Abstract
Women and female rodents are more responsive to the subjective effects of psychostimulant drugs of abuse compared to males. A growing body of literature supports a role for estradiol as a mechanism underlying these sex differences. However, little is known about the influence of acute elevations in levels of estradiol on drug conditioned behaviors. The aim of the present study was to evaluate the influence of an acute increase in systemic estradiol levels on the expression of cocaine conditioned place preference (CPP). Using a six day conditioning procedure, ovariectomized (OVX) female rats were conditioned with one of four doses of cocaine (2.5, 5, 10, or 15 mg/kg) to associate one of two large chambers of a CPP apparatus with cocaine or saline. Thirty minutes prior to the start of the CPP preference test, rats were pretreated with either 5 μg estradiol benzoate (EB) or peanut oil (PO). PO-treated rats expressed a significant preference for only the mid-range conditioning doses of cocaine (5 and 10 mg/kg). However, acute EB treatment resulted in a rightward shift in the cocaine dose-response curve; rats demonstrated a significant preference at only the moderate and high conditioning doses of cocaine (10 and 15 mg/kg). These findings demonstrate that acute elevations in estradiol may dampen the expression of conditioned responses to cocaine’s secondary rewards at lower conditioning doses of the drug and facilitate CPP at higher doses while estradiol deficiency decreases the threshold dose of cocaine necessary to induce CPP.
Keywords: Estrogen, Cocaine, Reward, Ovariectomy, Dose-response, Locomotion
1. Introduction
Cue reactivity and exposure to cues are important factors in continued drug use and relapse to former patterns of drug use (Childress et al., 1988; Epstein et al., 2009; O’Brien et al., 1990). Investigations into gender differences in reactivity to cocaine-associated cues have produced inconsistent results; some studies report greater cue reactivity in women (Elman et al., 2001; Robbins et al., 1999), one reports greater reactivity in men (Sterling et al., 2004), and another reports equivalent cue reactivity among men and women (Avants et al., 1995). Several methodological factors account for these discrepancies. A recent neuroimaging study high-lights the importance of simultaneously collecting neural data along with self-report data during specific periods of the menstrual cycle and reports greater brain reactivity to conditioned cocaine cues in mid-follicular phase women than in men who were current cocaine abusers even though their self-reported craving responses did not differ (Volkow et al., 2011). Overall, these data strongly suggest that exposure to cocaine-associated environmental conditioned stimuli stimulates and/or increases the desire to use drugs in drug dependent individuals and this desire to use may be further enhanced in women during phases of the menstrual cycle during which high levels of circulating estrogen are predominant (O’Brien et al., 1977).
Evidence from drug selt-administration studies in rodents has reliably demonstrated sex and hormonally modulated differences during all phases of the addiction process: acquisition, maintenance, and reinstatement (Anker and Carroll, 2011). Overall, these data show that female rats’ operant behavior is more robust than males’ during acquisition of cocaine self-administration, escalation of cocaine intake, and drug-primed and stress-induced reinstatement (Bard et al., 2000; Buffalari et al., 2012; Fuchs et al., 2005; Lynch et al., 2000; Lynch and Carroll, 1999; Roth and Carroll, 2004). Female rodents’ response to cocaine also varies with their estrous cycle. Female rats in the estrus phase of the cycle display increased motivation to self-administer cocaine (Roberts et al., 1989) and increases in the intensity of cocaine-induced stereotypic and locomotor activities (Quinones-Jenab et al., 1999). Taken together, these data suggest that increases in circulating levels of estradiol increase the motivation to self-administer cocaine and other behavioral subjective effects of the drug.
Studies manipulating circulating levels of estradiol in rodents have consistently demonstrated a key role for estrogen in enhancing the behavioral response to cocaine in females (Becker, 1999; Festa et al., 2004). For example, removal of endogenous ovarian hormones by ovariectomy (OVX) decreases acquisition rates of cocaine self-administration and cocaine-primed reinstatement of drug-seeking behavior. Moreover, replacement of estradiol, via chronic daily subcutaneous injections or via continuous release Silastic implant, restores cocaine self-administration rates to levels comparable with those of intact females’ (Frye, 2007; Larson et al., 2005; Lynch et al., 2001). In summary, most studies have consistently shown that chronic sustained elevations in levels of estradiol increases cocaine self-administration.
The conditioned place preference (CPP) paradigm is used to determine the conditioned rewarding effects of drugs in rodents because the contextual cues used within the paradigm acquire secondary appetitive properties when paired with an addictive drug. Very few studies have used the CPP paradigm to investigate sex differences in the conditioned rewarding effects of cocaine and fewer still have examined the role of ovarian hormones. To date, only three investigations have examined the activational effects of estradiol on conditioned cocaine reward (Russo et al., 2003; Segarra et al., 2010; Twining et al., 2013). Through the use of slightly different methodologies, each of these studies examined the role of prolonged elevations in estradiol levels on cocaine-induced CPP and has provided some fundamental insight into our understanding of the influence of estradiol on learning drug-context associations. However, until now, the issue of the effects of acute elevations of estradiol on cocaine-induced CPP has remained unsettled. Therefore, the purpose of the present study was to examine the influence of a single acute increase in systemic estradiol levels on the expression of cocaine CPP.
2. Methods
2.1. Subjects
Eighty-three experimentally naïve, adult (60 day old), female, Long Evans rats (University of Texas at Arlington vivarium) were triple housed with same-sex cage mates in a temperature and humidity-controlled environment under a 12 h reversed light/dark cycle with lights on at 7 p.m. and off at 7 a.m. All animals had free access to food and water throughout the study and were maintained and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the University of Texas at Arlington’s Institutional Animal Care and Use Committee (IACUC) in accordance with AAALAC standards.
2.2. Ovariectomy
Rats were anesthetized with a 2–3% isoflurane-oxygen vapor mixture and ovariectomized (OVX) using a dorsal approach. Briefly, both flanks were shaved and swabbed with Betadine. The skin was opened with a 5 mm incision along the midline just below the ribs, and a 10 mm incision was made through the muscle ∼1.5–2 cm lateral to the midline. The ovary was pulled through the incision. The tissue between the oviduct and uterus were clamped with a hemostat and a ligature was placed just below the hemostat. The ovary was removed with scissors and the hemostats released. This procedure was repeated on the contralateral side. Lastly, the muscle layer was sutured closed and the skin incision closed with 9 mm wound clips.
2.3. Vaginal lavage testing
Following a 4–5 day surgical recovery period, all rats underwent daily vaginal lavage testing for 8–10 consecutive days to confirm cessation of cycling. Vaginal secretion was collected with a plastic pipette filled with 10 μL of 0.9% NaCl− by inserting the tip into the rat vagina, but not deeply. Unstained material was observed under a light microscope. All ovariectomies performed were confirmed as complete and thus, no animals were eliminated on the basis of an imcomplete procedure.
2.4. Estradiol treatment
Animals were assigned to one of two groups of hormone treatment: 0.1 ml peanut oil - vehicle (OVX); or 5 μg 17β-Estradiol 3 benzoate (EB; Sigma-Aldrich, St Louis, MO) dissolved in 0.1 ml peanut oil. Hormone treatment was delivered via subcutaneous (s.c.) injection only once; 30 min prior the test for conditioned place preference (see Fig. 1).
Fig. 1.
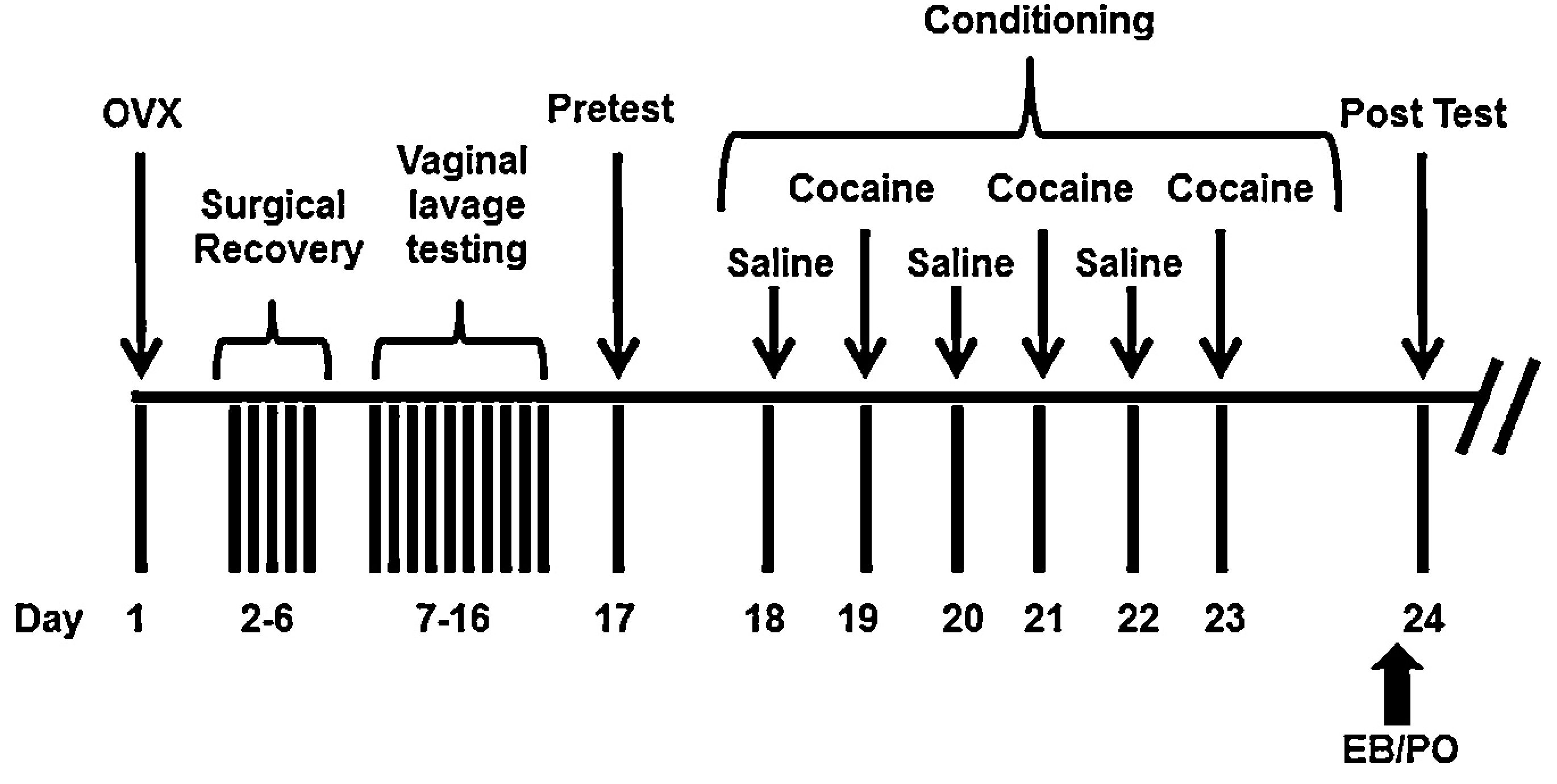
Timeline of experimental procedures. All animals undergo ovariectomy (Day1; OVX) followed by a five day surgical recovery period (Days 2–6) after which they are subjected to ten days of vaginal lavage testing to confirm completion of OVX. The conditioned place preference protocol commences with a PreTest (Day 17) during which rats freely explore the entire apparatus in a drug-free state. Conditioning for Saline/Cocaine occurred over days 18–23; on alternating days OVX rats received injections of 0.9% saline or one of four doses of cocaine (2.5, 5,10, or 15 mg/kg) and were confined to one chamber of the apparatus for 30 min. Thirty minutes prior to the test for conditioned preference (PostTest; Day 24), animals received an injection of EB (5 μg) or peanut oil (PO).
2.5. Cocaine conditioned place preference
The apparatus used to carry out the conditioned place preference (CPP) consists of two large chambers distinct in visual and tactile cues (wall color and floor material) that are connected by a small shuttle chamber (Med Associates, Georgia, VT). The two large contextually distinct chambers (8.25″W × 8.24″H × 26.75″L) are equipped with 16 infrared photobeam detectors for automated data collection. Behavioral testing began 12–14 days after OVX surgery; following confirmation of OVX. On Day 17 of experimentation, all rats underwent a preconditioning test (PreTest) allowing them to freely explore the entire apparatus for one 15-min session. Rats were then randomly assigned to saline/cocaine conditioning chambers for a total of six conditioning sessions. On each of the three saline conditioning days, animals received a 1 ml/kg intraperitoneal (i.p.) injection of 0.9% NaCl− (saline) and were confined to the previously assigned saline-paired chamber for 30 min. For each of the three cocaine conditioning days, animals received an i.p. injection of cocaine hydrochloride (or saline) at one of four doses (2.5, 5, 10, or 15 mg/kg; Sigma-Aldrich, St Louis, MO) at a volume of 1 ml/kg dissolved in 0.9% NaCl− and were confined to the cocaine-paired chamber for 30 min. Thirty minutes prior to the preference test (PostTest; Day 24), animals received a s.c injection of 5 μg EB in 0.1 mL or PO alone (see Fig. 1) and were again permitted free access to all chambers for 15 min. Total time spent in each chamber during the PreTest and PostTest was automatically recorded for subsequent statistical analyses using MedPC software (Med Associates, Georgia, VA).
2.6. Statistical analyses
Preference scores (CPP scores) for each animal were created by calculating the total time spent in the cocaine-paired chamber during the PreTest and subtracting it from the total time spent in the cocaine-paired chamber during the PostTest. These CPP scores were than analyzed using a 5 (cocaine conditioning dose) × 2 (hormone treatment) ANOVA. A statistically significant interaction (p < 0.05) was followed by post hoc analyses with Fisher’s lease significant differences tests to compare preference scores between hormone treatment groups within each dose.
Locomotor scores for each animal were created by subtracting the total movement counts during the PreTest from the total movement counts during the PostTest. The effects of estradiol on movement counts were examined using a 5 (Cocaine Dose) × 2 (Treatment) between subjects ANOVA. A statistically significant main effect or interaction (p < 0.05) was followed by post hoc analyses with Fisher’s least significant differences.
3. Results
To determine the extent to which an acute post-training elevation in estradiol affects cocaine-induced CPP, rats were conditioned with one of four doses of cocaine (2.5, 5,10, or 15 mg/kg) or saline and received an injection of EB or PO 30 min prior to the CPP acquisition test (Fig. 2A). A significant Dose × Treatment interaction F(4, 73) = 3.78, p < 0.05, partial n2 = 0.17 was observed and post hoc tests revealed that PO-treated rats demonstrated a significant preference for the cocaine-paired chamber at the 5 mg/kg and 10 mg/kg conditioning doses of cocaine (p < 0.05), but not at the lowest 2.5 or highest 15 mg/kg conditioning doses of the drug (p > 0.05). Acute EB-treated females demonstrated significant cocaine-induced CPP to the 10 mg/kg and 15 mg/kg conditioning doses of cocaine (p < 0.05) but not to the lower conditioning doses of the drug (2.5 or 5mg/kg; p > 0.05). Post hoc tests to examine differences between the hormone treatment groups within each dose revealed that PO-treated females had significantly higher CPP scores for the 5 and 10 mg/kg conditioning doses of cocaine compared to the EB-treated females (p < 0.05). Although, EB-treated females demonstrated significant preference for 15 mg/kg of cocaine while PO-treated females did not, there were no significant differences between the CPP scores of these treatment groups (p > 0.05; Fig. 2A).
Fig. 2.
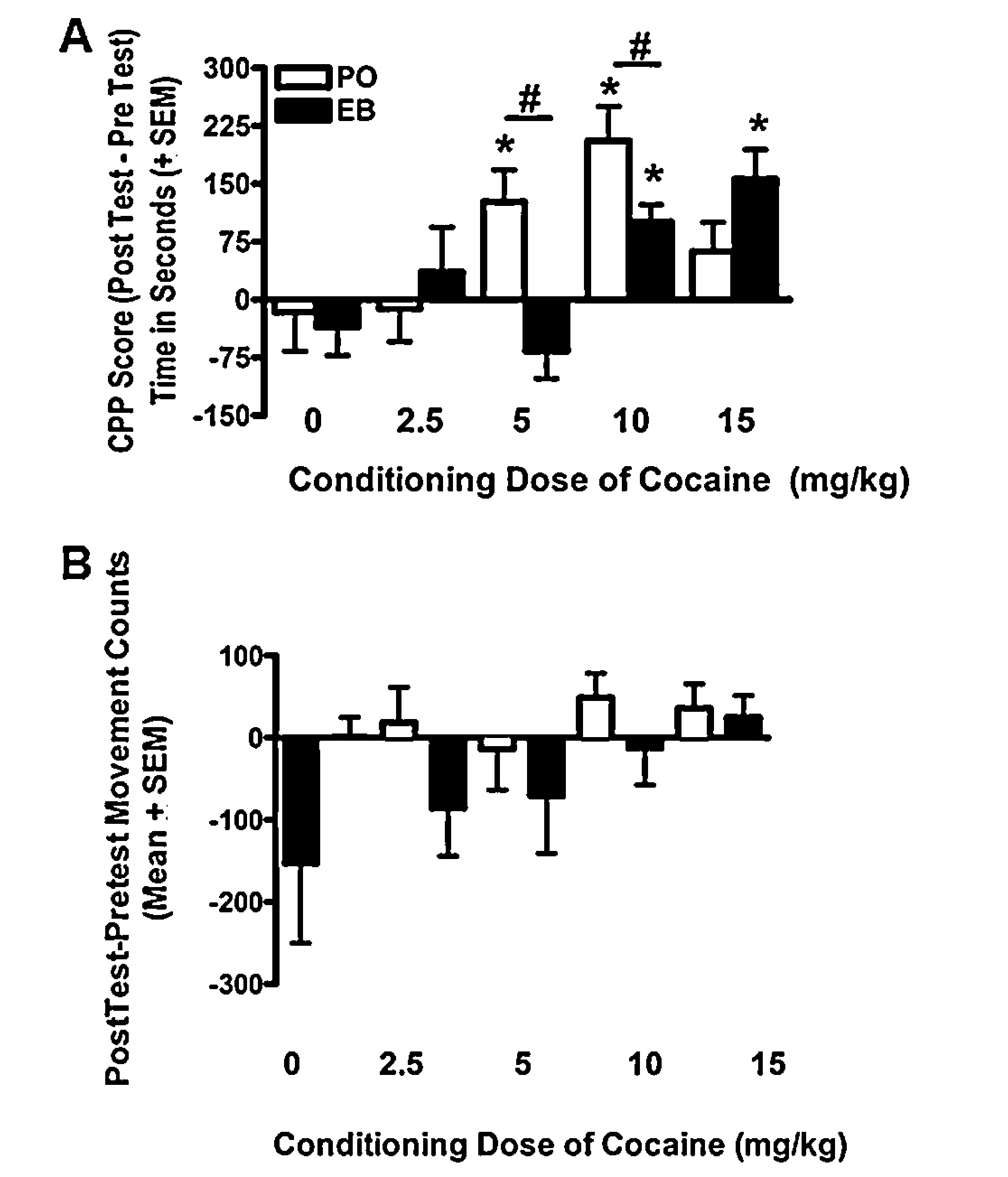
Cocaine-induced conditioned place preferance. (A) OVX rats were conditioned with one of five doses of cocaine (0, 2.5, 5, 10, or 15 mg/kg i.p.) or saline on alternating days for six consecutive days and then pretreated with a single s.c. injection of EB (5 μg) or peanut oil (PO) 30 min prior to the test for cocaine conditioned place preference (CPP; PostTest). PO-treated (white bars) rats expressed CPP to the 5 and 10 mg/kg conditioning doses of cocaine (p < 0.05), but not the 2.5 or 15 mg/kg doses. EB-treated rats (black bars) demonstrated an inhibition of CPP to the 5 mg/kg dose, but expressed a significant preference to the higher doses of cocaine (10 and 15 mg/kg; p < 0.05). n = 7–10 rats per group. * indicates a significant difference from zero (p < 0.05). ** indicates a significant difference between EB and PO treatment groups within a dose (p < 0.05). (B) Locomotor activity during the CPP PostTest as measured by total photobeam breaks over the 15 min testing period for PO treated (white bars) and EB-treated (gray bars) at each of the five conditioning doses of cocaine. Neither dose nor EB treatment prior to the CPP PostTest affected locomotor activity.
The 2-way ANOVA testing the effects of estradiol on cue induced locomotor activation during the Post-Test produced no significant main effect of Dose (p = .25), Treatment (p = .44), or Dose × Treatment interaction (p = .15). Overall, neither Treatment nor Dose alone altered locomotor response to cocaine cues during the Post Test. Furthermore, EB and PO treated females did not differ in their locomotor response to cocaine cues during the Post Test for any cocaine conditioning dose (Fig. 2B).
4. Discussion
The present study was conducted to investigate the influence of an acute post-training elevation in estradiol on cocaine-induced CPP. Our results show that the influence of post-training elevations in estradiol levels on expression of cocaine CPP was dependent upon the conditioning dose of cocaine; CPP was inhibited at lower and maintained at higher training doses of the drug. These data indicate that acute elevations in estradiol may facilitate or inhibit conditioned responses to cocaine’s secondary rewards.
Previous studies have demonstrated a role for estradiol on conditioned cocaine reward (Russo et al., 2003; Segarra et al., 2010; Twining et al., 2013). Through the use of slightly different methodology, each of these studies examined the role of chronic sustained elevations in estradiol levels on cocaine-induced CPP and has provided some fundamental insight into our understanding of the influence of estradiol on learning drug-context associations. Each of these studies used a chronic estradiol replacement method. Two of these investigations (Russo et al., 2003; Segarra et al., 2010) both employed the same general method of chronic continual estrogen replacement; slow release Silastic capsule implants were used, albeit the amounts of estradiol contained within the capsule were different (10% estradiol solution vs. a fixed amount of 4 mg of crystalline estradiol). In a more recent investigation byTwining et al. (2013), the investigators used chronic daily subcutaneous estradiol injections. Thus, for all three investigations the amount of estradiol administered and resultant circulating levels achieved were different and, not surprisingly, the behavioral results of these investigations are incongruent. Two of these studies report that chronic continual (Russo et al., 2003) and chronic intermittent (Twining et al., 2013) administration of estradiol does not influence the expression of cocaine-induced CPP in OVX rats beyond what is observed in non-estradiol treated controls. Interestingly, the data presented in Segarra et al. (2010) suggest that chronic continual lower dose estradiol treatment may result in enhanced cocaine-induced CPP in OVX rats.
Previous experiments conducted in our laboratory indicate that female rodents are more responsive to the environmental stimuli associated with cocaine reward compared to males (Bobzean et al., 2010). Data from self-administration studies in rodents suggest that circulating levels of estradiol are one of the fundamental underlying factors responsible for this sex difference (Hu and Becker, 2008; Lynch et al., 2001). However, until now, the direct effects of acute increases in circulating levels of estradiol on the salience of drug-associated cues remained unresolved. Because it is well established that estradiol is known to enhance many cocaine-related behaviors, such as locomotor activation and behavioral sensitization (Becker, 1990; Festa and Quinones-Jenab, 2004; Hu et a., 2004), we postulated that the effects of estradiol on the salience of drug-cues may be mediated by a more rapid mechanism. Thus, the hormone treatment paradigm used in this study was targeted to specifically assess the influence of an acute elevation of estradiol, similar to what would be observed during proestrus, on the expression of cocaine-induced CPP. The results presented herein, indicate dose-dependent expression of cocaine-induced CPP in both EB-treated and PO-treated groups. More specifically, neither EB-treated nor PO rats expressed CPP for the lowest conditioning dose of cocaine (2.5 mg/kg). PO rats expressed CPP for only the 5 and 10 mg/kg conditioning doses of cocaine, while the EB-treated rats expressed CPP for the 10 and 15 mg/kg conditioning doses and not for the 5 mg/kg dose. This suggests that the rewarding effects stimuli associated with higher versus lower doses of cocaine are differentially modulated by estradiol and that acute EB pretreatment shifts the cocaine dose-response curve to the right and inhibits the expression of cocaine CPP at lower conditioning doses of the drug. Our conclusions that acute EB treatment is responsible for altered CPP expression is further supported by the finding that acute EB and PO pre-treated females did not differ in their locomotor response to cocaine cues during the Post Test for any cocaine conditioning dose; thus, differences in CPP expression are not likely attributed to other conditioned treatment effects.
Previous investigations into underlying mechanisms indicate that estradiol increases dopamine release and increases the density of striatal dopamine uptake sites in the dorsal and ventral striatum; brain regions critical in mediating cocaine reward; (Becker and Beer, 1986; Becker et al., 1984; Becker and Ramirez, 1981; Di Paolo, 1994; Morissette et al., 1990). In this way the potentiated effects of estradiol on dopamine activities are implicated as a likely contributor to previously reported sex and hormone-related differences to cocaine. Our current study extends these findings by demonstrating that an acute elevation in estradiol occurring in the presence of drug-associated cues can influence conditioned behavioral responses to cocaine.
Interestingly, no CPP was seen in the 15 mg/kg PO treated rats, as has been previously reported by our group in 2010. Differences in methodology could account for this difference in findings. One of the problems associated with working with ovariectomized females is long-term reduction of female gonadal steroids (24 days in our experiments) animals undergo following surgery. The long term absence of estradiol has previously been shown to alter DA transmission, DA transporter, and D2 expression in the striatum of OVX female rats (Attali et al., 1997; Bazzett and Becker, 1994; Morissette et al., 1990). Acute and chronic EB treatment reversed the effects of OVX in these experiments. Such changes to mesolimbic DA systems could lead to a significant reduction in the salience of cocaine-paired cues. This supports our notion of the critical impact that estradiol is necessary for female behavioral responses to cocaine and cocaine-associated stimuli.
One mechanism by which estradiol produces its effects by genomic and non-genomic actions. Genomic estrogen receptors are ligand-activated transcription factors which reside in the cytosol and translocate to the nucleus upon ligand binding and dimerization and take several hours or even days to activate (Nilsson et al., 2001). More rapid effects are initiated via binding at nongenomic estrogen receptors localized on the cell membrane; membrane estrogen receptors (mERs) (Boulware et al., 2005; Mermelstein and Micevych, 2008; Micevych and Mermelstein, 2008). The signaling cascades initiated via mERS are some of the same that are initiated by dopamine at D1 receptors. Our findings that the estradiol treatment 30 min prior to behavior testing supports that estradiol’s effects in this study are likely modulated by rapid nongenomic actions. We believe that these behavioral data reflect the ability of estradiol to alter dopamine signaling and subsequently influence the salience of cocaine associated cues.
In conclusion, we found that the threshold conditioning dose of cocaine necessary for inducing cocaine CPP in PO rats is lower than in animals given acute EB. The fact that this effect can be blocked by administering EB 30 min prior to the preference test provides an intriguing avenue for future research to explore the underlying mechanisms of estradiol on brain reward systems.
Acknowledgements
The authors thank Dr. Angela Liegey Dougall for her help with the statistical analyses. The authors also thank Aliza K. DeNobrega, Aspen Samuel, and Alexandra Schiller for technical assistance.
References
- Anker JJ, Carroll ME, 2011. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr. Top. Behav. Neurosci 8, 73–96. [DOI] [PubMed] [Google Scholar]
- Attali G, Weizman A, Gil-Ad I, Rehavi M, 1997. Opposite modulatory effects of ovarian hormones on rat brain dopamine and serotonin transporters. Brain Res. 756,153–159. [DOI] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Kosten TR, Cooney NL, 1995. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict. Behav 20,215–224. [DOI] [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME, 2000. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Dev. Psychobiol 36,194–212. [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB, 1994. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res 637,163–172. [DOI] [PubMed] [Google Scholar]
- Becker JB, 1990. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci. Lett 118, 169–171. [DOI] [PubMed] [Google Scholar]
- Becker JB, 1999. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav 64,803–812. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME, 1986. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav. Brain Res 19, 27–33. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME, Robinson TE, 1984. Striatal dopamine release stimulated by amphetamine or potassium: influence of ovarian hormones and the light-dark cycle. Brain Res. 311,157–160. [DOI] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD, 1981. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 204, 361–372. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, Dennis TS, Addison BD, Perrotti LI., 2010. Influence of sex on reinstatement of cocaine-conditioned place preference. Brain Res. Bull. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG, 2005. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci 25, 5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE, 2012. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol. Behav 105, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP, 1988. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res. Monogr 84, 25–43. [PubMed] [Google Scholar]
- Di Paolo T, 1994. Modulation of brain dopamine transmission by sex steroids. Rev. Neurosci 5,27–41. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR, 2001. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am. J. Drug Alcohol Abuse 27,193–202. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL, 2009. Real-time electronic diaiy reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch. Gen. Psychiatry 66, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V, 2004. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm. Behav 46, 509–519. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V, 2004. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46, 672–687. [DOI] [PubMed] [Google Scholar]
- Frye CA, 2007. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol. Biochem. Behav 86,209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE, 2005. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 179,662–672. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB, 2008. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 94, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB, 2004. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29, 81–85. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME, 2005. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol. Biochem. Behav 82, 98–108. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME, 2000. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 152,132–139. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144, 77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME, 2001. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol. Biochem. Behav 68, 641–646. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Micevych PE, 2008. Nervous system physiology regulated by membrane estrogen receptors. Rev. Neurosci 19,413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG, 2008. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol. Neurobiol 38,66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Biron D, Di Paolo T, 1990. Effect of estradiol and progesterone on rat striatal dopamine uptake sites. Brain Res. Bull 25,419–422. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA, 2001. Mechanisms of estrogen action. Physiol. Rev 81, 1535–1565. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R, 1990. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict. Behav 15,355–365. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B, 1977. Conditioned narcotic withdrawal in humans. Science 195,1000–1002. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ, 1999. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav. Brain Res 101, 15–20. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP, 1999. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 53, 223–230. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ, 1989. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 98,408–411. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME, 2004. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol. Biochem. Behav 78,199–207. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V, 2003. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience 120, 523–533. [DOI] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menendez-Delmestre R, Puig-Ramos A, Torres-Diaz YM, 2010. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm. Behav 58,33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling RC, Dean J, Weinstein SP, Murphy J, Gottheil E, 2004. Gender differences in cue exposure reactivity and 9-month outcome. J. Subst. Abuse Treat 27, 39–44. [DOI] [PubMed] [Google Scholar]
- Twining RC, Tuscher JJ, Doncheck EM, Frick KM, Mueller D, 2013. 17beta-Estradiol is necessary for extinction of cocaine seeking in female rats. Learn. Mem 20, 300–306. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, Alia-Klein N, Wong C, 2011. Reduced metabolism in brain control networks following cocaine-cues exposure in female cocaine abusers. PLoS ONE 6, el6573. [DOI] [PMC free article] [PubMed] [Google Scholar]


