Abstract
Background
The H3N2 component of egg-based 2017–2018 influenza vaccines possessed an adaptive substitution that alters antigenicity. Several influenza vaccines include antigens that are produced through alternative systems, but a systematic comparison of different vaccines used during the 2017–2018 season has not been completed.
Methods
We compared antibody responses in humans vaccinated with Fluzone (egg-based, n = 23), Fluzone High-Dose (egg-based, n = 16), Flublok (recombinant protein–based, n = 23), or Flucelvax (cell-based, n = 23) during the 2017–2018 season. We completed neutralization assays using an egg-adapted H3N2 virus, a cell-based H3N2 virus, wild-type 3c2.A and 3c2.A2 H3N2 viruses, and the H1N1 vaccine strain. We also performed enzyme-linked immunosorbent assays using a recombinant wild-type 3c2.A hemagglutinin. Antibody responses were compared in adjusted analysis.
Results
Postvaccination neutralizing antibody titers to 3c2.A and 3c2.A2 were higher in Flublok recipients compared with Flucelvax or Fluzone recipients (P < .01). Postvaccination titers to 3c2.A and 3c2.A2 were similar in Flublok and Fluzone High-Dose recipients, though seroconversion rates trended higher in Flublok recipients. Postvaccination titers in Flucelvax recipients were low to all H3N2 viruses tested, including the cell-based H3N2 strain. Postvaccination neutralizing antibody titers to H1N1 were similar among the different vaccine groups.
Conclusions
These data suggest that influenza vaccine antigen match and dose are both important for eliciting optimal H3N2 antibody responses in humans. Future studies should be designed to determine if our findings directly impact vaccine effectiveness.
Clinical Trials Registration
Keywords: influenza, hemagglutinin, vaccine, antibodies
We measured antibody responses in humans vaccinated with Fluzone (egg-based), Fluzone High-Dose (egg-based), Flublok (recombinant protein–based), or Flucelvax (cell-based) during the 2017–2018 season. Our studies demonstrate that different influenza vaccine platforms elicit substantially different antibody responses in humans.
Seasonal influenza epidemics cause up to 650 000 deaths worldwide each year [1]. In the United States, both trivalent and quadrivalent influenza vaccines are available. These vaccines include antigens from an H1N1 strain, an H3N2 strain, and 1 or 2 influenza B strains. Most influenza vaccines contain antigens that are prepared from viruses grown in fertilized chicken eggs. This is not ideal because viral strains often acquire adaptive mutations that alter antigenicity when propagated in eggs [2–4]. This has been particularly problematic with contemporary H3N2 egg-adapted vaccine strains used since 2016, which are antigenically mismatched with circulating H3N2 strains [5, 6]. H3N2 vaccine effectiveness (VE) was very low during the 2016–2017 (33%; 95% confidence interval [CI], 23%–41%) and 2017–2018 (22%; 95% CI, 12%–31%) influenza seasons [7, 8].
Alternative technologies for producing influenza vaccine antigens include cell-based and recombinant protein–based strategies. We previously demonstrated that a recombinant hemagglutinin (HA)–based vaccine (Flublok) elicited higher neutralizing antibody titers against circulating 3c2.A H3N2 strains compared with egg-based (Fluzone) and cell-based (Flucelvax) vaccines in humans during the 2016–2017 season [5]. During that season, the Flublok vaccine possessed an H3 HA that was well matched to circulating 3c2.A H3N2 strains, whereas the Fluzone and Flucelvax vaccines possessed an H3 HA that was egg-adapted and antigenically mismatched [5]. During the 2017–2018 influenza season, an egg-adapted H3N2 strain continued to be used in the Fluzone vaccine; however, for the first time, a unique cell-based H3N2 strain was approved for use in the Flucelvax vaccine [9]. Nonetheless, an observational study in older adults demonstrated only small increases (11%; 95% CI, 8%–14%) in relative VE for the cell-based Flucelvax vaccine compared with egg-based Fluzone vaccine during the 2017–2018 season [10]. This comparative VE study did not evaluate the recombinant protein–based Flublok vaccine since so few people received this vaccine during the 2017–2018 season.
It is important to characterize antibody responses elicited by different influenza vaccines and determine how these antibody responses relate to differences in VE. To date, there has not been a comprehensive analysis of antibody responses elicited in humans who received different 2017–2018 influenza vaccines. Here, we completed immunogenicity studies using sera collected from humans vaccinated with Fluzone, Fluzone High-Dose, Flublok, or Flucelvax during the 2017–2018 influenza season.
METHODS
Study Population
We collected sera from 85 healthy adults (age 18–49 years) immediately before and 28 days after receipt of a 2017–2018 influenza vaccine. All participants received a single dose of 1 licensed influenza vaccine (Fluzone, Fluzone High-Dose, Flublok, or Flucelvax). All vaccinations and serum collections were completed at the University of Rochester, and all serological assays were completed with deidentified samples at the University of Pennsylvania. Informed consent was obtained from all participants. The study was approved by the University of Rochester and the University of Pennsylvania institutional review boards.
Vaccines
Fluzone, Fluzone High-Dose, Flublok, and Flucelvax were administered intramuscularly to participants. Supplementary Table 1 summarizes the differences between these vaccines. Fluzone and Fluzone High-Dose antigens were prepared in eggs, Flublok antigens were produced via a baculovirus expression system in insect cells, and Flucelvax antigens were produced in Madin-Darby canine kidney (MDCK) cells. Fluzone and Fluzone High-Dose included H3 antigens from an egg-adapted A/Hong Kong/4801/2014 H3N2 virus, Flublok contained recombinant H3 antigens based on the original A/Hong Kong/4801/2014 H3N2 virus, and Flucelvax included H3 antigens from the cell-based A/Singapore/GP2050/2015 virus. All vaccines included antigens from the A/Michigan/45/2015 H1N1 virus. Fluzone, Flublok, and Flucelvax were quadrivalent vaccines that contained antigens from B/Brisbane/60/2008 and B/Phuket/3073/2013, whereas Fluzone High-Dose was trivalent and only contained influenza B virus antigens from B/Brisbane/60/2008.
Viruses for Serological Testing
Viruses expressing the HA from A/Hong Kong/4801/2014 (3c2.A), A/Hong Kong/4801/2014 egg-adapted (3c2.A/egg), A/Singapore/GP2050/2015 cell-adapted (3c2.A/cell), and A/Pennsylvania/49/2018 (3c2.A2) were generated through reverse genetics [11]. Relative to the 3c2.A HA, the 3c2.A/egg HA possessed N96S, T160K, L194P, and D225N substitutions, and the 3c2.A/cell HA possessed R142K and Q197R substitutions. The 3c2.A2 strain is an antigenically advanced virus from 2018 that possessed the T131K, R142K, and R261Q HA substitutions relative to the 3c2.A strain. The neuraminidase (NA) from the wild-type 3c2.A strain was included in all reverse genetics–derived H3N2 viruses. Virus expressing HA and NA from the A/Michigan/45/2015 H1N1 virus was also generated through reverse genetics. Viruses were rescued after transfecting cocultures of 293T and MDCK-SIAT1 cells with plasmids (pHW2000- or pDZ-based) encoding all 8 influenza virus gene segments. Viruses were rescued using the internal genes from A/Puerto Rico/8/1934 in combination with HA and NA genes of interest. Transfection supernatants were harvested 3 days after transfection and stored at ˗80°C. Transfection supernatants from H3N2 viruses were used directly in experiments without expansion to minimize the chance of adaptive mutations, while the H1N1 virus was expanded once in fertilized chicken eggs prior to experiments. HA and NA genes of all viruses were sequenced to confirm that no additional mutations were introduced during these processes.
Serological Assays
Foci reduction neutralization tests (FRNTs) and enzyme-linked immunosorbent assays (ELISAs) were completed as described in detail in the Supplementary Materials. FRNT titers report the reciprocal of the highest dilution of sera that reduced the number of foci by at least 90% relative to control wells that had no serum added. ELISA measured anti-HA immunoglobulin (Ig) G concentrations using recombinant A/Hong Kong/4801/2014 HA protein as the coating antigen.
Statistical Analyses
Pre and postvaccination replicate titers are presented as geometric mean titers (GMTs). To investigate associations with prevaccination titers, scaled log2 prevaccination GMTs were regressed against vaccination history (receipt of influenza vaccine in neither, only 1, or both of the last 2 seasons, as a factor variable) and scaled year of birth. Linear models that included vaccination history and vaccine group (Flublok, Flucelvax, Fluzone, and Fluzone High-Dose) as factor variables and scaled year of birth and scaled log2 prevaccination titers as continuous variables were similarly fitted to log2 postvaccination GMTs. With the above variables included, sex had no significant effect on any absolute prevaccination or postvaccination titers and was thus excluded as a variable. The rate of seroconversion, defined as a 4-fold or greater ratio of postvaccination GMT to prevaccination GMT, was fitted via logistic regression with a logit link function, again adjusting for vaccination history, vaccine group, year of birth, and log2 prevaccination titer. For all regressions, a threshold of α = 0.05 was used, and associated P values are reported after Bonferroni corrections for multiple tests. We conducted all analyses with deidentified data using R 3.5.0 (R Foundation for Statistical Computing) and GraphPad Prism version 8 (GraphPad Software).
RESULTS
Participant Characteristics
We examined antibody responses in 85 participants (36 males and 49 females) pre and 28 days postvaccination during the 2017–2018 season. The median age of participants in our study at enrollment was 23 years (range, 18–49 years). Each participant was vaccinated with Fluzone (egg-based, n = 23), Fluzone High-Dose (egg-based, n = 16), Flublok (recombinant protein–based, n = 23), or Flucelvax (cell-based, n = 23). Fluzone, Flublok, and Flucelvax are quadrivalent vaccines (possessing H1, H3, and 2 influenza B HAs), whereas Fluzone High-Dose is a trivalent vaccine (possessing H1, H3, and 1 influenza B HA). Fluzone and Flucelvax include 15 μg of each HA, Flublok includes 45 μg of each HA, and Fluzone High-Dose includes 60 μg of each HA [12, 13]. Participants assigned to different vaccine groups did not differ significantly in age, sex, vaccination history, or prior enrollment (see Table 1).
Table 1.
Participant Profiles
| Assigned Treatment Group, No. (%) | ||||
|---|---|---|---|---|
| Flublok | Flucelvax | Fluzone | Fluzone High-Dose | |
| Number in group | 23 | 23 | 23 | 16 |
| Median age (range), y | 23 (20–43) | 25 (18–49) | 22 (19–49) | 23 (18–48) |
| Female sex | 15 (65) | 15 (65) | 12 (52) | 7 (44) |
| Prior season’s vaccination | ||||
| Neither of past 2 years | 2 (9) | 3 (13) | 5 (22) | 4 (25) |
| One of past 2 years | 4 (17) | 5 (22) | 5 (22) | 4 (25) |
| Both of past 2 years | 17 (74) | 15 (65) | 13 (57) | 8 (50) |
| Enrolled in 2016–2017 | 6 (26) | 8 (35) | 6 (26) | 0 (0) |
Differences between groups are not significant (P > .05) for all listed traits. See Table 2 for prevaccination titers in each group.
Antibody Responses Against Wild-type H3N2 Viruses
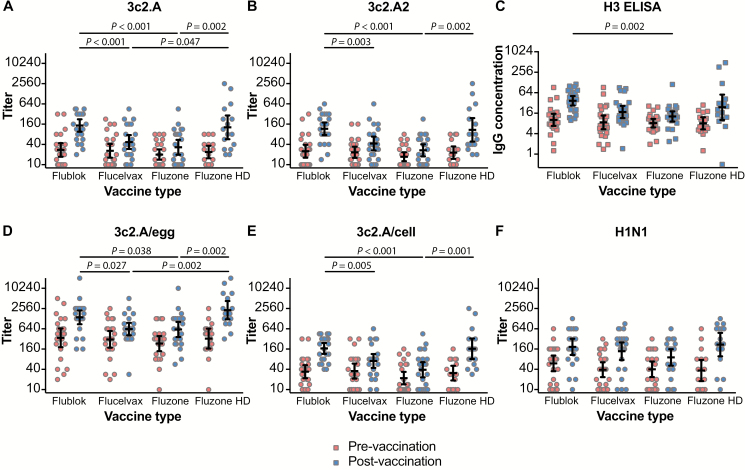
First, we completed neutralization assays (FRNTs) using wild-type 3c2.A and 3c2.A2 H3N2 viruses, which circulated in humans during the 2014–2015 and 2017–2018 seasons, respectively. We included a virus from the 2014–2015 season because the H3N2 components of 2017–2018 influenza vaccines were based on viruses that circulated during that season. We found that postvaccination titers to wild-type 3c2.A and 3c2.A2 viruses were approximately 3.9- to approximately 4.3-fold higher using sera from participants vaccinated with Flublok compared with participants vaccinated with Fluzone (P < .001 in adjusted analysis; Figure 1A, 1B, Table 2; Supplementary Table 2). Surprisingly, despite possessing a cell-based H3, Flucelvax elicited wild-type 3c2.A and 3c2.A2 H3N2 antibody titers that were similar to titers elicited by Fluzone and significantly lower compared with antibody titers elicited by Flublok (P < .001 and P = .003, respectively, in adjusted analysis; Figure 1A, 1B and Table 2; Supplementary Table 2). We also measured antibody titers using ELISAs coated with recombinant wild-type 3c2.A HA. Unlike FRNTs that only detect neutralizing antibodies, recombinant HA ELISAs detect both neutralizing and nonneutralizing antibodies. We found that anti-H3 ELISA titers closely mirrored FRNT titers. Participants who received Flublok had approximately 2.1- to approximately 3.0-fold higher wild-type 3c2.A HA ELISA titers compared with participants who received Flucelvax or Fluzone (P = .076 and P = .002, respectively, in adjusted analysis; Figure 1C and Table 2; Supplementary Table 2). Our observation that the 2017–2018 Fluzone and Flucelvax vaccines elicited weak FRNT and anti-H3 ELISA antibody titers against wild-type H3N2 viruses is consistent with the relatively low effectiveness of these vaccines during the 2017–2018 season [8, 10].
Figure 1.
Pre- and postvaccination titers in sera from individuals vaccinated with Flublok, Flucelvax, Fluzone, or Fluzone HD during the 2017–2018 season. Thick horizontal lines show the geometric mean titers and 95% confidence intervals. Significant P values (<.05) for postvaccination titers adjusted for prevaccination titers, year of birth, and vaccination history and adjusted for multiple comparisons are indicated above each graph. A, Neutralizing antibody titers (foci reduction neutralization test [FRNT]90) to wild-type 3c2.A virus. B, Neutralizing antibody titers (FRNT90) to wild-type 3c2.A2 virus. C, Hemagglutinin ELISA IgG concentrations to wild-type 3c2.A virus (mg/mL). D, Neutralizing antibody titers (FRNT90) to 3c2.A/egg virus. E, Neutralizing antibody titers (FRNT90) to 3c2.A/cell virus. F, Neutralizing antibody titers (FRNT90) to H1N1 virus. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HD, high-dose; Ig, immunoglobulin.
Table 2.
Geometric Mean Titers With 95% Confidence Intervals Before Vaccination (Pre) and 28 Days After Vaccination (Post) With Flublok, Flucelvax, Fluzone, or Fluzone High-Dose
| Flublok | Flucelvax | Fluzone | Fluzone High-Dose | |
|---|---|---|---|---|
| 3c2.A | ||||
| Pre | 27 (17–44) | 26 (16–41) | 20 (14–28) | 24 (16–36) |
| Post | 146 (95–224) | 47 (29–76) | 33 (20–55) | 129 (57–290) |
| 3c2.A2 | ||||
| Pre | 25 (16–39) | 23 (16–34) | 17 (13–23) | 23 (15–35) |
| Post | 117 (76–179) | 42 (27–68) | 27 (18–40) | 108 (49–242) |
| Enzyme-linked immunosorbent assay | ||||
| Pre | 10 (7–15) | 9 (5–14) | 8 (6–11) | 8 (5–12) |
| Post | 37 (27–51) | 17 (12–26) | 13 (9–18) | 24 (10–56) |
| 3c2.A/egg | ||||
| Pre | 345 (182–654) | 311 (176–549) | 233 (139–391) | 327 (165–647) |
| Post | 1401 (876–2242) | 630 (411–967) | 612 (366–1023) | 2297 (1230–4289) |
| 3c2.A/cell | ||||
| Pre | 34 (22––53) | 35 (21–59) | 22 (14–34) | 31 (19–51) |
| Post | 167 (115–243) | 71 (44–114) | 39 (23–65) | 164 (81–329) |
| H1N1 | ||||
| Pre | 60 (35–103) | 39 (23–64) | 40 (23–68) | 37 (18–76) |
| Post | 186 (107–323) | 138 (75–251) | 90 (51–159) | 217 (97–484) |
Effects of Increasing Antigen Dose on Antibody Responses Against Wild-type H3N2 Viruses
Given that Flublok and Flucelvax both possess HAs that are not egg-adapted, it is important to determine why the 2017–2018 formulation of Flublok, but not Flucelvax, elicited strong antibody responses against wild-type H3N2 viruses. Flublok possesses a wild-type HA and also has more HA antigen relative to Fluzone and Flucelvax. To determine if increased amounts of egg-adapted HA antigens elicit stronger antibody responses against wild-type 3c2.A viruses, we measured antibody responses in participants who received Fluzone High-Dose vaccine. The Fluzone High-Dose vaccine is not a perfect comparator to Flublok since it contains 4 times the amount of HA compared with Fluzone and Flucelvax, whereas Flublok contains only 3 times the amount of HA compared with these standard-dose vaccines. Nonetheless, the Fluzone High-Dose vaccine allowed us to analyze the effects of increasing vaccine dose on the induction of wild-type H3N2 antibody responses. We found that participants who received Fluzone High-Dose mounted significantly better FRNT antibody responses against wild-type H3N2 viruses compared with participants who received the standard dose of Fluzone (approximately 3.2-fold higher titers; 95% CI, 1.8–5.8; P = .002 for 3c2.A virus and approximately 3.2-fold higher titers,= 95% CI, 1.8–5.7; P = .002 for 3c2.A2 virus in adjusted analysis; Figures 1 and 2 and Table 2; Supplementary Table 2). Postvaccination FRNT titers to wild-type H3N2 viruses were similar between the Fluzone High-Dose and Flublok groups (Figure 1A, 1B and Table 2; Supplementary Table 2); however, a larger fraction of participants seroconverted to wild-type H3N2 viruses following vaccination with Flublok. Twelve participants in the Flublok group (52%) and 6 participants in the Fluzone High-Dose group (38%) seroconverted to 3c2.A virus, whereas 14 participants in the Flublok group (61%) and 6 participants in the Fluzone High-Dose group (38%) seroconverted to 3c2.A2 virus. These differences were marginally significant after adjusting for age, prevaccination titers, and vaccination history in logistic regression but not for multiple tests (P = .056 and P = .022, respectively, before Bonferroni correction). Taken together, these data suggest that vaccine antigen match and vaccine dose are both important in eliciting optimal antibody responses against contemporary wild-type H3N2 viruses.
Antibody Responses Against Egg-adapted and Cell-adapted H3N2 Viruses
Next, we completed FRNTs using the egg-adapted H3N2 strain that was in Fluzone and Fluzone High-Dose. Most participants had higher preexisting antibody titers against 3c2.A/egg H3N2 viruses compared with wild-type H3N2 viruses (Figure 1D and Table 2). This is unsurprising since most humans have already encountered antigenically similar viruses either through vaccination (the 2016–2017 H3N2 component was identical to the 2017–2018 H3N2 component) or through past infections (the 3c2.A/egg H3N2 virus is antigenically similar to H3N2 viruses that circulated prior to 2014). In our study, 71 of 85 (85%) participants had been vaccinated in at least 1 of the 2 prior seasons. Postvaccination titers against the 3c2.A/egg virus were approximately 2- to 3-fold higher in participants vaccinated with Flublok and Fluzone High-Dose compared with participants vaccinated with Fluzone or Flucelvax (P ≤ .04 in adjusted analysis; Figure 1D and Table 2; Supplementary Table 2). Postvaccination titers against the 3c2.A/egg virus were not significantly different between Flublok and Fluzone High-Dose recipients.
We also completed FRNTs with viruses that possessed the 3c2.A/cell HA that was in Flucelvax. The H3N2 component of the cell-based Flucelvax does not possess the same egg-adapted HA substitutions that are in Fluzone but does have several HA substitutions relative to the wild-type HA in Flublok (Supplementary Table 1). We expected to find that Flucelvax elicited higher titers to the 3c2.A/cell virus, but both Flucelvax and Fluzone elicited poor antibody responses against viruses with 3c2.A/cell HA (Figure 1E and Table 2). Similar to what we found in FRNTs using wild-type and egg-adapted 3c2.A viruses, Flublok elicited significantly higher antibody titers against 3c2.A/cell virus compared with both Flucelvax and Fluzone (approximately 2.6- and approximately 3.9-fold, respectively; P ≤ .005 in adjusted analysis; Supplementary Table 2). Fluzone High-Dose also elicited higher titers than Fluzone (approximately 3.2-fold, P = .001) and Flucelvax (approximately 2.1-fold), but differences with the latter were not significant in adjusted analysis after correction for multiple tests (P = .105; Figure 1E and Table 2; Supplementary Table 2).
Antibody Responses Against the H1N1 Component
We also completed FRNTs using the 2017–2018 H1N1 vaccine strain since this strain is antigenically similar among the different vaccines [9]. We found that all 4 vaccines elicited similar H1N1 FRNT antibody titers (Figure 1F and Table 2; Supplementary Table 2). Postvaccination titers to H1N1 were approximately 0.6-fold lower in sera from individuals who received Fluzone compared with Flublok and Flucelvax recipients, but these differences were not statistically significant (P = .061 and P = .090 after adjusting for age, prevaccination titer, and vaccination history, but not multiple tests). Postvaccination Fluzone titers also trended lower compared with those from Fluzone High-Dose recipients (P = .02 in Wilcoxon 1-sided rank sum test without multiple test adjustment; P = .007 after adjusting for age, prevaccination titer, and vaccination history, but not multiple tests). Postvaccination H1N1 titers were not significantly different in Flublok recipients compared with Flucelvax or Fluzone High-Dose recipients.
Prior Influenza Vaccination Is Associated With Decreased Postvaccination Antibody Titers
Many studies have reported a negative effect of prior vaccination on influenza vaccine immunogenicity [5, 14–18] and effectiveness [19–22], although such effects are clearly not universal [18, 23, 24]. We found that prior influenza vaccination is associated with higher prevaccination titers to 3c2.A/egg, 3c2.A/cell, and H1N1 viruses. However, when adjusted for multiple tests, these effects remained significant only for H1N1 (an approximately 3.2-fold increase; Supplementary Table 3). After adjusting via multivariate regression for initial titer, birth year, and vaccine group, influenza vaccination in previous seasons appeared to negatively affect postvaccination titers (Supplementary Table 2). Compared with people vaccinated in both prior seasons, newly vaccinated participants had approximately 2.4- to approximately 2.9-fold higher postvaccination titers to all viruses tested (Supplementary Figure 1, Supplementary Tables 2, and 4). Younger participants had higher prevaccination titers to most H3N2 viruses and H1N1 after adjusting for vaccination history (Supplementary Table 3). Younger individuals also had higher postvaccination titers to H1N1 (P < .001 in adjusted analysis), but no linear age effect was observed for postvaccination titers to any of the H3N2 viruses tested.
DISCUSSION
In this study, we found that influenza vaccine antigen match and dose each appear to influence antibody responses. We could not definitively separate the effects of antigen match vs antigen dose in our experiments since there were various antigen types and doses in each vaccine. Both vaccine types with increased antigen (Flublok, which has 3× antigen relative to standard vaccines, and Fluzone High-Dose, which has 4× antigen relative to standard vaccines) elicited robust antibody responses to multiple H3N2 viruses in our experiments. It is difficult to directly compare Fluzone High-Dose to Flublok since Fluzone High-Dose has more antigen relative to Flublok, while Flublok has an H3 with a better antigenic match to viruses that circulated during the 2017–2018 season.
The Fluzone High-Dose vaccine is typically used in individuals aged >65 years and has been shown to elicit strong antibody responses and have higher VE in this population relative to standard-dose vaccines [25]. We found that individuals in our study who were aged 18–49 years produced stronger antibody responses to wild-type 3c2.A viruses upon vaccination with Fluzone High-Dose compared with the standard dose of Fluzone. While seroconversion to wild-type 3c2.A viruses was lower in individuals who received Fluzone High-Dose compared with individuals who received Flublok, our studies raise the possibility that Fluzone High-Dose should be considered for use in younger individuals to partially circumvent problems associated with H3N2 egg-adaptive substitutions. One limitation of our study is that our sample size for each vaccine type was relatively small, and additional studies should explore the immunogenicity of higher-dose vaccines in nonelderly adults using larger sample sizes.
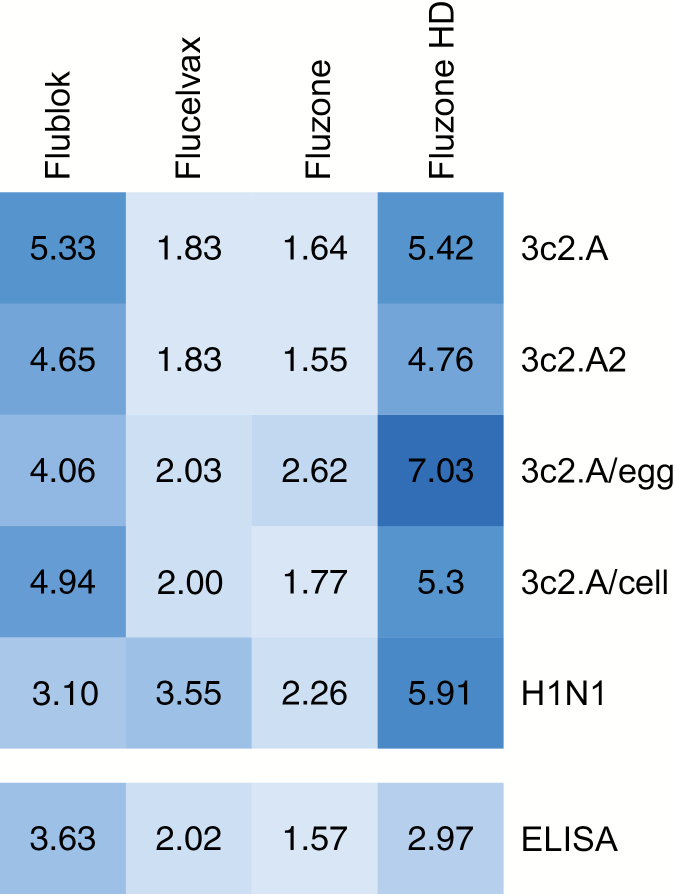
Further studies need to be completed to determine if current formulations of Flublok are more effective against 3c2.A H3N2 viruses relative to cell-based and egg-based vaccines. Flublok was not included in a recent VE comparative study [10] since so few people received this vaccine during the 2017–2018 season. Future studies also need to be completed to determine why the H3 component of Flucelvax appears to be poorly immunogenic relative to the H3 component of Flublok. It is possible that this simply relates to the different doses of antigen in each vaccine, but this seems unlikely since both vaccines elicited similar H1N1 responses (Figure 2). The different immunogenicity of the H1 and H3 components of Flucelvax might be due to modifications of these antigens as they were produced in MDCK cells. Previous studies have shown that HAs produced in mammalian cells have complex glycans [26, 27], which might mask key neutralizing sites and reduce overall immunogenicity [28]. Contemporary H3N2 viruses possess more glycosylation sites in the HA globular head compared with current H1N1 viruses [29], and it is possible that complex glycans on the globular head of the H3 component of the 2017–2018 Flucelvax vaccine decreased immunogenicity. Flublok includes HA antigens that were produced in insect cells, which have less complex glycosylation machinery [26, 27]. It is possible that the H3 component of Flublok is immunogenic because it possesses less complex glycans on the HA globular head domain, although further studies are required to mechanistically address this possibility. Studies to investigate the basis of reduced immunogenicity and possibly reduced VE after repeat vaccination will also likely be important since we found that the effects of repeat vaccination on titers were often as large as the effects of different vaccines (see Supplementary Table 2).
Figure 2.
Heat map of fold change in geometric mean neutralizing antibody levels (foci reduction neutralization test90) and ELISA titers (immunoglobulin G concentration) upon influenza vaccination during the 2017–2018 season. Postvaccination titers were divided by prevaccination titers to calculate fold change. Darker colors indicate higher fold changes. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HD, high-dose.
Taken together, we demonstrate that different vaccines that are currently being used in humans have different abilities to elicit neutralizing levels of antibodies against contemporary H3N2 virus strains. We strongly support ongoing efforts to measure the VE of these different vaccines to determine if our immunogenicity data are predictive of protection from community-acquired influenza virus infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Theresa Eilola and Madison Weirick for technical assistance.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (1R01AI113047 to S. E. H., 1R01AI108686 to S. E. H., and Centers of Excellence for Influenza Research and Surveillance HHSN272201400005C to A. B., D. J. T., S. C., and S. E. H.). S. E. H. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Potential conflicts of interest. S. E. H. reports receiving consulting fees from Sanofi Pasteur (who produces Fluzone, Fluzone High-dose, and Flublok), Lumen, Novavax, and Merck. A. B. reports receiving consulting fees from GlaxoSmithKline (GSK) and Merck and grant support from Merck, Pfizer, and Janssen unrelated to influenza vaccination. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Iuliano AD, Roguski KM, Chang HH, et al. ; Global Seasonal Influenza-associated Mortality Collaborator Network Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu NC, Zost SJ, Thompson AJ, et al. . A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog 2017; 13:e1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skowronski DM, Janjua NZ, De Serres G, et al. . Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza— the need for a universal influenza vaccine. N Engl J Med 2018; 378:7–9. [DOI] [PubMed] [Google Scholar]
- 5. Zost SJ, Parkhouse K, Gumina ME, et al. . Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine MZ, Martin ET, Petrie JG, et al. . Antibodies against egg- and cell-grown influenza A(H3N2) viruses in adults hospitalized during the 2017–2018 season. J Infect Dis 2019; DOI:10.1093/infdis/jiz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2004–2018 Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed 26 April 2019.
- 8. Rolfes MA, Flannery B, Chung J, et al. . Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; DOI:10.1093/cid/ciz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Candidate vaccine viruses and potency testing reagents for development and production of vaccines for use in the northern hemisphere 2017–2018 influenza season. Available at: http://www.who.int/influenza/vaccines/virus/candidates_reagents/2017_18_north/en/. Accessed 1 February 2019.
- 10. Izurieta HS, Chillarige Y, Kelman J, et al. . Relative effectiveness of cell-cultured and egg-based influenza vaccines among the U.S. elderly, 2017–18. J Infect Dis 2018; DOI:10.1093/infdis/jiy716. [DOI] [PubMed] [Google Scholar]
- 11. Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of hemagglutinin residues responsible for H3N2 antigenic drift during the 2014–2015 influenza season. Cell Rep 2015; 12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunkle LM, Izikson R, Patriarca PA, Goldenthal KL, Muse D, Cox MMJ. Randomized comparison of immunogenicity and safety of quadrivalent recombinant versus inactivated influenza vaccine in healthy adults 18–49 years of age. J Infect Dis 2017; 216:1219–26. [DOI] [PubMed] [Google Scholar]
- 13. Manini I, Domnich A, Amicizia D, et al. . Flucelvax (Optaflu) for seasonal influenza. Expert Rev Vaccines 2015; 14:789–804. [DOI] [PubMed] [Google Scholar]
- 14. Nabeshima S, Kashiwagi K, Murata M, Kanamoto Y, Furusyo N, Hayashi J. Antibody response to influenza vaccine in adults vaccinated with identical vaccine strains in consecutive years. J Med Virol 2007; 79:320–5. Available at: http://doi.wiley.com/10.1002/jmv.20801. Accessed 12 September 2019. [DOI] [PubMed] [Google Scholar]
- 15. Thompson MG, Naleway A, Fry AM, et al. . Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010–11. Vaccine 2016; 34:981–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26813801. Accessed 12 September 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leung VKY, Carolan LA, Worth LJ, et al. . Influenza vaccination responses: evaluating impact of repeat vaccination among health care workers. Vaccine 2017; 35:2558–68. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0264410X17303900. Accessed 12 September 2019. [DOI] [PubMed] [Google Scholar]
- 17. Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet (London, England) 1979; 1:33–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/83475. Accessed 12 September 2019. [DOI] [PubMed] [Google Scholar]
- 18. Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997; 15:1114–22. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9269055. Accessed 12 September 2019. [DOI] [PubMed] [Google Scholar]
- 19. Ohmit SE, Petrie JG, Malosh RE, et al. . Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013; 56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohmit SE, Thompson MG, Petrie JG, et al. . Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24235265. Accessed 12 September 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skowronski DM, Chambers C, Sabaiduc S, et al. . A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. Available at: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/ciw176. Accessed 12 September 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McLean HQ, Thompson MG, Sundaram ME, et al. . Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trieu M-C, Jul-Larsen Å, Sævik M, et al. . Antibody responses to influenza A/H1N1pdm09 virus after pandemic and seasonal influenza vaccination in healthcare workers: a 5-year follow-up study. Clin Infect Dis 2019; 68:382–92. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29893797. Accessed 12 September 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beyer WEP, de Bruijn IA, Palache AM, Westendorp RGJ, Osterhaus ADME. Protection against influenza after annually repeated vaccination. Arch Intern Med 1999; 159:182. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9927102. Accessed 12 September 2019. [DOI] [PubMed] [Google Scholar]
- 25. Robertson CA, DiazGranados CA, Decker MD, Chit A, Mercer M, Greenberg DP. Fluzone® high-dose influenza vaccine. Expert Rev Vaccines 2016; 15:1495–505. [DOI] [PubMed] [Google Scholar]
- 26. An Y, Rininger JA, Jarvis DL, et al. . Comparative glycomics analysis of influenza hemagglutinin (H5N1) produced in vaccine relevant cell platforms. J Proteome Res 2013; 12:3707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. An Y, Parsons LM, Jankowska E, Melnyk D, Joshi M. N-glycosylation of seasonal influenza vaccine hemagglutinins: implication for potency testing and immune processing. J Virol 2018; 93:pii: e01693–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tseng Y-C, Wu C-Y, Liu M-L, et al. . Egg-based influenza split virus vaccine with monoglycosylation induces cross-strain protection against influenza virus infections. Proc Natl Acad Sci 2019; 116:4200–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. York IA, Stevens J, Alymova IV. Influenza virus N-linked glycosylation and innate immunity. Biosci Rep 2019; 39:DOI:10.1042/BSR20171505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.