Abstract
Objective
To investigate the efficacy and safety of pembrolizumab in women with recurrent small cell neuroendocrine tumors of the lower genital tract.
Methods
We conducted an open-label, investigator-initiated phase II basket trial of pembrolizumab 200 mg intravenously every 3 weeks in patients with rare tumors (ClinicalTrials.gov: NCT02721732). The trial had prespecified cohorts, including small cell malignancies of extrapulmonary origin. Eligibility criteria included disease progression during standard treatment in the 6 months before study enrollment. Patients were enrolled from February 2017 to February 2019. The primary endpoint was the proportion of patients alive without progression at 27 weeks. Response to pembrolizumab was evaluated every 9 weeks (3 cycles) with radiographic imaging.
Results
Seven women with gynecologic extrapulmonary small cell carcinoma were enrolled, 6 with cervical and 1 with vulvar carcinoma. No patient was progression free at 27 weeks. At first radiologic assessment, 1 patient had stable disease, while 6 had progression. The single patient with stable disease at 6 weeks had disease progression at 14 weeks. The median progression-free interval was 2.1 months (range 0.8–3.3 months). Severe treatment-related adverse events (≥grade 3) were seen in 2 of 7 patients (29%); 1 patient had grade 3 asymptomatic elevation of serum alkaline phosphatase, and 1 had grade 3 asymptomatic elevation of serum alanine aminotransferase.
Conclusions
Pembrolizumab alone showed minimal activity in women with recurrent small cell neuroendocrine tumors of the lower genital tract. Treatment was well tolerated in the majority of study participants, and the rate of severe adverse events was low.
Introduction
High-grade neuroendocrine carcinomas of the cervix (small cell, large cell, and undifferentiated) account for < 2% of all newly diagnosed cervical cancers. These tumors are highly aggressive and have high rates of recurrence. Even though > 70% of women with high-grade neuroendocrine carcinoma of the cervix are diagnosed with early-stage disease, the 5-year survival rate for all patients with this disease is < 30% [1]. Consensus guidelines detailing recommended therapies for newly diagnosed patients have been published, but none of these guidelines offer options for recurrent disease [2, 3]. The National Comprehensive Cancer Network guidelines for treating cervical cancer specifically exclude high-grade neuroendocrine carcinoma [4]. Combination chemotherapy with topotecan, paclitaxel, and bevacizumab has emerged as a common regimen for recurrent small cell neuroendocrine carcinoma of the cervix but even with these drugs, median overall survival after first recurrence is < 10 months [5]. There are very few active regimens for women with recurrent disease, and new treatment options are desperately needed.
Many therapeutic approaches for treating women with high-grade neuroendocrine carcinomas of the cervix have been extrapolated from studies in small cell lung cancer as the diseases appear histologically alike with similar clinical behavior. Studies have demonstrated the activity of single-agent checkpoint inhibitors in treating recurrent small cell lung cancer. The KEYNOTE-028 study reported an objective response rate of 33% (1 complete response, 7 partial responses) for the anti-PD-1 antibody pembrolizumab in 24 patients with recurrent small cell lung cancer [6]. The CheckMate-032 study also showed good activity for PD-1 inhibitors in recurrent small cell lung cancer: 10 (10%) of 98 patients had a partial response to single-agent nivolumab, and an additional 22 (22%) had stable disease [7].
Immune checkpoint inhibitors have also shown promise in the most common types of cervical cancer. Over 98% of cervical cancers are of squamous, adenocarcinoma, or adenosquamous histologies. In 98 patients with such tumors, pembrolizumab demonstrated an overall response rate of 12% (3 complete responses and 9 partial responses) [8]. Nivolumab as a single agent has been explored in 2 different studies in cervical cancer. In a study of 19 patients, the objective response rate was 26% (3 complete responses and 2 partial responses), and another 8 patients (42%) had stable disease [9]. Results of a second study, however, were less impressive: only 1 (4%) of 25 evaluable patients achieved a partial response (duration of response, 3.8 months), and another 9 (36%) had stable disease [10]. The median duration of response for those with stable disease was only 5.7 months.
Although there is a single case report of nivolumab as an active agent in a woman with recurrent high-grade neuroendocrine carcinoma of the cervix [11], we identified no prospective studies evaluating the activity of PD-1/PD-L1 inhibitors in high-grade neuroendocrine carcinomas of the cervix in a search of PubMed. As part of a multi-arm basket trial for patients with rare tumors, we evaluated the safety and clinical efficacy of pembrolizumab in a cohort of women with small cell neuroendocrine carcinomas of the lower genital tract.
Methods
This phase II, open-label study of single-agent pembrolizumab (ClinicalTrials.gov: NCT02721732) was approved by both the US Food and Drug Administration and the Institutional Review Board at The University of Texas MD Anderson Cancer Center. All patients were enrolled at MD Anderson Cancer Center. Patients with recurrent or advanced rare tumors were enrolled into one of 10 cohorts: 1) squamous cell carcinoma of the skin, 2) small cell malignancies of extrapulmonary origin, 3) adrenocortical carcinoma, 4) medullary renal cell carcinoma, 5) carcinoma of unknown primary, 6) penile carcinoma, 7) vascular sarcoma, 8) testicular cancer, later relabeled as germ cell tumor, 9) paraganglioma-pheochromocytoma, and 10) other rare tumor histologies. Results of the entire study have been reported elsewhere [12]. Here, we report on the patients with gynecologic cancers enrolled in the prespecified extrapulmonary small cell carcinoma cohort.
To be eligible for cohort 2 of the umbrella trial, patients had to be ≥ 18 years old with histologically confirmed recurrent or metastatic extrapulmonary small cell malignancy. Patients had to have experienced disease progression during standard therapies within the previous 6 months. Patients treated with prior immunotherapy were excluded. Patients were eligible regardless of PD-L1 expression. In this report, we included only patients with extrapulmonary small cell carcinoma of gynecologic origin.
Pembrolizumab was administered at a fixed dose of 200 mg intravenously every 3 weeks. Patients remained on treatment until documented disease progression, completion of 24 months of treatment, withdrawal of consent or investigator’s decision to stop treatment, or unacceptable adverse event. Patients could be removed from the study for either clinical or radiologic disease progression.
The primary endpoint of the study was the proportion of patients who were alive and progression free at 27 weeks (9 cycles). Secondary endpoints included objective response rate (partial or complete response), clinical benefit rate ≥ 4 months (complete response, partial response, or stable disease), and safety and tolerability.
Tumor response was assessed every 9 weeks (3 cycles) using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and/or immune-related RECIST (irRECIST). Patients who had radiographic progression but clinically stable disease were allowed to continue pembrolizumab until confirmation of disease progression by a follow-up scan ≥ 4 weeks after initial documentation of progression. Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
When fresh or archived tissue was available, PD-L1 biomarker analysis was performed by Qualtek assay using Merck 22C3 antibody. An H-score (range, 0–300) was assigned on the basis of the percentage and intensity of membrane staining. Through recursive partitioning analysis, a score of 42.5 was identified as the optimal cut-off point for PD-L1 H-score. Tumorinfiltrating lymphocytes (TILs) within tumor nests were scored on a scale of 0 to 3, with 0 denoting absence and 3 representing intense intratumoral lymphocytic infiltration. Boardcertified pathologists performed all biomarker evaluations.
The study utilized a Simon’s optimal 2-stage design for each of the 10 cohorts. The first stage accrued 12 patients, and ≥3 patients had to meet the primary endpoint (nonprogression at 27 weeks) in order for the second stage, with accrual of an additional 13 patients, to be opened. In this report, we present data for the patients with gynecologic malignancies in cohort 2 (extrapulmonary small cell cancers). Descriptive statistics are used to summarize patient characteristics. Patients were included in the outcome analysis if they received ≥ 1 adequate onstudy tumor assessment and were included in the safety analysis if they received ≥ 1 dose of pembrolizumab. The best overall response was defined as the best response observed from the start of the treatment until disease progression or discontinuation of treatment for any reason. Waterfall plots were constructed to demonstrate best overall response based on irRECIST.
Results
Twelve patients with extrapulmonary small cell carcinoma were enrolled in this cohort between February 2017 and February 2019. Six patients had cervical cancer, 4 had prostate cancer, 1 had vulvar cancer, and 1 had small cell carcinoma of unknown primary. No patients in the cohort met the primary endpoint of nonprogression at 27 weeks, and therefore no further patients with extrapulmonary small cell carcinoma were enrolled.
Seven patients had a small cell carcinoma of gynecologic origin. Demographics for this group are summarized in Table 1. The median age was 41 years (range, 31–76), and the median number of prior therapies was 2 (range, 1–3).
Table 1:
Patient demographics
| Characteristic | n (%) |
|---|---|
| Primary tumor site | |
| Cervix | 6 (86) |
| Vulva | 1 (14) |
| Median age, years (range) | 41 (31–76) |
| Race | |
| Caucasian | 6 (86) |
| Hispanic | 1 (14) |
| ECOG performance status | |
| 0 | 2 (29) |
| 1 | 5 (71) |
| Number of prior therapies | |
| 1 | 2 (29) |
| 2 | 3 (43) |
| 3 | 2 (29) |
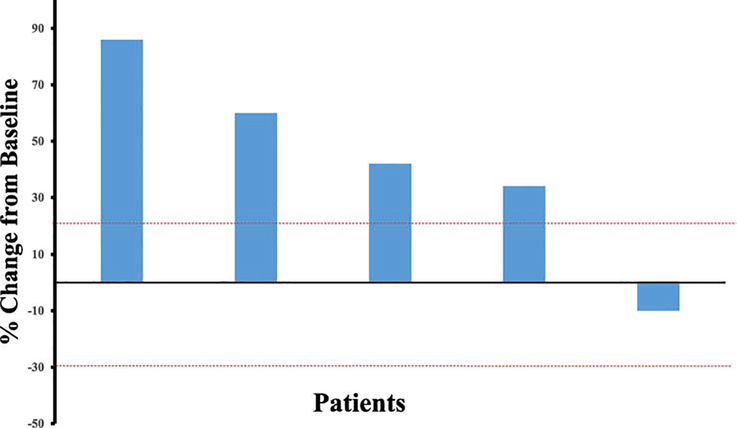
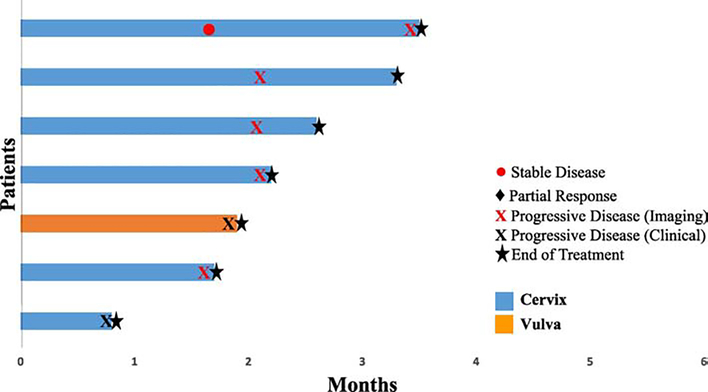
Two patients (28%), 1 with vulvar cancer and 1 with cervical cancer, had clinical progression prior to first imaging assessment. At first imaging assessment, 4 patients (57%) had immune-related progression by irRECIST criteria and 1 patient (14%) had stable disease. The patient with stable disease had imaging early at 6 weeks due to a suspicion of clinical progression but was noted to have 10% decrease in tumor size. However, at her next assessment, 8 weeks later, she was noted to have 40% increase in tumor size. The best overall responses are summarized in Figure 1. The median progression-free survival was 2.1 months (range, 0.8–3.3 months, 95% confidence interval, 0.8–3.2 months). Durations of response are shown in Figure 2. At the time of data analysis, none of the 7 patients were alive.
Figure 1:
Waterfall plot illustrating response to pembrolizumab in 5 evaluable patients with cervical cancer. Two patients (1 with vulvar cancer and 1 with cervical cancer) had clinical progression before first imaging assessment and are not included in this figure.
Figure 2:
Time to and duration of response to pembrolizumab in 7 evaluable patients
Treatment-related adverse events are summarized in Table 2. Fatigue and elevation of liver transaminases were the most commonly reported treatment-related adverse events. The majority of treatment-related adverse events were grade 1 or 2. Two patients had grade 3 adverse events: 1 had asymptomatic elevation of alkaline phosphatase, and the other had asymptomatic elevation of alanine aminotransferase. There were no grade 4 adverse events or treatment relateddeaths in this cohort.
Table 2:
Treatment-related adverse events during pembrolizumab therapy
| No. (%) of patients | ||
|---|---|---|
| Adverse event | ||
| Any grade | Grade ≥3 | |
| Fatigue | 2 (29) | 0 |
| Elevated ALT/AST | 2 (29) | 1 (14) |
| Elevated alkaline phosphatase | 1 (14) | 1 (14) |
| Arthralgia | 1 (14) | 0 |
| Maculopapular rash | 1 (14) | 0 |
ALT - alanine aminotransferase, AST - aspartate aminotransferase
Six patients (86%) had tissue available for analysis of PD-L1 membrane staining and assessment of the presence of TILs within tumor nests. On the basis of percentage and intensity of membrane staining, 2 patients had an H-score of 0, 3 patients had an H-score of 1 (indicating PD-L1 staining of 1+ in 1% of the tumor cells), and 1 patient had an H-score of 3 (indicating PD-L1 staining of 1+ in 3% of tumor cells). No patient had ≥ 2+ staining in any cells. All 6 patients had TILs within tumor nests; 4 patients had a score of 1, 1 patient had a score of 2, and 1 patient had a score of 3. A TIL score of ≥ 2 (representing high TILs) was noted in 2 (33%) of 6 patients.
Discussion
Our group recently reported a phase II study of pembrolizumab in patients with rare tumors. Anti-tumor activity was observed in patients with adrenal cortical carcinoma, paraganglioma, squamous cell carcinoma of skin, and cancers of unknown origin [12]. However, in this phase II clinical trial, no patients with gynecologic small cell neuroendocrine carcinoma showed response to single-agent pembrolizumab. One patient had stable disease at her first assessment, at 6 weeks, but was noted to have progression < 2 months later. The first imaging was performed early at 6 weeks as opposed to 9 weeks due to a clinical suspicion for progression. The other 6 patients had disease progression after only 2 to 3 cycles of treatment. As recurrent small cell carcinoma of the cervix is incurable and is associated with a median survival after first relapse of < 10 months, more clinical trials for women with this malignancy are desperately needed [5].
The relationship between biomarkers and response to PD-1/PD-L1 inhibitors has varied widely across disease sites, tumor histologies, and specific checkpoint inhibitors. In the study reported here, a PD-L1 score was assigned that was based on the percentage and intensity of membrane staining. No patient had more than 1+ staining in any tumor cells, and no patient had more than 3% of cells testing positive. There is no means to translate these findings into the more commonly used combined positive score (CPS) for PD-L1 protein expression, although presumably these modest findings from the Qualtek assay would be similar to a combined positive score of 0. In a separate study of 23 high-grade neuroendocrine carcinomas stained for PD-L1 expression, only 1 (4%) tested positive for the receptor (combined positive score ≥ 1) [13].
In KEYNOTE-028, in which investigators found an overall response rate of 33% in patients with small cell lung cancer treated with single-agent pembrolizumab, PD-L1 expression was a requirement for eligibility, so tumors from all 24 patients enrolled were positive for PD-L1 expression. In contrast, in Checkmate-032, in which investigators found a response rate of 10% in patients with recurrent small cell lung cancer, PD-L1 expression was not a requirement for inclusion, and in fact 86% of patients tested had PD-L1-negative tumors [7]. In that study, there was no relationship between response and PD-L1 expression.
For the most part, treatment strategies for small cell neuroendocrine carcinomas of the gynecologic tract have been borrowed from approaches that have proven successful in patients with small cell lung cancer [2]. However, data from our previous study of whole exome sequencing of small cell neuroendocrine tumors of the cervix show that these tumors may be more similar to squamous carcinomas and adenocarcinomas of the cervix than to small cell lung cancer [14]. In KEYNOTE-158, a study of single-agent pembrolizumab in women with recurrent squamous carcinoma, adenocarcinoma, or adenosquamous carcinoma, the only responses observed occurred in patients with PD-L1-expressing tumors [8]. In contrast, in Checkmate-358, a study of single-agent nivolumab in a similar patient population, responses did not seem to be related to the presence or absence of PD-L1 expression [9].
In addition to PD-L1 expression, tumor mutation burden has emerged as a potential predictor of response to immunotherapy. Clinical trials of checkpoint inhibitors in patients with melanoma and non-small cell lung cancer have shown improved survival for those with high tumor mutation burden [15–17]. Tumors that have a mutational load above 10 somatic mutations per megabase are considered most likely to be able to produce neoantigens that can be detected by T-cells [18]. Although a small number of small cell lung cancer and cervical cancer specimens have > 10 mutations per megabase, the overwhelming majority of these tumors will not be considered to harbor high mutational burden [18]. We previously evaluated tumor mutational burden in 15 small cell cervical cancer tumor specimens through whole exome sequencing and found a median of only 1.7 somatic mutations per megabase [14]. Another marker associated with high tumor mutation burden is high microsatellite instability. In our previous evaluation of 25 high-grade neuroendocrine cervical cancer tumor specimens, none were found to have high microsatellite instability [13].
Even though traditional biomarkers of immunotherapy response such as PD-L1 expression, microsatellite instability, and tumor mutational burden might suggest a lack of response to checkpoint inhibitors for high grade neuroendocrine carcinomas of the cervix, there may still be a role for immunotherapy in these tumors. Immunohistochemistry staining for PARP expression in small cell lung cancer specimens has predicted in vitro responses to PARP inhibitors [19]. Similarly, immunohistochemistry studies have demonstrated high PARP expression of >90% in small cell cervical cancer specimens [13]. DNA damage repair (DDR) inhibitors such as PARP inhibitors have been shown to upregulate PD-L1 expression and enhance responses to immunotherapy in cell lines [20]. We believe the combination of DDR and checkpoint inhibitors may be an active therapeutic approach for patients with high grade neuroendocrine carcinomas and will soon open a phase II basket trial of the PARP inhibitor niraparib and the anti-PD1 agent TSR-042 for neuroendocrine small cell carcinomas for any primary site.
One of the major limitations of this study is the small sample size. Performing clinical trials in rare tumors is challenging for many reasons, among them difficulty with recruiting patients and securing funding. Research in rare tumors requires recruiting from small populations of potential patients spread across large geographical areas, which makes trial accrual and completion difficult [21]. However, social media has emerged as a very effective means to “advertise” trials to focused groups of women with rare gynecologic cancers, potentially improving accrual [22]. In addition, one might consider opening trials at a few select centers chosen for geographic location to optimize access to patients from around the world. Small, innovative single-arm clinical trials focused on “pertinence, validity, and precision” could positively change therapeutic options for patients with rare diseases with only a relatively few patients enrolled in any single trial [21]. In this study, we were able to accrue 7 patients with small cell neuroendocrine carcinoma of the gynecologic tract in 2 years, an accrual rate much higher than the rate of < 2 patients per year that our institution uses to define a low-accruing trial. [23].
A second factor that can make performing clinical trials in rare tumors challenging is that drug companies may be hesitant to fund expensive studies for a small return on their investment even if drugs are Food and Drug Administration–approved for an indication in a specific rare tumor. Currently multiple drug companies are making drugs in the same class such as PD-1/PDL1 and PARP inhibitors. They therefore may be incentivized to expand indications for their drug into rare tumors as a means to differentiate themselves from a competitor’s compound. We may be in an era where pharmaceutical companies are more willing to explore supporting trials in rare malignancies.
Highlights.
Single-agent pembrolizumab had minimal antitumor activity against small cell neuroendocrine tumors of the gynecologic tract
Single-agent pembrolizumab had acceptable toxicity in patients with small cell neuroendocrine tumors of the gynecologic tract
Primary small cell neuroendocrine tumors of the gynecologic tract was associated with low PD-L1 expression
Acknowledgments
Supported by Merck and the NIH/NCI under award number P30CA016672, which supports the MD Anderson Clinical Trials Office.
Footnotes
Conflict of Interest Statement
Dr. Frumovitz reports personal fees from Stryker, grants and non-financial support from Astra Zeneca, personal fees from Biom’Up, outside the submitted work.
Dr. Jazaeri reports personal fees from Iovance Advisory Board Meeting, Nuprobe, Simcere, Pact Pharma; grants from Astra Zeneca, BMS, Iovance, Aravive, Pfizer, Immatics USA, Eli Lilly, and non-financial support from Astra Zeneca, outside the submitted work.
Dr. Karp reports other from Phosplatin Pharmaceutical Co., personal fees from Black Beret Life Sciences, other from AFFIGEN (Immunotherapy Startup Firm), grants from NIH Clinical Translational Science Award Grant, outside the submitted work.
Dr. Naing reports other from NCI, other from EMD Serono, other from MedImmune, other from Healios Onc. Nutrition;, other from Atterocor, other from Amplimmune, other from ARMO BioSciences, other from Eli Lilly, other from KaryopharmTherapeutics, other from Incyte, other from Novartis, other from Regeneron, other from Merck, other from BMS, other from Pfizer, other from CytomX Therapeutics, other from Neon Therapeutics, other from Calithera Biosciences, other from TopAlliance Biosciences, other from Kymab, other from PsiOxus, other from Immune Deficiency Foundation, other from Genome, outside the submitted work.
Dr. Rodon Ahnert reports personal fees and other from Novartis, personal fees from Eli Lilly, personal fees from Orion Pharmaceuticals, personal fees from Peptomyc, personal fees and other from Kelun Pharmaceuticals/Klus Pharma, personal fees and other from Spectrum Pharmaceuticals Inc., personal fees and other from Pfizer, personal fees from Roche Pharmceuticals, personal fees from Ellipses Pharma, personal fees from Certera, personal fees and other from Bayer, personal fees from Ionctura SA, other from European Journal of Cancer, other from VHIO/ Ministero De Empleo Y Seguridad Social, other from Chinese University of Hong Kong, other from SOLTI, other from Elsevier, other from GLAXOSMITHKLINE, other from ESMO, from Department of Defense, other from Merck Sharp & Dohme, other from Lousiania State University, other from Huntsman Cancer Institute, other from Cancer Core Europe, other from Karolinska Cancer Institute, other from King Abdullah International Medical Research Center, other from WIN Consortium, other from Janssen, other from Tocagen, other from Symphogen, other from BioAlta, other from GenMab, other from CytomX, other from Kelun-Biotech, other from Takea-Millenium, other from Ipsen, outside the submitted work.
Dr. Westin reports grants and personal fees from AstraZeneca, grants and personal fees from Clovis Oncology, grants and personal fees from GSK/Tesaro, grants and personal fees from Roche/Genentech, grants and personal fees from Novartis, grants from Cotinga Pharmaceuticals, grants from ArQule, grants from Bayer, personal fees from Merck, personal fees from Pfizer, personal fees from Eisai, personal fees from Circulogene, outside the submitted work.
Dr. Yap reports grants, personal fees and non-financial support from Merck, outside the submitted work.
Drs. Abonofal, Salvo, Xu and Zarifa have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cohen JG, Kapp DS, Shin JY, Urban R, Sherman AE, Chen LM, Osann K, Chan JK. Small cell carcinoma of the cervix: treatment and survival outcomes of 188 patients, Am J Obstet Gynecol. 203 (2010) 347 e1–6. [DOI] [PubMed] [Google Scholar]
- [2].Gardner GJ, Reidy-Lagunes D, Gehrig PA. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document, Gynecol Oncol. 122 (2011) 190–8. [DOI] [PubMed] [Google Scholar]
- [3].Satoh T, Takei Y, Treilleux I, Devouassoux-Shisheboran M, Ledermann J, Viswanathan AN, Mahner S, Provencher DM, Mileshkin L, Avall-Lundqvist E, Pautier P, Reed NS, Fujiwara K. Gynecologic Cancer InterGroup (GCIG) consensus review for small cell carcinoma of the cervix, Int J Gynecol Cancer. 24 (2014) S102–8. [DOI] [PubMed] [Google Scholar]
- [4].Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, Crispens MA, Damast S, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, Han E, Huh WK, Lurain JR, Mariani A, Mutch D, Nagel C, Nekhlyudov L, Fader AN, Remmenga SW, Reynolds RK, Tillmanns T, Ueda S, Wyse E, Yashar CM, McMillian NR, Scavone JL. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology, J Natl Compr Canc Netw. 17 (2019) 64–84. [DOI] [PubMed] [Google Scholar]
- [5].Frumovitz M, Munsell MF, Burzawa JK, Byers LA, Ramalingam P, Brown J, Coleman RL. Combination therapy with topotecan, paclitaxel, and bevacizumab improves progression-free survival in recurrent small cell neuroendocrine carcinoma of the cervix, Gynecol Oncol. 144 (2017) 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study, J Clin Oncol. 35 (2017) 3823–3829. [DOI] [PubMed] [Google Scholar]
- [7].Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jager D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial, Lancet Oncol. 17 (2016) 883–895. [DOI] [PubMed] [Google Scholar]
- [8].Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L, Zeigenfuss S, Pruitt SK, Leary A. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study, J Clin Oncol. 37 (2019) 1470–1478. [DOI] [PubMed] [Google Scholar]
- [9].Naumann RW, Hollebecque A, Meyer T, Devlin MJ, Oaknin A, Kerger J, Lopez-Picazo JM, Machiels JP, Delord JP, Evans TRJ, Boni V, Calvo E, Topalian SL, Chen T, Soumaoro I, Li B, Gu J, Zwirtes R, Moore KN. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial, J Clin Oncol. 37 (2019) 2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santin AD, Deng W, Frumovitz M, Buza N, Bellone S, Huh W, Khleif S, Lankes HA, Ratner ES, O’Cearbhaill RE, Jazaeri AA, Birrer M. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002), Gynecol Oncol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Paraghamian SE, Longoria TC, Eskander RN. Metastatic small cell neuroendocrine carcinoma of the cervix treated with the PD-1 inhibitor, nivolumab: a case report, Gynecol Oncol Res Pract. 4 (2017) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Naing A, Meric-Bernstam F, Stephen B, Karp DD, Hajjar J, Rodon Ahnert J, Piha-Paul SA, Colen RR, Jimenez C, Raghav KP, Ferrarotto R, Tu SM, Campbell M, Wang L, Sabir SH, Tapia C, Bernatchez C, Frumovitz M, Tannir N, Ravi V, Khan S, Painter JM, Abonofal A, Gong J, Alshawa A, McQuinn LM, Xu M, Ahmed S, Subbiah V, Hong DS, Pant S, Yap TA, Tsimberidou AM, Dumbrava EEI, Janku F, Fu S, Simon RM, Hess KR, Varadhachary GR, Amir Habra M. Phase 2 study of pembrolizumab in patients with advanced rare cancers, J Immunother Cancer. 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carroll M, Salvo G, Ramalingam P, Phoolcharoen N, Cardnell R, Byers LA, Frumovitz M. PARP and PD-L1 as Potential Therapeutic Targets for Women with Neuroendocrine Cervical Cancer, Gynecol Oncol. 156 (2020) e21–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hillman RT, Cardnell R, Fujimoto J, Byers LA, Futreal PA, Frumovitz M. Genomic landscape of small cell neuroendocrine cervical cancer, Society of Gynecologic Oncology Winter Meeting 2018 Snowmass, CO February 8–10, 2018. [Google Scholar]
- [15].Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma, N Engl J Med. 371 (2014) 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR Jr., Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA, CheckMate I. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer, N Engl J Med. 376 (2017) 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fujii T, Naing A, Rolfo C, Hajjar J. Biomarkers of response to immune checkpoint blockade in cancer treatment, Crit Rev Oncol Hematol. 130 (2018) 108–120. [DOI] [PubMed] [Google Scholar]
- [18].Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy, Science. 348 (2015) 69–74. [DOI] [PubMed] [Google Scholar]
- [19].Cardnell RJ, Feng Y, Diao L, Fan YH, Masrorpour F, Wang J, Shen Y, Mills GB, Minna JD, Heymach JV, Byers LA. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer, Clin Cancer Res. 19 (2013) 6322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, Li CW, Chou CK, Lim SO, Chang SS, Litton J, Arun B, Hortobagyi GN, Hung MC. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression, Clin Cancer Res. 23 (2017) 3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brown J, Naumann RW, Brady WE, Coleman RL, Moore KN, Gershenson DM. Clinical trial methodology in rare gynecologic tumor research: Strategies for success, Gynecol Oncol. 149 (2018) 605–611. [DOI] [PubMed] [Google Scholar]
- [22].Zaid T, Burzawa J, Basen-Engquist K, Bodurka DC, Ramondetta LM, Brown J, Frumovitz M. Use of social media to conduct a cross-sectional epidemiologic and quality of life survey of patients with neuroendocrine carcinoma of the cervix: a feasibility study, Gynecol Oncol. 132 (2014) 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tang C, Sherman SI, Price M, Weng J, Davis SE, Hong DS, Yao JC, Buzdar A, Wilding G, Lee JJ. Clinical Trial Characteristics and Barriers to Participant Accrual: The MD Anderson Cancer Center Experience over 30 years, a Historical Foundation for Trial Improvement, Clin Cancer Res. 23 (2017) 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]