Abstract
Background
Initiation of antiretroviral therapy (ART) often leads to weight gain. While some of this weight gain may be an appropriate return-to-health effect, excessive increases in weight may lead to obesity. We sought to explore factors associated with weight gain in several randomized comparative clinical trials of ART initiation.
Methods
We performed a pooled analysis of weight gain in 8 randomized controlled clinical trials of treatment-naive people living with human immunodeficiency virus (HIV) initiating ART between 2003 and 2015, comprising >5000 participants and 10 000 person-years of follow-up. We used multivariate modeling to explore relationships between demographic factors, HIV disease characteristics, and ART components and weight change following ART initiation.
Results
Weight gain was greater in more recent trials and with the use of newer ART regimens. Pooled analysis revealed baseline demographic factors associated with weight gain including lower CD4 cell count, higher HIV type 1 RNA, no injection drug use, female sex, and black race. Integrase strand transfer inhibitor use was associated with more weight gain than were protease inhibitors or nonnucleoside reverse transcriptase inhibitors (NNRTIs), with dolutegravir and bictegravir associated with more weight gain than elvitegravir/cobicistat. Among the NNRTIs, rilpivirine was associated with more weight gain than efavirenz. Among nucleoside/nucleotide reverse transcriptase inhibitors, tenofovir alafenamide was associated with more weight gain than tenofovir disoproxil fumarate, abacavir, or zidovudine.
Conclusions
Weight gain is ubiquitous in clinical trials of ART initiation and is multifactorial in nature, with demographic factors, HIV-related factors, and the composition of ART regimens as contributors. The mechanisms by which certain ART agents differentially contribute to weight gain are unknown.
Keywords: HIV, weight gain, obesity, antiretroviral therapy, ART
In this report, we use pooled data from randomized clinical trials to identify demographic-, human immunodeficiency virus-, and antiretroviral therapy (ART)–related risk factors for weight gain after the initiation of ART, highlighting the multifactorial nature of ART-associated weight gain.
(See the Editorial Commentary by Bares on pages 1390–2.)
Excess weight and obesity are an escalating global health concern, affecting an estimated 600 million adults and contributing to substantial morbidity and mortality through an increased risk of cardiovascular disease, diabetes, chronic kidney disease, nonalcoholic steatohepatitis, and cancer. An increasing prevalence of overweight and obesity has also been reported in people living with human immunodeficiency virus (PLWH), among whom initiation of antiretroviral therapy (ART) often leads to weight gain [1–7]. Although this weight gain may be a positive prognostic indicator in PLWH who are underweight at the time of ART initiation [6, 8, 9], among those in normal or overweight categories, weight gain may increase the risk of cardiovascular and metabolic diseases [7, 10, 11].
Possible mechanisms for ART-associated weight gain include a return-to-health phenomenon, especially in those with advanced human immunodeficiency virus (HIV) disease, with weight returning to a preillness baseline. The mechanism underlying the return-to-health phenomenon is incompletely understood, but likely results from the alleviation of HIV-associated inflammation and accelerated catabolism [12]. Treatment of HIV may also hasten resolution of opportunistic infections and gastrointestinal (GI) dysfunction that could adversely affect appetite and nutrient absorption. Additional factors associated with weight gain among PLWH include both demographic and HIV-specific characteristics, with greater weight gain observed in black people, women, and those with high pretreatment HIV RNA or low CD4 cell counts [2, 4–6, 13–15]. Specific ART regimens or drug classes have also been implicated in weight gain, with integrase strand transfer inhibitors (INSTIs) cited in 2 randomized studies [15, 16] and several retrospective cohort studies [2, 17, 18]. To further explore the demographic-, HIV-, and treatment-related contributors to weight gain, we conducted a pooled analysis of 8 randomized comparative clinical trials of initial ART. We also explored whether weight changes were associated with adverse metabolic effects.
METHODS
Study Design and Participants
Pooled analyses included 8 Gilead Sciences–sponsored trials of participants initiating ART (2003–2015) that satisfied the selection criteria of phase 3 stage, active-controlled design, enrollment of treatment-naive participants, and follow-up duration of at least 96 weeks; the trial designs, treatment arms, and dates are provided in Supplementary Table 1. One additional trial (trial 99–903) was included in individual trial analyses (Figure 1A and 1B) but was excluded from all pooled analyses due to inadequate frequency of weight monitoring. All participants provided informed consent and trials were undertaken in accordance with the Declaration of Helsinki and approved by central or site-specific review boards or ethics committees.
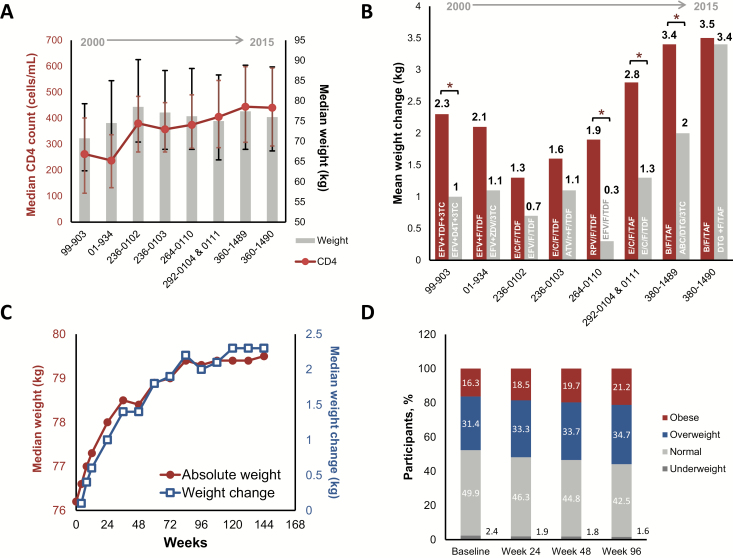
Figure 1.
Weight trends in participants initiating antiretroviral therapy. A, Baseline median CD4 cell count and median weight in the indicated clinical trials, which are ordered by date of trial initiation. Error bars represent the first through third quartiles. B, Mean weight change observed at the 48-week time point for the indicated trials, which are organized by date of initiation. Red bars are the investigational regimen, and gray bars are the comparator. *P < .05 by analysis of variance. C, Median weight (red) and median weight change (blue) over time in 8 pooled clinical trials. D, Body mass index category distributions over time in 8 pooled clinical trials. Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV/r, ritonavir-boosted atazanavir; B, bictegravir; C, cobicistat; D4T, stavudine; DTG, dolutegravir; E, elvitegravir; EFV, efavirenz; F, emtricitabine; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Procedures
All studies included a baseline visit and follow-up visits every 12 weeks through week 96. Height was collected at baseline; body weight and body mass index (BMI) calculations (weight in kilograms divided by height in meters, squared [kg/m2]) were performed at each visit. Analyses included only weights that were obtained while the participant was on randomized therapy. Laboratory evaluations including CD4 cell count and HIV type 1 (HIV-1) RNA were performed at each study visit. Serum glucose and lipid measurements were performed after an 8-hour fast. All laboratory evaluations were performed by Covance Laboratories (Indianapolis, Indiana).
Adverse events (AEs) were reported by the site investigators and were coded using the Medical Dictionary for Regulatory Activities (MedDRA); version varied depending on clinical trial. Diabetes-related treatment-emergent AEs reported by the study site investigators were identified by querying MedDRA terms found in the “hyperglycemia/new-onset diabetes mellitus (standardised MedDRA queries)” class (Supplementary Table 2 and Supplementary Methods).
Statistical Analyses
Statistical testing, multivariate models, and linear mixed-effect models were performed as described in the Supplementary Methods. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
Population, Demographics, and Baseline Disease Characteristics
In the pooled analyses of 8 phase 3 trials (Supplementary Table 1), 5680 treatment-naive participants initiated ART. At ART initiation, median BMI was 24.8 kg/m2; 16.3% were obese (BMI ≥30 kg/m2), 31.4% were overweight (BMI 25–29.9 kg/m2), and 52.3% were normal (18.5–24.9 kg/m2) or underweight (<18.5 kg/m2). Additional baseline weight and demographic data are summarized in Table 1, and baseline disease characteristics are summarized in Table 2.
Table 1.
Demographics and Baseline Characteristics by Study
| Clinical Trials | Combined | 934: EFV + FTC/TDF vs EFV + ZDV/3TC | 236–0102: E/C/F/TDF vs EFV/FTC/TDF | 236–0103: E/C/F/TDF vs ATV/r + F/TDF | 264–0110: RPV/FTC/TDF vs EFV/FTC/TDF | 292–0104, 292–0111: E/C/F/TAF vs E/C/F/TDF | 380–1489: B/F/TAF vs ABC/DTG/3TC | 380–1490: B/F/TAF vs DTG + F/TAF |
|---|---|---|---|---|---|---|---|---|
| Year first participant screened | … | 2003 | 2010 | 2010 | 2011 | 2012 | 2015 | 2015 |
| No. of participants | 5680 | 501 | 698 | 704 | 781 | 1728 | 629 | 639 |
| Age, y | ||||||||
| Mean (SD) | 37 (10.7) | 38 (9.5) | 38 (10.5) | 38 (10.2) | 37 (10.7) | 36 (10.6) | 34 (10.8) | 37 (11.9) |
| Median (Q1, Q3) | 35 (28, 44) | 37 (32, 42) | 37 (29, 45) | 38 (30, 45) | 36 (28, 45) | 34 (27, 43) | 32 (25, 41) | 34 (27, 45) |
| Sex at birth | ||||||||
| Male | 5018 (88.3) | 434 (86.6) | 621 (89.0) | 637 (90.5) | 725 (92.8) | 1469 (85.0) | 567 (90.1) | 565 (88.4) |
| Female | 662 (11.7) | 67 (13.4) | 77 (11.0) | 67 (9.5) | 56 (7.2) | 259 (15.0) | 62 (9.9) | 74 (11.6) |
| Race | ||||||||
| Asian | 290 (5.1) | 6 (1.2) | 16 (2.3) | 34 (4.8) | 21 (2.7) | 180 (10.4) | 16 (2.6) | 17 (2.7) |
| Black | 1471 (25.9) | 113 (22.6) | 197 (28.2) | 118 (16.8) | 191 (24.5) | 432 (25.0) | 226 (36.0) | 194 (30.4) |
| White | 3499 (61.6) | 296 (59.2) | 439 (62.9) | 524 (74.4) | 524 (67.3) | 982 (56.8) | 359 (57.3) | 375 (58.7) |
| Other | 415 (7.3) | 85 (17.0) | 46 (6.6) | 28 (4.0) | 43 (5.5) | 134 (7.8) | 26 (4.1) | 53 (8.3) |
| Unknowna | 4 (0.1) | 1 | 0 | 0 | 1 | 0 | 2 | 0 |
| Sex and race | ||||||||
| Male, black | 1161 (20.4) | 80 (16.0) | 155 (22.2) | 88 (12.5) | 156 (20.0) | 341 (19.7) | 184 (29.3) | 157 (24.6) |
| Male, non-black | 3853 (67.8) | 354 (70.7) | 466 (66.8) | 549 (78.0) | 567 (72.6) | 1128 (65.3) | 381 (60.6) | 408 (63.8) |
| Female, black | 310 (5.5) | 33 (6.6) | 42 (6.0) | 30 (4.3) | 35 (4.5) | 91 (5.3) | 42 (6.7) | 37 (5.8) |
| Female, non-black | 351 (6.2) | 33 (6.6) | 35 (5.0) | 37 (5.3) | 21 (2.7) | 168 (9.7) | 20 (3.2) | 37 (5.8) |
| Ethnicity | ||||||||
| Hispanic or Latino | 1119 (20.0) | 78 (15.6) | 166 (23.8) | 109 (15.8) | 132 (17.0) | 334 (19.4) | 137 (21.9) | 163 (25.5) |
| Not Hispanic or Latino | 4535 (80.0) | 423 (84.4) | 532 (76.2) | 581 (84.2) | 643 (83.0) | 1391 (80.6) | 489 (78.1) | 476 (74.5) |
| Unknowna | 24 | 0 | 0 | 14 | 5 | 2 | 3 | 0 |
| Baseline weight, kg | ||||||||
| No. | 5680 | 501 | 698 | 704 | 781 | 1728 | 629 | 639 |
| Mean (SD) | 78.9 (17.25) | 76.5 (14.93) | 81.3 (17.75) | 79.3 (16.57) | 79.2 (16.39) | 77.4 (17.09) | 80.2 (18.03) | 79.8 (19.18) |
| Median (Q1, Q3) | 76.2 (67.1, 87.5) | 74.8 (65.8, 85.4) | 78.5 (69.8, 90.2) | 77.1 (68.0, 87.5) | 76.2 (68.0, 88.0) | 75 (65.3, 86.6) | 77.4 (68.0, 88.8) | 76.1 (67.7, 88.4) |
| Baseline BMI, kg/m2 | ||||||||
| No. | 5674 | 496 | 698 | 704 | 780 | 1728 | 629 | 639 |
| Mean (SD) | 25.7 (5.20) | 25.0 (4.55) | 26.4 (5.58) | 25.6 (4.95) | 25.6 (4.70) | 25.5 (5.18) | 26.0 (5.53) | 26.0 (5.66) |
| Median (Q1, Q3) | 24.8 (22.2, 28.1) | 24.3 (22.0, 27.4) | 25.2 (22.7, 28.6) | 24.8 (22.2, 27.8) | 24.8 (22.4, 28.1) | 24.5 (21.8, 28.0) | 25 (22.4, 28.8) | 24.8 (22.2, 28.1) |
| Underweight: <18.5 | 136 (2.4) | 18 (3.6) | 13 (1.9) | 15 (2.1) | 16 (2.1) | 41 (2.4) | 21 (3.3) | 12 (1.9) |
| Normal: 18.5–24.9 | 2829 (50.0) | 266 (53.6) | 318 (45.6) | 355 (50.4) | 390 (50.0) | 891 (51.6) | 292 (46.4) | 317 (49.6) |
| Overweight: 25–29.9 | 1785 (31.4) | 154 (31.0) | 236 (33.8) | 236 (33.5) | 248 (31.8) | 527 (30.5) | 197 (31.3) | 187 (29.3) |
| Obese: ≥30 | 924 (16.3) | 58 (11.7) | 131 (18.8) | 98 (13.9) | 126 (16.2) | 269 (15.6) | 119 (18.9) | 123 (19.2) |
Data are presented as no. (%) unless otherwise indicated. One participant with missing race and 2 participants with missing ethnicity were excluded from the race, combination of sex and race, and ethnicity summary.
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV/r, ritonavir-boosted atazanavir; B, bictegravir; BMI, body mass index; C, cobicistat; DTG, dolutegravir; E, elvitegravir; EFV, efavirenz; F, emtricitabine; FTC, emtricitabine; Q1, first quartile; Q3, third quartile; RPV, rilpivirine; SD, standard deviation; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
aInquiry regarding race and ethnicity was not permitted at some study sites. Participants with unknown ethnicity were excluded from the denominator for percentage calculations.
Table 2.
Baseline Disease Characteristics by Study
| Clinical Trials | Combined | 934: EFV + FTC/TDF vs EFV + ZDV/3TC | 236–0102: E/C/F/TDF vs EFV/FTC/TDF | 236–0103: E/C/F/TDF vs ATV/r + F/TDF | 264–0110: RPV/FTC/TDF vs EFV/FTC/TDF | 292–0104, 292–0111: E/C/F/TAF vs E/C/F/TDF | 380–1489: B/F/TAF vs ABC/DTG/3TC | 380–1490: B/F/TAF vs DTG + F/TAF |
|---|---|---|---|---|---|---|---|---|
| HIV-1 RNA, log10 copies/mL | ||||||||
| No. | 5680 | 501 | 698 | 704 | 781 | 1728 | 629 | 639 |
| Mean (SD) | 4.65 (0.667) | 5.01 (0.538) | 4.75 (0.583) | 4.81 (0.614) | 4.79 (0.629) | 4.53 (0.674) | 4.42 (0.665) | 4.41 (0.698) |
| Median (Q1, Q3) | 4.69 (4.23, 5.08) | 5.04 (4.63, 5.36) | 4.76 (4.34, 5.15) | 4.87 (4.37, 5.19) | 4.79 (4.36, 5.22) | 4.58 (4.14, 4.96) | 4.47 (4.04, 4.87) | 4.44 (4.00, 4.87) |
| HIV-1 RNA, copies/mL | ||||||||
| ≤100 000 | 4020 (70.8) | 246 (49.1) | 466 (66.8) | 415 (58.9) | 508 (65.0) | 1338 (77.4) | 526 (83.6) | 521 (81.5) |
| >100 000 | 1660 (29.2) | 255 (50.9) | 232 (33.2) | 289 (41.1) | 273 (35.0) | 390 (22.6) | 103 (16.4) | 118 (18.5) |
| CD4 count, cells/μL | ||||||||
| No. | 5679 | 501 | 698 | 704 | 781 | 1727 | 629 | 639 |
| Mean (SD) | 401 (211.4) | 242 (163.9) | 386 (179.7) | 370 (170.1) | 391 (182.8) | 428 (217.7) | 464 (226.3) | 456 (244.4) |
| Median (Q1, Q3) | 382 (264, 513) | 229 (123, 322) | 380 (271 484) | 359 (270, 460) | 375 (284, 490) | 406 (288, 549) | 444 (307, 598) | 442 (291, 597) |
| CD4 count category, cells/μL | ||||||||
| <200 | 871 (15.3) | 209 (41.7) | 94 (13.5) | 91 (12.9) | 103 (13.2) | 228 (13.2) | 68 (10.8) | 78 (12.2) |
| ≥200 | 4808 (84.7) | 292 (58.3) | 604 (86.5) | 613 (87.1) | 678 (86.8) | 1499 (86.8) | 561 (89.2) | 561 (87.8) |
| IV drug use | ||||||||
| Yes | 87 (1.5) | 14 (2.8) | 22 (3.2) | 12 (1.7) | 10 (1.3) | 11 (0.6) | 9 (1.4) | 9 (1.4) |
| No | 5593 (98.5) | 487 (97.2) | 676 (96.8) | 692 (98.3) | 771 (98.7) | 1717 (99.4) | 620 (98.6) | 630 (98.6) |
| HIV disease status | ||||||||
| Asymptomatic | 4590 (80.8) | 57 (11.4) | 583 (83.5) | 576 (81.8) | 660 (84.6) | 1574 (91.5) | 572 (90.9) | 568 (88.9) |
| Symptomatica | 599 (10.5) | 241 (48.1) | 63 (9.0) | 73 (10.4) | 84 (10.8) | 87 (5.1) | 30 (4.8) | 21 (3.3) |
| AIDS | 483 (8.5) | 203 (40.5) | 52 (7.4) | 55 (7.8) | 36 (4.6) | 60 (3.5) | 27 (4.3) | 50 (7.8) |
| Unknownb | 8 | 0 | 0 | 0 | 1 | 7 | 0 | 0 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV/r, ritonavir-boosted atazanavir; B, bictegravir; C, cobicistat; DTG, dolutegravir; E, elvitegravir; EFV, efavirenz; F, emtricitabine; FTC, emtricitabine; HIV, human immunodeficiency virus; IV, intravenous; Q1, first quartile; Q3, third quartile; RPV, rilpivirine; SD, standard deviation; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
aDefined as any participant with symptoms attributable to HIV infection but without AIDS-defining criteria, as determined by the study site investigator.bParticipants with unknown HIV disease status were excluded from the denominator for percentage calculations.
Baseline weight was higher in the more recent trials, as was baseline CD4 cell count (Figure 1A). Weight gain occurred in all study arms. The magnitude of weight gain was larger in the more recent trials, and the investigational regimen was consistently associated with more weight gain than the comparator (Figure 1B). The international composition of the trials can be found in Supplementary Figure 1.
Weight Gain in Participants Initiating ART
In pooled analyses, the 96-week median weight gain was 2.0 kg (interquartile range [IQR], −.9 to 5.9), with the greatest rate of weight gain occurring during the initial 48 weeks (Figure 1C). Through 96 weeks, 48.6%, 36.6%, and 17.3% of participants had at least 3%, 5%, and 10% weight increase from baseline, respectively. Weight gain was not observed in all participants; 30.2% lost weight. The proportion of participants in overweight and obese BMI categories increased over time (Figure 1D).
Risk Factors for Any Weight Gain in Participants Initiating ART
Baseline CD4 cell count had the strongest association with weight gain in multivariate models; participants with a baseline CD4 count of <200/μL gained on average 2.97 kg more than participants with baseline CD4 count ≥200/μL (95% confidence interval [CI], 2.81–3.13; P < .001; Table 3). Furthermore, increases in CD4 cell count and weight over time were closely correlated (Supplementary Figure 3). Higher baseline HIV RNA (>100 000 copies/mL) was associated with a mean 0.96 kg greater weight gain (95% CI, .84–1.08; P < .001); participants with symptomatic HIV or AIDS gained 0.51 kg more than those with asymptomatic HIV (95% CI, .36–.65; P < .001). Participants who did not inject drugs at baseline gained 1.41 kg more than those who did (95% CI, .97–1.85; P < .001). Black race was associated with weight gain, with a mean 0.99 kg greater weight gain compared to participants of other races (95% CI, .87–1.11; P < .001). Female sex, age <50 years, and persons with baseline obesity had smaller but statistically significant correlations with weight gain (Table 3).
Table 3.
Risk Factors for Any Weight Gain in Individuals Initiating Antiretroviral Therapy
| Variable | Difference, kg | (95% CI) | P Value |
|---|---|---|---|
| CD4 count (<200 vs ≥200 cells/μL) | 2.97 | (2.81–3.13) | <.001 |
| IV drug use (no vs yes) | 1.41 | (.97–1.85) | <.001 |
| Race (black vs non-black) | 0.99 | (.87–1.11) | <.001 |
| HIV RNA (>100K vs ≤100K copies/mL) | 0.96 | (.84–1.08) | <.001 |
| Symptomatic HIV (yes vs no) | 0.51 | (.36–.65) | <.001 |
| Sex (female vs male) | 0.23 | (.07–.4) | .006 |
| Age (<50 vs ≥50 y) | 0.22 | (.07–.37) | .004 |
| BMI | |||
| Obese vs normal | 0.21 | (.06–.36) | .005 |
| Overweight vs normal | 0.24 | (−.36 to −.13) | <.001 |
Stepwise model selection was used to identify baseline risk factors associated with weight gain in individuals initiating antiretroviral therapy, resulting in the inclusion of the above 8 baseline risk factors in the mixed-effect model. Difference, 95% CI, and P values were determined from the mixed-effect model including these 8 baseline risk factors and visit as fixed effects and participants as a random effect.
Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; IV, intravenous.
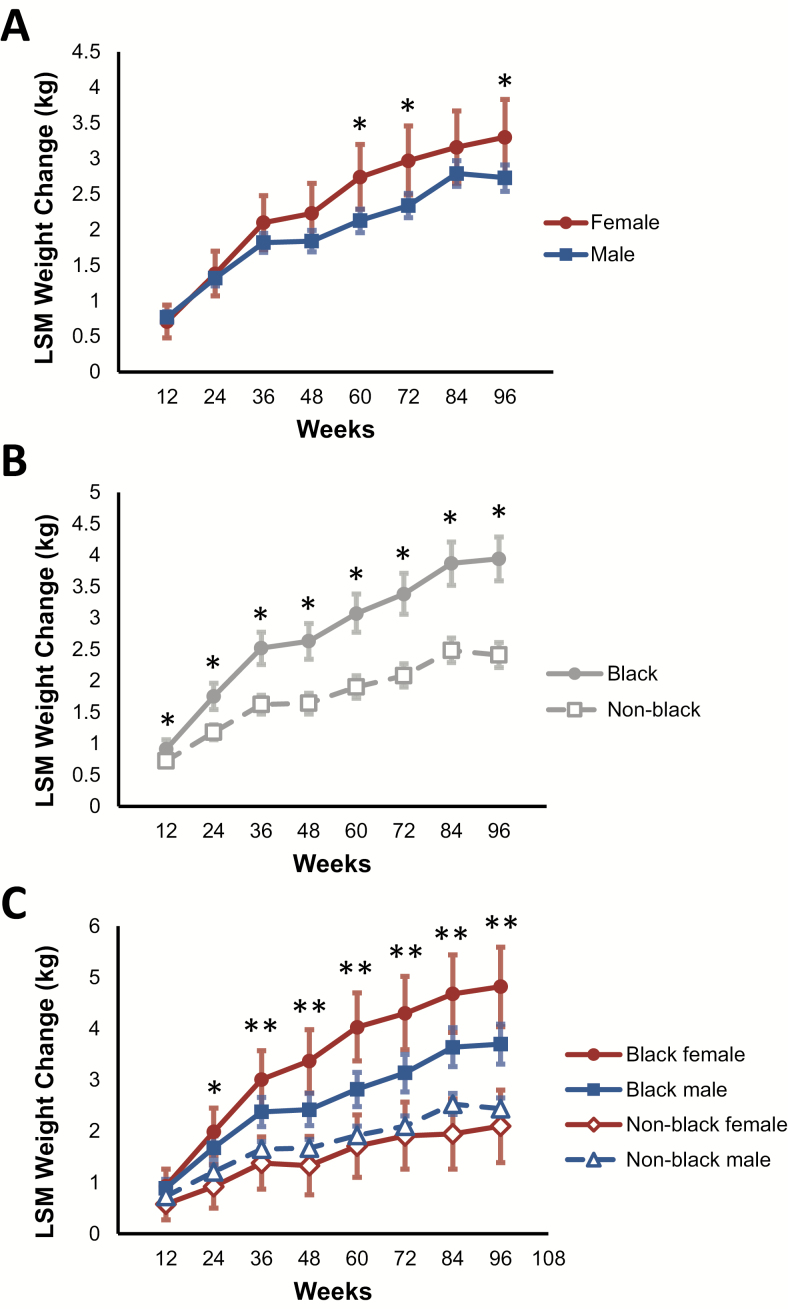
We further explored these findings by using longitudinal models to study the relationship between sex, race, and weight gain. Female participants gained more weight than male participants, and black participants gained significantly more weight than non-black participants (Figure 2A and 2B and Supplementary Table 3). Stratification by both sex and race revealed the greatest weight gain among black female participants, followed by black male participants (96-week difference between black females and black males, 1.12 kg [95% CI, .25–1.99]; P = .011; Figure 2C and Supplementary Table 3).
Figure 2.
Effect of sex and race on weight change in individuals initiating antiretroviral therapy. A, Least squares mean (LSM) weight change over time, stratified by sex. *P < .05 vs the comparator. B, LSM weight change over time, stratified by race (black vs non-black). *P < .05 vs the comparator. C, LSM weight change over time, stratified by both sex and race. *P < .05 for black females vs non-black females; **P < .05 for black females vs non-black females and for black females vs black males. P values for these comparisons are found in Supplementary Table 3.
Association of Antiretroviral Regimen Components With Any Weight Gain
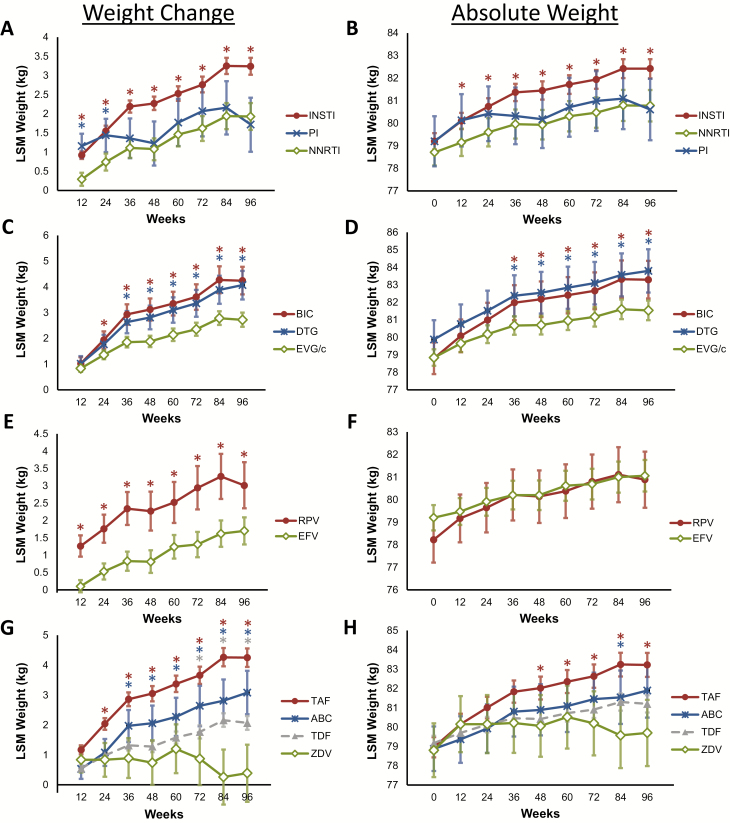
Longitudinal modeling of weight gain and ART third-agent class (INSTI, nonnucleoside reverse transcriptase inhibitor [NNRTI], or protease inhibitor [PI]) revealed weight gain in all 3 classes (96-week least squares mean weight gain: INSTI, 3.24 kg [95% CI, 3.02–3.46]; NNRTI, 1.93 kg [95% CI, 1.58–2.28]; PI, 1.72 kg [95% CI, 1.01–2.42]; Figure 3A). Participants taking INSTIs experienced the most weight gain (Figure 3A and Supplementary Table 4). Weight gain was similar between the NNRTI and PI treatment groups. Absolute weight followed a similar trend (Figure 3B and Supplementary Table 4).
Figure 3.
Weight change and absolute weight in participants initiating antiretroviral therapy. A and B, Least squares mean (LSM) weight change (A) and absolute weight (B) over time in all participants, stratified by third antiretroviral agent. C and D, LSM weight change (C) and absolute weight (D) in participants taking integrase strand transfer inhibitors (INSTIs), stratified by INSTI used. E and F, LSM weight change (E) and absolute weight (F) in all participants taking nonnucleoside reverse transcriptase inhibitors (NNRTIs), stratified by NNRTI used. G and H, LSM weight change (G) and absolute weight (H) in participants taking a nucleoside reverse transcriptase inhibitor (NRTI), stratified by NRTI used. Error bars depict the 95% confidence interval. Asterisks are color-coded to match the respective comparator and denote P ≤ .05 compared with NNRTIs (A and B), EVG/c (C and D), EFV (E and F), or ZDV (G and H). P values for these comparisons are found in Supplementary Table 3. Abbreviations: ABC, abacavir; BIC, bictegravir; DTG, dolutegravir; EFV, efavirenz; EVG/c, cobicistat-boosted elvitegravir; PI, protease inhibitor; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
We next assessed the association between weight gain and the specific INSTI used. Participants taking bictegravir (BIC) or dolutegravir (DTG) demonstrated similar weight gain, both greater than that in PLWH taking cobicistat-boosted elvitegravir (EVG/c) (96-week least squares mean weight gain: BIC, 4.24 kg [95% CI, 3.71–4.78]; DTG, 4.07 kg [95% CI, 3.51–4.62]; EVG/c, 2.72 kg [95% CI, 2.45–3.0]). We observed a similar trend when the analysis was performed using absolute weight (Figure 3C and 3D and Supplementary Table 4).
Among participants taking NNRTI-containing regimens, those taking rilpivirine (RPV) gained more weight compared to those taking efavirenz (EFV) (96-week least squares mean weight gain: RPV, 3.01 kg [95% CI, 2.35–3.68]; EFV, 1.7 kg [95% CI, 1.31–2.09]). Absolute weights were similar after an initial rapid increase in participants taking RPV (Figure 3E and 3F and Supplementary Table 4).
Finally, we assessed whether specific nucleoside reverse transcriptase inhibitors (NRTIs) were differentially associated with weight gain, using zidovudine (ZDV) as a reference. At 96 weeks, mean weight gains by NRTI were as follows: tenofovir alafenamide (TAF), 4.25 kg (95% CI, 3.94–4.56); abacavir (ABC), 3.08 kg (95% CI, 2.36–3.81); tenofovir disoproxil fumarate (TDF), 2.07 kg (95% CI, 1.84–2.30), as compared to ZDV 0.39 kg (95% CI, −.57 to 1.34). Absolute weight followed a similar trend (Figure 3G and 3H and Supplementary Table 4).
Risk Factors for ≥10% Weight Increase in Participants Initiating ART
To understand factors associated with more extreme weight gain, we stratified the pooled trial data into individuals who gained ≥10% body weight over 48 weeks (12.8% of participants) vs those who did not. Individuals with ≥10% weight gain were more likely to be female or black, have a lower baseline weight or BMI, have lower baseline CD4 cell count, and have higher baseline HIV-1 RNA (Table 4).
Table 4.
Demographics, Baseline Weight Characteristics, and Baseline Disease Characteristics by Weight Gain Category (≥10% or <10% Weight Increase Through 48 Weeks)
| Characteristic | Weight Increase ≥10% | Weight Increase <10% | P Value |
|---|---|---|---|
| No. (for demographics) | 728 | 4952 | |
| Age, y | .59 | ||
| Mean (SD) | 36 (10.8) | 37 (10.7) | |
| Median (Q1, Q3) | 35 (28, 44) | 35 (28, 44) | |
| Sex at birth | <.001 | ||
| Male | 613 (84.2) | 4405 (89) | |
| Female | 115 (15.8) | 547 (11) | |
| Race | <.001 | ||
| Asian | 29 (4.0) | 261 (5.3) | |
| Black | 239 (32.8) | 1232 (24.9) | |
| White | 406 (55.8) | 3093 (62.5) | |
| Other | 52 (7.1) | 363 (7.3) | |
| Unknowna | 2 | 2 | |
| Sex and race | |||
| Male | <.001 | ||
| Black | 178 (24.5) | 983 (19.9) | |
| Non-black | 433 (59.5) | 3420 (69.1) | |
| Female | .15 | ||
| Black | 61 (8.4) | 249 (5.0) | |
| Non-black | 54 (7.4) | 297 (6.0) | |
| Ethnicity | .58 | ||
| Hispanic or Latino | 138 (19) | 981 (19.8) | |
| Not Hispanic or Latino | 587 (80.6) | 3948 (79.8) | |
| Unknowna | 3 (0.4) | 21 (0.4) | |
| Baseline weight, kg | <.001 | ||
| No. | 728 | 4952 | |
| Mean (SD) | 75.2 (17.3) | 79.4 (17.2) | |
| Median (Q1, Q3) | 72 (63.5, 83.7) | 77 (67.9, 88.0) | |
| Baseline BMI, kg/m2 | <.001 | ||
| No. | 728 | 4946 | |
| Mean (SD) | 24.6 (5.1) | 25.9 (5.9) | |
| Median (Q1, Q3) | 23.4 (21.2, 26.6) | 24.9 (22.4, 28.3) | |
| Baseline BMI, kg/m2 | <.001 | ||
| Underweight: <18.5 | 34 (4.7) | 102 (2.1) | |
| Normal: 18.5–24.9 | 426 (58.5) | 2403 (50.6) | |
| Overweight: 25–29.9 | 176 (24.2) | 1609 (32.5) | |
| Obese: ≥30 | 92 (12.6) | 832 (16.8) | |
| HIV-1 RNA, log10 copies/mL | <.001 | ||
| No. | 727 | 4952 | |
| Mean (SD) | 4.97 (0.7) | 4.6 (0.65) | |
| Median (Q1, Q3) | 4.98 (4.54, 5.46) | 4.65 (4.19, 5.03) | |
| HIV-1 RNA, copies/mL | <.001 | ||
| ≤100 000 | 380 (52.2) | 3640 (73.5) | |
| >100 000 | 348 (47.8) | 1312 (26.5) | |
| CD4 count, cells/μL | <.001 | ||
| No. | 728 | 4952 | |
| Mean (SD) | 291 (220.9) | 417 (205.0) | |
| Median (Q1, Q3) | 270 (99, 432) | 393 (284, 523) | |
| CD4 count category, cells/mL | <.001 | ||
| <200 | 296 (40.7) | 575 (11.6) | |
| ≥200 | 431 (59.2) | 4377 (88.4) | |
| IV drug use | ns | ||
| Yes | 9 (1.2) | 78 (1.6) | |
| No | 719 (98.8) | 4874 (98.4) | |
| HIV disease status | <.001 | ||
| Asymptomatic | 475 (65.2) | 4115 (83.1) | |
| Symptomatic | 93 (12.8) | 506 (10.2) | |
| AIDS | 160 (22) | 323 (6.5) | |
| Unknown | 0 | 8 (0.2) |
Data are presented as no. (%) unless otherwise indicated. For categorical data, P value was from the Cochran–Mantel–Haenszel test (general association statistic was used for nominal data; row mean scores differ statistic was used for ordinal data). For continuous data, P value was from the 2-sided Wilcoxon rank-sum test.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; IV, intravenous; ns, not significant; Q1, first quartile; Q3, third quartile; SD, standard deviation.
aInquiry regarding race and ethnicity was not permitted at some study sites. Participants with unknown HIV race were excluded from the denominator for percentage calculations.
In multivariate regression models (Table 5), lower CD4 cell count and higher HIV-1 RNA were associated with greater odds of ≥10% weight gain (odds ratio [OR], 4.4 [95% CI, 3.60–5.27]; P < .001 and OR, 2.0 [95% CI, 1.65–2.37]; P < .001, respectively). Normal baseline BMI was associated with ≥10% weight gain when compared to individuals with overweight or obese baseline BMI (normal vs overweight: OR, 1.54 [95% CI, 1.27–1.87]; P < .001 and normal vs obese: OR, 1.66 [95% CI, 1.29–2.15]). Female sex and black race were associated with ≥10% weight gain (female vs male: OR, 1.54 [95% CI, 1.21–1.96]; P < .001 and black vs non-black: OR, 1.32 [95% CI, 1.10–1.59]; P = .003). More black women experienced ≥10% weight gain than non-black women (19.7% vs 12.4%; P < .001).
Table 5.
Risk Factors for Significant (≥10%) Weight Gain in Individuals Initiating Antiretroviral Therapy
| Variable | OR | (95% CI) | P Value |
|---|---|---|---|
| CD4 count (<200 vs ≥200 cells/all) | 4.36 | (3.6–5.27) | <.001 |
| HIV RNA (>100K vs ≤100K copies/mL) | 1.98 | (1.65–2.37) | <.001 |
| BMI | |||
| Normal vs overweight | 1.54 | (1.27–1.87) | <.001 |
| Normal vs obese | 1.66 | (1.29–2.15) | <.001 |
| Sex (female vs male) | 1.54 | (1.21–1.96) | <.001 |
| Race (black vs non-black) | 1.32 | (1.10–1.59) | .003 |
| Third ART agent | |||
| BIC/DTG vs EFV | 1.82 | (1.24–2.66) | .002 |
| EVG/c vs EFV | 1.36 | (1.04–1.78) | .026 |
| RPV vs EFV | 1.51 | (1.03–2.20) | .035 |
| ATV/r vs EFV | 0.92 | (.59–1.45) | .73 |
| NRTI | |||
| TAF vs ZDV | 1.75 | (1.04–2.95) | .034 |
| TDF vs ZDV | 1.19 | (.76–1.87) | .44 |
| ABC vs ZDV | 0.93 | (.47–1.8) | .82 |
| TAF vs ABC | 1.9 | (1.25–2.88) | .003 |
| TDF vs ABC | 1.29 | (.79–2.11) | .31 |
| TAF vs TDF | 1.47 | (1.14–1.90) | .003 |
Stepwise model selection was used to identify which baseline risk factors were associated with significant (≥10%) weight gain in individuals initiating ART. As a result, CD4 cell count, HIV RNA, BMI, sex, and race were selected. ORs and their 95% CIs and P values were from the logistic regression model including baseline categories of CD4 cell count, HIV-1 RNA, BMI, sex, and race as risk factors, with third agent and NRTIs as fixed effects.
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; ATV/r, ritonavir-boosted atazanavir; BIC, bictegravir; BMI, body mass index; CI, confidence interval; DTG, dolutegravir; EFV, efavirenz; EVG/c, cobicistat-boosted elvitegravir; HIV, human immunodeficiency virus; NRTI, nucleoside reverse transcriptase inhibitor; OR, odds ratio; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
We assessed the association between the specific third-agent drug and ≥10% weight gain (Table 5). Compared to EFV, the initiation of BIC or DTG (OR, 1.82 [95% CI, 1.24–2.66]; P = .002), EVG/c (OR, 1.36 [95% CI, 1.04–1.78]; P = .026), and RPV (OR, 1.51 [95% CI; 1.03–2.20]; P = .035), but not ritonavir-boosted atazanavir, was associated with an increased risk of ≥10% weight gain. Among the NRTIs, TAF (OR, 1.75 [95% CI, 1.04–2.95]; P = .034), but not ABC or TDF, was associated with increased risk for ≥10% weight gain compared to ZDV. TAF was also associated with an increased risk of ≥10% weight gain compared with ABC (OR, 1.90 [95% CI, 1.25–2.88]; P = .003) and TDF (OR, 1.47 [95% CI, 1.14–1.90]; P = .003).
Metabolic Impacts of Significant Weight Increase
Next, we evaluated whether ≥10% weight gain was associated with subsequent changes in fasting glucose or the incidence of treatment-emergent AEs related to diabetes or hyperglycemia. We found no significant difference in fasting glucose change between participants with ≥10% or <10% weight gain (96-week mean fasting glucose change for both groups was 3 mg/dL [95% CIs, 1.01–4.99 and 2.39–3.61, respectively]; Supplementary Figure 2A). The incidence rate of diabetes- or hyperglycemia-related AEs was higher in individuals with ≥10% weight gain compared with those without, although this difference was not statistically significant (1.01 per 100 person-years [PY] [95% CI, .59–1.74] and 0.67 per 100 PY [95% CI, .53–.85], respectively; P = .18). Low-density lipoprotein and triglycerides had similar small increases in both groups, whereas high-density lipoprotein (HDL) had a small but significant increase in participants with <10% weight increase compared to those with ≥10% weight increase (Supplementary Figure 2B–D). The ratio of total cholesterol to HDL was slightly higher in the ≥10% weight gain group (week 96 median, 3.7 [IQR, 2.9–4.6] vs 3.5 [IQR, 2.9–4.4]; P = .027). Blood pressure data are available for 3 trials (trials 264–0110, 380–1489, and 380–1490); no clinically significant changes were observed (the week 96 weighted mean change from baseline in systolic and diastolic blood pressure was 2.2 mm Hg and 1.5 mm Hg, respectively).
DISCUSSION
In our pooled analysis of 8 randomized clinical trials of ART-naive PLWH ranging from the years 2003 to 2019, we found that PLWH are initiating ART at a higher baseline weight and that many gain significant amounts of weight during the first 2 years of therapy. A mix of demographic, HIV disease–specific, and ART-specific factors were associated with weight increase from baseline and with more extreme (≥10%) weight gain.
Similar to other reports, we observed higher baseline weight in more recent studies of ART-naive PLWH, with median baseline BMIs at or near the overweight category [1–4]. Weight gain was common following ART initiation: About half of participants gained at least 3% body weight with a median weight gain of 2.0 kg over nearly 2 years of follow-up. This degree of weight gain mirrors the obesity trend observed in the National Health and Nutrition Examination Survey and the Coronary Artery Risk Development in Young Adults study, where the average American aged 20–40 years gained nearly 1 kg per year [19]. Accordingly, the distribution of BMI classes in trial participants shifted toward overweight and obese categories by trial conclusion, approaching the distribution seen in recent HIV cohort studies and in the general population (approximately one-third overweight, one-third obese) [4, 5, 19–21].
We did not observe a clinically significant metabolic impact of weight gain in our trials as measured by fasting glucose and investigator-reported AEs; however, this analysis is limited by duration of follow-up, a relatively small number of reported AEs, and the absence of more sensitive markers of glucose tolerance.
Black race and female sex were associated with weight gain, consistent with other studies [2, 5, 6, 13, 15]. This association was particularly notable among black females, who gained approximately twice as much weight as women of other races. The mechanism underlying this observation is unknown, but it mirrors the disproportionately high prevalence of obesity in black women in the United States [22], and both may be affected by similar factors. These findings are similar to prior studies reporting a concurrence of HIV and obesity risk in the black population [23], and highlight the need for increased obesity awareness, monitoring, and clinical intervention in this high-risk population.
We observed strong associations between weight gain and HIV disease characteristics. Disease stage, as reflected by low baseline CD4 cell count and high baseline HIV RNA, correlated with weight gain in our models of any weight gain and ≥10% weight gain, similar to other reports [2, 4–6, 15]. These findings support a contribution of the return-to-health phenomenon to weight gain in PLWH initiating ART. This effect may be desirable in some individuals, but could also contribute to excess weight gain in individuals with early-stage HIV disease and those with normal or above-normal BMI.
Our analyses revealed several important associations between weight gain and ART at the class and drug level. Examining the clinical trials individually, we observe greater weight gain in newer investigational regimens relative to the comparator, consistent with the findings reported by others [2, 24]. In our pooled analyses, INSTI-containing regimens were associated with more weight gain than NNRTI- or PI-based regimens, with DTG and BIC associated with more weight gain than EVG/c. Among NNRTIs, RPV was associated with more weight gain than EFV. Among NRTI pairs, TAF/emtricitabine (FTC) was associated with the most weight gain, ABC/lamivudine (3TC) and TDF/FTC with slightly less weight gain, and ZDV/3TC with weight stability. These findings are similar to the ADVANCE trial, in which DTG and TAF were associated with treatment-emergent obesity, whereas TDF/FTC/EFV was associated with treatment-emergent underweight status and a higher rate of treatment discontinuation [25]. Altogether, these findings establish a pattern of more weight gain with newer ART regimens, possibly reflecting better-tolerated, easier-to-take regimens [26].
The hypothesis that improved tolerability may contribute to weight gain in PLWH initiating ART is supported by clinical trial data comparing the GI tolerability of HIV regimens. INSTIs such as DTG, BIC, and RAL do not require boosting with cobicistat, which has been associated with nausea and diarrhea [27]. Among NNRTIs, RPV is better tolerated than EFV and should be taken with food, which may result in higher caloric intake [28]. In the case of NRTIs, early trials demonstrated more GI toxicity with ZDV compared with newer NRTIs, including ABC and TDF [26]. There is also evidence that TAF may be associated with better GI tolerability than ABC; in a study comparing BIC/FTC/TAF vs ABC/DTG/3TC, there was a lower incidence of nausea in the TAF-containing arm, a difference not observed in a study comparing BIC with DTG, both with TAF/FTC [29, 30].
If individual agents contribute to weight gain aside from tolerability, the mechanisms by which they do so is not known. For treatment-naive PLWH, some of the association between weight gain and INSTI-containing regimens could be their faster virologic control compared to older regimens [31]. Another explanation for drug-specific effects on weight could be off-target biological interactions. One such example is the observed interaction between DTG and melanocortin 4 receptor (MC4R), a receptor involved in the regulation of caloric intake by modulating leptin signaling in the central nervous system [32, 33]. This finding is intriguing as mutations in MC4R are associated with heritable obesity [33]. This potential mechanism requires further validation, and it remains unknown whether other INSTIs interact similarly.
Evaluating the effect of ART drugs on weight gain is confounded by HIV disease factors such as return to health; some of these limitations may be avoided by studying weight changes in preexposure prophylaxis (PrEP) trials. In the Iniciativa Profilaxis Pre-Exposición study comparing TDF/FTC to placebo, the TDF/FTC arm gained less weight than placebo, suggesting that TDF/FTC may have a mild weight-suppressive effect [34]. In the DISCOVER trial of TDF/FTC vs TAF/FTC for PrEP, weight gain at week 48 was 1.1 kg in the TAF/FTC arm with no change in the TDF/FTC arm [35]. Finally, in a phase 2 placebo controlled trial of cabotegravir for PrEP, both arms experienced about 1 kg of weight gain over 41 weeks, with no significant difference between arms [36]. Together these findings suggest that heathy participants taking TAF or an INSTI likely experience weight gain much like the general population, which contrasts with the weight-suppressive effect of TDF.
There are several limitations to our analyses. We do not have body composition data, so we cannot determine the anatomical distribution of the observed weight gain. However, data from recent studies suggest that ART-associated weight gain is generalized, with increases in subcutaneous fat, visceral fat, and lean mass [37]. Additionally, our assessment of GI tolerability relied solely on investigator-reported AEs, as more detailed and sensitive participant-reported outcome data are only available in more recent trials [29, 30]. Our analyses did not evaluate other potential contributors to weight gain such as psychiatric comorbidities, concomitant medications, diet, physical activity, or smoking. In the included trials, newer third agents were generally coadministered with newer NRTIs, making it challenging to completely disentangle the independent associations of individual agents with weight gain; thus, these associations should be interpreted with some caution. Finally, while we report 2-year data, the duration of follow-up may not be long enough to capture longer-term metabolic consequences of weight gain.
Collectively, our results suggest that there are demographic-, HIV- and treatment-related contributors to weight gain in PLWH. Our findings raise the possibility that modern ART regimens with improved tolerability and potency may lead to weight gain in some PLWH, necessitating increased clinical attention to the maintenance of healthy body weight, lifestyle modification, and exercise at ART initiation [38, 39]. Ongoing studies including the analysis of weight gain in ART switch trials may provide important insights by avoiding the contribution of return-to-health effects. Additional important areas for investigation include the magnitude, clinical significance, and biologic mechanisms of ART-related weight gain.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the individuals who participated in this these trials and their families, the principal investigators and their staff, the Gilead study staff, and Anna Kido (Gilead employee) for providing editorial assistance.
Financial support. This study was sponsored by Gilead Sciences, Inc (Gilead).
Potential conflicts of interest. P. S. reports grant support from Gilead, GlaxoSmithKline (GSK), and ViiV, and personal fees and scientific advisory board membership from Gilead Sciences, GSK, Janssen, Merck, and ViiV. K. E. reports consultancy for Gilead and ViiV. J. L. reports consultancy for Gilead and Merck. G. M. reports consultancy for Gilead, ViiV, and Merck, and grant support from Astellas, Roche, and Tetraphase. C. O. reports grant support and personal fees and nonfinancial support from Gilead, Merck Sharp & Dohme, Janssen, and ViiV. S. E. reports grant support, funding or honoraria for clinical research, and participation in advisory boards or talks in workshops or symposia from Gilead, AbbVie, MSD, Janssen, and ViiV. T. B. reports receipt of personal fees from Gilead, Merck, ViiV, and Theratechnologies. J. R. reports honoraria for consulting and educational events from Abivax, AbbVie, Cipla, Gilead, Janssen, Merck, and ViiV. X. W., C. C., L. Z., D. B., M. D., and K. M. are Gilead employees and hold stock in the company. H. S. reports study fees and honoraria for consultancy and speaking from Gilead, Janssen, MSD, and GlaxoSmithKline. F. P. reports grant support from Gilead, ViiV, and Janssen and receipt of personal fees from Gilead, ViiV, Janssen, and MSD. L. W. reports speaker/advisory fees from Gilead, ViiV, MSD, Janssen, Mylan, and Cipla, and clinical trial support from Gilead, Janssen, and ViiV. J. K. reports consultancy for Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Koethe JR, Jenkins CA, Lau B, et al. . North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakal DR, Coelho LE, Luz PM, et al. . Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother 2018; 73:2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS 2008; 22:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tate T, Willig AL, Willig JH, et al. . HIV infection and obesity: where did all the wasting go? Antivir Ther 2012; 17:1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses 2013; 29:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuh B, Tate J, Butt AA, et al. . Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Achhra AC, Mocroft A, Reiss P, et al. . D:A:D Study Group Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med 2016; 17:255–68. [DOI] [PubMed] [Google Scholar]
- 8. Koethe JR, Heimburger DC. Nutritional aspects of HIV-associated wasting in sub-Saharan Africa. Am J Clin Nutr 2010; 91:1138–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma A, Hoover DR, Shi Q, et al. . Relationship between body mass index and mortality in HIV-infected HAART users in the Women’s Interagency HIV Study. PLoS One 2015; 10:e0143740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herrin M, Tate JP, Akgün KM, et al. . Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morse CG, Kovacs JA. Metabolic and skeletal complications of HIV infection: the price of success. JAMA 2006; 296:844–54. [DOI] [PubMed] [Google Scholar]
- 12. Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol (Lausanne) 2018; 9:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCormick CL, Francis AM, Iliffe K, et al. . Increasing obesity in treated female HIV patients from sub-Saharan Africa: potential causes and possible targets for intervention. Front Immunol 2014; 5:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bares SH, Smeaton LM, Xu A, Godfrey C, McComsey GA. HIV-infected women gain more weight than HIV-infected men following the initiation of antiretroviral therapy. J Womens Health (Larchmt) 2018; 27:1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhagwat P, Ofotokun I, McComsey GA, et al. . Changes in waist circumference in HIV-infected individuals initiating a raltegravir or protease inhibitor regimen: effects of sex and race. Open Forum Infect Dis 2018; 5:ofy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waters L, Assoumou L, Rusconi S, et al. . Switch to dolutegravir (DTG) from a boosted protease inhibitor (PI/r) associated with significant weight gain over 48 weeks in NEAT-022, a randomised 96-week trial [abstract P102]. In: HIV Glasgow, Glasgow, UK, 28–31 October 2018. [Google Scholar]
- 17. Norwood J, Turner M, Bofill C, et al. . Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menard A, Meddeb L, Tissot-Dupont H, et al. . Dolutegravir and weight gain: an unexpected bothering side effect? AIDS 2017; 31:1499–500. [DOI] [PubMed] [Google Scholar]
- 19. Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003; 299:853–5. [DOI] [PubMed] [Google Scholar]
- 20. Hernandez D, Kalichman S, Cherry C, Kalichman M, Washington C, Grebler T. Dietary intake and overweight and obesity among persons living with HIV in Atlanta, Georgia. AIDS Care 2017; 29:767–71. [DOI] [PubMed] [Google Scholar]
- 21. Crum-Cianflone N, Roediger MP, Eberly L, et al. . Infectious Disease Clinical Research Program HIV Working Group Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frieden TR; Centers for Disease Control and Prevention CDC health disparities and inequalities report—United States, 2013. Foreword. MMWR Suppl 2013; 62:1–2. [PubMed] [Google Scholar]
- 23. Taylor BS, Liang Y, Garduño LS, et al. . High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr 2014; 65:e33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taramasso L, Ricci E, Menzaghi B, et al. . CISAI Study Group Weight gain: a possible side effect of all antiretrovirals. Open Forum Infect Dis 2017; 4:ofx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Venter WDF, Moorhouse M, Sokhela S, et al. . Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 26. Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res 2010; 85:201–9. [DOI] [PubMed] [Google Scholar]
- 27. Sherman EM, Worley MV, Unger NR, Gauthier TP, Schafer JJ. Cobicistat: review of a pharmacokinetic enhancer for HIV infection. Clin Ther 2015; 37:1876–93. [DOI] [PubMed] [Google Scholar]
- 28. van Lunzen J, Antinori A, Cohen CJ, et al. . Rilpivirine vs. efavirenz-based single-tablet regimens in treatment-naive adults: week 96 efficacy and safety from a randomized phase 3b study. AIDS 2016; 30:251–9. [DOI] [PubMed] [Google Scholar]
- 29. Gallant J, Lazzarin A, Mills A, et al. . Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017; 390:2063–72. [DOI] [PubMed] [Google Scholar]
- 30. Sax PE, Pozniak A, Montes ML, et al. . Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 31. Jacobson K, Ogbuagu O. Integrase inhibitor-based regimens result in more rapid virologic suppression rates among treatment-naive human immunodeficiency virus-infected patients compared to non-nucleoside and protease inhibitor-based regimens in a real-world clinical setting: a retrospective cohort study. Medicine (Baltimore) 2018; 97:e13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad 2019; 5:41–3. [PMC free article] [PubMed] [Google Scholar]
- 33. Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol 2006; 149:815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glidden DV, Mulligan K, McMahan V, et al. . Metabolic effects of preexposure prophylaxis with coformulated tenofovir disoproxil fumarate and emtricitabine. Clin Infect Dis 2018; 67:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hare CB, Coll J, Ruane P, et al. . The phase 3 DISCOVER study: daily F/TAF or F/TDF for HIV preexposure prophylaxis [abstract 104LB]. In: et al. , Seattle, WA, 4–7 March 2019. [Google Scholar]
- 36. Landovitz RJ, Zangeneh SZ, Chau G, et al. . Cabotegravir is not associated with weight gain in HIV-negative individuals: HPTN 077 [abstract 34LB]. In: et al. , Seattle, WA, 4–7 March 2019. [Google Scholar]
- 37. McComsey GA, Moser C, Currier J, et al. . Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis 2016; 62:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montoya JL, Jankowski CM, O’Brien KK, et al. . Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS 2019; 33:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lake JE, Stanley TL, Apovian CM, et al. . Practical review of recognition and management of obesity and lipohypertrophy in human immunodeficiency virus infection. Clin Infect Dis 2017; 64:1422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.