Abstract
Background:
Coronary no-reflow phenomenon in ST-segment elevation myocardial infarction (STEMI) is associated with a poor clinical outcome. Although its pathophysiology is not fully understood, a deregulated systemic inflammatory response plays an important role. We aimed to explore the relationship between platelet\lymphocyte ratio (PLR) and no-reflow in patients with acute STEMI who were treated with a primary percutaneous coronary intervention (PPCI).
Methods:
A total of 200 patients with STEMI undergoing PPCI were included in the study. Transthoracic echocardiographic examination was performed to assess left ventricular (LV) ejection fraction (EF) and wall motion score index. Blood samples were assayed for platelet and lymphocyte count before PPCI. No-reflow was defined as coronary blood flow thrombolysis in myocardial infarction grade ≤II.
Results:
No-reflow was observed in 58 (29%) of STEMI patients following PPCI. PLR was significantly higher in hypertensive patients compared to normotensive patients (144.7±91.6 vs. 109.1±47.1, respectively, P<0.001) and in the no-reflow group compared to the normal reflow group (214±93 vs. 101.6±51.3, respectively, P<0.0001). Logistic regression analysis revealed that PLR (β: 0.485, 95% CI: −0.006-0.001, P<0.002) and LV EF (β: 0.272, 95% CI: 0.009-0.034, P<0.001) were independent predictors of no-reflow after PPCI.
Conclusion:
Pre-procedural increase in PLR is predictive of the no-reflow phenomenon following PPCI in STEMI patients.
Relevance for Patients:
No reflow phenomenon is an unfavorable complication following PPCI in patients with acute STEMI. High pre-procedural PLR is an independent predictor of reperfusion failure and helps to identify patients who require prophylactic treatment.
Keywords: platelet/lymphocyte ratio, primary percutaneous coronary intervention, ST-segment elevation myocardial infarction
1. Introduction
In acute ST-segment elevation myocardial infarction (STEMI), primary percutaneous coronary intervention (PPCI), and stent implantation are the first choice of treatment [1,2]. However, earlier studies demonstrated a high incidence of coronary slow/no-reflow in 1-40% of the patients that may be associated with stoppage of myocardial perfusion restoration, whereby patients continued to suffer from the severe impairment [3,4]
No reflow is recorded in large registries based on thrombolysis in myocardial infarction (TIMI) flow grade, myocardial blush grade, ST resolution [5], myocardial contrast echocardiography, and cardiac magnetic resonance imaging that assessed microcirculatory dysfunction [6].
Several hypotheses have been formulated to describe the pathogenesis of no-reflow, including distal microembolization of thrombus fragments, swelling of endothelial cells caused by ischemia-reperfusion injury, and microvascular spasm [7-10]. A large number of studies have been carried out to investigate the predictors of slow/no-reflow phenomenon and the results showed that thrombosis burden, reperfusion time, and inflammatory factors are implicated [11-16].
Platelet activation plays a central role in the initiation and progression of atherosclerosis [17], and increased platelet activation is associated with major adverse cardiovascular consequences [18-20]. On the other hand, a low blood lymphocyte count has been shown to be related to worse cardiovascular outcomes in patients with coronary artery disease. The aim of this study was to explore the relationship between the platelet/lymphocyte ratio (PLR) and post-intervention TIMI flow in STEMI patients who have undergone PPCI.
2. Patients and Methods
This cross-sectional observational study was conducted on patients presented with STEMI and treated with PPCI between December 2017 and August 2019. We investigated 200 consecutive patients presented in two tertiary referral centers.
Patients with one or more of the following criteria were excluded from the study: Prior acute coronary syndrome, non-STEMI, unstable angina, STEMI duration more than 12 h, cardiogenic shock, treatment with thrombolytic therapy in the previous 24 h, estimated glomerular filtration rate <60 mL/min/1.73 m2 or renal dialysis, active systemic inflammatory diseases, or active treatment for specific conditions (including allergy, asthma, autoimmune diseases, glomerulonephritis, hepatitis, inflammatory bowel disease, and known malignancy).
All patients were reviewed for their risk profile, including smoking, hypertension, diabetes, dyslipidemia, and family history. Twelve leads electrocardiography (ECG), conventional echocardiography for evaluation of left ventricular (LV) function using ejection fraction (EF%), and wall motion score index (WMSI) were performed.
2.1. Blood analysis
Routine laboratory investigations included platelet, lymphocyte count, hemoglobin (HB), serum creatinine, cardiac biomarkers including troponin and creatine kinase myocardial band (CK-MB). Venous blood samples were drawn from antecubital veins immediately after patient evaluation and ECG recording.
Whole blood was analyzed on a Sysmex K-1000 and Sysmex XN-10 Automated Hematology Analyzer (Sysmex Corporation, Kobe, Japan) immediately following blood sampling. Whole blood was collected in ethylene diamine tetraacetic acid containers.
Before PPCI, all patients received 300 mg aspirin and 600 mg clopidogrel at the time of diagnosis before the intervention, and an intravenous bolus of unfractionated heparin 40-70 U/kg to achieve an activated clotting time of 200-250 s during the procedure. Coronary angiography was performed using standard techniques (Siemens Axiom Artis zee 2011 standard) encompassing a femoral approach with a 6-French guiding catheter. Direct stenting, balloon pre-dilatation, and the use of balloon pre-dilatation or post-dilatation, the type of stents, the use of tirofiban, and thrombus aspiration were at the operator’s discretion.
The TIMI flow grade was evaluated by two independent, experienced interventional cardiologists using quantitative cardiovascular angiographic software. The TIMI flow was assessed where TIMI 0 was defined as no antegrade filling of the culprit vessel, TIMI I was defined as sluggish filling and evacuation of the culprit vessel, TIMI II was defined as normal filling with sluggish evacuation, and TIMI III was defined as normal filling with normal evacuation.
Angiographic slow/no-reflow during PCI was defined as TIMI flow grade of ≤II during the procedure without evidence of dissection, residual stenosis, distal embolism, or vasospasm.
The study patients were divided into two groups based on the post-intervention infarct-related artery flow: The normal-reflow group included patients with post-intervention TIMI flow grade of III and the no-reflow group included patients with post-intervention TIMI flow grade 0, I, and II.
2.2. Statistical analysis
Data were collected, coded, revised, and entered into the Statistical Package for the Social Science (SPSS). Data were presented as mean±SD for continuous data and as number (%) for categorical data. Logistic regression analysis was used to assess the risk factors for coronary flow. The confidence interval was set to 95% and the margin of error accepted was set to 5%. P≤0.05 was considered statistically significant.
3. Results
Two hundred patients were enrolled in the study, their mean age was 52.9±11.1, body mass index was 27.6±2.5, 160 (80%) patients were male, 118 (59%) were smokers, 88 (44%) were diabetic, 102 (51%) were hypertensive, 35 (17.5%) were obese, and 41 (20.5%) were dyslipidemic. Twenty-eight patients (14%) had a positive family history, 123 (61.5%) had anterior STEMI, 75 (37.5%) had inferior STEMI, and 2 (1%) had lateral STEMI. Twelve (6%) patients had no risk factors, 52 (26%) had one risk factor, 69 (34.5%) had two risk factors, 51 (25.5%) had three risk factors, and 16 (8%) had four risk factors. The mean CK-MB was 104±67, the mean troponin was 7.8±3.2, the PLR was 14.22±11.2, the EF was 46.5%±7.7%, and the WMSI was 1.2±0.1 (Figure 1). Demographic and clinical characteristics of studied groups according to TIMI flow are presented in Table 1.
Figure 1. Coronary flow profile following primary percutaneous coronary intervention.
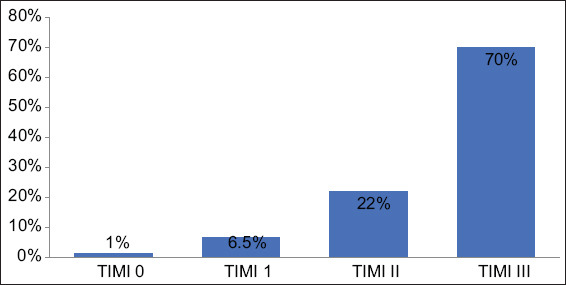
Table 1. Patients characteristics and risk factors.
| TIMI 0-II (n=58) | TIMI III (n=142) | P-value | |
|---|---|---|---|
| Male (%) | 49 (84.5%) | 111 (78.2%) | 0.2 |
| Female (%) | 9 (15.5%) | 31 (21.8%) | |
| Obese (%) | 9 (15.5%) | 26 (18.3%) | 0.84 |
| Diabetic (%) | 26 (44.8%) | 62 (43.7%) | 0.92 |
| HTN (%) | 31 (53.4%) | 71 (50%) | 0.87 |
| Dyslipidemia (%) | 7 (12.1%) | 34 (23.9%) | 0.23 |
| Smokers (%) | 37 (63.8%) | 81 (57%) | 0.22 |
| + ve family history (%) | 4 (6.8%) | 24 (16.9%) | 0.11 |
| Troponin (ng/mL) | 8.2±3 | 5.1±2.4 | 0.07 |
| CK-MB (IU/L) | 195±35 | 104±24 | 0.01 |
| Ejection fraction (%) | 40±6 | 56±4 | 0.03 |
| Platelet (×103/μL) | 345±114 | 228±84 | 0.0001 |
| Lymphocyte (×103/μL) | 1.73±0.5 | 2.2±0.9 | 0.02 |
| Platelet/lymphocyte ratio | 199.4±52 | 102±53 | 0.001 |
| Infarction site | |||
| Anterior (%) | 44 (35.7%) | 79 (64.2%) | 0.07 |
| Lateral (%) | 1 (50%) | 1 (50%) | |
| Inferior (%) | 13 (17.3%) | 62 (82.7%) | |
| Left anterior descending (%) | 44 (35.7%) | 79 (64.2%) | 0.4 |
| Left circumflex (%) | 3 (14.3%) | 18 (85.7%) | |
| Obtuse marginal 1 (%) | 1 (33.3%) | 2 (66.7%) | |
| Obtuse marginal 3 (%) | 0 (0.0%) | 1 (100%) | |
| Right coronary artery (%) | 10 (19.2%) | 42 (80.8%) |
PPCI to the culprit vessel was performed; 123 patients (61.5%) had percutaneous coronary intervention (PCI) to left anterior descending with 140 (70%) drug-eluting stents (DESs), 21 patients (10.5%) had PCI to left circumflex with 21 (10.5%) DESs, three patients (1.5%) had PCI to OM1 with 3 (1.5%) DESs, one patient (0.5%) had PCI to OM3 with 1 (0.5%) DES, and 52 (20%) patients had PCI to RCA with 52 (20%) DESs.
Coronary flow was graded using TIMI flow and showed that 2 (1%) patients had TIMI 0, 13 (6.5%) patients had TIMI I, 44 (22%) patients had TIMI II, and 141 (70.5%) patients had TIMI III.
We studied the relationship between platelet, lymphocyte, and PLR and risk factors (clinical and angiographic findings) after successful PCI. The platelet count was significantly higher in hypertensive patients compared to non-hypertensive patients; 271.5±111 versus 237.2±87.8, P<0.017, respectively, and in the no-reflow group compared to normal reflow group; 345±114 versus 228±84, P<0.0001, respectively. The lymphocyte count was significantly lower in the no-reflow group compared to the reflow group, 17.3±5% versus 25±9, P<0.0001. PLR was significantly elevated in hypertensive patients compared to non-hypertensive patients: 14.5±9.2 versus 10.9±4.7, P<0.001, respectively, and in the no-reflow group compared to the normal reflow group: 23.7±8 versus 9.1±5.3, P<0.001, respectively (Table 2).
Table 2. Platelet, lymphocytes, and PLR values in the study cohort.
| No. | Platelet | P-value | Lymphocyte | P-value | PLR | P-value | |
|---|---|---|---|---|---|---|---|
| Male | 160 | 248.7±98.7 | 0.9 | 2.3±0.9 | 0.8 | 125±73 | 0.4 |
| Female | 40 | 278.9±110 | 2.3±0.9 | 137±85 | |||
| Obese | 35 | 269.7±110 | 0.3 | 2.4±0.9 | 0.5 | 129±91 | 0.8 |
| Non-obese | 165 | 251.5±99.8 | 2.2±0.9 | 127±72 | |||
| Smokers | 118 | 252±97 | 0.6 | 2.3±0.9 | 0.4 | 125±75 | 0.6 |
| Non-smokers | 82 | 258.6±108.6 | 2.2±0.8 | 131±75 | |||
| Hypertensive | 102 | 271.5±111 | 0.01 | 2.2±0.8 | 0.9 | 145±92 | 0.001 |
| Non-hypertensive | 98 | 237.2±87.8 | 2.3±0.9 | 109±47 | |||
| Diabetic | 88 | 262.9±108 | 0.3 | 2.2±1.0 | 0.9 | 134±86 | 0.3 |
| Non-diabetic | 112 | 248.3±96.2 | 2.3±0.8 | 122±65 | |||
| Dyslipidemia | 41 | 237.7±78.9 | 0.2 | 2.2±0.8 | 123±58 | 0.6 | |
| No dyslipidemia | 159 | 259.1±106 | 2.3±0.9 | 0.4 | 128±79 | ||
| Family history | 28 | 244.6±101 | 0.6 | 2.6±0.9 | 0.06 | 104±59 | 0.8 |
| No family history | 172 | 256.3±102 | 2.2±0.8 | 131±77 | |||
| TIMI 0 | 3 | 373.6±126 | 0.00001 | 1.7±0.5 | 0.00001 | 285±124 | 0.00001 |
| TIMI I | 10 | 360.6±115 | 1.6±0.4 | 258±114 | |||
| TIMI II | 45 | 302.8±103.5 | 1.8±0.6 | 188±65 | |||
| TIMI III | 142 | 228.6±84.6 | 2.2±0.9 | 102±53 | |||
| No. of risk factors | 0.5 | ||||||
| 0 | 12 | 266.42±94.7 | 0.5 | 2.3±0.8 | 0.9 | 122±39 | |
| 1 | 52 | 233.9±93.9 | 2.3±0.8 | 112±59 | |||
| 2 | 69 | 265.7±107.1 | 2.3±1.0 | 134±79 | |||
| 3 | 51 | 256.0±104.2 | 2.2±0.7 | 135±97 | |||
| 4 | 16 | 261.8±101.3 | 2.4±1.0 | 127±76 | |||
| TVR: LAD | 123 | 253.8±103.3 | 0.7 | 2.2±0.8 | 0.07 | 130±73.31 | 0.6 |
| LCX | 25 | 270.4±93.7 | 2.5±0.8 | 122±76.4 | |||
| RCA | 52 | 249.3±102.7 | 2.4±1.1 | 121±79.8 |
TVR: Target vessel revascularization, LAD: Left anterior descending, LCX: Left circumflex, RCA: Right coronary artery
Pearson correlation coefficient was used to examine the relationship between platelet, lymphocyte, and PLR and the patients’ clinical and angiographic findings (Table 2). From all clinical and angiographic data, platelet counts showed a direct correlation to CK-MB (P<0.004) and TIMI flow (P<0.000), respectively. The total lymphocytic count was inversely correlated to TIMI flow (P<0.000) and directly correlated with HB (P<0.001). PLR ratio showed a direct correlation to CK-MB (P<0.006) and TIMI flow (P<0.0001) (Table 3).
Table 3. Correlation of PLR with patient’s characteristics.
| Platelet | Lymphocyte | PLR | |
|---|---|---|---|
| Age (years) | |||
| r | −0.113 | −0.100 | −0.023 |
| p | 0.111 | 0.158 | 0.741 |
| BMI (kg/m2) | |||
| r | 0.083 | 0.034 | 0.048 |
| p | 0.240 | 0.627 | 0.495 |
| EF (%) | |||
| r | −0.052 | −0.002 | −0.034 |
| p | 0.467 | 0.982 | 0.636 |
| Troponin (ng/mL) | |||
| r | 0.232 | −0.047 | 0.180 |
| P | 0.005 | 0.331 | 0.006 |
| CK-MB (IU/L) | |||
| r | 0.202 | −0.056 | 0.193 |
| p | 0.004 | 0.431 | 0.006 |
| WMSI | |||
| r | 0.079 | −0.057 | 0.091 |
| p | 0.267 | 0.419 | 0.198 |
| Cr (mg/dL) | |||
| r | −0.002 | −0.132 | 0.119 |
| p | 0.980 | 0.062 | 0.092 |
| TIMI flow | |||
| r | −0.434 | 0.339 | v0.599 |
| p | 0.000 | 0.000 | 0.000 |
| HB (g/dL) | |||
| r | 0.009 | 0.228 | −0.100 |
| p | 0.902 | 0.001 | 0.158 |
| Gensini score | |||
| r | −0.018 | −0.042 | 0.013 |
| p | 0.799 | 0.553 | 0.859 |
EF: Ejection fraction, WMSI: Wall motion score index, G: Gensini score, Cr: Creatinine, HB: Hemoglobin
Multivariate logistic regression analysis was employed to identify the independent predictors of TIMI flow in STEMI patients following PPCI. PLR (β: −0.485, 95% CI: −0.006-0.001, P<0.002) and EF % (β: 3.407, 95% CI: 0.009-0.034, P<0.001) were independent predictors of TIMI flow in STEMI patient after PPCI (Table 4 and Figure 2).
Table 4. Predictors of TIMI flow in STEMI patients after primary PCI.
| B | SE | β | t | P-value | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| HTN | 0.039 | 0.071 | 0.032 | 0.551 | 0.582 | −0.100 | 0.178 |
| Platelets (/mm3) | 0.000 | 0.001 | −0.073 | −0.564 | 0.573 | −0.002 | 0.001 |
| Lymphocyte (%) | 0.068 | 0.069 | 0.100 | 0.980 | 0.328 | −0.069 | 0.204 |
| PLR | −0.004 | 0.001 | −0.485 | −3.186 | 0.002 | −0.006 | −0.001 |
| BMI (kg/m2) | −0.021 | 0.014 | −0.087 | −1.509 | 0.133 | −0.047 | 0.006 |
| EF% | 0.021 | 0.006 | 0.272 | 3.407 | 0.001 | 0.009 | 0.034 |
| WMSI | 0.260 | 0.320 | 0.065 | 0.812 | 0.418 | −0.371 | 0.891 |
| Cr (mg/dl) | −0.056 | 0.118 | −0.028 | −0.479 | 0.633 | −0.289 | 0.176 |
Figure 2. Creatine kinase myocardial band levels stratified per platelet\lymphocyte ratio quartile.

4. Discussion
In the present study, PLR was an independent predictor of no-reflow in STEMI patients treated with PPCI. PLR was directly correlated to cardiac enzyme level (CK-MB) and showed higher values in hypertensive patients. STEMI patients that had regained normal coronary flow (TIMI III) had considerably lower platelet count and PLR but higher lymphocytic count compared to patients with slow or no flow.
Several studies have shown a relationship between the no-reflow phenomenon and increased inflammatory activity and proposed the PLR as a surrogate pro-thrombotic and inflammatory marker [21-23]. Our findings confirm the relation between PLR and the occurrence of no-reflow as a post-procedural complication following PPCI.
Although the pathophysiology of no-reflow has not been fully elucidated, its cause appears to be multifactorial. These factors include reperfusion injury from neutrophil aggregation, microvascular leukocytes, platelets plugging, complex interactions between neutrophils and platelets induced by the inflammatory process, distal embolization of culprit lesion, endothelial damage, and the production of reactive oxygen species [24-26].
PLR first gained attention in cancer patients as a marker for prognosis [27,28], after which it received growing interest with respect to its usefulness as a prognostic marker in cardiovascular medicine. The proposed mechanism of platelet involvement is platelet activation as a pivotal step of the inflammatory response in CAD and cardiovascular events [29,30]. During inflammation, a variety of inflammatory mediators (e.g., interleukin [IL]-1, IL-3, and IL-6) are released that stimulate megakaryocytes to proliferate and increase platelet levels in the circulation [31]. Activated platelets promote a pro-inflammatory environment by secreting cytokines and coagulation factors and they play a key role in the progression of atherosclerosis [32]. On the other hand, lymphocytes are responsible for providing a regulatory and quiescent pathway of inflammation [31,33].
Early risk stratification to detect patients at high risk of angiographic no-reflow is very important for its prevention in addition to early treatment using pharmacological and/or interventional strategy.
A recent analysis of eight pooled cohorts with a total of 6627 patients with acute coronary syndrome demonstrated that high PLR (>150) doubles the risk of in-hospital, all-cause, and cardiovascular mortality (pooled relative risk, 2.15; 95% CI, 1.73-2.67, 1.95, and 1.30-2.91, respectively) [34].
Prior studies demonstrated the association between PLR and cardiovascular events. Azabet et al. [35] showed higher PLR independently predicted 4-year mortality in non-STEMI patients, while Osadnik et al. [36] demonstrated the predictive value of PLR in patients with stable CAD undergoing PCI and stent implantation. Cho et al. [37] investigated the prognostic value of PLR and neutrophil-lymphocyte ratio (NLR) in patients without STEMI undergoing elective PCI with drug-eluting stents and showed PLR and NLR, alone and in combination, predicted long-term major adverse cardiovascular events.
In our study, we investigated 200 patients presented with STEMI and no previous history of the acute coronary syndrome. The patients were subjected to PPCI within 12 h of presentation and divided into two groups based on the TIMI flow grade: Normal reflow in 71% of patients with TIMI flow grade III, while no-reflow developed in 29% of patients with TIMI flow grade ≤II. Patients with no-reflow were predominantly male, hypertensive, had higher WMSI, PLR, and lower EF compared to those with normal flow. In contrast, other risk factors did not differ between groups. Using logistic regression analysis, PLR and EF were independent predictors of no-reflow after PPCI.
Similarly, Kurtul et al. [31] investigated 520 patients with acute STEMI and reported a lower rate of no-reflow (22% of patients). These patients were older than the patients who had regained normal coronary flow. PLR on the admission of ≥126 predicted the angiographic no-reflow with 73% sensitivity and 71% specificity. Moreover, PLR and stent length were independent predictors of no-reflow following PPCI.
Taken together, with the published data, the current study raises the potential role of inflammation theory and underscores the value of inflammatory markers in the pathophysiology of coronary circulation and no-reflow phenomenon. However, further studies are required to explain the exact mechanism of PLR in the pathogenesis of this phenomenon.
4.1. Study limitations
Our study has some limitations. First, the small sample size and there was no patient follow-up to examine the occurrence of adverse cardiac events and to explore the relationship between these cardiac events and PLR. Second, other inflammatory markers such as endothelin 1 and thromboxane A2 were not measured. Third, the incidence of no-reflow during PCI ranged widely from 1 to 41% [3-8]. While there are other studies that also demonstrate high rates of no-reflow, most large contemporary studies do not. The possible explanation for this difference might lie in the clinical and procedural characteristics and the application of a standardized definition of no-reflow. Although no-reflow is commonly recognized as transient, angiographically visible flow impairment despite epicardial coronary patency, other studies have included more liberal definitions, such as a failure to achieve TIMI III flow at the end of the procedure or decreased myocardial flow after PCI as shown by perfusion imaging [38,39]. Hence, it is not clear whether PLR would have the same prognostic information if the no-reflow rate was smaller. Finally, limited clinical application of PLR as it is not routinely measured before PPCI.
5. Conclusions
High PLR and lower EF are strong, independent predictors of no-reflow in STEMI patients undergoing PPCI. Assessment of PLR might be considered to address patient prognosis and serve as a useful biomarker in the risk stratification model.
Acknowledgments
I wish to thank Professor Ahmed Hassouna for his expertise in statistics and assistance throughout all phases of data analysis in this study. Furthermore, I would like to thank Doctor Amr Nasser Elsheikh for his expertise and assistance in English languish editing and revision of this manuscript.
References
- [1].O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, Lemos JA, et al. ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction:A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- [2].O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction:A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- [3].Piana RN, Paik GY, Moscucci M, Cohen DJ, Gibson CM, Kugelmass AD, et al. Incidence and Treatment of No-Reflow after Percutaneous Coronary Intervention. Circulation. 1994;89:2514–8. doi: 10.1161/01.cir.89.6.2514. [DOI] [PubMed] [Google Scholar]
- [4].Resnic FS, Wainstein M, Lee MK, Behrendt D, Wainstein RV, Ohno-Machado L, et al. No-reflow is An Independent Predictor of Death and Myocardial Infarction after Percutaneous Coronary Intervention. Am Heart J. 2003;145:42–6. doi: 10.1067/mhj.2003.36. [DOI] [PubMed] [Google Scholar]
- [5].Harrison RW, Aggarwal A, Ou FS, Klein LW, Rumsfeld JS, Roe MT, et al. Incidence and Outcomes of No-Reflow Phenomenon During Percutaneous Coronary Intervention Among Patients with Acute Myocardial Infarction. Am J Cardiol. 2013;111:178–84. doi: 10.1016/j.amjcard.2012.09.015. [DOI] [PubMed] [Google Scholar]
- [6].Galiuto L. Optimal Therapeutic Strategies in the Setting of Post-Infarct No Reflow:The Need for a Pathological Classification. Heart. 2004;90:123–5. doi: 10.1136/hrt.2003.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular Obstruction and the No-Reflow Phenomenon after Percutaneous Coronary Intervention. Circulation. 2008;117:3152–6. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- [8].Dong-Bao L, Qi H, Zhi L, Wei-Ying J. Predictors and Long-Term Prognosis of Angiographic Slow/No-Reflow Phenomenon During Emergency Percutaneous Coronary Intervention for ST-Elevated Acute Myocardial Infarction. Clin Cardiol. 2010;33:E7–12. doi: 10.1002/clc.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karimianpour A, Maran A. Advances in Coronary No-Reflow Phenomenon-A Contemporary Review. Curr Atheroscler Rep. 2018;20:44. doi: 10.1007/s11883-018-0747-5. [DOI] [PubMed] [Google Scholar]
- [10].Gupta S, Gupta M. No Re-Flow Phenomenon in Percutaneous Coronary Interventions in ST-Segment Elevation Myocardial Infarction. Indian Heart J. 2016;68:539–51. doi: 10.1016/j.ihj.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang K, Zhang J, Zhang N, Shen Y, Wang L, Gu R, et al. Combined Primary PCI with Multiple Thrombus Burden Reduction Therapy Improved Cardiac Function in Patients with Acute Anterior Myocardial Infarction. Int Heart J. 2019;60:27–36. doi: 10.1536/ihj.18-064. [DOI] [PubMed] [Google Scholar]
- [12].Zhou H, He XY, Zhuang SW, Zhuang SW, Wang J, Lai Y, et al. Clinical and Procedural Predictors of No-Reflow in Patients with Acute Myocardial Infarction after Primary Percutaneous Coronary Intervention. World J Emerg Med. 2014;5:96–102. doi: 10.5847/wjem.j.issn.1920-8642.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J, Chen Y, Wang C, Yang XC, Zhu XL, Zhou ZQ. Development and Validation of a Clinical Risk Score Predicting the No-Reflow Phenomenon in Patients Treated with Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction. Cardiology. 2013;124:153–60. doi: 10.1159/000346386. [DOI] [PubMed] [Google Scholar]
- [14].Bouleti C, Mewton N, Germain S. The No-Reflow Phenomenon:State of the Art. Arch Cardiovasc Dis. 2015;108:661–74. doi: 10.1016/j.acvd.2015.09.006. [DOI] [PubMed] [Google Scholar]
- [15].Watanabe Y, Sakakura K, Taniguchi Y, Yamamoto KK, Wada H, Momomura S, et al. Determinants of Slow Flow in Percutaneous Coronary Intervention to the Culprit Lesion of Non-ST Elevation Myocardial Infarction. Int Heart J. 2018;59:1237–45. doi: 10.1536/ihj.18-050. [DOI] [PubMed] [Google Scholar]
- [16].Fabris E, Kilic S, Schellings DA, Berg JM, Kennedy MW, van Houwelingen KG, et al. Long-Term Mortality and Prehospital Tirofiban Treatment in Patients with ST Elevation Myocardial Infarction. Heart. 2017;103:1515–20. doi: 10.1136/heartjnl-2017-311181. [DOI] [PubMed] [Google Scholar]
- [17].Pekdemir H, Polat G, Cin VG, Camsari A, Cicek D, Akkus MN, et al. Elevated Plasma Endothelin-1 Levels in Coronary Sinus During Rapid Right Atrial Pacing in Patients with Slow Coronary Flow. Int J Cardiol. 2004;97:35–41. doi: 10.1016/j.ijcard.2003.06.025. [DOI] [PubMed] [Google Scholar]
- [18].Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood Platelet Count and Function are Related to Total and Cardiovascular Death in Apparently Healthy Men. Circulation. 1991;84:613–7. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- [19].Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schömig A, et al. Relationship Between Platelet Count and 30-Day Clinical Outcomes after Percutaneous Coronary Interventions. Pooled Analysis of Four ISAR Trials. Thromb Haemost. 2007;98:852–7. [PubMed] [Google Scholar]
- [20].Vidwan P, Lee S, Rossi JS, Stouffer GA. Relation of Platelet Count to Bleeding and Vascular Complications in Patients Undergoing Coronary Angiography. Am J Cardiol. 2010;105:1219–22. doi: 10.1016/j.amjcard.2009.12.035. [DOI] [PubMed] [Google Scholar]
- [21].Ommen SR, Hammill SC, Gibbons RJ. The Relative Lymphocyte Count Predicts Death in Patients Receiving Implantable Cardioverter Defibrillators. Pacing Clin Electrophysiol. 2002;25:1424–8. doi: 10.1046/j.1460-9592.2002.01424.x. [DOI] [PubMed] [Google Scholar]
- [22].Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of Resected Ampullary Adenocarcinoma by Preoperative Serum CA19-9 Levels and Platelet-Lymphocyte Ratio. J Gastrointest Surg. 2008;12:1422–8. doi: 10.1007/s11605-008-0554-3. [DOI] [PubMed] [Google Scholar]
- [23].Wang D, Yang JX, Cao DY, Wan XV, Feng FZ, Huang HF, et al. Preoperative Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios as Independent Predictors of Cervical Stromal Involvement in Surgically Treated Endometrioid Adenocarcinoma. Onco Targets Ther. 2013;6:211–6. doi: 10.2147/OTT.S41711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kaya MG, Uyarel H, Akpek M, Kalay N, Ergelen M, Ayhan E, et al. Prognostic Value of Uric Acid in Patients with ST-Elevated Myocardial Infarction Undergoing Primary Coronary Intervention. Am J Cardiol. 2012;109:486–91. doi: 10.1016/j.amjcard.2011.09.042. [DOI] [PubMed] [Google Scholar]
- [25].Akpek M, Kaya MG, Yarlioglues M, Dogdu O, Ardic I, Sahin O, et al. Relationship Between Platelet Indices and Spontaneous Echo Contrast in Patients with Mitral Stenosis. Eur Echocardiogr. 2011;12:865–70. doi: 10.1093/ejechocard/jer159. [DOI] [PubMed] [Google Scholar]
- [26].Kaya MG, Akpek M, Elcik D, Kalay N, Yarlioglues M, Koc F, et al. Relation of Left Atrial Spontaneous Echocardiographic Contrast in Patients with Mitral Stenosis to Inflammatory Markers. Am J Cardiol. 2012;109:851–5. doi: 10.1016/j.amjcard.2011.11.010. [DOI] [PubMed] [Google Scholar]
- [27].Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, Gerger A, et al. The Elevated Preoperative Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis in Breast Cancer Patients. Br J Cancer. 2014;110:2524–30. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera Badillo FE, Hermanns T, et al. Prognostic role of Platelet to Lymphocyte Ratio in Solid Tumors:A Systematic Review and Meta-Analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–12. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- [29].Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, et al. Increased Platelet Reactivity and Circulating Monocyte-Platelet Aggregates in Patients with Stable Coronary Artery Disease. J Am Coll Cardiol. 1998;31:352–8. doi: 10.1016/s0735-1097(97)00510-x. [DOI] [PubMed] [Google Scholar]
- [30].Ault KA, Cannon CP, Mitchell J, Cahan J, Tracy RP, Novotny WF, et al. Platelet Activation in Patients after an Acute Coronary Syndrome:Results from the TIMI-12 Trial. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1999;33:634–9. doi: 10.1016/s0735-1097(98)00635-4. [DOI] [PubMed] [Google Scholar]
- [31].Kurtul A, Murat SN, Yarlioglues M, Duran M, Ergun G, Acikgoz SK, et al. Association of Platelet-to-Lymphocyte Ratio with Severity and Complexity of Coronary Artery Disease in Patients with Acute Coronary Syndromes. Am J Cardiol. 2014;114:972–8. doi: 10.1016/j.amjcard.2014.07.005. [DOI] [PubMed] [Google Scholar]
- [32].Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as Predictors of Vascular Risk:Is there a Practical Index of Platelet Activity?Clin Appl Thromb Hemost. 2003;9:177–90. doi: 10.1177/107602960300900301. [DOI] [PubMed] [Google Scholar]
- [33].Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the Blood Lymphocyte Count in Predicting Recurrent Instability and Death in Patients with Unstable Angina Pectoris. Am J Cardiol. 2000;86:449–51. doi: 10.1016/s0002-9149(00)00963-2. [DOI] [PubMed] [Google Scholar]
- [34].Li H, Zhou Y, Ma Y, Han S, Zhou L. The Prognostic Value of the Platelet:Lymphocyte Ratio in Acute Coronary Syndrome:A Systematic Review and Meta-Analysis. Kardiol Pol. 2017;75:666–73. doi: 10.5603/KP.a2017.0068. [DOI] [PubMed] [Google Scholar]
- [35].Azab B, Shah N, Akerman M, Han S, Zhou L. Value of Platelet/Lymphocyte Ratio as a Predictor of All-Cause Mortality after Non-ST-Elevation Myocardial Infarction. J Thromb Thrombolysis. 2012;34:326–34. doi: 10.1007/s11239-012-0718-6. [DOI] [PubMed] [Google Scholar]
- [36].Osadnik T, Wasilewski J, Lekston A, Strzelczyk J, Kurek A, Gonera M, et al. The Platelet-to-Lymphocyte Ratio as a Predictor of All-Cause Mortality in Patients with Coronary Artery Disease Undergoing Elective Percutaneous Coronary Intervention and Stent Implantation. J Saudi Heart Assoc. 2015;27:144–51. doi: 10.1016/j.jsha.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cho KI, Ann SH, Singh GB, Her AV, Shin ES. Combined Usefulness of the Platelet-to- Lymphocyte Ratio and the Neutrophil-to-Lymphocyte Ratio in Predicting the Long-Term Adverse Events in Patients Who Have Undergone Percutaneous Coronary Intervention with a Drug-Eluting Stent. PLoS One. 2015;10:e0133934. doi: 10.1371/journal.pone.0133934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Akasaka T, Yoshida K, Kawamoto T, Kaji S, Ueda Y, Yamamuro A, et al. Relation of Phasic Coronary Flow Velocity Characteristics with TIMI Perfusion Grade and Myocardial Recovery after Primary Percutaneous Transluminal Coronary Angioplasty and Rescue Stenting. Circulation. 2000;101:2361–7. doi: 10.1161/01.cir.101.20.2361. [DOI] [PubMed] [Google Scholar]
- [39].Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, et al. Distal Microcirculatory Protection During Percutaneous Coronary Intervention in Acute ST-Segment Elevation Myocardial Infarction:A Randomized Controlled Trial. JAMA. 2005;293:1063–72. doi: 10.1001/jama.293.9.1063. [DOI] [PubMed] [Google Scholar]


