Virtually every U.S. state has implemented a prescription drug monitoring program (PDMP) to address high-risk opioid-prescribing and opioid-seeking behaviors that have contributed to the opioid-overdose crisis. PDMPs — electronic databases that track dispensing of controlled substances — are intended to support clinical practice and monitoring efforts. But given that heroin and illicit synthetic opioids account for an increasing share of the 130 opioid-overdose deaths that occur daily in the United States, many stakeholders have expressed doubts about the utility of PDMPs as well as concerns regarding their potential unintended consequences.
Ever since Oklahoma established the first electronic PDMP in 1990, the number of states with PDMPs — which are backed by federal funding — has increased in tandem with opioid-overdose deaths: 17 states had such programs in 2000, and 49 had one by 2015. The lone holdout, Missouri, has repeatedly failed to implement a statewide provider-accessible PDMP due to privacy concerns. Several national data-sharing platforms also exist. The federal government conditions certain overdose-prevention funds for states on participation in RxCheck, its designated PDMP interstate data-sharing system. PDMP Interconnect, a network run by the National Association of Boards of Pharmacy, enables clinicians in 47 states to request prescribing data from other states.
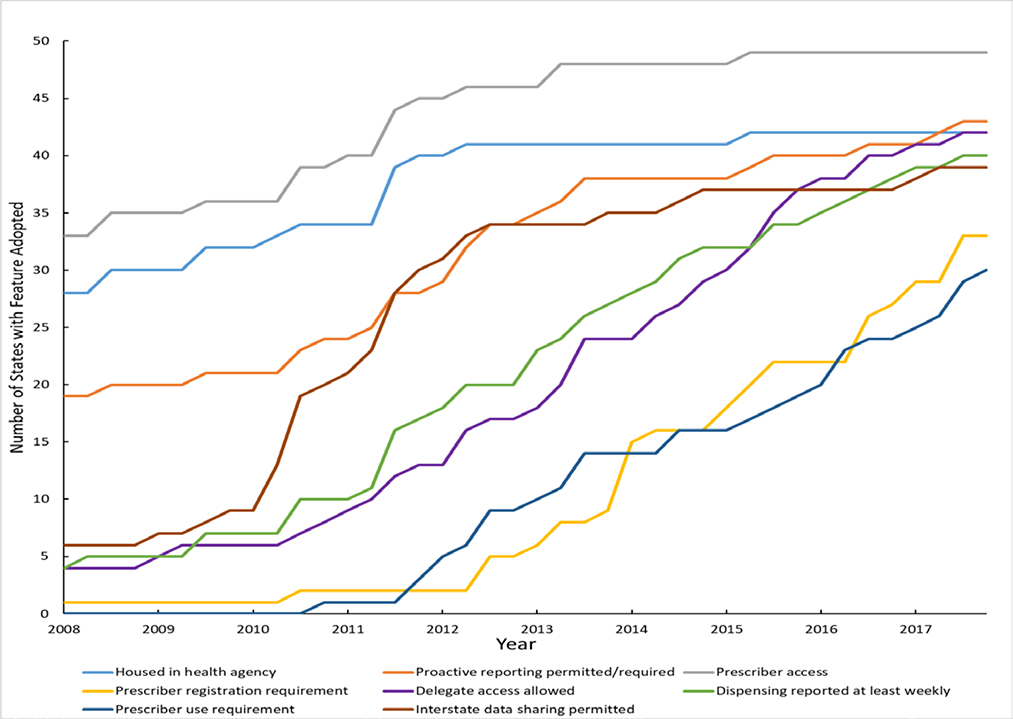
Momentum has favored stronger state PDMP features over time (see graph). Programs increasingly facilitate enhanced clinical use. Stakeholders see PDMPs as a resource for improving treatment decisions, reducing harmful polypharmacy (e.g., overlapping opioid and benzodiazepine prescribing), and identifying patients frequenting multiple prescribers or pharmacies for controlled substances — a practice known as multiple provider use.1,2 Most states originally made PDMP use optional but now require prescribers to register with and use the databases, while also allowing delegates (i.e., clinical staff who regularly access confidential patient data) to run queries on prescribers’ behalf. Despite these trends, heterogeneity among state PDMPs persists.
More is known about the effects of PDMPs than perhaps any other opioid-related policy. The overwhelming majority of PDMP evaluations have focused on prescribing outcomes; results from such studies have suggested that PDMPs may be associated with reduced dispensing of higher-schedule (and thus typically more addictive) opioid medications and lower rates of multiple provider use.3,4 More sophisticated evaluations that leveraged variations in PDMP features have revealed that mandatory-access provisions (including those that permit delegate access) and registration mandates are associated with reductions in multiple provider use and in the probability of patients receiving any opioid, receiving high-dose opioids, and having overlapping opioid prescriptions.4 Rigorous evaluations demonstrating the effects of PDMPs on nonfatal and fatal opioid overdoses are lacking.
But PDMPs remain controversial. Foremost among concerns is that such databases might induce a dramatic reduction in opioid-analgesic prescriptions without an equivalent increase in the provision of alternative treatments for patients dependent on opioids. As a result, patients may have untreated pain, and anecdotal evidence suggests that some of them may turn to illicit sources of opioids, including opioids containing synthetic fentanyl, or even become suicidal. Rigorous research on these associations is needed.
A second objection to PDMPs is that they infringe substantially on medical practice — a domain in which professional autonomy is paramount. Clinicians persistently voice concerns regarding the time required to use PDMPs; their poor integration with electronic health records (EHRs); the lack of timely access to data from other states; and registration and use mandates.1 Recent innovations — such as PDMP Interconnect and the implementation of more rapid data access, delegate access, and automated registration — aim to address some of these barriers. PDMP software is increasingly integrated directly into EHRs and often features built-in algorithms that flag high-risk dispensing patterns. Predetermined risk measures should be used with care, however, since algorithms may not account for all patient risk factors and are probably too prescriptive to dictate care.
A third objection involves PDMPs’ hybrid and somewhat competing goals. PDMPs were designed to support clinical decision making, but they also help law-enforcement agencies and medical boards identify prescribers and patients exhibiting troublesome behavior. Law-enforcement agencies’ access rights vary by state, with some states requiring agencies to obtain a court order based on probable cause that a crime was committed to obtain data for a particular patient or clinician. “Trolling” of databases by law-enforcement officers to identify cases of potential opioid overprescribing and multiple provider use could hypothetically chill prescribing and deter patients from seeking treatment with opioids, even when it is clinically appropriate. Expanding access to PDMP data also raises concerns that confidential information could be improperly disclosed and patient privacy invaded. Courts, however, have found that federal law-enforcement officers gaining access to PDMP data without probable cause doesn’t constitute a violation of constitutional privacy rights — so long as the data are adequately secured. Such trends could change, however, as data sharing becomes more widespread and offers increasingly comprehensive information about patients’ health conditions.
The newest source of controversy concerns whether the current nature of the opioid crisis undermines PDMP utility. PDMPs don’t track sales of heroin and illicit synthetic opioids — drugs responsible for a growing share of drug-overdose deaths in the United States (more than 28,000 and 15,000, respectively, in 2017). Modeling studies suggest that further lowering the incidence of prescription-opioid misuse will contribute only marginally to reducing overdoses, and that PDMPs could actually contribute to increased opioid-related deaths in the short-term.5 Such factors raise important questions about whether continued substantial investments in PDMPs — including federal funding prioritized in the 2018 SUPPORT for Patients and Communities Act for real-time reporting, interstate data sharing, and clinical-workflow integration — should be favored over policies aimed at reducing harms associated with illicit drugs and improving social determinants of health, for example.
So what role should PDMPs play moving forward? I believe concerns about PDMPs, although substantial and credible, don’t justify wholesale abandonment of these rich tools from which we derive substantial clinical and law-enforcement benefit. PDMPs, along with other policy levers, have contributed to continuous reductions in opioid prescriptions in the United States since 2011, and further reductions may be warranted. Policies that require prescribers to check PDMP data but permit some clinical discretion regarding what to do with such information may be preferable to blunt policies that restrict the amount of prescription opioids supplied regardless of individual patient characteristics.
Still, PDMPs could be more carefully calibrated to maximize clinician, patient, and population interests. Usability and utility for clinicians could continue to be improved in some states. States could integrate nonpharmaceutical data, such as data on emergency-department admissions and emergency-responder incidents, into PDMPs to highlight additional risk factors for controlled-substance use, including prior nonfatal overdoses. When PDMP information indicates potential opioid misuse, clinicians could discuss concerns with their patients, carefully supervise prescribing and dispensing, and facilitate addiction treatment. Clinical insights gleaned from PDMP data should form the beginning of enhanced patient engagement, rather than the end of an encounter. Mandated checks or universal EHR integration would allow PDMP data to be regularly used in patient care and could reduce disparities that may arise if biases dictate when clinicians check such data.
To preserve prescriber autonomy and patient privacy, I believe access to individually identifiable PDMP records by law-enforcement officers should be limited to circumstances when probable cause supports a specific need. Robust access to deidentified data for law-enforcement agencies and researchers, however — preferably written into the law — can facilitate pattern identification and public health interventions. Researchers should continue to rigorously evaluate PDMPs’ effects, paying particular attention to unintended consequences including unfavorable tapering or prescription cessation, differential effects on patients and prescribers based on their characteristics, substitution of illicit substances, and opioid-related overdoses and suicides. Although the role of PDMPs in preventing illicit-opioid overdoses may currently be limited, leveraging PDMP data appropriately could allow these programs to have enduring benefits in terms of safe prescribing and dispensing of opioids and other controlled substances, such as benzodiazepines, that contribute to the larger U.S. drug-overdose crisis.
Figure. Prescription Drug Monitoring Program Feature Implementation, 2018–2017.
States with Selected Prescription Drug Monitoring Program Features, 2008–2017. *
*Data are from the author’s primary legal research and the Prescription Drug Abuse Policy System. “Housed in a health agency” means the agency in charge of PDMP operation is a state department of health, board of pharmacy, or professional licensing body. “Prescriber registration requirement” means the PDMP automatically enrolls prescribers or there is a requirement that prescribers enroll in (i.e., obtain login information for) the PDMP. “Prescriber use requirement” is a requirement that prescribers access PDMP data under specified clinical circumstances. “Proactive reporting permitted or required” means the PDMP operating agency is either required or permitted to proactively identify outlier prescribing, dispensing, or purchasing and report findings to professional licensing bodies, prescribers, dispensers, or law-enforcement officials. “Prescriber access” indicates prescribers can access PDMP data when registered with the system.
Footnotes
Disclosure forms provided by the author are available at NEJM.org.
References
- 1.Haffajee RL, French CA. Provider perceptions of system-level opioid prescribing and addiction treatment policies. Curr Opin Psychol 2019;30:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutkow L, Smith KC, Lai AY, Vernick JS, Davis CS, Alexander GC. Prescription drug monitoring program design and function: A qualitative analysis. Drug Alcohol Depend 2017;180:395–400. [DOI] [PubMed] [Google Scholar]
- 3.Wilson MN, Hayden JA, Rhodes E, Robinson A, Asbridge M. Effectiveness of prescription monitoring programs in reducing opioid prescribing, dispensing, and use outcomes: a systematic review. J Pain 2019. doi: 10.1016/j.jpain.2019.04.007.4. [DOI] [PubMed] [Google Scholar]
- 4.Mauri AI, Townsend TN, Haffajee RL. The association of state opioid misuse prevention policies with patient and provider related outcomes: a scoping review. Millbank Q (forthcoming 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitt AL, Humphreys K, Brandeau ML. Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am J Public Health 2018;10:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]