Abstract
Late onset Alzheimer disease (LOAD) is traditionally considered as a separate disease from vascular dementia (VAD). However, growing evidence suggests that β-amyloid (Aβ) accumulation, that initiates LOAD-related neurodegeneration, is preceded by vascular events. Previous in vitro studies showed that β-secretase 1 (BACE1), the key-enzyme of amyloidogenesis, is upregulated by cerebrovascular insult; moreover, its activity is increased both in brain and serum of LOAD patients. We aimed to investigate whether BACE1 serum activity is altered also in dementias related, or not, to cerebrovascular disease. Thus, we evaluated serum BACE1 activity in a sample of individuals, including patients with LOAD (n. 175), VAD (n. 40), MIXED (LOAD/VAD) dementia (n. 123), other types of dementia (n. 56), and healthy Controls (n. 204). We found that BACE1 was significantly higher not only in LOAD (+ 30%), but also in VAD (+ 35%) and MIXED dementia (+ 22%) (p < 0.001 for all), but not in the other types of dementia (+ 10%). Diagnostic accuracy was 77% for LOAD, 83% for VAD, and 77% for MIXED dementia. In conclusion, we showed for the first time that the increase in peripheral BACE1 activity is a common feature of LOAD and VAD, thus underlying a further pathogenic link between these two forms of dementia.
Subject terms: Neuroscience, Biomarkers, Diseases
Introduction
Alzheimer’s disease (AD) and vascular dementia (VAD) are the most common causes of dementia syndrome, with AD accounting for about 70% of cases1. Although both pathologies lead to cognitive impairment, AD and VAD have been traditionally considered as two distinct diseases2. In fact, if from one side Amyloid-β (Aβ) formation and deposition in neuritic plaques and neurofibrillary tangles (NFT, due to hyperphosphorylation of tau protein) are the main pathophysiological hallmarks of AD, VAD is an heterogeneous group of brain disorders caused by cerebrovascular deterioration, with cortical/subcortical ischemic infarctions and leukoaraiosi3.
In contrast with the idea of AD and VAD as different disorders, there is abundant evidence that highlights the involvement of cerebrovascular disease in AD dementia3–5. This consideration mostly applies for the late onset form of AD (LOAD), a multifactorial and complex disease where a number of different abnormalities concur to cause the pathophysiological traits4,6. In particular, it has been proposed that vascular dysregulation might play an important role in LOAD development, and could contribute to Aβ deposition, functional impairment, and brain atrophy4,7. For instance, it has been amply proved that ischemic and neurodegenerative pathology coexist and reciprocally interact in LOAD, impacting the clinical presentation of the disease and it is well-known that LOAD and VAD share important cardiometabolic and lifestyle risk factors8.
Despite the convergent experimental and epidemiological evidence supporting the implication of vascular abnormalities in LOAD pathogenesis3,4,9, the mechanisms and the cause-effect nature of this interaction is still unclear. The most convincing mechanistic hypotheses are based on the observations that cerebral hypoperfusion may enhance Aβ formation and aggregation8,10,11. Increase in Aβ burden caused by vascular factors could occur through the impairment of the clearance (vascular pathway is estimated to be a major route of removal of Aβ from the brain12) and/or in the production of the peptide11. Relevant to this context, in vivo and in vitro studies showed that hypoperfusion/hypoxia, and induced oxidative stress, may facilitate Aβ production by activating the APP cleavage enzyme β-secretase 1 (BACE1)13,14. This is the key-enzyme of the amyloidogenic processing of APP, catalyzing the rate-limiting initial cleavage at the β site of APP; the subsequent cleavage by γ-secretase leads to the generation of Aβ. BACE1 has been recently found to be increased in the brain/CSF(cerebrospinal fluid) of patients with LOAD or mild cognitive impairment (MCI)15,16. BACE1 is present also in serum, as recently demonstrated by our group17 and Shen et al.18 in two recent studies. In particular, we found that LOAD patients has significantly higher levels of serum BACE1 activity compared to controls, and this difference is independent of possible confounders including age, gender, and other risk factors for dementia17. Based on this corollary of evidences, in the present study we investigated whether an increase in serum BACE1 activity is a specific feature of LOAD or might also characterize VAD. To this purpose, we evaluated the serum levels of BACE1 in a large sample of elderly individuals (n. 598) including patients affected by LOAD, VAD, mixed LOAD-VAD dementia (MIXED), and other type of dementia, in comparison with cognitively healthy subjects.
Results
Demographic and main clinical characteristics of the population sample
In Table 1 are reported the main demographic and clinical characteristics of the subjects included into the study according to diagnosis. Controls were younger (p < 0.001 for all post-hoc tests) and presented a lower prevalence of female gender (p < 0.01) compared to all the other groups; a similar trend was observed for comorbidities (in particular hypertension and cardiovascular disease, CVD). Years of formal education, as well as MMSE, IADL and BADL score were higher in Controls compared with all dementia groups.
Table 1.
Principal characteristics of the sample according to diagnosis.
| Characteristics | Controls (n:204) | Load (n:175) | VAD (n:40) | Mixed (n:123) | Other dementias (n:56) |
|---|---|---|---|---|---|
| Age (years)* | 74 ± 5 | 78 ± 6a | 79 ± 7a | 80 ± 5a | 77 ± 6a |
| Female gender (%)* | 49 | 68a | 73a | 70a | 67a |
| Formal Education (years)* | 11 (8–13) | 5 (3–5)a | 2 (3–6)a,b | 5 (4–5)a | 5 (5–9)a |
| MMSE score (/30)* | 27 (26–30) | 21 (18–23)a | 20 (16–23)a | 20 (16–23)a | 22 (18–25)a |
| -Current smoker (%) | 7 | 5 | 12a | 5 | 5 |
| Comorbidities | |||||
| Hypertension (%)* | 41 | 67a | 79a | 59a | 56a |
| Diabetes (%) | 8 | 13 | 22a | 17 | 15 |
| CVD (%)* | 5 | 8 | 19a,b | 21a,b | 15 |
| Functional status | |||||
| -IADLs* | 7 (5–8) | 3 (2–6)a | 4 (2–6)a | 3 (1–6)a | 4 (1–7)a |
| -BADLs* | 6 (5–6) | 6 (5–6)a | 5 (4–6)a | 5 (4–6)a | 5 (4–6)a |
| BACE1 (kU/L)# | 15 (12–18) | 21 (16–27)a | 23 (18–32)a | 19 (17–26)a | 17 (15–20)b,c,d |
Continuous variables are expressed as mean ± SEM or median (interquartile range). Categorical variable are expressed as percentage within group.
CVD cardiovascular diseases, MMSE mini mental state examination, LOAD late onset Alzheimer’s disease, IADL instrumental activities of daily living, BADL basic activity of daily living.
Post-hoc test: ap < 0.05 vs controls; bp < 0.05 versus LOAD; cp < 0.05 versus VAD; dp < 0.05 versus MIXED.
Serum BACE1activity across the study groups
As displayed in Fig. 1, compared with Controls, serum BACE1 activity was significantly higher in LOAD (+ 30%), VAD (+ 35%) and MIXED dementia (+ 22%) (p < 0.001 for all post-hoc comparisons), but not in the Other Dementias group (+ 12%; p > 0.10).
Figure 1.
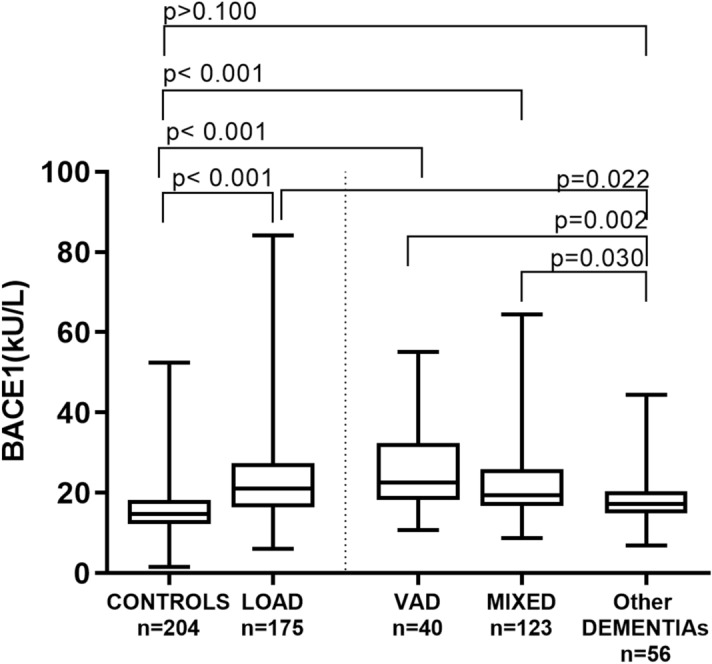
Serum BACE1 activity in Controls, LOAD, VaD, MIXED dementia, and other dementia. Serum BACE1 activity is significantly higher in LOAD (+ 30%), VAD (+ 35%) and MIXED dementia (+ 22%) (p < 0.001 for all post-hoc comparisons), but not in the Other Dementias group (+ 12%; p > 0.10).
As the second step, we evaluated the possible influence of potential confounding factors on the association between BACE1 activity and the diagnosis of LOAD, VAD, or MIXED dementia.
We first checked the age parameter, as it was significantly different between groups (see Table 1) and positively correlated with BACE1 activity (r: 0.269, p < 0.001). To abrogate the potential effect of age on BACE1 outcomes, we performed a further comparison after the exclusion of younger controls; the resulting sub-sample (n. 476 subjects) included groups with similar age (ANOVA p:0.20). As reported in Table 2, the differences between Controls and the other groups of patients were substantially unchanged. Of note, similar results were obtained when we compared younger controls with the two most homogeneous and numerous subsets of Other Dementia (i.e. Frontotemporal Dementia, n = 13; Lewy Body disease /Parkinson’s dementia, n = 13) (supplementary Table S1).
Table 2.
Levels of serum BACE1 activity in controls, LOAD, VAD, MIXED, and other dementias after exclusion of younger controls.
| Characteristics | Controls (n:82) | Load (n:175) | VAD (n:40) | Mixed (n:123) | Other dementias (n:56) |
|---|---|---|---|---|---|
| Age (years) | 79 ± 4 | 78 ± 6 | 79 ± 7 | 80 ± 5 | 77 ± 6 |
| BACE1 (kU/L)# | 16 (13–20) | 21 (16–27)a | 23 (18–32)a | 19 (17–26)a | 17 (15–20)b,c,d |
Continuous variables are expressed as mean ± SEM or median.
Post-hoc test: ap < 0.05 versus controls; bp < 0.05 versus LOAD; cp < 0.05 versus VAD; dp < 0.05 versus MIXED.
The potential confounding effect of gender on BACE1 was also evaluated due to the significant difference in gender across the sample groups (Table 1). In line with our previous analysis16, BACE1 was significantly higher in women (women: 19.2 kU/L vs men, 16.9 kU/L; p < 0.01). Despite this gender gap, the difference in BACE1 activity between Controls and LOAD, VAD or MIXED dementia was similar in women and men (Supplementary Table S2). As confirmation of these results, multivariable logistic regression analysis showed that higher BACE1 activity (IV quartile) was associated with the diagnosis of LOAD, VAD, and MIXED dementia after adjustment for age and gender (Supplementary Table S3).
At variance of age and gender, comorbidities and smoking did not affect BACE1 levels, and thus were not examined as potential confounders (data not shown).
Diagnostic accuracy evaluation
Finally, the diagnostic accuracy of BACE1 was evaluated by ROC curves and the area under ROC curves (AUC) calculation (Fig. 2) using the best compromises between sensitivity and specificity for the diagnosis of LOAD, VAD and MIXED dementia, with a cut-off of BACE1 activity of 16.9, 18.0, and 17.00, respectively. AUC values were 0.772 (Fig. 2a, sensitivity/specificity: 73/70%) for LOAD, 0.831 (Fig. 2b, sensitivity/specificity: 85/75%) for VAD, 0.771 (Fig. 2c, sensitivity/specificity: 74/71%) for MIXED dementia, and 0.773 (Fig. 2d, sensitivity/specificity: 75/70%) for the group including the 3 diseases all together (Supplementary Table S4). We also calculated the positive and negative predictive values, which were 67% and 75% for LOAD, 40% and 96% for VAD, and 60% and 81% for MIXED dementia, respectively.
Figure 2.
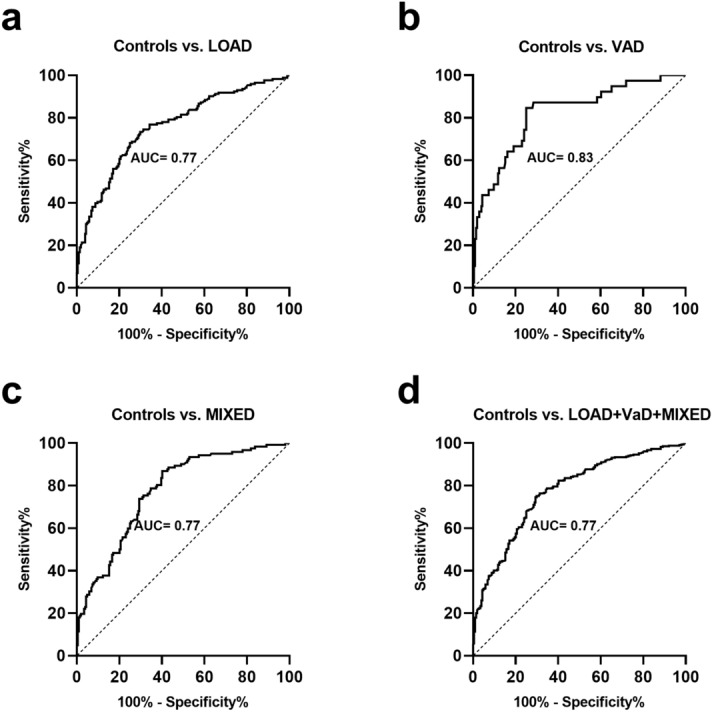
Receiver operating characteristic (ROC) curves for BACE1 activity for the diagnosis of LOAD, VaD, Mixed dementia, and the grouped LOAD + VaD + MIXED dementia. The calculated AUC values are 0.772 (Panel a, sensitivity/specificity: 73/70%) for LOAD, 0.831 (Panel b, sensitivity/specificity: 85/75%) for VAD, 0.771 (Panel c, sensitivity/specificity: 74/71%) for MIXED dementia, and 0.773 (Panel d, sensitivity/specificity: 75/70%) for the group including the 3 diseases all together.
Discussion
The present study was conceived as the logical continuation of our previous findings showing that BACE1 serum activity is increased in LOAD compared to Controls17. To this aim, we extended the investigation to a much larger population sample (n. 598), including not only a higher number of Controls and LOAD, but also other types of dementia.
Our efforts led us not only to confirm our previous results, but also to novel findings that might add to the current knowledge on the relationship between BACE and dementia. As a matter of fact, we showed that the increase in BACE1 serum activity is not a specific feature of LOAD, but also occurs in VAD and MIXED dementia. On the contrary, patients with other forms of dementia (such as Lewy body disease, frontotemporal, etc.) did not exhibit a significant alteration in BACE1 activity. Last but not least, BACE1 showed to perform even better in discriminating VAD from Controls when compared to MIXED dementia and LOAD (diagnostic accuracy values: 0.831, 0.772, 0.771, respectively).
Our data might appear contradictory at first glance. Indeed, BACE1 is classically ascribed as the key enzyme of amyloidogenic pathway that drives LOAD and not VAD pathogenesis. BACE1 is indispensable for the generation of Aβ since germline deletion of BACE1 gene abolishes Aβ deposition, preventing the subsequent development of amyloid-associated pathologies19–21. Moreover, several lines of evidence show that both protein levels and BACE1 activity are elevated in the brain regions affected by AD22,23. Although it might be challenging to establish from post-mortem tissue whether a specific change is a late or early event or an epiphenomenon in disease pathogenesis24, Cole et al. showed that BACE1 elevation was correlated with amyloid pathology in mouse models both in the absence (model: Tg2576) and presence (5XFAD) of significant neuronal loss24,25. In agreement with an early involvement of BACE1 in LOAD, enzyme activity was found to be higher in CSF and plasma of MCI converters compared to non-converters16,18,26.
On the other hand, there is a wealth of convergent data (“in vitro” and “in vivo” models) clearly suggesting that a vascular insult up-regulates BACE113,24,27,28. Owing this data, the observed elevation of BACE1 in VAD and MIXED dementia is not surprising, but rather expected. BACE1 is currently described as a stress-response protein, sensitive to factors that can tackle energy metabolism in the brain24,27. These perturbing factors are often a direct consequence of cerebral micro/macro infarctions, particularly of chronic/acute cerebral hypo-perfusion. It is well known that this phenomenon gives rise to several interconnected abnormalities including hypoxia, inflammation, glucose/lipid dyshomeostasis and oxidative stress, which are all putative modulators of BACE1 expression and activity. In this frame, an emblematic example is the effect of hypoxia on BACE1 expression, that may occur by release of ROS from dysfunctional mitochondria and by activation of Hypoxia-inducible factor 1 (HIF-1), the master regulator of oxygen homeostasis13,28,29. The induced elevation in BACE1 may in turn affect vascular integrity and function, due to the well-recognized vasoactive proprieties of Aβ24.
We are aware that our cross-sectional findings do not allow to precisely define the role of BACE1 in dementia pathogenesis. However, our data led us to hypothesize that BACE1 might have a role in dementia that is far beyond its significance as possible biomarker. Based on our result, we propose that BACE1 might represent an important mechanistic link between VAD and LOAD. The overlap in symptomatology, pathological traits and risk factors has led some authors to propose that VAD and LOAD are similar diseases30. Others are more cautious about the idea of an equivalence between these two diseases, and suggest that vascular abnormalities might be the earliest and strongest pathologic factor, thus preceding Aβ formation/deposition4,31. This has been elegantly shown by Iturria-Medina et al. through the reconstruction of LOAD-abnormality trajectories by integrating brain imaging biomarkers and blood/CSF biological descriptors4.
Thus, by combining data pointing to a precocious alteration of BACE1 in LOAD18,24,25 with data of multifactorial-driven analysis4 and our current results, it is tempting to speculate a temporal cascade of events in LOAD (Fig. 3) in which vascular and other abnormalities induce BACE1 alteration. This, in presence of a concomitant defective Aβ clearance, would be followed by deposition of Aβ and neurodegeneration. Certainly, other factors might modulate all these pathogenetic steps. The fact remains that 1) a significant increase in BACE 1 activity seems to be a common phenomenon in LOAD and VAD; 2) the increase in BACE1 serum activity is absolutely comparable in LOAD and VAD, suggesting the possibility that other factors downstream Beta-amyloid production might direct brain pathology toward LOAD rather than VAD.
Figure 3.
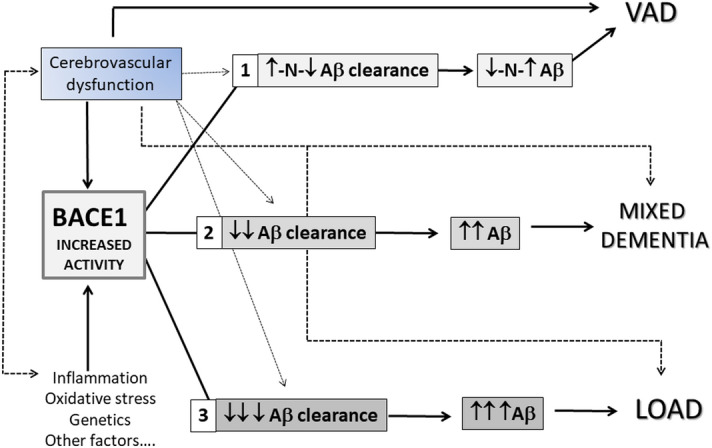
Potential impact of increased BACE1 activity in the pathogenesis of VaD, MIXED and LOAD. Cerebrovascular dysfunctions and/or other endogen/exogenous factors (e.g. oxidative stress, genetic predisposition, etc.) may cause increase in BACE1 activity leading to an increase in Aβ formation. This, in turn, can be accompanied by different levels of Aβ clearance efficiency and, as a consequence, by different levels of Aβ brain accumulation. The scenarios that can take place from different combination of increased BACE1/Aβ clearance effectiveness might predispose elderly individuals to different type of dementia: 1) VaD: partially impaired or unaffected Aβ clearance – mild increase/unchanged levels of Aβ 2) MIXED: partially impaired Aβ clearance—moderate to high levels of Aβ 3) LOAD: impaired Aβ clearance- high levels of Aβ.
However, it is noteworthy that our diagnoses were not based on biomarkers; as a consequence, it cannot be excluded that AD pathology might be present in patients with probable VAD (it was expected instead that AD pathology would be present in MIXED dementia). With regards to this crucial point, it has to be emphasized that NINDS-AIREN criteria for probable VAD have a low sensitivity (about 20–60%) but a high specificity (about 90–99%) as reported by clinical and neuropathological studies32,33. Thus, while we might have missed some VAD diagnosis, the number of false positive would be very low. To confirm this, at autopsy a small percentage (10–15%) of demented patients with pure vascular damage and no AD pathology are found34–36. In this light, it appears unlikely that serum BACE1 is high in VAD as much as in LOAD just as a consequence of a low diagnostic specificity. Rather, the finding of elevated BACE1 levels in both LOAD and VAD (but not in other types of dementia) might have alternative explanations: 1. BACE-1 dysregulation together with AD pathology accumulation might be the cornerstone of both LOAD (no/minimal cerebrovascular disease) and VAD (significant cerebrovascular disease); at this point, the real existence of pure VAD from the nosological point of view could be at least questionable. This would be in line with the finding that all of patients with signs of cerebrovascular pathology at autopsy also had some concomitant neurodegenerative disease, particularly AD37. 2. On the other hand, since about 50% of AD patients also have cerebrovascular disease at autopsy38, the theoretical possibility exists that high BACE-1 serum levels might be actually driven by VAD and not by LOAD (shift of perspective).
Finally, we should also acknowledge two other important limitations of the study. First, the study was cross-sectional, thereby precluding our ability to establish any cause/effect relationship between BACE1 and cognitive impairment/dementia. Second, we cannot exclude that biases or unmeasured confounders might have also a role in the development of the pathologies. However, we took in consideration several potential confounders (age, gender, hypertension, diabetes, CVD, etc.) and the observed increase in BACE1 activity was independent from those factors.
In conclusion, we have demonstrated for the first time that BACE1 serum activity is increased in VAD and MIXED dementia as much as in LOAD, compared with healthy Controls; this increase is significant and is independent of possible measured confounders. On the contrary, BACE1 activity was not increased in other forms of dementia. Our findings further underline the possible shared characteristics between LOAD and VAD, highlighting the potential role of BACE1in the early pathogenesis of these two common types of dementia.
Material and methods
Subjects
A total of 598 older subjects referring to the Day Service for Cognitive Decline of University of Ferrara, or the Casa Sollievo della Sofferenza, San Giovanni Rotondo (Italy) were enrolled into the present study. The study sample included:
175 patients with mild to moderate probable LOAD determined by the National Institute on Aging–Alzheimer’s Association (NIA-AA) workgroups criteria39. Mini Mental State Examination (MMSE) range: 18–23; Clinical Dementia Rating (CDR) range: 1–2;
40 patients with probable VAD, according to National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINDS-AIREN) criteria33. MMSE range: 16–23; CDR range: 1–2
123 patients with MIXED dementia; in these patients a definite diagnosis of LOAD or VAD was not possible since they presented both the characteristics VAD (e.g. significant vascular disease, focal neurological signs) and LOAD (e.g. memory impairment, type of progression). MMSE range: 16–23; CDR range: 1–2.
56 patients with other dementias (13 Lewy Body disease/Parkinson’s dementia, 13 frontotemporal dementia, 8 condition related to psychiatric conditions, 5 neoplasm/metastasis, 2 hydrocephalus, 2 Fahr’s syndrome, 2 alcohol related, 1 post syphilis, 1 hypothyroidism, 9 not defined). MMSE range: 18–25; CDR range: 1–2
204 healthy individuals (Controls) without any evidence of dementia and without functional disabilities attributable to cognitive impairment. MMSE range: 26–30.
Of note, 131 Controls and 115 LOAD were already examined in a previous study17.
There was no evidence of acute illnesses at the time of clinical observation and blood sampling. No subject was taking Nonsteroidal anti-inflammatory drug (NSAIDS), antibiotics, or steroids at the time of recruitment.
General and neuropsychological examination including MMSE and items geriatric depression scale (GDS), CDR, and MMSE was carried as previously described40. Functional status was also evaluated though basic activities of daily living (BADL) and instrumental activity of daily living (IADL). Personal data and medical history (e.g. hypertension, coronary heart disease, stroke, diabetes, chronic obstructive pulmonary disease) were collected by trained personnel41. Clinical chemistry analyses were routinely performed to exclude causes of secondary cognitive impairment including serum B-12 vitamin and folate, liver, kidney and thyroid function tests, blood cell count, and arterial oxygen saturation. The study was carried out in accordance with the guidelines provided by Declaration of Helsinki (World Medical Association, https://www.wma.net) and it was approved by the Local Ethic Committee of "Casa Sollievo della Sofferenza", San Giovanni Rotondo (protocol n. 3,877/DS) and Local Ethic Committee of "Azienda Arcispedale S. Anna", Ferrara (protocol n. 170,579). Signed informed consent, which was written in compliance with local and national ethical guidelines, was obtained from each patient prior to the inclusion into the study.
BACE1 activity assay
Peripheral blood samples were collected by venipuncture into VACUTAINER tubes without anticoagulant after overnight fasting. After 30 min of incubation at room temperature, the blood samples were centrifuged at 4,650×g for 20 min and sera were collected and stored in single-use aliquots at − 80 °C until analysis.
The assay was carried out as previously described17. Briefly, 100 μL of substrate (i.e. H–K(Dabsyl)SEVNLDAEFRC(MAL-LY)-NH2 which was synthetized as described in17), at a final concentration of 30 μM in the assay, was added to the wells of a black flat-bottom microplate.
After pre-incubation for 10 min at 37 °C, the reaction was started by the addition of 5 μL of sample (wild-type enzyme or undiluted serum) in triplicate. The fluorescence was read every 30 s for 20 min using excitation and emission wavelengths of 430 nm and 520 nm, respectively, in a Tecan Infinite M200 (TECAN GROUP, Switzerland) microplate reader. The reaction rates were converted from relative fluorescence units (RFU) per minute to enzyme units (U) by interpolation with a standard curve constructed using known concentrations of the wild-type enzyme.
Statistical analysis
Continuous variables were expressed as mean (standard deviation—SD) or median (interquartile range) when they were normally and non-normally distributed, respectively. Means were compared by t-test or ANOVA (with Sidak post-hoc test for multiple comparison); medians were compared by Kruskal–Wallis/Mann–Whitney U test. Proportion were compared by the χ2 test. Correlations were analyzed by Pearson’s and Spearman’s test according to their normal/non-normal distribution. Multivariable logistic regression analysis (using the highest quartile value as cut-off) was performed to determine the impact of covariates on the relationship between the variables of interest. A receiver operating characteristic (ROC) curve was performed to evaluate the diagnostic accuracy of the examined biomarker in discriminating controls from cases. Analyses were performed by SPSS for Windows statistical package, version 13.0.
Supplementary information
Acknowledgements
The authors would like to thank Dr. Cristina Bosi and Dr. Juana M. Sanz for her technical support and assistance. Additionally, we thank the research team for the help and logistic support.
Author contributions
G.Z.: conceptualization, writing original draft, data analysis, review and editing; A.T.: writing original draft, data analysis, review and editing; V.R.: data analysis, data curation, validation, review and editing; R.G.: data analysis, data curation, review and editing, Formal analysis; S.P.: data analysis, investigation, review and editing, validation; S.B.: data analysis, investigation, data curation, review and editing; A.G.: investigation, review and editing; A.P.: investigation, review and editing; D.S.: data curation, investigation, review and editing; G.V.: data analysis, data curation, writing, review and editing; C.C.: conceptualization, writing original draft, data analysis, review and editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72168-3.
References
- 1.Prince M, et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers. Res. Ther. 2016;8:23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iemolo F, et al. Pathophysiology of vascular dementia. Immun. Ageing. 2009;6:13. doi: 10.1186/1742-4933-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Torre JC. Alzheimer disease as a vascular disorder. Stroke. 2002;33:1152–1162. doi: 10.1161/01.STR.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 4.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breteler MM. Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol. Aging. 2000;21:153–160. doi: 10.1016/S0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 6.Cervellati C, et al. Oxidative challenge in Alzheimer’s disease: state of knowledge and future needs. J. Investig. Med. 2016;64:21–32. doi: 10.1136/jim-2015-000017. [DOI] [PubMed] [Google Scholar]
- 7.De La Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 8.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelleher RJ, Soiza RL. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: is Alzheimer’s a vascular disorder? Am. J. Cardiovasc. Dis. 2013;3:197–226. [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Alloza M, et al. Cerebrovascular lesions induce transient β-amyloid deposition. Brain. 2011;134:3697–3707. doi: 10.1093/brain/awr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mawuenyega KG, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellano JM, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc. Natl. Acad. Sci. USA. 2012;109:15502–15507. doi: 10.1073/pnas.1206446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guglielmotto M, et al. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1α. J. Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 14.Mouton-Liger F, et al. Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2α pathway. Biochim. Biophys. Acta. 2012;1822:885–896. doi: 10.1016/j.bbadis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 16.Zhong Z, et al. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch. Gen. Psychiatry. 2007;64:718–726. doi: 10.1001/archpsyc.64.6.718. [DOI] [PubMed] [Google Scholar]
- 17.Cervellati C, et al. Serum beta-secretase 1 (BACE1) activity as candidate biomarker for late-onset Alzheimer’s disease. GeroScience. 2020;42:159–167. doi: 10.1007/s11357-019-00127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y, et al. Increased plasma beta-secretase 1 may predict conversion to Alzheimer’s disease dementia in individuals with mild cognitive impairment. Biol. Psychiatry. 2018;83:447–455. doi: 10.1016/j.biopsych.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai H, et al. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 20.Das B, Yan R. Role of BACE1 in Alzheimer’s synaptic function. Transl. Neurodegener. 2017;6:23. doi: 10.1186/s40035-017-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McConlogue L, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J. Biol. Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- 22.Yang LB, et al. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease [1] Nat. Med. 2003 doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 23.Li R, et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SL, Vassar R. Linking vascular disorders and Alzheimer’s disease: Potential involvement of BACE1. Neurobiol. Aging. 2009;30:1535–1544. doi: 10.1016/j.neurobiolaging.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakley H, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexopoulos P, et al. Cerebrospinal fluid BACE1 activity and sAβPPβ as biomarker candidates of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2018;45:152–161. doi: 10.1159/000488481. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Na R, Gu M, Richardson A, Ran Q. Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer’s disease. J. Neurochem. 2008;107:197–207. doi: 10.1111/j.1471-4159.2008.05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X, et al. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc. Natl. Acad. Sci. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamagno E, Guglielmotto M, Monteleone D, Tabaton M. Amyloid-β production: major link between oxidative stress and BACE1. Neurotox. Res. 2012;22:208–219. doi: 10.1007/s12640-011-9283-6. [DOI] [PubMed] [Google Scholar]
- 30.Launer L. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res. Rev. 2002;1:61–77. doi: 10.1016/S0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 31.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010 doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiederkehr S, Simard M, Fortin C, van Reekum R. Comparability of the clinical diagnostic criteria for vascular dementia: a critical review. Part I. J. Neuropsychiatry Clin. Neurosci. 2008;20:150–161. doi: 10.1176/jnp.2008.20.2.150. [DOI] [PubMed] [Google Scholar]
- 33.Román GC, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/WNL.43.2.250. [DOI] [PubMed] [Google Scholar]
- 34.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 36.Knopman DS, et al. Vascular dementia in a population-based autopsy study. Arch. Neurol. 2003;60:569–575. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- 37.Nolan KA, Lino MM, Seligmann AW, Blass JP. Absence of vascular dementia in an autopsy series from a dementia clinic. J. Am. Geriatr. Soc. 1998;46:597–604. doi: 10.1111/j.1532-5415.1998.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 38.Toledo JB, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellazzi M, et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016;81:356–363. doi: 10.1016/j.biocel.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Cervellati C, et al. Serum paraoxonase and arylesterase activities of paraoxonase-1 (PON-1), mild cognitive impairment, and 2-year conversion to dementia: a pilot study. J. Neurochem. 2015;135:395–401. doi: 10.1111/jnc.13240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


