Abstract
Background
To optimize utility of laboratory testing for Clostridiodes difficile infection (CDI), the 2017 Infectious Diseases Society of America–Society for Healthcare Epidemiology of America (IDSA-SHEA) clinical practice guidelines recommend excluding patients from stool testing for C. difficile if they have received laxatives within the preceding 48 hours. Sparse data support this recommendation.
Methods
Patients with new-onset diarrhea (≥3 bowel movements in any 24-hour period in the 48 hours before stool collection) and a positive stool C. difficile nucleic acid amplification test were enrolled. Laxative use within 48 hours before stool testing, severity of illness (defined by 4 distinct scoring methods), and clinical outcomes were recorded.
Results
209 patients with CDI were studied, 65 of whom had received laxatives. There were no significant differences in the proportion of patients meeting severe CDI criteria by 4 severity scoring methods in patients receiving versus not receiving laxatives (66.2% vs 56.3%, respectively; P = .224) by IDSA-SHEA, the primary scoring system. Similar rates of serious outcomes attributable to CDI, including death, intensive care unit admission, and colectomy, were observed in the laxative and no laxative groups.
Conclusions
Our study found similar rates of severe CDI and serious CDI-attributable clinical outcomes in CDI-diagnosed patients who did or did not receive laxatives. Precluding recent laxative users from CDI testing, as proposed by the IDSA-SHEA guideline, carries a potential for harm due to delayed diagnosis and treatment.
Keywords: Clostridiodes difficile infection, Clostridiodes difficile colonization, laxative, diarrhea
Clostridiodes difficile infection severity and outcomes did not differ between those who had or had not received laxatives within 48 hours prior to diagnosis. These findings challenge the IDSA-SHEA guideline to exclude patients receiving laxatives from C. difficile testing.
(See the Editorial Commentary by Rock and Maragakis on pages 1479–80.)
In the United States, Clostridiodes difficile is estimated to cause approximately 500 000 infections and 30 000 deaths annually [1]. Clostridiodes difficile infection (CDI) diagnosis rates continue to be at historic highs, partly due to inappropriate specimens sent for C. difficile testing and the use of highly sensitive assays such as nucleic acid amplification tests (NAATs) [2]. NAATs can detect asymptomatic C. difficile colonization, which may be present in as many as 22% of hospitalized inpatients [3]. Diarrhea is also common, observed in 12.4–32.9% of inpatients; yet, CDI is the cause in less than 7.4% of cases [4, 5]. Public reporting of hospital CDI rates and implementation of pay-for-performance measures have further increased pressure to control and prevent CDI and distinguish asymptomatic colonization from true infection.
To increase the relevance of a positive test, the 2017 Infectious Diseases Society of America–Society for Healthcare Epidemiology of America (IDSA-SHEA) clinical practice guidelines emphasize testing only patients likely to have true CDI. The guidelines recommend that laboratories reject formed specimens, that testing only be sent for patients with at least 3 episodes of loose stool in a 24-hour period, and that patients be excluded from stool testing if a laxative was given within the preceding 48 hours [6]. Evidence cited to support the laxative-use restriction consists only of observations that many patients tested for CDI have recently received laxatives. Specifically, the guidelines refer to a study that found that 19% of a cohort who tested positive for C. difficile had also received laxatives within 48 hours prior to diagnosis, but the impact of laxative use on CDI diagnosis was not examined [7]. Moreover, the guidelines do not define meaningful laxative use (eg, total number of doses) nor allow room for clinical judgment when other features signal infection.
Still, many institutions have adopted the recommendation to exclude patients receiving laxatives from C. difficile testing [8, 9]. Out of concern that this restriction may delay or miss diagnoses, this study was performed to test the a priori primary hypothesis that among patients with NAAT-positive C. difficile, clinical outcomes and severity of illness do not differ between those who have or have not received laxatives.
METHODS
Hospital inpatients at Beth Israel Deaconess Medical Center (Boston, Massachusetts) were enrolled between 21 June 2016 and 6 July 2018. During this time period, there were no restrictions on provider ordering of C. difficile testing for patients receiving laxatives, although our microbiology laboratory would routinely reject formed stool specimens. Eligible patients were 18 years or older with a positive clinical stool C. difficile NAAT result, were initiating CDI therapy, and had acute diarrhea, defined as (1) documentation of 3 or more unformed bowel movements during any 24 hours in the 48 hours before stool collection or (2) persistent diarrhea in the 48 hours before stool collection per medical notes. In the majority of cases definition “1” was applied. Patients were excluded if they had chronic diarrhea, if there was any doubt about the presence of diarrhea, if the specimen volume was insufficient or older than 72 hours, if they had received CDI treatment for more than 48 hours prior to stool collection, or if they had a colostomy [10]. During the study period, the C. difficile testing method at our institution was NAAT. In order to collect toxin data for research purposes, enrolled patients also had stool tested for C. difficile toxins A and B with an ultrasensitive quantitative single molecule array (Simoa) immunoassay, which can separately detect and quantify C. difficile toxins A and B over a 5-log range of concentrations with a clinical cutoff of 20 pg/mL in diluted stool samples [10].
Data Collection
Clinical outcomes and laboratory findings were gathered through chart review and patient phone calls. Outcomes assessed during the 40 days after diagnosis included the following: CDI recurrence (diarrhea that resolved for 48 hours off CDI therapy, but recurred and was documented as recurrence in provider notes) or severe outcomes including intensive care unit (ICU) admission, colectomy, and death. Two independent physicians who were unaware of laxative status determined whether severe outcomes were attributable to CDI, with discrepancies adjudicated by a third physician reviewer. Comorbidities were evaluated using the Charlson comorbidity index and immunocompromised status was defined as in Figure 1 [11]. Laboratory characteristics including peak white blood cell count (WBC), peak creatinine, and albumin nadir were recorded within 5 days preceding and 2 days following stool collection. If performed within 1 week of diagnosis, colonoscopy or flexible sigmoidoscopy reports were reviewed for the finding of pseudomembranes. The presence of colitis or ileus on abdominal imaging (abdominal X-ray or computed tomography) was noted if obtained within 48 hours of CDI diagnosis. Temperature of 38.0°C or higher, systolic blood pressure lower than 100 mm Hg, and peak lactate values were recorded within 24 hours of diagnosis. Abdominal tenderness was considered present if documented in a physician-administered physical examination the day prior to or the day of specimen collection. Receipt of antibiotics (within 48 hours) or laxatives (specific agents and number of doses within 24-, 48-, and 72-hour windows) prior to CDI diagnosis were determined from the electronic medication administration record.
Figure 1.
Criteria for immunocompromise. Abbreviation: HIV, human immunodeficiency virus.
Definition of Severe Clostridiodes difficile Infection
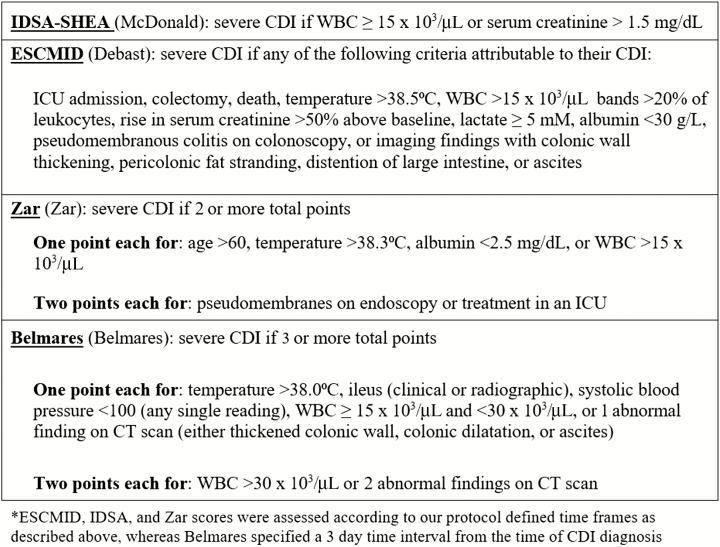
A severe CDI outcome was defined as any one of the following outcomes attributable to CDI: ICU admission, colectomy, or death. Severity of CDI was assessed using 4 severity scores: IDSA-SHEA, European Society of Microbiology and Infectious Diseases (ESCMID), Zar, and Belmares (Figure 2) [6, 11–14].
Figure 2.
Criteria for severe CDI by scoring system. ESCMID [12], IDSA-SHEA [6], and Zar [13] scores were assessed according to our protocol-defined time frames as described in the figure, whereas Belmares [14] specified a 3-day time interval from the time of CDI diagnosis. Abbreviations: CDI, Clostridiodes difficile infection; CT, computed tomography; ESCMID, European Society of Microbiology and Infectious Diseases; ISDA-SHEA, Infectious Diseases Society of America–Society for Healthcare Epidemiology of America; WBC, white blood cell count.
Statistical Analysis
Descriptive statistics included median and interquartile range (IQR) for continuous variables and frequency and percentages for categorical variables. Continuous and discrete variables were compared between groups using the Mann-Whitney U test and the chi-square or Fisher’s exact test, respectively. Results were considered statistically significant when P < .05. All statistical analyses were performed using SPSS 23.0 software (IBM Corporation). Additional details are described in the Supplementary Materials.
RESULTS
Of a total of 209 patients, 65 (31.1%) received at least 1 dose of laxative (LAX-48 group) and 144 (68.9%) patients received no laxatives (NO-LAX-48 group) within 48 hours prior to collection of the stool sample used for CDI diagnosis. Table 1 displays demographic and clinical characteristics, illustrating that the groups were demographically similar in baseline age, race, hospital unit at diagnosis, and immunocompromised host status (Table 1). Clinical parameters including peak WBC, lactate, fever, hypotension, and acute kidney injury did not differ significantly between the groups. In addition to acute diarrhea, most patients (82% of LAX-48 and 75% of NO-LAX-48) had at least 1 other clinical feature consistent with infection: fever, WBC of 15 × 103/mL or greater, hypotension, or abdominal tenderness. Nearly half of LAX-48 patients (47.7%) and over one-third of NO-LAX-48 patients (36.4%) exhibited a peak WBC of 15 × 103/mL or higher; this difference was not statistically significant. Radiographic findings of colitis and colonoscopic findings of pseudomembranes were not different between the groups; however, imaging and endoscopy were not performed for all patients. Antibiotic receipt within the preceding 48 hours was common in both groups, with a similar distribution by antibiotic class (Supplementary Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients Receiving Laxatives (LAX-48) or Not Receiving Laxatives (NO-LAX-48) Within 48 Hours Prior to Clostridiodes difficile Infection Diagnosis
| LAX-48 (n = 65) | NO-LAX-48 (n = 144) | P | |||
|---|---|---|---|---|---|
| Patient Characteristics | na | % | na | % | |
| Male (%) | 34 | 52.3 | 64 | 44.4 | .299 |
| Median (IQR) age, years | 61 (47–70) | 66 (54–77) | .944 | ||
| Race | .390 | ||||
| White | 48 | 73.8 | 97 | 67.4 | |
| African American | 9 | 13.8 | 20 | 13.9 | |
| Asian | 2 | 3.1 | 8 | 5.6 | |
| Hispanic | 2 | 3.1 | 11 | 7.6 | |
| Pacific Islander | 1 | 1.5 | 6 | 4.2 | |
| Unknown | 3 | 4.6 | 2 | 1.4 | |
| Hospital unit at diagnosis | .610 | ||||
| ICU | 8 | 12.3 | 16 | 11.1 | |
| Medical/surgical | 50 | 76.9 | 102 | 70.8 | |
| Oncology | 6 | 9.2 | 23 | 16.0 | |
| ED | 1 | 1.5 | 3 | 2.1 | |
| ICU stay within 1 week prior to diagnosis | 16 | 24.6 | 25 | 17.4 | .260 |
| Major surgery within 1 week prior to diagnosis | 9 | 13.8 | 9 | 6.3 | .107 |
| Immunocompromised | 18 | 27.7 | 51 | 35.4 | .341 |
| Charlson comorbidity index, median (IQR) | 3 (1–4) | 2 (1–4) | .053 | ||
| History of prior CDI | 13 | 20.0 | 44 | 30.6 | .132 |
| Number of prior CDI episodes | .300 | ||||
| 1 | 10 | 15.4 | 26 | 18.1 | |
| 2 | 2 | 3.1 | 11 | 7.6 | |
| ≥3 | 1 | 1.5 | 7 | 4.9 | |
| Stool consistency | .716 | ||||
| Liquid | 36 | 55.4 | 86 (n = 143) | 60.1 | |
| Semiformed | 28 | 43.1 | 56 (n = 143) | 39.2 | |
| Formedb | 1 | 1.5 | 1 (n = 143) | 0.7 | |
| Abdominal tenderness | 10 | 15.4 | 28 | 19.4 | .564 |
| Temperature ≥38.0°C within 24 hours of diagnosis | 12 | 18.5 | 33 | 22.9 | .586 |
| Systolic BP <100 mm Hg within 24 hours of diagnosis | 29 | 44.6 | 57 | 39.6 | .545 |
| Renal replacement therapy at baseline | 7 | 10.8 | 9 | 6.3 | .270 |
| AKI (peak Cr ≥1.5× baseline) | 6 (n = 60) | 10.0 | 14 (n = 141) | 9.9 | 1.000 |
| Median (IQR) WBC peak –5 days to +2 days of diagnosis, × 103/mL | 14.5 (7–18.7) | 12 (8–19.7) (n = 143) | .277 | ||
| WBC ≥15 × 103/mL | 31 | 47.7 | 52 (n = 143) | 36.4 | .130 |
| Median (IQR) albumin nadir –5 days to +2 days of diagnosis, g/dL | 3.0 (2.5–3.3) (n = 45) | 3.2 (2.7–3.6) (n = 121) | .045 | ||
| Median (IQR) lactate peak within 24 hours, mmol/L | 1.6 (1.2–1.8) (n = 11) | 1.6 (1.2–2.4) (n = 74) | .808 | ||
| Colitis on imaging | 7 (n = 21) | 33.3 | 42 (n = 78) | 53.8 | .140 |
| Colonoscopy or flexible sigmoidoscopy with pseudomembranes | 0 (n = 5) | 0.0 | 1 (n = 5) | 20.0 | 1.000 |
| BI/NAP1/027 strain | 11 | 16.9 | 19 | 13.2 | .524 |
| Received antibiotics within 48 hours prior to diagnosis | 40 | 61.5 | 77 | 53.5 | .296 |
Abbreviations: AKI, acute kidney injury; BP, blood pressure; CDI, Clostridiodes difficile infection; Cr, creatinine; ED, emergency department; ICU, intensive care unit; IQR, interquartile range; WBC, white blood count.
aUnless otherwise noted in parentheses for variables where not all patients had data obtained.
bConsidered as formed by our researchers, but previously categorized as unformed when accepted for testing by the microbiology lab.
Median Simoa toxin A + B levels did not differ significantly: 167.3 (IQR, 8.5–13 627) pg/mL for LAX-48 versus 214.4 (IQR, 6.7–15 671) pg/mL for NO-LAX-48 (P = .667). The proportion of patients with toxin A + B levels greater than 20 pg/mL also did not differ between the 2 groups (67.2% LAX-48 versus 63.4% NO-LAX-48; P = .639). Eleven of 65 (16.9%) LAX-48 patients and 19 of 144 (13.2%) NO-LAX-48 patients were positive for the BI/NAP1/027 strain (P = .524).
There were no statistically significant differences in severity of illness between the LAX-48 and NO-LAX-48 groups for each of the 4 severity grading systems (Table 2). The majority of patients in both groups met criteria for severe CDI by IDSA-SHEA (66.2% LAX-48 vs 56.3% NO-LAX-48; P = .224) and ESCMID (61.5% LAX-48 vs 60.4% NO-LAX-48; P = 1). Clinical outcomes were also compared; rates of death within 40 days (including CDI-attributable deaths) and ICU admission within 40 days (including CDI-attributable ICU admissions) were not different. One patient in each group required a colectomy due to severe CDI. A composite measure of severe outcomes also failed to show a difference between groups.
Table 2.
Severity of Illness and Clinical Outcomes in Patients Who Did Receive Laxatives (LAX-48) or Did Not Receive Laxatives (NO-LAX-48) Within 48 Hours Prior to Clostridiodes difficile Infection Diagnosis
| LAX-48 (n = 65) | NO-LAX-48 (n = 144) | P | |||
|---|---|---|---|---|---|
| Outcomes | na | % | na | % | |
| Severe CDI by IDSA-SHEA [6] | 43 | 66.2 | 81 | 56.3 | .224 |
| Severe CDI by ESCMID [12] | 40 | 61.5 | 87 | 60.4 | 1.000 |
| Severe CDI by Zar et al [13] | 33 | 50.8 | 67 | 46.5 | .654 |
| Severe CDI by Belmares et al [14] | 8 | 12.3 | 22 | 15.3 | .673 |
| Composite of severe attributable outcomes: ICU admission, colectomy, or death within 40 days of diagnosis | 7 | 10.8 | 13 | 9.0 | .800 |
| Death within 40 days | 3 | 4.6 | 12 | 8.3 | .401 |
| CDI contributing or primary cause | 1 | 1.5 | 4 | 2.8 | .659 |
| ICU stay within 40 days | 11 | 16.9 | 26 | 18.1 | 1.000 |
| CDI contributing or primary cause | 7 | 10.8 | 13 | 9.0 | .744 |
| Colectomy | 1 | 1.5 | 1 | 0.7 | .526 |
| CDI recurrence within 40 days | 2 | 3.1 | 8 | 5.6 | .728 |
| Median (IQR) length of hospital admission after CDI diagnosis, days | 8 (4–16) | 5 (3–11) | .031 | ||
| Median (IQR) days to resolution of diarrhea | 8 (3–15) (n = 52) | 5 (3–12) (n = 117) | .074 | ||
Abbreviations: CDI, Clostridiodes difficile infection; ESCMID, European Society of Microbiology and Infectious Diseases; ICU, intensive care unit; IDSA, Infectious Diseases Society of America; IQR, interquartile range; SHEA, Society for Healthcare Epidemiology of America.
aUnless otherwise noted in parentheses for variables where not all patients had data obtained.
Time to resolution of diarrhea was not significantly different, with a median time to resolution of 8 days versus 5 days in the LAX-48 and NO-LAX-48 groups, respectively (P = .074). However, a significantly longer length of hospital stay was observed following CDI diagnosis in the LAX-48 group (median, 8 days vs 5 days for the NO-LAX-48 group; P = .031). When analyzed using 24- or 72-hour time windows for laxative administration prior to CDI diagnosis, findings mirrored those of the 48-hour group, except for a marginally significantly longer length of time to resolution of diarrhea in the LAX-24 group compared with the NO-LAX-24 group (median, 8 vs 5 days; P = .042) (Supplementary Tables 2 and 3).
Table 3 characterizes the types of laxatives administered to the LAX-48 cohort. Table 4 displays the distribution of total number of laxative doses received by patients in the cohort.
Table 3.
Laxatives Received Within 48 Hours of Diagnosis
| Patients (N = 65) | ||
|---|---|---|
| n | % | |
| Received bulk laxatives (range: 1–4 doses) | 49 | 75 |
| Colace (1–4) | 48 | 74 |
| Psyllium (1–3) | 2 | 3 |
| Received stimulant laxatives (range: 1–4 doses) | 33 | 51 |
| Senna (1–4) | 31 | 48 |
| Bisacodyl (1) | 5 | 8 |
| Received osmotic laxatives (range: 1–6 doses) | 26 | 40 |
| Polyethylene glycol (1–2) | 14 | 22 |
| Lactulose (1–6) | 11 | 17 |
| Magnesium citrate (1) | 1 | 2 |
| Magnesium oxide (1) | 1 | 2 |
Table 4.
Distribution of Laxative Doses Received 48 Hours Prior to Clostridiodes difficile Infection Diagnosis
| Patients (N = 65) | ||
|---|---|---|
| No. of Doses | n | % |
| 1 | 15 | 23 |
| 2 | 12 | 18 |
| 3 | 8 | 12 |
| 4 | 7 | 11 |
| 5 | 9 | 14 |
| 6 | 9 | 14 |
| 7 | 1 | 2 |
| 8 | 3 | 5 |
| 9 | 1 | 2 |
Discussion
Highly sensitive NAAT testing for C. difficile may detect patients who are colonized with C. difficile but who have an alternative explanation for diarrhea. In order to improve test performance, the 2017 IDSA-SHEA guidelines recommend against testing for CDI if a patient has received a laxative within the preceding 48 hours [6], citing a prospective study that included clinical presentation (diarrhea severity) along with C. difficile assay results to improve test performance [7]. Of note, that important study did not propose laxative use as an absolute exclusion criterion for CDI testing. Of 150 enrolled patients, 18.7% had received laxatives within 48 hours prior to CDI diagnosis; the authors cautioned that even clinically relevant diarrhea may have another, noninfectious, cause and called for validated criteria for when to test for CDI. Other studies corroborate a rate of laxative use in hospitalized patients as high as 44% within 48 hours prior to C. difficile testing [15]. Similarly, we observed that 31.1% of our patients had received at least 1 laxative dose in the 48 hours prior to testing. However, the fact that both CDI and laxatives can cause diarrhea appears to be the basis for the recommendation to exclude patients taking laxatives from C. difficile testing, as no compelling data indicate that laxative administration precludes or decreases the risk of CDI. On the contrary, other well-established causes of diarrhea predispose patients to a higher risk of CDI (inflammatory bowel disease, enteral tube feeding, and intensive cancer chemotherapy) [16–18]. Furthermore, laxatives are often utilized in situations independently known to increase the risk of CDI, including surgery and hospitalization [19].
Other authors have reviewed the association between laxative use and testing for CDI, observing that many patients who were tested for CDI concomitantly received laxatives [7, 15]. Excluding patients receiving laxatives lowers rates of CDI detection [9], but this may simply reflect that reductions in testing will generate fewer diagnoses. Other authors have argued that diarrhea is noninfectious in patients who are C. difficile NAAT positive receiving laxatives because clinical illness appears mild or indistinguishable from patients who are C. difficile NAAT negative [8, 9]. However, these studies included a substantial cohort (43.7–66.6%) who failed to meet a clinical definition of diarrhea (despite receiving laxatives in many cases) and so were unlikely to have true CDI [8, 9, 20]. One study found that clinical complication rates were not significantly different in patients with cancelled C. difficile test orders compared with patients with negative C. difficile test results [9]. However, when comparing clinical outcomes, the authors did not differentiate patients with orders cancelled for laxative use from those with orders cancelled for lack of clinical diarrhea. Ahmad et al [21] suggested that rapid resolution of diarrhea after diagnosis in patients with NAAT-positive C. difficile receiving laxatives (41% of patients had resolution within 48 hours of starting therapy in their study) indicates that diarrhea is noninfectious. However, it seems reasonable to expect the resolution of diarrhea within several days if appropriate CDI therapy is initiated.
Many institutions have already adopted the recommendation to avoid testing in patients receiving laxatives, some even incorporating test restrictions into electronic ordering systems to enforce guideline adherence [8, 9]. Providers appear to lack awareness of bowel regimen in many cases; only 78% of ordering providers were aware of bowel medications at the time they ordered C. difficile testing in 1 study, and as many as 52% of patients continued to receive laxatives for more than 24 hours even after a diagnosis of CDI in another study [21, 22]. Yet, in practice, providers override over 75% of these alerts, doing so deliberately for patients on laxatives in cases involving a stable baseline bowel regimen or presence of risk factors for CDI [8, 22]. The IDSA-SHEA guidelines offer no guidance on reconciling CDI risk factors, signs, or symptoms with the recommended testing restriction and fail to define meaningful laxative use (ie, consideration of baseline bowel regimen, type of laxative and number of doses, or agents with cathartic effects not deployed intentionally for laxative properties, such as oral contrast for computed tomography scans).
Our investigation compared outcomes and illness severity between patients with and without laxative use in the 48 hours prior to CDI diagnosis. The results are striking; there was no difference in the severity of illness or the rate of attributable adverse outcomes (CDI recurrence, ICU stay, colectomy, and death) between the LAX-48 and NO-LAX-48 groups. The groups did not differ in markers of clinical severity including fever, hypotension, leukocytosis, and colitis on imaging. Most patients in both groups met IDSA-SHEA and ESCMID criteria for classification as severe CDI. Median stool toxin concentrations, time to resolution of diarrhea, and the rates of severe CDI by all 4 severity scoring methods did not differ significantly by laxative status. There was a significant difference in longer length of stay following CDI diagnosis in the LAX-48 group (8 vs 5 days; P = .031), possibly indicating that patients on laxatives had even more substantial illness and complicated hospital stays.
If LAX-48 patients had been excluded from testing as recommended by the IDSA-SHEA guidelines, diagnosis of CDI would have been missed in nearly one-third of this cohort, 66.2% of whom met criteria for severe CDI by IDSA-SHEA scoring methods—including 1 death, 1 patient who required a colectomy, and 7 patients who required treatment in an ICU due to CDI. It is likely that some of the other LAX-48 patients would also have suffered additional adverse outcomes due to delayed or missed diagnoses and treatment.
There are several important limitations to this study. This is a single-site study with a relatively small sample size. However, there appears to be a trend, although not significant, towards more severe CDI-related illness in the LAX-48 group, as evidenced by more patients with hypotension, acute kidney injury, and WBC peak of 15 × 103/mL or higher, and who met severe criteria by IDSA-SHEA, ESCMID, and Zar scoring. It is also possible that, during the study period, clinicians had already begun to adopt laxative-related recommendations and were already delaying testing patients on laxatives until they appeared sicker; however, the majority of our cohort was enrolled before the updated IDSA-SHEA guidelines were published [6]. In practice, providers may override the recommendation for test exclusion in patients who have more severe illness or signs consistent with infection [22]. This could render the laxative cohort who underwent testing more likely to have true CDI. Regardless, if the guidelines had been followed, all of the laxative cases with severe outcomes would have had missed or delayed diagnoses.
We also recognize that established severity scoring methods for C. difficile are imperfect, but they are used in clinical practice. Lack of specificity in scoring criteria applies to both cohorts, regardless of laxative status, and we observed no significant differences using 4 separate CDI severity scores. We also found no differences in severe CDI-attributable clinical outcomes.
Importantly, we only studied patients with clinically significant diarrhea. It is possible that laxative recipients without confirmed diarrhea but with positive stool NAAT testing may have lower rates of severe CDI or severe CDI-related clinical outcomes. We fully support the IDSA-SHEA recommendation that CDI testing be confined to patients with clinically significant diarrhea. However, our study findings indicate that a history of recent laxative use cannot be used as a surrogate for the absence of CDI.
In conclusion, we found no difference in underlying patient characteristics, clinical presentation of CDI, CDI attributable outcomes, or CDI severity by established society guidelines in patients with clinically significant diarrhea who received laxatives within 48 hours preceding CDI diagnosis compared with patients who did not receive laxatives. There is a need for larger multisite studies to further investigate this issue. Clostridiodes difficile infection remains a diagnosis that requires both clinical and laboratory assessment, and the entire clinical picture must be considered when deciding whether a patient warrants testing and treatment. Our findings lead us to recommend that IDSA-SHEA guidelines to limit CDI testing to those with clinically significant diarrhea be emphasized; conversely, the recommendation to exclude CDI testing in patients who have received laxatives within 48 hours should be re-evaluated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all patients who participated in this study, Ted Allister for preparation of tables for the manuscript, and Christopher McCoy.
Financial support. This work was supported by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (grant number 1R01AI116596-01 to N. R. P. and C. P. K.), and Institut Mérieux (to N. R. P. and C. P. K.). C. D. A has received an NIH Loan Repayment through the National Institute of Allergy and Infectious Diseases. Simoa assays were performed by bioMérieux.
Potential conflicts of interest. C. P. K. has acted as a paid consultant to Artugen, Facile Therapeutics, First Light Biosciences, Finch, Matrivax, Merck, Seres Health, and Vedanta and has received grant support from Merck. C. D. A. has acted as a paid consultant to Merck and Roche and has received grant support from Merck. N. P. has acted as a paid speaker for Singulex. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). 2016. Available at: http://hcupnet.ahrq.gov/. Accessed 4 February 2019. [PubMed] [Google Scholar]
- 3. Curry SR, Muto CA, Schlackman JL, et al. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis 2013; 57:1094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McFarland LV. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control 1995; 23:295–305. [DOI] [PubMed] [Google Scholar]
- 5. Garey KW, Graham G, Gerard L, et al. Prevalence of diarrhea at a university hospital and association with modifiable risk factors. Ann Pharmacother 2006; 40:1030–4. [DOI] [PubMed] [Google Scholar]
- 6. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of American (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubberke ER, Han Z, Bobo L, et al. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol 2011; 49:2887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bilinskaya A, Goodlet KJ, Nailor MD. Evaluation of a best practice alert to reduce unnecessary Clostridium difficile testing following receipt of a laxative. Diagn Microbiol Infect Dis 2018; 92:50–5. [DOI] [PubMed] [Google Scholar]
- 9. Truong CY, Gombar S, Wilson R, et al. Real-time electronic tracking of diarrheal episodes and laxative therapy enables verification of Clostridium difficile clinical testing criteria and reduction of Clostridium difficile infection rates. J Clin Microbiol 2017; 55:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollock NR, Banz A, Chen X, et al. Comparison of Clostridioides difficile stool toxin concentrations in adults with symptomatic infection and asymptomatic carriage using an ultrasensitive quantitative immunoassay. Clin Infect Dis 2019; 68:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 12. Debast SB, Bauer MP, Kuijper EJ; European Society of Clinical Microbiology and Infectious Diseases European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20(Suppl 2):1–26. [DOI] [PubMed] [Google Scholar]
- 13. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 14. Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, Johnson S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect 2007; 55:495–501. [DOI] [PubMed] [Google Scholar]
- 15. Buckel WR, Avdic E, Carroll KC, Gunaseelan V, Hadhazy E, Cosgrove SE. Gut check: Clostridium difficile testing and treatment in the molecular testing era. Infect Control Hosp Epidemiol 2015; 36:217–21. [DOI] [PubMed] [Google Scholar]
- 16. Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med 1998; 129:1012–9. [DOI] [PubMed] [Google Scholar]
- 17. Martinelli M, Strisciuglio C, Veres G, et al. ; Porto IBD Working Group of European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Clostridium difficile and pediatric inflammatory bowel disease: a prospective, comparative, multicenter, ESPGHAN study. Inflamm Bowel Dis 2014; 20:2219–25. [DOI] [PubMed] [Google Scholar]
- 18. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis 1993; 17:109–13. [DOI] [PubMed] [Google Scholar]
- 19. Carter KA, Malani AN. Laxative use and testing for Clostridium difficile in hospitalized adults: an opportunity to improve diagnostic stewardship. Am J Infect Control 2019; 47:170–4. [DOI] [PubMed] [Google Scholar]
- 20. Banaei N, Anikst V, Schroeder LF. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:2368–9. [DOI] [PubMed] [Google Scholar]
- 21. Ahmad SM, Blanco N, Dewart CM, Dobosz A, Malani AN. Laxative use in the setting of positive testing for Clostridium difficile infection. Infect Control Hosp Epidemiol 2017; 38:1513–5. [DOI] [PubMed] [Google Scholar]
- 22. Kinlay J, Sandora TJ. A qualitative study to identify reasons for Clostridium difficile testing in pediatric inpatients receiving laxatives or stool softeners. Am J Infect Control 2017; 45:539–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.