Abstract
Behavioral neuroendocrinology has a rich history of using diverse model organisms to elucidate general principles and evolution of hormone-brain-behavior relationships. The oxytocin and vasopressin systems have been studied in many species, revealing their role in regulating social behaviors. Oxytocin and vasopressin receptors show remarkable species and individual differences in distribution in the brain that have been linked to diversity in social behaviors. New technologies allow for unprecedented interrogation of the genes and neural circuitry regulating behaviors, but these approaches often require transgenic models and are most often used in mice. Here we discuss seminal findings relating the oxytocin and vasopressin systems to social behavior with a focus on non-traditional animal models. We then discuss the potential of using CRISPR/Cas9 genome editing to examine the roles of genes and enable circuit dissection, manipulation and activity monitoring of the oxytocin and vasopressin systems. We believe that it is essential to incorporate these genetic and circuit level techniques in comparative behavioral neuroendocrinology research to ensure that our field remains innovative and attractive for the next generation of investigators and funding agencies.
Keywords: CRISPR, neuropeptides, social behavior, prairie vole, genome editing, vasopressin receptor, oxytocin receptor, species differences, optogenetics, chemogenetics
Graphical abstract
1. Introduction
1.1. The value of a comparative perspective
Behavioral neuroendocrinology has a rich tradition using comparative approaches to discover mechanisms giving rise to diversity in behavior and to elucidate general principles of hormone-brain-behavior relationships. Social behaviors are particularly diverse among vertebrates and provide exciting opportunities to investigate the evolution of proximate mechanisms of behavior. The oxytocin (OXT) and arginine vasopressin (AVP) systems have emerged as key regulators of social and reproductive behaviors and variation in these systems contribute to the extraordinary diversity in social behaviors across and within species. Indeed, both neuropeptide systems are potential pharmacological targets or biomarkers for disorders of social function, including autism (DeMayo et al. 2019; Parker et al. 2019; Oztan et al. 2020). Much of the growth in interest in the OXT/AVP systems in relation to behavior is attributable to the success of the comparative approach, with key discoveries being made in sheep, hamsters, voles and finches. Research into OXT/AVP systems provides an example of the critical value of maintaining diversity in study organisms in behavioral neuroendocrinology. In this review, we will highlight the contribution of comparative research and innovation in the surge in interest in OXT/AVP systems and social behavior, and discuss our vision to ensure that OXT/AVP research maintains prominence in behavioral neuroendocrinology for decades to come.
Seventy years ago, Frank Beach warned that the intensive focus on the albino rat was detrimental to comparative psychology (Beach 1950). As noted by several colleagues, the concern of excessive focus on few model organisms (e.g. mice) and behaviors is no less a concern for behavioral neuroendocrinology today (Phelps et al. 2010; Lambert et al. 2019; Thompson 2020). Indeed, modern behavioral neuroscience, like neuroscience in general, focuses tremendous resources and attention to a few model organisms, and we and others have argued for preserving diversity in study species (Blumstein et al. 2010; Yartsev 2017; Johnson and Young 2018; Gallant and O’Connell 2020). After all, some of the most important discoveries of neuroscience were made using non-traditional species, including action potentials in giant squid neurons, long-term potentiation in Aplysia, and light-sensitive proteins from alga for optogenetics. Exciting advances have been made in understanding neural mechanisms underlying motivated behavior, fear, and learning and memory using precisely defined conditioning paradigms, albeit in relatively few species. This is understandable considering the ease by which behavior in these paradigms is quantified (e.g. number of lever presses, nose pokes, startle intensity), and tractability of certain model species to technically challenging techniques, such as cell type specific circuit manipulation (Datta et al. 2019). Research in a greater variety of animal species provides opportunities to study a broader range of behaviors relevant to humans, such as pair bonding, paternal care, empathy and grieving (Bosch et al. 2016; Burkett et al. 2016; Bendesky et al. 2017; Walum and Young 2018). We believe there is tremendous interest among young investigators for utilizing non-traditional species to study a diversity of behaviors. However, unprecedented advances in technology to manipulate genes and circuits in select model organisms is equally enticing to next generation scientists. A solution to this problem is to adapt these technologies to diverse species.
1.2. Technological innovation in behavioral neuroendocrinology
Behavioral neuroendocrinology has historically used a wide variety of model organisms and approaches to study hormone-brain-behavior relationships, by focusing on hormones and neuropeptides. Success in the field has been fueled by technical developments, such as hormone assays, autoradiography, and lesions, which are applicable to many species (Balthazart 2020). In the 1990’s, genetic engineering in mice emerged, resulting in increasing focus on mice to understand molecular mechanisms of behavior (Rissman et al. 1999). However, we are optimistic that there will be a resurgence of comparative studies in behavioral neuroscience now that techniques such as viral-mediated gene transfer and CRISPR-mediated genome editing make genetic and circuit manipulation possible in virtually any species (Amadei et al. 2017; Tanaka et al. 2018; Horie et al. 2019; Yokoi et al. 2020). OXT/AVP behavioral neuroendocrinology is poised to combine gene/circuit manipulation techniques with comparative neuroscience and remain at the forefront of behavioral neuroscience. We predict the study of other neuroendocrine systems will thrive by incorporation of these new technologies, keeping our field competitive for funding and attractive for the next generation of scientists. We highlight selected past accomplishments in OXT/AVP comparative research and highlight some future directions we feel our field is heading. We identify genetic, circuit and behavioral technical opportunities that will strengthen OXT/AVP research in diverse species. We hope our contribution to this special issue (McCormick 2020) will inspire young aspiring and established investigators alike to invigorate comparative behavioral neuroendocrinology, keeping the field at the forefront of innovation in neuroscience.
2. Oxytocin, vasopressin and social behavior
2.1. A brief summary of findings
The first studies linking OXT to social behavior demonstrated that in parallel to stimulating birth and milk ejection during lactation, OXT initiated maternal responsiveness and maternal bonding in rats and sheep (Pedersen et al. 1982; Kendrick et al. 1987). Later studies in monogamous prairie voles demonstrated a role of OXT in pair bonding as well (Williams et al. 1994; Young and Wang 2004). In parallel, AVP was found to regulate scent marking and territorial aggression in hamsters (Albers 2012), and selective aggression and pair bonding in prairie voles (Winslow et al. 1993). These neuropeptide systems were then linked to vocal communication in fish (Goodson and Bass 2000), and flock size preferences in finches (Goodson et al. 2009). More recent studies have explored the roles of these peptides in consoling behavior in voles (Burkett et al. 2016), social vigilance in Peromyscus californicus (Duque-Wilckens et al. 2018) and mate choice in medaka fish (Yokoi et al. 2015; Yokoi et al. 2020). We will not attempt to summarize all studies linking these neuropeptides to social behaviors, but highlight a few concepts emerging in the last decades.
2.2. Receptor distribution and diversity in social behavior
Prairie voles have been particularly useful for understanding the role of OXT and AVP in regulating social behaviors. Prairie vole are socially monogamous while meadow voles are not, and OXT and AVP promote pair bonding in prairie voles (Johnson and Young 2015; Walum and Young 2018). While the voles have similar distributions of OXT and AVP in the brain, they differ markedly in distribution of receptors that respond to the peptides, OXTR and AVPR1a, respectively (Young and Wang 2004). The first study to demonstrate a relationship between brain receptor distribution and behavior used transgenic technology to create mice that express prairie vole Avpr1a in a pattern resembling that of vole brain. These mice responded to AVP with increased affiliative behavior (Young et al. 1999). In one of the first studies to use viral vector gene transfer to study behavior, we showed that expressing the prairie vole Avpr1a in ventral pallidum of meadow voles elicited partner preference behavior (Lim et al. 2004). Subsequent viral vector mediated over-expression or inhibition studies in voles confirmed the role of variation in receptor expression in regulating pair bonding behaviors (Gobrogge et al. 2009; Keebaugh and Young 2011; Barrett et al. 2013; Keebaugh et al. 2015). Likewise, oxytocin receptors in lateral septum correlates with flock size preference in different species of estrildid finches (Goodson et al. 2009). Within prairie voles, robust individual variation in OXTR density in the nucleus accumbens (NAc) affects resilience to early-life neglect on later life pair bonding (Barrett et al. 2015), while variation in AVPR1A expression in retrosplenial cortex predicts space use and sexual fidelity in males in seminaturalistic enclosures (Okhovat et al. 2015). Genetics studies revealed a role for genetic polymorphisms in generating individual variation in OXTR and AVPR1A density and social behaviors in voles (Hammock and Young 2005; Okhovat et al. 2015; King et al. 2016). While genetic regulation of OXT and AVP genes are highly conserved, such that puffer fish genome sequences are faithfully transcribed in OXT neurons of transgenic mice (Gilligan et al. 2003), expression patterns of receptor genes are much more labile, and subject to natural selection through accumulation of mutations, a mechanism key to diversity in social behaviors.
Receptor autoradiography has been used to characterize OXTR and AVPR1A distribution in the brains of a variety of species (Beery et al. 2008), lending support that diversity in brain receptor expression contributes to behavioral diversity. Comparative studies show that OXTR/AVPR1a distribution in sensory areas is associated with the dominant modality of social communication, such that OXTR is distributed in olfactory pathways in rodents, but in visual pathways in primates (Freeman et al. 2014; Freeman et al. 2014; Freeman and Young 2016). The mapping of OXTR and AVPR1a distribution guided site-specific behavioral pharmacology, ultimately leading to a better understanding of the circuitry involved in many social behaviors. However, one limitation of OXTR/AVPR1A research is the lack of antibodies that give reliable, reproducible staining across species to facilitate molecular and cellular characterization of peptide sensitive neurons, limiting understanding of OXT/AVP sensitive circuits responsible for regulating behaviors.
2.3. Social salience and the flow of social information
One theme that has emerged from OXT/AVP research is that these peptides modulate the salience and reinforcing value of social stimuli (Walum and Young 2018). OXT acts in olfactory bulb of mice to increase signal to noise of social cues, and in the auditory cortex to enhance response to pup vocalizations (Marlin et al. 2015; Oettl et al. 2016). OXT interacts with dopamine and serotonin systems to facilitate social reward (Dolen et al. 2013; Hung et al. 2017; Borland et al. 2018). These properties make OXT an attractive candidate for improving social cognition in autism (DeMayo et al. 2019). These peptides modulate a highly conserved ‘social behavior network’, or ‘social salience neural network’, consisting of several interconnected brain areas across vertebrate species Goodson. OXTR signaling facilitates the flow of sociosensory information across this network by coordinating neural activity across the network (Johnson et al. 2016; Johnson et al. 2017; Johnson and Young 2017). Thus, OXT has been called the “grease” of the social brain (Walum and Young 2018). Variation in OXTR and AVPR1a expression across these networks likely produces variation in social behaviors between physiological states and across species.
OXT and AVP modulate social behaviors in an often sex-, context-, and species-dependent manner. They facilitate parental nurturing and maternal aggression (Bosch and Neumann 2012), social approach and social vigilance (Duque-Wilckens et al. 2018; Williams et al. 2020) depending on context and site of action. The ability to manipulate specific circuits would enhance our ability to investigate the full complexity of OXT/AVP action. Some genetic tools have become available to manipulate these neuropeptide systems, including viral vectors that faithfully drive transgene expression in OXT neurons under the control of the OXT promoter. These tools have been used to optogenetically stimulate OT neurons in Mandarin voles (He et al. 2019) and to label OXT projections in prairie voles (Bosch et al. 2016). Unfortunately, viral vectors to drive transgene expression selectively in OXTR and AVPR1a neurons are not available, and likely will not become available, because of the nature of the regulatory control of receptor expression.
3. Future directions with a focus in genome editing
3.1. The challenge and a solution
There are many areas of potential growth in OXT/AVP research, including epigenetics, and imaging (Perkeybile et al. 2019; Andari et al. 2020). But here we focus on one advance that we believe has the greatest potential for accelerating OXT/AVP comparative research in this age of circuit neuroscience; genome editing. The ability to manipulate or monitor activity of specific cell types in mice has accelerated discovery in behavioral neuroscience and these techniques are attracting the brightest future researchers to mouse behavioral neuroscience. The lack of ability to manipulate genomes of non-traditional organisms is perhaps the biggest threat to comparative behavioral neuroendocrinology. We are confident that this limitation can now be overcome. Recent advances in CRISPR/Cas9 technologies now enable the targeted editing of genomes in a wide variety of species and could be a boon for behavioral neuroendocrinology. In the remainder of this review, we describe the use of genomic editing in non-traditional models and detail how genomic editing in combination with other innovative approaches can facilitate mechanistic understanding of OXT/AVP signaling on genetic and circuit levels. While we focus on OXT and AVP, these principles apply to all behavioral neuroendocrinology.
3.2. CRISPR/Cas9 and comparative research
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 technology provides a means for introducing errors or specific DNA sequences in a targeted manner in a range of species through injection of proteins and targeting nucleic acids into embryos (Figure 1A) (Jinek et al. 2012; Cong et al. 2013). In species where embryos are not easily accessible, like birds or reptiles, the generation of transgenic animals is still challenging (London 2020).
Figure 1.
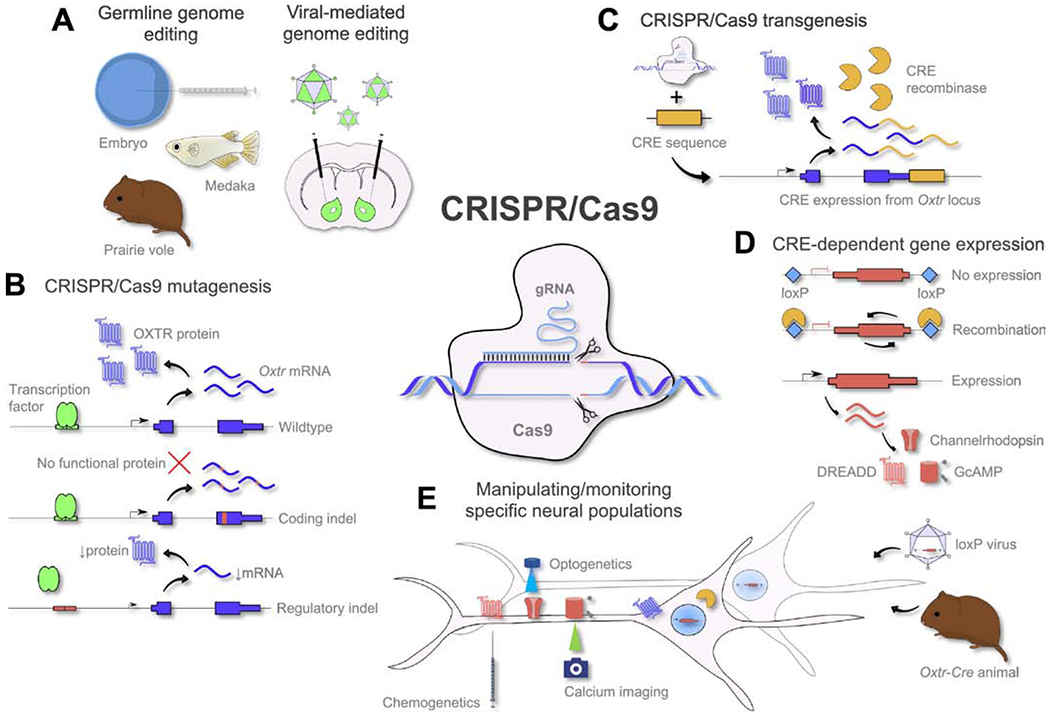
Use of the CRISPR/Cas9 toolkit for comparative studies of OXT and AVP signaling. A) (Left) CRISPR/Cas9 components can be injected into the embryo to generate germline transgenic animals, as has been done for prairie vole (Horie et al. 2018) and medaka fish (Yonoi et al. 2020). (Right) CRISPR/Cas9 components can be packaged into viral vector to enable genomic editing of neural population with spatiotemporal control. B) After induction of double-stranded breaks in the DNA, non-perfect repair mechanisms result in small indels, that can be used to disrupt the coding sequence of Oxtr, or regulatory elements that control Oxtr expression. C) CRISPR/Cas9 can be used to place the Cre sequence after Oxtr or Avpr1a to generate Oxtr-Cre or Avpr1a-Cre transgenic animals. D) CRE recombinase inverts genetic sequences that are flanked by loxP sites, and is exploited to specifically express DREADD, channelrhodopsin and GcAMP in CRE-positive cells. E) Specific expression of transgenes allows for the manipulation and monitoring of neural circuits with (sub)cellular resolution.
CRISPR/Cas9 technology is derived from the innate adaptive immune system of prokaryotes that serves to deactivate foreign nucleic acid sequences. The CRISPR/Cas9 system consists of a guide RNA (gRNA) homologous to the target gene that guides the Cas9, an RNA guided endonuclease, to any genomic locus flanked by the sequence NGG. In its simplest form, the CRISPR/Cas9 system induces double-stranded breaks in DNA, leading to small insertions and deletions (indels, typically <10 nucleotides), because of imperfect DNA repair. CRISPR/Cas9 allows editing of coding and non-coding sequences, to create null mutations (knockout), or disrupt transcription factor binding cites (Figure 1B). CRISPR/Cas9 also enables the insertion of genetic sequences in a targeted manner (knockin) (Figure 1C).
3.2. Knockouts and Cre Recombinase Knockins
The construction of transgenic animals by CRISPR/Cas9 could greatly facilitate the comparative study of OT/AVP function on a molecular level. CRISPR/Cas9 has been used to generate Oxtr knockout prairie voles and medaka fish (Horie et al. 2019; Yokoi et al. 2020), providing insight into OXT function in these species. Male medaka compete for females and court indiscriminately, while females selectively choose mates. Oxtr mutant males prefer familiar mates and females to mate indiscriminately, suggesting OXTR facilitates adaptive mate choices in this species.
Cre recombinase knockin mice are useful for expressing transgenes in specific cell types, allowing cell-type specific circuit manipulation. An exciting use of CRISPR/Cas9 is to insert Cre coding sequence in tandem with OXT/AVP or OXTR/AVPR1a so that Cre recombinase is expressed only cells that express the targeted gene (Figure 1C). By injecting viral vectors with Cre-dependent transgenes (e.g. fluorescent proteins, channel rhodopsin, DREADDs, GcAMP) into the brain of Cre transgenic animals, the transgene will only be expressed in neurons that express the targeted gene (e.g. Oxtr, Figure 1D). An Oxtr-Cre animal would enable the expression of any Cre-dependent gene only in Oxtr neurons in any brain region (Figure 1E). When crossed with a Cre-dependent reporter transgenic animal (e.g. GFP), all Oxtr neurons in the brain could be mapped (Hidema et al. 2016; Newmaster et al. 2020).
While CRISPR/Cas9 facilitates the generation of transgenic animals, it remains a laborious task requiring in vitro fertilization technology. An alternative approach that is viable for a broader range of species is to deliver CRISPR/Cas9 components via viral vectors directly into the brain (Swiech et al. 2015). Adeno-associated viral vector (AAV) mediated CRISPR/Cas9 offers spatiotemporal control over genomic editing, depending on injection parameters, but provides less control over the cell types targeted, although specific promoters and AAV serotypes may bias editing to selected cell types (e.g. glia vs neurons). To illustrate this approach, we generated AAV9-CRISPR/Cas9 and to target the prairie vole Oxtr coding sequence. Six weeks after injection we observed ~60% reduction in OXTR binding using receptor autoradiography compared to using a control guide RNA (gRNA) vector (Figure 2).
Figure 2.
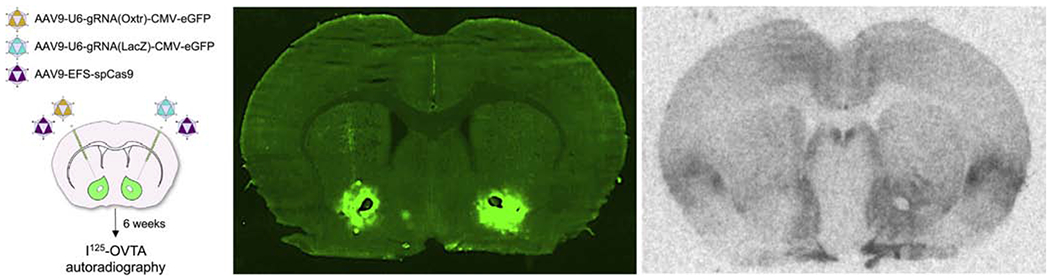
AAV-mediated CRISPR/Cas9 can be used in adult prairie vole to reduce OXTR expression. (Left) Schematic illustrating the procedure in which AAV-CRISPR/Cas9 was used to perturb OXTR expression in the nucleus accumbens. (Middle) Image of untreated, fresh-frozen slice depicting viral-mediated eGFP expression. (Right) I125-OVTA autoradiography was performed on the adjacent slice, showing a reduction in OXTR binding after CRISPR/Cas9 mutagenesis (left hemisphere, arrow) in comparison to the control site (right hemisphere).
3.3. Probing genetic regulation of Oxtr and Avpr1a
Human genetic studies have linked single nucleotide polymorphisms (SNPs) in OXTR and AVPR1A with variation in social behavior, brain function and psychiatric disorders (Skuse et al. 2014; LoParo and Waldman 2015; Hernandez et al. 2020). In prairie voles, multiple polymorphisms in noncoding regions of Avpr1a and Oxtr genes robustly predict region-specific expression of brain receptors and social behaviors (Okhovat et al. 2015; King et al. 2016). An important question with translational implications is which SNPs are functional and how do they alter expression in brain. CHIP-seq to determine SNP alignment with chromatin enhancers and repressors based on histone modification, and ATAC-seq to align polymorphisms with accessible chromatin can provide evidence of which SNPs are most likely functionally influencing expression. Viral-mediated CRISPR/Cas9 is an ideal tool to prove the causal relationship between non-coding SNPs and expression of Oxtr and Avpr1a, as CRISPR/Cas9 can be adapted to induce double stranded breaks at the SNP locus with the appropriate guide RNA. By introducing indels at the candidate SNP, it would be possible ascertain its effect on expression. This approach could be useful to disrupt transcription factor binding sites in putative regulatory regions to study neuropeptide or receptor regulation (Figure 1B).
3.4. Labeling OXT/AVP system circuits
For many species, we know where OXT and AVP act to mediate a behavior but we know very little about circuits involved. The development of CRISPR Oxtr-Cre or Avpr1a-Cre animals would allow the tracing of the inputs and projections of any particular receptor population. For example, by injection Cre-dependent green fluorescent protein (GFP) viral vector into NAc of Oxtr-Cre animals, one could map the projections of NAc OXTR neurons. Alternatively, infusion of Cre-dependent retrograde viral tracers into NAc would reveal all OXTR neurons that project to NAc. This information is valuable for going beyond individual brain regions and considering circuits in relation to neuropeptide regulation of behavior.
3.5. Monitoring Circuit Activity
Ultimately, it will be important to understand how OT/AVP signaling influences circuit activity to modulate behaviors. Fiber photometry and intracranial miniscopes to monitor activity of neurons by detecting fluorescence from transgenically expressed Ca2+-sensors (e.g. GcAMPs) are revolutionizing behavioral neuroscience from a circuit perspective (Chen et al. 2013). By injecting AAV’s expressing GcAMPs into specific brain regions it is possible to monitor cellular activity with a high degree of spatial and temporal resolution (Figure 1D, E). This technique has been used to investigate ensemble activity of NAc neurons in relation to partner versus stranger approach in prairie voles (Scribner et al. 2020). Sensors for detecting neurotransmitters in vivo using similar techniques are also rapidly becoming available (Ma et al. 2018; Sun et al. 2018).
When CRE-dependent GcAMP AAV’s are combined with Cre transgenic animals, it becomes possible to monitor activity of specific cell types during behavior or stimulation. This approach was recently used in Oxt-Cre mice to monitor activity of hypothalamic OXT neurons during social interactions (Resendez et al. 2020). It would be feasible to monitor the activity of any population of Oxtr neurons in voles using CRISPR generated Oxtr-Cre animals.
3.6. Manipulating neural activity and behavioral outcomes
Recent developments in opto- and chemogenetics now make it possible to activate and/or inhibit neural network activity in behaving animals. Optogenetics works by activating light-sensitive membrane proteins by shining light via optical cables on a brain region, thereby activating or inhibiting neural firing (Boyden et al. 2005) . Chemogenetics using DREADDs, works by activating engineered muscarinic receptors (coupled to either Gs,Gq or Gi-proteins) with a physiologically “inactive” form of clozapine, clozapine-N-oxide (CNO) (Armbruster et al. 2007). CNO can be infused systemically, or locally and is extremely adaptable to behavioral paradigms. Both opto- and chemogenetics are easily combined with viral-mediated approaches, and are adaptable to non-traditional animal models (Figure 1D, E). We recently showed that excitatory prefrontal cortex neurons modulate NAc neuron activity to promote pair bonding in prairie voles (Amadei et al. 2017). These techniques become more powerful through manipulation of specific neurons using selective promoters or Cre transgenic animals. Numerous studies have used opto- or chemogenetics to manipulate OXT neurons in mice (Oettl et al. 2016; Ferretti et al. 2019; Resendez et al. 2020) and mandarin vole (He et al. 2019) using AAV and an OXT promoter to limit expression to OXT neurons. When combined with CRISPR generated Oxtr-Cre or Avpr1a-cre transgenics, it is possible to activate of inhibit OXTR or AVPR1a neurons (Tan et al. 2019).
Conclusion
OXT/AVP behavioral neuroendocrinology has benefited tremendously from innovative, comparative research. The field must continue to embrace a diversity of model organisms while at the same time take advantage of the revolutionary neuroscience tools that allow precise interrogation of genes and circuits. We hope that CRISPR/Cas9 will enhance the genetic tractability of many non-traditional animal models and attract the most talented young neuroscientists to the behavioral neuroendocrinology tradition.
Highlights.
Comparative approaches invigorate behavioral neuroendocrinology
Important discoveries of OXT and AVP function were made in non-traditional models
CRISPR techniques allows targeted genome editing in non-traditional models
CRISPR allows interrogation of functional polymorphisms regulating Oxtr and Avpr1a
CRISPR transgenics allow manipulation and monitoring of cell-type specific circuits
Acknowledgments
Preparation of this manuscript was supported by a 2017 NARSAD Young Investigator Grant (ID 26058) from the Brain & Behavior Research Foundation to AB and NIH grants P50MH100023, R01MH112788 and R21MH114151 to LJY and OD P51OD011132 to YNPRC.
‘AB is supported by a 2017 NARSAD Young Investigator Grant (ID 26058) from the Brain & Behavior Research Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The author have no conflict of interest to declare.
References cited:
- Albers HE, 2012. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav 61, 283–292. [DOI] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, Ryan SJ, Walum H, Rainnie DG, Young LJ, Liu RC, 2017. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Nishitani S, Kaundinya G, Caceres GA, Morrier MJ, Ousley O, Smith AK, Cubells JF, Young LJ, 2020. Epigenetic modification of the oxytocin receptor gene: implications for autism symptom severity and brain functional connectivity. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 104, 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, 2020. How technical progress reshaped behavioral neuroendocrinology during the last 50 years… and some methodological remarks. Horm Behav 118, 104682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ, 2015. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry 5, e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ, 2013. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav 63, 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA, 1950. The Snark was a Boojum. American Psychologist 5, 115–124. [Google Scholar]
- Beery AK, Lacey EA, Francis DD, 2008. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). J Comp Neurol 507, 1847–1859. [DOI] [PubMed] [Google Scholar]
- Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao S, Peterson BK, He MX, Dulac C, Hoekstra HE, 2017. The genetic basis of parental care evolution in monogamous mice. Nature 544, 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein DT, Ebensperger LA, Hayes LD, Vasquez RA, Ahern TH, Burger JR, Dolezal AG, Dosmann A, Gonzalez-Mariscal G, Harris BN, Herrera EA, Lacey EA, Mateo J, McGraw LA, Olazabal D, Ramenofsky M, Rubenstein DR, Sakhai SA, Saltzman W, Sainz-Borgo C, Soto-Gamboa M, Stewart ML, Wey TW, Wingfield JC, Young LJ, 2010. Toward an integrative understanding of social behavior: new models and new opportunities. Front Behav Neurosci 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Grantham KN, Aiani LM, Frantz KJ, Albers HE, 2018. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology 95, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ, 2016. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID, 2012. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61, 293–303. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K, 2005. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ, 2016. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F, 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Anderson DJ, Branson K, Perona P, Leifer A, 2019. Computational Neuroethology: A Call to Action. Neuron 104, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMayo MM, Young LJ, Hickie IB, Song YJC, Guastella AJ, 2019. Circuits for social learning: A unified model and application to Autism Spectrum Disorder. Neurosci Biobehav Rev 107, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC, 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, Trainor BC, 2018. Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol Psychiatry 83, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti V, Maltese F, Contarini G, Nigro M, Bonavia A, Huang H, Gigliucci V, Morelli G, Scheggia D, Manago F, Castellani G, Lefevre A, Cancedda L, Chini B, Grinevich V, Papaleo F, 2019. Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Curr Biol 29, 1938–1953 e1936. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ, 2014. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology 45, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ, 2014. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus). Neuroscience 273, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ, 2016. Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. J Neuroendocrinol 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant JR, O’Connell LA, 2020. Studying convergent evolution to relate genotype to behavioral phenotype. J Exp Biol 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan P, Brenner S, Venkatesh B, 2003. Neurone-specific expression and regulation of the pufferfish isotocin and vasotocin genes in transgenic mice. J Neuroendocrinol 15, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z, 2009. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A 106, 19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH, 2000. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature 403, 769–772. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA, 2009. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science 325, 862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Young LJ, 2005. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308, 1630–1634. [DOI] [PubMed] [Google Scholar]
- He Z, Young L, Ma XM, Guo Q, Wang L, Yang Y, Luo L, Yuan W, Li L, Zhang J, Hou W, Qiao H, Jia R, Tai F, 2019. Increased anxiety and decreased sociability induced by paternal deprivation involve the PVN-PrL OTergic pathway. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Lawrence KE, Padgaonkar NT, Inada M, Hoekstra JN, Lowe JK, Eilbott J, Jack A, Aylward E, Gaab N, Van Horn JD, Bernier RA, McPartland JC, Webb SJ, Pelphrey KA, Green SA, Geschwind DH, Bookheimer SY, Dapretto M, 2020. Imaging-genetics of sex differences in ASD: distinct effects of OXTR variants on brain connectivity. Transl Psychiatry 10, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidema S, Fukuda T, Hiraoka Y, Mizukami H, Hayashi R, Otsuka A, Suzuki S, Miyazaki S, Nishimori K, 2016. Generation of Oxtr cDNA(HA)-Ires-Cre Mice for Gene Expression in an Oxytocin Receptor Specific Manner. J Cell Biochem 117, 1099–1111. [DOI] [PubMed] [Google Scholar]
- Horie K, Inoue K, Suzuki S, Adachi S, Yada S, Hirayama T, Hidema S, Young LJ, Nishimori K, 2019. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm Behav 111, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dolen G, Malenka RC, 2017. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, Young LJ, 2016. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav 79, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Riefkohl PC, Young LJ, 2017. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm Behav 87, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ, 2015. Neurobiological mechanisms of social attachment and pair bonding. Curr Opin Behav Sci 3, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ, 2017. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev 76, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ, 2018. Evolutionary diversity as a catalyst for biological discovery. Integr Zool 13, 616–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ, 2015. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci 10, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ, 2011. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Hormones and Behavior 60, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, 1987. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology 46, 56–61. [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ, 2016. Variation in the Oxytocin Receptor Gene Predicts Brain Region-Specific Expression and Social Attachment. Biol Psychiatry 80, 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert K, Kent M, Vavra D, 2019. Avoiding Beach’s Boojum Effect: Enhancing bench to bedside translation with field to laboratory considerations in optimal animal models. Neurosci Biobehav Rev 104, 191–196. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ, 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757. [DOI] [PubMed] [Google Scholar]
- London SE, 2020. Gene manipulation to test links between genome, brain and behavior in developing songbirds: a test case. J Exp Biol 223. [DOI] [PubMed] [Google Scholar]
- LoParo D, Waldman ID, 2015. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry 20, 640–646. [DOI] [PubMed] [Google Scholar]
- Ma L, Jongbloets BC, Xiong WH, Melander JB, Qin M, Lameyer TJ, Harrison MF, Zemelman BV, Mao T, Zhong H, 2018. A Highly Sensitive A-Kinase Activity Reporter for Imaging Neuromodulatory Events in Awake Mice. Neuron 99, 665–679 e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’Amour J A, Chao MV, Froemke RC, 2015. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, 2020. Introduction to the special issue: 50th anniversary of Hormones and Behavior: Past accomplishments and future directions in behavioural neuroendocrinology. Horm Behav 122, 104751. [DOI] [PubMed] [Google Scholar]
- Newmaster KT, Nolan ZT, Chon U, Vanselow DJ, Weit AR, Tabbaa M, Hidema S, Nishimori K, Hammock EAD, Kim Y, 2020. Quantitative cellular-resolution map of the oxytocin receptor in postnatally developing mouse brains. Nat Commun 11, 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, Kelsch W, 2016. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron 90, 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM, 2015. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science 350, 1371–1374. [DOI] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Constantino JN, Parker KJ, 2020. Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proc Natl Acad Sci U S A 117, 10609–10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Fung LK, Garner JP, Hardan AY, 2019. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ Jr., 1982. Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, Karaoli T, Gregory SG, Mohammadi N, Epstein L, Bales KL, Connelly JJ, 2019. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps SM, Campbell P, Zheng DJ, Ophir AG, 2010. Beating the boojum: comparative approaches to the neurobiology of social behavior. Neuropharmacology 58, 17–28. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Namboodiri VMK, Otis JM, Eckman LEH, Rodriguez-Romaguera J, Ung RL, Basiri ML, Kosyk O, Rossi MA, Dichter GS, Stuber GD, 2020. Social Stimuli Induce Activation of Oxytocin Neurons Within the Paraventricular Nucleus of the Hypothalamus to Promote Social Behavior in Male Mice. J Neurosci 40, 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC, 1999. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res 835, 80–90. [DOI] [PubMed] [Google Scholar]
- Scribner JL, Vance EA, Protter DSW, Sheeran WM, Saslow E, Cameron RT, Klein EM, Jimenez JC, Kheirbek MA, Donaldson ZR, 2020. A neuronal signature for monogamous reunion. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimaki T, Binder EB, Young LJ, 2014. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci U S A 111, 1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G, Li Y, 2018. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174, 481–496 e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F, 2015. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol 33, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Singhal SM, Harden SW, Cahill KM, Nguyen DM, Colon-Perez LM, Sahagian TJ, Thinschmidt JS, de Kloet AD, Febo M, Frazier CJ, Krause EG, 2019. Oxytocin Receptors Are Expressed by Glutamatergic Prefrontal Cortical Neurons That Selectively Modulate Social Recognition. J Neurosci 39, 3249–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sun F, Li Y, Mooney R, 2018. A mesocortical dopamine circuit enables the cultural transmission of vocal behaviour. Nature 563, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, 2020. An updated field guide for snark hunting: Comparative contributions to behavioral neuroendocrinology in the era of model organisms. Horm Behav 122, 104742. [DOI] [PubMed] [Google Scholar]
- Walum H, Young LJ, 2018. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci 19, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Duque-Wilckens N, Ramos-Maciel S, Campi KL, Bhela SK, Xu CK, Jackson K, Chini B, Pesavento PA, Trainor BC, 2020. Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS, 1994. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J Neuroendocrinol 6, 247–250. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. [DOI] [PubMed] [Google Scholar]
- Yartsev MM, 2017. The emperor’s new wardrobe: Rebalancing diversity of animal models in neuroscience research. Science 358, 466–469. [DOI] [PubMed] [Google Scholar]
- Yokoi S, Naruse K, Kamei Y, Ansai S, Kinoshita M, Mito M, Iwasaki S, Inoue S, Okuyama T, Nakagawa S, Young LJ, Takeuchi H, 2020. Sexually dimorphic role of oxytocin in medaka mate choice. Proc Natl Acad Sci U S A 117, 4802–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Okuyama T, Kamei Y, Naruse K, Taniguchi Y, Ansai S, Kinoshita M, Young LJ, Takemori N, Kubo T, Takeuchi H, 2015. An essential role of the arginine vasotocin system in mate-guarding behaviors in triadic relationships of medaka fish (Oryzias latipes). PLoS Genet 11, e1005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR, 1999. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400, 766–768. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, 2004. The neurobiology of pair bonding. Nat Neurosci 7, 1048–1054. [DOI] [PubMed] [Google Scholar]


