Abstract
Background
Chemoprophylaxis vaccination with sporozoites (CVac) with chloroquine induces protection against a homologous Plasmodium falciparum sporozoite (PfSPZ) challenge, but whether blood-stage parasite exposure is required for protection remains unclear. Chloroquine suppresses and clears blood-stage parasitemia, while other antimalarial drugs, such as primaquine, act against liver-stage parasites. Here, we evaluated CVac regimens using primaquine and/or chloroquine as the partner drug to discern whether blood-stage parasite exposure impacts protection against homologous controlled human malaria infection.
Methods
In a Phase I, randomized, partial double-blind, placebo-controlled study of 36 malaria-naive adults, all CVac subjects received chloroquine prophylaxis and bites from 12–15 P. falciparum–infected mosquitoes (CVac-chloroquine arm) at 3 monthly iterations, and some received postexposure primaquine (CVac-primaquine/chloroquine arm). Drug control subjects received primaquine, chloroquine, and uninfected mosquito bites. After a chloroquine washout, subjects, including treatment-naive infectivity controls, underwent homologous, PfSPZ controlled human malaria infection and were monitored for parasitemia for 21 days.
Results
No serious adverse events occurred. During CVac, all but 1 subject in the study remained blood-smear negative, while only 1 subject (primaquine/chloroquine arm) remained polymerase chain reaction–negative. Upon challenge, compared to infectivity controls, 3/3 chloroquine arm subjects displayed delayed patent parasitemia (P = .01) but not sterile protection, while 3/11 primaquine/chloroquine subjects remained blood-smear negative.
Conclusions
CVac-primaquine/chloroquine is safe and induces sterile immunity to P. falciparum in some recipients, but a single 45 mg dose of primaquine postexposure does not completely prevent blood-stage parasitemia. Unlike previous studies, CVac-chloroquine did not produce sterile immunity.
Clinical Trials Registration
Keywords: chemoprophylaxis vaccination with sporozoites, malaria, Plasmodium falciparum, primaquine, chloroquine
Chemoprophylaxis vaccination with sporozoites (CVac) with chloroquine and primaquine is safe and induces sterile immunity to Plasmodium falciparum in some recipients, but does not completely prevent blood-stage parasitemia. Unlike previous studies, CVac-chloroquine alone did not produce sterile immunity.
Inoculation with radiation-attenuated Plasmodium falciparum (Pf) sporozoites (SPZ) that arrest during liver-stage development confers sterile protective immunity to subsequent PfSPZ challenges in humans, but this requires high doses [1–6]. Conversely, inoculation with wild-type PfSPZ parasites during chemoprophylaxis (chemoprophylaxis vaccination [CVac]) with chloroquine (CQ) or mefloquine induces sterile immunity against homologous infections with a much smaller parasite inoculum [7–12]. CQ and mefloquine specifically kill blood-stage parasites, so protective, CVac-induced immune responses may target SPZ, liver, or blood-stage antigens. Defining the life cycle–specific antigens targeted by protective immune responses is vital to tailoring a highly protective malaria vaccine. In this Phase I safety and tolerability study, we investigated the drug efficacy of postexposure primaquine (PQ) to kill liver-stage parasites [13] and the vaccine efficacy of standard CVac (with CQ) versus CVac plus postexposure PQ to induce protection against controlled human malaria infection (CHMI).
METHODS
This randomized, partial double-blind, placebo-controlled, Phase I study evaluated the safety, tolerability, and protective efficacy of CVac-CQ or CVac-PQ/CQ in 18–50-year-old healthy men and non-pregnant women at a single site in Seattle, Washington, from January–December 2012. The study was monitored by an independent Safety Monitoring Committee and medical monitor, who was approved by the Western Institutional Review Board and the University of Washington Institutional Review Board, and was conducted under Food and Drug Administration (FDA) Investigational New Drug Application 14752. The trial followed Good Clinical Practice guidelines and institutional procedures.
Study Design
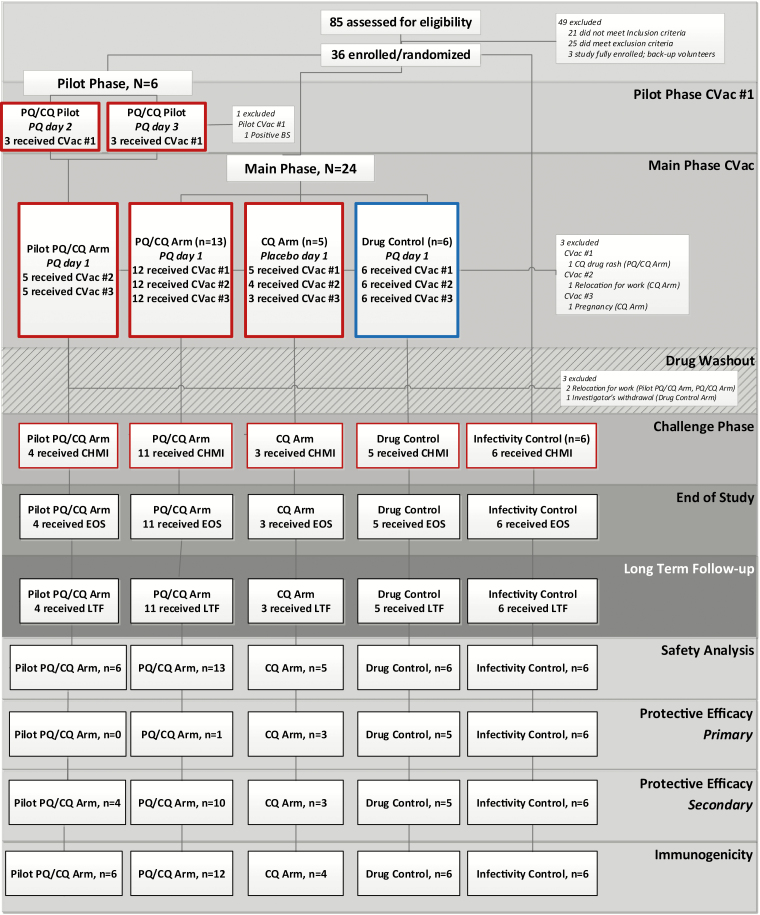
We enrolled 36 healthy, malaria-naive adult subjects. Eight days before first CVac mosquito bites, all subjects received 600 mg CQ base loading dose, then 12 weekly 300 mg doses including each day prior to PfSPZ inoculation. We randomly assigned 6 subjects to the open-label CVac pilot phase (to evaluate the optimal timing for PQ administration); they received weekly CQ, 12–15 infectious mosquito bites (PfSPZ+), and PQ on Day 2 (n = 3) or Day 3 (n = 3) post-PfSPZ+ (Pilot CVac-PQ/CQ; Figure 1; Supplementary Figure S1). This regimen of PfSPZ+ followed by drug administration comprised 1 CVac “dose.” Following the first CVac dose in the pilot phase, 24 subjects were randomized to the partially double-blind, placebo-controlled main phase, and received 3 CVac doses at 4-week intervals. Of these, 13 subjects received weekly CQ, 12–15 PfSPZ+, and PQ at 1 day post-PfSPZ+ (CVac-PQ/CQ); 5 received weekly CQ, 12–15 PfSPZ+, and a PQ placebo at 1 day post-PfSPZ+ (CVac-CQ); and 6 received weekly CQ, non-infectious mosquito bites (PfSPZ-), and PQ at 1 day post PfSPZ- (drug control). Those in the pilot phase joined the main phase subjects at the second CVac administration. At 9 weeks after last CVac mosquito bite, 6 additional infectivity controls were enrolled, and protective efficacy was assessed by homologous CHMI via 5 PfSPZ+.
Figure 1.
Trial profile. The boxes outlined in red represent the receipt of Pf SPZ+ mosquito bites, and those outlined in blue represent the receipt of Pf SPZ- mosquito bites. All CVac subjects received CQ from 8 days prior to the first iteration of mosquito bites to 20 days following the third mosquito bite exposure. Approximately 5 weeks elapsed between the discontinuation of CQ and CHMI to allow drug concentrations to decrease to subtherapeutic concentrations. “Received CVac” was defined as undergoing Pf SPZ± mosquito bites and receiving PQ/placebo. The EOS was defined as the final post-CHMI study visit (study Day 182; 35 days post-CHMI). LTF visits at 3 and 6 months post-CHMI were optional. Abbreviations: -, noninfectious mosquito bites; +, infectious mosquito bites; CHMI, controlled human malaria infection; CQ, chloroquine; CVac, chemoprophylaxis vaccination with sporozoites; EOS, end of study; LTF, long-term follow-up; Pf, Plasmodium falciparum; PQ, primaquine; SPZ, sporozoites.
Participants
Malaria-naive, healthy, adult men or non-pregnant women were eligible if they provided informed consent, were available for the study duration, passed an “Assessment of Understanding” questionnaire, and met the study inclusion/exclusion criteria. The full inclusion/exclusion criteria are in the Supplementary Appendix (SA; pages 5–8).
Study Products
Laboratory-reared Anopheles stephensi mosquitoes, originally from the Walter Reed Army Institute of Research and historically established for use in CHMI [14–16], were produced under phase-appropriate Good Manufacturing Practices conditions. PfSPZ+ and PfSPZ- mosquitoes were maintained in separate facilities, according to standard practices [17]. Either 12–15 (CVac phase) PfSPZ± or 5 (CHMI phase) PfSPZ+ mosquito bites were administered using standard procedures (SA, pages 9–10) [18].
For the PQ/CQ arm, three 26.3mg PQ phosphate (equivalent to 15 mg PQ base; Sanofi-Synthelabo, Inc., New York, NY) tablets were encapsulated in a single gelatin capsule (45 mg PQ base) by University of Washington Investigational Drug Services, placed in brown bottles, and labeled for each subject. Only a single PQ dose was administered, following FDA guidance. For the CQ arm, visually identical capsules with lactose excipient only were similarly prepared. CQ phosphate (West-ward Pharmaceutical Corp., Eatontown, NJ) 500 mg tablets (equivalent to 300 mg CQ base) were maintained in the manufacturer’s original packaging until dispensed.
All subjects received standard antimalarial treatment after PfSPZ+, at the time of either patent parasitemia, withdrawal, or the end of the challenge phase (Day 168–170), using fixed-dose atovaquone and proguanil hydrochloride (Malarone, GlaxoSmithKline), according to the labelled dose regimen. All study medications were administered orally as directly observed therapy with food.
Study Randomization and Blinding Procedures
Study randomization, enrollment, and blinding procedures are available in the SA (page 8).
Clinical Evaluation
Subjects were evaluated on site for safety, including for malaria symptoms on Days 6–10, following PfSPZ± mosquito bites. The full procedures are described in the SA (pages 8–9). Medically qualified study personnel were available 24 hours per day for unscheduled visits. All subjects were monitored for local and systemic reactogenicity for ≥1 hour after each mosquito exposure and each first dose of a study drug (Supplementary Table S1).
Study Objectives and Endpoints
Safety and Reactogenicity
The primary outcome was CVac safety and tolerability. Adverse event (AE) severity grading was per protocol-established toxicity grading tables, based on US FDA guidelines for vaccine trials [19], with laboratory AEs based on institutional normal reference ranges (Supplementary Table S2). Each AE’s relationship to the investigational products (CQ, PQ/placebo, mosquito bites, blood-stage malaria, or any combination thereof) was designated as not related, possibly related, or definitely related. Solicited AEs related to CQ, PQ/placebo, or mosquito bites were captured for 6 days postexposure; those related to malaria infection were captured for 28 days during CVac and 35 days during CHMI (Supplementary Table S1). Further details on AE reporting are in the SA (page 9).
Stage-specific Exposure and Protective Efficacy
The pilot phase primary efficacy objective was PQ timing post-PfSPZ+ that prevented blood-stage parasitemia (negative by thick blood smear [TBS] and by quantitative reverse transcription-polymerase chain reaction [qRT-PCR] [20]; procedure details are in the SA, page 11). The decision for the main phase PQ timing followed a prespecified algorithm (Supplementary Figure S2).
The main phase primary CVac efficacy objective was the prevention of blood-stage parasitemia (defined above) during CVac (PQ/CQ) and following CHMI (all arms).
Immunogenicity
Humoral and cell-mediated immune responses to pre-erythrocytic (circumsporozoite protein [CSP]) and blood-stage antigens (apical membrane antigen 1; merozoite surface protein 1) were assessed by enzyme-linked immunosorbent assay and enzyme-linked immune absorbent spot (details are in the SA, pages 10–11).
Drug Assays
CQ and desethyl-chloroquine levels were measured in whole-blood spots at various timepoints (SA, page 11) by high-performance liquid chromatography at the Walter Reed Army Institute of Research, Division of Experimental Therapeutics, as previously described [21].
Mosquito Infectivity
Mosquito bites were administered by standard procedures. Qualitative measures of the SPZ load and number of infective bites were recorded during CVac and CHMI (details are in the SA, pages 9–10).
Sample Size and Statistical Analysis
The sample size and statistical analysis information are in the SA (pages 11–15).
RESULTS
We screened 85 subjects, and 36 were enrolled and evaluable for safety (Figure 1). The baseline characteristics were well-balanced between arms (Table 1); most participants were young (mean age, 28.6 years; standard deviation, ± 7.1), fit (body mass index, 22.7 kg/m2; standard deviation, ± 2.1), Caucasian adults, equally men and women (Table 1).
Table 1.
Study Population Demographics and Baseline Characteristics
| Pilot Phase | Main Phase | Infectivity Control, n = 6 | Total, n = 36 | |||
|---|---|---|---|---|---|---|
| PQ/CQ, n = 6 | PQ/CQ, n = 13 | CQ, n = 5 | Drug Control, n = 6 | |||
| Gender, n (%) | ||||||
| Male | 2 (33.3%) | 7 (53.8%) | 3 (60.0%) | 3 (50.0%) | 3 (50.0%) | 18 (50.0%) |
| Female | 4 (66.7%) | 6 (46.2%) | 2 (40.0%) | 3 (50.0%) | 3 (50.0%) | 18 (50.0%) |
| Age, years | ||||||
| Mean (±SD) | 27.3 (3.2) | 30.2 (7.9) | 22.4 (2.8) | 32.8 (10.2) | 27.2 (4.4) | 28.6 (7.1) |
| BMI, kg/m2 | ||||||
| Mean (±SD) | 22.6 (1.0) | 22.3 (2.0) | 22.2 (2.5) | 23.4 (2.6) | 23.6 (2.5) | 22.7 (2.1) |
| Race, n (%) | ||||||
| White | 5 (83.3%) | 13 (100.0%) | 5 (100.0%) | 3 (50.0%) | 6 (100.0%) | 32 (88.9%) |
| Black | 1 (16.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.8%) |
| Asian | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33.3%) | 0 (0%) | 2 (5.6%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.7%) | 0 (0%) | 1 (2.8%) |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.7%) | 1 (16.7%) | 2 (5.6%) |
| Not Hispanic or Latino | 6 (100.0%) | 13 (100.0%) | 5 (100.0%) | 5 (83.3%) | 5 (83.3%) | 34 (94.4%) |
| Study completion, n (%) | ||||||
| CVac #1 | 6 (100.0%) | 12 (92.3%) | 5 (100.0%) | 6 (100.0%) | N/A | 29 (96.7%) |
| CVac #2 | 5 (83.3%) | 12 (92.3%) | 4 (80.0%) | 6 (100.0%) | N/A | 27 (90.0%) |
| CVac #3 | 5 (83.3%) | 12 (92.3%) | 3 (60.0%) | 6 (100.0%) | N/A | 26 (86.7%) |
| CHMI | 4 (66.7%) | 11 (84.6%) | 3 (60.0%) | 5 (83.3%) | 6 (100.0%) | 30 (80.6%) |
| End of study | 4 (66.7%) | 11 (84.6%) | 3 (60.0%) | 5 (83.3%) | 6 (100.0%) | 30 (80.6%) |
Abbreviations: BMI, body mass index; CHMI, controlled human malaria infection; CQ, chloroquine; CVac, chemoprophylaxis vaccination with sporozoites; N/A, not applicable; PQ, primaquine; SD, standard deviation.
There were 3 subjects enrolled in each of 2 pilot phase PQ/CQ arms; 5/6 pilot phase PQ/CQ subjects joined the main phase subjects at CVac #2 (8 weeks post–pilot phase CVac #1), and 4/6 completed CHMI and end of study. A single PfSPZ+ pilot PQ/CQ subject receiving PQ on Day 3 was withdrawn and treated per protocol, secondary to a positive TBS 8 days after the first PfSPZ+ mosquito exposure, when qRT-PCR–measured densities also peaked (Supplementary Figure S3).
We randomly assigned 24 main phase subjects, and 21 (87.5%) completed 3 CVacs; 23 subjects (pilot phase, n = 4; main phase, n = 19) and 6 infectivity controls proceeded to homologous CHMI (Figure 1; Table 1). A single PQ/CQ subject was excluded from the protective efficacy analysis due to inconsistent TBS (positive) and qRT-PCR (negative) results post-CHMI.
Safety and Reactogenicity During Chemoprophylaxis Vaccination Phase
Overall, both regimens (CVac-PQ/CQ and CVac-CQ alone) were safe and well tolerated, with no serious AEs (Table 2). Individual (Table 2) and overall AE frequencies (Supplementary Table S3) were similar in all study arms. Mosquito bite–related local reactogenicity did not differ in frequency (22/23 [96%] vs 6/6 [100%], respectively) or severity between PfSPZ+ and PfSPZ-, and did not increase with repeated exposure (Supplementary Table S4). Systemic reactogenicity related to mosquito bites was infrequent. CQ was well tolerated, with the frequency of CQ-related solicited AEs decreasing with successive CVac in all arms, likely due to frequent AE reporting after the initial CQ loading dose (Table 2). A single subject withdrew after a Grade 3, CQ-associated AE after the loading dose (drug eruption). The most common CQ-related AEs included nausea, poor quality sleep, headache, and tinnitus (Supplementary Table S5). PQ-related AEs occurred less frequently than CQ-related AEs in all study arms, with similar frequencies over repeated CVac doses (Table 2). PQ-associated AE reporting was not significantly different between PQ-receiving groups (pilot PQ/CQ, PQ/CQ, and drug controls; 8/24, 33%) and the PQ placebo–receiving CQ subjects (3/5, 60%; Supplementary Table S6).
Table 2.
Overview of Adverse Events and Other Indicators and Contributors of Reactogenicity in the Chemoprophylaxis Vaccination With Sporozoites Phase Study Population
| CVac Phase | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CVac #1 | CVac #2 | CVac #3 | ||||||||||
| Pilot PQ/ CQ, n = 6 | PQ/CQ, n = 13a | CQ, n = 5 | Drug Control, n = 6 | Pilot PQ/CQ, n = 5 | PQ/CQ, n = 12 | CQ, n = 4 | Drug Control, n = 6 | Pilot PQ/ CQ, n = 5 | PQ/CQ, n = 12 | CQ, n = 4b | Drug Control, n = 6 | |
| Any solicited AE | 5 (83) | 13 (100) | 4 (80) | 6 (100) | 4 (80) | 12 (100) | 3 (75) | 6 (100) | 2 (40) | 11 (92) | 2 (50) | 6 (100) |
| Any SAE | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Any unsolicited AE | 6 (100) | 7 (54) | 1 (20) | 5 (83) | 0 (0) | 5 (42) | 2 (50) | 3 (50) | 3 (60) | 2 (17) | 2 (50) | 3 (50) |
| Any AE related to: | ||||||||||||
| Chloroquine | 3 (50) | 11 (85) | 3 (60) | 4 (67) | 1 (20) | 4 (33) | 0 (0) | 3 (50) | 1 (20) | 2 (17) | 0 (0) | 2 (33) |
| Primaquine/placeboc | 2 (33) | 2 (17) | 2 (40) | 1 (17) | 1 (20) | 2 (17) | 1 (25) | 1 (17) | 1 (20) | 3 (25) | 1 (25) | 0 (0) |
| Local mosquito bites | 5 (83) | 12 (100) | 4 (80) | 5 (83) | 4 (80) | 11 (92) | 2 (50) | 6 (100) | 3 (60) | 11 (92) | 3 (75) | 4 (67) |
| Systemic mosquito bites | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0 (0) | 1 (17) |
| Malariad | 2 (33) | 3 (25) | 0 (0) | 1 (17) | 1 (20) | 4 (33) | 0 (0) | 2 (33) | 1 (20) | 3 (25) | 0 (0) | 0 (0) |
| Combination | 4 (67) | 2 (17) | 0 (0) | 0 (0) | 2 (40) | 2 (17) | 0 (0) | 0 (0) | 2 (40) | 6 (50) | 0 (0) | 0 (0) |
| Any lab abnormalities | 3 (50) | 5 (38) | 0 (0) | 1 (17) | 3 (60) | 4 (33) | 0 (0) | 1 (17) | 1 (20) | 7 (58) | 0 (0) | 0 (0) |
| Any related AE by grading: | ||||||||||||
| Grade 1, mild | 5 (83) | 13 (100) | 4 (80) | 6 (100) | 4 (80) | 10 (83) | 2 (50) | 5 (83) | 3 (60) | 10 (83) | 3 (75) | 3 (50) |
| Grade 2, moderate | 3 (50) | 4 (31) | 2 (40) | 4 (67) | 1 (20) | 5 (42) | 0 (0) | 3 (50) | 1 (20) | 5 (42) | 0 (0) | 3 (50) |
| Grade 3, severe | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 1 (20) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0 (0) | 0 (0) |
| Peak parasite density during CVac by qRT-PCR, parasites/mL | ||||||||||||
| Any positive qPCR | 6 (100) | 8 (67) | 5 (100) | 0 (0) | 4 (80) | 9 (75) | 1 (25) | 0 (0) | 3 (60) | 5 (42) | 0 (0) | 0 (0) |
| Detectable to <102 | 1 (17) | 4 (33) | 0 (0) | 0 (0) | 0 (0) | 4 (33) | 0 (0) | 0 (0) | 2 (40) | 3 (25) | 0 (0) | 0 (0) |
| ≥102 to <103 | 0 (0) | 2 (17) | 1 (20) | 0 (0) | 4 (80) | 4 (33) | 1 (25) | 0 (0) | 1 (20) | 2 (17) | 0 (0) | 0 (0) |
| ≥103 to <104 | 4 (67) | 2 (17) | 4 (80) | 0 (0) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ≥104 | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Data are number of unique subjects (%), unless stated otherwise.
Abbreviations: AE, adverse event; CQ, chloroquine; CVac, chemoprophylaxis vaccination with sporozoites; PQ, primaquine; qPCR, quantitative polymerase chain reaction; qRT-PCR, quantitative reverse transcription polymerase chain reaction; SAE, severe adverse event; SPZ, sporozoites.
aThe safety population for PQ/CQ arm for the first CVac was 13 for the CQ dose evaluation only, as a subject was withdrawn from the study prior to any further study interventions.
bThe safety population for the CQ arm for CVac #3 was 4, but only 3 subjects were evaluable for parasite density, as a subject was withdrawn secondary to pregnancy on the day of PQ/placebo dosing (Day 1 post–SPZ exposure) and treated.
cPQ-receiving arms: pilot PQ/CQ, PQ/CQ, and drug controls; placebo-receiving arm: CQ.
dSPZ-receiving arms: pilot PQ/CQ, PQ/CQ, and CQ; non–SPZ receiving arm: drug controls.
Malaria-related symptoms during the CVac phase were reported from all arms but did not appear to be associated with either qRT-PCR positivity or peak parasitemia (Table 2; Supplementary Table S7).
Laboratory abnormalities occurred in all study arms except the CQ-only arm (Table 2; Supplementary Table S8) and were mainly Grade 1 (mild; 32 of 39 total lab AEs during CVac, 82%); decreased hemoglobin and anemia were the most common abnormalities (Supplementary Table S8). Most unsolicited AEs were Grade 1 (mild; 59/68 total unsolicited AEs during CVac, 87%), and all AEs that were Grade 2 or higher (9/68, 13%) were deemed not related (Supplementary Table S9).
More detailed summaries of mosquito-, CQ-, PQ-, malaria-, and combination-associated AEs are in the SA (Supplementary Tables S4–S10).
Safety and Reactogenicity Following Controlled Human Malaria Infection
Safety during and after mosquito-based CHMI was similar to previous reports, with most subjects (28/29; 97%) experiencing at least 1 symptom following a challenge (Table 3). Further details regarding safety post-CHMI are in the SA (Supplementary Tables S11–S14).
Table 3.
Overview of Adverse Events and Other Indicators and Contributors of Reactogenicity in the Challenge Phase Study Population
| Pilot PQ/CQ, n = 4 | PQ/CQ, n = 11/10a | CQ, n = 3 | Drug Control, n = 5 | Infectivity Control, n = 6 | |
|---|---|---|---|---|---|
| Challenge Phase | |||||
| Safety | |||||
| Any solicited AE | 4 (100) | 10 (91) | 3 (100) | 5 (100) | 6 (100) |
| Any SAE | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Any unsolicited AEb | 0 (0) | 10 (91) | 2 (67) | 4 (80) | 5 (83) |
| Any AE related to: | |||||
| Local mosquito bites | 4 (100) | 10 (91) | 1 (33) | 4 (80) | 5 (83) |
| Systemic mosquito bites | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) |
| Malaria | 4 (100) | 10 (91) | 3 (100) | 5 (100) | 6 (100) |
| Combination: malaria/malaria treatment | 0 (0) | 4 (36) | 2 (67) | 4 (80) | 5 (83) |
| Any laboratory abnormalities | 4 (100) | 8 (73) | 3 (100) | 4 (80) | 4 (67) |
| Any malaria related AE by grading: | |||||
| Grade 1, mild | 4 (100) | 10 (91) | 3 (100) | 5 (100) | 6 (100) |
| Grade 2, moderate | 3 (75) | 7 (64) | 2 (67) | 4 (80) | 5 (83) |
| Grade 3, severe | 1 (25) | 4 (36) | 2 (67) | 3 (60) | 3 (50) |
| Protective efficacy | |||||
| TBS+ | 4 (100) | 7 (70) | 3 (100) | 5 (100) | 6 (100) |
| Prepatent period, days, median (IQR)c | 11 (10–11) | 11 (9–undefined) | 14 (13–16) | 9 (9–11) | 9 (8–9) |
| Pre-subpatent period, days, median (IQR)d | 7 (7–7) | 7 (6–10) | 7 (7–10) | 6 (6–6) | 6.5 (6–7) |
| Symptomatic subjects at time of malaria diagnosis | 1 (25) | 4 (50) | 2 (67) | 1 (20) | 4 (67) |
| Estimated median parasite density by qRT-PCR at the time of TBS+, parasites/mL (IQR)e | 3.6 × 104 (2.0 × 104 to 4.7 × 104) | 1.1 × 104 (2.2 × 103 to 8.1 × 104) | 8.6 × 104 (2.1 × 104 to 1.1 × 105) | 4.9 × 104 (2.5 × 104 to 9.1 × 104) | 9.6 × 103 (4.6 × 103 to 2.9 × 104) |
| Estimated peak parasite density by qRT-PCR, parasites/mL | |||||
| Any positive qRT-PCR | 4 (100) | 8 (80)f | 3 (100) | 5 (100) | 6 (100) |
| Detectable to <102 | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| ≥102 to <103 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ≥103 to <104 | 0 (0) | 2 (20) | 0 (0) | 0 (0) | 1 (17) |
| ≥104 to <105 | 4 (100) | 3 (30) | 2 (67) | 3 (60) | 4 (67) |
| ≥105 | 0 (0) | 2 (20) | 1 (33) | 2 (40) | 1 (17) |
Data are the number of unique subjects (%), unless stated otherwise.
Abbreviations: +, positive; AE, adverse event; CHMI, controlled human malaria infection; CQ, chloroquine; CVac, chemoprophylaxis vaccination with sporozoites; IQR, interquartile range; PQ, primaquine; rRNA, ribosomal ribonucleic acid; qRT-PCR, qualitative real-time polymerase chain reaction; SAE, serious adverse event; TBS, thick blood smear.
aTBS/qRT-PCR data were censored for 1 PQ/CQ main phase vaccinated subject who was completely asymptomatic post-CHMI but was positive by TBS at a single time point. This subject was 18S rRNA negative at all time points post-CHMI and was TBS negative at all other time points post-CHMI, such that the positive TBS was deemed to be a false positive. The safety data were not censored (n = 11).
bUnsolicited AEs were any systemic or local symptom that was not solicited for per protocol; laboratory values were considered separately from solicited and unsolicited AEs.
cKaplan-Meier estimates of number of days from CHMI to time of first positive TBS.
dKaplan-Meier estimate of number of days from CHMI to first positive qRT-PCR count (>20 parasites/mL).
eParasite count from qRT-PCR on day of first positive TBS. Excludes the 3 PQ/CQ subjects who did not have a positive TBS following CHMI.
fA single subject in the PQ/CQ main phase arm was qRT-PCR positive at a single time point (day 10.5 post-CHMI, 58 parasites/mL), but was never TBS positive, was never qRT-PCR positive again, and was not included in the time-to-positivity analysis.
Stage-specific Parasite Exposure
Pilot Phase Postexposure Primaquine Dosing Determination
Pilot phase PQ testing was performed to determine whether blood-stage parasitemia could be suppressed by administering PQ on Day 2 or 3 after PfSPZ inoculation. All pilot phase subjects developed subpatent parasitemia by qRT-PCR during the first CVac, with 1 subject becoming TBS positive (Supplementary Figure S3). Thereafter, either PQ or placebo was subsequently administered 1 day (~36 hours) after PfSPZ±, per protocol (Supplementary Figure S2).
Main Phase Subpatent Parasitemia
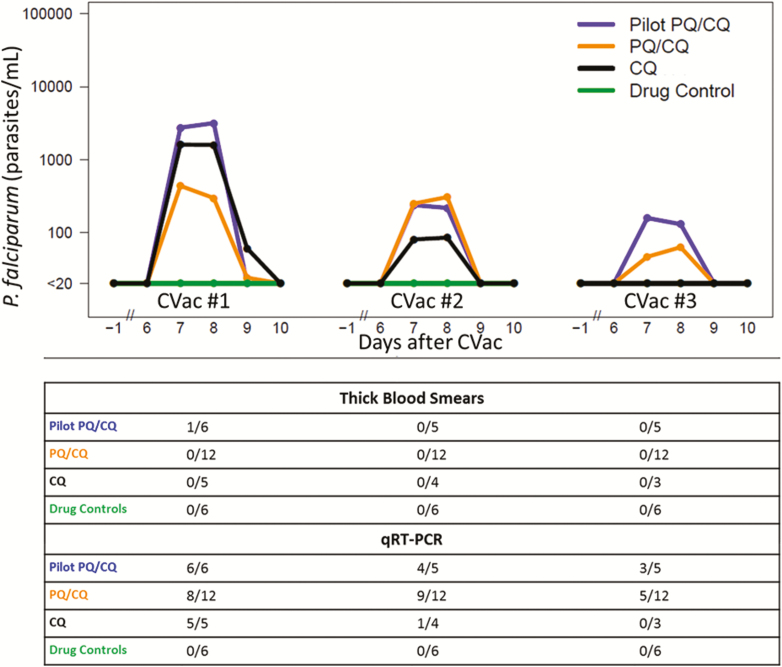
During CVac #1, 8/12 (67%) PQ/CQ subjects and 5/5 (100%) CQ subjects developed subpatent parasitemia (Figure 2; Supplementary Figure S4). Peak parasite densities and durations of parasitemia in the CQ arm were similar to previous reports [11] and, as expected, diminished with subsequent administrations. All CQ arm subjects remained qRT-PCR negative during CVac #3 (Table 2; Figure 2; Supplementary Figure S4).
Figure 2.
CVac-phase parasite densities for all study arms. The estimated mean parasites/mL by qRT-PCR are shown for the combined pilot phase PQ/CQ arm (blue line), main phase PQ/CQ Arm (orange line), CQ arm (black line), and drug control arm (green line) following the first, second, and third PfSPZ± mosquito bite exposures. Time is shown relative to each PfSPZ± mosquito bite exposure, which were spaced 28 days apart. The cumulative number of subjects positive in each arm by thick blood smear and/or qRT-PCR over eligible subjects during that CVac are shown for each corresponding CVac phase. Abbreviations: -, noninfectious mosquito bites; +, infectious mosquito bites; CQ, chloroquine; CVac, chemoprophylaxis vaccination with sporozoites; Pf, Plasmodium falciparum; PQ, primaquine; qRT-PCR, qualitative real-time polymerase chain reaction; SPZ, sporozoites.
PQ dosing on Day 1 prevented subpatent parasitemia in 4/12 (33%) subjects during CVac #1; amongst positive patients, there was a trend toward a lower mean peak parasite density during CVac #1 in the PQ/CQ arm, as compared to the CQ arm (721 vs 1833, respectively; P = .06; Mann-Whitney test). However, unlike in the CQ arm, the overall proportion that was parasitemic, the durations of parasitemia, and the mean peak parasite density in the PQ/CQ arm did not change appreciably from the first to second CVac dose (Figure 2; Supplementary Figure S4). All but 1 PQ/CQ arm participant became qRT-PCR positive sometime during the CVac phase. As expected, all drug control subjects who received PfSPZ- mosquito bites remained qRT-PCR negative throughout.
Except for the 1 TBS-positive subject during the pilot PQ/CQ (Day 8 post-PfSPZ+, the mosquito bite’s estimated density was 12 094 parasites/mL), no other positive TBS were reported in any arm during the CVac phase (Figure 2; Supplementary Figure S4).
Protective Efficacy
There were 29 subjects who underwent CHMI: 4 pilot PQ/CQ and 19 main phase subjects (11 PQ/CQ, 3 CQ, and 5 drug control), and 6 infectivity controls. A single main phase PQ/CQ subject with discordant TBS and qRT-PCR results was excluded from the protective efficacy analysis, leaving 28 subjects evaluable for protective efficacy.
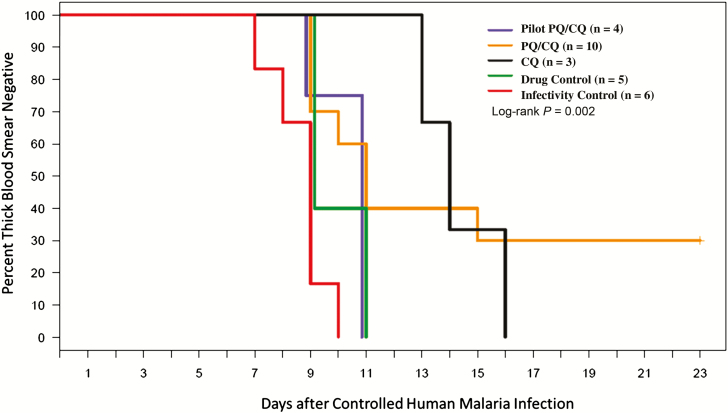
Patent parasitemia developed in all infectivity controls (6/6) by 10 days post-CHMI, and developed by Day 11 for all pilot PQ/CQ (4/4) and drug control (5/5) subjects (Figure 3). All CQ subjects (3/3) were TBS positive by Day 16, which was a significant delay (P = .01) compared to the infectivity controls. There were 3 PQ/CQ subjects who remained TBS negative postchallenge; the time to TBS positivity was not delayed for those PQ/CQ subjects who developed positive TBS (10.8 days), compared to controls (1 PQ/CQ subject remained TBS negative until Day 15). The difference in the time to patent parasitemia between the main phase PQ/CQ and CQ arms was nonsignificant, regardless of whether the main PQ/CQ arm was analyzed with or without the pilot PQ/CQ arms (P values = .92 and .78, respectively). The differences between the arms in mean estimated parasite densities by qRT-PCR on the day of the first positive TBS were not statistically significant (P values > .45 for all comparisons; Table 3). The median times to subpatent parasitemia post-CHMI were similar for the 5 arms (6–7 days; Table 3). The mean parasite counts per day illustrate an appreciable delay in the growth kinetics for the CQ arm, relative to the other arms (Supplementary Figure S5).
Figure 3.
Kaplan-Meier curve showing the percentage of subjects who remained TBS-negative following homologous CHMI. The pilot phase PQ/CQ arm (blue line), main phase PQ/CQ arm (orange line), CQ arm (black line), drug control arm (green line), and infectivity control arm (red line) are shown. The P value reported is for the log-rank test, comparing all 5 arms. The CQ arm compared to the combined controls (drug and infectivity controls; n = 12) is P = .004 and compared to the infectivity controls alone (n = 6) is P = .01. The CQ arm compared to the combined PQ/CQ arms (pilot and main) is P = .74. Abbreviations: CHMI, controlled human malaria infection; CQ, chloroquine; PQ, primaquine; TBS, thick blood smear.
Mosquito Infectivity
The average number of infective bites, mosquito infectivity (measured by the salivary gland SPZ rating, percent infective bites, and percent infective bites with 4+ SPZ ratings), and total number of bites did not differ between arms during each CVac, between the 3 CVac administrations when treatment arms were pooled, or between arms upon CHMI (Supplementary Table S15). Mosquitoes had higher average SPZ ratings per infective bite (P < .001) and percent infective bites with 4+ ratings (P = .01) at CHMI versus CVac phase (Supplementary Table S15). These measurements of mosquito infectivity did not differ between the 3 protected and 7 unprotected PQ/CQ main subjects.
Chloroquine and Desethyl-Chloroquine Concentrations and Immunology
CQ and desethyl-chloroquine assay results (Supplementary Figure S6) and immunology assessments (Supplementary Figure S7; Supplementary Table S16) are described in the SA (pages 15–16).
DISCUSSION
CVac using CQ can confer durable sterilizing immunity against homologous Plasmodium infections [11], but the role of immune responses to pre-erythrocytic versus blood-stage parasites in protection is unclear. Here, we assessed PQ for preventing blood-stage parasitemia during CVac and compared CQ and PQ/CQ for conferring homologous, sterile immunity.
Both the CVac-CQ and CVac-PQ/CQ regimens were well tolerated, and no subject developed clinically significant patent parasitemia during the CVac phases, though 1 subject developed TBS positivity that warranted preemptive treatment. In early human trials, a single administration of 30 mg of PQ at 1 or 3 days after infective mosquito bites prevented blood-stage parasitemia in 10/10 and 9/10 subjects, respectively [13], suggesting that postexposure PQ effectively arrests liver-stage parasite development and prevents blood-stage antigen exposure. However, subpatent parasitemia had not been evaluated in prior studies. We anticipated that PQ would be similarly effective at preventing blood-stage parasitemia; however, all but 1 subject became qRT-PCR–positive during CVac-PQ/CQ administrations. Hypothetically, CQ could alter the PQ metabolism, thereby reducing activity; however, available human evidence suggests that CQ may potentiate PQ activity [22].
PQ appeared to reduce, but not completely ablate, liver-stage infections. During the first CVac, subpatent parasitemia appeared in all 5 CVac-CQ arm subjects, versus two-thirds (8/12) of CVac-PQ/CQ participants, with lower peak parasite densities in the latter, suggesting that postexposure PQ reduced the liver parasite burden.
Upon CHMI, 3 main phase CVac-PQ/CQ but no CVac-CQ subjects displayed sterilizing immunity. Previously, CVac-CQ conferred high sterilizing immunity against a homologous challenge [11, 12] that was dose-dependent, raising questions about SPZ dosing in this study. Notably, only 1 subject remained qRT-PCR negative throughout CVac-PQ/CQ administration; hence, we could not assess our primary efficacy endpoint in subjects whose CVac exposure was limited exclusively to pre-erythrocytic parasites. A caveat for our trial is the relatively small sample size (it is typical of CHMI studies to limit the risk of P. falciparum exposure); therefore, our findings should be confirmed in future studies.
Whereas some in the CVac-PQ/CQ group attained sterile immunity, the CVac-CQ group demonstrated greater blood-stage immunity. CVac-CQ (but not CVac-PQ/CQ) yielded progressive reductions in parasite densities with each dose, similar to previous reports [7, 10, 11]. Unlike previous studies [11], CVac-CQ did not confer sterile immunity, but delayed patent parasitemia. The blood-stage parasite exposure during CVac was similar to that in other studies, and hence does not explain the absence of sterile immunity. The SPZ inoculum per mosquito was higher during CHMI than CVac, based on mosquito salivary gland SPZ ratings (Supplementary Table S15), which may have overwhelmed protective immune responses. However, the SPZ burden may not correlate with the time to parasitemia or parasite density of first-wave parasitemia [23]. Since SPZ inocula can vary between centers or within centers over time, mosquito parasite burdens should be more carefully monitored and systematically reported by the CHMI community. Differences in SPZ inocula may explain varying results between centers that are testing interventions and prioritizing candidates to advance to field trials.
CVac-immunized subjects mounted antibody responses against CSP without differences between groups. How this relates to or impacts sterile immunity remains unclear; CVac-CQ antibodies are known to inhibit SPZ traversal of hepatocytes in vitro and reduce the liver-stage parasite burden in a humanized mouse model [24]. In murine Plasmodium berghei CVac models, CD8+ T cells were absolutely required for protection [25, 26], and protected against SPZ but not a blood-stage challenge [25]. Here, T cell responses to CSP-specific and liver-stage antigen-1–specific peptide pools were very limited. Innate immune responses were not measured but should be tested in future studies, since CVac induces interferon γ-producing γδT and natural killer cells that persist for >1 year [27].
Although CVac PQ/CQ induced sterile protective immunity in only 3/10 subjects, this is an important first step to extending the CVac model to interrogate differences between liver- and blood-stage antigen exposures and roles in malaria immunity. Our results indicate that a single dose of 45 mg PQ is insufficient to consistently prevent blood-stage infection in this model. Likewise, the results suggest the timing of PQ administration correlates with the degree of subsequent breakthrough parasitemia. As might be anticipated, pilot phase PQ/CQ subjects receiving PQ on Day 3 had higher breakthrough subpatent parasitemia than those receiving PQ on Day 2. Likewise, PQ/CQ subjects receiving PQ on Day 1 in the main phase had the lowest parasite density.
Overall, CVac-PQ/CQ is well tolerated and induces sterilizing immunity in some participants, but does not ablate liver-stage parasites. Recently, CVac-CQ induced potent sterilizing immunity to homologous but not heterologous parasites [28], suggesting that improvements to CVac are needed. We are now assessing CVac regimens that completely kill liver-stage parasites and will test their activity against both homologous and heterologous parasites in humans. Furthermore, this study highlights the importance of standardizing SPZ inocula when using the CHMI model as a tool for product development.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors participated in the design, implementation or analysis, or interpretation of the study and in the development of this manuscript. All authors had full access to the data and gave final approval before submission. S. A. H., S. C. M., J. G. K., P. E. D. drafted the manuscript, and S. A. H. was responsible for submission of the publication.
Acknowledgments. The authors thank the study volunteers for their participation in the study. They also thank Christine Johnston, MD, MPH; Michael Stern, ARNP; Lauren Asaba, ARNP; Claire Stevens, MA, PA; Darcie Somera; Sol Bockelie; Julie Thach; Emma Robinson; Mark Drummond; Pavlina Barcal; Scott Caparelli; Kirsten Hauge; Sharanya Rajagopal; Reina Hibbert; Lisa Dellino; Sara Leffler; Tracie VonGoedert; Ami Davis; Michael Swift; and Sepideh Kittleson for their contributions throughout the study. They thank Joshua Schiffer, MD; Margaret Green, MD, MPH; Robert Choi, MD; Malaika Little, MD, MPH; Tara Perti, MD; Paul Bollyky, MD; T. Nui Pholsena, ARNP; Amy Anderson, ARNP; Jenni Gallagher, RN; Anna Hess, RN; David Breton, RN; Jean Zatochill, RN; E. Houston LeBron, MA; and Camilla Wilson, MA for their contributions to this study during the controlled human malaria infection phase. The authors thank the C3 Research Associates (Ron Carozza, PharmD; Bonnie Hein) for their assistance with pharmacovigilance and safety monitoring for the study; the University of Washington Investigational Drug Services for their assistance with primaquine and placebo blinding; and the Western Institutional Review Board and University of Washington Institutional Review Board. They thank Axio Research, specifically April Slee, for help with data management; Heather Kain and Will Betz for their entomology and blood smear reading expertise during the study; Jackie Williams for microscopy expertise during controlled human malaria infection; Judy Epstein, MD, for assisting with acquiring primaquine for the study; and J. Patrick Gorres for his assistance in editing and preparing the manuscript for publication.
Disclaimer. Bill and Melinda Gates Foundation representatives periodically reviewed the development and progress on the study but were not involved in the study conduct or data analysis. The authors received no remuneration for the development of the present manuscript.
Financial support. This work was supported by the Bill and Melinda Gates Foundation and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number ZIA AI001170-05).
Potential conflicts of interest. Z. M. received salary support through a contract from Sanofi Pasteur, awarded to Fred Hutch to provide statistical analysis, but did not receive personal fees/independent consulting fees. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society of Tropical Medicine & Hygiene, Atlanta, Georgia, 11–15 November 2012. Symposium #91; Poster #187.
References
- 1. Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 1973; 266:169–77. [DOI] [PubMed] [Google Scholar]
- 2. Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight 2017; 2:e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 2002; 185:1155–64. [DOI] [PubMed] [Google Scholar]
- 4. Ishizuka AS, Lyke KE, DeZure A, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, Sell KW. Letter: sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg 1974; 68:258–9. [DOI] [PubMed] [Google Scholar]
- 6. Seder RA, Chang LJ, Enama ME, et al. ; Vaccine Research Center 312 Study Team. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359–65. [DOI] [PubMed] [Google Scholar]
- 7. Bijker EM, Bastiaens GJ, Teirlinck AC, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci USA 2013; 110:7862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bijker EM, Schats R, Obiero JM, et al. Sporozoite immunization of human volunteers under mefloquine prophylaxis is safe, immunogenic and protective: a double-blind randomized controlled clinical trial. PLOS One 2014; 9:e112910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bijker EM, Teirlinck AC, Schats R, et al. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis 2014; 210:1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mordmüller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017; 542:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009; 361:468–77. [DOI] [PubMed] [Google Scholar]
- 12. Roestenberg M, Teirlinck AC, McCall MB, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 2011; 377:1770–6. [DOI] [PubMed] [Google Scholar]
- 13. Arnold J, Alving AS, Hockwald RS, et al. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, P-F-6 strain). J Lab Clin Med 1955; 46:391–7. [PubMed] [Google Scholar]
- 14. Chulay JD, Schneider I, Cosgriff TM, et al. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg 1986; 35:66–8. [DOI] [PubMed] [Google Scholar]
- 15. Epstein JE, Rao S, Williams F, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis 2007; 196:145–54. [DOI] [PubMed] [Google Scholar]
- 16. Vanderberg JP. Reflections on early malaria vaccine studies, the first successful human malaria vaccination, and beyond. Vaccine 2009; 27:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Society of Tropical Medicine and Hygiene. Arthropod containment guidelines version 3.1, a project of the American Committee of Medical Entomology of the American Society of Tropical Medicine and Hygiene. Deerfield, Illinois:Mary Ann Liebert, Inc; 2001. [Google Scholar]
- 18. Talley AK, Healy SA, Finney OC, et al. Safety and comparability of controlled human Plasmodium falciparum infection by mosquito bite in malaria-naïve subjects at a new facility for sporozoite challenge. PLOS One 2014; 9:e109654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Food and Drug Administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Available at: https://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm074775.htm. Accessed 16 August 2017.
- 20. Murphy SC, Prentice JL, Williamson K, et al. Real-time quantitative reverse transcription PCR for monitoring of blood-stage Plasmodium falciparum infections in malaria human challenge trials. Am J Trop Med Hyg 2012; 86:383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patchen LC, Mount DL, Schwartz IK, Churchill FC. Analysis of filter-paper-absorbed, finger-stick blood samples for chloroquine and its major metabolite using high-performance liquid chromatography with fluorescence detection. J Chromatogr 1983; 278:81–9. [DOI] [PubMed] [Google Scholar]
- 22. Alving AS, Arnold J, Hockwald RS, Clayman CB, Dern JR, Beutler E, Flanagan CL. Potentiation of the curative action of primaquine in vivax malaria by quinine and chloroquine. J Lab Clin Med 1955; 46:301–6. [PubMed] [Google Scholar]
- 23. Walk J, van Gemert GJ, Graumans W, Sauerwein RW, Bijker EM. Mosquito infectivity and parasitemia after controlled human malaria infection. Am J Trop Med Hyg 2018; 98:1705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Behet MC, Foquet L, van Gemert GJ, et al. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar J 2014; 13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis MD, Pfeil J, Heiss K, Mueller AK. CD8(+) T cells mediate robust stage-specific immunity to P. berghei under chemoprophylaxis and this protective environment is not downregulated by the presence of blood-stage infection. PLOS One 2014; 9:e88117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brando C, Richardson JH, Murphy J, Ockenhouse CF, Kamau E. Phenotypic characterization of Plasmodium berghei responsive CD8+ T cells after immunization with live sporozoites under chloroquine cover. Malar J 2014; 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teirlinck AC, McCall MB, Roestenberg M, et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLOS Pathog 2011; 7:e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walk J, Reuling IJ, Behet MC, et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med 2017; 15:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.