Abstract
Phaeochromocytoma and paraganglioma (PPGL) are chromaffin cell tumours that require timely diagnosis because of their potentially serious cardiovascular and sometimes life- threatening sequelae. Tremendous progress in biochemical testing, imaging, genetics and pathophysiological understanding of the tumours has far-reaching implications for physicians dealing with hypertension and more importantly affected patients. Because hypertension is a classical clinical clue for PPGL, physicians involved in hypertension care are those who are often the first to consider this diagnosis. However, there have been profound changes in how PPGLs are discovered; this is often now based on incidental findings of adrenal or other masses during imaging and increasingly during surveillance based on rapidly emerging new hereditary causes of PPGL. We therefore address the relevant genetic causes of PPGLs and outline how genetic testing can be incorporated within clinical care. In addition to conventional imaging (CT, MRI), new functional imaging approaches are evaluated. The novel knowledge of genotype-phenotype relationships, linking distinct genetic causes of disease to clinical behaviour and biochemical phenotype, provides the rationale for patient-tailored strategies for diagnosis, follow-up and surveillance. Most appropriate preoperative evaluation and preparation of patients are reviewed, as is minimally invasive surgery. Finally we discuss risk factors for developing metastatic disease and how they may facilitate personalised follow-up. Experts from the European Society of Hypertension have prepared this position document which summarises the current knowledge in epidemiology, genetics, diagnosis, treatment and surveillance of PPGL.
Condensed abstract
Phaeochromocytomas and paragangliomas (PPGL) are rare causes of secondary hypertension. The unpredictable serious effects of tumoural surges of catecholamines into the circulation result in significant cardiovascular mortality and morbidity when the diagnosis is delayed or missed. After publication of the Endocrine Society Clinical Practice Guideline on PPGL in 2014, relevant new data have been published reflecting further scientific advances impacting on patient care. This position statement, written by the working group on Endocrine Hypertension of the European Society of Hypertension, provides an update in epidemiology, genetics, diagnosis and therapeutic and surveillance strategies for patients with PPGLs.
Keywords: phaeochromocytoma, paraganglioma, catecholamines, metanephrines, imaging
Introduction
Phaeochromocytomas and sympathetic paragangliomas (PPGL) are rare neuroendocrine tumours. Eighty to eighty-five percent are phaeochromocytomas arising from the adrenal medulla. Fifteen to twenty percent are sympathetic paraganglioma arising from the sympathetic ganglia in thorax, abdomen, and pelvis [1]. In the head and neck region, paragangliomas have a parasympathetic origin (HNPGLs) [2]. The catecholamine phenotype of phaeochromocytomas is characterised by production of adrenaline accompanied by varying amounts of noradrenaline (adrenergic phenotype) in about 50% of cases; other phaeochromocytomas or sympathetic paragangliomas produce predominantly or exclusively noradrenaline (noradrenergic phenotype) but sometimes with significant amounts of dopamine as the catecholamine biosynthetic end-product (dopaminergic phenotype) [3]
Among the few epidemiological studies on PPGLs most are from the previous century. Prevalences in autopsy studies amount to 0.05%, indicating that the diagnosis of PPGL is missed during life in substantial numbers of patients [4, 5]. In addition, the reported median delay in the diagnosis is about 3 years [6]. PPGLs are considered as a ‘white raven’ with quoted prevalences varying from 0.2% to 0.6% in hypertensive patients to less than 0.05% in the general population. The annual incidence of PPGLs is about 5 cases per million per year. The most recent nationwide study performed in the Netherlands demonstrated an age-standardized incidence rate of 0.46 (95% CI: 0.39–0.53) and 0.11 (95% CI: 0.09–0.13) per 100,000 person years for phaeochromocytoma and sympathetic paraganglioma, respectively [7]. These incidences are higher than reported in earlier studies [8, 9]; in line with this the Dutch study noted a near doubling of the incidence from 1995 to 2015, likely reflecting higher rates of detection in part due to increased use and improvement of imaging modalities [7, 10]. Indeed, two recent retrospective series of patients with PPGLs reported that nearly two thirds were discovered as incidentalomas [11, 12]. Increasing numbers of individuals and family members in whom germline mutations for one of the known PPGL susceptibility genes have been detected are now also being routinely screened for PPGL, thus also impacting detected incidence [13, 14].
The working group on Endocrine Hypertension of the European Society of Hypertension prepared this consensus document to review the available knowledge on genetics, diagnosis, treatment and surveillance of PPGL. We also provide a perspective on the next challenges and future directions of research on this field.
Clinical presentation and indications for PPGL screening
The heterogeneous clinical presentation and signs and symptoms of PPGLs are well established, but this is near exclusively based on retrospective series with most studies involving no comparisons to patients in whom PPGLs were suspected and excluded [15–17]. Invariably patients in these earlier studies were those diagnosed based on a presentation of signs and symptoms of catecholamine excess. As mentioned earlier PPGLs are now increasingly diagnosed as incidentalomas or during surveillance screening due to genetic risk or past history of the tumours. In a recent prospective multicentric series of 245 patients with PPGLs, reasons for disease discovery included signs and symptoms in 37% of patients, incidentalomas in 36% and surveillance in 27% of patients [18]. Prevalences of PPGLs for the latter situations are much higher than among patients tested due to signs and symptoms of presumed catecholamine excess. Among adrenal incidentaloma about 7% represent phaeochromocytoma [19], whereas among unselected patients tested for PPGLs prevalences are less than 1% [20], indicating even lower prevalences among those tested due to signs and symptoms. Thus, the clinical presentation of the patient in terms of prevalence or risk for PPGL is a key first consideration for personalised approaches for diagnosis and management.
Signs and symptoms
The diverse and variable signs and symptoms of PPGL are related to the amounts, proportions and continuous or episodic secretory nature of noradrenaline, adrenaline and dopamine released by the tumours. Paroxysmal symptoms with a high variety of severity and frequency are a characteristic feature, presumably due to episodic secretory activity. Such symptoms may occur spontaneously or may be provoked by triggers, including exercise, abdominal pressure, large meals, medications (glucocorticosteroids, ephedrine, phenylephrine, ACTH, phenothiazines, amphetamine, metoclopramide, antidepressants, some anaesthetics), stress, alcohol and food (e.g. tyramine in some cheeses) [15, 21–24].
In the above and other circumstances, PPGLs can present with life-threatening cardiovascular manifestations including hypertensive crisis, myocardial infarction, brady- and tachyarrhythmias, Takotsubo cardiomyopathy and acute heart failure. Rarely, patients with a PPGL present with low blood pressure or even with circulatory shock that may occasionally precede a multisystem crisis. PPGLs should therefore be considered in patients with cardiovascular events that cannot be explained by other mechanisms [25, 26].
Recent studies have shown that the classic signs and symptoms occur more rarely than previously assumed. A recent meta-analysis showed that headache, palpitations and sweating occurred in 60%, 59% and 52% of patients whereas other symptoms occurred at a much lower frequency [27]. A further prospective study found that no single symptom occurred in more than 65% of patients with PPGL, including those tested because of signs and symptoms of presumed catecholamine excess. Moreover, for several symptoms there were little or no differences between patients in whom tumours were confirmed versus excluded (Table 1) [18].
Table 1.
Signs and symptoms in patients with and without PPGL enrolled in the full study cohort (N=2017) and three different patient sub-populations according to their clinical presentation§
| All patients | Signs & symptoms | Incidentaloma | Surveillance | |||||
|---|---|---|---|---|---|---|---|---|
| PPGL | No PPGL | PPGL | No PPGL | PPGL | No PPGL | PPGL | No PPGL | |
| N | 243 | 1774 | 89 | 1170 | 88 | 387 | 66 | 217 |
| BMI (kg/m2) | 25* | 28 | 25* | 28 | 25* | 29 | 25* | 27 |
| Heart rate (b/min) | 79* | 72 | 81* | 72 | 78* | 73 | 77* | 70 |
| Pallor (%) | 26* | 13 | 37* | 15 | 21* | 10 | 18* | 7 |
| Sweating (%) | 46* | 28 | 55* | 30 | 47* | 25 | 32* | 21 |
| Palpitations (%) | 46* | 36 | 65* | 44 | 46* | 22 | 21 | 19 |
| Tremor (%) | 25* | 15 | 33* | 18 | 21* | 8 | 18* | 6 |
| Nausea /vomiting (%) | 21* | 11 | 26* | 12 | 18 | 11 | 20* | 10 |
| Symptom score ≥3** | 42 | 18 | 56 | 21 | 40 | 13 | 28 | 1 |
| Hypertension (%) | 83 | 85 | 95 | 93 | 80 | 78 | 71 | 58 |
| Headaches (%) | 38 | 39 | 46 | 45 | 39 | 29 | 27 | 25 |
| Weakness (%) | 42* | 35 | 51* | 36 | 34 | 32 | 42 | 32 |
| Panic/anxiety (%) | 25 | 25 | 37 | 32 | 19 | 14 | 15* | 7 |
| Flushing (%) | 20 | 23 | 21 | 27 | 23 | 17 | 14 | 14 |
| Constipation (%) | 14* | 9 | 17* | 9 | 10 | 9 | 14 | 7 |
The full study cohort of the PMT study (Prospective Monoamine-Producing Tumor study)y (modified from [18]).
PPGL, Phaeochromocytoma or paraganglioma
significant differences between patients with and without PPGL;
symptom score: one point for each sign: BMI < 25 kg/m2, heart rate ≥85 beats/min, pallor, sweating, palpitations, tremor and nausea; a negative point for obesity;
Hypertension, even paroxysmal is also not a specific sign of PPGL [27]. However, ambulatory blood pressure in PPGL patients may show large BP variation and a decreased nocturnal BP fall, or even a non-dipping BP pattern and BP elevation during the night [28–30]. Indiscriminate testing for PPGL in patients with uncomplicated hypertension is not indicated due to lack of cost-effectiveness.
Patients with PPGL are characterized not only by a higher frequency of palpitations but also by a higher heart rate as compared with patients without PPGL [18, 27]. Diabetes and prediabetes are other signs of the hyperadrenergic state. Moreover PPGL patients are more often characterized by lower BMI compared to those without PPGL [15, 21, 22, 31]. However, obesity does not exclude a PPGL. Clinical features that differ between patients in whom PPGLs have been confirmed versus excluded include BMI, heart rate, pallor, sweating, palpitations, tremor and nausea (actively asked to patients) [18]. From these signs and symptoms a score system has been devised (one point for each sign: BMI<25kg/m2, heart rate ≥85 beats/min, pallor, sweating, palpitations, tremor and nausea and a negative point for obesity) that enables stratification of patients (symptom score ≥ or <3) according to increased or decreased likelihood of tumours (Table 1) [18]. This thereby enables further personalised approaches for diagnosis and management based on refining estimates of disease risk based on prevalence according to clinical features.
Head and neck paragangliomas
Head and neck paragangliomas (HNPGLs) can be sporadic or can occur as part of hereditary syndromes in up to 40% with variable penetrance and increased risk for tumour multiplicity. Most HNPGLs do not produce significant amounts of catecholamines so that presence of signs of symptoms of catecholamine excess rarely represents a mode for their discovery. They are more often discovered during imaging studies or revealed by the presence of a temporal bone or cervical mass with in advanced stages causing compression or infiltration of adjacent structures, thus leading to hearing loss, pulsatile tinnitus, dysphagia, and cranial nerve palsies [32].
Adrenal incidentaloma
All patients with adrenal incidentaloma with tumour density >10 HU should be screened for PPGL even in the absence of hypertension [19, 33–36]. This is also justified by the finding that hypertension among patients with adrenal incidentalomas is not more frequent in patients with PPGL than in those without PPGL [18]. It should be emphasized that a diagnosis of PPGL should be also considered in the differential diagnosis of incidentally discovered retroperitoneal masses, especially when some features of catecholamine excess are present.
Surveillance screening
Routine screening for PPGL is recommended in patients with known mutations predisposing for PPGL, syndromic features suggesting hereditary PPGL and a previous history of PPGL. In these patients, signs and symptoms of PPGL are less often expressed mostly due to the small size of their tumours detected earlier than in other cases [18, 37].
Screening for PPGL is recommended in patients with:
– Signs and symptoms of PPGL: spontaneous or provoked by use of medications or by other triggers
– Cardiovascular events (including Takotsubo cardiomyopathy) with signs/symptoms indicative for PPGL
– Adrenal incidentaloma (with or without hypertension) if density is ≥ 10 HU
– Lean subjects (BMI<25 kg/m2) with diabetes type 2 with or without sign/symptoms of catecholamine excess.
– Carrier of a germline mutation in one of the PPGL susceptibility genes
– Syndromic features suggesting genetically determined or syndromic PPGL
– Previous history or family history of PPGL
Biochemical Diagnosis of Phaeochromocytoma and Paraganglioma
Over the past two decades improved understanding of catecholamine metabolism has led to a shift in emphasis from catecholamines to plasma or urinary metanephrines for biochemical diagnosis. Improved analytical accuracy attained using liquid chromatography with electrochemical detection (LC-ECD) or tandem mass spectrometry (LC-MS/MS) have also been instrumental to improved biochemical diagnosis of PPGL [38, 39]. Biochemical proof of a PPGL is a prerequisite for the next diagnostic step of imaging, except for seriously ill patients in the intensive care unit in whom biochemical testing is insufficiently reliable. These patients should go directly to imaging [22, 40]. This also applies to patients in whom a PPGL is suspected but who are known not to produce catecholamines.
What is the preferred initial biochemical test?
For first-line screening, measurements of plasma or urinary free nornetanephrine and metanephrine are the most accurate tests to detect or exclude a PPGL [22, 41]. Inclusion of 3-methoxytyramine is useful for detecting dopamine-producing PPGLs when measured in plasma but not urine. Both plasma and urinary free metabolites have near maximal negative predictive values (>99%) at similar specificities (94%) [41–43]. Measurements in plasma show similar diagnostic accuracy as those in urine in patients at low risk, such as those tested due to signs and symptoms, but are superior to those in urine in patients at high risk for a PPGL, such as those tested due to an incidentaloma or as part of surveillance screening [41]. Currently, most routine laboratories still measure deconjugated metanephrines (free and conjugated metanephrines) in 24-hour urine. This causes more false-positive test results than with free metanephrines, supporting a shift to measurements of free metanephrines.
LC-MS/MS is presently the preferred assay method because of its optimal analytical accuracy, cost-effectiveness and minimal analytical interference from drugs [39, 42]. If local normative data are lacking, published reference intervals may be applied [44]. Of note, immunoassays often underestimate plasma metanephrines, negatively impacting diagnostic performance [45].
How to perform blood and urine collections?
Amine rich foods have no relevant effect on plasma or urinary free metanephrines but do increase plasma free and urinary 3-methoxytyramine [46]. Therefore, blood sampling for plasma metanephrines is preferred in the fasting state to circumvent false-positive test results.
Problems with analytical interference are minimal for LC-MS/MS, but several drugs may cause pharmacodynamic interference and cause falsely elevated test results [13]. Some antihypertensive drugs cause analytical interference with liquid chromatography methods that employ electrochemical or fluorometric detection, but not mass spectrometry. Pharmacodynamic interference is independent of the assay; typical examples are drugs that inhibit noradrenaline reuptake such as antidepressants (Table 2). Interrupting such medications is not usually required, but may be considered after initial testing yields positive results.
Table 2.
Medications that may cause falsely-elevated results for plasma and urinary metanephrines due to pharmacodynamic interference
| Plasma and urine | ||||||||
|---|---|---|---|---|---|---|---|---|
| Normetanephrine | Metanephrine | 3-Methoxytyramine | ||||||
| Antidepressants | ++ | - | - | |||||
| Phenoxybenzamine | ++ | - | - | |||||
| MAO-inhibitors | ++ | ++ | - | |||||
| Sympathomimetics | + | + | - | |||||
| Levodopa | - | - | +++ | |||||
MAO = monoamine oxidase;
clear increase;
mild increase;
no increase
Blood should be collected on ice. Preservatives such as hydrochloric acid are not required for urinary metanephrines [47]. Measurement of urinary creatinine excretion is useful to verify completeness of 24-hour collections. It is important to take blood samples after at least 20 minutes of supine rest as elevated sympathetic activity, associated with sitting, may elicit false-positive test results [48, 49]. As physical activity may also result in significant and sustained increases of plasma metanephrines, strenuous exercise before sampling should be avoided [50]. If drawing blood samples after supine rest is not feasible, measurement of 24-hour urinary free metanephrines is an acceptable alternative [22] [44].
Personalised interpretation of test results
In addition to strict compliance of blood sampling after supine rest, correct and reliable interpretation of test results can only be achieved using appropriate reference intervals, also being established after supine rest [22, 41]. Use of personalised age-specific reference intervals for plasma normetanephrine and gender-specific reference intervals for urinary metanephrines optimises the diagnostic performance [44]. In patients with chronic kidney disease, higher specific upper cut-off values should be applied [51].
The nearly maximal negative predictive value of both plasma and urine tests implies that negative test results virtually exclude a PPGL. Exceptions include patients with tumours that are either small or those such as some HNPGLs that do not synthesize catecholamines; most of these do not cause signs and symptoms of catecholamine excess.
In patients tested because of hereditary risk, choice of test and interpretation of test results should take into account the nature of the impacted gene. In patients with PPGLs due to mutations of RET, NF1 and related genes involved in kinase signalling the focus should be on both metanephrine and normetanephrine since almost always the first and invariably both metabolites are elevated [3]. In contrast, for mutations of genes that result of stabilisation of hypoxia-inducible factors and epigenetic silencing of phenylethanolamine N-methyltransferase, the enzyme that converts noradrenaline into adrenaline (e.g., mutations of VHL and SDHx genes) the focus should be on normetanephrine since these tumours do not produce adrenaline. For patients with SDHx mutations or in whom there is the possibility of metastatic disease plasma measurements should include 3-methoxytyramine.
Interpretations of a positive test result should also take into account pre-test prevalence (e.g., 7% in a patient with incidentaloma) and features about the patient that can be further used to assess likely risk of disease [18]. Such considerations can then be used to assess post-test probability of a tumour based on both the extent of increase of a specific metabolite above the cut-offs and whether that increase is also accompanied by an increase in another metabolite [41]. Increments in any plasma metabolite in excess of two-fold above the upper cutoff or increases in two or more metabolites are very rare in patients without PPGLs, but occur in over 80% of patients with PPGL. In such patients the likelihood of a PPGL is sufficiently high to justify imaging studies regardless of pre-test prevalence. In other patients with smaller increases of single metabolites post-test probability must consider pre-test risk. In such cases the clonidine suppression test can be helpful to differentiate false- from true-positive test results [52, 53].
Falsely-elevated test results [48, 54] can occur in patients with any clinical condition associated with chronically elevated sympathetic activity such as heart failure or obstructive sleep apnea syndrome. Inappropriate sampling conditions, drug interferences and incorrectly applied reference intervals are other situtations that can lead to false-positive results. Interpretation of test results in such patients requires care since elevations can reach levels that would otherwise indicate high probability of disease and justify imaging for a tumour.
Imaging of phaeochromocytoma and paraganglioma
Imaging of patients with a PPGL is important at every step of their management. Anatomic imaging by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is usually sufficient to locate a tumour and proceed to surgery. However, combined with functional imaging there is added value in improved specificity, sensitivity (detection of metastases and multifocality) and links with targeted radionuclide therapy. The optimal choice of a radiopharmaceutical depends heavily on tumour genotype and biology, which are linked to location (sympathetic vs parasympathetic, adrenal vs extra-adrenal), size and biochemical phenotype as detailed in the recently updated European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 (Table 3) [55].
Table 3.
Proposed clinical algorithm for nuclear imaging investigations in phaeochromocytoma and paraganglioma
| First choice | Second choice | Third choice | |
|---|---|---|---|
| Phaeochromocytoma (sporadic) | 18F-FDOPA or 123I-MIBG | 68Ga-DOTA-SSA | 18F-FDG |
| Hereditary phaeochromocytoma (except SDHx): NF1/RET/VHL/MAX | 18F-FDOPA | 123I-MIBG or 68Ga-DOTA-SSA | 18F-FDG |
| Extra-adrenal sympathetic and/or multifocal and/or metastatic and/or SDH mutation | 68Ga-DOTA-SSA | 18F-FDG and 18F-FDOPA | 18F-FDG and 123I-MIBG or 18F-FDG and 111In-SSA/99mTc-SSA |
| Head and neck paraganglioma (sporadic) | 68Ga-DOTA-SSA | 18F-FDOPA | 111In-SSA/99mTc-SSA |
(adapted from the 2019 EANM/SNMMI guidelines [55])
Phaeochromocytomas
The radiological appearance of phaeochromocytoma on anatomical imaging is variable. The tumours can be homogeneous or heterogeneous, including features of necrosis, haemorrhage, cystic changes and calcifications. In representative areas of the tumour, however, unenhanced attenuation on CT is almost invariably >10 Hounsfield units as opposed to most adrenal adenomas [34, 36, 56]. Contrast washout is unreliable in an individual patient to distinguish between phaeochromocytoma and adenoma. On MRI, a high intensity (bright) T2-weighted signal is typical for phaeochromocytoma, but this occurs in only one third of tumours [57, 58]. Additional functional imaging can be useful in patients with bilateral masses or a high risk of multifocality (e.g., due to a hereditary syndrome) or in a critical care setting and renal insufficiency. 18F-FDOPA positron emission tomography (PET), 68Ga-DOTA-somatostatin analogue (SSA) PET might then be performed [13, 55](Table 3). When PET is unavailable, 123I-MIBG scintigraphy can be a next alternative, though with recognition that its impact on clinical decision making is limited [59–61]. Functional imaging is also recommended for excluding metastatic disease particularly in large phaeochromocytomas (>5.0 cm) and in patients with SDHA/B/D mutations. In these cases, 18F-FDOPA or 68Ga-DOTA-SSA PET can be performed depending on the genotype.
In a first study in six patients with a MAX mutation, it is suggested that the use of 18F-FDOPA is preferred over 68Ga-DOTA-SSA due to better tumour-to-adrenal uptake ratio (Table 3). [62]. This is important since these tumours can be multifocal within the adrenals and very small in size. This may help to select potential candidates for subtotal cortex-sparing adrenalectomy. For the same reason, CT is preferred over MRI due to spatial resolution, especially for patients without contraindications for the use of ionizing radiations such as patients with germline mutation in a PPGL susceptibility gene.
Sympathetic paragangliomas
There are several typical locations for extra-adrenal PPGLs: 1. the peri-aortic region, particularly the Zuckerkandl body as example of a paraganglion located at the root of the inferior mesenteric artery; 2. the sympathetic plexus of the urinary bladder, the kidneys and the heart; and 3. Alongside the sympathetic ganglia in the posterior mediastinum. It is first important in these situations to differentiate a PPGL from other neurogenic tumours, sarcomas and pathologically enlarged lymph nodes. The specificity of functional imaging together with metanephrines are very helpful in distinguishing these entities. In patients with confirmed or suspected SDH mutation, 68Ga-DOTA-SSA is the imaging modality of choice. (Table 3). When PPGL is caused by VHL or HIF2A mutations, 18F-FDOPA appears to be superior to 68Ga-DOTA-SSA [63, 64].
Head and neck paragangliomas
Functional imaging is highly sensitive for detecting multiple tumours and facilitates their subsequent location by anatomical imaging with dedicated protocols. Based on the most recent studies, 68Ga-DOTA-SSA is today considered the first-choice radiopharmaceutical [60, 65–67]. Anatomic imaging with MRI and temporal bone CT serves as the first-line approach for locoregional staging. MR angiography sequences are particularly sensitive for detecting tumours, especially 3D time-of-flight and 4D MR acquisitions [60, 68].
Metastatic PPGL
The excellent results obtained with 68Ga-DOTA-SSA PET in sporadic and SDH-related PPGL have simplified the imaging approach to patients with metastatic disease [69–72]. 68Ga-DOTA-SSA PET/CT is also very useful for follow-up and assessment of responses to therapies, complementary to anatomic imaging which remains the gold standard (RECIST evaluation). 18F-FDG and 18F-FDOPA PET may serve as alternatives in this setting (Table 3). 68Ga-DOTA-SSA or 123I-MIBG can also be used for selecting patients for targeted radionuclide therapy with 177Lu-DOTATATE or 131I-MIBG, respectively.
Genetics
PPGL has the highest known heritability rate among all human tumours. Up to 40% of patients with this disease carry germline mutations in one of the twenty-five known susceptibility genes, a proportion that increases every year as mutations impacting new genes are found. Somatic mutations in some of the same genes or other genes known to initiate tumorigenesis can be found in an additional 30–40% of patients with sporadic disease (Table 4) [73, 74] [75, 76]. Genetic alterations as a whole therefore currently explain up to 80% of all cases. Other somatic mutations, such as in ATRX and TERT genes may have a secondary role in influencing disease progression with potential prognostic significance. Based on these and other accumulating data, it is recommended to consider genetic studies for any patient diagnosed with PPGL [77, 78].
Table 4.
Characteristics of the gene mutations in phaeochromocytoma and paraganglioma
| Gene | Type of gene | Inheritance | Germline mutation | Somatic mutation | Mos. | GD | Predominant tumour location | Related syndrome; Associated tumours/features |
|---|---|---|---|---|---|---|---|---|
| SDHB | TSG | AD | 10% | <1% | NR | Yes | TA>HN>PCC | Carney-Stratakis syndrome; PGL4; ccRCC, GIST; PA |
| SDHD | TSG | AD (P) | 9–10% | <1% | NR | Yes | HN>TA>PCC | Carney-Stratakis syndrome; PGL1; ccRCC; GIST; PA |
| SDHC | TSG | AD | <1–5% | <1% | Yes | Yes | HN>TA>PCC | Carney-Stratakis syndrome; PGL3; clear cell renal carcinoma; GIST; PA |
| SDHA | TSG | AD | 3% | <1% | NR | NR | TA>>PCC | PGL6; Leigh syndrome (homozygous germline mutations); ccRCC; GIST; PA |
| SDHAF2 | TSG | AD (P) | <1% | NR | NR | NR | HN>>PCC | PGL2 |
| FH | TSG | AD | 1% | NR | NR | NR | PCC+TA>HN | Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) |
| VHL | TSG | AD | 7–10% | 10% | Yes | Yes | PCC (Bil PCC 50%)>>>TA, HN | von Hippel-Lindau syndrome; 10–25% of patients present PPGL |
| HIF2A (or EPAS1) | O | ? | ? | 5–7% | Yes | - | TA>PCC | Familial erythrocytosis type 4; Pacak-Zhuang syndrome; polycythemia; somatostatinoma. |
| NF1 | TSG | AD | <3–5% | 14–25% | Yes | Yes | PCC 95% (Bil PCC 16%)>TA | von Recklinghausen’s disease |
| RET | O | AD | 5–10% | 10% | NR | - | PCC (Bil PCC 50–80%)>>>TA, HN | Multiple endocrine neoplasia type 2 (MEN2) |
| TMEM127 | TSG | AD | <1% | NR | NR | NR | PCC (Bil PCC 33–39%)> TA, HN | PGL5/FPCC1; ccRCC |
| MAX | TSG | AD (P) | <1% | <2% | NR | Yes | PCC (Bil PCC 68%)>PGL | PGL7/FPCC2; PA, renal oncocytoma |
| HRAS | O | - | NR | 7–10% | NR | - | PCC>PGL | Costello syndrome (in case of germline mutations) |
| MDH2 | TSG | AD | <1% | NR | NR | NR | TA | Early-Onset Severe Encephalopathy (homozygous germline mutations) |
| SLC25A11 | TSG | AD | <1% | NR | NR | NR | TA-PGL>>>HN PGL | NR |
| DLST | TSG | AD | <1% | NR | NR | NR | TA-PGL | NR |
TSG, tumour suppressor gene; O, oncogene; ?, not clear; AD, autosomal dominant; AD (P), autosomal dominant with paternal inheritance (maternal imprinting);
NR, not reported; Mos., mosaic; GD, gross deletions; PGL, paraganglioma; PCC, phaeochromocytoma; A, abdominal PGL; TA, thoraco-abdominal PGL;
HN, head and neck PGL; Bil, Bilateral; GIST, gastrointestinal stromal tumour; ccRCC, clear cell renal cell carcinoma; PA, pituitary adenoma
Although genetic testing has traditionally been performed on DNA acquired from blood or buccal swabs, recent data suggest a potential benefit of PPGL mutational screening on tumour DNA (conveniently selected by a pathologist in order to avoid excessive contamination with normal tissue) [79, 80]. If a mutation in the tumour is found, the second step is to search for that same unique mutation in germline DNA. This not only enables discrimination of inherited from sporadic disease but also allows for targeted germline testing, and as detailed later, the available tumour material can also help to determine the pathogenicity of variants of unknown significance. Although somatic genetic testing has yet to be routinely employed, it should become the future procedure of choice for any index patient in whom tumor material is available.
Late age of onset or the lack of family history of the disease do not exclude a germline mutation [79, 80]. This mainly reflects the low penetrance of some gene mutations such as those of SDHA [81] and to inheritance models modified by imprinting or other mechanisms, which result in generation-skipping of clinical manifestations of the disease for a specific gene. This for example is the case for SDHD [82]. An individual who inherits a pathogenic variant affecting the maternal gene is at low risk of developing the disease compared to high risk for inheritance of the paternal allele [83]. Although pediatric cases usually involve germline mutations, for those presenting with a single tumour more than 20% are explained by somatic mutations [79, 80].
It is recommended that all patients with PPGL be engaged in shared decision making for genetic testing [22]. There is a long list of known PPGL driver genes, the most prevalent include SDHB, SDHD, VHL, RET and NF1 with lower prevalences for SDHA, SDHAF2, MAX, and TMEM127 (Table 4). Although all PPGLs and all genotypes have a potential for developing metastatic disease, mutations in the SDHB gene are associated with the highest risk of metastatic disease (30–70%). The clinical presentation of a patient does not always predict the underlying genotype [79, 80]; thus, a gene by gene screening is not always the most effective and efficient path for identifying mutations. Instead, targeted Next Generation Sequencing (NGS) is now the recommended approach by enabling testing of all relevant genes in a single panel [84].
The information from the genetic test is critical not only to the patient, but also to the relatives who may carry the same germline mutation; thus genetic screening for the same mutation should be offered to family members at risk for the mutation. In such cases the minimum age for performing genetic testing should be performed depends on the specific causal gene and nature of surveillance indicated for mutation carriers. While this age is well established at five years for VHL syndrome [85], there remains debate for other hereditary causes of PPGL. Based on a review of the literature comprising 105 pediatric cases, the proposed minimum age for starting genetic testing is 5 years for SDHB- and 10 years for SDHA-, SDHC- and SDHD-related PPGLs [86].
While NGS carries advantages over former methods for genetic diagnosis, it also generates large amounts of data in need of interpretation [87]. In particular, although many genetic variants have been characterized, others are inevitably classified as variants of unknown significance (VUS). Such variants may require functional analyses, complementary tests performed in tumour material and checks for the presence of the variant in an appropriate control population [84]. Close collaboration between the genetic laboratory and clinicians is essential to interpret the potential meaning of the such variants. Clinical presentation, including age at diagnosis, family history, biochemistry, tumour location and presence of metastatic disease can also be critical for classifying VUS. For instance, a pathogenic variant in SDHB is characterized by loss of SDHB protein expression in the tumour on immunohistochemical analysis, elevated tumour tissue succinate and a noradrenergic biochemical profile [3, 88, 89]. The genomic features related to each genotype can also be considered in the final classification of a genetic variant [78, 90]. Classification of a VUS as pathogenic remains difficult and it is not always possible to characterize a genetic variant [91]. In such scenarios, genetic testing should not be offered to family members.
In patients in whom no mutation is found, whether further suspicion of an underlying germline mutation is warranted should take into account specific characteristics of the patient or tumour, such as loss of SDHB immunohistochemistry or abnormal metabolite profiles [92]. If characteristics are recognisable as linked to a specific gene, other mutational mechanisms (i.e. deep intronic variants or epimutations) in specific candidate genes can be explored. Nevertheless, a proportion of patients or tumours remain genetically unclassified; these provide candidates for identifying mutations in new tumour-susceptibility genes.
Presurgical and surgical management of phaeochromocytoma and paraganglioma
Treatment of patients with a PPGL requires a multidisciplinary team of dedicated specialists in centres that have extensive experience in management of these complex patients. This approach ensures the most favourable outcome [13].
Surgical resection is the only curative treatment for PPGL. Surgery itself, however, may evoke a massive release of catecholamines from a PPGL into the circulation, resulting in potentially life-threatening cardiovascular complications, including hypertensive crises, cardiac arrhythmias, myocardial infarction, pulmonary oedema and multi-organ failure [93, 94]. In addition, the rapid drop in catecholamines after resection of the PPGL may result in severe hypotension [95]. For prevention of these cardiovascular complications treatment with antihypertensive drugs before surgery is recommended [22]. Even though the evidence underpinning these recommendations for optimal presurgical treatment is almost entirely based on observational studies and expert opinion, the published studies that question this approach do not provide sufficient solid evidence that surgery without appropriate presurgical preparation is safe and feasible. [96–98].
Presurgical administration of α-adrenergic receptor blockers is considered as the treatment of first choice [22]. Frequently prescribed drugs are phenoxybenzamine, a nonselective and non-competitive α1- and α2-adrenergic receptor blocker, and doxazosin, a selective and competitive α1-adrenergic receptor blocker. These drugs (dosed twice daily) are initiated at least 7 to 14 days before surgery in gradually increasing dosages until blood pressure targets are achieved (distinct recommendations on drug dosing can be found elsewhere, e.g. Lenders 2014). Retrospective studies comparing the efficacy of these two α-adrenergic receptor blockers have yielded conflicting results [99–102]. The only randomised controlled trial comparing presurgical treatment with either phenoxybenzamine or doxazosin did not find any difference in the total duration of blood pressure levels outside the predefined target range during surgery [103]. It was found, however, that phenoxybenzamine was associated with more hemodynamic stability during surgery [103]. Although there is no consensual definition of hemodynamic instability, several studies have demonstrated that hemodynamic instability is associated with a higher risk of adverse perioperative events [104–106]. It should be noted that normotensive patients with a PPGL with normal or elevated levels of catecholamines or metanephrines may also be at increased risk of intraoperative hemodynamic instability and should therefore receive presurgical α-blockade as well [107, 108].
Calcium channel blockers may be added to an α-adrenergic receptor blocker for further improvement of blood pressure control [23, 101, 103]. These drugs have also been used as the treatment of first choice for presurgical treatment in patients with PPGL [109, 110]. In general, however, calcium channel blockers are not recommended as monotherapy unless in case of normal or mildly elevated preoperative blood pressure levels or severe orthostatic hypotension during α-adrenergic receptor blocker [13].
The drug metyrosine (α-methyl-paratyrosine) inhibits tyrosine hydroxylase, the enzyme catalyzing the rate limiting step in catecholamine biosynthesis. It is usually prescribed in combination with an α-adrenergic receptor blocker [111, 112]. Disadvantages of metyrosine are the limited availability and side effects such as sedation.
Presurgical treatment with β-adrenergic receptor blockers is required for presentations of tachycardia, which can be a direct result of catecholamine hypersecretion by the PPGL or occur as reflex tachycardia after the initiation of α-adrenergic receptor blockers. It is of utmost importance that treatment with β-adrenergic receptor blockers should only be initiated in a patient already receiving an α-adrenergic receptor blocker; if not there is risk of a hypertensive crisis due to unopposed stimulation of α−adrenergic receptors with ensuing peripheral vasoconstriction [113]. Labetalol is not recommended for presurgical treatment as it provides a combination of selective α1- and nonselective β-adrenergic receptor blockade with a higher potency for β- than α1-adrenergic receptors [114]. Several cases of worsening hypertension or hypertensive crises have been described after administration of labetalol [115–117].
The optimal presurgical blood pressure and heart rate targets have not been established firmly, as no randomised comparative studies have been conducted. A seated blood pressure target <130/80 mmHg and an upright systolic blood pressure > 90 mmHg have been suggested previously [13]. The last recommendation has been corroborated by the finding that an upright systolic blood pressure < 90 mmHg was associated with more hemodynamic instability [102, 103]. Suggested heart rate targets are 60–70 and 70–80 bpm in seated and upright position, respectively [13].
Mortality rates for PPGL resection have dropped impressively from about 40% in the past to 0–3% in contemporary series [103, 104, 118, 119]. It is likely that the improved perioperative outcome is the combined result of presurgical administration of α-adrenergic receptor blockers and major technical advances in both anaesthesiology and surgery. In view of their efficacy in controlling catecholamine induced symptoms and signs, minimal side effects and the lack of randomised placebo-controlled trials, α-adrenergic receptor blockers remain the mainstay of presurgical medical treatment.
It is advised to use a high-sodium intake during treatment with α-adrenergic receptor blockers in order to reduce the risk of orthostatic hypotension before surgery and hypotension during the postoperative period [22]. In addition, intravenous administration of 1–2 L of saline during the last 24 hours of surgery is commonly performed [22, 23, 120]. Supportive evidence for this practice is limited and based on a few retrospective studies [121, 122].
Minimally invasive adrenalectomy is the preferred surgical approach [123]. Transabdominal laparoscopic and retroperitoneoscopic adrenalectomy are the most frequently used techniques. The surgical approach does not significantly affect the intraoperative hemodynamics [124]. Minimally invasive adrenalectomy is associated with lower blood loss and shorter postoperative length of stay compared to open adrenalectomy [125, 126].
In patients with some hereditary syndromes such as MEN2, partial adrenalectomy should be considered as this can preserve adrenocortical function, thus circumventing steroid replacement [127]. In patients in whom extensive surgery results in bilateral adrenalectomy adequate steroid replacement should be initiated in a timely manner.
Head and neck paragangliomas
Although most HNPGLs do not synthesize significant amounts of catecholamines, symptomatic patients with a HNPGL should have presurgical adrenergic blockade. If completely asymptomatic, one can exempt these patients from presurgical blockade. It has to be noted that there is no strong scientific evidence for this approach.
Although surgery is the only curative treatment option for HNPGLs, radical surgical resection is not always possible or is not the preferred option. Advanced tumours, as stratified by specific radiological classification systems, may encroach adjacent structures and removal may therefore cause potentially serious complications. Options other than surgery that might be considered are: wait-and-scan, stereotactic radiosurgery, and conventional radiotherapy or more recently somatostatin-receptor directed targeted radiotherapy for progressive locally advanced tumours [32, 128, 129]. Patient-tailored treatment decisions should be reached in a multidisciplinary setting and requires optimal localisation and staging, knowledge of the genetic status and disease course [32].
Post-surgical follow-up
Surgical resection of a PPGL does not guarantee cure. There may be residual tumour tissue, another PPGL or distant metastases. Even when surgery results in complete resection, there remains risk of local recurrence, metastatic recurrence or a new PPGL. Thus, post-surgical follow-up and continued surveillance is essential [14].
For the patients with residual disease, including metastasis, the nature of post-surgical follow-up and the frequency of clinical, biochemical and imaging follow-up is best established by a multidisciplinary team and specially the physician in charge of the patient. Nature of follow-up depends on the degree of hormonal excess as well as the location and size of the tumour. However, most PPGLs including many metastatic cases only progress slowly [130].
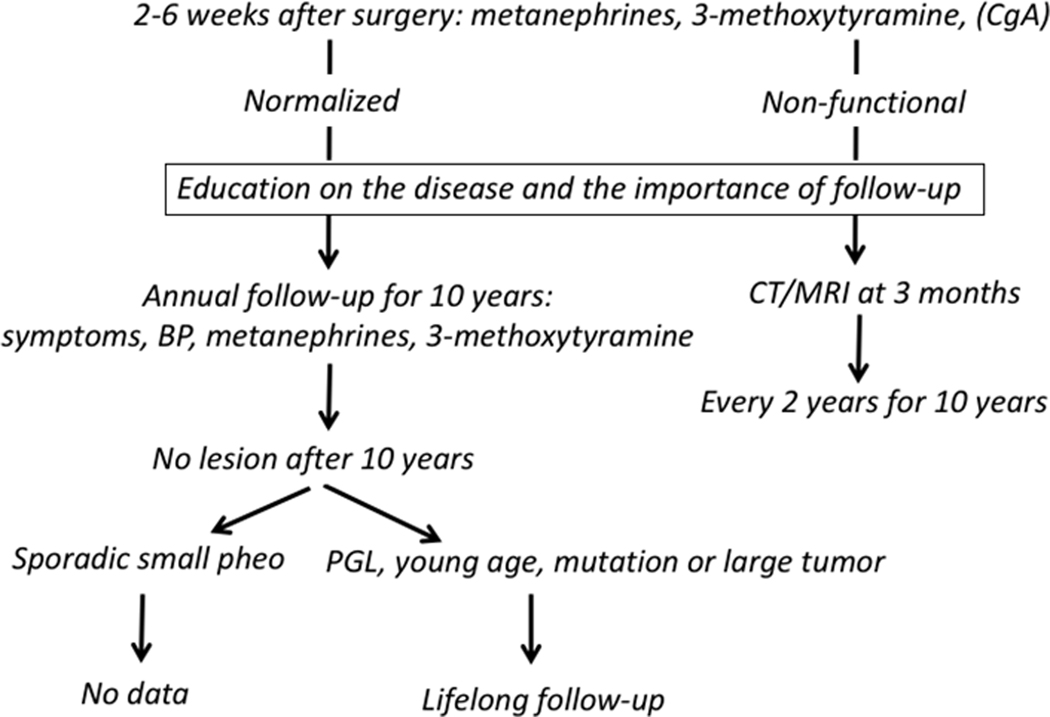
For patients who have undergone surgery, the first issue is to establish whether surgical resection was complete. The European guideline recommends measurements of metanephrine, normetanephrine and 3-methoxytyramine two to six weeks after recovery from surgery according to the nature of elevated concentrations before surgery (Figure 1) [14]. Earlier measurements after surgery can also be informative. Metanephrines in the normal range after surgery document complete resection and no anatomical imaging is required [41]. Of note, concentrations of metanephrine, the metabolite of adrenaline, are decreased after unilateral and bilateral adrenalectomy compared to reference values [131]. In cases of post-surgically elevated metanephrines or of a non-functional PPGL imaging may be performed 3 and 6 months after surgery to document completeness of resection [14].
Figure 1. Flow chart for guiding follow-up after surgery.
After 10 years of follow-up there are insufficient data to provide any recommendation about follow-up of patients with a sporadic small (< 5 cm) phaeochromocytoma. Young patients or patients with a sympathetic paraganglioma, a mutation or a large tumour (≥ 5 cm) should have lifelong follow-up.
If the patient is tumour free at post-operative evaluation, an explanation for the need and nature of follow-up should be provided for the patients. Patients should first be informed that there is a small risk of malignancy and that pathology cannot distinguish benign and metastatic PPGL. In addition, information should be provided about any results of genetic testing that may contribute further to need for follow-up.
Risk of recurrence has been described to be one per 100 person-years [132]. With further doubling of risk in patients with genetic or syndromic disease [132]. Although follow-up is recommended for all patients, studies establishing any impact of follow-up on outcomes are scarce. Nevertheless, there is consensus that follow-up should be performed in all patients during the first ten years after surgery. Available data are insufficient to assess any potential values of post-surgical follow-up beyond ten years. For patients with functional PPGLs it is commonly accepted that follow-up should include clinical examination and annual assessment of metanephrines (Figure 1). For patients in whom primary tumours were non-functional (e.g. HNPGLs) it is proposed that imaging studies should be performed every two years.
Depending on presentation there is considerable scope for personalised approaches to follow-up. Patients with large tumours, paragangliomas, a childhood presentation, multifocal disease or SDHB mutations have a high risk for metastatic progression ([133–135]. These patients may particularly benefit from long-term and more strict annual surveillance, including measurements of plasma 3-methoxytyramine, in order to detect recurrent disease at an earlier stage amenable to therapeutic intervention. For all patients at hereditary risk there is need for annual surveillance with measurements of catecholamine O-methylated metabolites that can be individualized according to genotype-phenotype relationships as well as any additional syndromic features. In patients with VHL syndrome, for example, MRI should be performed every two years to search for kidney, pancreas and other potential tumours [136]. In patients with SDHx mutations, imaging should also be performed to search for non-functional paragangliomas, including HNPGLs.
Metastatic phaeochromocytoma/paraganglioma
Metastatic PPGL is defined by the occurrence of metastatic lesions either in lymph nodes or bones, lung or liver (WHO) [137]. Approximately 10–20% patients with these tumours develop metastatic lesions, about 10–15% at initial diagnosis of the primary tumour [138]. About half of patients with metastatic disease harbour PPGL susceptibility genes mutations, most frequently SDHB, and 50–70% will be considered as apparently sporadic [138, 139]. More than 90% of these PPGLs are functional, with most of them having a noradrenergic and/or dopaminergic phenotype [76, 134, 140].
Clinically, patients with metastatic PPGL usually present similarly as patients with non-metastatic PPGL. However, due to a high tumour burden, many patients present with severe hypertension or fluctuation of blood pressure due to episodic catecholamine secretion from metastatic lesions; associated with this there may be catecholamine-induced end-organ dysfunction (e.g. ileus, severe constipation, organ ischemia, drug-resistant hypertension and tachyarrhythmia) [141]. Although the development of metastatic PPGL cannot be predicted by a magic circulating biomarker, the presence of SDHA/C/B/D, FH, MDH2, SLC25A11 and HIF2A gene mutations, large tumour size (> 5 cm) or multifocality, a noradrenergic and/or dopaminergic biochemical phenotype, or previously detected metastatic lesions (even surgically cured), are risk factors tightly linked to higher incidence of future metastases [138–140, 142]. Metastatic behavior of the most aggressive SDHB-related PPGLs was also uniquely associated with their specific mutation types and the size of the primary tumour. Meanwhile, the age at initial diagnosis, functional phenotype and the presence of multiple tumours or metastatic disease predict overall survival [140].
Currently, the best imaging approach for metastatic PPGL detection is to use 68Ga-DOTA-SSA PET which was found to be highly sensitive as described above [66, 128, 143]. 18F-FDG and 18F-FDOPA serve as the second and third options, respectively [55]. If any surgical procedure is planned, anatomic imaging using CT and MR is necessary.
Treatment options for metastatic PPGLs are limited but require personalised considerations. Before any treatment of metastatic PPGL is initiated, a physician must perform appropriate staging of disease and establish disease progression (usually by imaging over a 3-month interval). Exceptions involve patients with numerous liver, lung, and brain metastatic lesions in whom therapy should be initiated immediately. In patients with rapidly progressive disease, chemotherapy is preferable. Classic CVD (cyclophosphamide, vincristine, dacarbazine) chemotherapy may be effective, especially in patients harbouring SDHx gene mutations: up to 70% of patient will respond compared to 30% with apparently sporadic PPGL [144–147]. Other approaches include temozolomide, temozolomide together with capecitabine, temozolomide with PARP inhibitors, tyrosine kinase inhibitors, and cold somatostatin analogues (for review, see Nölting et al [148].
For moderately progressive metastatic disease, radiotherapeutic approaches should be considered; these include 177Lu-DOTATATE (Lutathera) and 131I-MIBG, the latter either high-specific-activity 131I-MIBG (Azedra) or conventional 131I-MIBG [149–152]. With Azedra, a patient will receive usually a high dose of 131I-MIBG (5 mCi/kg) in either one or two doses 2–3 months apart. Present data suggest that efficacy of Azedra and Lutathera is similar, around 90% [128]. External beam radiation is used especially for metastatic bone lesions and to alleviate pain or to treat lesions associated with possible spinal cord compression [148]. All patients with metastatic PPGL require lifetime follow-up including annual measurements of metanephrines and re-staging using either anatomic or functional imaging modalities. This follow-up frequency may be altered and can be much shorter depending on tumour burden, disease progression, and the patient’s clinical presentation.
Personalized management of metastatic PPGL and future directions of research
For metastatic disease we have witnessed new molecular subclassification of these tumours, involving the Wnt pathway, MAML3 fusion and CSDE1 genes. Larger-scale studies, some by utilizing the sophisticated link between TCGA/other databases and available bioinformatics tools, discovered clearer predictors of metastatic disease [78, 153]. These include the ATRX and SDHB mutations as well as telomerase activation or TERT structural rearrangement [154].
The immediate future directions of research tightly linked to clinical practice more than ever before will require multi-institutional and large-scale collaborations in order to obtain solid, usable, and easily transferrable results to patients. Promising uses of PARP inhibitors together with temozolomide, bone seeking calcium mimetic alpha emitter (223RaCl2; Xofigo®), various approaches for somatostatin receptors-targeted radiotherapy, immunotherapy, and radioimmunotherapy are on the horizon [155]. In the near future, treatment options will also take advantage of new schedules, timing paradigms, potential synergistic combinations, new bioinformatics tools, and artificial intelligence. Furthermore, the search has already begun to discover new targets, biomarkers, oncometabolites, lincRNAs, and signalling pathways that can be used for tumour identification, pathogenesis, localization and treatment. Precision medicine recommendation cards, especially for rare tumours, will soon be available to practicing physicians.
Personalised management
The diagnosis, management and therapy of patients with PPGL can benefit from personalised approaches requiring close multidisciplinary clinical teams of endocrinologists or internists, geneticists, clinical chemists, radiologists, specialists in nuclear medicine, surgeons, pathologists and when needed oncologists. Only working together in such teams can patients with PPGLs receive optimal personalized care at each step in their management journey from initial testing to surgical intervention and follow-up or treatment of metastatic disease. As outlined in the preceding sections such personalised care starts with consideration of the actual presentation of the patient, according to specific signs and symptoms, findings of an incidentaloma, or if testing is carried out as part of surveillance due to hereditary risk or previous disease. From individualized assessments of disease risk and subsequent biochemical test results, patients can be stratified according to post-test probabilities of disease for immediate imaging studies, further evaluation or exclusion of a tumour. As also outlined, major advances in genomics have revealed the rich hereditary background of PPGLs that has been instrumental in guiding personalised approaches to biochemical testing, imaging studies, therapeutic interventions and disease follow-up. Genomic information is now also allowing us to identify specific tumourigenic pathways, relevant for development of potential therapeutic targets for metastatic or inoperable PPGL. We are now at a critical cross-road where clinicians who first see these patients, such as those involved in hypertension care, should be aware of progress in the field and best approaches to provide optimal management of their patients in whom PPGLs are detected.
Consensus point:
Increased incidence of PPGLs likely reflects the changing mode of discovery of these tumours; the spectrum of indications for biochemical testing now includes not only signs and symptoms but also an incidentally detected adrenal mass and genetic causes or syndromes for which periodic surveillance is mandatory
Prevalence of PPGL in patients presenting with adrenal incidentaloma, mutations of tumour susceptibility genes, specific syndromic features and family or past history of a PPGL is relatively high compared to patients presenting with signs and symptoms alone
Likelihood of a PPGL can be assessed from the combination of prevalence (e.g. low in patients with clinical signs and symptoms, high in patients with adrenal incidentaloma) and presence of clinical features (e.g. palpitations)
Sustained hypertension can not be considered as a highly specific sign of a PPGL as it is also very prevalent in patients without PPGL
In non-emergency situations biochemical testing should precede imaging whenever a PPGL is suspected
Measurements of plasma or urinary free metanephrines are the most accurate tests to detect or exclude a PPGL
LC-MS/MS is presently the preferred assay method because of its optimal analytical accuracy, cost-effectiveness and minimal analytical interference from drugs
For measurements of plasma or urinary free metanephrines, no dietary precautions are necessary but an overnight fast is required when measurements include plasma 3-methoxytyramine
To circumvent false-positive test results, testing for plasma free metanephrines requires blood sampling after at least 20 minutes of supine rest. If not possible, urinary measurements of free metanephrines provide an alternative
Plasma or urinary metanephrines in the normal range reliably exclude a PPGL in symptomatic patients
With some exceptions plasma metanephrines in excess of twice the upper reference limit indicate a high likelihood of a PPGL and the patient can proceed to imaging studies
Nearly all phaeochromocytomas have unenhanced attenuation on CT (>10 Hounsfield units; Contrast washout is unreliable in an individual patient to distinguish between phaeochromocytoma and adenoma
Functional imaging is essential in patients with a high risk of recurrence or multifocal disease
The optimal choice of the radiopharmaceutical for functional imaging depends on genotype, biochemical phenotype, size and location
MRI angiography and 68Ga-DOTA-SSA PET are considered as first-choice imaging modalities in patients with HNPGLs
68Ga-DOTA-SSA PET imaging is the preferred imaging modality in patients with metastatic disease and is mandatory for selecting patients amenable for 177Lu-DOTATATE treatment
Currently nearly 80% of all PPGLs can be explained by genetic alterations and therefore all patients with a PPGL should be considered for genetic testing
Lack of family history for PPGL does not preclude the presence of a germline mutation
Next generation sequencing (NGS) is the preferred technique to analyse all relevant genes in a single test
Suspicion of a variant of unknown significance requires complementary testing to establish whether a mutation is pathogenetic
Presurgical medical preparation using an α-adrenergic receptor blocker remains the mainstay for preventing life-threatening peri-operative cardiovascular complications
Normotensive patients with a PPGL should also receive presurgical medical treatment as they are also at increased risk of hemodynamic instability
Both doxazosin and phenoxybenzamine are effective for pre-operative treatment
β-adrenergic blockade for treatment of tachyarrhythmias should not be started before instalment of α-adrenergic blockade
Minimally invasive adrenalectomy is the preferred surgical approach
All patients who have been operated for a PPGL should be followed up annually for at least 10 years
Follow-up should at least include assessment of symptoms, blood pressure, and plasma or urinary free metanephrines
The first follow-up should be at 2–6 weeks after surgery to verify completeness of surgical resection. In case of persistent abnormal biochemical test results, imaging studies are indicated
Patients at high-risk for recurrent disease such as the young subjects, those with a paraganglioma, germline mutation, and patients with an extra-adrenal or large tumour, should be followed up lifelong
SDHx mutations, tumour size larger than 5 cm, multifocality and noradrenergic/ dopaminergic biochemical phenotypes are risk factor for developing metastatic disease
40–50% of patients with metastatic PPGL harbour SDHx mutations
Staging of metastatic PPGL should first be carried out using 68Ga-DOTATATE PET/CT
Rapidly progressing metastatic PPGLs should be treated with chemotherapy (CVD preferable); slowly growing ones can be treated with radiotherapy using either Lutathera or 131I-MIBG therapy
Acknowledgments
Source of Support / Funding
J.W.M. Lenders and G. Eisenhofer were supported by the Deutsche Forschungsgemeinschaft (DFG) within the CTC/Transregio 205/1, project B12, ‘The Adrenal: Central Relay in Health and Disease’.
T. Zelinka was supported by Ministry of Health of the Czech Republic, grants # 16–30345A and NV19–01-00083 and research programs of Charles University (PROGRES Q25 and Q28).
K. Pack was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Abbreviations list
- PPGL
Phaeochromocytoma and Paraganglioma
- PGL
Paraganglioma
- HNPGL
Haed/neck paraganglioma
- BP
Blood pressure
- BMI
Body mass index
- LC-ECD
Liquid chromatography with electrochemical detection
- LC-MS/MS
Liquid chromatography with tandem mass spectrometry
- CT
Computed tomography
- PET
Positron emission tomography
- MRI
Magnetic Resonance Imaging
- CGA
Chromogranin A
- VUS
Variants of unknown significance
- NGS
Next generation sequencing
- MAO
Monoamine oxidase
- VHL
von Hippel-Lindau
- MEN2
Multiple Endocrine Neoplasia type 2
- RET
REarranged during Transfection
- NF1
NeuroFibromatosis type 1
- SDHA
Succinate Dehydrogenase subunit A
- SDHB
Succinate Dehydrogenase subunit B
- SDHC
Succinate Dehydrogenase subunit C
- SDHD
Succinate Dehydrogenase subunit D
- SDHAF2
Succinate Dehydrogenase Complex Assembly Factor 2
- TMEM127
Transmembrane domain protein 127
- MAX
MYC Associated factor X
- FH
Fumarate Hydratase
- MDH2
Malate Dehydrogenase 2
- HRAS
Harvey Rat Sarcoma viral oncogene homolog
- HIF2α
Hypoxia Inducible Factor 2α
- DLST
DihydroLipoamide S-Succinyl Transferase
- SLC25A11
Solute Carrier Family 25 Member 11
- ATRX
Alpha-Thalassemia/mental Retardation syndrome X-linked
- MAML3
MAsterMind-Like 3
- CSDE1
Cold-Shock Domain containing E1
- TERT
TElomerase Reverse Transcriptase
- TCGA
The Cancer Genome Atlas
- 123I-MIBG
123I-metaiodobenzylguanidine
- 18F-FDOPA
18F-fluorodihydroxy-phenylalanine
Footnotes
For the remaining authors none were declared.
Conflict of interest statement: The authors state that there are no conflicts of interest regarding the publication of this article.
References
- 1.DeLellis R, Lloyd R, Heitz P, Eng C. Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 2.Williams MD, Tischler AS. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Paragangliomas. Head and neck pathology. 2017; 11:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenhofer G, Klink B, Richter S, Lenders JW, Robledo M. Metabologenomics of Phaeochromocytoma and Paraganglioma: An Integrated Approach for Personalised Biochemical and Genetic Testing. The Clinical biochemist Reviews / Australian Association of Clinical Biochemists. 2017; 38:69–100. [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil AR, Blok BH, Koelmeyer TD, Burke MP, Hilton JM. Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Australian and New Zealand journal of medicine. 2000; 30:648–52. [DOI] [PubMed] [Google Scholar]
- 5.Lo CY, Lam KY, Wat MS, Lam KS. Adrenal pheochromocytoma remains a frequently overlooked diagnosis. American journal of surgery. 2000; 179:212–5. [DOI] [PubMed] [Google Scholar]
- 6.Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. The Journal of clinical endocrinology and metabolism. 2005; 90:2110–6. [DOI] [PubMed] [Google Scholar]
- 7.Berends AMA, Buitenwerf E, de Krijger RR, Veeger N, van der Horst-Schrivers ANA, Links TP et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: A nationwide study and systematic review. European journal of internal medicine. 2018; 51:68–73. [DOI] [PubMed] [Google Scholar]
- 8.Stenstrom G, Svardsudd K. Pheochromocytoma in Sweden 1958–1981. An analysis of the National Cancer Registry Data. Acta medica Scandinavica. 1986; 220:225–32. [PubMed] [Google Scholar]
- 9.Andersen GS, Toftdahl DB, Lund JO, Strandgaard S, Nielsen PE. The incidence rate of phaeochromocytoma and Conn’s syndrome in Denmark, 1977–1981. Journal of human hypertension. 1988; 2:187–9. [PubMed] [Google Scholar]
- 10.Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA : the journal of the American Medical Association. 2012; 307:2400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falhammar H, Kjellman M, Calissendorff J. Initial clinical presentation and spectrum of pheochromocytoma: a study of 94 cases from a single center. Endocrine connections. 2018; 7:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber LM, Hartman RP, Thompson GB, McKenzie TJ, Lyden ML, Dy BM et al. Pheochromocytoma Characteristics and Behavior Differ Depending on Method of Discovery. The Journal of clinical endocrinology and metabolism. 2019; 104:1386–93. [DOI] [PubMed] [Google Scholar]
- 13.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2014; 99:1915–42. [DOI] [PubMed] [Google Scholar]
- 14.Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW et al. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. European journal of endocrinology / European Federation of Endocrine Societies. 2016; 174:G1–G10. [DOI] [PubMed] [Google Scholar]
- 15.Manger WM. The protean manifestations of pheochromocytoma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2009; 41:658–63. [DOI] [PubMed] [Google Scholar]
- 16.Manger W, Gifford R. Clinical and Experimental pheochromocytoma. Cambridge, MA, USA: Blackwell Science; 1996. [Google Scholar]
- 17.Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocrine reviews. 2003; 24:539–53. [DOI] [PubMed] [Google Scholar]
- 18.Geroula A, Deutschbein T, Langton K, Masjkur JR, Pamporaki C, Peitzsch M et al. Pheochromocytoma and paraganglioma: Clinical feature based disease probability in relation to catecholamine biochemistry and reason for disease suspicion. European journal of endocrinology / European Federation of Endocrine Societies. 2019. [DOI] [PubMed] [Google Scholar]
- 19.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European journal of endocrinology / European Federation of Endocrine Societies. 2016; 175:G1–G34. [DOI] [PubMed] [Google Scholar]
- 20.Brain KL, Kay J, Shine B. Measurement of urinary metanephrines to screen for pheochromocytoma in an unselected hospital referral population. Clinical chemistry. 2006; 52:2060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. Journal of hypertension. 2006; 24:2331–9. [DOI] [PubMed] [Google Scholar]
- 22.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2014; 99:1915–42. [DOI] [PubMed] [Google Scholar]
- 23.Pacak K. Preoperative management of the pheochromocytoma patient. The Journal of clinical endocrinology and metabolism. 2007; 92:4069–79. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K. Adverse drug reactions in patients with phaeochromocytoma: incidence, prevention and management. Drug safety : an international journal of medical toxicology and drug experience. 2007; 30:1031–62. [DOI] [PubMed] [Google Scholar]
- 25.Zelinka T, Petrak O, Turkova H, Holaj R, Strauch B, Krsek M et al. High incidence of cardiovascular complications in pheochromocytoma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2012; 44:379–84. [DOI] [PubMed] [Google Scholar]
- 26.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. Journal of hypertension. 2011; 29:2049–60. [DOI] [PubMed] [Google Scholar]
- 27.Soltani A, Pourian M, Davani BM. Does this patient have Pheochromocytoma? a systematic review of clinical signs and symptoms. J Diabetes Metab Disord. 2015; 15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelinka T, Pacak K, Widimsky J Jr., Characteristics of blood pressure in pheochromocytoma. Annals of the New York Academy of Sciences. 2006; 1073:86–93. [DOI] [PubMed] [Google Scholar]
- 29.Petrak O, Rosa J, Holaj R, Strauch B, Kratka Z, Kvasnicka J et al. Blood Pressure Profile, Catecholamine Phenotype and Target Organ Damage in Pheochromocytoma/Paraganglioma. The Journal of clinical endocrinology and metabolism. 2019. [DOI] [PubMed] [Google Scholar]
- 30.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Journal of hypertension. 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 31.An Y, Reimann M, Masjkur J, Langton K, Peitzsch M, Deutschbein T et al. Adrenomedullary function, obesity and permissive influences of catecholamines on body mass in patients with chromaffin cell tumours. Int J Obes (Lond). 2019; 43:263–75. [DOI] [PubMed] [Google Scholar]
- 32.Taieb D, Kaliski A, Boedeker CC, Martucci V, Fojo T, Adler JR Jr., et al. Current approaches and recent developments in the management of head and neck paragangliomas. Endocrine reviews. 2014; 35:795–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannelli M, Colagrande S, Valeri A, Parenti G. Incidental and metastatic adrenal masses. Seminars in oncology. 2010; 37:649–61. [DOI] [PubMed] [Google Scholar]
- 34.Canu L, Van Hemert JAW, Kerstens MN, Hartman RP, Khanna A, Kraljevic I et al. CT Characteristics of Pheochromocytoma: Relevance for the Evaluation of Adrenal Incidentaloma. The Journal of clinical endocrinology and metabolism. 2019; 104:312–8. [DOI] [PubMed] [Google Scholar]
- 35.Ctvrtlik F, Tudos Z, Szasz P, Sedlackova Z, Hartmann I, Schovanek J et al. Characteristic CT features of pheochromocytomas - probability model calculation tool based on a multicentric study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2019; 163:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buitenwerf E, Korteweg T, Visser A, Haag C, Feelders RA, Timmers H et al. Unenhanced CT imaging is highly sensitive to exclude pheochromocytoma: a multicenter study. European journal of endocrinology / European Federation of Endocrine Societies. 2018; 178:431–7. [DOI] [PubMed] [Google Scholar]
- 37.Mannelli M, Lenders JW, Pacak K, Parenti G, Eisenhofer G. Subclinical phaeochromocytoma. Best practice & research Clinical endocrinology & metabolism. 2012; 26:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jong WH, Graham KS, van der Molen JC, Links TP, Morris MR, Ross HA et al. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clinical chemistry. 2007; 53:1684–93. [DOI] [PubMed] [Google Scholar]
- 39.Eisenhofer G. Impact of LC-MS/MS on the laboratory diagnosis of catecholamine-producing tumors. Trends in Analytical Chemistry. 2016. [Google Scholar]
- 40.Amar L, Eisenhofer G. Diagnosing phaeochromocytoma/paraganglioma in a patient presenting with critical illness: biochemistry versus imaging. Clinical endocrinology. 2015; 83:298–302. [DOI] [PubMed] [Google Scholar]
- 41.Eisenhofer G, Prejbisz A, Peitzsch M, Pamporaki C, Masjkur J, Rogowski-Lehmann N et al. Biochemical Diagnosis of Chromaffin Cell Tumors in Patients at High and Low Risk of Disease: Plasma versus Urinary Free or Deconjugated O-Methylated Catecholamine Metabolites. Clinical chemistry. 2018; 64:1646–56. [DOI] [PubMed] [Google Scholar]
- 42.Boyle JG, Davidson DF, Perry CG, Connell JM. Comparison of diagnostic accuracy of urinary free metanephrines, vanillyl mandelic Acid, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma. The Journal of clinical endocrinology and metabolism. 2007; 92:4602–8. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Xiao H, Zhou X, Huang X, Li Y, Xiao H et al. Accuracy of Plasma Free Metanephrines in the Diagnosis of Pheochromocytoma and Paraganglioma: A Systematic Review and Meta-Analysis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2017; 23:1169–77. [DOI] [PubMed] [Google Scholar]
- 44.Eisenhofer G, Peitzsch M, Kaden D, Langton K, Mangelis A, Pamporaki C et al. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clinica chimica acta; international journal of clinical chemistry. 2019; 490:46–54. [DOI] [PubMed] [Google Scholar]
- 45.Weismann D, Peitzsch M, Raida A, Prejbisz A, Gosk M, Riester A et al. Measurements of plasma metanephrines by immunoassay vs liquid chromatography with tandem mass spectrometry for diagnosis of pheochromocytoma. European journal of endocrinology / European Federation of Endocrine Societies. 2015; 172:251–60. [DOI] [PubMed] [Google Scholar]
- 46.de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP. Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors. The Journal of clinical endocrinology and metabolism. 2009; 94:2841–9. [DOI] [PubMed] [Google Scholar]
- 47.Willemsen JJ, Ross HA, Lenders JW, Sweep FC. Stability of urinary fractionated metanephrines and catecholamines during collection, shipment, and storage of samples. Clinical chemistry. 2007; 53:268–72. [DOI] [PubMed] [Google Scholar]
- 48.Boyd J, Leung AA, Sadrzadeh H, Pamporaki C, Pacak K, Deutschbein T et al. A high rate of modestly elevated plasma normetanephrine in a population referred for suspected PPGL when measured in a seated position. European journal of endocrinology / European Federation of Endocrine Societies. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darr R, Kuhn M, Bode C, Bornstein SR, Pacak K, Lenders JW et al. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: a systematic review. Endocrine. 2017; 56:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deutschbein T, Unger N, Jaeger A, Broecker-Preuss M, Mann K, Petersenn S. Influence of various confounding variables and storage conditions on metanephrine and normetanephrine levels in plasma. Clinical endocrinology. 2010; 73:153–60. [DOI] [PubMed] [Google Scholar]
- 51.Pamporaki C, Prejbisz A, Malecki R, Pistrosch F, Peitzsch M, Bishoff S et al. Optimized Reference Intervals for Plasma Free Metanephrines in Patients With CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2018; 72:907–9. [DOI] [PubMed] [Google Scholar]
- 52.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR et al. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. The Journal of clinical endocrinology and metabolism. 2003; 88:2656–66. [DOI] [PubMed] [Google Scholar]
- 53.Darr R, Lenders JW, Stange K, Kindel B, Hofbauer LC, Bornstein SR et al. [Diagnosis of pheochromocytoma and paraganglioma: the clonidine suppression test in patients with borderline elevations of plasma free normetanephrine]. Deutsche medizinische Wochenschrift. 2013; 138:76–81. [DOI] [PubMed] [Google Scholar]
- 54.Yu R, Nissen NN, Chopra P, Dhall D, Phillips E, Wei M. Diagnosis and treatment of pheochromocytoma in an academic hospital from 1997 to 2007. The American journal of medicine. 2009; 122:85–95. [DOI] [PubMed] [Google Scholar]
- 55.Taieb D, Hicks RJ, Hindie E, Guillet BA, Avram A, Ghedini P et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. European journal of nuclear medicine and molecular imaging. 2019; 46:2112–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buitenwerf E, Berends AMA, van Asselt ADI, Korteweg T, Greuter MJW, Veeger NJM et al. Diagnostic Accuracy of Computed Tomography to Exclude Pheochromocytoma: A Systematic Review, Meta-analysis, and Cost Analysis. Mayo Clinic proceedings Mayo Clinic. 2019; 94:2040–52. [DOI] [PubMed] [Google Scholar]
- 57.Jacques AE, Sahdev A, Sandrasagara M, Goldstein R, Berney D, Rockall AG et al. Adrenal phaeochromocytoma: correlation of MRI appearances with histology and function. European radiology. 2008; 18:2885–92. [DOI] [PubMed] [Google Scholar]
- 58.Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR American journal of roentgenology. 2013; 201:825–33. [DOI] [PubMed] [Google Scholar]
- 59.Rao D, van Berkel A, Piscaer I, Young WF, Gruber L, Deutschbein T et al. Impact of 123 I-MIBG scintigraphy on clinical decision making in pheochromocytoma and paraganglioma. The Journal of clinical endocrinology and metabolism. 2019. [DOI] [PubMed] [Google Scholar]
- 60.Archier A, Varoquaux A, Garrigue P, Montava M, Guerin C, Gabriel S et al. Prospective comparison of (68)Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. European journal of nuclear medicine and molecular imaging. 2016; 43:1248–57. [DOI] [PubMed] [Google Scholar]
- 61.Gild ML, Naik N, Hoang J, Hsiao E, McGrath RT, Sywak M et al. Role of DOTATATE-PET/CT in preoperative assessment of phaeochromocytoma and paragangliomas. Clinical endocrinology. 2018. [DOI] [PubMed] [Google Scholar]
- 62.Taieb D, Jha A, Guerin C, Pang Y, Adams KT, Chen CC et al. 18F-FDOPA PET/CT Imaging of MAX-Related Pheochromocytoma. The Journal of clinical endocrinology and metabolism. 2018; 103:1574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janssen I, Chen CC, Zhuang Z, Millo CM, Wolf KI, Ling A et al. Functional Imaging Signature of Patients Presenting with Polycythemia/Paraganglioma Syndromes. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017; 58:1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weisbrod AB, Kitano M, Gesuwan K, Millo C, Herscovitch P, Nilubol N et al. Clinical utility of functional imaging with (1)(8)F-FDOPA in Von Hippel-Lindau syndrome. The Journal of clinical endocrinology and metabolism. 2012; 97:E613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kroiss A, Putzer D, Frech A, Decristoforo C, Uprimny C, Gasser RW et al. A retrospective comparison between 68Ga-DOTA-TOC PET/CT and 18F-DOPA PET/CT in patients with extra-adrenal paraganglioma. European journal of nuclear medicine and molecular imaging. 2013; 40:1800–8. [DOI] [PubMed] [Google Scholar]
- 66.Janssen I, Chen CC, Taieb D, Patronas NJ, Millo CM, Adams KT et al. 68Ga-DOTATATE PET/CT in the Localization of Head and Neck Paragangliomas Compared with Other Functional Imaging Modalities and CT/MRI. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016; 57:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han S, Suh CH, Woo S, Kim YJ, Lee JJ. Performance of (68)Ga-DOTA-Conjugated Somatostatin Receptor-Targeting Peptide PET in Detection of Pheochromocytoma and Paraganglioma: A Systematic Review and Metaanalysis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2019; 60:369–76. [DOI] [PubMed] [Google Scholar]
- 68.Gravel G, Niccoli P, Rohmer V, Moulin G, Borson-Chazot F, Rousset P et al. The value of a rapid contrast-enhanced angio-MRI protocol in the detection of head and neck paragangliomas in SDHx mutations carriers: a retrospective study on behalf of the PGL.EVA investigators. European radiology. 2016; 26:1696–704. [DOI] [PubMed] [Google Scholar]
- 69.Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P et al. Superiority of [68Ga]-DOTATATE PET/CT to Other Functional Imaging Modalities in the Localization of SDHB-Associated Metastatic Pheochromocytoma and Paraganglioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015; 21:3888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jha A, Ling A, Millo C, Gupta G, Viana B, Lin FI et al. Superiority of (68)Ga-DOTATATE over (18)F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx )-related pheochromocytoma and paraganglioma in the pediatric population. European journal of nuclear medicine and molecular imaging. 2018; 45:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kan Y, Zhang S, Wang W, Liu J, Yang J, Wang Z. (68)Ga-somatostatin receptor analogs and (18)F-FDG PET/CT in the localization of metastatic pheochromocytomas and paragangliomas with germline mutations: a meta-analysis. Acta radiologica. 2018; 59:1466–74. [DOI] [PubMed] [Google Scholar]
- 72.Kong G, Schenberg T, Yates CJ, Trainer A, Sachithanandan N, Iravani A et al. The role of 68Ga-DOTA-Octreotate (GaTate) PET/CT in follow-up of SDH-associated pheochromocytoma and paraganglioma (PPGL). The Journal of clinical endocrinology and metabolism. 2019. [DOI] [PubMed] [Google Scholar]
- 73.Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nature reviews Endocrinology. 2015; 11:101–11. [DOI] [PubMed] [Google Scholar]
- 74.Dahia PL. Pheochromocytomas and Paragangliomas, Genetically Diverse and Minimalist, All at Once! Cancer cell. 2017; 31:159–61. [DOI] [PubMed] [Google Scholar]
- 75.Cascon A, Remacha L, Calsina B, Robledo M. Pheochromocytomas and Paragangliomas: Bypassing Cellular Respiration. Cancers (Basel). 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma. Endocrine-related cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buffet A, Ben Aim L, Leboulleux S, Drui D, Vezzosi D, Libe R et al. Positive Impact of Genetic Test on the Management and Outcome of Patients With Paraganglioma and/or Pheochromocytoma. The Journal of clinical endocrinology and metabolism. 2019; 104:1109–18. [DOI] [PubMed] [Google Scholar]
- 78.Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer cell. 2017; 31:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curras-Freixes M, Inglada-Perez L, Mancikova V, Montero-Conde C, Leton R, Comino-Mendez I et al. Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. Journal of medical genetics. 2015; 52:647–56. [DOI] [PubMed] [Google Scholar]
- 80.Ben Aim L, Pigny P, Castro-Vega LJ, Buffet A, Amar L, Bertherat J et al. Targeted next-generation sequencing detects rare genetic events in pheochromocytoma and paraganglioma. Journal of medical genetics. 2019; 56:513–20. [DOI] [PubMed] [Google Scholar]
- 81.Burnichon N, Briere JJ, Libe R, Vescovo L, Riviere J, Tissier F et al. SDHA is a tumor suppressor gene causing paraganglioma. Human molecular genetics. 2010; 19:3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baysal BE. Mitochondrial complex II and genomic imprinting in inheritance of paraganglioma tumors. Biochimica et biophysica acta. 2013; 1827:573–7. [DOI] [PubMed] [Google Scholar]
- 83.Burnichon N, Mazzella JM, Drui D, Amar L, Bertherat J, Coupier I et al. Risk assessment of maternally inherited SDHD paraganglioma and phaeochromocytoma. Journal of medical genetics. 2017; 54:125–33. [DOI] [PubMed] [Google Scholar]
- 84.Group NGSiPS Toledo RA, Burnichon N, Cascon A, Benn DE, Bayley JP et al. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nature reviews Endocrinology. 2017; 13:233–47. [DOI] [PubMed] [Google Scholar]
- 85.Lahlou-Laforet K, Consoli SM, Jeunemaitre X, Gimenez-Roqueplo AP. Presymptomatic genetic testing in minors at risk of paraganglioma and pheochromocytoma: our experience of oncogenetic multidisciplinary consultation. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2012; 44:354–8. [DOI] [PubMed] [Google Scholar]
- 86.Wong MY, Andrews KA, Challis BG, Park SM, Acerini CL, Maher ER et al. Clinical Practice Guidance: Surveillance for phaeochromocytoma and paraganglioma in paediatric succinate dehydrogenase gene mutation carriers. Clinical endocrinology. 2019; 90:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Curras-Freixes M, Pineiro-Yanez E, Montero-Conde C, Apellaniz-Ruiz M, Calsina B, Mancikova V et al. PheoSeq: A Targeted Next-Generation Sequencing Assay for Pheochromocytoma and Paraganglioma Diagnostics. J Mol Diagn. 2017; 19:575–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gill AJ, Benn DE, Chou A, Clarkson A, Muljono A, Meyer-Rochow GY et al. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Human pathology. 2010; 41:805–14. [DOI] [PubMed] [Google Scholar]
- 89.Papathomas TG, Oudijk L, Persu A, Gill AJ, van Nederveen F, Tischler AS et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2015; 28:807–21. [DOI] [PubMed] [Google Scholar]
- 90.Castro-Vega LJ, Letouze E, Burnichon N, Buffet A, Disderot PH, Khalifa E et al. Multi-omics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat Commun. 2015; 6:6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calsina B, Curras-Freixes M, Buffet A, Pons T, Contreras L, Leton R et al. Role of MDH2 pathogenic variant in pheochromocytoma and paraganglioma patients. Genetics in medicine : official journal of the American College of Medical Genetics. 2018; 20:1652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richter S, Gieldon L, Pang Y, Peitzsch M, Huynh T, Leton R et al. Metabolome-guided genomics to identify pathogenic variants in isocitrate dehydrogenase, fumarate hydratase, and succinate dehydrogenase genes in pheochromocytoma and paraganglioma. Genetics in medicine : official journal of the American College of Medical Genetics. 2019; 21:705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plouin PF, Duclos JM, Soppelsa F, Boublil G, Chatellier G. Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: analysis of 165 operations at a single center. The Journal of clinical endocrinology and metabolism. 2001; 86:1480–6. [DOI] [PubMed] [Google Scholar]
- 94.Siddik-Sayyid SM, Dabbous AS, Shaaban JA, Daaboul DG, Baraka AS. Catastrophic cardiac hypokinesis and multiple-organ failure after surgery in a patient with an undiagnosed pheochromocytoma: emergency excision of the tumor. Journal of cardiothoracic and vascular anesthesia. 2007; 21:863–6. [DOI] [PubMed] [Google Scholar]
- 95.Namekawa T, Utsumi T, Kawamura K, Kamiya N, Imamoto T, Takiguchi T et al. Clinical predictors of prolonged postresection hypotension after laparoscopic adrenalectomy for pheochromocytoma. Surgery. 2016; 159:763–70. [DOI] [PubMed] [Google Scholar]
- 96.Boutros AR, Bravo EL, Zanettin G, Straffon RA. Perioperative management of 63 patients with pheochromocytoma. Cleveland Clinic journal of medicine. 1990; 57:613–7. [DOI] [PubMed] [Google Scholar]
- 97.Groeben H, Nottebaum BJ, Alesina PF, Traut A, Neumann HP, Walz MK. Perioperative alpha-receptor blockade in phaeochromocytoma surgery: an observational case series. Br J Anaesth. 2017; 118:182–9. [DOI] [PubMed] [Google Scholar]
- 98.Lentschener C, Baillard C, Dousset B, Gaujoux S. Dogma Is Made to Be Broken. Why Are We Postponing Curative Surgery to Administer Ineffective Alpha Adrenoreceptor Blockade in Most Patients Undergoing Pheochromocytoma Removal? Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2019; 25:199. [DOI] [PubMed] [Google Scholar]
- 99.Kocak S, Aydintug S, Canakci N. Alpha blockade in preoperative preparation of patients with pheochromocytomas. International surgery. 2002; 87:191–4. [PubMed] [Google Scholar]
- 100.Prys-Roberts C, Farndon JR. Efficacy and safety of doxazosin for perioperative management of patients with pheochromocytoma. World journal of surgery. 2002; 26:1037–42. [DOI] [PubMed] [Google Scholar]
- 101.Weingarten TN, Cata JP, O’Hara JF, Prybilla DJ, Pike TL, Thompson GB et al. Comparison of two preoperative medical management strategies for laparoscopic resection of pheochromocytoma. Urology. 2010; 76:508 e6–11. [DOI] [PubMed] [Google Scholar]
- 102.Bruynzeel H, Feelders RA, Groenland TH, van den Meiracker AH, van Eijck CH, Lange JF et al. Risk Factors for Hemodynamic Instability during Surgery for Pheochromocytoma. The Journal of clinical endocrinology and metabolism. 2010; 95:678–85. [DOI] [PubMed] [Google Scholar]
- 103.Buitenwerf E, Osinga TE, Timmers HJLM, Lenders JWM, Feelders RA, Eekhoff EMW et al. Randomized controlled trial on the efficacy of pretreatment with phenoxybenzamine or doxazosin on hemodynamic control during pheochromocytoma resection. The Journal of clinical endocrinology and metabolism. 2019; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Livingstone M, Duttchen K, Thompson J, Sunderani Z, Hawboldt G, Sarah Rose M et al. Hemodynamic Stability During Pheochromocytoma Resection: Lessons Learned Over the Last Two Decades. Annals of surgical oncology. 2015; 22:4175–80. [DOI] [PubMed] [Google Scholar]
- 105.Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. Journal of anesthesia. 2018; 32:167–73. [DOI] [PubMed] [Google Scholar]
- 106.Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP et al. Effects of Intraoperative Fluid Management on Postoperative Outcomes: A Hospital Registry Study. Annals of surgery. 2018; 267:1084–92. [DOI] [PubMed] [Google Scholar]
- 107.Scholten A, Vriens MR, Cromheecke GJ, Borel Rinkes IH, Valk GD. Hemodynamic instability during resection of pheochromocytoma in MEN versus non-MEN patients. European journal of endocrinology / European Federation of Endocrine Societies. 2011; 165:91–6. [DOI] [PubMed] [Google Scholar]
- 108.Lafont M, Fagour C, Haissaguerre M, Darancette G, Wagner T, Corcuff JB et al. Per-operative hemodynamic instability in normotensive patients with incidentally discovered pheochromocytomas. The Journal of clinical endocrinology and metabolism. 2015; 100:417–21. [DOI] [PubMed] [Google Scholar]
- 109.Lebuffe G, Dosseh ED, Tek G, Tytgat H, Moreno S, Tavernier B et al. The effect of calcium channel blockers on outcome following the surgical treatment of phaeochromocytomas and paragangliomas. Anaesthesia. 2005; 60:439–44. [DOI] [PubMed] [Google Scholar]
- 110.Brunaud L, Boutami M, Nguyen-Thi PL, Finnerty B, Germain A, Weryha G et al. Both preoperative alpha and calcium channel blockade impact intraoperative hemodynamic stability similarly in the management of pheochromocytoma. Surgery. 2014; 156:1410–7; discussion7–8. [DOI] [PubMed] [Google Scholar]
- 111.Wachtel H, Kennedy EH, Zaheer S, Bartlett EK, Fishbein L, Roses RE et al. Preoperative Metyrosine Improves Cardiovascular Outcomes for Patients Undergoing Surgery for Pheochromocytoma and Paraganglioma. Annals of surgical oncology. 2015; 22 Suppl 3:S646–54. [DOI] [PubMed] [Google Scholar]
- 112.Butz JJ, Weingarten TN, Cavalcante AN, Bancos I, Young WF Jr.,, McKenzie TJ et al. Perioperative hemodynamics and outcomes of patients on metyrosine undergoing resection of pheochromocytoma or paraganglioma. International journal of surgery. 2017; 46:1–6. [DOI] [PubMed] [Google Scholar]
- 113.Sibal L, Jovanovic A, Agarwal SC, Peaston RT, James RA, Lennard TW et al. Phaeochromocytomas presenting as acute crises after beta blockade therapy. Clinical endocrinology. 2006; 65:186–90. [DOI] [PubMed] [Google Scholar]
- 114.MacCarthy EP, Bloomfield SS. Labetalol: a review of its pharmacology, pharmacokinetics, clinical uses and adverse effects. Pharmacotherapy. 1983; 3:193–219. [DOI] [PubMed] [Google Scholar]
- 115.Briggs RS, Birtwell AJ, Pohl JE. Hypertensive response to labetalol in phaeochromocytoma. Lancet. 1978; 1:1045–6. [DOI] [PubMed] [Google Scholar]
- 116.Feek CM, Earnshaw PM. Hypertensive response to labetalol in phaeochromocytoma. British medical journal. 1980; 281:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Navaratnarajah M, White DC. Labetalol and phaeochromocytoma. Br J Anaesth. 1984; 56:1179. [DOI] [PubMed] [Google Scholar]
- 118.Apgar V, Papper EM. Pheochromocytoma. Anesthetic management during surgical treatment. AMA archives of surgery. 1951; 62:634–48. [PubMed] [Google Scholar]
- 119.Kinney MA, Narr BJ, Warner MA. Perioperative management of pheochromocytoma. Journal of cardiothoracic and vascular anesthesia. 2002; 16:359–69. [DOI] [PubMed] [Google Scholar]
- 120.van der Horst-Schrivers AN, Kerstens MN, Wolffenbuttel BH. Preoperative pharmacological management of phaeochromocytoma. The Netherlands journal of medicine. 2006; 64:290–5. [PubMed] [Google Scholar]
- 121.Deoreo GA Jr., Stewart BH, Tarazi RC, Gifford RW Jr. Preoperative blood transfusion in the safe surgical management of pheochromocytoma: a review of 46 cases. The Journal of urology. 1974; 111:715–21. [DOI] [PubMed] [Google Scholar]
- 122.Desmonts JM, le Houelleur J, Remond P, Duvaldestin P. Anaesthetic management of patients with phaeochromocytoma. A review of 102 cases. Br J Anaesth. 1977; 49:991–8. [DOI] [PubMed] [Google Scholar]
- 123.Stefanidis D, Goldfarb M, Kercher KW, Hope WW, Richardson W, Fanelli RD et al. SAGES guidelines for minimally invasive treatment of adrenal pathology. Surgical endoscopy. 2013; 27:3960–80. [DOI] [PubMed] [Google Scholar]
- 124.Vorselaars W, Postma EL, Mirallie E, Thiery J, Lustgarten M, Pasternak JD et al. Hemodynamic instability during surgery for pheochromocytoma: comparing the transperitoneal and retroperitoneal approach in a multicenter analysis of 341 patients. Surgery. 2018; 163:176–82. [DOI] [PubMed] [Google Scholar]
- 125.Lang B, Fu B, OuYang JZ, Wang BJ, Zhang GX, Xu K et al. Retrospective comparison of retroperitoneoscopic versus open adrenalectomy for pheochromocytoma. The Journal of urology. 2008; 179:57–60; discussion [DOI] [PubMed] [Google Scholar]
- 126.Tiberio GA, Baiocchi GL, Arru L, Agabiti Rosei C, De Ponti S, Matheis A et al. Prospective randomized comparison of laparoscopic versus open adrenalectomy for sporadic pheochromocytoma. Surgical endoscopy. 2008; 22:1435–9. [DOI] [PubMed] [Google Scholar]
- 127.Rossitti HM, Soderkvist P, Gimm O. Extent of surgery for phaeochromocytomas in the genomic era. The British journal of surgery. 2018; 105:e84–e98. [DOI] [PubMed] [Google Scholar]
- 128.Taieb D, Jha A, Treglia G, Pacak K. Molecular imaging and radionuclide therapy of paraganglioma and pheochromocytoma. Endocrine-related cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Satapathy S, Mittal BR, Bhansali A. Peptide Receptor Radionuclide Therapy in the Management of Advanced Pheochromocytoma and Paraganglioma: A Systematic Review and Meta-analysis. Clinical endocrinology. 2019. [DOI] [PubMed] [Google Scholar]
- 130.Hescot S, Leboulleux S, Amar L, Vezzosi D, Borget I, Bournaud-Salinas C et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. The Journal of clinical endocrinology and metabolism. 2013. [DOI] [PubMed] [Google Scholar]
- 131.Osinga TE, van den Eijnden MH, Kema IP, Kerstens MN, Dullaart RP, de Jong WH et al. Unilateral and bilateral adrenalectomy for pheochromocytoma requires adjustment of urinary and plasma metanephrine reference ranges. The Journal of clinical endocrinology and metabolism. 2013; 98:1076–83. [DOI] [PubMed] [Google Scholar]
- 132.Amar L, Lussey-Lepoutre C, Lenders JW, Djadi-Prat J, Plouin PF, Steichen O. MANAGEMENT OF ENDOCRINE DISEASE: Recurrence or new tumors after complete resection of pheochromocytomas and paragangliomas: a systematic review and meta-analysis. European journal of endocrinology / European Federation of Endocrine Societies. 2016; 175:R135–45. [DOI] [PubMed] [Google Scholar]
- 133.King KS, Prodanov T, Kantorovich V, Fojo T, Hewitt JK, Zacharin M et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011; 29:4137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. European journal of cancer. 2012; 48:1739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pamporaki C, Hamplova B, Peitzsch M, Prejbisz A, Beuschlein F, Timmers HJ et al. Characteristics of Pediatric vs adult Pheochromocytomas and Paragangliomas. The Journal of clinical endocrinology and metabolism. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. European journal of human genetics : EJHG. 2011; 19:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocrine pathology. 2017; 28:213–27. [DOI] [PubMed] [Google Scholar]
- 138.Turkova H, Prodanov T, Maly M, Martucci V, Adams K, Widimsky J Jr., et al. Characteristics and Outcomes of Metastatic Sdhb and Sporadic Pheochromocytoma/Paraganglioma: An National Institutes of Health Study. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016; 22:302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hamidi O, Young WF Jr., Iniguez-Ariza NM, Kittah NE, Gruber L, Bancos C et al. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. The Journal of clinical endocrinology and metabolism. 2017; 102:3296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hescot S, Curras-Freixes M, Deutschbein T, van Berkel A, Vezzosi D, Amar L et al. Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. The Journal of clinical endocrinology and metabolism. 2019; 104:2367–74. [DOI] [PubMed] [Google Scholar]
- 141.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005; 366:665–75. [DOI] [PubMed] [Google Scholar]
- 142.Mei L, Khurana A, Al-Juhaishi T, Faber A, Celi F, Smith S et al. Prognostic Factors of Malignant Pheochromocytoma and Paraganglioma: A Combined SEER and TCGA Databases Review. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2019; 51:451–7. [DOI] [PubMed] [Google Scholar]
- 143.Janssen I, Chen CC, Millo CM, Ling A, Taieb D, Lin FI et al. PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. European journal of nuclear medicine and molecular imaging. 2016; 43:1784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008; 113:2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fishbein L, Ben-Maimon S, Keefe S, Cengel K, Pryma DA, Loaiza-Bonilla A et al. SDHB mutation carriers with malignant pheochromocytoma respond better to CVD. Endocrine-related cancer. 2017; 24:L51–L5. [DOI] [PubMed] [Google Scholar]
- 146.Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clinical endocrinology. 2014; 81:642–51. [DOI] [PubMed] [Google Scholar]
- 147.Deutschbein T, Fassnacht M, Weismann D, Reincke M, Mann K, Petersenn S. Treatment of malignant phaeochromocytoma with a combination of cyclophosphamide, vincristine and dacarbazine: own experience and overview of the contemporary literature. Clinical endocrinology. 2015; 82:84–90. [DOI] [PubMed] [Google Scholar]
- 148.Nolting S, Ullrich M, Pietzsch J, Ziegler CG, Eisenhofer G, Grossman A et al. Current Management of Pheochromocytoma/Paraganglioma: A Guide for the Practicing Clinician in the Era of Precision Medicine. Cancers (Basel). 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L et al. Efficacy and Safety of High-Specific-Activity (131)I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2019; 60:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jimenez C, Erwin W, Chasen B. Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine. Cancers (Basel). 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O et al. Efficacy of Peptide Receptor Radionuclide Therapy for Functional Metastatic Paraganglioma and Pheochromocytoma. The Journal of clinical endocrinology and metabolism. 2017; 102:3278–87. [DOI] [PubMed] [Google Scholar]
- 152.Vyakaranam AR, Crona J, Norlen O, Granberg D, Garske-Roman U, Sandstrom M et al. Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with (177)Lu-DOTATATE. Cancers (Basel). 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Crona J, Taieb D, Pacak K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocrine reviews. 2017; 38:489–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Job S, Draskovic I, Burnichon N, Buffet A, Cros J, Lepine C et al. Telomerase Activation and ATRX Mutations Are Independent Risk Factors for Metastatic Pheochromocytoma and Paraganglioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019; 25:760–70. [DOI] [PubMed] [Google Scholar]
- 155.Caisova V, Li L, Gupta G, Jochmanova I, Jha A, Uher O et al. The Significant Reduction or Complete Eradication of Subcutaneous and Metastatic Lesions in a Pheochromocytoma Mouse Model after Immunotherapy Using Mannan-BAM, TLR Ligands, and Anti-CD40. Cancers (Basel). 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]