Abstract
Background
Respiratory illnesses is the most common manifestation of Coronavirus disease 2019 (COVID-19); however, myocardial injury has recently emerged as a frequent complication.
Methods
An observational, longitudinal, prospective, and multicenter study of hospitalized Mexican patients was made. We assessed the prevalence of myocardial injury and its relationship with complications and mortality.
Results
254 COVID-19 patients were included. Their average age was 53.8 years old, 167 (65.7%) were male and 87 (34.3%) female. According to troponin levels, two populations were generated, those with and without myocardial injury. There was no difference in gender or age between both groups. However, there was a greater proportion of obesity and hypertension in myocardial injury group. Multivariate logistic regression analysis revealed that obesity (OR 2.029, 95% CI 1.039–3.961; p = 0.038), arterial oxygen saturation <90% (OR 2.250, 95% CI 1.216–3.560; p = 0.025), and systolic blood pressure <90 mmHg (OR 2.636, 95% CI 1.530–4.343; p = 0.042), were directly related to higher levels of troponins. Multivariate cox proportional hazards analysis showed that primary endpoint (mortality) was determined by overweight/obesity (OR 1.290, 95% CI 0.115–0.730; p = 0.009), ferritin levels (OR 1.001, 95% CI 1.000–1.001; p < 0.001), myocardial injury (OR 3.764, 95% CI 1.307–10.838; p = 0.014), septic shock (OR 4.104, 95% CI 1.142–14.132; p = 0.024), acute respiratory distress syndrome (OR 3.001, 95% CI 1.008–10.165; p = 0.040), and treatment with Hydroxychloroquine/Azithromycin (OR 0.357, 95% IC 0.133–0.955; p = 0.040). Secondary endpoint (Mechanical ventilation risk) was associated to the same factors.
Conclusions
Myocardial injury represents an increased risk of complications and death in Mexican hospitalized patients with COVID-19.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Myocardial injury, Troponins
1. Introduction
Until a few years ago, infection by coronaviruses had been in control. On December 31, 2019, China reported a cluster of cases of pneumonia [1]. On January 7, 2020, Chinese health authorities, through high-throughput sequencing from lower respiratory tract samples has revealed a novel coronavirus that was named 2019 novel coronavirus (2019-nCoV), also named Severe Acute Respiratory Syndrome (SARS)-Coronavirus (CoV)-2 (SARS-CoV-2) [2].
The rapid spread of coronavirus disease 2019 (COVID-19) warrants intense surveillance and isolation protocols to prevent further transmission. At the time of preparing this manuscript, the World Health Organization (WHO) last information June 16, 2020 has reported 7,941,791 confirmed cases, 434,796 confirmed deaths, and 216 countries affected. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. At the same time, Mexico reports 150,264 confirmed cases and 17,580 deaths. https://coronavirus.gob.mx/datos/.
Although the respiratory tract is the main system affected by SARS-CoV-2, the cardiovascular (CV) system has been involved in this disease. Four putative and direct mechanisms of acute cardiac injury due to COVID-19 have been proposed: (i) viral angiotensin-converting enzyme 2 (ACE-2)-mediated direct damage; (ii) hypoxia-induced myocardial injury; (iii) cardiac microvascular damage due to perfusion defects, vessel hyperpermeability, or angiospasm; and (iv) systemic inflammatory response syndrome including cytokine storm, dysregulated immunocytes, and uncontrolled inflammation [3]. Also, the SARS-CoV-2 viral RNA presence was detected in a series of heart samples that underwent autopsy, suggesting a direct invasion of the virus and probably direct heart damage [4], [5].
Myocardial injury defined by cardiac enzymes elevation has been found in approximately 8–12% of positive cases [6], [7]. Some studies support the use of high-sensitivity cardiac Troponin I (hs-cTnI) as a prognostic predictor for patients with COVID-19. A recent meta-analysis showed that cardiac injury has an independent adverse prognostic value in COVID-19. It suggest that the increased value of high sensitive troponin might be useful in the risk stratification of COVID-19 patients and should be included in the routine assessment [8].
Our study aim to clarify the incidence of myocardial injury in the Mexican population and to know its relationship between in-hospital complications and death.
2. Methods
2.1. Study population
The study was carried out in two hospitals in Mexico; beneficiaries of the Centro Médico Nacional “20 de Noviembre” ISSSTE in Mexico City and beneficiaries of the Hospital Regional de Alta Especialidad ISSSTE in the state of Puebla.
-
-
The inclusion criteria were: Both genders, over 18-year-old patients, and patients confirmed test result of COVID-19 with positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR). Patients who did not undergo testing, had no record of testing in the collaborative database, or had a negative test were not included in the present study.
-
-
The exclusion criteria were: Patients under 18-year-old, pregnancy, and patients with COVID-19 without informed consent.
The protocol, informed consent, study procedures and measurements were approved by the bioethics committee of hospitals involved. Prior written informed consent was obtained from each patient to be included in the study. We certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
2.2. Study design and protocol
An analytical, observational, longitudinal, prospective and multicenter study was designed.
The initial sample size was based on a positive RT-PCR test for COVID-19 (n = 380). We exclude 100 patients because they were not hospitalized and 26 more patients because they were transferred to other hospitals. The total of 126 patients were excluded. Total population included in this study was 254 hospitalized patients admitted between March 2020 and April 2020.
Demographic analysis and biochemical characterization of total population was performed. After that, two patient populations were generated accord to hs-cTnI levels, those with myocardial injury and without myocardial injury. Samples of hs-cTnI and pro-B-type Natriuretic Peptide (NT pro-BNP) was taken at hospital admission and repeated seven days later. This process was done to monitor the dynamic changes of these variables and evaluate the possibility of new myocardial injury cases.
An ECG was performed in all patients at the time of hospital admission, after that the ECG was repeat in patients presenting chest pain, troponin elevation levels or telemetry disturbances.
The primary endpoint was to determine the prevalence of myocardial injury and its relationship with mortality in COVID-19 patients. The secondary endpoint was to determine if there exists a higher risk of complications, such as the necessity of mechanical ventilation therapy in this group.
2.3. Definition and data collection
-
–
Diagnosis of COVID-19: Initially, any subject with symptoms related to COVID-19 was be considered a suspect for COVID-19 infection. The diagnosis of COVID-19 was confirmed as positive result for nasopharyngeal swab and respiratory pathogen nucleic acid test with high-throughput sequencing or real-time reverse transcriptase polymerase chain reaction.
-
–
Diagnosis of myocardial injury: Myocardial injury was defined as an elevated serum level of hs-cTnI greater than the 99th percentile upper reference limit recommended cut-off of 17.5 ng/L (pg/mL) by the Access hs-cTnI assay (Beckman Coulter) [9], [10].
2.4. Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences program for Windows, version 25 (SPSS, Chicago, IL). All continuous variables were determined. The normality of distribution was determined by performing the Kolmogorov-Smirnov test. Normally distributed variables were described as the means ± standard deviation, and the skewed distributed variables were expressed as the median and interquartile range (IQR). We compared the normally distributed continuous variables using the Student t-test and skewed distributed variables using the Mann-Whitney U test. The difference between categorical variables was expressed as number (%) and compared by the Chi-Square test. We perform a multivariate logistic regression analysis to identify independent predictors of increased troponin value and cox regression analysis to identify the primary endpoints' predictors. Survival curves were plotted using the Kaplan-Meier method and compared between patients with vs without myocardial injury using the log-rank test. Statistical charts were generated using Excel 2016 (Microsoft). For all the statistical analyses, P < 0.05 was considered significant.
3. Results
3.1. Demographic, clinical and biochemical characteristics
The demographics and clinical characteristics of COVID-19 patients were summarized in Table 1.
Table 1.
Clinical characteristics, treatment and outcomes of COVID-19 total patients and subdivided in with or without myocardial injury.
|
Total (n = 254) |
Myocardial injury (+) (n = 73) |
Myocardial injury (−) (n = 181) |
p value | |
|---|---|---|---|---|
| Age (years) | 53.8 SD 12.7 | 54.3 SD 10.7 | 53.6 SD 13.1 | NS |
| Male n (%) Female n (%) |
167 (65.7) 87 (34.3) |
47 (64.5) 26 (35.5) |
120 (66.2) 61 (33.8) |
NS |
| BMI (Kg/Mm2) | 29.8 SD 7.7 | 29.5 SD 9.0 | 26.5 SD 9.0 | 0.014 |
|
Comorbidities n (%) Coronary Heart Disease Autoimmune Diseases COPD Overweight or Obesity Smoking Hypertension Diabetes mellitus Chronic Kidney Disease ACE2i or ARBs treatment |
14 (5.5) 6 (2.4) 1 (0.4) 158 (62.2) 38 (15.0) 90 (35.4) 80 (31.5) 2 (0.8) 70 (27.6) |
5 (6.8) 1 (1.4) 0 (0.0) 60 (82.2) 14 (19.2) 34 (45.3) 27 (37.0) 1 (1.4) 20 (27.4) |
9 (5.0) 5 (2.8) 1 (0.6) 98 (54.1) 24 (13.2) 56 (31.0) 53 (29.3) 1 (0.6) 50 (27.6) |
NS NS NS 0.001 NS 0.014 NS NS NS |
|
Symptoms n (%) Fever Odynophagia Dyspnea Headache Cough Diarrhea Myalgia or Arthralgia Tiredness Rhinorrhea |
220 (86.6) 130 (51.2) 220 (86.6) 126 (49.6) 211 (83.0) 52 (20.5) 203 (79.9) 220 (86.6) 52 (20.5) |
54 (74.0) 30 (41.1) 70 (95.9) 23 (31.5) 54 (74.0) 14 (19.2) 57 (78.0) 62 (84.9) 7 (9.6) |
166 (92.8) 100 (55.2) 150 (82.9) 103 (56.9) 157 (86.7) 38 (20.9) 146 (80.6) 158 (87.3) 45 (24.8) |
0.004 NS 0.020 0.001 NS NS NS NS 0.004 |
|
Complications n (%) Acute Kidney Injury Renal Replacement Therapy ARSD Septic Shock Arrhythmias (ST, SB, AF) (n%) |
64 (25.2) 23 (9.0) 133 (52.3) 82 (32.2) 20 (7.9) |
36 (49.3) 11 (15.0) 49 (67.1) 50 (68.5) 20 (27.4) |
27 (14.9) 12 (6.6) 84 (46.4) 32 (17.6) 0 (0%) |
<0.001 0.003 0.009 <0.001 <0.001 |
| Hospitalization days Mechanical Ventilation (%) Mechanical Ventilation days |
12.0 SD 3.0 133 (52.3) 9.0 SD 1.5 |
11.5 SD 2.8 49 (67.1) 10 SD 1.5 |
12.0 SD 3.0 84 (46.4) 9.0 SD 1.5 |
NS <0.001 NS |
|
Treatment n (%) HCQ + Azithromycin High dose Steroids VTE prophylaxis |
139 (54.7) 105 (41.3) 73 (28.7) |
50 (68.5) 49 (67.1) 50 (68.5) |
89 (49.1) 56 (30.9) 85 (46.9) |
<0.001 <0.001 <0.001 |
| Death n (%) | 89 (35.0) | 46 (63.0) | 43(23.7) | <0.001 |
Each value represents the mean ± SD or the number (%). p < 0.05 was considered statistically significant. Chronic obstructive pulmonary disease (COPD), Angiotensin-converting enzyme 2 inhibitors (ACE2i), Angiotensin II receptor blockers (ARBs), acute respiratory distress syndrome (ARDS), Sinus tachycardia (ST), Sinus bradycardia (SB), Atrial fibrillation (AF), Hydroxychloroquine (HCQ), venous thromboembolism (VTE).
A total 254 patients with confirmed COVID-19 test were included in the final analysis. The median age was 53.8 SD 12.7, 167 (65.7%) were male, and 87 (34.3%) were female, a total of 89 (35.0%) patients died, and 165 (64.9%) survived to discharge.
A sample of hs-cTnI was taken at hospital admission, diagnosing 64 patients with myocardial injury. After seven days of hospitalization, a new determination was made, adding 9 more patients to the diagnosis, obtaining a total population of 73 (28.7%) patients with myocardial injury, and 181 (71.3%) without myocardial injury.
After comparing this two populations, we found that there was no significant difference in gender or age between both groups. Regarding comorbidities, a significant difference in myocardial injury group was found for obese and hypertension patients. Coronary Heart Disease (CHD), chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), chronic kidney disease (CKD); autoimmune diseases and smoking did not demonstrate differences in both groups.
Regarding biochemical variables (Table 2), we found in patients with myocardial injury lower levels of leukocyte count, lymphocytes and albumin, but higher levels of high-sensitivity C-reactive Protein (hs-CRP), Creatine kinase-MB (CK-MB), Ferritin, Glucose, Lactate Dehydrogenase (LDH), Creatinine, Blood Urea Nitrogen (BUN), Aspartate Aminotransferase (AST), NT pro-BNP and hs-cTnI.
Table 2.
Laboratory findings of total patients and subdivided in with or without myocardial injury.
| Laboratory findings | Total (n = 254) |
Myocardiacl injury (+) (n = 73) |
Myocardial injury (−) (n = 181) |
p value |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 14.6(13.4–15.5) | 14.7 (13.5–15.7) | 14.6(13.4–15.4) | NS |
| Platelets count (× 103/μL) | 207(166.0–275.0) | 199(165.3–240) | 222.0(166.0–280.0) | NS |
| Leukocytes count (× 103/μL) | 7.8(5.7–11.0) | 9.5(7.5–18.8) | 10.2(7.4–13.9) | <0.001 |
| Neutrophils (× 103/μL) | 6.1(3.9–9.1) | 7.9(6.4–11.5) | 5.6(3.5–8.6) | <0.001 |
| Lymphocytes (× 103/μL) | 0.8(0.6–1.2) | 0.7(0.5–1.2) | 0.9(0.7–1.3) | <0.021 |
| hs-CRP (mg/L) | 126.6(61.3–200.1) | 188.2(127.0–220.7) | 103.4(50.2–194.0) | <0.001 |
| CK-MB (ng/mL) | 2.3(0.7–7.1) | 4.5(2.0–12.8) | 1.3(0.7–7.1) | <0.001 |
| Fibrinogen (g/L) | 5.5(4.3–6.69 | 5.3(4.3–6.3) | 5.6(4.3–6.6) | NS |
| ESR (mm/hr) | 40.5(30.0–48.0) | 35.0(30.0–47.0) | 41(23.0–48.0) | NS |
| Ferritin (ng/mL) | 690(376.0–1168.0) | 939(428.1–1354.0) | 583(350.0–1141.5) | <0.001 |
| Glucose (mg/dL) | 109.5(89.0–148.0) | 128.4(89.0–170.4) | 109.0(88.0–139.0) | 0.033 |
| Sodium (mmol/L) | 137.0(134.0–140) | 137.0(132.4–140.0) | 137.0(134.0–140.0) | NS |
| Potassium (mmol/L) | 4.0(3.7–4.4) | 4.1(3.8–4.4) | 4.0(3.7–4.4) | NS |
| Albumin (g/dL) | 3.5 SD 0.5 | 3.2 SD 0.5 | 3.6 SD 0.4 | <0.001 |
| LDH (U/L) | 397(280.0–501.0) | 430.8(344.4–527.1) | 346.0(271.8–485.0) | <0.001 |
| Creatinine (mg/dL) | 0.9(0.7–1.1) | 1.0(0.8–1.2) | 0.8(0.7–1.0) | 0.002 |
| BUN (mg/dL) | 16.9(13.0–29.2) | 21.0(14.9–29.2) | 15.4(12.0–21.0) | <0.001 |
| Total Cholesterol (mg/dL) | 114.0(92.0–139.0) | 105.5883.0–139.0) | 117.0(100.0–139.0) | NS |
| Triglycerides (mg/dL) | 130.0(98.3–183.3) | 145.0(101.8–196.5) | 125.0(96.8–182.8) | NS |
| LDL (mg/dL) | 72.8(53.1–93.4) | 37.6(30.6–60.3) | 77.6(66.0–96.1) | <0.001 |
| ALT (U/L) | 43.0(26.0–60.2) | 43.7(24.5–88.0) | 40.8(26.0–59.0) | NS |
| AST (U/L) | 45.3(31.0–65.5) | 58.7(35.8–84.5) | 43.5(29.6–60.0) | 0.008 |
| D-dimer (μg/mL) | 0.6(0.4–1.3) | 0.6(0.3–3.5) | 0.6(0.4–1.3) | NS |
| hs-cTnI (pg/mL) | 9(5.0–17) | 34.9(22.2–95.7) | 9.0(4.3–9.0) | <0.001 |
| hs-cTnI after 7 day (pg/mL) | 6.5(3.0–24.8) | 66.3(20.4–208.0) | 3.9(2.5–6.1) | <0.001 |
| NT pro-BNP (pg/mL) | 196.0(52.0–2095.0) | 704.5(246.0–2130.8) | 100.0(42.6–361.0) | <0.001 |
| NT pro-BNP after 7 day (pg/mL) | 230.0(101.5–1563.0) | 2250.0(596.0–9408.5) | 129.0(68.8–581.3) | 0.004 |
Each value represents the median (interquartile range), mean ± SD or the number (%), p < 0.05 was considered statistically significant. Hemoglobin (Hb), Creatine kinase-MB (CK-MB), high-sensitivity C-reactive Protein (hs-CRP), Erythrocyte Sedimentation Rate (ESR), Lactate Dehydrogenase (LDH), Blood Urea Nitrogen (BUN), Low-Density Lipoprotein (LDL), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), high-sensitivity cardiac Troponin I (hs-cTnI), N-terminal pro-B-type Natriuretic Peptide (NT pro-BNP).
Baseline electrocardiographic characteristics in the myocardial injury group were: mean heart rate of 80 ± 40 bpm and mean Bazett-corrected QT interval of 420 ± 40 ms. The majority were in normal sinus rhythm; only 10 patients were detected with sinus tachycardia, 6 patients with sinus bradycardia, and 4 developed atrial fibrillation. Repolarization abnormalities such as symmetric T-wave inversion was found in 15 patients. There was no evidence of ST-segment elevation, atrial premature contractions, ventricular premature contractions, or atrioventricular block. In the group, without myocardial injury, the mean heart rate was 80 ± 15 bpm, and the Bazett-corrected QT interval 400 ± 20 ms. Only 5 patients showed symmetric T-wave inversion, and there was also no evidence of arrhythmias.
Multivariate logistic regression analysis (Table 3), shows that the independent predictors related to increasing troponin levels were: overweight/obesity (OR 2.029, 95% CI 1.103–3.96; p 0.038), arterial oxygen saturation at admission <90% (OR 2.250, 95% CI 1.216–3.560; p = 0.025), and systolic blood pressure at admission <90 mmHg (OR 2.636, 95% CI 1.530–4.343; p = 0.042). It also showed that previous treatment with angiotensin-converting enzyme 2 inhibitors (ACE2i) or ARBs (Angiotensin II receptor blockers) was not related to increased troponin levels (OR 1.347, 95% CI 0.665–2.722; p 0.408).
Table 3.
Multivariate logistic regression analysis of independent predictors related to increased troponin levels.
| Variables | Odds ratio | 95% CI | p |
|---|---|---|---|
| Age | 1.007 | 0.980–1.035 | 0.607 |
| Male gender | 0.828 | 0.430–1.593 | 0.572 |
| Hypertension | 1.718 | 0.895–3.298 | 0.104 |
| Diabetes mellitus | 1.260 | 0.639–2.485 | 0.505 |
| Smoking | 1.507 | 0.645–3.520 | 0.343 |
| Overweight/Obesity | 2.029 | 1.039–3.961 | 0.038 |
| Dyspnea | 3.805 | 1.051–13.772 | 0.042 |
| Hearth Rate > 100 (At hospital admission) |
1.016 | 0.960–1.354 | 0.923 |
| Arterial oxygen saturation <90% (At hospital admission) |
2.250 | 1.216–3.560 | 0.025 |
| Systolic blood pressure <90 mmHg (At hospital admission) |
2.636 | 1.530–4.343 | 0.042 |
| ACE2i or ARBs treatment | 1.347 | 0.665–2.722 | 0.408 |
CI: Confidence interval, Angiotensin-converting enzyme 2 inhibitors (ACE2i), Angiotensin II receptor blockers (ARBs). p < 0.05 was considered statistically significant.
The proportion of administered therapy was: hydroxychloroquine (HCQ) + azithromycin in 68.5% of myocardial injury group and 49.1% without myocardial injury, high dose steroids (prednisone 30–100 mg/day or equivalent) in 67.1% of myocardial injury group and 30.9% without myocardial injury and, anticoagulation therapy for Venous Thromboembolism (VTE) prophylaxis was administered to 68.5% of myocardial injury group and 46.9% patients without myocardial injury, according to the next dosage [11]:
-
(a)
For all non-critically ill hospitalized, standard dose VTE prophylaxis was administered (enoxaparin 40 mg subcutaneous once a day).
-
(b)
For critically ill patients was administered enoxaparin 0.5 mg/kg subcutaneous twice daily.
Critically ill patients was defined as meeting any one of following items: Respiratory rate ≥30 breaths/min; arterial oxygen saturation ≤93% at rest; PaO2/FiO2 ≤300 mm Hg [12].
3.2. Clinical outcomes
Complications such as Acute Respiratory Distress Syndrome (ARDS), acute kidney injury (AKI), septic shock, and arrhythmias were more common in patients with myocardial injury. Also, greater proportions of patients with myocardial injury required invasive mechanical ventilation (p < 0.001) and Renal Replacement Therapy (RRT) (p = 0.003), but hospitalization days were no different. At the end, patients with myocardial injury had higher mortality (46 of 73 [63.0%] vs 43 of 181 [23.7%]; p < 0.001).
Cox regression analysis (Table 4) was performed to identify demographic, electrocardiographic and biochemical predictors of the main endpoints. Accord to death risk the next variables were related: Overweight/obesity (OR 1.290, 95% CI 0.115–0.730; p = 0.009), ferritin levels (OR 1.001, 95% CI 1.000–1.001; p < 0.001), hs-cTnI levels (OR 1.002, 95% CI 1.001–1.003; p = 0.004), myocardial injury (OR 3.764, 95% CI 1.307–10.838; p = 0.014), localized T-wave inversion (ischemia) (OR 3.642, 95% CI 1.850–6.443; p = 0.002), septic shock (OR 4.104, 95% CI 1.142–14.132; p = 0.024), and ARDS (OR 3.001, 95% CI 1.008–10.165; p = 0.040), while treatment with HCQ/Azithromycin (OR 0.357, 95% CI 0.133–0.955; p = 0.040) and male gender (OR 0.545, 95% CI 0.159–0.833; p = 0.017), were related with less mortality risk. Accord to mechanical ventilation risk the next variables were related: Overweight/obesity (OR 1.293, 95% CI 0.112–0.768; p = 0.013), myocardial injury (OR 9.999, 95% CI 2.772–36.738; p = 0.001), localized T-wave inversion (ischemia) (OR 2.480, 95% CI 1.430–5.355; p = 0.020)septic shock (OR 8.094, 95% CI 1.533–42.790, p = 0.014) and ARDS (OR 7.085, 95% CI 1.263–26.540; p = 0.001) while treatment with HCQ/Azithromycin (OR 0.204, 95% CI 0.063–0.602; p = 0.008) and steroids (OR 0.342, 95% CI 0.122–0.959; p = 0.018) were related with less risk.
Table 4.
Cox regression analysis identifying predictors of mechanical ventilation and mortality risk.
|
Mortality risk |
Mechanical Ventilation Risk |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.018 | 0.976–1.062 | 0.414 | 1.005 | 0.980–1.045 | 0.833 |
| Male gender | 0.545 | 0.159–0.833 | 0.017 | 1.245 | 0.561–2.177 | 0.611 |
| Hypertension | 0.691 | 0.259–1.778 | 0.444 | 0.833 | 0.406–1.546 | 0.708 |
| Diabetes mellitus | 1.784 | 1.349–4.003 | 0.160 | 0.692 | 0.825–2.835 | 0.385 |
| Overweight or Obesity | 1.290 | 0.115–0.730 | 0.009 | 1.293 | 0.112–0.768 | 0.013 |
| NT pro-BNP | 1.000 | 1.000–1.000 | 0.469 | 1.000 | 1.000–1.000 | 0.989 |
| Ferritin | 1.001 | 1.000–1.001 | <0.001 | 1.000 | 1.000–1.001 | 0.172 |
| hs-CRP (mg/L) | 1.001 | 0.998–1.004 | 0.966 | 0.996 | 0.998–1.004 | 0.052 |
| hs-cTnI (pg/mL) | 1.002 | 1.001–1.003 | 0.004 | 1.001 | 1.001–1.003 | 0.158 |
| Lymphocytes (× 103/μL) | 0.928 | 0.534–1.613 | 0.335 | 2.315 | 0.682–3.467 | 0.159 |
| D-dimer (μg/mL) | 0.971 | 0.915–1.030 | 0.971 | 0.970 | 0.914–0.994 | 0.290 |
| Myocardial injury | 3.764 | 1.307–10.838 | 0.014 | 9.999 | 2.772–36.738 | 0.001 |
| Localized T-wave inversion | 3.642 | 1.850–6.443 | 0.002 | 2.480 | 1.430–5.355 | 0.020 |
| Septic Shock | 4.104 | 1.142–14.132 | 0.024 | 8.094 | 1.533–42.790 | 0.014 |
| ARDS | 3.001 | 1.008–10.165 | 0.040 | 7.085 | 1.263–26.540 | 0.001 |
| In-hospital hemodialysis | 0.347 | 0.121–0.998 | 0.050 | 0.567 | 0.177–1.815 | 0.339 |
| HCQ/Azithromycin | 0.357 | 0.133–0.955 | 0.040 | 0.204 | 0.063–0.602 | 0.008 |
| Steroids | 1.273 | 0.532–3.022 | 0.581 | 0.342 | 0.122–0.959 | 0.018 |
| Anticoagulant therapy | 0.370 | 0.123–1.112 | 0.077 | 0.191 | 0.048–0.757 | 0.992 |
High-sensitivity C - reactive protein (hs-CRP), high-sensitivity cardiac Troponin I (hs-cTnI), N-terminal pro-B-type Natriuretic Peptide (NT pro-BNP), (ARDS) Acute respiratory distress syndrome, (HCQ) Hydroxychloroquine.
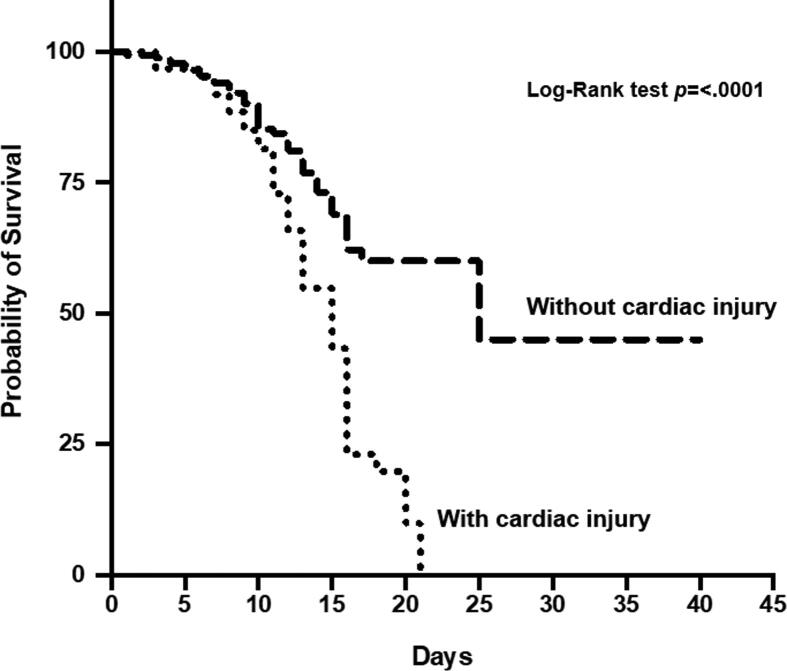
After comparing the Kaplan-Meier curve with the Log Rank test, the survival (days) of both populations showed a significant difference in favor of more prolonged survival in patients without myocardial injury (Fig. 1).
Fig. 1.
Kaplan-Meier curves with log-rank tests of Survival Analysis. Kaplan-Meier curve with log Rank test shows the survival (days) of both populations. A significant difference was found in favor of more prolonged survival in patients without myocardial injury.
4. Discussion
Our study's total population was 254 patients, where the most affected sex was male 167 (65.7%). Similar findings are observed in other studies, such as the one observed and described by Chen et al., where 68% of the cases with COVID-19 were men [13]. Studies in Italy and the United States show a higher proportion of men affected, up to 70% [14], [15]. Only one study was found which contrasts with this information, that described by Han et al. which evaluated 273 patients of which 64% were women. [16]
As for the age range most affected by SARS-CoV-2, our population had an average age of 53, similar value to studies carried out in Asian population where the most affected age ranges are from 35 to 55 years [17], However, the average age of SARS-CoV-2 patients can be variable; for example in the United States, the average that has been observed is 29 years [14] and in Italy, it is approximately 80 years [15].
Of the total population, 73 patients (28.7%) presented myocardial injury, a figure higher than those reported in the international literature (7.2–19.7%) [18].Of those diagnosed with myocardial injury, 64 fulfilled the diagnosis at the time of hospital admission and only 9 patients after seven days of hospitalization; It should be emphasized that most patients were admitted with myocardial injury, suggesting myocardial injury prior to systemic complications. Unlike previous studies where they observed altered biomarkers of heart damage at the midpoint of hospitalization and immediately before death [18], [19].
While the increase in troponins determines the existence or not of myocardial injury, the interpretation of this biomarker requires careful integration with a range of other clinical factors [symptoms, history of CVD and other comorbidities, electrocardiogram (ECG) changes, etc.]. Besides the dynamic nature of this biomarker should be considered [20].
A relationship has been shown between electrocardiographic findings and mortality in patients with COVID-19, among these findings are alterations of repolarization as the inversion of the T wave of ischemic type. [21] This same electrocardiographic finding was the most frequent in our study, and it was related to increased mortality and mechanical intubation risk.
Some reports mention a mild increase in troponins in patients with COVID-19 and a pre-existing CHD or CKD [22], however, in our study, the percentage of the population with a history of CHD and CKD was minimal, without statistical significance in favor of myocardial injury. This finding leads us to believe that troponin's elevation was secondary to the SARS-CoV-2 infection and not secondary to previous comorbidities.
The prevalence of comorbidities is also an important factor in patients evaluated. The most frequent pathologies in COVID-19 patients were: obesity (62.2%), systemic arterial hypertension (35.4%) and diabetes (31.5%); when comparing these findings with the Chinese population where prevalence of 20.7% of hypertensive patients as well as 10.5% of diabetes were observed [23], [24], we can see that the Mexican population tends to associate more comorbidities, which means a worsening of the prognosis.
In the population with myocardial injury was found more prevalence of hypertension (45.3%) and obesity (82.2%), figures that match those reported in several previous series [18], [25], [26]; it is interesting to mention that the prevalence of diabetes in the myocardial injury group was not statistically significant. Data that contrast with the reported records where diabetes has been identified as significant comorbidity for myocardial injury and mortality. [15], [25]
The clinical presentation of COVID-19 is very similar to that reported in other case series, with the main symptoms being fever, dyspnea, tiredness and cough. [13], [17].
At the biochemical level, the elevation of leukocytes, neutrophils, ferritin, DHL, hs-CRP, AST, creatinine, BUN, hs-cTnI, NT pro-BNP and decreased lymphocyte count, and albumin, are similar to those observed in the international literature [26], [27], [28], [29]. The detection of inflammatory markers simultaneously with cardiac enzymes reveals a strong relationship between the myocardial lesion and the inflammatory process.
No drugs are formally approved for COVID-19, although some have been tried out. In our study was administered azithromycin + HCQ based on the hypothesis that these drugs had efficacy in the treatment of COVID-19 [30], [31]. Patients who underwent treatment showed less mortality risk even though current data do not support hydroxychloroquine and azithromycin for prophylaxis or treatment of COVID-19 [32].
Interest in corticosteroid therapy in COVID-19 has been rekindled after the results from Randomized Evaluation of COVID-19 therapy (RECOVERY) Trial. This is the only randomized controlled trial that has shown a significant reduction of death by 35% in ventilated patients and by 20% amongst patients on supplemental oxygen therapy with the dexamethasone [33]. Our study found that patients under assisted mechanical ventilation showed less mortality risk in those who underwent high dose steroids, regardless of the type of steroid.
Anticoagulant therapy with low molecular weight heparin appears to be associated with better prognosis in severe COVID‐19 patients, especially in patients with severe disease or high ferritin levels. [34], [35] In our study, no difference was found with this treatment. It is also important to mention that no thromboembolism episode was evidenced.
The overall mortality of our population with COVID-19 was 35.0%. This is a high proportion compared to other studies in China, where mortality is reported as 4.3–28%. [13], [19], [26]
Mortality in patients with myocardial injury was approximately 2.5 times higher, 46 (63.0%) vs 43 (23.7%) p (<0.001); reporting higher mortality than in previous studies where the myocardial injury is associated with up to 51.2% mortality [18]. Therefore, myocardial injury in patients with COVID-19 is strongly associated with worse outcomes, the same results are reported in Chinese population studies [18], [25], [36]. Survival in days from hospital admission was significantly superior in patients without myocardial injury vs. patients with myocardial injury.
5. Conclusions
Myocardial injury in COVID-19 Mexican patients is associated with an increase in systemic complications and mortality. Some demographic and biochemical predictors are primarily associated with these outcomes and must be taken into consideration during the hospital stay.
We consider incorporating cardiac biomarkers to their routine laboratory panel for COVID-19 is vital because serial measurements facilitate the understanding of results as compared to single point measurements.
Randomized trials are urgently needed to investigate specifically treatment modalities to reduce the incidence and mortality associated with COVID-19 related acute myocardial injury.
CRediT authorship contribution statement
Aquino Bruno Heberto: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration. Plata Corona Juan Carlos: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration. Castro Rubio José Antonio: Writing - original draft, Writing - review & editing, Visualization. Pulido Pérez Patricia: Methodology, Software, Formal analysis, Data curation. Torres Rasgado Enrique: Methodology, Software, Formal analysis, Data curation. Morales Portano Julieta Danira: Resources, Supervision, Project administration, Funding acquisition. Gómez Álvarez Enrique Benito: Resources, Supervision, Project administration, Funding acquisition. Merino Rajme José Alfredo: Resources, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
Acknowledgement of grant support
To Josefina Edaly Galindo Ballinas for helping to translate this article.
This research was sponsored by resources of hospitals involved: Centro Médico Nacional 20 de Noviembre ISSSTE, CD.MX and Hospital Regional de Alta Especialidad ISSSTE Puebla.
Author’s contributions and consent for publication
As the corresponding author, I declare that all authors in this paper contribute substantially in the work design, analysis, data interpretation, drafting, and intellectual content. Finally all authors approved this version to be published.
Author’s agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Manuscript
We declare this manuscript has not been published before; that it is not under consideration for publication anywhere else; that its publication has been approved by all co-authors, if any, as well as by the responsible authorities – tacitly or explicitly – at the institute where the work has been carried out. The publisher will not be held legally responsible should there be any claims for compensation.
Ethics approval
This research was approved by bioethics committee of hospitals involved. We certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants (or their parents) included in the study, they also consent use the info in this research and for publish their data.
References
- 1.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosyns B., Lochy S., Luchian M.L., Gimelli A., Pontone G., Allard S.D. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur. Heart J. Cardiovasc. Imaging. 2020 doi: 10.1093/ehjci/jeaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S.S., Cheng C.W., Fu C.L., Chan Y.H., Lee M.P., Chan J.W. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. 2003;108(15):1798–1803. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 8.Kollias A., Kyriakoulis K.G., Destounis A., Stergiou G.S., Syrigos K. Cardiac injury and prognosis in COVID-19: Methodological considerations and updated meta-analysis. J. Infect. 2020 doi: 10.1016/j.jinf.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G., Ferrari A., Gandini G., Gelati M., Lo Cascio C., Salvagno G.L. Analytical evaluation of the new Beckman Coulter Access high sensitivity cardiac troponin I immunoassay. Clin. Chem. Lab. Med. 2017;56(1):157–161. doi: 10.1515/cclm-2017-0350. [DOI] [PubMed] [Google Scholar]
- 10.Pretorius C.J., Tate J.R., Wilgen U., Cullen L., Ungerer J.P.J. A critical evaluation of the Beckman Coulter Access hsTnI: Analytical performance, reference interval and concordance. Clin. Biochem. 2018;55:49–55. doi: 10.1016/j.clinbiochem.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J. Thromb. Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020;1–4 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajema K.L., Oster A.M., McGovern O.L., Lindstrom S., Stenger M.R., Anderson T.C. Persons evaluated for 2019 novel coronavirus - United States, January 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(6):166–170. doi: 10.15585/mmwr.mm6906e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porcheddu R., Serra C., Kelvin D., Kelvin N., Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J. Infect. Dev. Ctries. 2020;14(2):125–128. doi: 10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- 16.Han H., Xie L., Liu R., Yang J., Liu F., Wu K. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A. Fourth universal definition of myocardial infarction (2018) J. Am. Coll. Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 21.McCullough S.A., Goyal P., Krishnan U., Choi J.J., Safford M.M., Okin P.M. Electrocardiographic findings in coronavirus disease-19: insights on mortality and underlying myocardial processes. J. Card. Fail. 2020;26(7):626–632. doi: 10.1016/j.cardfail.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulut C., Kato Y. Epidemiology of COVID-19. Turk. J. Med. Sci. 2020;50(Si-1):563–570. doi: 10.3906/sag-2004-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol. Arch. Intern. Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 25.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou B., She J., Wang Y., Ma X. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji D., Zhang D., Xu J., Chen Z., Yang T., Zhao P. Prediction for progression risk in patients with COVID-19 Pneumonia: the CALL Score. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 30.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 31.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A.K., Majumdar S., Singh R., Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician's perspective. Diabetes Metab. Syndr. 2020;14(5):971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]