Abstract
The severe infection is becoming a significant health problem which threaten the lives of patients and the safety and economy of society. In the way of finding new strategy, antimicrobial peptides (AMPs) - an important part of host defense family, emerged with tremendous potential. Up to date, huge numbers of AMPs has been investigated from both natural and synthetic sources showing not only the ability to kill microbial pathogens but also propose other benefits such as wound healing, anti-tumor, immune modulation. In this review, we describe the involvements of AMPs in biological systems and discuss the opportunity in developing AMPs for clinical applications. In the detail, their properties in antibacterial activity is followed by their application in some infection diseases and cancer. The key discussions are the approaches to improve biological activities of AMPs either by modifying chemical structure or incorporating into delivery systems. The new applications and perspectives for the future of AMPs would open the new era of their development.
Abbreviations: AMPs, antimicrobial peptides; BAI, biomaterial-associated infection; BLES, bovine lipid extract surfactant; BMP, bone morphogenetic protein; CARG, compounded annual growth rate; CF, cystic fibrosis; CL, contact lens; COPD, chronic obstructive pulmonary disease; CRAMP, cathelin-related antimicrobial peptides; FCS, fetal calf serum; FDA, Food and Drug Administration; GI tract, gastrointestinal tract; HBD-1, human beta-defensin-1; HIV, human immunodeficiency viruses; LPS, lipopolysaccharides; LTA, lipoteichoic acid; MDR, MULTIDRUG RESIStant; MIC, minimum inhibitory concentration; PAMPs, pathogen-associated molecular patterns; PLGA, poly(lactic-co-glycolic acid); PS, phospholipid phosphatidylserine; RCM, ring-closing metathesis; ROS, reactive oxygen species; SDF, silver diamine flouride; SNPs, single-nucleotide polymorphisms; SSTIs, skin and soft tissue infections; STAMP, specifically targeted antimicrobial peptide; TKR, total hip and knee replacement; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis inducing ligand
Keywords: Antimicrobial peptides (AMPs), Antimicrobial resistance, Infectious diseases, Anticancer agent, Medical devices, Cosmetic ingredients, Peptide drugs
Graphical abstract
1. Introduction
The antibiotic drug resistance and shortage of new antibacterial drugs putted medical care at risk of severe infections. In the quest of new antimicrobial strategies, antimicrobial peptides (AMPs) have showed a great potential. AMPs have been widely investigated in both mechanisms of action and new therapeutic applications [1,2]. Most of clinical study until now, are focusing on its antimicrobial properties and the possible for topical administration. Nonetheless, many recent reports suggested that AMPs also show promising characteristics for wound recovery, anti-cancer, as well as new cosmetic ingredients [[3], [4], [5]].
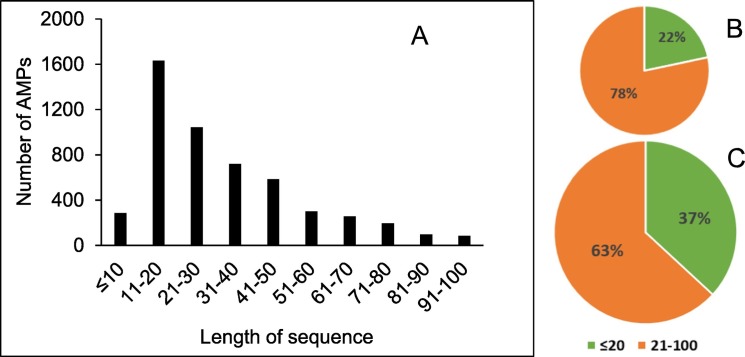
Though AMPs are potential in drug development they still have several undesirable properties for clinical application. Natural peptides are usually unstable in gastrointestinal track and other body fluids, poor absorption and distribution while fast metabolic degradation and excretion, which result in low bioavailability [6,7]. Their flexible structures also may possibly induce interactions with unintended components, which could result in undesirable side-effects. Furthermore, according to the DRAMP Database, there are approximately 67% known antimicrobial peptides from all sources and particularly 78% from human source compose of more than 20 amino acid residues [[8], [9], [10], [11], [12]], whereas many of them including several dominant residues [8] (Fig. 1 ). This matter of length and composition make their preparation challenging and costly.
Fig. 1.
Distribution of AMPs by length of sequence according to the DRAMP Database. (A) The number of AMPs categorized by length of sequence, Percentage of AMPs in (B) human, (C) all source.
To overcome these challenges, recently both chemical and bioengineering strategies have been proposed to develop potent, selective and metabolically stable peptide preparations with cheaper price and less tendency to trigger undesired side-effects. These innovative techniques together with new findings in biological roles make antimicrobial peptides become an emerging category for clinical applications [[13], [14], [15]]. In this review, the development of AMPs as therapeutic agents is summarized in which their properties and applications are discussed. Moreover, the delivery of them and the chemical modification are also introduced to pave the way of translating them to the clinical practice.
2. AMPs as alternative of conventional antibiotics for infectious diseases
As the World Health Organization (WHO) has widely announced in its global report on surveillance, antimicrobial resistance in recent decades has become one of the biggest threats to the global public health, mostly because of the extensive use of classical antibiotics in health care systems, animal production and in the community. If this problem is not well-controlled, even common infections or minor injuries can be life-threatening. In fact, antimicrobial-resistant infections cause at least 700,000 deaths each year globally including more than 23,000 in the USA and 175,000 in the EU [16]. Thus, using antibiotic appropriately is not enough to keep up with the resistant bacteria. Therefore, it is urgent need to develop new alternative antibiotics. Human body produces AMPs as the part of the innate immune response to prevent bacteria infection and/or inhibit the growth of microorganisms, which can be promising candidates for that purpose since they possess many advantages.
2.1. Rapid and broad-spectrum antibacterial activity
AMPs showed broad antimicrobial spectrum against both classes of bacteria (Gram-negative and Gram-positive species). They exhibited the direct action through either perturbation of bacteria membrane or inhibition of intracellular functions. Generally, the positive charge of AMPs was considered to be a critical factor for the selectively interaction to the anionic bacteria membrane instead of the human zwitterionic membrane while the hydrophobic part is important for the effective interaction with the hydrophobic interior of bacteria cell membrane [[17], [18], [19]]. After reaching a certain threshold concentration, AMPs can induces pore formation and also disrupt membrane of bacteria organelles when it goes inside the cell. A variety of disrupted models includes barrel-stave, carpet-stave, toroidal or disordered toroidal pores [2,20,21]. Moreover, many AMPs are also reported to inhibit protein and nucleic acid synthesis, block enzyme activities or induce apoptosis-like death through generating reactive oxygen species (ROS) such as NK-18 [22], buferin II [23], lactoferricin B [24], respectively.
Besides, the rapid antimicrobial action of AMPs was also widely known [[25], [26], [27]]. For examples, an antimicrobial peptide mBjAMP1 can completely kill the Gram-positive bacteria Staphylococcus aureus (S. aureus) within 30 min and the Gram-negative bacteria Escherichia coli (E. coli) within 3 h [28]; Another antimicrobial peptide PaDBS1R1 at 6.25 μM even kill all of the S. aureus after 30 min and E. coli after 5 min [29]; In a more extensive investigation, an antimicrobial peptide isolated from chicken (CHAP) exhibited potent and fast activity against 19 strains of bacteria including drug-resistant strains as CHAP showed 50–94% of its inhibition activity to almost all the tested bacteria species after only 30 min [30].
2.2. Indirect activity
In addition to inducing cell death directly, AMPs modulated the host immune defense system to clear infection, which includes promoting inflammation through enhancement of nucleic acid recognition and activation of immunocytes [31,32]. Interestingly, AMPs can also prevent the strong inflammation by neutralizing Pathogen-Associated Molecular Patterns (PAMPs), which mainly includes the endotoxin lipopolysaccharides (LPS) and lipoteichoic acid (LTA) [31,33,34].
2.3. Antiviral, antifungal and antiprotozoal activities
AMPs exhibited their antiviral activity via several mechanisms, for examples, binding to the negatively charged heparan sulfate in viral surface to prevent viral docking [35,36], interacting directly with viral envelope to induce disruption or in some cases inhibiting viral gene expression [37]{Sinha, 2003 #174}. Thus AMPs can be an potential therapeutic option for the treatment of life-threatening viral infection, such as COVID-19 [38], MERS-CoV [39], vaccinia virus [40].
Regarding to fungi and yeast cells, AMPs in the most common mode of action demonstrated selective activity by inducing perturbation of their negatively charged membranes [41]. Furthermore, several specific intracellular mechanisms have been proposed, for examples DNA damage of Dermaseptin S3 and Magainin 2 MAG-2 [42] or targeting energized mitochondrion in the case of human basic salivary antimicrobial peptide Histatin 5 [43].
The antiprotozoal property also mostly involve the membrane interaction event, which results in the modification of membrane fluidity or disruption [44,45]. Besides, there are some other mechanisms include intracellular targets, such as inducing necrotic and apoptotic-like processes, inhibiting mitochondrial ATP synthesis or causing bioenergetics exhaustion [46]. Moreover, some AMPs interestingly showed the ability to interact selectively with infected enthythrocytes over host cell [[47], [48], [49]]. Therefore, AMPs represented a new class of peptide inhibitors that can possibly not only block the attachment and entry of human pathogenic protozoa and viruses but also prevent their growth and replication in the host cells.
2.4. Anti-biofilm activity
Many bacterial species form biofilms to become significantly more resistant to conventional antibiotics [50]. Recently, biofilm inhibitory property of AMPs has been widely reported, for examples, IDR-1018 displayed broad spectrum anti-biofilm activity by targeting an important signal in biofilm development, which is bacterial guanosine pentaphosphate (p)ppGpp [51]. Besides, the human antimicrobial peptide LL- 37 prevents biofilm by influencing the downregulation of crucial genes for biofilm, inhibiting initial bacteria attachment and stimulating twitching motility [52]. Regarding biofilm formation on the surface of PMMA bone cement by several species of bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Pseudomonas aeruginosa and Escherichia coli, antimicrobial peptides and antibiotics showed comparable in antibiofilm activity [53].
2.5. Lower risk of antimicrobial resistance
Since AMPs possessing various functions in killing microbes, the possibility of antimicrobial species to develop resistance is limited [2,54]. Furthermore, the direct antimicrobial action may be affected by some resistant bacteria but the inflammatory properties are unaffected. Recently, a systematic study suggested very low resistance level of AMPs by point mutations and gene amplification while antibiotic-resistant bacteria did not exhibit cross-resistance to AMPs [55]. In fact, AMPs have been existing for thousands of years as an important component in the host defense systems of human and other creatures [56,57].
2.6. Low propensity to develop toxicity
Along with high selectivity, many reports suggested that AMPs have low propensity to induce toxicity when administrated in both topical and parenteral routes [13,[58], [59], [60], [61]]. In fact, none of publications until now reported any severe undesirable effects in animals treated with AMPs. One of the big problems of conventional antibiotics that makes the outcome of infection worsen is the release of pathogen-associated molecular patterns (PAMPs), which can cause septic shock as reported in ciprofloxacin [62,63]. Some AMPs, however, were even reported to prevent the endotoxin-induced sepsis in both in vitro and in mouse model [64].
3. Applications of AMPs in some infectious diseases
3.1. Wound healing and skin infections
Our skin is the first barrier that constantly exposed to various pathogens. Therefore, it is not surprised that Skin and Soft Tissue Infections (SSTIs) rank among the most common microbial infections in human [65]. Nonetheless, the therapy for SSTIs and wound treatment is challenged by the emerging of antibiotic resistance. Thus, AMPs and their derivatives can serve as new therapeutic option. In fact, AMPs were produced by both skin microbiota and keratinocyte as an important part of defense line against invasive infectious agents.
Since skin pathogens not only include just bacteria species but also virus, fungi and protozoa, AMPs with such a broad-spectrum can be more useful compared to traditional antibiotics. Furthermore, due to its low ability to permeable into blood stream, AMPs preparations have the benefit of high concentration at target side for topical administration. Especially in wounds, AMPs not only prevent pathogen proliferation and biofilm development in lesional skin but also promote wound healing through modulating the cell migration, chemotaxis, cytokine release and angiogenesis [54,66]. Thus, these beneficial effects suggest AMPs as potential source for the treatment of both infective and non-infective wounds [3,67,68].
In fact, most of peptide-related antibiotics approved by FDA until now for infected skin indication are in topical application, for examples Tyrothricin, Bacitracin and Gramicidin [69] while many are currently in clinical trial phases, such as LL37 for hard-to-heal venous leg ulcers, omiganan for treatment of rosacea and severe inflammatory acne vulgaris or pexiganan cream for diabetic foot infection [54].
3.2. Respiratory diseases
According to the WHO, lower respiratory infection in 2016 became the fourth worldwide leading cause of death and the first in low-income country. Together with pneumonia and bronchitis, infections in lower respiratory tract also markedly contribute to chronic inflammatory lung disorders such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and asthma [70]. However, antibiotic treatment - the most effective medical intervention is facing a growing problem of microbial resistance.
In our respiratory system, AMPs were produced by neutrophils airway epithelial cells to prevent the continuous exposure to various inhaled pathogens. The roles of AMPs are shown through the broad-spectrum antimicrobial activity including multidrug resistant (MDR) bacteria and those present in biofilms as well as the indirect activities in shaping the lung immune response and improving wound healing by promoting angiogenesis and inducing airway epithelial cell repair. In fact, the serum level of human cathelicidin antimicrobial peptide LL-37 in bacterial lung infected patients was reported to be significantly higher than that of healthy volunteers [71].
In the opposite side, the lack of AMPs could seriously weaken the protection ability. In mice, the deficiency of cathelin-related antimicrobial peptides (CRAMP) leads to the consequence of more sensitive to bacterial pneumonia infection. Some in vivo studies also provided the role of human LL-37 and murine CRAMP in host defense to prevent influenza A respiratory viral infection. Moreover, vitamin D deficiency could result in low LL-37 expression may increase tuberculosis infections susceptibility. Therefore, besides the therapy that enhances local production, there are two possible ways for using AMPs in respiratory infection, which is direct application and AMPs – surfactant combination to improve local conditions. Recently, amphibian antimicrobial peptide Esculentin (1−21) with potent activity on both planktonic and biofilm form of Pseudomonas aeruginosa exerted the ability to prolong survival of mouse models with pulmonary infection [72]. Moreover, the direct application of the antimicrobial peptide D-BMAP18 showed positive in vitro result, however, the peptide lost its potency in a murine lung infection model of Pseudomonas aeruginosa though showing resistance to pulmonary proteases [73]. Another potential treatment therapy is the combination of conventional surfactant with AMPs. Following this strategy, several publications have reported the impact of different types of surfactants on the structure, activity and toxicity of AMPs [[74], [75], [76]]. Recently, bovine lipid extract surfactant (BLES) – a surfactant compound was supplemented with antimicrobial peptides CATH-2, however, though showing promising in vitro activity, this combination failed to show bactericidal activity in vivo [77,78].
3.3. Eyes diseases
Microbial keratitis has several challenges for treatment, which in fact is the most common cause of corneal blindness in the world. However, the efficacy of conventional antibiotics is rapidly reducing due to the involvement of microbial resistance. Keratitis is frequently poly-microbial with bacterial infections in combination with fungal, parasitic or both, which licensed drugs for those two are few and far between. Thus, AMPs have the potential ability to answers to almost all the challenges listed. Furthermore, AMPs are also proven to induce corneal wound healing in eyes [78,79].
In addition to the consequence of blindness after cornea infections, another serious problem is the limitation of donor human corneas for transplant surgery. Such AMPs can be alternatives to antibiotics with potent killing efficacy and immunomodulatory property. Moreover, earlier control of infection will have a huge impact on eye banks and engineered corneas development.
Besides some accepted by FDA like Bacitracin, Gramicidin or Polymyxin E (Colistin), many other AMPs are still being developed. For examples, a topical eye drops formulation containing LyeTxI-b recently has showed effective in the treatment of keratitis with resistant bacterial while no signs of ocular toxicity found. In more details, LyeTxI-b could eliminate bacteria and reduce both inflammatory cellular activity and biofilm viability in the eyes [80]. In development of artificial corneal skirt, the corneas with AMP-immobilized Titan (via a cross-linker – polydopamine) compared to those with unprotected Titan implants, showed lower incidence and lesser extent of bacterial infection [81].
Furthermore, the combination of a certain AMP with other antibiotics, ophthalmic corticosteroids or AMPs also holds promise in dealing with wide range of eyes problems to enhance the effectiveness of treatment while reducing the dose required. Such a proper combinations can help limit the toxicity of both antibiotics and corticosteroids, which can cause ocular surface damage and more scarring itself [82].
3.4. Gastrointestinal diseases
3.4.1. Stomach infection by Helicobacter pylori
Helicobacter pylori (or H. pylori) causes the most morbidity and prevalence of chronic infection in the human stomach [83]. This Gram-negative bacterium specifically hijacks immune responses including evading the host defense shield and selectively compromising AMPs production to allow colonization [[84], [85], [86], [87]]. Moreover, H. pylori can also promote (hepatic) malignancy both inside and outside of the gastrointestinal (GI) tract, even if it translocates across the intestinal barrier or not [88].
In fact, the resistance problem of H. pylori to antibiotics such as clarithromycin, amoxicillin or metronidazole is well-documented, especially in developing world including Africa [89,90]. Nonetheless, the drug-resistance emergence, the cost as well as the side effects of conventional antibiotics and proton-pump inhibitors on intestinal track give chance to develop AMPs as alternative therapy. Since AMPs are usually highly susceptible to intestinal enzymes and hardly absorbed through GI tract, the main advantages of its application include minimizing the adverse effect on systemic or intestinal microbiota composition. Furthermore, changing properties of membrane components to avoid interaction of AMPs could lead to the consequence of failing to colonize in the highly acidic stomach environment. Recently, more than 22 AMPs with positively charge and mostly having alpha-helical structure were demonstrated to show anti-H. pylori properties in vitro [91]. However, to be success in vivo, the essential requirements of AMPs applications includes having long duration staying in stomach while being relatively stable and remaining their antimicrobial potency in gastric environment. An example is a antimicrobial peptide TP4 displayed potent antimicrobial activity against H. pylori strains both in vitro and in vivo while high doses did not cause any detected toxicity in mice or rabbits [92]. In addition, Zhang and colleagues suggested that manganin was also a potent antimicrobial peptides against H. pylori and it became more effective when administrated in the nanoparticles form [93].
3.4.2. Intestinal infection
Human intestines are frequently exposed to the threats of various pathogenic microorganisms. Thus, along with enzymes and friendly bacteria, AMPs are commonly produced in intestinal mucosa as an important part of intestinal barriers. Therefore, the balance between intestinal microbes and host AMPs keeps the intestinal tract healthy [94].
Recently, some studies suggest that disruption of the gut microbiota by regular antibiotic treatment can weaken mucosal barrier and lead to invade of pathogens and intestinal inflammation [95]. Moreover, antibiotic administered in early life may cause long-term consequences on immune response as well as metabolic homeostasis and gut microbiota composition [96,97]. Those problems together with the widespread problem of bacterial resistance lead to an urgent need for more effective and safe antimicrobial agents.
Several efforts for antimicrobial peptide development are in progress, for instance, coprisin – an natural peptide derived from Korean dung beetle were demonstrated to prevent inflammation as well as mucosal damage in Clostridium difficile infection [98]. Besides, cathelicidin-WA was introduced to inhibit inflammation while enhance intestinal barrier function in weaned piglets and mice, which open a potential therapy for the treatment of diarrhea and other intestinal diseases [16].
It should be noted that one of the big challenges for oral application is the low stability of AMPs in gastric acid and various protease in gastrointestinal system. However, it could be solved by many strategies, such as chemical modifications including non-natural amino acids incorporation, cyclic peptides or pseudo-peptides [99]; nano delivery systems [100,101] or AMP-antibiotic combinations [102]. Furthermore, AMPs can also be delivered to the site of intestinal infection by engineering the probiotic bacteria [103].
3.5. Bone and joint infection
Bone infections (also called osteomyelitis) can lead to many consequences such as protracted infected lesions, necrotic bone formation, implant loosening and surgical failure, which usually prolong and require and enormous treatment efforts [104]. As over 1.5 million total hip and knee replacement (TKR) procedures performed annually in the United States, bone infection became the most severe and devastating risk related to orthopedic implants [105,106]. Furthermore, the annual treatment cost in the US for prosthetic joint infection is estimated to exceed $1.62 billion by 2020 [107]. Regarding to all orthopedic subspecialties, the incidences of infection range from 0.1% to 30% with a cost of $17,000–$150,000 per patient as reported by the 2018 International Consensus Meeting on Musculoskeletal Infection [108].
In fact, local or systemic antibiotics are popular for removal surgery and the treatment of bone-related infections [[109], [110], [111]]. Nonetheless, it not only results in increased emergence of antibiotic resistance [111] but also induces osteolysis, for instance, gentamicin inhibits osteoblast viability or vancomycin prevents osteoblast proliferation at certain concentrations [112]. Thus, the discovery of new antimicrobial agents to cure infection while improves or at least be harmless to osseointegration should be clinically significant. Therefore, antimicrobial peptides are potential for joint and bone infection treatment, which is commonly complicated because of the resistance to the classical antibiotics and the formation of bacteria biofilm.
Bormann et al. reported high antimicrobial potency of some AMPs against bacteria both incorporated in a biofilm or internalized into cells with no harm to human osteoblasts [113]. Furthermore, Hui Li et al. demonstrated that KR-12 - a fragment of human antimicrobial peptide cathelicidin LL-37 improves the osteogenic differentiation of human bone marrow mesenchymal stem cells via stimulating BMP/SMAD signaling [114]. Interestingly, Pavel Melicherˇcík et al. reported that some antimicrobial peptides had lower minimum inhibitory concentrations (MICs) value than the two traditional antibiotics vancomycin and gentamincin but was found to be significantly more effective than those antibiotics when separately loaded into the calcium phosphate cement. Moreover, AMP-loaded poly(methyl methacrylate)-based bone cement can also prevent microbial adhesion as well as subsequent formation of biofilm on their surface as compared to vancomycin and control loaded cement [60].
Regarding toxicity, Pavel and colleagues suggested no signs of local or systemic side effects in laboratory rats has found since their infected femurs were treated with antimicrobial peptide [59]. Although these studies demonstrated the multiple benefits of AMPs for bone and implant-related infections, still there are several issues needed to examine, including the permeability of AMPs into the surrounding bone tissues; the stability and efficacy of AMPs in bone environment as well as carrier materials; the long-term effect on osteoblast proliferation.
3.6. Oral diseases
Antimicrobial peptides produced by gingival epithelial cells contribute the first line of defense system against wide range of oral pathogens. The importance of AMPs were reported in many reports such as type 1 diabetes patients having single-nucleotide polymorphisms (SNPs) in genes encoding HBD-1 are associated with high rates of Candida albicans carriage [115]. Moreover, patients with deficient cathelicidin peptide (LL-37) and human neutrophil peptides generally experience oral infections and periodontitis (severe periodontal diseases) [116]. The concentrations of human neutrophil peptides 1 to 3 in children saliva without caries are much higher than those with caries [115]. These interesting findings suggest that normal amount of antimicrobial peptide concentrations can control the growth of oral bacteria and fungi, thus preventing caries and other types of infections.
Dental caries (also known as tooth decay or cavities) is one of the most common global chronic diseases [117]. Several therapies have been developed for preventing tooth decay, however, all current anti-caries approaches have their disadvantages. For instance, sodium fluoride toothpaste - the most popular one is nonselective intervention [118]. Silver diamine fluoride (SDF) option is simple, non-invasive with inexpensive equipment and low risk of spreading infection. However, SDF treatment is not a complete therapy for caries risk and its drawbacks include unpleasant metallic taste, mucosal and gingival irritation as well as the black staining of the tooth surfaces [119].
Most of cavities are caused by Streptococcus mutans, a Gram-positive bacteria produces lactic acid that responsible to reduce the microenvironment pH, which leading to teeth damage through the erosion of enamel. For the treatment strategy, C16G2 - a Specifically Targeted Antimicrobial Peptide (STAMP) have demonstrated to be effective in preventing S.mutans while showing no side effect to other noncariogenic oral streptococci and human host cells. In fact, C16G2 is currently in phase II clinical study for mouth wash, which encourage the attempts to develop novel selective cariogenic inhibitors for tooth decay control [120,121]. Besides, pH-responsive antimicrobial peptides is another great approach could be considered [122,123].
Oral mucositis and candidiasis are common complications in patients receiving chemotherapy and radiation treatment. Lesions of oral infection are not only painful, significantly affect nutritional support as well as oral hygiene but also increase risk of local and systemic infection [124]. In fact, there is no drug approved by FDA for preventing or treating radiation and chemotherapy-related oral mucositis. A topical antimicrobial peptide called Iseganan originally showed promising in reducing mucositis symptoms but failed to show efficacy at phase III clinical study [125,126].
Oral candiasis frequently caused by Candida albicans fungus can be a serious problem for those wearing dentures [127] or with a weakened immune system, for examples children, elderly people and patients taking steroids, radiation treatment, having AIDS or having type 1 diabetes [[128], [129], [130]]. Since oral antifungal drugs have many possible undesirable side effects including mouth irritation, diarrhea, nausea or even more serious such as irregular heart rhythm, live failure, etc., topical application of AMPs holds great opportunities as novel oral diseases treatment. Until now, the mouth rinse product containing antimicrobial peptide PAC113 from Pacgen Biopharmaceuticals just passed phase II clinical trial for the treatment of oral candidiasis in HIV patients [126,131].
4. AMPs – a new potential candidate for cancer treatment
Structurally, cancer and normal cells are distinguished by a number of differences including greater negative charge membrane of malignant cells due to presenting anionic molecules like phospholipid phosphatidylserine (PS), O-glycosylated mucins, sialylated gangliosides and heparin sulfate. Moreover, the cancer cell membranes are more fluid than common mammalian cells. Those featured trait opens a big window for anticancer peptides can selectively and effectively act on tumor cell membranes resulting in rapid disruption, pore formation, ion channel modification and permeation [132]. Despite of the diversity of tertiary structures such as α-helical, β-sheet or combination, most of anticancer peptides share a couples of common properties including positively charged, amphipathic structure [133]. The interaction of cationic anticancer peptides and certain lipids like plasma membrane phospholipid phosphatidylinositol 4,5-bisphosphate is reported to explain the mechanism behind the selective anti-cancer therapy of NaD1—isolated from the ornamental tobacco Nicotiana alata. This binding quickly induces blebbing of tumor cells which causes the death afterward [134]. Polybia-MP1 was also well studied for the interaction with PS on the membrane and PS was found to impact on the lytic properties of MP1 [135]. In another study, it is suggested that MP1 could form the pore on the cell membrane by the support of combination of PS and phosphatidylethanolamine (PE) lipids [136] which have a mutual relationship and together alter the membrane of cancer cells [137]. The investigation of AMP and cell membrane are supported effectively by studying the interaction between AMP and artificial lipid bilayers vesicles with variety of lipid compositions. Those studies suggested a lot of insights the real scenarios to develop AMPs as anticancer agents [[138], [139], [140]].
On the other way of approach, AMPs are able to internalize the cells and interact with intracellular compartments such as mitochondria and induce cell apoptosis [141]. Peptide m2386 from a lactic acid bacterium Lactobacillus casei ATCC334, induced the expression of Fas and TRAILR1 death receptors on the cell surface of treated SW480 cells and also kill cancer cells via the programmed cell death pathway [142]. The apoptosis was also enhanced in cancer cells after exposure to Parasporin, a non-insecticidal crystalline protein of Bacillus thuringiensis [143], Hexokinase II - derived cell-penetrating peptide [144] and more.
LTX-315 - a cationic antimicrobial peptide with oncolytic properties is even found to not only kill the cancer cells but also reprogram the tumor microenvironment with a decrease of immunosuppressive Tregs and myeloid-derived suppressor cells and increase frequency of polyfunctional T helper type 1/type 1 cytotoxic T cells which is very promising for anti-cancer immunotherapy [145]. Immunotherapy for cancer treatment is getting closer to the clinical practice in which AMPs are also gaining their role as an important component of evolutionary biology [146,147].
5. Approaches to improve biological activities of AMPs
5.1. Chemical approaches
5.1.1. Common structural requirements of AMPs
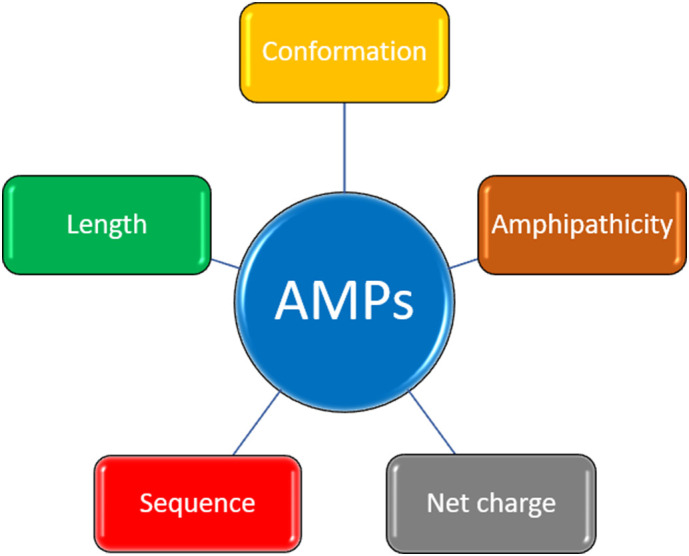
The feature of an AMPs is demonstrated in Fig. 2 . The most common conformation of AMPs is alpha helix structure [18,56], which is stabilized by three mains factors: the hydrogen bonding interaction between >N-H and > C=O, the hydrophobic interactions and the electrostatic interaction from the side chains. In fact, most antimicrobial peptides form a random coil structure in aqueous solutions but turn into an alpha helical conformation in membrane mimicking solutions, positioning the hydrophobic residues on one side of the helix and hydrophilic residues on the other side. Several wide-known antimicrobial peptides possessing alpha-helical structure including LL37, Esculentin-2, Magainins, Polybia-MP1 [8,13,56,148]. Beta sheet is characterized by the presence of an antiparallel beta-sheet, mostly stabilized by disulfide bonds. Some β-sheet antimicrobial peptides: Tachyplesins, Protegrins, Bactenecin [9,149,150]. Linear/extended peptides lack classical secondary structures, generally due to their high content of proline and/or glycine [[151], [152], [153]]. In fact, natural AMPs can have more than one type of secondary structure, for example Human beta defensins (HBDs) contain a combination of alpha helices and β-sheet [12,56,57].
Fig. 2.
Several structural aspects in antimicrobial peptides (AMPs).
As the most common reported mechanism of actions of AMPs is membrane-lysis, the peptide sequences need to be long enough to span or insert into the hydrophobic layer of the membrane. Moreover, the lengths of AMPs are also important to maintain the required conformation and generally longer chain is better for forming secondary structure. Nonetheless, long sequence also means there are a certain percentage of dominant residues and higher cost of production. In fact, some studies suggested that the derived short version of many natural AMPs can have more potent antimicrobial activity while showing lower hemolysis effect [[154], [155], [156], [157]].
Positively charge is a common property of AMPs provided by cationic amino acids such as Lysine, Arginine or the amino group at N-terminus, which also is a component of the hydrophilicity part in AMPs [158,159]. The net positive charge and the distribution of charged residues is important for the initial binding step as well as the selectivity with the negatively charged membrane of bacteria species [160], thus, too high cationic density can cause the reversed effects: less potent antimicrobial activity but more hemolytic activity [161]. In fact, most of the natural AMPs have the net charge from +2 to +10 [9] and some studies suggested the optimal net charge should depend on the length of sequence [[162], [163], [164]].
Hydrophobicity is crucial for the interaction with the hydrophobic layer in bacteria membrane. Therefore, too low may result in weak bacteria membrane interaction and less permeability effect, however, highly hydrophobicity can induce hemolysis - one of the most common side effect [165,166]. Thus, the percentage of hydrophobic residues is normally in the range of 40–60%. Furthermore, the higher hydrophilicity of AMPs can help reduce the hemolytic activities, however it may prevent the hydrophobic interaction with bacteria membranes. In fact, the killing activity and safety of AMPs depends on the balance hydrophobicity-hydrophilicity, especially to Gram negative species with thick and more complicated membrane components than Gram positive species. The optimal ratio between the two properties (hydrophobicity and hydrophilicity) can maximize the antimicrobial benefit while minimize the adverse side effect on human red blood cells [161].
There are several amino acids that have been extensively studied due to its striking impact on the structure or activity of antimicrobial peptides. Proline and Glycine provides more flexibility for the peptide structure, which can help induce the hemolytic activity [151,163]. Tryptophan (W) is demonstrated to facilitate the interaction with bacteria membrane when it was put in the interface between hydrophobic face and hydrophilic face of the amphipathic helices [151,161,167]. Cysteine - an amino acid contains sulfur in its side chain which is strongly reactive. It could easily be oxidized to form a dimer, therefore generating a disulfide bond between two cysteines. These disulfide bridges are strongly hydrophobic and important for the structures of many natural antimicrobial peptides [168,169]. It also can improve the resistance toward proteolytic degradation [170].
5.1.2. Chemical modifications
5.1.2.1. D-enantiomer
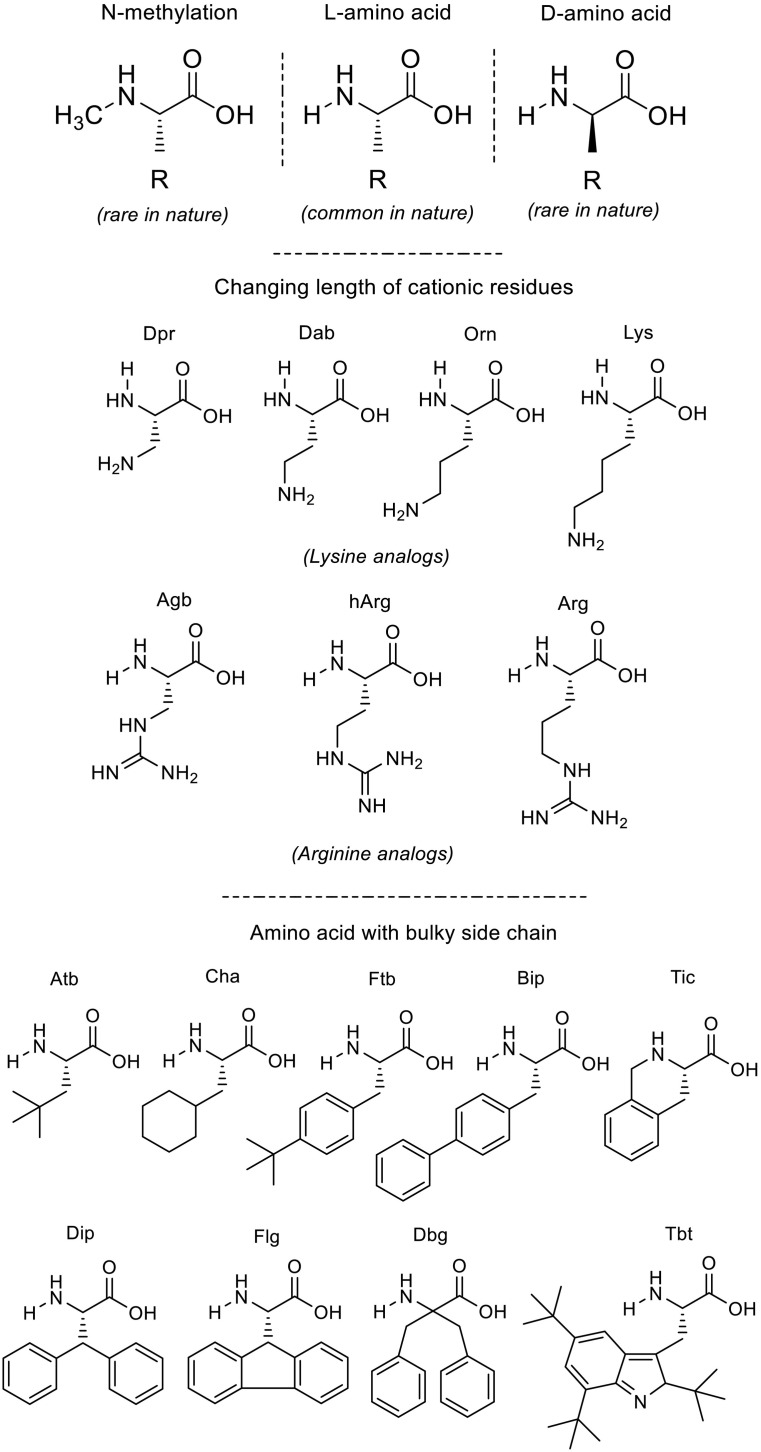
Linear peptides are often suffered from the protease degradation which is secreted by human, bacteria or fungi. Therefore, the most advantage of using D-amino acids is to significantly improve peptide stability against protease digestion, which can increase the retention of antimicrobial activity. Thus, in some cases, it promoted the MICs [171,172]. Nonetheless, it should be noted that the cost of production is major disadvantages of this strategy. This type of modification is illustrated in the top of Fig. 3 .
Fig. 3.
Examples of chemical strategy in generating non-canonical amino acids.
5.1.2.2. Modification of side-chain length
Modify the length of side-chain is another common strategy for cationic residues that improves the susceptibility to proteolysis, especially against trypsin digestion [173,174]. However, in term of the antimicrobial activity, this type of modification seem not to influence much on the potency [175]. Examples of Lysine and Arginine and their analogs having different side-chain lengths are shown in the middle of Fig. 3.
5.1.2.3. N-methylation residues
Though this strategy can help enhance the stability against protease and slightly increase hydrophobicity, their application on antimicrobial peptides until now still rare [176].
5.1.2.4. Bulky residues
Incorporation of non-genetically coded amino acids opens the opportunity for the development of extremely short active peptides with improved pharmaceutical properties [177,178]. Examples are displayed in the bottom of Fig. 3.
5.1.2.5. Terminal capping
Usually, the acetylation of N-terminal would increase the helical content and peptide stability, however, it also reduces the total net positive charge of AMPs. Therefore, though this modification showed effect on higher perturbation in the case of mastoparan-like peptide [179], it is not always good for biological activity as demonstrated in the case of stapled peptides [161,180]. Regarding C-terminal, amidation of its carboxyl group was proven to be a good strategy to prevent metabolic degradation and improve antimicrobial potency [6,[181], [182], [183]].
5.1.2.6. Cross-link containing peptides
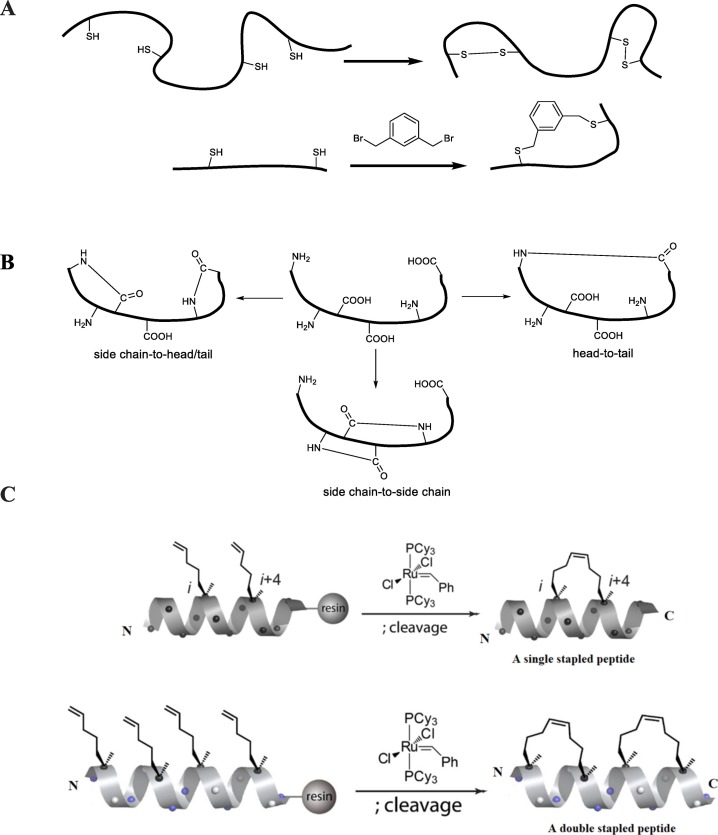
Crosslinks are generally used for making macrocyclic peptides or stabilizing peptide secondary structures. Peptide cyclization with limited conformational flexibility not only provides large surface area, which could enhance membrane-binding affinity but also prevents protease degradation. Until now, there are three common types of cross-linking construction: All-hydrocarbon stapling system [184,185], disulfide bonds and its modification [[186], [187], [188], [189]] and lactam-bridge [[190], [191], [192]] (Fig. 4 ). Among them, thiol and disulfide groups are usually highly chemically reactive which makes it sensitive to oxidation and aggregation [193] while lactam-bridge strategy is not stable to metabolic degradation [194]. Recently, all-hydrocarbon stapling system has been attracting lots of attentions since it has introduced to effectively stabilize the alpha-helical structure and serve as a part of hydrophobic component. This strategy can improve the antimicrobial activity, stability against trypsin while the undesirable side effect on human red blood cells could be manipulated by sequence modifications [162,185,195]. In more details, this system contains alpha-methyl, alpha-n-alkenyl amino acids and then the macrocyclic hydrocarbon cross-link was formed by ruthenium-mediated ring-closing metathesis (RCM) on the resin-bound [196].
Fig. 4.
Examples of cyclization systems. (A) Disulfide bonds and its mimics, (B) Lactam bridges, (C) All-hydrocarbon stapling systems.
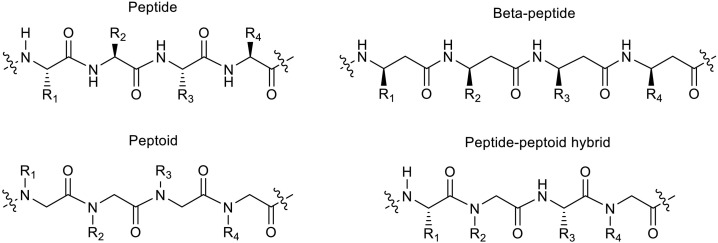
5.1.2.7. Pseudo-peptides
Peptidomimetics generally mimic the structure, activity and selectivity of antimicrobial peptides while improves plasma half-life. The most popular pseudo-peptide forms in this category is peptoids and beta-peptide [[197], [198], [199], [200], [201]] (Fig. 5 ). Another promising approach in peptidomimetics is merged structures of native peptides and pseudo-peptides which results in hybrid structures [202,203].
Fig. 5.
Some pseudo-peptide structures for development of antimicrobial agents.
5.2. Delivery systems
The potential of AMP triggers a huge attention, biologically. However, the way to bring those AMPs from bench to bed still requires much more effort, firstly, how drugs are administered to patients. In general, AMPs like other peptides are undergone a couple of challenges including low permeability due to large molecular size, short residence time at the absorption site and quick degradation due to variety of enzymes or body fluids [204]. In the context of this section, we discuss about the obstacles by individual administration routes and respective solutions, up to date, to enhance the bioavailability of AMPs.
5.2.1. Systemic absorption
5.2.1.1. Transdermal delivery
Developed since long time ago, transdermal benefits patients by the painless administration, ease of termination, also the sustained release, and avoidance of first-pass metabolism regarding the substance [205]. However, being as the first and most important barrier to protect body, human skin turns out to be the challenges for this administration route. The outmost layer of the skin -stratum corneum consisting of non-living cells, corneocytes and multiple lipid bilayers restricts the diffusion of drugs. Following that, before being absorpt to blood circulation, the drugs need to overcome the difficulties in epidermis - the layer lacking blood vessels, as well [206]. The approaches to advance transdermal administration are divided into two categories: enhancing drug diffusion through the intact skin and physically disrupt and bypass the barrier [207].
5.2.1.2. Oral delivery
Oral delivery offers patients the most convenient in usage, thus being the most widely used route. The development of delivery technology is able to facilitate the oral administration of AMPs, but it still needs to be improved to overcome challenges. Firstly, AMPs are the big molecules which restrains them being transported across the GI tract walls [204]. Another practical barrier is the intestinal mucus, above the intestinal epithelial, containing the glycosylated proteins forming a steric shield to limit molecular diffusion or epithelium interaction of biologics [208]. Last but not least, the assemblage of bile salts, gastric acids and proteases at different stages even can degrade or structurally disturb biologics resulting in eliminating their function before the absorption [209]. To address those difficulties, the advantages of delivery technology are able to control over dissolution rate, residence time; preserve molecules from chemical hazards; enhance the absorbance using absorption and permeation enhancers [205]. A couple of advanced systems is also developed such as nanoparticles, hydrogels, mucoadhesive matrixes [210]. On the other hand, medical device types like microneedles or intestinal patches offer biologics ability to bypass those challenges [211,212].
5.2.1.3. Inhalation delivery
The large surface area and widely distributed vascularization in the lungs offer a rapid systemic absorption for biologics via inhalation route. In order to reach to the lung, the formulations should be delivered deep into lung which required a crucial optimization of size, density, shape and other physical properties [213]. Following by that, the biologics will interact with pulmonary surfactant containing lipid and protein secreted by type II alveolar cells. In addition, the presence of enzymes and mucus layer also can dampen out the biologics resulting in limited absorption [214]. A couple of strategies could be approached including mucopenetrative, mucoadhensive methods permeation enhancers, cell-penetrating peptides or improving particles properties [215].
5.2.1.4. Subcutaneous delivery
Still maintaining a high bioavailability of substance but improving the acceptance and compliance of patients comparing to intravenous route, subcutaneous delivery is attracting the attention in biological delivery system development. The main challenge of this administration route is penetrating through extracellular matrix where physiological components like protein, antibody, immune cells may interact and aggregated active molecules. This way of drug administration is also limited by the injected volume [216]. The development of polymeric particles advances the delivery by controlling release, physically separating the biologic from the subcutaneous microenvironment and improved dispersion using hyaluronidases. The other stimuli-responsive like glucose, pH or target systems also improve the diffusion and absorption of biologics [217].
5.2.2. Topical application
Generally, compared to systemic absorption, topical administration can minimize systematically adverse such as hemolytic or allergy while less prone to be degraded by enzymes. In fact, most of AMPs in the clinical phase until now are prepared in topical applications, especially for oral and skin disease indications. This route of administration in many cases required the effective penetration of antimicrobial peptides into the skin. As AMPs mostly consist of long sequences which limits its large molecules to be absorbed, several strategies have been applied to improve the bioavailability, for examples antimicrobial hydrogels [218], lipid-based nanocarriers [219], poly(lactic-co-glycolic acid (PLGA) nanoparticles [220] or gold/silver nanoparticles conjugation [[221], [222], [223]]. In addition, the importance of delivery formulation can be shown in protecting AMPs from degradation or maintaining AMPs activity in such conditions as high salt concentration, low pH or dryness. Furthermore, not only the penetration ability but also the released kinetic from topical formulation to the surrounding area need to be assessed.
6. AMPs and other health care applications
6.1. AMPs in the development of medical devices
The medical devices are becoming a hall-mark in modern health care systems with varieties of types such as implantable devices, artificial heart valves, catheters [224] (Table 1 ). Over the past century, the more popular of this artificial aid is, the more risk is increased. Most of those interacts with one or some opened wounds of human body that is threaten by infection during treatment. Staphylococcus aureus and Staphylococcus epidermidis are the most common causative microorganisms that were found in biomaterial-associated infections (BAI) [225]. Those bacteria strains are able to develop biofilms on the surface of devices, and/or form tissue colonizations then possibly promote antimicrobial resistance to dampen the efficacy of antibiotic treatment [226].
Table 1.
Applications of antimicrobial peptides on medical devices development.
| AMPs | Surface/Devices | Antimicrobial activity | Ref. |
|---|---|---|---|
| Esc(1–21) | Contact lens | Disrupt the bacterial biofilm Reduce the number of bacterial cells within 20 min and bacterial adhesion to the CL surface (77%–97% reduction) in 24 h |
[237] |
| RRWRIVVIRVRRC | Catheter | The antimicrobial peptide coating prevented both Gram-positive and Gram-negative bacterial adhesion by up to 99.9% and inhibited planktonic bacterial growth by up to 70%. In vivo, the peptide coating was tested in a mouse urinary catheter infection model. |
[238] |
| Indolicidin | Gold nanoparticles | Prevent Candida albicans to form biofilms and impairs preformed mature biofilms | [239] |
| GL13K | TiO2 nanotubes | Kill both F. nucleatum and P. gingivalis | [240] |
| FK-16 | Titanium surface | Anti-adhesion and biofilm inhibition capabilities against both S. aureus and E. coli. | [241] |
| OP-145 | Polymer-Lipid Encapsulation MatriX | Effective in killing S. aureus and inhibiting biofilm | [242] |
| KYE28 | Poly(ethyl acrylate-co-methacrylic acid) microgels | Anti-inflammatory effects on human monocytes Potent antifouling properties |
[243] |
In the detail, bacteria tend to attach to the surface of medical devices then proliferate and create an extracellular matrix containing bacteria, bacterial exopolysaccharides, proteins, extracellular DNA, and host proteins [227]. This complex matrix restrains antibiotic drugs from interaction with bacteria and attenuate their activity to target pathogen. In another approach, bacteria invade surrounding tissue and form bacterial colonization which is known as small colony variants. Those small “outbreak” gives more troubles for antimicrobial compounds [228]. Last but not least, along with the abuse of antimicrobials, many strains were found to resistant with one or several drugs. Interestingly, those bacteria are evolved frequently in the hospital environment where all of devices-based interventions are taken place [229].
AMPs are expected to be candidates which can harmonize the implant area by interact with pathogen membranes inducing depolarization, destabilization, and/or disruption of the bacterial plasma membrane [230]. Furthermore, AMPs are also known to manipulate immune system which indirectly eradicate pathogens [231] or escalate wound healing [232], angiogenesis process [233]. By using AMPs, the medical devices are expected to be protected via three different mechanisms [234]. Firstly, the surface of devices is modifying by charge, hydrophilicity using hydrophilic polymers or conjugate with polymer chains to form antifouling surfaces [234,235]. In addition, AMPs are decorated on surfaces of medical devices to kill bacteria directly [235]. However, this approach may be limited because those AMPs can only inhibit replication of nearby bacteria and leave tissue-related issue unsolved. This drawback could be addressed by release systems by which the amount of antimicrobials is maintained and delivered to surrounding area at sufficient concentration to clean up any residual bacteria [236].
6.2. AMPs as new cosmetic ingredients
AMPs as an essential part in maintaining a healthy skin hold promising potential in the development of new cosmetics. Some beneficial features of AMPs for cosmetic application include (i) clearance of skin pathogens through direct killing action and indirect immune modulatory, which is important for many skin problems, for examples, acne management due to inhibition of Propionibacterium acnes and inflammation control; (ii) wound healing promotion through multiple means covering cell proliferation, cell migration, angiogenesis, etc. [54,[244], [245], [246]]; (iii) anti-fibrogenic effect [[247], [248], [249]], interestingly, some reports suggested that AMPs can suppress the excessive collagen synthesis following injury, thus indicated the excellent benefit for recovering wound without scarring [68]; (iv) oxidative stress management [250,251]. Among those desirable characteristics, the most frequent is the improvement of tissue repair, followed by cell proliferation and migration, angiogenesis, anti-inflammation and other characteristics [4]. Recently, novel AMPs with multiples interested properties for cosmetic application can be mentioned as LL-37, temporin-1TL, Esculentin-1a, Psoriasin (S100a7), LLKKK18, SR-0397 [4]. However, though possessing various advantages, application of AMPs in cosmetic industry is still rare, which mainly because of high production cost, low stability and poor understanding in mechanism of action.
7. Conclusions and future perspectives
Infection and cancer remain to be great challenges of human being. The race to an effective therapy is limited by the quick alternation of viruses, bacterium and cancer cells resulting in drug resistance. In this review, we summarized the knowledge about AMPs serving as the base for modification toward better efficacy in treatment.
The potential of AMPs in regulating immune system is expanding. Playing a key role in natural evolution, AMPs might be an important breakthrough in which AMPs are acting for direct therapies or indirect via immune-modulation. For example, a cathelicidin antimicrobial peptide D-CATH2–2 has been proven to modulate the immune systems and therefore partially support to the protection of newborn chickens later against E. coli infection challenge without any sign of harmful strong inflammatory response [252]. Thus, AMPs should be in call for a new paradigm of infection and cancer treatment and prevention, exploiting immune system.
Until now various chemical adjustment has been introduced to make AMPs shorter and promote its biological activities and stability, mostly. Besides, the mentioned delivery systems, on one hand, help to protect AMPs from hazardous environment, on the other hand, elevate the activity of them. In fact, though some synthetic peptides showed relatively stable and potent in several proposed conditions such as trypsin digestion or presence of salt and human serum [161,253,254], it should be noted that there are still various proteases and other components (salt, DNA, pH, mucins, etc.) in body fluids that can effect on the stability and activity of peptides. Of note, many AMPs displayed promising in vitro, however unable to show activity in vivo [73,255,256]. Therefore, the ability of AMPs that maintain its specificity as well as antimicrobial potential in real conditions critical point or the success of peptides.
Since conventional antibiotics are not only suffering from the threat of antimicrobial resistant but also being reported to cause long-lasting side effects such as multisystem toxicity syndromes by quinolone and fluoroquinolone antibiotics [96,97,257,258], AMPs were one of the most promising candidates for development of new alternative therapy. Some studies has reported the potential of antimicrobial peptides in combination with others antimicrobial peptides or conventional therapy [259]. Such a synergistic combination can help to increase the potency while reduce the therapeutic dose, side effects and the threats of drug resistance. In term of safety, many studies in vivo suggested almost no toxicity in laboratory mice at the low levels of AMPs. This combination approach, could avoid the usage of high AMP levels that may cause side effects such as hemolysis, inflammation with uncontrolled immune cell recruitment or unintentionally binding.
However, many challenges have been preventing the clinical application of AMPs, such as poor “absorption, distribution, metabolism, excretion”, bulky size, sensitive to the ambient conditions. Consequently, many AMPs were failed or denied approval in Phase III clinical trials, such as Pexiganan (MSI-78) for treatment of impetigo and diabetic foot ulcers, Iseganan (IB-367) for mouth rinse to prevent infection in patient receiving chemotherapy, Locilex (22-amino acid magainin) for diabetic foot ulcers, Omiganan for catheter infection. Several failures in the past have not discouraged efforts to put this product line into practical use. In contract, more and more AMPs are being tested with expanding list of potential applications for both human and animals [[260], [261], [262], [263]]. Therefore, it is very important to develop chemical modifications, bioengineering strategies and combination approaches which are strongly needed to improve the pharmaceutical properties of potential AMPs.
Overall, we believe with new advances in finding solutions that improve AMPs design as well as peptide production technologies, AMPs will provide a strong impact to the medical practices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Phenikaa University Foundation for Science and Technology Development.
References
- 1.Mookherjee N., Anderson M.A., Haagsman H.P., Davidson D.J. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Disc. 2020;19:311–332. doi: 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- 2.Magana M, Pushpanathan M, Santos AL, Leanse L, Fernandez M, Ioannidis A, et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Diseas. [DOI] [PubMed]
- 3.Pfalzgraff A., Brandenburg K., Weindl G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018;9:281. doi: 10.3389/fphar.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alencar-Silva T., Braga M.C., Santana G.O.S., Saldanha-Araujo F., Pogue R., Dias S.C., et al. Breaking the frontiers of cosmetology with antimicrobial peptides. Biotechnol. Adv. 2018;36:2019–2031. doi: 10.1016/j.biotechadv.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 5.da Silva A.M.B., Silva-Gonçalves L.C., Oliveira F.A., Arcisio-Miranda M. Pro-necrotic activity of cationic mastoparan peptides in human glioblastoma multiforme cells via membranolytic action. Mol. Neurobiol. 2018;55:5490–5504. doi: 10.1007/s12035-017-0782-1. [DOI] [PubMed] [Google Scholar]
- 6.Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa F., Teixeira C., Gomes P., Martins M.C.L. In: Antimicrobial Peptides: Basics for Clinical Application. Matsuzaki K., editor. Springer Singapore; Singapore: 2019. Clinical application of AMPs; pp. 281–298. [Google Scholar]
- 8.Pham T.K., Kim D.-H., Lee B.-J., Kim Y.-W. Truncated and constrained helical analogs of antimicrobial esculentin-2EM. Bioorg. Med. Chem. Lett. 2013;23:6717–6720. doi: 10.1016/j.bmcl.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Kang X., Dong F., Shi C., Liu S., Sun J., Chen J., et al. DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci. Data. 2019;6:148. doi: 10.1038/s41597-019-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S., Bao J., Lao X., Zheng H. Novel 3D structure based model for activity prediction and design of antimicrobial peptides. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S., Fan L., Sun J., Lao X., Zheng H. Computational resources and tools for antimicrobial peptides. J. Pept. Sci. 2017;23:4–12. doi: 10.1002/psc.2947. [DOI] [PubMed] [Google Scholar]
- 12.Fan L., Sun J., Zhou M., Zhou J., Lao X., Zheng H., et al. DRAMP: a comprehensive data repository of antimicrobial peptides. Sci. Rep. 2016;6 doi: 10.1038/srep24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mourtada R., Herce H.D., Yin D.J., Moroco J.A., Wales T.E., Engen J.R., et al. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 2019;37:1186–1197. doi: 10.1038/s41587-019-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordström R., Malmsten M. Delivery systems for antimicrobial peptides. Adv. Colloid Interf. Sci. 2017;242:17–34. doi: 10.1016/j.cis.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Kazemzadeh-Narbat M., Lai B.F.L., Ding C., Kizhakkedathu J.N., Hancock R.E.W., Wang R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials. 2013;34:5969–5977. doi: 10.1016/j.biomaterials.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Yi H., Zhang L., Gan Z., Xiong H., Yu C., Du H., et al. High therapeutic efficacy of cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 2016;6 doi: 10.1038/srep25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shai Y. Mode of action of membrane active antimicrobial peptides. Pept. Sci. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y., Huang J., Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein & Cell. 2010;1:143–152. doi: 10.1007/s13238-010-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Z., Vasil A.I., Hale J.D., Hancock R.E.W., Vasil M.L., Hodges R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Pept. Sci. 2008;90:369–383. doi: 10.1002/bip.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahlapuu M., Håkansson J., Ringstad L., Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen L.T., Haney E.F., Vogel H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trend. Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Yan J., Wang K., Dang W., Chen R., Xie J., Zhang B., et al. Two hits are better than one: membrane-active and DNA binding-related double-action mechanism of NK-18, a novel antimicrobial peptide derived from mammalian NK-Lysin. Antimicrob. Agent. Chemother. 2013;57:220–228. doi: 10.1128/AAC.01619-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park C.B., Kim H.S., Kim S.C. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 1998;244:253–257. doi: 10.1006/bbrc.1998.8159. [DOI] [PubMed] [Google Scholar]
- 24.Lee B., Hwang J.S., Lee D.G. Antibacterial action of lactoferricin B like peptide against Escherichia coli: reactive oxygen species-induced apoptosis-like death. J. Appl. Microbiol. 2020;129(2):287–295. doi: 10.1111/jam.14632. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Koh J.-J., Liu S., Lakshminarayanan R., Verma C.S., Beuerman R.W. Membrane active antimicrobial peptides: translating mechanistic insights to design. Front. Neurosci. 2017;11 doi: 10.3389/fnins.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raheem N., Straus S.K. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front. Microbiol. 2019;10:2866. doi: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazzaro B.P., Zasloff M., Rolff J. Antimicrobial peptides: application informed by evolution. Science. 2020;368 doi: 10.1126/science.aau5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam J., Yun H., Rajasekaran G., Kumar S.D., Kim J.I., Min H.J., et al. Structural and functional assessment of mBjAMP1, an antimicrobial peptide from Branchiostoma japonicum, revealed a novel α-hairpinin-like scaffold with membrane permeable and DNA binding activity. J. Med. Chem. 2018;61:11101–11113. doi: 10.1021/acs.jmedchem.8b01135. [DOI] [PubMed] [Google Scholar]
- 29.Irazazabal L.N., Porto W.F., Fensterseifer I.C.M., Alves E.S.F., Matos C.O., Menezes A.C.S., et al. Fast and potent bactericidal membrane lytic activity of PaDBS1R1, a novel cationic antimicrobial peptide. Biochim. Biophys. Acta Biomembr. 2019;1861:178–190. doi: 10.1016/j.bbamem.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Hu F., Wu Q., Song S., She R., Zhao Y., Yang Y., et al. Antimicrobial activity and safety evaluation of peptides isolated from the hemoglobin of chickens. BMC Microbiol. 2016;16:287. doi: 10.1186/s12866-016-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L.-j., Gallo R.L. Antimicrobial peptides. Curr. Biol. 2016;26 doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Hancock R.E.W., Sahl H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 33.Mookherjee N., Brown K.L., Bowdish D.M.E., Doria S., Falsafi R., Hokamp K., et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 34.Brandenburg K., Heinbockel L., Correa W., Lohner K. Peptides with dual mode of action: killing bacteria and preventing endotoxin-induced sepsis. Biochim. Biophys. Acta Biomembr. 2016;1858:971–979. doi: 10.1016/j.bbamem.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Yasin B., Wang W., Pang M., Cheshenko N., Hong T., Waring A.J., et al. θ Defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 2004;78:5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krepstakies M., Lucifora J., Nagel C.-H., Zeisel M.B., Holstermann B., Hohenberg H., et al. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J. Infect. Dis. 2012;205:1654–1664. doi: 10.1093/infdis/jis273. [DOI] [PubMed] [Google Scholar]
- 37.Sinha S., Cheshenko N., Lehrer R.I., Herold B.C. NP-1, a rabbit α-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 2003;47:494–500. doi: 10.1128/AAC.47.2.494-500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elnagdy S., AlKhazindar M. The potential of antimicrobial peptides as an antiviral therapy against COVID-19. ACS Pharmacol. Transl. Sci. 2020;3(4):780–782. doi: 10.1021/acsptsci.0c00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East respiratory syndrome (MERS): a review. J. Infect. Public Health. 2018;11:9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohan K.V.K., Rao S.S., Atreya C.D. Antiviral activity of selected antimicrobial peptides against vaccinia virus. Antivir. Res. 2010;86:306–311. doi: 10.1016/j.antiviral.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matejuk A., Leng Q., Begum M.D., Woodle M.C., Scaria P., Chou S.T., et al. Peptide-based antifungal therapies against emerging infections. Drugs Future. 2010;35:197. doi: 10.1358/dof.2010.035.03.1452077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morton C.O., Hayes A., Wilson M., Rash B.M., Oliver S.G., Coote P. Global phenotype screening and transcript analysis outlines the inhibitory mode(s) of action of two amphibian-derived, α-helical, cationic peptides on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2007;51:3948–3959. doi: 10.1128/AAC.01007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helmerhorst EJ, Breeuwer P, van't Hof W, Walgreen-Weterings E, Oomen LCJM, Veerman ECI, et al. The cellular target of Histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274 (1999)7286–91. [DOI] [PubMed]
- 44.Giovati L., Ciociola T., Magliani W., Conti S. Antimicrobial peptides with antiprotozoal activity: current state and future perspectives. Future Med. Chem. 2018;10:2569–2572. doi: 10.4155/fmc-2018-0460. [DOI] [PubMed] [Google Scholar]
- 45.Biswaro L.S., da Costa Sousa M.G., Rezende T.M.B., Dias S.C., Franco O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018;9:855. doi: 10.3389/fmicb.2018.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandeira I.C.J., Bandeira-Lima D., Mello C.P., Pereira T.P., De Menezes R.R.P.P.B., Sampaio T.L., et al. Antichagasic effect of crotalicidin, a cathelicidin-like vipericidin, found in Crotalus durissus terrificus rattlesnake’s venom gland. Parasitol. 2018;145:1059–1064. doi: 10.1017/S0031182017001846. [DOI] [PubMed] [Google Scholar]
- 47.Pretzel J., Mohring F., Rahlfs S., Becker K. In: Yellow Biotechnology I: Insect Biotechnologie in Drug Discovery and Preclinical Research. Vilcinskas A., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2013. Antiparasitic Peptides; pp. 157–192. [Google Scholar]
- 48.Giovati L., Santinoli C., Mangia C., Vismarra A., Belletti S., D’Adda T., et al. Novel activity of a synthetic decapeptide against Toxoplasma gondii Tachyzoites. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adade C.M., Oliveira I.R.S., Pais J.A.R., Souto-Padrón T. Melittin peptide kills Trypanosoma cruzi parasites by inducing different cell death pathways. Toxicon. 2013;69:227–239. doi: 10.1016/j.toxicon.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Römling U., Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 51.Reffuveille F, de la Fuente-Núñez C, Mansour S, Hancock REW. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 58 (2014)5363–71. [DOI] [PMC free article] [PubMed]
- 52.Overhage J., Campisano A., Bains M., Torfs E.C.W., Rehm B.H.A., Hancock R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008;76:4176–4182. doi: 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volejníková A., Melicherčík P., Nešuta O., Vaňková E., Bednárová L., Rybáček J., et al. Antimicrobial peptides prevent bacterial biofilm formation on the surface of polymethylmethacrylate bone cement. J. Med. Microbiol. 2019;68:961–972. doi: 10.1099/jmm.0.001000. [DOI] [PubMed] [Google Scholar]
- 54.Mangoni M.L., McDermott A.M., Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp. Dermatol. 2016;25:167–173. doi: 10.1111/exd.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spohn R., Daruka L., Lázár V., Martins A., Vidovics F., Grézal G., et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019;10:4538. doi: 10.1038/s41467-019-12364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang G., Li X., Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44 doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waghu F.H., Barai R.S., Gurung P., Idicula-Thomas S. CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016;44 doi: 10.1093/nar/gkv1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mourtada R., Herce H.D., Yin D.J., Moroco J.A., Wales T.E., Engen J.R., et al. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotech. 2019;37:1186–1197. doi: 10.1038/s41587-019-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melichercik P., Cerovsky V., Nesuta O., Jahoda D., Landor I., Ballay R., et al. Testing the efficacy of antimicrobial peptides in the topical treatment of induced osteomyelitis in rats. Folia Microbiol (Praha) 2018;63:97–104. doi: 10.1007/s12223-017-0540-9. [DOI] [PubMed] [Google Scholar]
- 60.Melichercik P., Nesuta O., Cerovsky V. Antimicrobial peptides for topical treatment of osteomyelitis and implant-related infections: study in the spongy bone. Pharmaceuticals (Basel) 2018;11 doi: 10.3390/ph11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slaninová J., Mlsová V., Kroupová H., Alán L., Tůmová T., Monincová L., et al. Toxicity study of antimicrobial peptides from wild bee venom and their analogs toward mammalian normal and cancer cells. Peptides. 2012;33:18–26. doi: 10.1016/j.peptides.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Brandenburg K., Schürholz T. Lack of new antiinfective agents: passing into the pre-antibiotic age? World J. Bio. Chem. 2015;6:71–77. doi: 10.4331/wjbc.v6.i3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brandenburg K., Andrä J., Garidel P., Gutsmann T. Peptide-based treatment of sepsis. Appl. Microbiol. Biotechnol. 2011;90:799–808. doi: 10.1007/s00253-011-3185-7. [DOI] [PubMed] [Google Scholar]
- 64.Brandenburg K., Heinbockel L., Correa W., Lohner K. Peptides with dual mode of action: killing bacteria and preventing endotoxin-induced sepsis. Biochim. Biophys. Acta. 2016;1858:971–979. doi: 10.1016/j.bbamem.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Sunderkötter C., Becker K. Frequent bacterial skin and soft tissue infections: diagnostic signs and treatment. J. Dtsch. Dermatol. Ges. 2015;13:501–526. doi: 10.1111/ddg.12721. [DOI] [PubMed] [Google Scholar]
- 66.Ramos R., Silva J.P., Rodrigues A.C., Costa R., Guardão L., Schmitt F., et al. Wound healing activity of the human antimicrobial peptide LL37. Peptides. 2011;32:1469–1476. doi: 10.1016/j.peptides.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Guo L., McLean J.S., Yang Y., Eckert R., Kaplan C.W., Kyme P., et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7569–7574. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sørensen O.E. Antimicrobial peptides in cutaneous wound healing. Antimicrob. Pept. 2016:1–15. [Google Scholar]
- 69.Costa F., Teixeira C., Gomes P., Martins M.C.L. Clinical application of AMPs. Adv. Exp. Med. Biol. 2019;1117:281–298. doi: 10.1007/978-981-13-3588-4_15. [DOI] [PubMed] [Google Scholar]
- 70.Hiemstra P.S., Amatngalim G.D., van der Does A.M., Taube C. Antimicrobial peptides and innate lung defenses: role in infectious and noninfectious lung diseases and therapeutic applications. Chest. 2016;149:545–551. doi: 10.1378/chest.15-1353. [DOI] [PubMed] [Google Scholar]
- 71.Majewski K., Kozłowska E., Żelechowska P., Brzezińska-Błaszczyk E. Serum concentrations of antimicrobial peptide cathelicidin LL-37 in patients with bacterial lung infections. Cent. Eur. J. Immunol. 2018;43:453–457. doi: 10.5114/ceji.2018.81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luca V., Stringaro A., Colone M., Pini A., Mangoni M.L. Esculentin(1−21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cell. Mol. Life Sci. 2013;70:2773–2786. doi: 10.1007/s00018-013-1291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mardirossian M., Pompilio A., Degasperi M., Runti G., Pacor S., Di Bonaventura G., et al. D-BMAP18 antimicrobial peptide is active in vitro, resists to pulmonary proteases but loses its activity in a murine model of Pseudomonas aeruginosa lung infection. Front. Chem. 2017;5:40. doi: 10.3389/fchem.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H., Sun J., Xin X., Xu W., Shen J., Song Z., et al. Modulating self-assembly behavior of a salt-free peptide amphiphile (PA) and zwitterionic surfactant mixed system. J. Colloid Interface Sci. 2016;467:43–50. doi: 10.1016/j.jcis.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H., Yu M., Song A., Song Y., Xin X., Shen J., et al. Modulating hierarchical self-assembly behavior of a peptide amphiphile/nonionic surfactant mixed system. RSC Adv. 2016;6:9186–9193. [Google Scholar]
- 76.Liu K., Yang L., Peng X., Gong H., Wang J., Lu J.R., et al. Effects of conventional surfactants on the activity of designed antimicrobial peptide. Langmuir. 2020;36(13):3531–3539. doi: 10.1021/acs.langmuir.0c00032. [DOI] [PubMed] [Google Scholar]
- 77.Banaschewski B.J., Veldhuizen E.J., Keating E., Haagsman H.P., Zuo Y.Y., Yamashita C.M., et al. Antimicrobial and biophysical properties of surfactant supplemented with an antimicrobial peptide for treatment of bacterial pneumonia. Antimicrob. Agents Chemother. 2015;59:3075–3083. doi: 10.1128/AAC.04937-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDermott A.M. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009;41:60–75. doi: 10.1159/000187622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Griffith G.L., Kasus-Jacobi A., Pereira H.A. Bioactive antimicrobial peptides as therapeutics for corneal wounds and infections. Adv Wound Care (New Rochelle) 2017;6:175–190. doi: 10.1089/wound.2016.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva C.N.D., Silva F.R.D., Dourado L.F.N., Reis P., Silva R.O., Costa B.L.D., et al. A new topical eye drop containing LyeTxI-b, a synthetic peptide designed from a Lycosa erithrognata venom toxin, was effective to treat resistant bacterial keratitis. Toxins (Basel) 2019;11 doi: 10.3390/toxins11040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan X.W., Goh T.W., Saraswathi P., Nyein C.L., Setiawan M., Riau A., et al. Effectiveness of antimicrobial peptide immobilization for preventing perioperative cornea implant-associated bacterial infection. Antimicrob. Agents Chemother. 2014;58:5229–5238. doi: 10.1128/AAC.02859-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohammed I., Said D.G., Dua H.S. Human antimicrobial peptides in ocular surface defense. Prog. Retin. Eye Res. 2017;61:1–22. doi: 10.1016/j.preteyeres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Jäger S., Stange E.F., Wehkamp J. Antimicrobial peptides in gastrointestinal inflammation. Int. J. Inflam. 2010;2010:910283. doi: 10.4061/2010/910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bauer B., Wex T., Kuester D., Meyer T., Malfertheiner P. Differential expression of human beta defensin 2 and 3 in gastric mucosa of Helicobacter pylori-infected individuals. Helicobacter. 2013;18:6–12. doi: 10.1111/hel.12000. [DOI] [PubMed] [Google Scholar]
- 85.McGee D.J., George A.E., Trainor E.A., Horton K.E., Hildebrandt E., Testerman T.L. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37. Antimicrob. Agents Chemother. 2011;55:2897–2904. doi: 10.1128/AAC.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lina T.T., Alzahrani S., Gonzalez J., Pinchuk I.V., Beswick E.J., Reyes V.E. Immune evasion strategies used by Helicobacter pylori. World J. Gastroenterol. 2014;20:12753–12766. doi: 10.3748/wjg.v20.i36.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nuding S., Gersemann M., Hosaka Y., Konietzny S., Schaefer C., Beisner J., et al. Gastric antimicrobial peptides fail to eradicate Helicobacter pylori infection due to selective induction and resistance. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dias Bastos P.A., Lara Santos L., Pinheiro Vitorino R.M. How are the expression patterns of gut antimicrobial peptides modulated by human gastrointestinal diseases? A bridge between infectious, inflammatory, and malignant diseases. J. Pept. Sci. 2018;24 doi: 10.1002/psc.3071. [DOI] [PubMed] [Google Scholar]
- 89.Jaka H., Rhee J.A., Östlundh L., Smart L., Peck R., Mueller A., et al. The magnitude of antibiotic resistance to Helicobacter pylori in Africa and identified mutations which confer resistance to antibiotics: systematic review and meta-analysis. BMC Infect. Dis. 2018;18:193. doi: 10.1186/s12879-018-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Savoldi A., Carrara E., Graham D.Y., Conti M., Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterol. 2018;155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. (e17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neshani A., Zare H., Akbari Eidgahi M.R., Hooshyar Chichaklu A., Movaqar A., Ghazvini K. Review of antimicrobial peptides with anti-Helicobacter pylori activity. Helicobacter. 2019;24 doi: 10.1111/hel.12555. [DOI] [PubMed] [Google Scholar]
- 92.Narayana J.L., Huang H.-N., Wu C.-J., Chen J.-Y. Efficacy of the antimicrobial peptide TP4 against Helicobacter pylori infection: in vitro membrane perturbation via micellization and in vivo suppression of host immune responses in a mouse model. Oncotarget. 2015;6:12936–12954. doi: 10.18632/oncotarget.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X.-L., Jiang A.-M., Ma Z.-Y., Li X.-B., Xiong Y.-Y., Dou J.-F., et al. The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-helicobacter pylori activity in vitro and in vivo. Molecules (Basel, Switzerland) 2015;20:3972–3985. doi: 10.3390/molecules20033972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rivas-Santiago B., Serrano C.J., Enciso-Moreno J.A. Susceptibility to infectious diseases based on antimicrobial peptide production. Infect. Immun. 2009;77:4690–4695. doi: 10.1128/IAI.01515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wlodarska M., Willing B., Keeney K.M., Menendez A., Bergstrom K.S., Gill N., et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011;79:1536–1545. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho I., Yamanishi S., Cox L., Methé B.A., Zavadil J., Li K., et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fouhse J.M., Yang K., More-Bayona J., Gao Y., Goruk S., Plastow G., et al. Neonatal exposure to amoxicillin alters Long-term immune response despite transient effects on gut-microbiota in piglets. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang J.K., Hwang J.S., Nam H.J., Ahn K.J., Seok H., Kim S.-K., et al. The insect peptide coprisin prevents Clostridium difficile-mediated acute inflammation and mucosal damage through selective antimicrobial activity. Antimicrob. Agents Chemother. 2011;55:4850–4857. doi: 10.1128/AAC.00177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luca G., Rossella De M., Lucia C. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010;16:3185–3203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 100.Biswaro L.S., da Costa Sousa M.G., Rezende T.M.B., Dias S.C., Franco O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018;9:855. doi: 10.3389/fmicb.2018.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin-Feng Y., Hong Y., Yan-Zhi Z., Ming X. Metabolism of peptide drugs and strategies to improve their metabolic stability. Curr. Drug Metab. 2018;19:892–901. doi: 10.2174/1389200219666180628171531. [DOI] [PubMed] [Google Scholar]
- 102.Nuding S., Frasch T., Schaller M., Stange E.F., Zabel L.T. Synergistic effects of antimicrobial peptides and antibiotics against Clostridium difficile. Antimicrob. Agents Chemother. 2014;58:5719. doi: 10.1128/AAC.02542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geldart K., Forkus B., McChesney E., McCue M., Kaznessis Y.N. pMPES: a modular peptide expression system for the delivery of antimicrobial peptides to the site of gastrointestinal infections using probiotics. Pharmaceuticals (Basel) 2016;9:60. doi: 10.3390/ph9040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hogan A., Heppert V.G., Suda A.J. Osteomyelitis. Arch. Orthop. Traum. Su. 2013;133:1183–1196. doi: 10.1007/s00402-013-1785-7. [DOI] [PubMed] [Google Scholar]
- 105.Maradit Kremers H., Larson D.R., Crowson C.S., Kremers W.K., Washington R.E., Steiner C.A., et al. Prevalence of total hip and knee replacement in the United States. J. Bone Joint Surg. Am. 2015;97:1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Masters E.A., Trombetta R.P., de Mesy Bentley K.L., Boyce B.F., Gill A.L., Gill S.R., et al. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019;7:20. doi: 10.1038/s41413-019-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kurtz S.M., Lau E., Watson H., Schmier J.K., Parvizi J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012;27:61–65. doi: 10.1016/j.arth.2012.02.022. (e1) [DOI] [PubMed] [Google Scholar]
- 108.Schwarz E.M., Parvizi J., Gehrke T., Aiyer A., Battenberg A., Brown S.A., et al. 2018 international consensus meeting on musculoskeletal infection: research priorities from the general assembly questions. J. Orthop. Res. 2019;37:997–1006. doi: 10.1002/jor.24293. [DOI] [PubMed] [Google Scholar]
- 109.Walter G., Kemmerer M., Kappler C., Hoffmann R. Treatment algorithms for chronic osteomyelitis. Dtsch. Arztebl. Int. 2012;109:257–264. doi: 10.3238/arztebl.2012.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bernard L., Dinh A., Ghout I., Simo D., Zeller V., Issartel B., et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385:875–882. doi: 10.1016/S0140-6736(14)61233-2. [DOI] [PubMed] [Google Scholar]
- 111.Spellberg B., Lipsky B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2011;54:393–407. doi: 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clerc N.L., Verillaud B., Duet M., Guichard J.-P., Herman P., Kania R. Skull base osteomyelitis: incidence of resistance, morbidity, and treatment strategy. Laryngoscope. 2014;124:2013–2016. doi: 10.1002/lary.24726. [DOI] [PubMed] [Google Scholar]
- 113.Bormann N., Koliszak A., Kasper S., Schoen L., Hilpert K., Volkmer R., et al. A short artificial antimicrobial peptide shows potential to prevent or treat bone infections. Sci. Rep. 2017;7:1506. doi: 10.1038/s41598-017-01698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li H., Zhang S., Nie Be Du Z., Long T., Yue B. The antimicrobial peptide KR-12 promotes the osteogenic differentiation of human bone marrow stem cells by stimulating BMP/SMAD signaling. RSC Adv. 2018;8:15547–15557. doi: 10.1039/c8ra00750k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dale B.A., Tao R., Kimball J.R., Jurevic R.J. Oral antimicrobial peptides and biological control of caries. BMC Oral Health. 2006;6:S13. doi: 10.1186/1472-6831-6-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pütsep K., Carlsson G., Boman H.G., Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 117.Selwitz R.H., Ismail A.I., Pitts N.B. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 118.Guo L., Edlund A. Targeted antimicrobial peptides: a novel technology to eradicate harmful Streptococcus Mutans. J. Calif. Dent. Assoc. 2017;45:557–564. [PMC free article] [PubMed] [Google Scholar]
- 119.Horst J.A., Ellenikiotis H., Milgrom P.L. UCSF protocol for caries arrest using silver Diamine fluoride: rationale, indications and consent. J. Calif. Dent. Assoc. 2016;44:16–28. [PMC free article] [PubMed] [Google Scholar]
- 120.Kaplan C.W., Sim J.H., Shah K.R., Kolesnikova-Kaplan A., Shi W., Eckert R. Selective membrane disruption: mode of action of C16G2, a specifically targeted antimicrobial peptide. Antimicrob. Agents Chemother. 2011;55:3446–3452. doi: 10.1128/AAC.00342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo L., McLean J.S., Yang Y., Eckert R., Kaplan C.W., Kyme P., et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7569–7574. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alvares D.S., Viegas T.G., Ruggiero Neto J. Lipid-packing perturbation of model membranes by pH-responsive antimicrobial peptides. Biophys. Rev. 2017;9:669–682. doi: 10.1007/s12551-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alvares D.S., Viegas T.G., Ruggiero Neto J. The effect of pH on the lytic activity of a synthetic mastoparan-like peptide in anionic model membranes. Chem. Phys. Lipids. 2018;216:54–64. doi: 10.1016/j.chemphyslip.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 124.Lalla R.V., Sonis S.T., Peterson D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008;52:61–viii. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donnelly J.P., Bellm L.A., Epstein J.B., Sonis S.T., Symonds R.P. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect. Dis. 2003;3:405–412. doi: 10.1016/s1473-3099(03)00668-6. [DOI] [PubMed] [Google Scholar]
- 126.Fjell C.D., Hiss J.A., Hancock R.E.W., Schneider G. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 127.Daniluk T., Tokajuk G., Stokowska W., Fiedoruk K., Sciepuk M., Zaremba M., et al. Occurrence rate of oral Candida albicans in denture wearer patients. Adv. Med. Sci. 2006;51:77. [PubMed] [Google Scholar]
- 128.Jurevic R.J., Bai M., Chadwick R.B., White T.C., Dale B.A. Single-nucleotide polymorphisms (SNPs) in human β-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J. Clin. Microbiol. 2003;41:90–96. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kiyoura Y., Tamai R. Innate immunity to Candida albicans. Jpn Dent Sci Rev. 2015;51:59–64. [Google Scholar]
- 130.Calderone R.A., Fonzi W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 131.Katarzyna E.G., Małgorzata D. Antimicrobial Peptides Under Clinical Trials. Curr. Top. Med. Chem. 2017;17:620–628. doi: 10.2174/1568026616666160713143331. [DOI] [PubMed] [Google Scholar]
- 132.Baxter A.A., Lay F.T., Poon I.K., Kvansakul M., Hulett M.D. Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cell. Mol. Life Sci. 2017;74:3809–3825. doi: 10.1007/s00018-017-2604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ciumac D., Gong H., Hu X., Lu J.R. Membrane targeting cationic antimicrobial peptides. J. Colloid Interface Sci. 2019;537:163–185. doi: 10.1016/j.jcis.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 134.Poon I.K., Baxter A.A., Lay F.T., Mills G.D., Adda C.G., Payne J.A., et al. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. Elife. 2014;3 doi: 10.7554/eLife.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alvares D.S., Neto J.R., Ambroggio E.E. Phosphatidylserine lipids and membrane order precisely regulate the activity of Polybia-MP1 peptide. Biochim. Biophys. Acta Biomembr. 2017;1859:1067–1074. doi: 10.1016/j.bbamem.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 136.Leite N.B., Aufderhorst-Roberts A., Palma M.S., Connell S.D., Neto J.R., Beales P.A. PE and PS lipids synergistically enhance membrane poration by a peptide with anticancer properties. Biophys. J. 2015;109:936–947. doi: 10.1016/j.bpj.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ishii H., Mori T., Shiratsuchi A., Nakai Y., Shimada Y., Ohno-Iwashita Y., et al. Distinct localization of lipid rafts and externalized phosphatidylserine at the surface of apoptotic cells. Biochem. Biophys. Res. Commun. 2005;327:94–99. doi: 10.1016/j.bbrc.2004.11.135. [DOI] [PubMed] [Google Scholar]
- 138.dos Santos Cabrera M.P., Alvares D.S., Leite N.B., Monson de Souza B., Palma M.S., Riske K.A., et al. New insight into the mechanism of action of wasp mastoparan peptides: lytic activity and clustering observed with giant vesicles. Langmuir. 2011;27:10805–10813. doi: 10.1021/la202608r. [DOI] [PubMed] [Google Scholar]
- 139.Alvares D.S., Wilke N., Neto J.R., Fanani M.L. The insertion of Polybia-MP1 peptide into phospholipid monolayers is regulated by its anionic nature and phase state. Chem. Phys. Lipids. 2017;207 (:38–48. doi: 10.1016/j.chemphyslip.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 140.dos Santos Cabrera M.P., Arcisio-Miranda M., Gorjao R., Leite N.B., de Souza B.M., Curi R., et al. Influence of the bilayer composition on the binding and membrane disrupting effect of Polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity. Biochem. 2012;51:4898–4908. doi: 10.1021/bi201608d. [DOI] [PubMed] [Google Scholar]
- 141.Deslouches B., Di Y.P. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017;8 doi: 10.18632/oncotarget.16743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsai T.-L., Li A.-C., Chen Y.-C., Liao Y.-S., Lin T.-H. Antimicrobial peptide m2163 or m2386 identified from Lactobacillus casei ATCC 334 can trigger apoptosis in the human colorectal cancer cell line SW480. Tumor Biol. 2015;36:3775–3789. doi: 10.1007/s13277-014-3018-2. [DOI] [PubMed] [Google Scholar]
- 143.Chubicka T., Girija D., Deepa K., Salini S., Meera N., Raghavamenon A.C., et al. A parasporin from Bacillus thuringiensis native to peninsular India induces apoptosis in cancer cells through intrinsic pathway. J. Biosci. 2018;43:407–416. [PubMed] [Google Scholar]
- 144.Woldetsadik A.D., Vogel M.C., Rabeh W.M., Magzoub M. Hexokinase II–derived cell-penetrating peptide targets mitochondria and triggers apoptosis in cancer cells. FASEB J. 2017;31:2168–2184. doi: 10.1096/fj.201601173R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamazaki T., Pitt J., Vetizou M., Marabelle A., Flores C., Rekdal Ø., et al. The oncolytic peptide LTX-315 overcomes resistance of cancers to immunotherapy with CTLA4 checkpoint blockade. Cell Death Differ. 2016;23:1004. doi: 10.1038/cdd.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu X., Wu F., Ji Y., Yin L. Recent advances in anti-cancer protein/peptide delivery. Bioconjug. Chem. 2018;30:305–324. doi: 10.1021/acs.bioconjchem.8b00750. [DOI] [PubMed] [Google Scholar]
- 147.Roudi R., Syn N.L., Roudbary M. Antimicrobial peptides as biologic and immunotherapeutic agents against cancer: a comprehensive overview. Front. Immunol. 2017;8:1320. doi: 10.3389/fimmu.2017.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leite N.B., dos Santos Alvares D., de Souza B.M., Palma M.S., Ruggiero Neto J. Effect of the aspartic acid D2 on the affinity of Polybia-MP1 to anionic lipid vesicles. Eur. Biophys. J. 2014;43:121–130. doi: 10.1007/s00249-014-0945-1. [DOI] [PubMed] [Google Scholar]
- 149.Aumelas A., Mangoni M., Roumestand C., Chiche L., Despaux E., Grassy G., et al. Synthesis and solution structure of the antimicrobial peptide protegrin-1. Euro. J. Biochem. 1996;237:575–583. doi: 10.1111/j.1432-1033.1996.0575p.x. [DOI] [PubMed] [Google Scholar]
- 150.Yonezawa A., Kuwahara J., Fujii N., Sugiura Y. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry. 1992;31:2998–3004. doi: 10.1021/bi00126a022. [DOI] [PubMed] [Google Scholar]
- 151.Mishra A.K., Choi J., Moon E., Baek K.-H. Tryptophan-rich and proline-rich antimicrobial peptides. Molecules (Basel, Switzerland) 2018;23:815. doi: 10.3390/molecules23040815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mahlapuu M., Håkansson J., Ringstad L., Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016;6 doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hancock REW. Peptide antibiotics. The Lancet. 349 (1997)418–22. [DOI] [PubMed]
- 154.Qu P., Gao W., Chen H., Li D., Yang N., Zhu J., et al. The central hinge link truncation of the antimicrobial peptide fowlicidin-3 enhances its cell selectivity without antibacterial activity loss. Antimicrob. Agents Chemother. 2016;60:2798. doi: 10.1128/AAC.02351-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kwon J.Y., Kim M.K., Mereuta L., Seo C.H., Luchian T., Park Y. Mechanism of action of antimicrobial peptide P5 truncations against Pseudomonas aeruginosa and Staphylococcus aureus. AMB Express. 2019;9:122. doi: 10.1186/s13568-019-0843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kanthawong S., Bolscher J.G.M., Veerman E.C.I., van Marle J., Nazmi K., Wongratanacheewin S., et al. Antimicrobial activities of LL-37 and its truncated variants against Burkholderia thailandensis. Int. J. Antimicrob. 2010;36:447–452. doi: 10.1016/j.ijantimicag.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 157.Akbari R., Hakemi Vala M., Hashemi A., Aghazadeh H., Sabatier J.-M., Pooshang Bagheri K. Action mechanism of melittin-derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids. 2018;50:1231–1243. doi: 10.1007/s00726-018-2596-5. [DOI] [PubMed] [Google Scholar]
- 158.Luong H.X., Kim D.-H., Lee B.-J., Kim Y.-W. Effects of lysine-to-arginine substitution on antimicrobial activity of cationic stapled heptapeptides. Arch. Pharm. Res. 2018;41:1092–1097. doi: 10.1007/s12272-018-1084-5. [DOI] [PubMed] [Google Scholar]
- 159.Lei J., Sun L., Huang S., Zhu C., Li P., He J., et al. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019;11:3919–3931. [PMC free article] [PubMed] [Google Scholar]
- 160.Leite N.B., da Costa L.C., dos Santos Alvares D., dos Santos Cabrera M.P., de Souza B.M., Palma M.S., et al. The effect of acidic residues and amphipathicity on the lytic activities of mastoparan peptides studied by fluorescence and CD spectroscopy. Amino Acids. 2011;40:91–100. doi: 10.1007/s00726-010-0511-9. [DOI] [PubMed] [Google Scholar]
- 161.Dinh T.T.T., Kim D.-H., Luong H.X., Lee B.-J., Kim Y.-W. Antimicrobial activity of doubly-stapled alanine/lysine-based peptides. Bioorg. Med. Chem. Lett. 2015;25:4016–4019. doi: 10.1016/j.bmcl.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 162.Luong H.X., Kim D.-H., Mai N.T., Lee B.-J., Kim Y.-W. Mono-substitution effects on antimicrobial activity of stapled heptapeptides. Arch. Pharm. Res. 2017;40:713–719. doi: 10.1007/s12272-017-0922-1. [DOI] [PubMed] [Google Scholar]
- 163.Luong H.X., Kim D.-H., Lee B.-J., Kim Y.-W. Antimicrobial and hemolytic activity of stapled heptapeptide dimers. Bull. Kor. Chem. Soc. 2016;37:1199–1203. [Google Scholar]
- 164.Jiang Z., Vasil A.I., Hale J.D., Hancock R.E.W., Vasil M.L., Hodges R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers. 2008;90:369–383. doi: 10.1002/bip.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen Y., Guarnieri M.T., Vasil A.I., Vasil M.L., Mant C.T., Hodges R.S. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007;51:1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schmidtchen A., Pasupuleti M., Malmsten M. Effect of hydrophobic modifications in antimicrobial peptides. Adv. Colloid Interf. Sci. 2014;205:265–274. doi: 10.1016/j.cis.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 167.Dinh T.T., Kim D.-H., Lee B.-J., Kim Y.-W. De novo design and their antimicrobial activity of stapled amphipathic helices of heptapeptides. Bull. Kor. Chem. Soc. 2014;35:3632–3636. [Google Scholar]
- 168.Dias RdO, Franco O.L. Cysteine-stabilized αβ defensins: from a common fold to antibacterial activity. Peptides. 2015;72:64–72. doi: 10.1016/j.peptides.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 169.Tailor R.H., Acland D.P., Attenborough S., Cammue B.P., Evans I.J., Osborn R.W., et al. A novel family of small cysteine-rich antimicrobial peptides from seed of Impatiens balsamina is derived from a single precursor protein. J. Biol. Chem. 1997;272:24480–24487. doi: 10.1074/jbc.272.39.24480. [DOI] [PubMed] [Google Scholar]
- 170.Shin Y.P., Park H.J., Shin S.H., Lee Y.S., Park S., Jo S., et al. Antimicrobial activity of a halocidin-derived peptide resistant to attacks by proteases. Antimicrob. Agents Chemother. 2010;54:2855–2866. doi: 10.1128/AAC.01790-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohamed M.F., Brezden A., Mohammad H., Chmielewski J., Seleem M.N. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci. Rep. 2017;7:6953. doi: 10.1038/s41598-017-07440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zaet A., Dartevelle P., Daouad F., Ehlinger C., Quiles F., Francius G., et al. D-cateslytin, a new antimicrobial peptide with therapeutic potential. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang J., Song J., Yang Z., He S., Yang Y., Feng X., et al. Antimicrobial peptides with high proteolytic resistance for combating gram-negative Bacteria. J. Med. Chem. 2019;62:2286–2304. doi: 10.1021/acs.jmedchem.8b01348. [DOI] [PubMed] [Google Scholar]
- 174.Bagheri M., Arasteh S., Haney E.F., Hancock R.E. Tryptic stability of synthetic bactenecin derivatives is determined by the side chain length of cationic residues and the peptide conformation. J. Med. Chem. 2016;59:3079–3086. doi: 10.1021/acs.jmedchem.5b01740. [DOI] [PubMed] [Google Scholar]
- 175.Arias M., Piga K.B., Hyndman M.E., Vogel H.J. Improving the activity of Trp-rich antimicrobial peptides by Arg/Lys substitutions and changing the length of cationic residues. Biomolecules. 2018;8 doi: 10.3390/biom8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li W., Sun Z., O’Brien-Simpson N.M., Otvos L., Reynolds E.C., Hossain M.A., et al. The effect of selective D- or N(alpha)-methyl arginine substitution on the activity of the proline-rich antimicrobial peptide, Chex1-Arg20. Front. Chem. 2017;5:1. doi: 10.3389/fchem.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Solanas C., de la Torre B.G., Fernández-Reyes M., Santiveri C.M., Jiménez M.A., Rivas L., et al. Sequence inversion and phenylalanine surrogates at the beta-turn enhance the antibiotic activity of gramicidin S. J. Med. Chem. 2010;53:4119–4129. doi: 10.1021/jm100143f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haug B.E., Stensen W., Stiberg T., Svendsen J.S. Bulky nonproteinogenic amino acids permit the design of very small and effective cationic antibacterial peptides. J. Med. Chem. 2004;47:4159–4162. doi: 10.1021/jm049582b. [DOI] [PubMed] [Google Scholar]
- 179.Alvares D.S., Wilke N., Ruggiero Neto J. Effect of N-terminal acetylation on lytic activity and lipid-packing perturbation induced in model membranes by a mastoparan-like peptide. Biochim. Biophys. Acta Biomembr. 2018;1860:737–748. doi: 10.1016/j.bbamem.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 180.Dinh T.T., Kim D.H., Nguyen T.Q., Lee B.J., Kim Y.W. N-capping effects of stapled heptapeptides on antimicrobial and hemolytic activities. Bull. Kor. Chem. Soc. 2015;36:2511–2515. [Google Scholar]
- 181.Dennison S.R., Mura M., Harris F., Morton L.H.G., Zvelindovsky A., Phoenix D.A. The role of C-terminal amidation in the membrane interactions of the anionic antimicrobial peptide, maximin H5. Biochim. Biophys. Acta Biomembr. 2015;1848:1111–1118. doi: 10.1016/j.bbamem.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 182.Čeřovský V., Buděšínský M., Hovorka O., Cvačka J., Voburka Z., Slaninová J., et al. Lasioglossins: three novel antimicrobial peptides from the venom of the Eusocial bee Lasioglossum laticeps (Hymenoptera: Halictidae) ChemBioChem. 2009;10:2089–2099. doi: 10.1002/cbic.200900133. [DOI] [PubMed] [Google Scholar]
- 183.Sforça M.L., Oyama S., Canduri F., Lorenzi C.C.B., Pertinhez T.A., Konno K., et al. How C-terminal carboxyamidation alters the biological activity of peptides from the venom of the eumenine solitary wasp. Biochemistry. 2004;43:5608–5617. doi: 10.1021/bi0360915. [DOI] [PubMed] [Google Scholar]
- 184.Gaillard V., Galloux M., Garcin D., Eleouet J.F., Le Goffic R., Larcher T., et al. A short double-stapled peptide inhibits respiratory syncytial virus entry and spreading. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02241-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Luong H.X., Kim D.-H., Lee B.-J., Kim Y.-W. Antimicrobial activity and stability of stapled helices of polybia-MP1. Arch. Pharm. Res. 2017;40:1414–1419. doi: 10.1007/s12272-017-0963-5. [DOI] [PubMed] [Google Scholar]
- 186.Cui H.K., Guo Y., He Y., Wang F.L., Chang H.N., Wang Y.J., et al. Diaminodiacid-based solid-phase synthesis of peptide disulfide bond mimics. Angew Chem. Int. Ed. Engl. 2013;52:9558–9562. doi: 10.1002/anie.201302197. [DOI] [PubMed] [Google Scholar]
- 187.Abdel Monaim S.A.H., Somboro A.M., El-Faham A., de la Torre B.G., Albericio F. Bacteria hunt Bacteria through an intriguing cyclic peptide. ChemMedChem. 2019;14:24–51. doi: 10.1002/cmdc.201800597. [DOI] [PubMed] [Google Scholar]
- 188.Di Bonaventura I., Baeriswyl S., Capecchi A., Gan B.H., Jin X., Siriwardena T.N., et al. An antimicrobial bicyclic peptide from chemical space against multidrug resistant gram-negative bacteria. Chem Commun (Camb) 2018;54:5130–5133. doi: 10.1039/c8cc02412j. [DOI] [PubMed] [Google Scholar]
- 189.Kale S.S., Villequey C., Kong X.-D., Zorzi A., Deyle K., Heinis C. Cyclization of peptides with two chemical bridges affords large scaffold diversities. Nat. Chem. 2018;10:715–723. doi: 10.1038/s41557-018-0042-7. [DOI] [PubMed] [Google Scholar]
- 190.Bagheri M., Amininasab M., Dathe M. Arginine/tryptophan-rich cyclic alpha/beta-antimicrobial peptides: the roles of hydrogen bonding and hydrophobic/hydrophilic solvent-accessible surface areas upon activity and membrane selectivity. Chemistry. 2018;24:14242–14253. doi: 10.1002/chem.201802881. [DOI] [PubMed] [Google Scholar]
- 191.Bartoloni M., Jin X., Marcaida M.J., Banha J., Dibonaventura I., Bongoni S., et al. Bridged bicyclic peptides as potential drug scaffolds: synthesis, structure, protein binding and stability. Chem. Sci. 2015;6:5473–5490. doi: 10.1039/c5sc01699a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dartois V., Sanchez-Quesada J., Cabezas E., Chi E., Dubbelde C., Dunn C., et al. Systemic antibacterial activity of novel synthetic cyclic peptides. Antimicrob. Agents Chemother. 2005;49:3302–3310. doi: 10.1128/AAC.49.8.3302-3310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang J., Chen H., Vlahov I.R., Cheng J.-X., Low P.S. Evaluation of disulfide reduction during receptor-mediated endocytosis by using FRET imaging. Proc. Natl. Acad. Sci. 2006;103 doi: 10.1073/pnas.0601455103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bourne D.G., Jones G.J., Blakeley R.L., Jones A., Negri A.P., Riddles P. Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Appl. Environ. Microbiol. 1996;62:4086–4094. doi: 10.1128/aem.62.11.4086-4094.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dinh T.T., Kim D.H., Luong H.X., Lee B.J., Kim Y.W. Antimicrobial activity of doubly-stapled alanine/lysine-based peptides. Bioorg. Med. Chem. Lett. 2015;25:4016–4019. doi: 10.1016/j.bmcl.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 196.Kim Y.-W., Grossmann T.N., Verdine G.L. Synthesis of all-hydrocarbon stapled α-helical peptides by ring-closing olefin metathesis. Nat. Protoc. 2011;6:761–771. doi: 10.1038/nprot.2011.324. [DOI] [PubMed] [Google Scholar]
- 197.Bolt H.L., Eggimann G.A., Jahoda C.A.B., Zuckermann R.N., Sharples G.J., Cobb S.L. Exploring the links between peptoid antibacterial activity and toxicity. MedChemComm. 2017;8:886–896. doi: 10.1039/c6md00648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mojsoska B., Carretero G., Larsen S., Mateiu R.V., Jenssen H. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci. Rep. 2017;7 doi: 10.1038/srep42332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hansen P.R., Munk J.K. In: Peptide Synthesis and Applications. Jensen K.J., Tofteng Shelton P., Pedersen S.L., editors. Humana Press; Totowa, NJ: 2013. Synthesis of antimicrobial peptoids; pp. 151–159. [Google Scholar]
- 200.Liu D., DeGrado W.F. De novo design, synthesis, and characterization of antimicrobial β-peptides. J. Am. Chem. Soc. 2001;123:7553–7559. doi: 10.1021/ja0107475. [DOI] [PubMed] [Google Scholar]
- 201.Godballe T., Nilsson L.L., Petersen P.D., Jenssen H. Antimicrobial β-peptides and α-peptoids. Chem. Biol. Drug Des. 2011;77:107–116. doi: 10.1111/j.1747-0285.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 202.Greco I., Emborg A.P., Jana B., Molchanova N., Oddo A., Damborg P., et al. Characterization, mechanism of action and optimization of activity of a novel peptide-peptoid hybrid against bacterial pathogens involved in canine skin infections. Sci. Rep. 2019;9:3679. doi: 10.1038/s41598-019-39042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Molchanova N., Hansen P.R., Damborg P., Nielsen H.M., Franzyk H. Lysine-based α-peptide/β-peptoid peptidomimetics: influence of hydrophobicity, fluorination, and distribution of cationic charge on antimicrobial activity and cytotoxicity. ChemMedChem. 2017;12:312–318. doi: 10.1002/cmdc.201600553. [DOI] [PubMed] [Google Scholar]
- 204.Kumar Malik D., Baboota S., Ahuja A., Hasan S., Ali J. Recent advances in protein and peptide drug delivery systems. Curr. Drug Deliv. 2007;4:141–151. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 205.Anselmo A.C., Gokarn Y., Mitragotri S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 206.Prausnitz M.R., Mitragotri S., Langer R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004;3:115. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 207.Schoellhammer C.M., Blankschtein D., Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin. Drug Del. 2014;11:393–407. doi: 10.1517/17425247.2014.875528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cone R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 209.Morishita M., Peppas N.A. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today. 2006;11:905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 210.Brayden D.J., Alonso M.-J. Oral delivery of peptides: opportunities and issues for translation. Adv. Drug Deliv. Rev. 2016;106 (:193–195. doi: 10.1016/j.addr.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 211.Traverso G., Schoellhammer C.M., Schroeder A., Maa R., Lauwers G.Y., Polat B.E., et al. Microneedles for drug delivery via the gastrointestinal tract. J. Pharm. Sci. 2015;104:362–367. doi: 10.1002/jps.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Choonara B.F., Choonara Y.E., Kumar P., Bijukumar D., du Toit L.C., Pillay V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014;32:1269–1282. doi: 10.1016/j.biotechadv.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 213.Bosquillon C., Lombry C., Preat V., Vanbever R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release. 2001;70:329–339. doi: 10.1016/s0168-3659(00)00362-x. [DOI] [PubMed] [Google Scholar]
- 214.Yang W, Peters JI, Williams III RO. Inhaled nanoparticles—a current review. Int. J. Pharm. 356 (2008)239–47. [DOI] [PubMed]
- 215.Shoyele S.A., Cawthorne S. Particle engineering techniques for inhaled biopharmaceuticals. Adv. Drug Deliv. Rev. 2006;58:1009–1029. doi: 10.1016/j.addr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 216.Turner M.R., Balu-Iyer S.V. Challenges and opportunities for the subcutaneous delivery of therapeutic proteins. J. Pharm. Sci. 2018;107:1247–1260. doi: 10.1016/j.xphs.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 218.Yang K., Han Q., Chen B., Zheng Y., Zhang K., Li Q., et al. Antimicrobial hydrogels: promising materials for medical application. Int. J. Nanomedicine. 2018;13:2217–2263. doi: 10.2147/IJN.S154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Niu Z., Conejos-Sánchez I., Griffin B.T., O’Driscoll C.M., Alonso M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016;106 (:337–354. doi: 10.1016/j.addr.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 220.Chereddy K.K., Her C.-H., Comune M., Moia C., Lopes A., Porporato P.E., et al. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J. Control. Release. 2014;194:138–147. doi: 10.1016/j.jconrel.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 221.Rajchakit U., Sarojini V. Recent developments in antimicrobial-peptide-conjugated gold nanoparticles. Bioconjug. Chem. 2017;28:2673–2686. doi: 10.1021/acs.bioconjchem.7b00368. [DOI] [PubMed] [Google Scholar]
- 222.Pal I., Bhattacharyya D., Kar R.K., Zarena D., Bhunia A., Atreya H.S. A peptide-nanoparticle system with improved efficacy against multidrug resistant bacteria. Sci. Rep. 2019;9:4485. doi: 10.1038/s41598-019-41005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chowdhury R., Ilyas H., Ghosh A., Ali H., Ghorai A., Midya A., et al. Multivalent gold nanoparticle–peptide conjugates for targeting intracellular bacterial infections. Nanoscale. 2017;9:14074–14093. doi: 10.1039/c7nr04062h. [DOI] [PubMed] [Google Scholar]
- 224.Kwakman P.H., Zaat S.A. Biomat. Asso. Infect. Springer; 2013. Preventive measures against transcutaneous device infections; pp. 229–248. [Google Scholar]
- 225.Zimmerli W., Trampuz A., Ochsner P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 226.Riool M., de Breij A., Drijfhout J.W., Nibbering P.H., Zaat S.A. Antimicrobial peptides in biomedical device manufacturing. Front. Chem. 2017;5:63. doi: 10.3389/fchem.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 228.Zaat S.A. Biomat. Asso. Infect. Springer; 2013. Tissue colonization in biomaterial-associated infection; pp. 175–207. [Google Scholar]
- 229.Magiorakos A.P., Srinivasan A., Carey R., Carmeli Y., Falagas M., Giske C., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 230.Pasupuleti M., Schmidtchen A., Malmsten M. Antimicrobial peptides: key components of the innate immune system. Crit. Rev. Biotechnol. 2012;32:143–171. doi: 10.3109/07388551.2011.594423. [DOI] [PubMed] [Google Scholar]
- 231.Mansour SC, de la Fuente-Núñez C, Hancock RE. Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 21 (2015)323–9. [DOI] [PubMed]
- 232.Nakatsuji T., Gallo R.L. Antimicrobial peptides: old molecules with new ideas. J. Invest. Dermatol. 2012;132:887–895. doi: 10.1038/jid.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Salvado M.D., Di Gennaro A., Lindbom L., Agerberth B., Haeggström J.Z. Cathelicidin LL-37 induces angiogenesis via PGE2–EP3 signaling in endothelial cells, in vivo inhibition by aspirin. Arterioscler Throm Vasc Biol. 2013;33:1965–1972. doi: 10.1161/ATVBAHA.113.301851. [DOI] [PubMed] [Google Scholar]
- 234.Busscher H.J., van der Mei H.C., Subbiahdoss G., Jutte P.C., van den Dungen J.J., Zaat S.A., et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci. Transl. Med. 2012;4:153rv10–rv10. doi: 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- 235.Keum H., Kim J.Y., Yu B., Yu S.J., Kim J., Jeon H., et al. Prevention of bacterial colonization on catheters by a one-step coating process involving an antibiofouling polymer in water. ACS Appl Mater Inter. 2017;9:19736–19745. doi: 10.1021/acsami.7b06899. [DOI] [PubMed] [Google Scholar]
- 236.Emanuel N., Rosenfeld Y., Cohen O., Applbaum Y.H., Segal D., Barenholz Y. A lipid-and-polymer-based novel local drug delivery system—BonyPidTM: from physicochemical aspects to therapy of bacterially infected bones. J. Control. Release. 2012;160:353–361. doi: 10.1016/j.jconrel.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 237.Casciaro B., Dutta D., Loffredo M.R., Marcheggiani S., McDermott A.M., Willcox M.D., et al. Esculentin-1a derived peptides kill Pseudomonas aeruginosa biofilm on soft contact lenses and retain antibacterial activity upon immobilization to the lens surface. Pept. Sci. 2018;110 doi: 10.1002/bip.23074. [DOI] [PubMed] [Google Scholar]
- 238.Yu K., Lo J.C., Yan M., Yang X., Brooks D.E., Hancock R.E., et al. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials. 2017;116:69–81. doi: 10.1016/j.biomaterials.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 239.de Alteriis E., Maselli V., Falanga A., Galdiero S., Di Lella F.M., Gesuele R., et al. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018;11:915. doi: 10.2147/IDR.S164262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li T., Wang N., Chen S., Lu R., Li H., Zhang Z. Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int. J. Nanomedicine. 2017;12:2995. doi: 10.2147/IJN.S128775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mishra B., Wang G. Titanium surfaces immobilized with the major antimicrobial fragment FK-16 of human cathelicidin LL-37 are potent against multiple antibiotic-resistant bacteria. Biofouling. 2017;33:544–555. doi: 10.1080/08927014.2017.1332186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.de Breij A., Riool M., Kwakman P.H., de Boer L., Cordfunke R.A., Drijfhout J.W., et al. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J. Control. Release. 2016;222:1–8. doi: 10.1016/j.jconrel.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 243.Nyström L., Strömstedt A.A., Schmidtchen A., Malmsten M. Peptide-loaded microgels as antimicrobial and anti-inflammatory surface coatings. Biomacromol. 2018;19:3456–3466. doi: 10.1021/acs.biomac.8b00776. [DOI] [PubMed] [Google Scholar]
- 244.Sørensen O.E. In: Antimicrobial Peptides: Role in Human Health and Disease. Harder J., Schröder J.-M., editors. Springer International Publishing; Cham: 2016. Antimicrobial peptides in cutaneous wound healing; pp. 1–15. [Google Scholar]
- 245.Chung E.M.C., Dean S.N., Propst C.N., Bishop B.M., van Hoek M.L. Komodo dragon-inspired synthetic peptide DRGN-1 promotes wound-healing of a mixed-biofilm infected wound. NPJ Biofilms and Microbiomes. 2017;3:9. doi: 10.1038/s41522-017-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jung Kim D., Lee Y.W., Park M.K., Shin J.R., Lim K.J., Cho J.H., et al. Efficacy of the designer antimicrobial peptide SHAP1 in wound healing and wound infection. Amino Acids. 2014;46:2333–2343. doi: 10.1007/s00726-014-1780-5. [DOI] [PubMed] [Google Scholar]
- 247.Yoo J.H., Ho S., Tran D.H.-Y., Cheng M., Bakirtzi K., Kubota Y., et al. Antifibrogenic effects of the antimicrobial peptide cathelicidin in murine colitis-associated fibrosis. Cell Mol. Gastroenterol. Hepatol. 2015;1:55–74.e1. doi: 10.1016/j.jcmgh.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Park H.J., Cho D.H., Kim H.J., Lee J.Y., Cho B.K., Bang S.I., et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J. Invest. Dermatol. 2009;129:843–850. doi: 10.1038/jid.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gauglitz G.G., Bureik D., Zwicker S., Ruzicka T., Wolf R. The antimicrobial peptides psoriasin (S100A7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts. Skin Pharmacol. Phys. 2015;28:115–123. doi: 10.1159/000363579. [DOI] [PubMed] [Google Scholar]
- 250.Guo C., Hu Y., Li J., Liu Y., Li S., Yan K., et al. Identification of multiple peptides with antioxidant and antimicrobial activities from skin and its secretions of Hylarana taipehensis, Amolops lifanensis, and Amolops granulosus. Biochimie. 2014;105:192–201. doi: 10.1016/j.biochi.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 251.Borquaye L.S., Darko G., Ocansey E., Ankomah E. Antimicrobial and antioxidant properties of the crude peptide extracts of Galatea paradoxa and Patella rustica. SpringerPlus. 2015;4:500. doi: 10.1186/s40064-015-1266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cuperus T., van Dijk A., Matthijs M.G., Veldhuizen E.J., Haagsman H.P. Protective effect of in ovo treatment with the chicken cathelicidin analog D-CATH-2 against avian pathogenic E. coli. Sci. Rep. 2016;6 doi: 10.1038/srep26622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rajasekaran G., Kumar S.D., Yang S., Shin S.Y. The design of a cell-selective fowlicidin-1-derived peptide with both antimicrobial and anti-inflammatory activities. Eur. J. Med. Chem. 2019;182 doi: 10.1016/j.ejmech.2019.111623. [DOI] [PubMed] [Google Scholar]
- 254.Lai Z., Tan P., Zhu Y., Shao C., Shan A., Li L. Highly stabilized α-helical coiled coils kill gram-negative bacteria by multicomplementary mechanisms under acidic condition. ACS Appl. Mater. Inter. 2019;11:22113–22128. doi: 10.1021/acsami.9b04654. [DOI] [PubMed] [Google Scholar]
- 255.Gordon Y.J., Romanowski E.G., McDermott A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005;30:505–515. doi: 10.1080/02713680590968637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Vaara M. New approaches in peptide antibiotics. Curr. Opin. Pharmacol. 2009;9:571–576. doi: 10.1016/j.coph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 257.Tennyson Lauren E., Averch Timothy D. An update on fluoroquinolones: the emergence of a multisystem toxicity syndrome. Urol. Practice. 2017;4:383–387. doi: 10.1016/j.urpr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 258.Millar N.L., Siebert S., McInnes I.B. Europe rules on harm from fluoroquinolone antibiotics. Nature. 2019;566:326. doi: 10.1038/d41586-019-00619-7. [DOI] [PubMed] [Google Scholar]
- 259.Yu G., Baeder D.Y., Regoes R.R., Rolff J. Combination effects of antimicrobial peptides. Antimicrob. Agents Chemother. 2016;60:1717–1724. doi: 10.1128/AAC.02434-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Magana M., Pushpanathan M., Santos A.L., Leanse L., Fernandez M., Ioannidis A., et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020;20(9):216–230. doi: 10.1016/S1473-3099(20)30327-3. [DOI] [PubMed] [Google Scholar]
- 261.Mookherjee N., Anderson M.A., Haagsman H.P., Davidson D.J. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 2020:1–22. doi: 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- 262.Li Z., Hu Y., Yang Y., Lu Z., Wang Y. Antimicrobial resistance in livestock: antimicrobial peptides provide a new solution for a growing challenge. Anim. Front. 2018;8:21–29. doi: 10.1093/af/vfy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang S., Zeng X., Yang Q., Qiao S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016;17:603. doi: 10.3390/ijms17050603. [DOI] [PMC free article] [PubMed] [Google Scholar]