimulated at 37 °C for 24 h. Data representative of three independent experiments. Data shown as mean ± SE unstimulated (unst): cells unstimulated during priming culture and recall. (D) Viable cell proportions after 3 d of activation at temperatures shown. Data representative of three independent experiments. Data shown as mean ± SE. ns, not significant, **P < 0.001, ***P < 0.0001.
While in culture, viable cell frequencies were unaffected at 39 °C, but they were modestly reduced at 40 °C (Fig. 1D). Therefore, subsequent comparisons were between 37 °C and 39 °C priming temperatures.
We confirmed that responding CD4 T cell activation and proliferation was unchanged between 37 °C and 39 °C. Induction CD69 and CD25, estimated by qRT-PCR 6-h postpriming, was comparable at 37 °C and 39 °C (SI Appendix, Fig. S3 A and B). Cell-surface CD25 expression at 24 h of priming also remained comparable (SI Appendix, Figs. S3C and S4) as did cell viability even up to 72 h (SI Appendix, Fig. S3 D and E), IL2 production by responding CD4 T cells at 24 h (SI Appendix, Fig. S3F), and the extent of proliferation at 72 h in CFSE-labeled NCD4T cells (SI Appendix, Fig. S3G).
We also examined if this effect of fever led to effector T cells expressing both Th1 and Th2 cytokines. After priming as above at 37 °C or 39 °C and a 24-h rest in IL2 at 37 °C, cells were challenged with phorbol myristate acetate (PMA)+ionomycin for 6 h at 37 °C in the presence of brefeldin A and were stained for intracellular Th1 (IFNg) versus Th2 (IL4+IL5+IL13) cytokines. While IFNg-expressing cells went down and IL4/5/13-expressing cells went up in frequency upon priming at 39 °C, there was no increase in Th1+Th2 cells (SI Appendix, Figs. S5 A and B and S6).
We tested if human NCD4T cells showed a similar fever effect. Human NCD4T cells purified from healthy volunteer peripheral blood mononuclear cells (PBMCs; CD4+CD25-CD45RO) were primed at 37 °C or 39 °C using plate-bound anti-CD3+anti-CD28 mAbs, followed by a 24-h rest at 37 °C in IL2, were restimulated with plate-bound anti-CD3+anti-CD28 at 37 °C, and cytokine levels measured in 24-h culture supernatants. Consistently, human CD4 T cells showed a Th2 switch when primed at 39 °C with lower IFNg production and higher IL13 production on recall (SI Appendix, Fig. S5C).
Thus, both mouse and human naive CD4 T cells showed Th2-skewed differentiation when primed in vitro at moderate fever temperature without any alterations in activation, proliferation, or cell survival.
Altered Expression Levels of Tbet and GATA3 Transcription Factors during CD4 T Cell Priming at Fever Temperature.
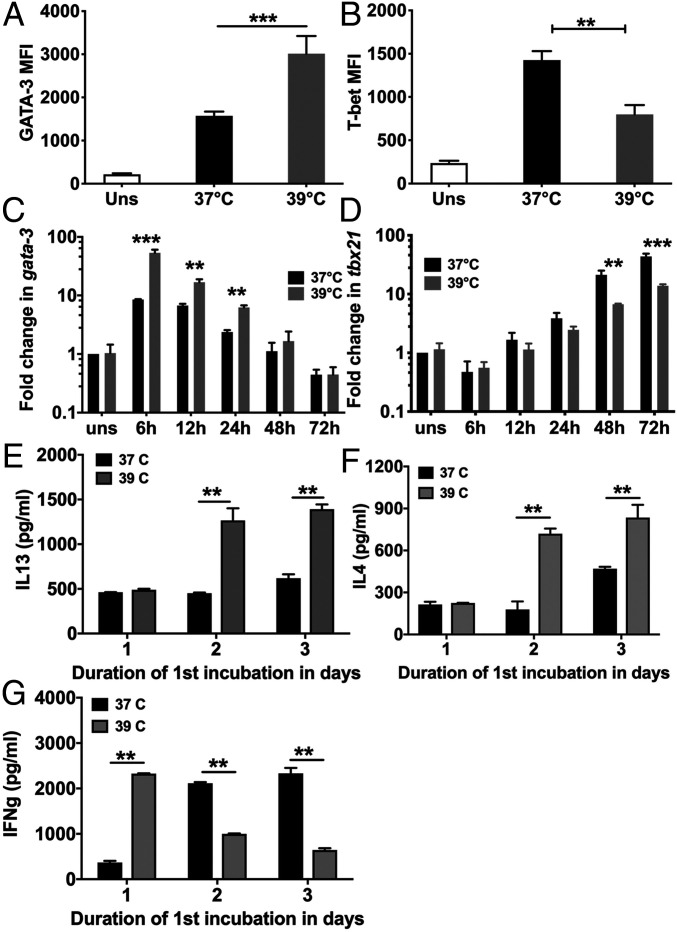
Since the transcription factors Tbet (Tbx21) and GATA3, respectively, regulate Th1 and Th2 differentiation trajectories of responding CD4 T cells (25), we examined the effect of fever temperature on the expression of these factors. Mouse NCD4T cells were stimulated with plate-coated anti-CD3+anti-CD28 at 37 °C or 39 °C, and intracellular GATA3 and Tbet protein levels were estimated at 72 h by flow cytometry. GATA3 protein levels were higher and Tbet levels lower in CD4 T cells primed at 39 °C (SI Appendix, Fig. S7 and Fig. 2 A and B).
Fig. 2.
Alterations in GATA-3 and T-bet expression levels and cytokine levels during NCD4 T cell priming at fever temperature (A and B). MFI levels for GATA3 (A) and T-bet (B) in NCD4 cells primed at indicated temperatures for 3 d, permeabilized and stained. Uns, unstimulated NCD4 cells. Mean ± SE, n = 4. (C and D) Time kinetics of Gata3 (C) and Tbx21 (D) mRNA up-regulation at indicated temperatures in NCD4 T cells stimulated with anti-CD3+anti-CD28. Fold increases over unstimulated NCD4 T cells at 37 °C shown. Mean ± SE, n = 3–5 for different time points. (E–G) Mouse NCD4 cells were primed at 37 °C/39 °C for 1 or 2 d before switching to 39 °C/37 °C, rested for one day, and restimulated. Control groups were primed for 3 d at 37 °C or 39 °C. Culture superntant concentrations of IL13 (E), IL4 (F), and IFNg (G) shown. Mean ± SE, n = 4. **P < 0.001; ***P < 0.0001.
We also measured Gata3 and Tbx21 transcript levels by qRT-PCR at varying time points during priming. Their transcript levels showed different trajectories over the 72-h priming period (Fig. 2 C and D), and fever temperature caused enhanced Gata3 and reduced Tbx21 expression (Fig. 2 C and D).
Since time courses of Tbet and GATA3 induction in responding CD4 T cells were distinct, we next asked what priming time period was sufficient for eliciting the temperature-mediated Th2 switch. Mouse NCD4 T cells were primed with plate-coated anti-CD3+anti-CD28 at 37 °C or 39 °C for 1 or 2 d and then switched to the other temperature for the remainder of the 3-d priming period, while control groups of cells were also primed at 37 °C or 39 °C for the entire 3-d period (priming scheme shown in SI Appendix, Fig. S8). After the 3-d priming, cells were rested for a day in IL2 at 37 °C, restimulated with plate-bound anti-CD3+anti-CD28 for 24 h, and cytokine levels determined in culture supernatants (Fig. 2 E–G). Fever temperature for the first priming day alone was insufficient for either reduction in IFNg commitment or increased IL4/IL13 commitment. An additional priming day at 39 °C was, however, sufficient to mediate a shift to IL4/IL13 commitment. Nonetheless, fever temperature on the first priming day was essential, although not sufficient for Th2 switching, since shifting cells to 39 °C after a first day at 37 °C did not lead to any Th2 increase. However, such a fever exposure after the first day was, indeed, sufficient to decrease IFNg commitment (Fig. 2 E–G). These findings were consistent with the early induction of GATA3 and the relatively later induction of Tbet in these priming cultures.
We also tested if these fever consequences were applicable to γ/δ T cells by inducing the proliferation and differentiation of γ/δ T cells from human PBMCs at 37 °C or 39 °C using a specific ligand for human γ/δ T cells 1-hydroxy-2-methyl-2-buten-4-yl 4-diphosphate (HDMAPP) along with IL2. After 12 d, the cultures were predominantly (>90%) γ/δ T cells, and 39 °C cultures showed higher messenger RNA (mRNA) levels of GATA3 and IL13 and lower levels of Tbet and IFNg than 37 °C cultures (SI Appendix, Fig. S9).
Role of TRPV Channels in Fever Sensing by Responding CD4 T Cells.
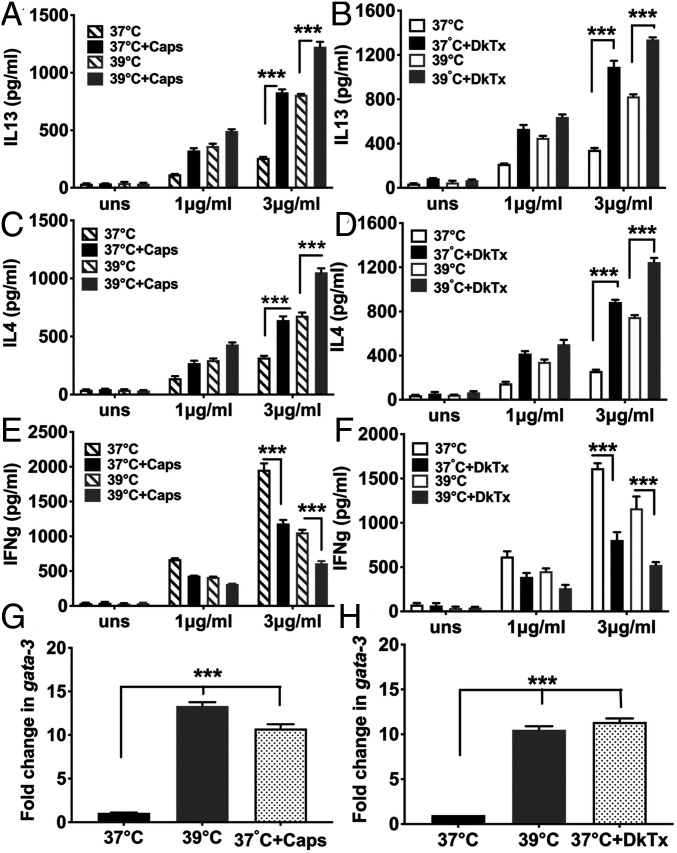
We next asked if TRPV channels were involved in thermosensing during CD4 T cell differentiation. We tested two different TRPV1 agonists for the ability to phenocopy fever temperature in Th1/Th2 balance alteration in responding CD4 T cells, capsaicin (Caps) (26) and a more TRPV1-specific agonist, the double knot toxin (DkTx) from the earth tiger tarantula (27). When NCD4T cells were primed using plate-coated anti-CD3+anti-CD28 in the presence or absence of Caps or DkTx at 37 °C, there was significant enhancement of IL4 and IL13 commitments and reduction in IFNg commitment, comparable to that in cells primed at 39 °C (Fig. 3 A–F). Furthermore, when NCD4T cells were activated at 37 °C for 6 h in the presence or absence of Caps or DkTx, Gata3 expression levels were increased to a similar degree as in cells primed at 39 °C (Fig. 3 G and H). Thus, TRPV1 agonism phenocopied fever temperature in altering the Th1/Th2 balance of responding CD4 T cells.
Fig. 3.
TRPV activation phenocopies fever-mediated Th2-skewed programming of responding NCD4 T cells (A–F). Levels of secreted IL13 (A and B), IL4 (C and D), or IFNg (E and F) by NCD4 cells primed at 37 °C or 39 °C in the presence or absence of Caps, panels (A, C, and E) or DkTx (panels [B, D, and F] and recalled with indicated concentrations of anti-CD3. uns, unstimulated NCD4 cells. Mean ± SE, n = 3. (G and H) Fold increase in gata3 mRNA levels induced by fever temperature, Caps, or DkTx in CD4 T cells activated for 6 h with plate-bound anti-CD3+anti-CD28. Data normalized to signals from cells activated at 37 °C. Mean ± SE, n = 3. ***P < 0.0001.
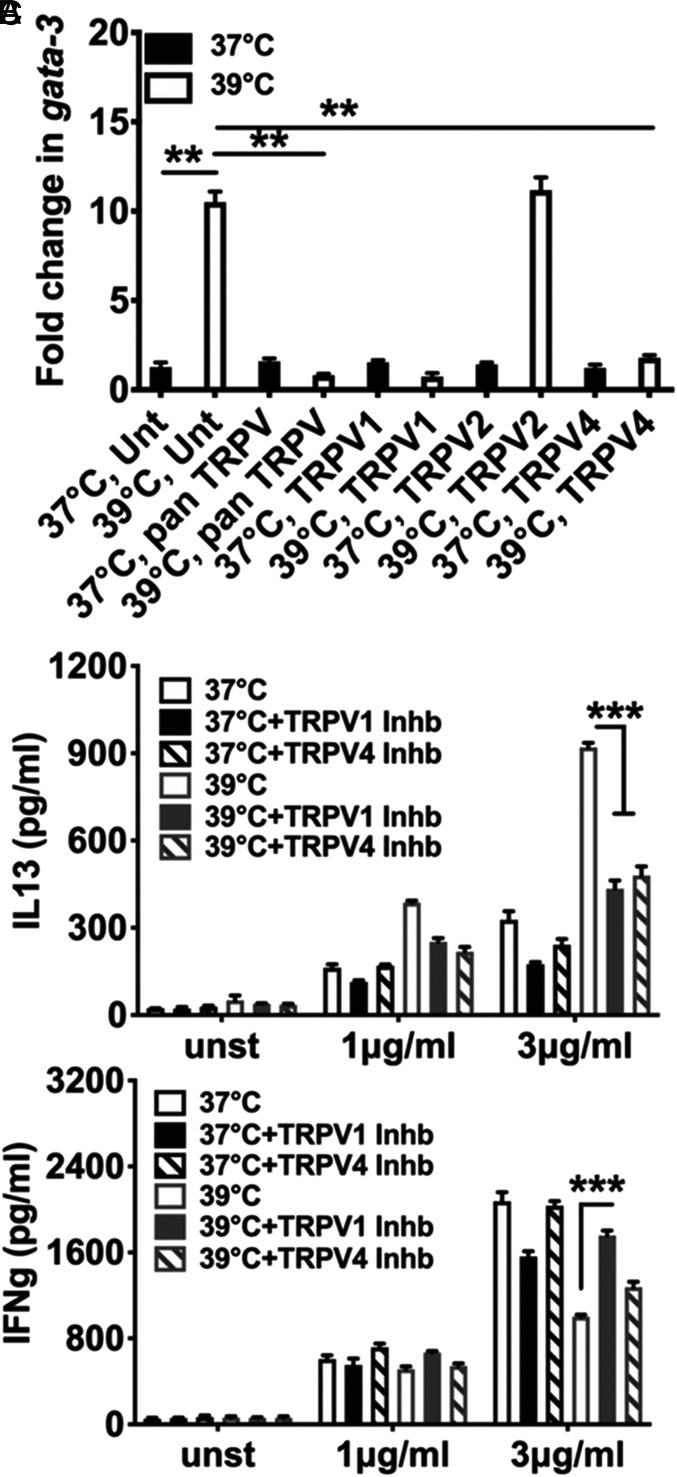
We next asked if TRPV members were actually involved in fever sensing by responding CD4 T cells. NCD4 T cells were subjected to activation for 6 h with anti-CD3+anti-CD28 at 37 °C or 39 °C in the presence or absence of various TRPV inhibitors, and Gata3 transcript levels were measured. None of the TRPV inhibitors used substantially modified Gata3 expression at 37 °C (Fig. 4A). However, a pan-TRPV inhibitor, ruthenium red, inhibited Gata3 up-regulation at 39 °C as did a TRPV1-specific inhibitor and a TRPV4-specific inhibitor, although a TRPV2-specific inhibitor did not do so (Fig. 4A), indicating that TRPV1 and TRPV4 were involved in fever sensing by responding CD4 T cells.
Fig. 4.
TRPV1 and TRPV4 are critical for fever-mediated Th2-skewed reprogramming of responding NCD4 T cells (A). Fold change in Gata3 mRNA levels in the presence or absence of TRPV inhibitors as indicated at 37 °C or 39 °C. NCD4 cells were stimulated for 6 h with plate-bound anti-CD3+anti-CD28. Fold increases in levels above those in cells activated at 37 °C without any TRPV agonists are shown. Unt, untreated with any TRPV agonist. Mean ± SE, n = 3. (B and C) Levels of secreted IL13 (B) and IFNg (C) in 24-h culture supertatants after restimulation of NCD4 cells primed at 37 °C or 39 °C in the presence or absence of TRPV1 or TRPV4 inhibitors (Inhb) for 3 d. Mean ± SE, n = 3. **P < 0.001; ***P < 0.0001.
Furthermore, when NCD4 T cells were primed with anti-CD3+anti-CD28 at 37 °C or 39 °C in the presence or absence of various TRPV inhibitors for 3 d and their Th1/Th2 commitment determined, neither TRPV1 or TRPV4 inhibition during priming altered the Th1/Th2 balance at 37 °C (Fig. 4 B and C), but inhibition of either TRPV1 or TRPV4 during priming substantially blocked the fever-induced Th2 switch in commitment (Fig. 4 B and C).
We tested if this involvement of TRPV channels involved fever temperature-mediated modulation of their expression. When Trpv1, Trpv2, Trpv3, and Trpv4 gene transcript levels were estimated by qRT-PCR in RNA from unstimulated naive T cells as well as cells activated at either 37 °C or 39 °C for 3 h, none of the Trpv genes showed any modulation either by T cell activation or between 37 °C and 39 °C (SI Appendix, Fig. S10). Thus, fever temperature sensing in responding CD4 T cells required signaling via TRPV1 and TRPV4.
Role of Notch Signaling in Fever Temperature-Mediated Th2 Switch.
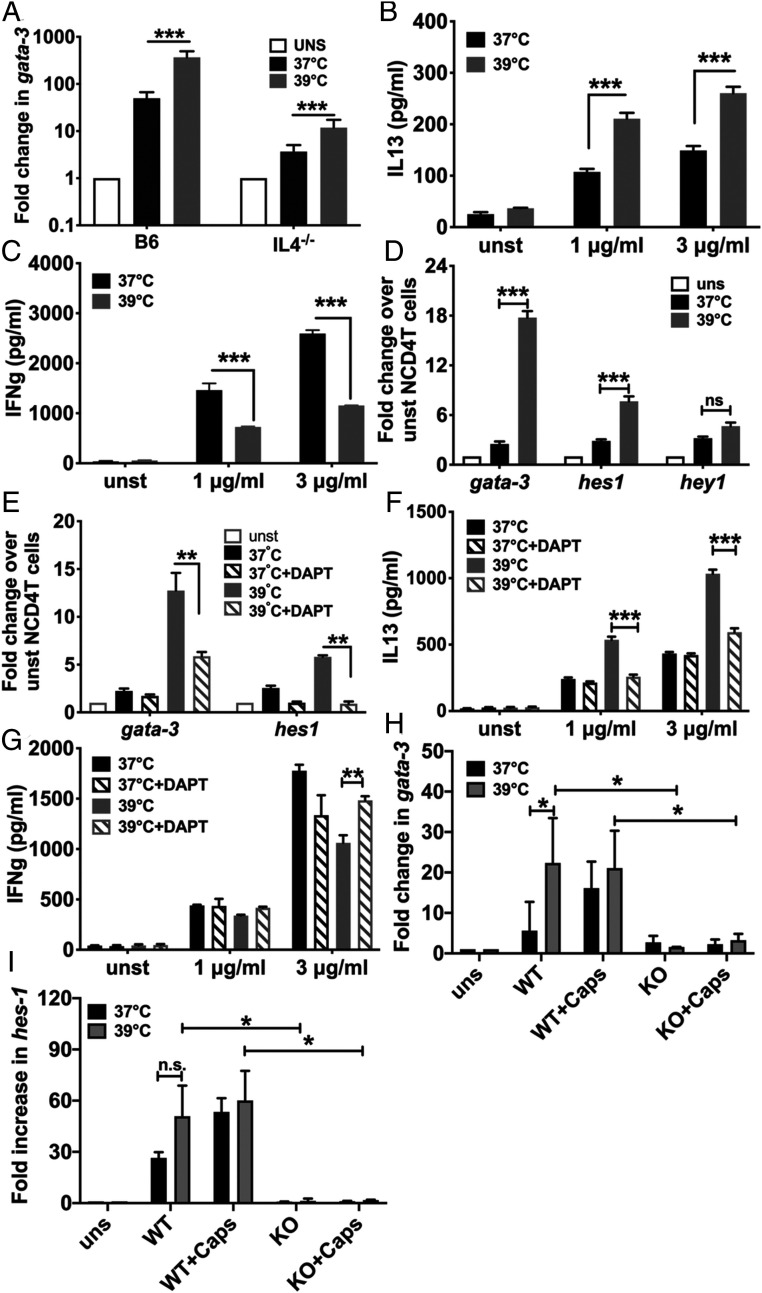
The signaling pathway/s modulated by fever temperature for the Th2 switch in responding CD4 T cells were of interest. Early low-level production of IL4 from responding CD4 T cells is significant for Th2 commitment (28). Therefore, NCD4T cells from WT and IL4-null mice were primed with plate-bound anti-CD3+anti-CD28 at 37 °C or 39 °C. At 6 h of activation, fever temperature led to hyperinduction of Gata3 mRNA expression in IL4-deficient CD4 T cells as well, albeit at lower levels (Fig. 5A). When these CD4 T cells were primed for 3 d, the fever temperature-mediated Th2 cytokine switch also persisted despite the absence of IL4 (Fig. 5 B and C).
Fig. 5.
Fever-mediated Th2-skewing of activated CD4 T cell programming is IL4 independent but Notch dependent (A). Up-regulation of Gata3 in wild-type (WT) and IL4−/− NCD4 cells activated at 37 °C or 39 °C with anti-CD3+anti-CD28 for 6 h. Mean ± SE; n = 3. UNS: unstimulated. (B and C) IL13 (B) and IFNg (C) levels in culture supernatants during recall response of NCD4 T cells from IL4−/− mice primed at 37 °C or 39 °C. Mean ± SE; n = 3. unst: unstimulated. (D) Change in Gata3, Hes1, and Hey1 mRNA levels of NCD4 T cells activated at 37 °C or 39 °C for 6 h. uns, unstimulated NCD4 T cells. Mean ± SE; n = 3. (E) Change in gata3 and Hes1 mRNA levels of NCD4 T cells activated at 37 °C or 39 °C in the presence or absence of Notch inhibitor DAPT for 6 h. unst, unstimulated NCD4 T cells. Mean ± SE; n = 3. (F and G) IL13 (F) and IFNg (G) levels in culture supernatants during recall response of NCD4 T cells activated at 37 °C or 39 °C in the presence or absence of DAPT. Mean ± SE; n = 3. (H and I). Change in Gata3 (H) and Hes1 (I) mRNA levels of NCD4 T cells from WT and Notch1−/− (knockout; KO) mice activated at 37 °C or 39 °C in the presence or absence of Caps for 6 h. uns, unstimulated NCD4 T cells. Mean ± SE; n = 3. ns, not significant, *P < 0.05; **P < 0.001; ***P < 0.0001.
An IL4-independent mechanism mediating Gata3 expression, the Notch signaling pathway, which induces Gata3 transcription in CD4 T cells from an upstream start site (29), was next tested for its involvement in fever-mediated Th2 switching. When NCD4T cells were primed with plate-bound anti-CD3+anti-CD28 at 37 °C or 39 °C, RNA extracted 6 h later and various transcript levels quantified, it was evident that fever temperature led to hyperinduction of not only Gata3, but also of, at least, one Notch-target gene Hes1 (30) (Fig. 5D), although the levels of another Notch-target gene Hey1 were not increased to the same extent (Fig. 5D), suggesting increased Notch signaling at fever temperature in responding CD4 T cells.
Notch cleavage by γ-secretase is crucial for activation, and γ-secretase inhibitors, such as N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) are used to block Notch signaling (31). We tested if DAPT could block the fever-mediated Th2 switch in CD4 T cells by priming NCD4T cells with plate-bound anti-CD3+anti-CD28 at 37 °C or 39 °C in the presence or absence of DAPT. At 6-h postactivation, DAPT did not alter Gata3 levels at 37 °C, but, at 39 °C, it significantly suppressed the hyperinduction of Gata3 as well as of Hes1 (Fig. 5E). When these CD4 T cells were primed for 3 d and functionally tested, it was again apparent that, while DAPT did not alter the Th1/Th2 balance of commitment at 37 °C, it substantially suppressed the fever-mediated Th2 switch (Fig. 5 F and G).
We next tested the role of the Notch1 molecule in these events by priming NCD4T cells from WT or Notch1-deficient mice with plate-coated anti-CD3+anti-CD28 at 37 °C or 39 °C with or without Caps for 6 h, and various transcript levels were quantified. Notch1-deficient CD4 T cells showed abrogation in the hyperinduction of both Gata3 and Hes1 mediated by either fever temperature or by Caps (Fig. 5 H and I).
We also tested if Notch pathway involvement involved fever temperature-mediated modulation of Notch and/or Notch ligand gene expression. Transcript levels of various Notch and Notch ligand genes were measured by qRT-PCR in unstimulated naive T cells as well as cells activated for 3 h at either 37 °C or 39 °C. None of these genes showed any substantial modulation either by T cell activation or between 37 °C and 39 °C with the exception of Dll1 which was induced by T cell activation but showed no effect of fever temperature (SI Appendix, Fig. S11).
Together, these data showed that the fever temperature-induced TRPV1-mediated Th2 switch in CD4 T cells was dependent on activation of Notch1 signaling.
Transcriptional Analysis of Naive CD4 T Cells Stimulated at 37 °C or 39 °C.
Since fever temperatures initiated early signaling events culminating in Gata3 hyperexpression, we also undertook global gene expression analysis at a very early time point, 3-h postactivation, using RNA from unstimulated naive T cells as well as cells activated at either 37 °C or 39 °C (32). While several thousand genes were differentially expressed in activated cells (at either temperature) compared to naive T cells (8,350 at 39 °C and 7,711 at 37 °C; SI Appendix, Fig. S12 and Table S1), the transcriptomes of activated cells at 37 °C and 39 °C were very similar (SI Appendix, Fig. S13) with only 445 genes differentially expressed. Gene set enrichment analysis showed that, as expected, the major gene ontology term enriched in the fever group consisted mainly of heat shock-induced chaperones (SI Appendix, Fig. S13B). The heatmap for normalized read counts for heat shock proteins (SI Appendix, Fig. S13C) as well as the enrichment plot (SI Appendix, Fig. S13D) showed enrichment of several heat shock proteins in the fever group. On the other hand, several distinct functional terms were down-regulated at 39 °C, most notably, terms associated with ribosome function and assembly, with membrane targeting of proteins, and with mitochondrial oxidative phosphorylation (SI Appendix, Fig. S13 B–E). When we analyzed the up-regulated and down-regulated genes separately using the g:profiler tool (33), chaperone-mediated protein folding was again enriched in the fever group (SI Appendix, Fig. S13G). Additionally, cell signaling pathways, such as the mitogen-activated protein kinase, NF-κB, and IL17 signaling pathways were enriched in the fever group (SI Appendix, Fig. S13G), some of which have been associated with Th2 differentiation (34, 35). Genes associated with the p53 pathway were enriched among the genes significantly down-regulated at 39 °C (SI Appendix, Fig. S13H).
IL12-Dependent Blockade of Fever Temperature-Mediated Th2 Skewing during DC-Mediated T-Cell Priming.
Our experiments so far used APC-free NCD4T cell stimulation via plate-coated anti-CD3+anti-CD28. However, physiological T cell activation involves APCs, particularly, DCs. Fever temperatures modify DC behavior, and LPS-activated DCs produce larger amounts of IL12 at fever temperatures (36). We next examined the possibility of interestingly complex fever-mediated modulation of T cell-APC cross talk.
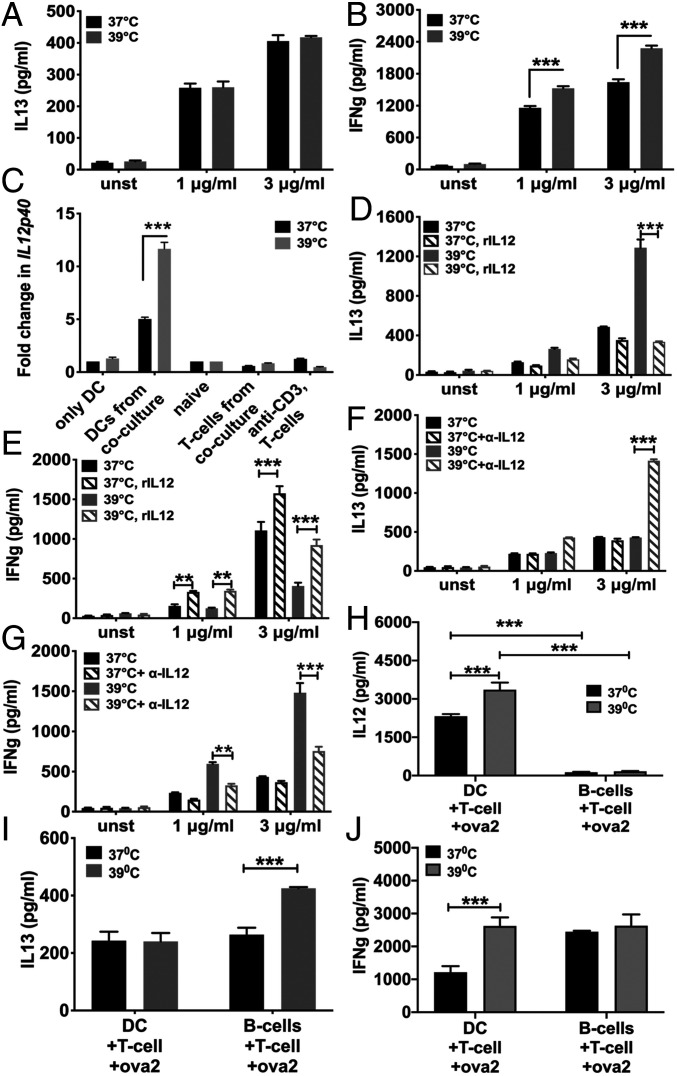
Using T cell receptor (TCR)-transgenic mice expressing the OTII TCR recognizing a chicken ovalbumin peptide (OVA323-329; Ova2) with major histocompatibility complex class II (H2-Ab), OTII NCD4T cells were either primed with plate-bound anti-CD3+anti-CD28 at 37 °C or 39 °C without APCs or with congeneic C57BL/6 bone marrow-derived DCs (BMDCs) in coculture along with Ova2 peptide at 37 °C or 39 °C for 3 d. Responding CD4 T cells were then separated, rested for a day in IL2, and restimulated with homologous stimuli either plate-bound anti-CD3+anti-CD28 or DCs pulsed with titrating dose of Ova2 peptide (SI Appendix, Fig. S2) at 37 °C for 24 h to measure cytokines in culture supernatants. OTII CD4 T cells primed with DCs+Ova2 peptide at 37 °C and 39 °C showed equivalent levels of IL13 commitment (Fig. 6A), indicating no fever-induced Th2 switch. Instead, they showed modest enhancement of IFNg commitment at fever temperature (Fig. 6B).
Fig. 6.
Fever-mediated Th2-skewed programming of responding CD4 T cells is blocked by fever-mediated IL12 production from APCs (A and B) IL13 (A) and IFNg (B) levels in culture supernatants during recall response of OT-II NCD4 T cells when activated at 37 °C or 39 °C in the presence of BMDCs and Ova2 peptide. Mean ± SE; n = 3. (C) Change in IL12p40 mRNA levels of different cell types either cultured alone or purified from cocultures as indicated after activation at 37 °C or 39 °C for 6 h. mRNA levels for DCs were normalized to unstimulated BMDCs cultured at 37 °C whereas those for T cells were normalized to unstimulated NCD4 T cells. Mean ± SE; n = 3. (D and E) IL13 (D) and IFNg (E) levels in culture supernatants during recall response of OT-II NCD4 T cells when activated at 37 °C or 39 °C with plate-bound anti-CD3+anti-CD28 with or without added rIL12 during priming. Mean ± SE; n = 3. (F and G) IL13 (F) and IFNg (G) levels in culture supernatants during recall response of OT-II NCD4 T cells when activated at 37 °C or 39 °C in the presence of BMDCs and Ova2 peptide with or without anti-IL12 added during priming. Mean ± SE; n = 3. (H) IL12 levels in culture supernatants during recall response of OT-II NCD4 T cells when activated at 37 °C or 39 °C in the presence of either BMDCs or LPS-activated B cells as APCs and Ova2 peptide during priming. Mean ± SE; n = 3. (I and J) IL13 (I) and IFNg (J) levels in culture supernatants during recall response of OT-II NCD4 T cells when activated at 37 °C or 39 °C in the presence of either BMDCs or LPS-activated B cells as APCs and Ova2 peptide during priming cultures. Mean ± SE; n = 3. **P < 0.001; ***P < 0.0001.
We next tested a role for IL12 in this situation. OTII NCD4T cells were activated with DCs and Ova2 peptide at 37 °C or 39 °C for 6 h, T cells and DCs separated by sorting, and relative IL12p40 mRNA expression levels assessed using qRT-PCR. IL12p40 mRNA expression was found only in DCs and not in T cells, and IL12p40 levels in DCs were significantly higher at 39 °C than at 37 °C (Fig. 6C).
It was, thus, likely that more IL12 from fever-exposed DCs blocked fever-induced Th2 switching in responding T cells. Recombinant IL12 (rIL12), added to NCD4T cells primed with plate-bound anti-CD3+anti-CD28 at 37 °C or 39 °C without APCs, blocked the fever-mediated Th2 switch (Fig. 6 D and E). Furthermore, neutralizing anti-IL12 monoclonal antibody, added in the OTII NCD4T cell+DC+Ova2 priming cultures at 37 °C or 39 °C, showed little effect on the Th1/Th2 balance achieved at 37 °C but led to substantial Th2 shift at 39 °C even in DC-driven T cell activation (Fig. 6 F and G).
We also examined outcomes of priming CD4 T cells using non-IL12-producing APCs other than DCs by using LPS-activated B cell blasts or DCs as APCs to prime OTII NCD4T cells with Ova-2 peptide at 37 °C or 39 °C. When culture supernatants from these 3-d cultures were tested for IL12, it was observed that IL12 was detectable only in DC-containing cultures, and fever temperature led to enhanced IL12 production (Fig. 6H). When the responding CD4T cells from these cultures were rested in IL2 for 24 h and restimulated at 37 °C with DCs and Ova-2 peptide, the fever-associated enhancement of IL13 commitment was evident in primed T cells from LPS-B cell blast-driven cultures but not as expected in those from DC-driven cultures (Fig. 6 I and J).
These results showed that the fever-mediated Th2 switch was abrogated in the presence of IL12-producing APCs, suggesting that fever temperatures can induce a number of interacting effects on different cell types, leading to complex emergent outcomes depending on the precise local circumstances involved. The complexity of these interactions would depend on whether or not the same signaling pathways were involved in fever sensing in T cells versus APCs. We, therefore, tested if Caps as a TRPV agonist could mimic the effects of fever temperature on IL12 expression from activated DCs and if the γ-secretase inhibitor DAPT could modify these effects. While LPS-mediated DC activation led to enhanced IL12 production at 39 °C compared to 37 °C, Caps did not show such an effect nor did DAPT affect the enhanced IL12 response of LPS-activated DCs at fever temperature (SI Appendix, Fig. S14), indicating that fever sensing by DCs was unlikely to involve the TRPV or Notch pathways.
Modulation of Th1/Th2 Balance in CD4 T Cell Responses In Vivo.
Thus, CD4 T cells sensed fever via TRPVs with resultant Th2 differentiation, while DCs sensed fever independent of TRPVs and modified Th2 differentiation direction via IL12. We next examined if fever could modulate the direction of CD4 T cell responses upon in vivo immunization. Most pyrogenic triggers (such as LPS) alter priming microenvironments in fever-independent ways, making interpretation difficult. Therefore, we used two separate interventions in vivo. First, we used Caps treatment along with immunization to examine TRPV-mediated modulation. Second, since fever responses in vivo require prostaglandin E2 binding to hypothalamic neuronal EP3 receptors (37), we used sulprostone, a strong and relatively specific EP3 agonist (albeit with weak EP1 agonism) (38) in vivo along with immunization to look for any alteration of the resultant Th1/Th2 balance. Mice were immunized subcutaneously (s.c.) with Ova with alum as the adjuvant, so as to minimize the role of IL12 (39). Seven days later, draining lymph node cells were restimulated with Ova, and IL13 and IFNg levels in culture supernatants determined. In preliminary experiments, while IL13 levels remained unchanged, IFNg levels showed a trend to decline in mice treated with either Caps or sulprostone with an increase in the IL13/IFNg ratio as the Th2/Th1 balance (SI Appendix, Fig. S15), although only the sulprostone group showed statistically significant changes. These data validated, to some extent, the physiological significance of the findings in vitro.
Discussion
Since fever responses to infection are ubiquitous (40), it appears plausible that there would be immunological consequences of fever. Yet, most studies of fever-mediated modulation of immune responses address innate immune cells, although there has been recent work on T cells (18, 19). Our data now show that moderate fever substantially modifies naive CD4 T cell responses via TRPV family members, such as TRPV1 and TRPV4, and that Notch signaling is a major pathway downstream of thermosensing driving the change in differentiation. Thus, CD4 T cell responses generated during fever would be expected to show a Th2 shift as, indeed, they can do in vivo. However, our data with APC-mediated priming complicate this scenario since fever also induces increased IL12 production from APCs, leading to Th2 skewing blockade consistent with previously reported findings in vivo (36). In effect, the extent of fever and the relative availability of IL12-making APCs would together constitute a complex matrix determining a number of possible outcomes, providing flexibility.
It is notable that fever temperatures compatible with organismal viability do not change gross parameters of CD4 T cell responses; neither activation nor proliferation are significantly affected, and cell viability is only marginally affected at severe fever levels. Thus, the fever response of responding CD4 T cells is a highly specific modulation. It is interesting to speculate on the selection pressure for the evolution of a fever-responsive TRPV-Notch pathway in T cells.
Our data showing that responding CD4 T cells distinguish between 38 °C and 39 °C for triggering this pathway generate another dimension of this evolutionary question. Both species we have tested, mouse and human, have approximately similar normal body temperatures. Do other homeotherms show such responses, and are they finely calibrated to detect the distinctions between the normal versus the fever temperatures for each species? In fact, does the much wider temperature tolerance of poikilothermic animals and their propensity to "behavioral fever" (3) lead to any differences in the fever behavior of their T cells? Central to these issues is the question of the identity of the molecular sensors that initiate this response. It is surprising that, even in the extensive studies addressing innate immune function modification by fever temperatures, this question has not been rigorously addressed.
Our data strongly suggest that TRPV family members, particularly, TRPV1 and TRPV4, are involved in CD4 T cell thermosensing. In addition to Caps, the DkTx toxin, widely acknowledged to be TRPV1-specific, phenocopies the effects of fever temperature, and a TRPV1 antagonist blocks the effects of fever temperature, although this issue remains to be formally proven with genetically Trpv1-deficient T cells. This effect likely does not involve modulation of TRPV expression levels since those are unaltered by either T cell activation or by fever temperature. The involvement of both TRPV1 and TRPV4 may be significant. Current understanding of TRPV1-mediated thermosensing involves detection of somewhat higher temperatures from 41 °C (41), while the thermal threshold of TRPV4 is ∼30 °C (42, 43). How do TRPV1 and TRPV4 detect and respond to 39 °C, and what is the structural basis of such detection? TRPVs function as calcium channels (44), calcium signaling is important for Th2 commitment (45), and both TRPV1 and TRPV4 have been shown to function in CD4 T cells to modify TCR-mediated signaling (23). The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells (23, 46–48). There is also evidence from other cell lineages that TRPV1 and TRPV4 can function in tandem with each other whether via heterodimerization or by mutual regulation of expression and/or signaling (49, 50). Since the TRPVs are involved, our data also bring up an interesting dimension of TRPV evolution so as to enable appropriate fever responses in species with different normal and fever temperatures.
Our data indicate that fever-induced TRPV-Notch-mediated modulation of GATA3 expression is a specific and major pathway for Th2 switching of CD4 T cell responses. However, this is not the only fever-mediated modulation to be detected in responding CD4 T cells. While it is tempting to ascribe the down-modulation of Tbet to the known reciprocal regulation by enhanced GATA3 levels (25), our time-course data showing that shifting responding CD4 T cell from 37 °C to 39 °C on the second day of priming reduces IFNg commitment without enhancing IL13 commitment suggest that there may be yet more subtle fever-mediated modulations that remain to be found, possibly, as a consequence of the early global transcriptomic differences that we have observed.
The Notch signaling pathway can induce Gata3 transcription in CD4 T cells independent of IL4 (25). Our data using a γ-secretase inhibitor as well as CD4 T cells from Notch1-deficient mice clearly show that Notch1 signaling is crucial for fever- and TRPV1-mediated hyperinduction of Gata3 expression and Th2 skewing, although formal demonstration of Notch1 activation by detection of its active component as well as detailed examination of the activation, proliferation, and differentiation of Notch1-deficient T cells at fever temperature remain to be performed. Notch activation, at least, as measured by Hes1 expression is not really notable at 37 °C yet is prominent at 39 °C. The obvious question brought up by these data is the basis for Notch1 activation at fever temperature especially since these are homotypic responding CD4 T cell cultures with no other cell type involved. So, are Notch ligands and/or Notch1 itself expressed at higher levels at fever temperatures on T cells? This is unlikely since fever temperature did not modulate transcript levels for any of them. Notch pathway activation upon TCR-mediated stimulation has been extensively shown in homogenous T cell populations with debate about whether this is initiated by ligand-dependent or ligand-independent Notch activation (51, 52). Thus, it is possible that fever- and TRPV-mediated signals may induce Notch1 activation, possibly, dependent on alteration of its trafficking during endocytotic recycling, a phenomenon recently reported in a number of situations (53–55) especially since TRPV channels are known to be regulators of endocytotic trafficking (56).
Our RNA-sequencing (RNA-seq) analysis at 3 h of activation provides a more global snapshot of fever-induced modulation of the gene expression landscape. Clearly, heat shock protein mRNAs were major transcripts prominent in fever. A recent study found fever-mediated enhancement of polarized Th17 differentiation (19) and suggested heat shock protein involvement in this effect. Interestingly, our pathway analysis also finds up-regulation of the Th17 signaling pathway at febrile temperature even in our unpolarized priming conditions. The roles heat shock proteins might play in the Th2 differentiation we observe remain to be investigated. Another interesting class of mRNAs that show relatively lower levels at febrile temperature are those of mitochondrial proteins, and mitochondrial function is important for the activation and fate determination of T cells (57). Furthermore, T cell activation is associated with immediate up-regulation of ribosomal proteins with increased ribosome biogenesis and translation potential (58). While ribosome biogenesis does increase at febrile temperature when compared to unstimulated naive T cells, these levels are significantly lower than at 37 °C, although it is unclear if such changes in ribosome biogenesis play any role in T cell fate determination.
LPS-activated DCs have been shown to produce higher levels of IL12 at fever temperatures (36), IL12 is a contributory signal for Th1 commitment (59), and, in fact, it has been reported that CD4 T cell responses in vivo at fever temperatures tend to show a Th1 bias (10, 60), in contrast to our data with APC-free priming of CD4 T cells in vitro. Our data using APC-based CD4 T cell priming in vitro confirmed this since fever temperature did not induce a Th2 switch during DC-mediated priming; instead, there was a modest enhancement of IFNg commitment. Our data also show that when DCs and responding CD4 T cells interact, DCs produce substantial amounts of IL12, and this IL12 induction is further enhanced by fever as previously shown (10). However, IL12 blockade or use of non-IL12-producing APCs was sufficient for fever-mediated Th2 switching to occur even in APC-based priming.
Together, these data indicate that fever temperatures in vivo are likely to induce a Th2 switch in responding CD4 T cells, but this outcome is likely to be modulated further by the presence and concentration of IL12 in the microenvironment. A major question is as follows: How relevant are these in vitro phenomena in vivo, especially given the likely complexity indicated by our finding that, while CD4 T cells sense fever via TRPV1 and shift their commitment toward GATA3 using the Notch pathway, while DCs sense fever independent of TRPV1 and Notch and redirect responding CD4 T cells away from GATA3 via IL12? We used immunization in vivo with alum as an adjuvant using the reported finding that alum tends to antagonize IL12 production from DCs (39) so as to minimize the role of IL12 in the immunization. Under these circumstances, both Caps and sulprostone, an EP3 agonist with nonimmune pyrogenic effects, tended to shift the resultant T cell responses in vivo toward Th2 commitment, although, in our experiments, so far only the sulprostone-mediated modulation was statistically significant.
Thus, it is entirely plausible that our findings have functional significance in vivo. Clearly, a quantitative balance in the CD4 T cell-intrinsic versus DC-mediated fever effects would be expected to give rise to a tuning of the Th1/Th2 balance generated in vivo under febrile conditions. The fact that the fever-sensing pathways in CD4 T cells and DCs are different would contribute to a complex matrix of potential interactions and outcomes. Furthermore, our findings of a Th2 shift in CD4 T cells primed by B cell APCs at fever temperature raises even more complex possibilities in vivo. While B cells do not commonly function as priming APCs in vivo for CD4 T cells, they have, indeed, been reported to do so in a number of specific situations, such as with a nanoparticle-mediated immunogen (61), as well as in infectious situations in which febrile responses would be expected, such as in the priming of follicular helper T (Tfh) cells in lymphocytic choriomeningitis virus (62) and malarial infections (63). This raises interesting possibilities of fever-driven modulatory interactions shifting resultant Th1/Th2 balances with consequences for infection outcomes. Clearly, the evolutionary genesis, molecular mechanisms, and functional consequences of these complex pathways of fever temperature sensing in CD4 T cells differentiating in response to immune activation in specific infectious circumstances are likely to be of great interest.
Materials and Methods
Mice.
All mouse strains (details in the SI Appendix) used were from the Jackson Laboratory, Bar Harbor, ME, USA, and maintained in the Small Animal Facility of the National Institute of Immunology. Mice of either sex aged 6–10 wk were used for all experiments. Mice were euthanized by cervical dislocation when indicated. All experiments used three to six mice per group.
Mice were maintained and used in accordance with the guidelines and with the prior approval of the duly constituted Institutional Animal Ethics Committee of the National Institute of Immunology.
Mouse Immunization and Restimulation Assays.
Mice were immunized s.c. with OVA in alum with daily sulprostone, Caps, or vehicle as indicated. On day 7, cells from draining lymph nodes were OVA stimulated in vitro, and levels of IL13 and IFNg in the culture supernatants were assayed (details in the SI Appendix).
PBMCs.
PBMCs were separated by density gradient centrifugation from venous blood collected from healthy young adult volunteers of either sex (age range 23–30 y) after consent.
All human experiments were carried out in accordance with the guidelines and with the prior approval of the duly constituted Institutional Human Ethics Committee of the National Institute of Immunology, New Delhi, or the Indian Institute of Science Education and Research (IISER) Ethics Committee for Human Research (IECHR) of the IISER, Pune.
Flow Cytometric Purification of Naive CD4 T Cells.
Mouse naive CD4 T cells were sorted as CD4+CD44-CD62L+CD25-, while naive human CD4 T cells were sorted as CD4+CD45RO-CD25 under sterile conditions (details in the SI Appendix) (BD FACSAria III; BD Biosciences, CA). Sorted populations showed >95% purity.
Priming and/or Restimulation of CD4 T Cells In Vitro.
These assays were performed as previously described (details in the SI Appendix). Briefly, naive CD4 T cells were activated by plate-coated anti-CD3 and anti-CD28 for 72 h at temperatures as mentioned. Cells were then harvested, rested in IL2 for 24 h at 37 °C, and used for subsequent recall assays at 37 °C. For detection of intracellular cytokines and transcription factors, cells were treated with phorbol myristate acetate and ionomycin for 6 h and brefeldin A for the last 3 h before permeabilization and staining with anti-cytokine and anti-TF antibodies. For analyzing secreted cytokines, cells were restimulated as indicated for 24 h, and cytokines were assayed in culture supernatants.
Human naive CD4 T cells were similarly primed, using 50 international units/mL IL2 during the rest period.
For experiments to test γ/δ T cells, an earlier published protocol (details in the SI Appendix) was used with further optimization. PBMCs were incubated with IL2 and HDMAPP in 24-well plates. Cells were supplemented with IL2 every third day, subcultured on day 6, and harvested on day 12 and processed for qRT-PCR.
For priming TCR-transgenic OTII naive CD4 T cells, Ova2 peptide and syngeneic BMDCs prepared as described below were used as alternative to anti-CD3 and anti-CD28 as above both for priming and for restimulation.
RNA-Seq Analysis of Activated CD4 T Cells.
Naive CD4 T cells were purified as above from C57BL/6 mice, activated with plate-bound anti-CD3+anti-CD28 as above at 37 °C or 39 °C for 3 h, and subjected to RNA-seq analysis (details in the SI Appendix).
Generation of BMDCs and LPS-Activated B Cell Blasts.
Mouse BM cells were cultured with granulocyte/macrophage colony-stimulating factor to obtain BMDCs (details in the SI Appendix), which were either used in T cell priming assays as above or were stimulated with LPS and supernatants tested for IL12 secretion.
Spleen cells were stimulated with LPS for 60 h, live cells isolated, confirmed to be >90% B cells, and used as APCs where indicated.
Enzyme-Linked Immunosorbent Assays for Cytokines.
As described previously, secreted cytokines were detected by commercially available cytokine detection kits (eBiosciences; details in the SI Appendix).
Flow Cytometric Staining and Data Analysis.
Staining and data analysis were performed as described previously (details in the SI Appendix). Flow cytometric data were collected (FACSVerse; BD Biosciences) and analyzed with FlowJo software (Treestar, Ashland, OR).
qRT-PCR Assays.
The qRT-PCR reactions were performed (Eppendorf Mastercycler or Bio-Rad CFX96) for a total of 30–40 cycles as described previously (details in the SI Appendix). Cycle threshold (Ct) values obtained were normalized with L7/GAPDH (mouse)/18S (human) values, and ΔΔCt values were calculated (details in the SI Appendix) and plotted to show enhancement in the signal above unstimulated T cell values.
Statistical Methodology.
For statistical analysis, Student’s t test or two-way ANOVA test were used as indicated. Values of P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Mr. Inder Jit Singh for help with animal maintenance and Mr. Rajesh Kumar for extensive help with electronic cell sorting. The study was supported, in part, by Grants from the Department of Biotechnology (to A.G. BT/PR12849/MED/15/35/2009; to V.B. BT/PR14420/Med/29/213/2010; and to S.R. BT/PR‐14592/BRB/10/858/2010), from the Department of Science and Technology, Government of India (to V.B. S.R./SO/HS‐0005/2011 and EMR/2015/001074; to S.R. SB/SO/HS/210/2013), and from the Arkansas Biosciences Institute to J.M.D. G.M. acknowledges the award of a Research Associateship from the Department of Biotechnology, Government of India. The National Institute of Immunology was supported by the Department of Biotechnology, Government of India.
Footnotes
Competing interest statement: S.R. is a nonexecutive director of Ahammune Biosciences Private Limited, Pune, India and a member of the scientific advisory boards of Curadev Pharma Private Limited, NOIDA, India, and Mynvax Private Limited, Bangalore, India. The other authors have no competing interests to declare.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922683117/-/DCSupplemental.
Data Availability.
The RNA-seq data generated and analyzed in the paper have been made available by submission to the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/ (accession no. GSE152757).
References
- 1.Chai Z., Gatti S., Toniatti C., Poli V., Bartfai T., Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: A study on IL-6-deficient mice. J. Exp. Med. 183, 311–316 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans S. S., Repasky E. A., Fisher D. T., Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 15, 335–349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boltaña S. et al., Behavioural fever is a synergic signal amplifying the innate immune response. Proc. Biol. Sci. 280, 20131381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulman C. I., et al. , The effect of antipyretic therapy upon outcomes in critically ill patients: A randomized, prospective study. Surg. Infect. 6, 369–375 (2005). Correction in: Surg. Infect.11, 495 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Thorne S. H. et al., Effects of febrile temperature on adenoviral infection and replication: Implications for viral therapy of cancer. J. Virol. 79, 581–591 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osawa E., Muschel L. H., Studies relating to the serum resistance of certain gram-negative bacteria. J. Exp. Med. 119, 41–51 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Launey Y., Nesseler N., Mallédant Y., Seguin P., Clinical review: Fever in septic ICU patients–Friend or foe? Crit. Care 15, 222 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takada Y. et al., Granulocyte-colony stimulating factor enhances anti-tumour effect of hyperthermia. Int. J. Hyperthermia 16, 275–286 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Rice P. et al., Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J. Immunol. 174, 3676–3685 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Yan X. et al., Fever range temperature promotes TLR4 expression and signaling in dendritic cells. Life Sci. 80, 307–313 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Basu S., Srivastava P. K., Fever-like temperature induces maturation of dendritic cells through induction of hsp90. Int. Immunol. 15, 1053–1061 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Ostberg J. R., Repasky E. A., Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol. Immunother. 55, 292–298 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostberg J. R., Dayanc B. E., Yuan M., Oflazoglu E., Repasky E. A., Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J. Leukoc. Biol. 82, 1322–1331 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Lee C.-T., Zhong L., Mace T. A., Repasky E. A., Elevation in body temperature to fever range enhances and prolongs subsequent responsiveness of macrophages to endotoxin challenge. PLoS One 7, e30077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans S. S. et al., Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood 97, 2727–2733 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Wang W. C. et al., Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J. Immunol. 160, 961–969 (1998). [PubMed] [Google Scholar]
- 17.Lin C. et al., Fever promotes T lymphocyte trafficking via a thermal sensory pathway involving heat shock protein 90 and α4 integrins. Immunity 50, 137–151.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mace T. A. et al., Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. J. Leukoc. Biol. 90, 951–962 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X. et al., Febrile temperature critically controls the differentiation and pathogenicity of T helper 17 cells. Immunity 52, 328–341.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Dhaka A., Viswanath V., Patapoutian A., Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135–161 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Kunert-Keil C., Bisping F., Krüger J., Brinkmeier H., Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7, 159 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iida T., Shimizu I., Nealen M. L., Campbell A., Caterina M., Attenuated fever response in mice lacking TRPV1. Neurosci. Lett. 378, 28–33 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Bertin S. et al., The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4+ T cells. Nat. Immunol. 15, 1055–1063 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warwick C. A., Shutov L. P., Shepherd A. J., Mohapatra D. P., Usachev Y. M., Mechanisms underlying mechanical sensitization induced by complement C5a: The roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain 160, 702–711 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang E. S., Szabo S. J., Schwartzberg P. L., Glimcher L. H., T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307, 430–433 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Caterina M. J. et al., The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Bohlen C. J. et al., A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141, 834–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noben-Trauth N., Hu-Li J., Paul W. E., Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J. Immunol. 165, 3620–3625 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Fang T. C. et al., Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27, 100–110 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borggrefe T., Oswald F., The notch signaling pathway: Transcriptional regulation at notch target genes. Cell. Mol. Life Sci. 66, 1631–1646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsauskas-Kuprys R., Zlobin A., Osipo C., Gamma secretase inhibitors of Notch signaling. OncoTargets Ther. 6, 943–955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arimbasseri G. A., Rath S., Umar D., Febrile temperature change modulates CD4 T cell differentiation via a TRPV channel-regulated Notch-dependent pathway. Gene expression omnibus (GEO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE152757. Deposited 18 June 2020. [DOI] [PMC free article] [PubMed]
- 33.Reimand J., Kull M., Peterson H., Hansen J., Vilo J., g:Profiler–A web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 35, W193–W200 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goplen N. et al., ERK1 is important for Th2 differentiation and development of experimental asthma. FASEB J. 26, 1934–1945 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kannan Y., Wilson M. S., TEC and MAPK kinase signalling pathways in T helper (T(H)) cell development, T(H)2 differentiation and allergic asthma. J. Clin. Cell. Immunol. 12, 11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatzfeld-Charbonnier A. S. et al., Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J. Leukoc. Biol. 81, 1179–1187 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furuyashiki T., Narumiya S., Roles of prostaglandin E receptors in stress responses. Curr. Opin. Pharmacol. 9, 31–38 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Moreno J. J., Eicosanoid receptors: Targets for the treatment of disrupted intestinal epithelial homeostasis. Eur. J. Pharmacol. 796, 7–19 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Mori A. et al., The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur. J. Immunol. 42, 2709–2719 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Walter E. J., Hanna-Jumma S., Carraretto M., Forni L., The pathophysiological basis and consequences of fever. Crit. Care 20, 200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caterina M. J., Rosen T. A., Tominaga M., Brake A. J., Julius D., A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398, 436–441 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Güler A. D. et al., Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 22, 6408–6414 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Story G. M., The emerging role of TRP channels in mechanisms of temperature and pain sensation. Curr. Neuropharmacol. 4, 183–196 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gees M., Colsoul B., Nilius B., The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2, a003962 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faries M. B. et al., Calcium signaling inhibits interleukin-12 production and activates CD83(+) dendritic cells that induce Th2 cell development. Blood 98, 2489–2497 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Ghoneum M. H. et al., Inhibition of TRPV1 channel activity in human CD4+ T cells by nanodiamond and nanoplatinum liquid, DPV576. Nanomaterials (Basel) 8, 770 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertin S., Raz E., Transient receptor potential (TRP) channels in T cells. Semin. Immunopathol. 38, 309–319 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majhi R. K. et al., Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J. 282, 2661–2681 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Lakk M. et al., Polymodal TRPV1 and TRPV4 sensors colocalize but do not functionally interact in a subpopulation of mouse retinal ganglion cells. Front. Cell. Neurosci. 12, 353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ota H. et al., TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PLoS One 8, e65751 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bheeshmachar G. et al., Evidence for a role for notch signaling in the cytokine-dependent survival of activated T cells. J. Immunol. 177, 5041–5050 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Steinbuck M. P., Winandy S., A review of notch processing with new insights into ligand-independent notch signaling in T-cells. Front. Immunol. 9, 1230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmer W. H., Deng W.-M., Ligand-independent mechanisms of notch activity. Trends Cell Biol. 25, 697–707 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Borgne R., Regulation of Notch signalling by endocytosis and endosomal sorting. Curr. Opin. Cell Biol. 18, 213–222 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Le Bras S., Loyer N., Le Borgne R., The multiple facets of ubiquitination in the regulation of notch signaling pathway. Traffic 12, 149–161 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Goswami C. et al., TRPV1 acts as a synaptic protein and regulates vesicle recycling. J. Cell Sci. 123, 2045–2057 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Buck M. D. et al., Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166, 63–76 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asmal M. et al., Production of ribosome components in effector CD4+ T cells is accelerated by TCR stimulation and coordinated by ERK-MAPK. Immunity 19, 535–548 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Athie-Morales V., Smits H. H., Cantrell D. A., Hilkens C. M. U., Sustained IL-12 signaling is required for Th1 development. J. Immunol. 172, 61–69 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Peng J. C. et al., Monocyte-derived DC primed with TLR agonists secrete IL-12p70 in a CD40-dependent manner under hyperthermic conditions. J. Immunother. 29, 606–615 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Hong S. et al., B cells are the dominant antigen-presenting cells that activate naive CD4+ T cells upon immunization with a virus-derived nanoparticle antigen. Immunity 49, 695–708.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Barnett L. G. et al., B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J. Immunol. 192, 3607–3617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arroyo E. N., Pepper M., B cells are sufficient to prime the dominant CD4+ Tfh response to Plasmodium infection. J. Exp. Med. 217, e20190849 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data generated and analyzed in the paper have been made available by submission to the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/ (accession no. GSE152757).