Abstract
Recent work has transformed our ideas about the neural mechanisms, behavioral consequences and effective therapies for amblyopia. Since the 1700’s, the clinical treatment for amblyopia has consisted of patching or penalizing the strong eye, to force the “lazy” amblyopic eye, to work. This treatment has generally been limited to infants and young children during a sensitive period of development. Over the last 20 years, we have learned much about the nature and neural mechanisms underlying the loss of spatial and binocular vision in amblyopia, and that a degree of neural plasticity persists well beyond the sensitive period. Importantly, the last decade has seen a resurgence of research into new approaches to the treatment of amblyopia both in children and adults, which emphasize that monocular therapies may not be the most effective for the fundamentally binocular disorder that is amblyopia. These approaches include perceptual learning, video game play and binocular methods aimed at reducing inhibition of the amblyopic eye by the strong fellow eye, and enhancing binocular fusion and stereopsis. This review focuses on the what we’ve learned over the past 20 years or so, and will highlight both the successes of these new treatment approaches in labs around the world, and their failures in clinical trials. Reconciling these results raises important new questions that may help to focus future directions.
INTRODUCTION
Amblyopia is a common neurodevelopmental abnormality that results in physiological alterations in the visual pathways and impaired vision in one eye, less commonly in both. It reflects a broad range of neural, perceptual, oculomotor and clinical abnormalities that can occur when normal visual development is disrupted early in life. This review focusses on unilateral amblyopia.
Aside from refractive error, amblyopia is the most common cause of vision loss in infants and young children. It causes a constellation of perceptual deficits in the vision of the amblyopic eye, including a loss of visual acuity, position acuity and contrast sensitivity, particularly at high spatial frequencies, as well as increased internal noise and prolonged manual and saccadic reaction times. There are also perceptual deficits in the strong eye, such as certain types of motion perception, reflecting altered neural responses and functional connectivity in visual cortex (Ho et al., 2005). Beyond the perceptual deficits are a range of visuo-motor deficits and potential psychological sequelae. Conventional clinical treatment in young children consists of correction of any refractive error and patching of the strong eye. Compliance with patching is challenging, and many amblyopic children fail to achieve normal acuity or stereopsis even after extended periods of treatment.
The broad goal of this review is to provide an update on the substantial progress made over the last 25 years or so in understanding the neural basis of amblyopia, the explosion of both randomized clinical trials and new experimental approaches to treatment that may improve compliance and outcomes, and evidence that amblyopic adults may still show some benefit of treatment. Why now? Although there have been a number of reviews on selected aspects of amblyopia, including a Special Issue of Visual Neuroscience (2018; referenced throughout this review), the year 2020 seems to be an appropriate time to take a close look at some of these new findings, approaches and possible future directions for recovering 20/20 (or at least improved) vision in amblyopia.
Amblyopia, and its association with strabismus (misalignment of the two eyes) was first described by Le Cat in the eighteenth century (as cited by Revel, 1971), and Cont Du Buffon in 1743 described what is still today the most widely prescribed treatment for amblyopia – occlusion of the strong eye. He also suggested “equalizing” vision in the two eyes with lenses by blurring the strong eye (cited by Revel, 1971).
Amblyopia has a prevalence of about 2–4 % in children (Ciuffreda, Levi & Selenow, 1991). Aside from refractive error, amblyopia is the most frequent cause of vision loss in infants and young children. Amblyopia reflects the damage that results when normal visual development is disrupted. In the clinical setting, this damage is generally expressed as a loss of visual acuity in an apparently healthy eye, despite appropriate refractive correction. However, there is a great deal of evidence showing that amblyopia results in a broad range of neural, perceptual, oculomotor and clinical abnormalities (for reviews see Kiorpes, 2006; Levi, 2006).
Amblyopia is almost always associated with a history of abnormal binocular visual development early in life. Specifically the main risk factors for amblyopia are three: a constant unilateral strabismus (one eye turned all or most of the time), usually in the form of esotropia (eye turn inward), anisometropia (unequal refractive error in the two eyes) or, much less common in developed countries, cataract (either in one or both eyes). A fourth, related risk factor is persistent high hyperopia (far-sightedness – Anker et. Al., 2003; Atkinson et al., 1996; Sjoestrand & Abrahamsson, 1996). It is important to note that these risk factors frequently co-occur (e.g., strabismus and hyperopic anisometropia; esotropia and high hyperopia) (Hunter & Cotter, 2018). If these risk factors occur late in life, though, amblyopia does not develop. The severity of the amblyopia is related to the degree of imbalance between the two eyes and the age at which the amblyogenic factor occurred. It is unclear how these factors interact, but it is clear that different early visual experiences result in different functional losses in amblyopia (McKee, Levi & Movshon, 2003 – Fig. 1), and an important aspect is the presence or absence of binocular function (see Maurer & McKee, 2018 for a recent review).
Fig. 1. Amblyopia functional loss ‘map’.
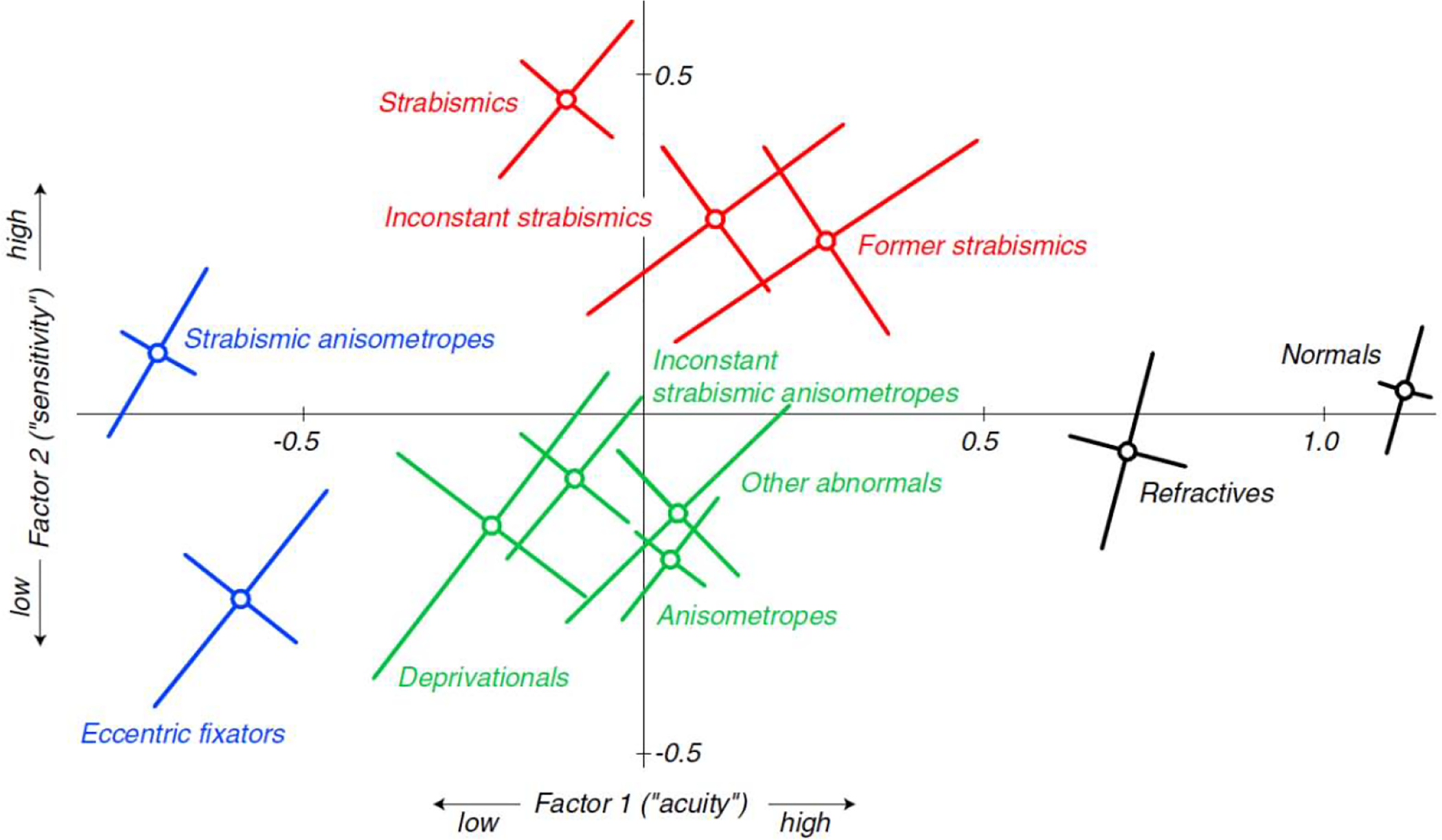
Mean locations of 11 clinically defined categories in two-factor space for a large population of patients (427) with amblyopia or its risk factors. Factor 1 is closely related to three different acuity measures, and Factor 2 is closely related to the two contrast sensitivity measures. The diagonal bars show one standard error of the mean measured along the principal axes of the elliptical distributions. From McKee, Levi & Movshon, 2003.
Sensitive Period for the development of amblyopia:
Amblyopia generally does not develop after age 6 to 8 years of age (Worth, 1903; von Noorden, 1981), leading to the suggestion that there is a “sensitive period” for the development of amblyopia. However, in humans the age of onset is difficult to determine, and the effects of treatment make it hard to obtain a clear picture of the “natural history” of the development of amblyopia. Consequently, much of our current understanding of the development of amblyopia derives from animal studies (see Boothe et al., 1985 for a review) and from retrospective reviews of clinical records (e.g., von Noorden, 1981). Advances in infant testing have provided more direct data on the development of naturally occurring amblyopia in humans (Mohindra et al., 1979; Maurer et al., 1983; Jacobson et al., 1981; Birch, 1983; Maurer et al., 1999; Norcia et al., 2015) and monkeys (Kiorpes and Boothe, 1981; Kiorpes et al., 1984, 1989) and provide strong evidence that amblyopia is induced by early deprivation.
Anatomical structures and behavioral and physiological functions that develop early may be less susceptible to the effects of abnormal visual input, than those that develop more slowly (Levi & Carkeet, 1993). This hypothesis can account for why different structures and functions may be vulnerable to visual deprivation at different times during development. For example, the sensitive period in layer IVc (the input layer) of the cortex of monkeys is shorter than that of other layers (LeVay et al., 1980), and there is evidence that different visual functions are affected when one eye lid is sutured shut is at different times. Visual functions that develop early are more immune to pattern deprivation, while those that develop late are at greater risk and remain susceptible for the longest time (Levi & Carkeet, 1993)
The upper limit for susceptibility of excitatory binocular interactions appears to be later than that for acuity or contrast sensitivity in monkeys and may extend to at least 7 or 8 years in humans. Interocular transfer studies provide an indirect estimate of the period of susceptibility of binocular connections in humans with strabismus (Banks et al., 1975; Hohmann and Creutzfeldt, 1975), and suggest that they are highly vulnerable during the first year and a half of life and remain at risk until at least age 7 years.
Amblyopia is classified by the accompanying amblyogenic factor (which is generally considered to be the cause), most commonly as strabismic amblyopia, anisometropic amblyopia, mixed amblyopia (i.e., both strabismus and anisometropia are present at the time of diagnosis), or less commonly form deprivation amblyopia (due to cataract, ptosis or corneal opacity) or meridianal amblyopia (due to high uncorrected astigmatism, in which the shape of the cornea results in different refractive error in the two meridians, and as a result, reduced vision in the more out of focus meridian).
While this classification by condition is clinically useful for descriptive purposes, an important and long-standing question is whether different causative factors (i.e., different early visual experiences) result in different functional losses. As shown in Fig. 1, rather than classification by accompanying amblyogenic factor, classification by visual function shows a map of visual deficits, in which the strabismic group, shown in red, has a different pattern of visual loss than the anisometropic group, shown in green (McKee et al., 2003). This study used a limited range of functional vision tests, and a different or more extensive set of tests may result in a different map. Ideally, the most useful classification scheme would also provide important prognostic information that could help guide treatment (Maurer & McKee, 2018).
BEHAVIORAL, OCULO-MOTOR & NEURAL CONSEQUENCES
Behavioral deficits:
Amblyopia results in a constellation of behavioral deficits when viewing is with the amblyopic eye. These include a loss of visual acuity, position acuity and contrast sensitivity, particularly at high spatial frequencies, as well as increased internal noise and prolonged manual and saccadic reaction times to visual stimuli (McKee et al. 2016). These deficits are usually greatest in central vision. There is both behavioral and physiological evidence that the amblyopic deficit may be amplified downstream in the brain (see Levi, 2006; 2013; Kiorpes, 2006, 2019 for reviews). Thus amblyopes suffer not only from ‘low-level’ deficits in acuity and contrast, but also more impairments in more complex visual processing (Sharma, Levi & Klein, 2000; Kiorpes, 2006; Levi, 2006; Farzin & Norcia, 2011; Wong-Kee-You, Wei & Hou, 2020). These include processing stimuli defined by contrast or motion (sometimes referred to as “second-order” because they are not defined by changes in luminance), contour integration, temporal and spatial problems, and reduced attentional capacity (Popple & Levi, 2008). Amblyopic vision also appears to have increased random noise (Levi & Klein, 2003; Levi Klein & Chen, 2008; Li et al., 2008). This is true even when viewing with the preferred eye (Levi, Klein & Chen, 2008), suggesting that this elevation of random noise, as well as other deficits when viewing with the preferred eye (e.g., Ho et al, 2005), is of central origin, likely related to the absence of correlated binocular visual experience early in life (Kind, Mitchell, Ahmed, Blakemore, Bonhoeffer & Sengpiel, 2002; McKee, Levi & Movshon, 2003).
The lack of correlated binocular visual experience early in life may lead to suppression of the amblyopic eye by the fellow eye. For more than a century, suppression has been implicated as an important characteristic and possibly a cause of amblyopia (Worth, 1903) and loss of stereopsis. This notion is based on clinical (von Noorden, 1990), psychophysical (Levi, Harwerth & Smith, 1979; 1980; Smith, Levi, Manny, Harwerth & White, 1985; Hess, 1991; Harrad & Hess, 1992; Baker, Meese & Hess, 2008; Maehara, Thompson, Mansouri, Farivar & Hess, 2011; Mansouri, Thompson & Hess, 2008; Ding, Klein & Levi, 2013; Ding & Levi, 2014; Hess, Thompson & Baker, 2014; Hess & Thompson, 2015) and physiological (Harrad, Sengpiel & Blakemore, 1996; Sengpiel & Blakemore, 1996; Bi et al., 2011) evidence. Indeed, as discussed below, binocular suppression in area V2 is proportional to the depth of amblyopia (Bi et al., 2011). It is important to note that there remain many questions about the role, occurrence and nature of suppression in amblyopia (Barrett, Panesar, Scally and Pacey, 2012; Holopigian, Blake & Greenwald, 1986; Vedamurthy et al., 2015), and whether suppression is different in anisometropia and strabismus – passive in anisometropia, where the amblyopic eye’s image is blurred, but active in strabismus, in order to avoid diplopia.
Under normal everyday viewing conditions with both eyes open, the most common deficit in amblyopia is reduced stereoscopic depth perception. Reducing the vision of one eye (in neurotypical persons) by blurring, filtering or reducing contrast degrades stereoacuity (Westheimer & McKee, 1980; Donzis, Rappazzo, Burde, Gordon, 1983; Legge & Gu, 1989; Menon, Bansal & Prakash, 1997), and degrading vision in one eye has a more deleterious effect on stereopsis than by degrading both eyes (Westheimer & McKee, 1980; Legge & Gu, 1989).
Persons with amblyopia frequently manifest deficits in visually-guided hand movements (similar to occluding vision of one eye neurotypical persons (see Levi, Knill & Bavelier, 2015 for a review), resulting from impaired stereopsis, rather than to reduced visual acuity (Suttle, Melmoth, Finlay, Sloper and Grant, 2011; Melmoth, Finlay, Morgan and Grant, 2009; Niechwiej-Szwedo et al, 2012a & b; Grant, Melmoth, Morgan and Finlay, 2007), fixation instability (Subramanian, Jost and Birch, 2013), or poor vergence control (Melmoth, Storoni, Todd, Finlay, & Grant, 2007). A recent study suggests that impaired stereopsis may also affect the gaze strategies used during walking across complex terrains, suggesting that stereopsis is important for the visuomotor control of locomotion (Bonnen et al., 2019).
Oculo-motor deficits:
Many amblyopes also show poor fixation stability when viewing with the amblyopic eye (Chung et al., 2015). Specifically, when attempting steady fixation, the amblyopic eye (particularly in strabismic amblyopes) drifts constantly, producing retinal image motion, and makes frequent microsaccades. Importantly, there has been a recent see-change in our understanding of the role of fixational eye-movements in vision [Martinez-Conde, Otero-Milan & Macknik, 2013; Rucci & Victor, 2015). As noted by Rucci & Victor (2015), “Fixational eye-movements are not a ‘bug’ but a feature: an integral and crucial stage of information processing, which enables the retina to represent space in a temporal fashion and to begin the process of feature extraction.” Moreover, recent work suggests a tight coupling between visual sensitivity and fixational eye movements in normal vision (Scholes et al., 2015). Fixation stability is abnormal in amblyopic eyes: compared to the fellow eye, the amblyopic eye shows large slow drifts and microsaccades as large as 1 degree (Schor & Hallmark, 1978; Ciuffreda, Kenyon & Stark, 1979; Subramanian, Jost & Birch, 2008; González et al., 2012), and recent work has shown that the fixational eye movements of the amblyopic eye may account for more than half of the variance in the amblyopic observers’ visual acuity(Chung et al., 2015).
Persons with strabismic amblyopia also have long latencies when making a saccade to a peripheral target with their amblyopic eyes. In a large sample (n = 145), McKee et al., (2016) found that strabismic amblyopes have a latency about 40 – 80 msec longer when viewing with the amblyopic eye than with the fellow eye. Persons with anisometropic amblyopia also have longer latencies when viewing with their amblyopic eyes, but the difference is significantly smaller than that of the strabismic amblyopes. What accounts for the sluggishness of saccadic eye movements? We hypothesize that for anisometropic amblyopes the increased response time simply reflects the reduced contrast sensitivity that is characteristic of amblyopic vision. However, our recent work suggests that this is not sufficient to explain the results of strabismics. For strabismic amblyopes saccadic reaction time is substantially delayed with the amblyopic eye relative to the fellow eye, even with perceptually matched contrast (Gambacorta, et al., 2018). An intriguing recent hypothesis is that poor fixation stability with its increased frequency of microsaccades may affect the dynamics and the spatial allocation of selective attention in individuals with amblyopia (Verghese, McKee & Levi, 2019).
Neural deficits:
The neural deficits in amblyopia are complex. They depend on the timing and nature of early abnormal visual experience, which differs in anisometropes and strabismics (Fig 1; McKee, Levi & Movshon, 2003; Levi, McKee & Movshon, 2011). Much of what we know about these neural deficits comes from studies of animals such as cats and mice. However, because of the close homology between the visual systems of humans and non-human primates, we focus mainly on neural deficits in both naturally occurring and experimental amblyopia in non-human primates.
In non-human primate models of amblyopia, the deficits are first seen in striate (area V1) and other visual cortical areas rather than in the retina or the lateral geniculate nucleus. For example, there is a striking loss of neurons that can be excited by both eyes (binocular neurons), and to a lesser extent a reduction in the number of neurons that can be driven by the deprived/amblyopic eye in area V1 (Blakemore & Vital-Durand, 1986; for recent reviews see Kiorpes & Daw, 2018; Kiorpes, 2019). In addition, some V1 neurons show reduced contrast sensitivity and spatial resolution (Kiorpes et al., 1998; Bi et al., 2011). However, these physiological losses of sensitivity and resolution in V1 do not provide a simple explanation of the behavioral losses (Levi, 2013). Considerations of noise, noise correlations, pooling and the weighting of information also play important roles in making perceptual decisions (Shadlen, Britten, Newsome & Movshon, 1996; Cohen & Newsome, 2009). More recent work that takes these into account show both increased neural response variability (noise) in V1 (Acar, Kiorpes, Movshon, 2019) and V2 (Wang et al., 2017) and higher correlations between neurons driven by the amblyopic eye in V1 of amblyopic monkeys. Increased noise and increased noise correlations reduce the visual information that is passed to downstream visual areas through the amblyopic eye. Moreover, the excitatory-inhibitory balance is altered in early visual cortex of amblyopic non-human primates (Hallum et al., 2017). Importantly some of the most profound effects of monocular deprivation can be substantially mitigated by brief periods of unrestricted binocular vision (Zhang et al., 2011) – a finding that has important implications for treatment.
There is also evidence for additional abnormalities in higher visual areas. For example, neurons in area V2 of amblyopic primates integrate visual information over larger areas than those of normal primates (Tao et al., 2014). Substantial binocular suppression of the amblyopic eye by the fellow eye is seen not only in V1 but also in area V2 (Bi et al., 2011; Wang et al. al, 2017; Shooner et al., 2017). In addition neural populations in the Middle Temporal area (area MT, or V5) have reduced sensitivity to motion, commensurate with the known behavioral deficits in motion perception.
Human imaging studies have shown both structural and functional changes. Structural abnormalities are present in the lateral geniculate nucleus (Barnes et al., 2010; Hess et al., 2009), which shows reduced activity and connectivity with V1 on functional imaging (Li et al., 2011). The striking physiological changes V1 cascade downstream (Imamura et al, 1997; Goodyear, Nicolle, Humphrey & Menon, 2000; Barnes et al., 2001; Muckli et al., 2006; Lerner et al., 2003; 2006; Bonhomme et al., 2006; Conner, Odom, Schwartz & Mendola, 2007; Li, Dumoulin, Mansouri, & Hess, 2007; Ho & Giaschi, 2009; Secen, Culham, Ho & Giaschi, 2011; Mendola et al., 2018), even reaching areas concerned with attention and decision making (Farzin & Norcia, 2011), where they may be amplified. Functional mapping of amblyopic cortex suggests that parafoveal regions of space activate cortical areas that normally represent the fovea (Muckli et al., 2006; Conner, Odom, Schwartz & Mendola, 2007a & b; Clavagnier et al., 2015), and functional connectivity of visual areas appears to be altered in an eccentricity dependent manner (Mendola et al., 2018).
Genetics.
Amblyopia is a non-genetic condition, but amblyogenic factors may have a genetic basis. For example, strabismus is often clustered in families, and a meta-analysis of twin studies suggests that genetic factors are necessary to cause strabismus (Wilmer & Backus, 2009). There is also a genetic contribution to refractive error (Dirani et al., 2006; Hammond et al., 2001), which may be a risk factor for strabismus and amblyopia if not corrected.
TREATMENT
For centuries the main approach to the treatment of amblyopia consisted of optical correction of any refractive error (to ensure a clearly focused retinal image in each eye) and occlusion or penalization of the “strong” eye, thus “forcing” the brain to use the input from the “weaker” amblyopic eye (Worth, 1903). However, it is only in the last twenty years or so that a number of large-scale clinical trials have provided detailed evidence for the efficacy of this approach.
Sensitive periods for treatment of amblyopia.
Amblyopia does not develop after about 6 to 8 years of age (Worth, 1903; von Noorden, 1981) and currently is treated almost exclusively in young children. The idea that there is a critical or sensitive period for treatment can be traced back to Claude Worth, who hypothesized that the reduced vision of the amblyopic eye represented arrested sensory development. Worth suggested that the presence of a “sensory obstacle” (e.g., unilateral strabismus) halted the development of visual acuity (“amblyopia of arrest”), so the depth of amblyopia is a direct function of the age of onset of the “sensory obstacle”, and, if amblyopia of arrest persisted, a further loss of visual acuity could occur (“amblyopia of extinction”) due to inhibition of the weak eye by the fellow eye. In Worth’s view, delaying treatment meant that only the loss due to amblyopia of extinction could be recovered. Based on his observations in almost 1000 strabismic amblyopes, Worth concluded that both the age of onset and the length of time the obstacle was in place influenced the outcome of treatment.
The notion of a critical or sensitive period was cemented by the work of Hubel & Wiesel (1970; Wiesel, 1982), and the many studies that followed (Berardi et al, 2000; Hensch & Quinlan, 2018). Early monocular deprivation (lid suture) in kittens and monkeys results in substantial neural alterations, in particular a change in ocular dominance in the visual cortex and a profound loss of vision. These effects only occur during a critical or sensitive period. In kittens this sensitive period begins at about 4 weeks and lasts for 3–4 months. The losses can be reversed by suturing the previously open eye, but only if the reverse suture is performed within the sensitive period (Movshon & Van Sluyters, 1981; Blakemore & Van Sluyters, 1974). The notion that amblyopia in humans cannot be treated beyond age 7 or 8 years seems to stem from the idea that treatment for amblyopia was only effective during the early critical period for visual development, when the neural system is plastic [Fig. 2 – blue curve].
Fig. 2. The sensitive period.
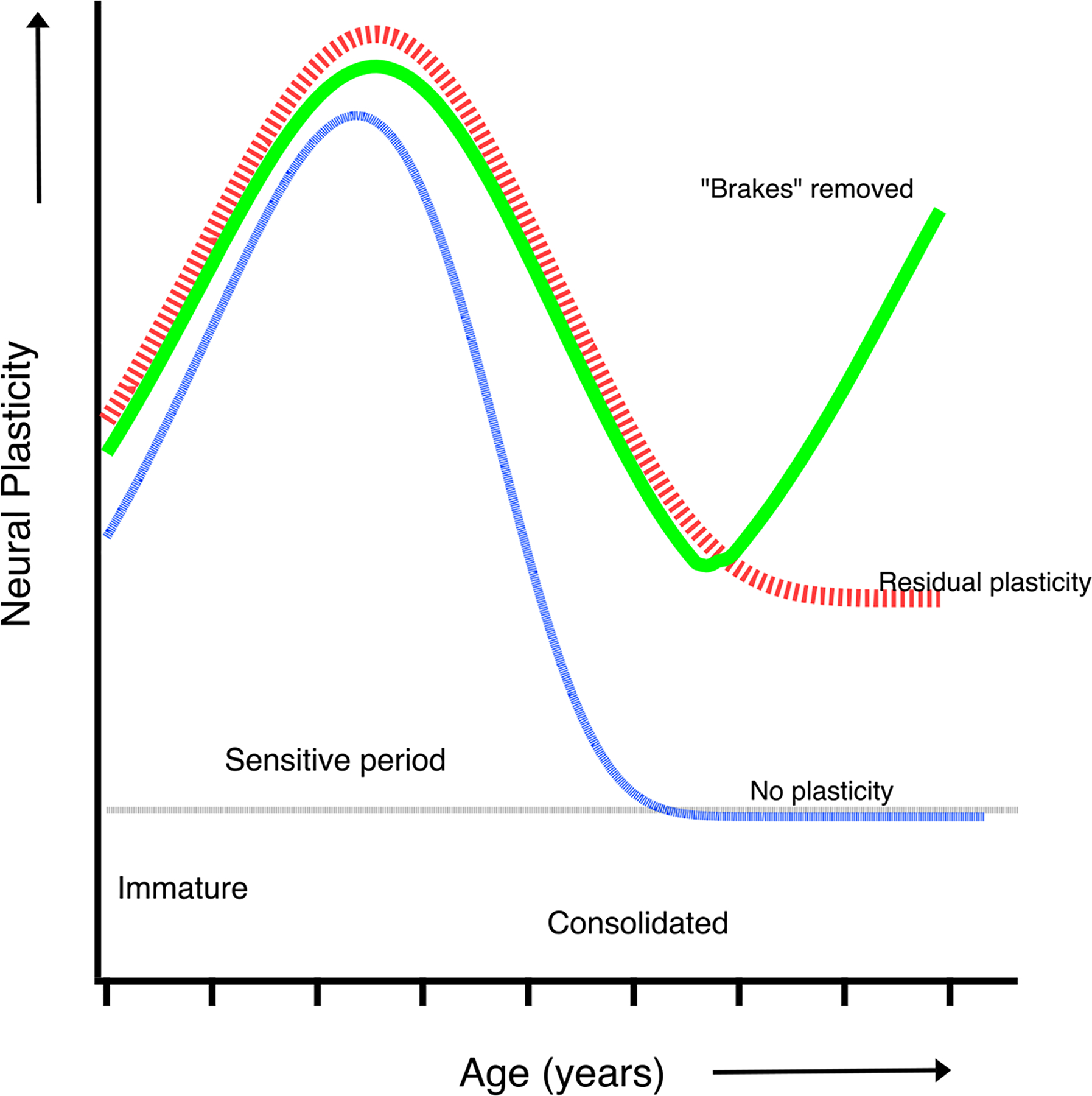
This cartoon illustrates several hypotheses regarding neural plasticity during development. The blue curve shows plasticity increasing during early development when the neural system is immature, peaking and rapidly diminishing to zero when function is consolidated. The red curve is similar, but with residual plasticity extending into adulthood. The green curve illustrates removal of the factors (brakes) that limit plasticity and restore levels of neural plasticity in adults.
More recent studies in humans and nonhuman primates suggest that rather than an abrupt and complete drop in plasticity, there may be some residual plasticity that extends into adulthood (for a recent review see Hensch & Quinlan, 2018 - Fig. 2 – red curve). Moreover, there maybe multiple anatomical and physiological sensitive periods for development: visual functions that develop early (e.g. scotopic sensitivity) may be less susceptible to the effects of deprivation than late developing functions (e.g. stereopsis) (Harwerth et al., 1987; 1990]. Furthermore, the sensitive period for recovery may not be the same as the period of susceptibility. Studies in humans suggest that binocular connections may remain susceptible until at least the age of 7 years [Banks et al., 1975; Hohmann & Creutzfeldt, 1975]. In the following paragraphs we will explore the question of neural plasticity and whether there is a sensitive period for recovery of function. Additionally, there is evidence from studies in mice that it is possible to remove the “brakes” that limit plasticity and restore a degree of neural plasticity in adults (Morishita & Hensch, 2008; Hensch & Quinlan, 2018 – Fig. 2 green curve)
The sensitive period(s) for the developing amblyopia has been considered to suggest that there is also a sensitive period for its treatment. Thus, clinical treatment was often limited to young children. However, this view has been widely refuted (Kupfer, 1957; Levi & Polat, 1996; Levi, Polat & Hu, 1997; Holmes & Levi, 2018 and see below). Large scale randomized clinical trials show that treatment may be effective in older (13 to 17 years) children who have not been previously treated (Pediatric Eye Disease Investigator Group, 2005), and a substantial number of studies have shown significant and long-lasting effects of experimental treatments in adults. For a recent evidenced-based review, see Piano & Simmers (2019).
Moreover, evidence suggests that the visual system retains a degree of neural plasticity well into adulthood (Bavelier et al., 2010). For example, adults who lose vision in the non-amblyopic eye may spontaneously recover vision in amblyopic eyes in (Vereecken & Brabant, 1984; Rahi, Logan, Borja, Timms, Russell-Eggitt, & Taylor, 2002). This can be seen most dramatically in older adults with long-standing amblyopia who have lost their strong eye through injury or disease. El Mallah et al. (2000) showed that in amblyopic patients who develop macular degeneration in their strong eye, the visual acuity of the amblyopic eye spontaneously improves. There are other reports of recovery of vision in amblyopic eyes in adults who lose vision in the non-amblyopic eye (Vereecken & Brabant, 1984; Rahi, Logan, Borja, Timms, Russell-Eggitt, & Taylor, 2002). An important implication of these studies is that the connections from the amblyopic eye may be suppressed rather than destroyed. A reduction of input from the strong eye would unmask these existing connections as occurs in adult cats with retinal lesions (Chino et al., 1992; Heinen & Skavenski, 1999; but see Smirnakis et al., 2005). These findings strongly implicate suppression of the amblyopic eye by the fellow eye. However, removing the strong eye is not a viable option for treating patients; but, as discussed below (Future directions), temporary inactivation of the fellow eye promotes anatomical recovery in cats that were aged beyond the critical period peak, and without detriment to the inactivated eye (DiCostanzo et al., 2020).
Indeed, it seems likely that the sensitive periods for producing amblyopia and for recovery are supported by different mechanisms of plasticity. There is evidence that mechanisms supporting the production of amblyopia are from those that promote recovery from amblyopia (Kaneko et al., 2008; Cho et al., 2009; Ranson et al., 2012). The dissociation between impairment and recovery processes suggests that the mechanisms mediating the two forms of plasticity are different, despite the fact that in animal models the critical period profile of impairment and recovery is largely overlapping, as has been revealed in cats (Olson and Freeman, 1980; Blakemore and Van Sluyters, 1974) and non-human primates (von Noorden, 1979; Blakemore, Garey & Vital Durand, 1978). Thus, while recovery from amblyopia is possible at older ages, development of amblyopia seems not to be.
Optical (refractive) treatment.
As implied by the clinical definition of amblyopia, correcting refractive error alone does not initially result in normal visual acuity in the amblyopic eye. However, a number of clinical trials have shown that over a period of 10 to as much as 30 weeks, optical treatment by itself significantly improves visual acuity in both anisometropic and strabismic amblyopes, with about one third of the cases resolving just with this optical correction (Stewart et al., 2004a & b; Cotter et al., 2006, 2007, 2012; Asper et al., 2018). While the effects were strongest in younger children, older children (up to 17) also showed improvements with optical correction alone (Asper et al., 2018; Gao et al., 2018a). Moreover, more than a third of the children who received optical treatment improved their 3-D stereovision by a factor of two or more.
Occlusion and penalization of the strong eye:
This has been the ‘gold standard’ treatment for the treatment of unilateral amblyopia since the French naturalist and botanist George-Louis Leclerc, Comte de Buffon in 1743 wrote that the weak eye could regain its strength by occluding the strong eye [For a detailed history of the treatment of amblyopia, see Louden & Simonsz, (2005)]. Occlusion generally refers to patching the strong fellow eye, typically with an opaque patch. Penalization of the strong eye can be achieved in a number of ways. One could place a lens or filter to blur its vision. Alternatively, one can use atropine drops to eliminate accommodation in the strong eye, forcing the patient to use their amblyopic eye for near activities such as reading and writing, while still allowing the strong eye to be in focus at far distances.
Clinical trials have established that this approach is often quite successful, leading to improved visual acuity in the amblyopic eye. However, there is considerable individual variability in the response to patching (Holmes & Levi, 2018). Figure 4 summarizes data from 3 large Randomized Clinical Trials (randomized clinical trials) by the PEDIG group in children between about 3 and 7 years of age. Several points emerge. First, most of the almost 800 patients acuity improved. Second, even in this group with a narrow range of ages, a range typical of the time when many amblyopic patients are treated, there is a huge range of outcomes for any age. Although current data indicate that patients actually wear the patch about 50% of the prescribed time, this variability in outcome was not simply a matter of compliance, as studies using monitored occlusion show a similar variability in outcome (Stewart et al., 2004a & b). At this stage there is no way of predicting which patients will improve and which will not.
Fig. 4. Improvement in visual acuity vs hours of training.
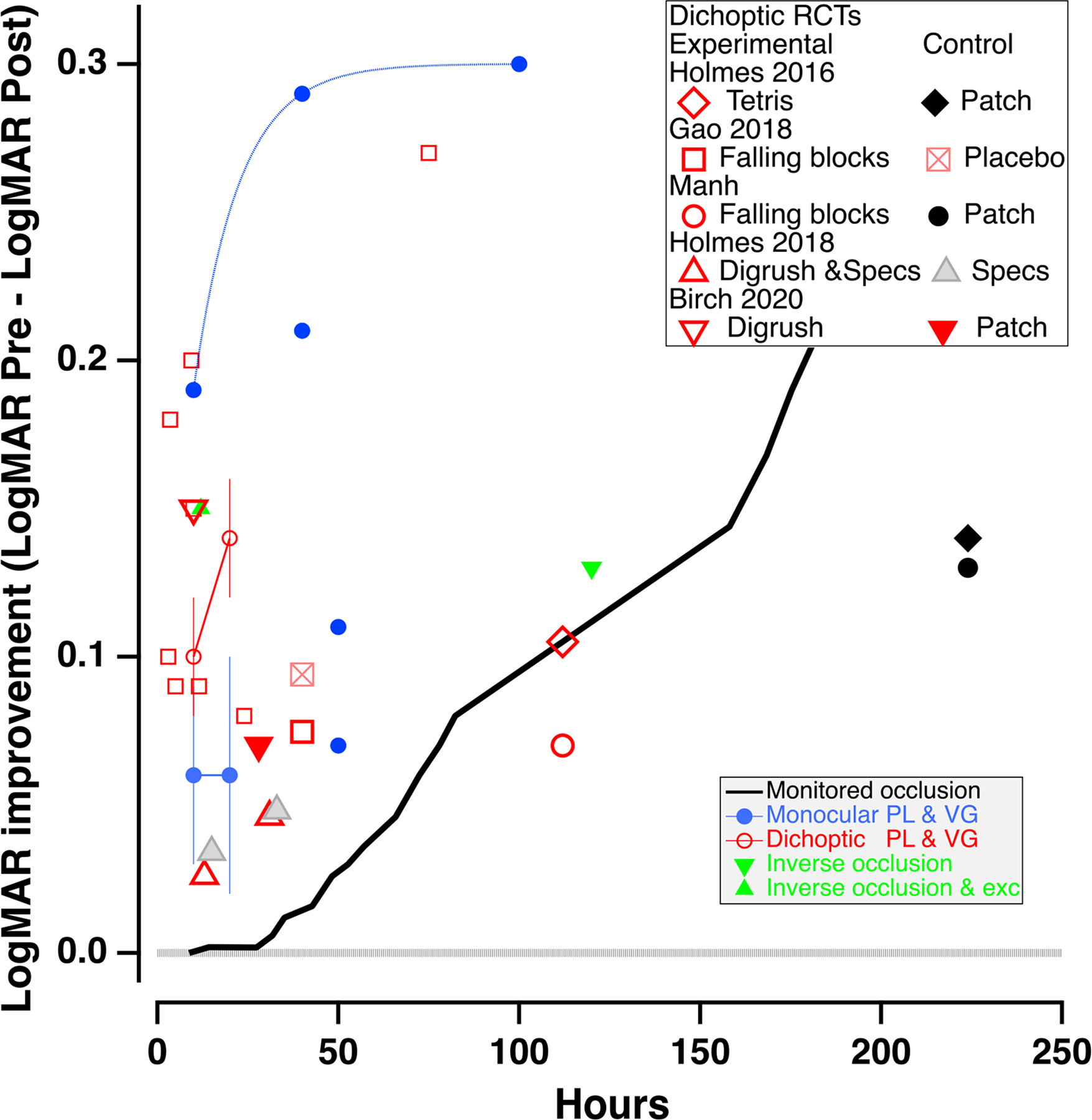
VA improvement data (pre-post) from a broad range of experimental treatment studies in adults and children with amblyopia, as a function of hours of treatment. Small blue solid circles represent monocular perceptual learning or video game treatment, and the small red open circles indicate dichoptic/binocular treatment, replotted from Gambacorta et al., 2018 (fig. 6. Error bars are the SEMs). The black line shows the time course of monitored occlusion (solid line from Stewart et al., 2007). The solid green triangles are from studies using inverse occlusion (Zhou et al., 2019, inverted triangle) and inverse occlusion plus exercise (upright triangle, Lunghi et al., 2018). The large symbols show the results of recent large scale RCTs of dichoptic/binocular treatment, for both the experimental and control groups. The dotted horizontal line extending from 0.0 on the Y axis indicates no improvement in visual acuity.
While patching and penalization can be effective, they have significant limitations. Their gains accrue very slowly. Patching requires about 170 hours for two lines of improvement in visual acuity for 4-year-olds, and more than 200 hours for a similar improvement for 6-year-olds (Stewart, 2007 – thick black line in Fig. 4). For children older than 7 it may require over 400 hours (Fronius, Cirina, Ackermann, Kohnen & Diehl, 2014). Patching itself may lead to reduced binocular vision and stereopsis, and to psycho-social problems such as a loss of self-esteem (Webber, Wood, Gole & Brown, 2008). The latter can make compliance a challenge. Any treatments (including optical treatment) that reduce the amount or duration of patching may improve compliance, as discussed further below. Even long periods of treatment may not result in normal acuity or stereopsis in a substantial proportion of amblyopic children (Birch and Stager, 2006; Birch et al., 2004; Repka et al., 2003; Repka et al., 2004; Repka et al., 2005; Rutstein et al., 2010; Stewart et al., 2004b; Wallace et al., 2006; Woodruff et al., 1994a). Morever, when vision is successfully restored to normal, visual acuity regresses within the first year of treatment in about 25% of patients (Holmes et al., 2004). Consequently, there have been a number of attempts over the last two decades to develop more effective and efficient approaches for treating amblyopia, using perceptual learning and videogame techniques (see Levi & Li, 2009; Levi, 2012; Birch, 2013; Tsirlin et al., 2015; Hess & Thompson, 2015; Levi, Knill & Bavelier, 2015 for reviews).
There is an effect of age over the range shown in Fig. 3, however the average data (large filled symbols) do not capture the huge range of individual outcomes (small dots), and we do not have a good predictor for prognosis in single subjects. An important factor is previous treatment. In the 13 to 17 year olds, those patients who had not been previously treated benefited much more than those who had been. Finally, while these results suggest the limits of conventional treatment, a review of the literature makes it clear that improvement in adults can exceed the predictions of the function shown in Figure 3. The open diamonds replot the remarkable results reported by Carl Kupfer (1957) from seven young adults with strabismic amblyopia, with initial acuity ranging from 20/70 to hand motion only, following 6 weeks of hospitalization during which they underwent complete, constant occlusion of the strong eye combined with intensive fixation training. [Note that the Kupfer study was not a randomized clinical trial, and therefore may have been subject to experimenter bias, placebo effects, etc.]
Fig. 3. Change in visual acuity vs. Age of Treatment.
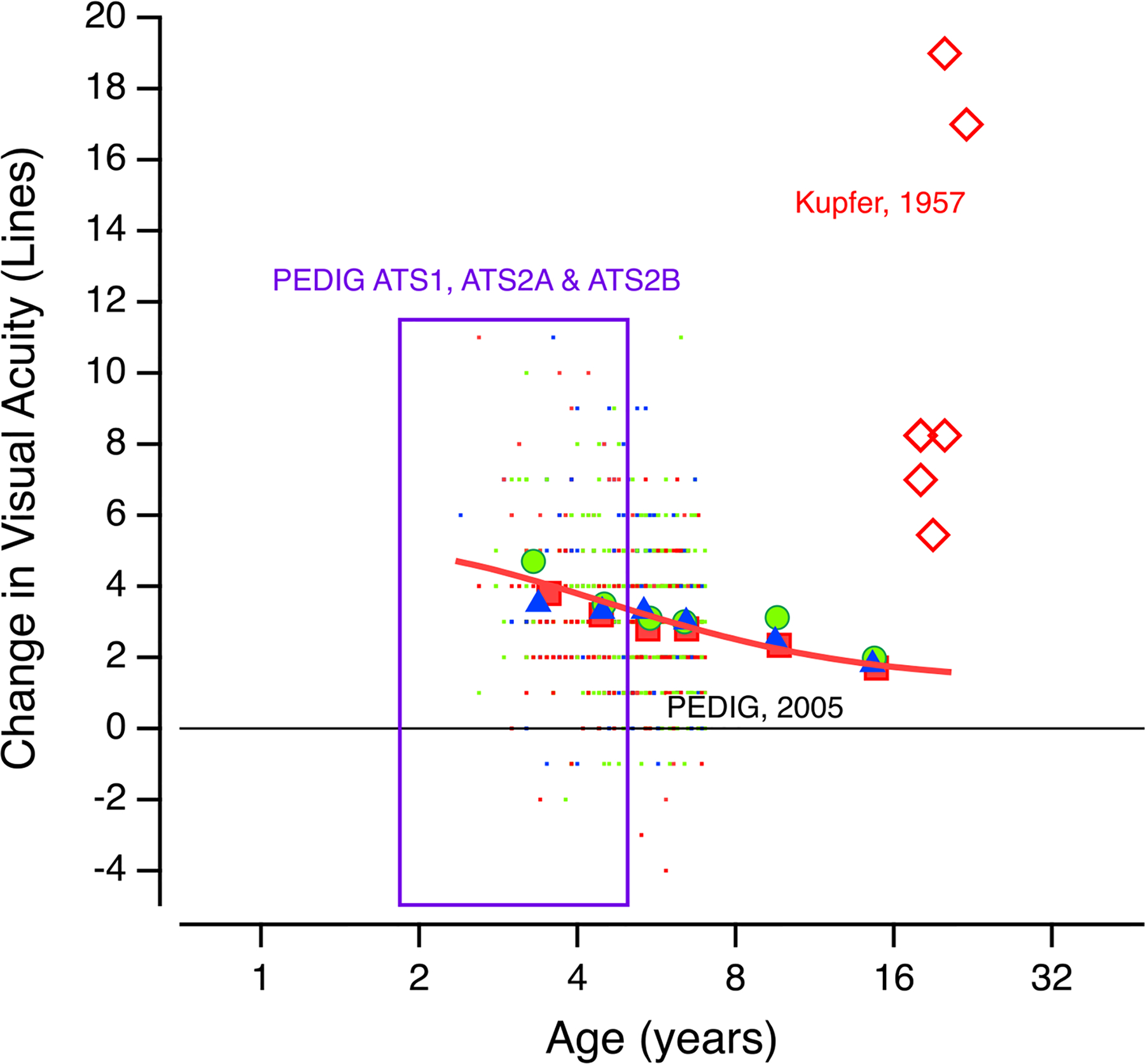
Summary of 3 clinical trials: Trial ATS1 compared daily atropine to occlusion for at least 6 hours/day; trial ATS2A compared 6 hours/day versus full-time patching for severe amblyopia and trial ATS2B compared 6 hours versus 2 hours/day of patching in patients with moderate amblyopia. The tiny dots are the individual data from ≈ 800 patients; the large symbols show the mean idata in ≈ 1 year bins; included also are the mean data from an additional study (PEDIG 2005) that examined the effectiveness of treatment in children ages 7 to 17. A positive change in acuity is improvement. (after Holmes and Levi, 2018). Also shown here (red diamonds) are data from a small group of adult amblyopes who underwent intensive treatment (see text) from Kupfer, 1957. The red line is a fit to the mean PEDIG data.
EXPERIMENTAL TREATMENTS: TARGETS, MECHANISMS AND FUTURE DIRECTIONS
Optimizing Occlusion and Penalization.
There have been many attempts to increase the effectiveness of treatment for amblyopia, as adjuncts to patching and penalization. They include subcutaneous injection of strychnine, electrical stimulation of the retina and optic nerve, flashing lights, red filters and rotating gratings (reviewed by Revell, 1971), administration of Levodopa (Leguire et al., 1993; Levi, 1994 and Wang, Li & Li, 2020 for a recent meta-analysis) and Transcranial Magnetic Stimulation (TMS – Thompson, Mansouri, Koski & Hess, 2008).
Some of the recent attempts have stemmed from the successful recovery from visual deprivation in rodents. One example is the study by Legas et al., (2019) that showed no benefit from use of Citalopram, a serotonin reuptake inhibitor that promoted recovery in rodents (Vetencourt et al., 2008). Similarly, while the administration of physostigmine promoted recovery from visual deprivation in rodents (Morishita et al., 2010), it failed to translate to humans (Chung et al., 2017). Other experimental treatments that seemed to succeed in rodents, such as exercise and dark exposure, also appear to be limited in their ability to promote recovery in human adults or higher order mammals (Holman et al., 2018; Campana, Fongoni, Astle & McGraw, 2020).
As noted in the Lasker report on amblyopia (Mitchell and Sengpiel, 2018), cross-species studies are needed to mitigate translation failures, and to avoid elevating frustration among frontline caregivers. Importantly, these failures highlight the importance of appropriate animal models, ideally non-human primates, to avoid costly failures.
A full discussion of the many attempts to increase the effectiveness of treatment for amblyopia is beyond this review; however, few have been subjected to rigorous scrutiny, and those that were often failed to stand up to it (e.g. Tytla & LaBow-Daily, 1981). Below we focus on three more promising recent approaches: Perceptual Learning, Videogame play and binocular/dichoptic training. Unlike treatments such as dark exposure and pharmacological interventions, which aim to enhance the level of developmental plasticity at a particular age in order to set the stage for recovery (green line in Figure 2), these treatments are aimed at using available plasticity mechanisms (red line in Figure 2). In this way the therapies may logically combine for synergistic benefit.
Perceptual Learning (PL).
Performance on sensory tasks can be improved through repeated and extensive practice. This is called perceptual learning (for reviews see Dosher & Lu, 2017; Buonomano & Merzenech, 1998). Perceptual Learning was originally defined as “Any relatively permanent and consistent change in the perception of a stimulus array following practice or experience with this array…” (Gibson, 1967). The key idea is that practicing challenging, near-threshold visual tasks results in long-term improvements in performing those tasks. This has been shown for a large variety of visual tasks in neurotypical adults [Dosher & Lu, 2017]. Over the last 25 years, there have been dozens of small-scale studies of PL in amblyopia, involving hundreds of patients. The extant studies (see Levi & Li, 2009; Levi, 2012; Tsirlin et al., 2015 for recent reviews and a meta analysis) show improvements through PL on a broad range of tasks, including Vernier acuity, contrast detection, letter identification, position discrimination, spatial frequency discrimination, grating acuity, letter acuity and motion coherence, among others (small blue solid symbols in Fig. 4, replotted from Gambacorta et al., 2018). Most of these studies have reported that amblyopic patients improve in the trained task, although the amount of improvement varies substantially both between tasks and between individuals. Importantly, most studies also found that the improvement transferred, at least partially, to improved visual acuity by a mean of 1 to 2 lines. This improvement occurred regardless of the training task, whether training was monocular or binocular, the patient’s age (over a fairly limited range) or the type of amblyopia (Levi & Li, 2009; Levi, 2012; Tsirlin et al., 2015).
During PL, amblyopic patients must attend and make fine discriminations with their amblyopic eyes, under challenging conditions. They are exposed to the same stimuli repeatedly and receive feedback. Thus, PL likely involves learning to attend to and use the most salient or reliable information for the task when viewing with the amblyopic eye. Indeed, when the strong eye is patched, normal everyday life may require attention and fine visual discriminations using the amblyopic eye, and this may account, at least in part, for the success of patching. However, PL provides intensive, active, supervised visual experience with feedback, requiring attention and action using the amblyopic eye, and thus may be more efficient.
As shown by the black line in Fig. 4, improvement during occlusion is slow. Visual acuity in 6–8 year old children improves by a factor of about 1.6 after about 240 hours of patching (Stewart et al., 2007). Perceptual learning may be faster and more efficient than patching, by as much as a factor of 8. It results in improvements of 1 –2 lines of acuity in a much shorter time. Combining occlusion with PL may reduce the duration of patching, thus reducing the emotional consequences of patching (Kvarnstrom, Jakobsson & Lennerstrand, 2001).
There are two important limitations of perceptual learning: specificity and boredom. Specificity refers to the training effects being confined to the trained stimulus and task. If improvements are limited to the trained stimulus, condition and/or task, then PL would have very little value for amblyopia. Fortunately, perceptual learning has a broader ‘bandwidth’ in amblyopia than in normal vision (Huang et al., 2008). Many different tasks transfer to improved visual acuity (Levi, 2012; Tsirlin et al., 2015) and other functions including stereoacuity and counting the number of briefly presented stimuli (Sharma et al., 2000). However, this transfer to acuity is often only partial. In some cases this may be due to the fact that the training improved performance for a specific orientation or tilt and not others, and that this limits improvements with stimuli such as letters that have multiple orientations.
The second limitation is that PL (as performed in laboratories) is, by its nature, highly repetitious, and may lead to boredom, making it less than ideal for clinical implementation. It is also important to note that this approach to the treatment of amblyopia has not yet been subjected to the scrutiny of a randomized clinical trial, which presumably will have to precede its broad deployment as a treatment. For these reasons, PL has not translated to clinical practice
Playing Video Games.
An alternative approach is video game play. In neurotypical observers playing action video games causes the brain to develop an optimal perceptual template for the task at hand (see Bavelier et al., 2012 for a review). However, unlike PL, action video games have varied demands and visual experiences, training the brain to learn to make optimal use of the stimulus information, and promoting a broad transfer of learning. This raises the possibility that playing such games could result in improved visual performance in amblyopia.
About ten years ago, we (Li et al., 2011) asked amblyopic adults to play a commercial first-person shooter videogame (Medal of Honor: Pacific Assault) with their fellow eye patched. All showed improvements in visual acuity, from about 13 to 44%. Two subjects with mild amblyopia improved to 20/20! Unlike normal subjects, whose vision does not improve after playing a non-action game (Tetris), amblyopic observers also improved when playing a non-action game (SimCity) (Li et al., 2011; 2015). Interestingly, video game play and for perceptual learning lead to similar improvements in visual acuity with (Fig. 4).
Other visual functions, including counting briefly presented dots, Vernier acuity and attentional blink also improve following videogame play [Li et al., 2011; 2015]. Some amblyopic subjects show improved stereoacuity after perceptual learning of a stereo task (Ding & Levi, 2011), action video game play (Li et al., 2011), or viewing 3-D movies (Li et al., 2018) similar to the improvements in stereopsis with monocular perceptual learning (Levi, Knill & Bavelier, 2015). Note, that these unmasked cohort studies have not been subjected to rigorous, large randomized clinical trials, and therefore we do not know whether not they lead to larger improvements than dichoptic treatment or clinical patching regimens.
Commercial videogames (like the ones used by Levi & Li, 2011, 2015) provide a very rich, engaging and dynamic environment and both challenge and reward the player, enabling them to succed. Furthermore, action game play has also been shown to enhance visuo-spatial selective attention, aspects of visual short-term memory, and the ability to select a target in an ever-changing stream of stimuli in neurotypical subjects (Bavelier et. Al., 2012), and may therefore benefit persons with amblyopia, who have both low-level and high level deficits (Sharma et al., 2000; Wong-Kee-You, Wei & Hou, 2020). It has been suggested that playing action videogame may promote neural plasticity (Morishita & Hensch, 2008; Bavelier et al., 2010) because they release neuromodulators associated with arousal and reward (Koepp et al., 1998; Baroncelli, Maffei & Sale, 2011). The importance of action to amblyopic learning is not clear, though, since non-action video games can also confer some benefit (Li et al., 2011; 2015). Importantly, patients are more likely to comply with playing an immersive videogame than with patching or perceptual learning.
Dichoptic Treatment.
As noted earlier, suppression has been implicated as a feature and possibly a cause, of amblyopia and loss of stereopsis. Another approach to treat amblyopia is to reduce this suppression by training under binocular/dichoptic conditions. A key aspect of this binocular treatment is to provide different stimuli to the two eyes. The amblyopic eye receives a more intense stimulus than the fellow eye, by reducing the contrast, the luminance or both of the image presented to the strong eye.
Over the past decade there have been a large number of unmasked cohort studies using dichoptic perceptual learning and videogame play to train adults and children with amblyopia. These have generally documented improvements in visual acuity that are similar to those obtained with monocular training (Birch et al., 2015; Hess & Thompson, 2015; Knox et al., 2012; Gambacorta et al., 2018; Vedamurthy et al., 2015A & b; Herbison et al.,2013; 2016; Li et al. 2014 – small open red symbols in Fig. 4, replotted from Gambacorta et al., 2018) although there appears to be an additional benefit for improved stereopsis in strabismic (but not anisometropic) amblyopia (Vedamurthy et al., 2015; Levi, Knill & Bavelier, 2015).
The efficacy of perceptual learning and video game play (both monocular and binocular) have been replicated by a number of small-scale studies (small symbols in Fig. 4) and generally result in, on average, a 1 to 2-line improvement in visual acuity both in children (as shown in Fig. 4) and in adults (Levi & Li, 2009; Levi, 2012; Tsirlin et al., 2015). The reason(s) for this 1 to 2 line improvement remain unclear. One hypothesis that it reflects changes in the ability to attend to (or upweight) the relevant signals and ignore the noise when viewing with the amblyopic eye in order to improve decision making (see Verghese, McKee & Levi, 2019 for a discussion), rather than low level changes in early visual cortex.
Recently, the binocular approach has been subjected to randomized clinical trials (Holmes et al., 2016; Manh et al., 2018; Kelly et al., 2016; Gao et al., 2018b). The results from these trials are shown by the large red symbols in Fig. 4, for both the experimental and control groups, and the results have been mixed, and sometimes disappointing. Although each of the these studies showed some improvement, two had improvements of less than 1 line, and the effects were no better and possibly slightly worse than the control conditions of patching (large black symbols), continued spectacle wear (large gray triangle), or presenting identical images to the two eyes (square with X). A more recent RCT with 48 children aged 4 to 10 either patched or playing a contrast rebalanced dichoptic game (Dig Rush), showed a larger improvement after 2 weeks for the game group (1.5 lines – large open red inverse triangles) than for the patched group (0.7 lines – large filled red inverse triangles; Birch et al., 2020).
There may be a number of reasons for the mixed outcomes of these clinical trials. On the one hand, many of the prior small-scale studies did not include appropriate or even any control groups, raising the possibility of placebo effects, Hawthorne effects and regression toward the mean. Small-scale studies often carefully select patients most likely to succeed, for example excluding patients with strabismus (or with large angle strabismus), and including subjects strongly motivated to volunteer. Not all small-scale studies included prolonged refractive adaptation, which as seen from the Holmes (2018) study had as much effect as the treatment. The larger clinical trials generally used the same fixed settings for the interocular contrast ratio for all participants, whereas the small-scale studies used individualized settings to determine the interocular contrast ratio. Many small-scale studies used stereoscopes or prisms to carefully align the images to the two eyes, which is critical for binocular combination with strabismus, but some of the clinical trials did not. Finally, it is not clear whether the stimuli used in the clinical trials and small-scale studies were equally engaging. Nevertheless, even “DigRush” had rather poor compliance, even though it was purportedly the most “engaging” of the games used in the clinical trials (Holmes, 2018): only 56% completed more than 75% of the prescribed game play.
Training stereopsis directly:
A number of recent laboratory studies have focused on training stereopsis directly in individuals with amblyopia as well as in non-amblyopic strabismics, with some success via perceptual learning (Ding & Levi, 2011; Astle, McGraw & Webb, 2011; Xi et al., 2014) videogame play (Vedamurthy et al., 2016) and viewing 3D movies (Li et al., 2018). For a review see Levi, Knill & Bavelier (2016), and a recent small RCT using perceptual learning embedded in a video-game format suggests that this may be a useful method for improving stereopsis in patients with amblyopia (Portela-Camino et al., 2018).
Homeostatic Plasticity.
A counterintuitive approach, based on the notion of homeostatic plasticity (the mechanism that seeks to balance neural excitation and inhibition), is to patch the amblyopic eye – i.e., inverse occlusion. Patching the amblyopic eye was employed to treat amblyopia with eccentric fixation in Europe in the 1960s and subsequently abandoned, because it was found to be less effective than direct occlusion. For example, von Noorden (1965) reported that ≈ 49% of patients improved 20 20/30 or better with occlusion of the sound eye, compared with 24% who improved with inverse occlusion, and 10% got worse. However, there is strong evidence that brief periods of monocular pattern deprivation can increase the sensitivity of the deprived eye and reduce that of the non-deprived eye by temporarily modulating the ocular dominance balance (Zhou et al., 2013; 2019; Lunghi et al., 2019). This is considered a form of ocular dominance plasticity. Based on the assumption that multiple episodes of short-term patching of the amblyopic eye could result in long-term alterations in interocular balance in favor of the amblyopic eye (Zhou et al., 2013; 2019; Lunghi et al., 2019). The green inverted triangle shows the effect of 2 hours a day of inverse occlusion for 2 months (Zhou et al., 2019, triangle) in patients age 10 to 35. The green upright triangle shows the effects of just six 2-hour sessions of inverse occlusion while exercising for 6 (Lunghi et al., 2019). They suggest that the physical activity might have played a role in the improvement (Lunghi & Sale, 2015); however, the effect of exercise on ocular dominance plasticity in normal vision has been disputed (Zhou et al., 2017; Finn et al., 2018).
It seems clear from Fig. 4 that at least some of these new treatment modalities can provide a rapid but modest improvement in vision with the amblyopic eye of, on average, 1 to 2 lines. Note that like conventional treatment, there are very substantial differences in the amount of improvement, with some patients achieving as much as 4 lines (a factor of ≈ 2.5) or more. Is a 1 – 2-line improvement worth the effort? For patients with deep amblyopia a small improvement in acuity is unlikely to make a noticeable difference to their vision and quality of life under everyday circumstances. However, for patients with mild amblyopia (20/40 – 20/60), the improvement may be sufficient to achieve some degree of binocular fusion and even stereopsis.
Future directions:
It also seems critical to develop engaging methods that will result in better compliance. One possibility is the use of dichoptically presented movies or other video content (Li et al., 2014; Bossi et al., 2017). At this stage it is not clear whether active gaming is necessary, or if simply controlled visual input will provide the same “bang for the buck”. It is also clear that we need better outcome measures, including better quantitative measures of stereopsis, hand-eye coordination, and quality of life to more fully assess the effects of treatment (Holmes & Levi, 2018). Importantly, it seems unlikely that any of these methods will completely replace patching or penalization; rather they may provide important adjuncts to conventional treatment that may speed the recovery of vision.
There is also ongoing research into other new treatments that can alter the balance between neural excitation and inhibition and restore plasticity beyond the sensitive period (See Bavelier et al., 2010; Baroncelli, Maffei & Sale, 2011; Hess & Thompson, 2015, for reviews). Acetylcholine plays important roles in visual cortical plasticity and perceptual learning (reviewed in Kang et al., 2014). One study found that donepezil a cholinesterase inhibitor that increases synaptic levels of acetylcholine, increased the magnitude and specificity of perceptual learning of motion direction discrimination in healthy humans (Rokem & Silver, 2010), and these effects were still evident 15 months later (Rokem & Silver, 2013). Unfortunately, recent studies show no such benefit of donepezil for perceptual learning of letter identification or crowding in patients with amblyopia (Chung et al., 2017), or of selective serotonin reuptake inhibitors such as citalopram (Legas et al., 2019). Another interesting pharmacologic observation is that intravitreal injections of tetrodotoxin results in faster recovery of amblyopia in cats (Fong et al., 2016), without detriment to the inactivated eye (DiCostanzo et al., 2020). Finally, rodent models also suggest a role for exercise, environmental enrichment and dark rearing; however, it is unclear how these relate to human amblyopia. Ultimately there may be a future role for combining new pharmacologic agents, behavioral techniques and other methods such as transcranial magnetic stimulation, and transcranial direct current stimulation (Thompson et al., 2008; Speigel et al., 2013) to improve the treatment of amblyopia in both children and adults.
ACKNOWLEDGEMENTS
Supported by grants from the National Eye Institute (NEI) RO1 EY020976 and R21 EY030609). Many thanks to Avigael Aizenman for her critical comments on an earlier version of this manuscript, and to two anonymous referees for their insightful and constructive comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acar K, Kiorpes L, Movshon JA and Smith MA (2019). Altered functional interactions between neurons in primary visual cortex of macaque monkeys with experimental amblyopia. J Neurophysiol 122(6): 2243–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker S, Atkinson J, Braddick O, Ehrlich D, Hartley T, Nardini M and Wade J (2003). Identification of infants with significant refractive error and strabismus in a population screening program using noncycloplegic videorefraction and orthoptic examination. Invest Ophthalmol Vis Sci 44(2): 497–504. [DOI] [PubMed] [Google Scholar]
- Asper L, Watt K and Khuu S (2018). Optical treatment of amblyopia: a systematic review and meta-analysis. Clin Exp Optom 101(4): 431–442. [DOI] [PubMed] [Google Scholar]
- Astle AT, McGraw PV, & Webb BS (2011). Recovery of stereo acuity in adults with amblyopia. BMJ Case Reports 10.1136/bcr.07.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Robier B, Anker S, Ehrlich D, King J, Watson P and Moore A (1996). Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye (Lond) 10 (Pt 2): 189–198. [DOI] [PubMed] [Google Scholar]
- Baker DH, Meese TS and Hess RF (2008). Contrast masking in strabismic amblyopia: attenuation, noise, interocular suppression and binocular summation. Vision Res 48(15): 1625–1640. [DOI] [PubMed] [Google Scholar]
- Banks MS, Aslin RN, & Letson RD (1975). Sensitive period for the development of human binocular vision. Science 190, 675–677. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Hess RF, Dumoulin SO, Achtman RL and Pike GB (2001). The cortical deficit in humans with strabismic amblyopia. J Physiol 533(Pt 1): 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Li X, Thompson B, Singh KD, Dumoulin SO and Hess RF (2010). Decreased gray matter concentration in the lateral geniculate nuclei in human amblyopes. Invest Ophthalmol Vis Sci 51(3): 1432–1438. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Maffei L and Sale A (2011). New perspectives in amblyopia therapy on adults: a critical role for the excitatory/inhibitory balance. Front Cell Neurosci 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett BT, Panesar GK, Scally AJ and Pacey IE (2012). A limited role for suppression in the central field of individuals with strabismic amblyopia. PLoS One 7(5): e36611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Green CS, Pouget A and Schrater P (2012). Brain plasticity through the life span: learning to learn and action video games. Annu Rev Neurosci 35: 391–416. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y and Hensch TK (2010). Removing brakes on adult brain plasticity: from molecular to behavioral interventions. The Journal of neuroscience 30(45): 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, & Maffei L (2000) Critical periods during sensory development. Curr Opin Neurobiol, 10, 138–45. [DOI] [PubMed] [Google Scholar]
- Bi H, Zhang B, Tao X, Harwerth RS, Smith EL 3rd and Chino YM (2011). Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cereb Cortex 21(9): 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE (1983) Assessment of binocular function during infancy. Ophthalmic Pediatrics and Genetics, 2, 43–50. [Google Scholar]
- Birch EE (2013). Amblyopia and binocular vision. Prog Retin Eye Res 33: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Li SL, Jost RM, Morale SE, De La Cruz A, Stager D Jr., Dao L, & Stager DR Sr. (2015). Binocular iPad treatment for amblyopia in preschool children. J AAPOS, 19 (1), 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch E, Williams C, Hunter J and Lapa MC (1997). Random dot stereoacuity of preschool children. ALSPAC Children in Focus Study Team. J Pediatr Ophthalmol Strabismus 34(4): 217–222; quiz 247–218. [DOI] [PubMed] [Google Scholar]
- Birch EE and Stager DR Sr. (2006). Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS 10(5): 409–413. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR Sr., Berry P and Leffler J (2004). Stereopsis and long-term stability of alignment in esotropia. J AAPOS 8(2): 146–150. [DOI] [PubMed] [Google Scholar]
- Birch EE, et al. (2020). Baseline and Clinical Factors Associated with Response to Amblyopia Treatment in a Randomized Clinical Trial Optom Vis Sci 2020;97:316–323. doi: 10.1097/OPX.0000000000001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C and Van Sluyters RC (1974). Experimental analysis of amblyopia and strabismus. Br J Ophthalmol 58(3): 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C and Vital-Durand F (1986). Effects of visual deprivation on the development of the monkey’s lateral geniculate nucleus. J Physiol 380: 493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Garey LJ, and Vital-Durand F (1978) The physiological effects of monocular deprivation and their reversal in the monkey visual cortex. J Physiol, 283: 223–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme GR, Liu GT, Miki A, Francis E, Dobre MC, Modestino EJ, Aleman DO and Haselgrove JC (2006). Decreased cortical activation in response to a motion stimulus in anisometropic amblyopic eyes using functional magnetic resonance imaging. J AAPOS 10(6): 540–546. [DOI] [PubMed] [Google Scholar]
- Bonnen K, Matthis JS, Gibaldi A, Banks MS, Levi DM & Hayhoe (2019). J. Vis, Vol.19, 178b. doi: 10.1167/19.10.178b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi M, Tailor VK, Anderson EJ, Bex PJ, Greenwood JA, Dahlmann-Noor A and Dakin SC (2017). Binocular Therapy for Childhood Amblyopia Improves Vision Without Breaking Interocular Suppression. Invest Ophthalmol Vis Sci 58(7): 3031–3043. [DOI] [PubMed] [Google Scholar]
- Bruce A, Pacey IE, Bradbury JA, Scally AJ and Barrett BT (2013). Bilateral changes in foveal structure in individuals with amblyopia. Ophthalmology 120(2): 395–403. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, & Merzenich MM (1998) Cortical plasticity: from synapses to maps. Annu Rev Neurosci, 21, 149–86. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Dobson V and Teller DY (1985) Postnatal development of vision in human and non-human primates. Ann. Rev. Neurosci, 8, 495–545. [DOI] [PubMed] [Google Scholar]
- Campana G, Fongoni L, Astle A & McGraw PV. (2020). Does physical exercise and congruent visual stimulation enhance perceptual learning? Ophthalmic and Physiological Optics. 2020 July 12. doi: 10.1111/opo.12712. [DOI] [PubMed] [Google Scholar]
- Chino YM, Kaas JH, Smith EL, Langston AL, & Cheng H (1992) Rapid reorganization of cortical maps in adult cats following restricted deafferation in retina. Vision Research, 32, 789–796. [DOI] [PubMed] [Google Scholar]
- Cho KK, Knibnik L, Philpot BD, & Bear MF (2009). The ratio of NR2A/B NMDA receptor subunits determines the qualities of ocular dominance plasticity in visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 106, 5377–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Kumar G, Li RW and Levi DM (2015). Characteristics of fixational eye movements in amblyopia: Limitations on fixation stability and acuity? Vision Res 114: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Li RW, Silver MA & Levi DM Donepezil does not enhance perceptual learning in adults with amblyopia: a pilot study. Frontiers in Neuroscience, 2017. 11 (448); 10.3389/fnins.2017.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Levi DM & Selenow A (1991). Amblyopia: Basic and Clinical Aspects. Stoneham, MA: Butterworth-Heinemann. [Google Scholar]
- Ciuffreda KJ, Kenyon RV & Stark L (1979). Fixational eye movements in amblyopia and strabismus. Journal of the American Optometric Association, 50, 1251–1258. 21. [PubMed] [Google Scholar]
- Clavagnier S, Dumoulin SO and Hess RF (2015). Is the Cortical Deficit in Amblyopia Due to Reduced Cortical Magnification, Loss of Neural Resolution, or Neural Disorganization? J Neurosci 35(44): 14740–14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR and Newsome WT (2009). Estimates of the contribution of single neurons to perception depend on timescale and noise correlation. J Neurosci 29(20): 6635–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner IP, Odom JV, Schwartz TL and Mendola JD (2007). Retinotopic maps and foveal suppression in the visual cortex of amblyopic adults. J Physiol 583(Pt 1): 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner IP, Odom JV, Schwartz TL and Mendola JD (2007). Monocular activation of V1 and V2 in amblyopic adults measured with functional magnetic resonance imaging. J AAPOS 11(4): 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Edwards AR, Arnold RW, Astle WF, Barnhardt CN, Beck RW, Birch EE, Donahue SP, Everett DF, Felius J, Holmes JM, Kraker RT, Melia BM, Repka MX, Wallace DK, Weise KK and Pediatric G Eye Disease Investigator (2007). Treatment of strabismic amblyopia with refractive correction. Am J Ophthalmol 143(6): 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Foster NC, Holmes JM, Melia BM, Wallace DK, Repka MX, Tamkins SM, Kraker RT, Beck RW, Hoover DL, Crouch ER 3rd, Miller AM, Morse CL and Suh DW (2012). Optical treatment of strabismic and combined strabismic-anisometropic amblyopia. Ophthalmology 119(1): 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Pediatric G Eye Disease Investigator, Edwards AR, Wallace DK, Beck RW, Arnold RW, Astle WF, Barnhardt CN, Birch EE, Donahue SP, Everett DF, Felius J, Holmes JM, Kraker RT, Melia M, Repka MX, Sala NA, Silbert DI and Weise KK (2006). Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology 113(6): 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCostanzo NR, Crowder NA, Kamermans BA, Duffy KR (2020) Retinal and optic nerve integrity following monocular inactivation for the treatment of amblyopia. Frontiers Systems Neuroscience doi:org/ 10.3389/fnsys.2020.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Klein SA and Levi DM (2013). Binocular combination in abnormal binocular vision. J Vis 13(2): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J and Levi DM (2011). Recovery of stereopsis through perceptual learning in human adults with abnormal binocular vision. Proc Natl Acad Sci U S A 108(37): E733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J and Levi DM (2014). Rebalancing binocular vision in amblyopia. Ophthalmic Physiol Opt 34(2): 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirani M, Chamberlain M, Garoufalis P, Chen C, Guymer RH and Baird PN (2006). Refractive errors in twin studies. Twin Res Hum Genet 9(4): 566–572. [DOI] [PubMed] [Google Scholar]
- Donzis PB, Rappazzo JA, Burde RM and Gordon M (1983). Effect of binocular variations of Snellen’s visual acuity on Titmus stereoacuity. Arch Ophthalmol 101(6): 930–932. [DOI] [PubMed] [Google Scholar]
- Dosher B and Lu ZL (2017). Visual Perceptual Learning and Models. Annu Rev Vis Sci 3: 343–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EI Mallah MK, Chakravarthy U, & Hart PM (2000). Amblyopia: is visual loss permanent? British Journal of Ophthalmology, 84, 952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Norcia AM: Impaired visual decision-making in individuals with amblyopia. J Vis 2011, 11:1–10 10.1167/11.14.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AE, Baldwin AS, Reynaud A & Hess RF (2018). Visual plasticity and exercise revisited: no evidence for a “cycling lane”. bioRxiv preprint doi: 10.1101/448498 [DOI] [PubMed] [Google Scholar]
- Fong MF, Mitchell DE, Duffy KR and Bear MF (2016). Rapid recovery from the effects of early monocular deprivation is enabled by temporary inactivation of the retinas. Proc Natl Acad Sci U S A 113(49): 14139–14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronius M, Cirina L, Ackermann H, Kohnen T and Diehl CM (2014). Efficiency of electronically monitored amblyopia treatment between 5 and 16 years of age: new insight into declining susceptibility of the visual system. Vision Res 103: 11–19. [DOI] [PubMed] [Google Scholar]
- Gambacorta C, Nahum M, Vedamurthy I, Bayliss J, Jordan J, Bavelier D and Levi DM (2018). An action video game for the treatment of amblyopia in children: A feasibility study. Vision Res 148: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambacorta C, Ding J, McKee SP, & Levi DM (2018). Both saccadic and manual responses in the amblyopic eye of strabismics are irreducibly delayed. J Vis, 18 (3), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao TY, Anstice N, Babu RJ, Black JM, Bobier WR, Dai S, Guo CX, Hess RF, Jenkins M, Jiang Y, Kearns L, Kowal L, Lam CSY, Pang PCK, Parag V, South J, Staffieri SE, Wadham A, Walker N, Thompson B and Binocular T Treatment of Amblyopia Using Videogames Study (2018a). Optical treatment of amblyopia in older children and adults is essential prior to enrolment in a clinical trial. Ophthalmic Physiol Opt 38(2): 129–143. [DOI] [PubMed] [Google Scholar]
- Gao TY, Guo CX, Babu RJ, Black JM, Bobier WR, Chakraborty A, Dai S, Hess RF, Jenkins M, Jiang Y, Kearns LS, Kowal L, Lam CSY, Pang PCK, Parag V, Pieri R, Raveendren RN, South J, Staffieri SE, Wadham A, Walker N, Thompson B and Team BS (2018b). Effectiveness of a Binocular Video Game vs Placebo Video Game for Improving Visual Functions in Older Children, Teenagers, and Adults With Amblyopia: A Randomized Clinical Trial. JAMA Ophthalmol 136(2): 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E 1963. Perceptual learning. Annu Rev Psychol, 14, 29–56. [DOI] [PubMed] [Google Scholar]
- González EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ (2012). Eye position stability in amblyopia and in normal binocular vision. Investigative Ophthalmology & Visual Science, 53, 5386–5394. [DOI] [PubMed] [Google Scholar]
- Goodyear BG, Nicolle DA, Humphrey GK and Menon RS (2000). BOLD fMRI response of early visual areas to perceived contrast in human amblyopia. J Neurophysiol 84(4): 1907–1913. [DOI] [PubMed] [Google Scholar]
- Grant S, Melmoth DR, Morgan MJ and Finlay AL (2007). Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci 48(3): 1139–1148. [DOI] [PubMed] [Google Scholar]
- Hallum LE, Shooner C, Kumbhani RD, Kelly JG, Garcia-Marin V, Majaj NJ, Movshon JA and Kiorpes L (2017). Altered Balance of Receptive Field Excitation and Suppression in Visual Cortex of Amblyopic Macaque Monkeys. J Neurosci 37(34): 8216–8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CJ, Snieder H, Gilbert CE and Spector TD (2001). Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci 42(6): 1232–1236. [PubMed] [Google Scholar]
- Harrad RA and Hess RF (1992). Binocular integration of contrast information in amblyopia. Vision Res 32(11): 2135–2150. [DOI] [PubMed] [Google Scholar]
- Harrad R, Sengpiel F and Blakemore C (1996). Physiology of suppression in strabismic amblyopia. Br J Ophthalmol 80(4): 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL III, Duncan GC, Crawford MLJ and von Noorden GK (1987) Multiple sensitive periods in the development of the primate visual system. Science, 232, 235–238. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL III, Duncan GC, Crawford MLJ and von Noorden GK (1990) Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav. Brain Res, 41, 179–198. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, & Skavenski AA (1991). Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Experimental Brain Research, 83, 670–674. [DOI] [PubMed] [Google Scholar]
- Hensch TK & Quinlan EM (2018). Critical periods in amblyopia. Visual Neuroscience (2018), 35, e014, 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison N, MacKeith D, Vivian A, Purdy J, Fakis A, Ash IM, Cobb SV, Eastgate RM, Haworth SM, Gregson RM, & Foss AJ (2016). Randomised controlled trial of video clips and interactive games to improve vision in children with amblyopia using the I-BiT system. Br J Ophthalmol, 100 (11), 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison N, Cobb S, Gregson R, Ash I, Eastgate R, Purdy J, Hepburn T, MacKeith D, Foss A, & group I.B.s. (2013). Interactive binocular treatment (I-BiT) for amblyopia: results of a pilot study of 3D shutter glasses system. Eye (Lond), 27 (9), 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF (1991). The site and nature of suppression in squint amblyopia. Vision Res 31(1): 111–117. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Gole G and Mullen KT (2009). Deficient responses from the lateral geniculate nucleus in humans with amblyopia. Eur J Neurosci 29(5): 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Thompson B and Baker DH (2014). Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic Physiol Opt 34(2): 146–162. [DOI] [PubMed] [Google Scholar]
- Hess RF and Thompson B (2015). Amblyopia and the binocular approach to its therapy. Vision Res 114: 4–16. [DOI] [PubMed] [Google Scholar]
- Ho CS, Giaschi DE, Boden C, Dougherty R, Cline R and Lyons C (2005). Deficient motion perception in the fellow eye of amblyopic children. Vision Res 45(12): 1615–1627. [DOI] [PubMed] [Google Scholar]
- Ho CS and Giaschi DE (2009). Low- and high-level first-order random-dot kinematograms: evidence from fMRI. Vision Res 49(14): 1814–1824. [DOI] [PubMed] [Google Scholar]
- Hohmann A, & Creutzfeldt OD (1975) Squint and the development of binocularity in humans. Nature, 254, 613–614. [DOI] [PubMed] [Google Scholar]
- Holman KD, Duffy KR, Mitchell DE (2018) Short periods of darkness fail to restore visual or neural plasticity in adult cats. Visual Neuroscience doi: 10.1017/S0952523817000335 [DOI] [PubMed] [Google Scholar]
- Holmes JM, Beck RW, Kraker RT, Astle WF, Birch EE, Cole SR, Cotter SA, Donahue S, Everett DF, Hertle RW, Keech RV, Paysse E, Quinn GF, Repka MX, Scheiman MM and Pediatric G Eye Disease Investigator (2004). Risk of amblyopia recurrence after cessation of treatment. J AAPOS 8(5): 420–428. [DOI] [PubMed] [Google Scholar]
- Holmes JM and Levi DM (2018). Treatment of amblyopia as a function of age. Vis Neurosci 35: E015. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Manh VM, Lazar EL, Beck RW, Birch EE, Kraker RT, Crouch ER, Erzurum SA, Khuddus N, Summers AI, Wallace DK, & Pediatric Eye Disease Investigator, G. (2016). Effect of a Binocular iPad Game vs Part-time Patching in Children Aged 5 to 12 Years With Amblyopia: A Randomized Clinical Trial. JAMA Ophthalmol, 134 (12), 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopigian K, Blake R and Greenwald MJ (1988). Clinical suppression and amblyopia. Invest Opthalmol Vis Sci 29(3): 444–451. [PubMed] [Google Scholar]
- Huang CB, Zhou Y and Lu ZL (2008). Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci U S A 105(10): 4068–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH and Wiesel TN (1970) The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. (Lond), 206, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D and Cotter S (2018). Early diagnosis of amblyopia. Vis Neurosci 35: E013. [DOI] [PubMed] [Google Scholar]
- Imamura K, Richter H, Fischer H, Lennerstrand G, Franzen O, Rydberg A, Andersson J, Schneider H, Onoe H, Watanabe Y and Langstrom B (1997). Reduced activity in the extrastriate visual cortex of individuals with strabismic amblyopia. Neurosci Lett 225(3): 173–176. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, & Stryker MP (2008). Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron, 58, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Huppé-Gourgues F, & Vaucher E (2014). Boosting visual cortex function and plasticity with acetylcholine to enhance visual perception. Front. Syst. Neurosci, 8, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN, & Birch EE (2016). Binocular iPad Game vs Patching for Treatment of Amblyopia in Children: A Randomized Clinical Trial. JAMA Ophthalmol, 134 (12), 1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind PC, Mitchell DE, Ahmed B, Blakemore C, Bonhoeffer T and Sengpiel F (2002). Correlated binocular activity guides recovery from monocular deprivation. Nature 416(6879): 430–433. [DOI] [PubMed] [Google Scholar]
- Kiorpes L 2006. Visual processing in amblyopia: Animal Studies. Strabismus, 14, 3–10. [DOI] [PubMed] [Google Scholar]
- Kiorpes L (2019). Understanding the development of amblyopia using macaque monkey models. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, & Boothe RG (1981) Naturally occurring strabismus in monkeys (Macaca nemestrina). Invest. Ophthalmol. Vis. Sci, 20, 257–263. [PubMed] [Google Scholar]
- Kiorpes L, Carlson MR, Alfi D, & Boothe RG (1989) Development of visual acuity in experimentally strabismic monkeys. Clin. Vision Sci, 4, 95–106. [Google Scholar]
- Kiorpes L and Daw N (2018). Cortical correlates of amblyopia. Vis Neurosci 35: E016. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR and Movshon JA (1998). Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci 18(16): 6411–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox PJ, Simmers AJ, Gray LS, & Cleary M (2012). An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci, 53 (2), 817–824. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM (1998). Evidence for Striatal Dopamine Release During a Video Game. Nature, 393 (6682), 266–8 [DOI] [PubMed] [Google Scholar]
- Kupfer C (1957). Treatment of amblyopia exanopsia in adults; a preliminary report of seven cases. Am J Ophthalmol 43(6): 918–922. [DOI] [PubMed] [Google Scholar]
- Kvarnstrom G, Jakobsson P and Lennerstrand G (2001). Visual screening of Swedish children: an ophthalmological evaluation. Acta Ophthalmol Scand 79(3): 240–244. [DOI] [PubMed] [Google Scholar]
- Legas AK, Black JM, Russell BR, Kydd RR, Thompson B (2019) The effect of combined patching and citralopram on visual acuity in adults with amblyopia: a randomized, crossover, placebo-controlled trial. Neural Plasticity 10.1155/2019/5857243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE and Gu Y (1989). Stereopsis and contrast. Vision Res 29(8): 989–1004. [DOI] [PubMed] [Google Scholar]
- Leguire LE, Rogers GL, Bremer DL, Walson PD, McGregor ML 1993. Levodopa/carbidopa for childhood amblyopia. Invest. Ophthalmol. Vis. Sci 34, 3090–3095. [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Malach R, Harel M, Leiba H, Stolovitch C and Pianka P (2006). Selective fovea-related deprived activation in retinotopic and high-order visual cortex of human amblyopes. Neuroimage 33(1): 169–179. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Pianka P, Azmon B, Leiba H, Stolovitch C, Loewenstein A, Harel M, Hendler T and Malach R (2003). Area-specific amblyopic effects in human occipitotemporal object representations. Neuron 40(5): 1023–1029. [DOI] [PubMed] [Google Scholar]
- Levay S, Wiesel TN and Hubel DH (1980) The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol, 191, 1–5. [DOI] [PubMed] [Google Scholar]
- Levi DM 1994. Pathophysiology of binocular vision and amblyopia. Curr. Opin. Ophthalmol 5, 3–10. [PubMed] [Google Scholar]
- Levi DM 2006. Visual processing in amblyopia: human studies. Strabismus 14, 11–19. [DOI] [PubMed] [Google Scholar]
- Levi DM (2012). Prentice award lecture 2011: removing the brakes on plasticity in the amblyopic brain. Optom Vis Sci 89(6): 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM (2013). Linking assumptions in amblyopia. Vis Neurosci 30(5–6): 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, & Carkeet A (1993). Amblyopia: A consequence of abnormal visual development In: Simons K (Ed.) Early Visual Development, Normal and Abnormal (pp. 391–408). Oxford, UK: Oxford University Press. [Google Scholar]
- Levi DM, Harwerth RS and Smith EL 3rd (1979). Humans deprived of normal binocular vision have binocular interactions tuned to size and orientation. Science 206(4420): 852–854. [DOI] [PubMed] [Google Scholar]
- Levi DM, Harwerth RS and Smith EL (1980). Binocular interactions in normal and anomalous binocular vision. Doc Ophthalmol 49(2): 303–324. [DOI] [PubMed] [Google Scholar]
- Levi DM, & Klein SA (1986) Sampling in spatial vision. Nature, 320, 360–2. [DOI] [PubMed] [Google Scholar]
- Levi DM and Klein SA (2003). Noise provides some new signals about the spatial vision of amblyopes. J Neurosci 23(7): 2522–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Klein SA and Chen I (2008). What limits performance in the amblyopic visual system: seeing signals in noise with an amblyopic brain. J Vis 8(4): 1 1–23. [DOI] [PubMed] [Google Scholar]
- Levi DM, Knill DC and Bavelier D (2015). Stereopsis and amblyopia: A mini-review. Vision Res 114: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, McKee SP and Movshon JA (2011). Visual deficits in anisometropia. Vision Res 51(1): 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM and Li RW (2009). Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Res 49(21): 2535–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, & Polat U (1996). Neural plasticity in adults with Amblyopia. Proceedings of the National Academy of Sciences of the United States of America, 93, 6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U, & Hu YS (1997). Improvement in vernier acuity in adults with amblyopia. Practice makes better. Investigative Ophthalmology and Visual Science, 38, 1493–1510. [PubMed] [Google Scholar]
- Lewis TL and Maurer D (2005). Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol 46(3): 163–183. [DOI] [PubMed] [Google Scholar]
- Li SL, Jost RM, Morale SE, Stager DR, Dao L, Stager D, & Birch EE (2014). A binocular iPad treatment for amblyopic children. Eye (Lond), 28 (10), 1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Klein SA & Levi DM (2008) Prolonged Perceptual Learning of Positional Acuity in Adult Amblyopia: Perceptual Template Retuning Dynamics. J. Neurosci, 28, 14223–14229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Dumoulin SO, Mansouri B and Hess RF (2007). Cortical deficits in human amblyopia: their regional distribution and their relationship to the contrast detection deficit. Invest Ophthalmol Vis Sci 48(4): 1575–1591. [DOI] [PubMed] [Google Scholar]
- Li X, Mullen KT, Thompson B and Hess RF (2011). Effective connectivity anomalies in human amblyopia. Neuroimage 54(1): 505–516. [DOI] [PubMed] [Google Scholar]
- Li RW, Ngo C, Nguyen J and Levi DM (2011). Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol 9(8): e1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Ngo CV and Levi DM (2015). Relieving the attentional blink in the amblyopic brain with video games. Sci Rep 5: 8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Tran KD, Bui JK, Antonucci MM, Ngo CV and Levi DM (2018). Improving Adult Amblyopic Vision with Stereoscopic 3-Dimensional Video Games. Ophthalmology 125(10): 1660–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon SE and Simonsz HJ (2005). The history of the treatment of amblyopia. Strabismus 13(2): 93–106. [DOI] [PubMed] [Google Scholar]
- Lunghi C & Sale A (2015). A cycling lane for brain rewiring. Curr. Biol; 25:R1122–R1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara G, Thompson B, Mansouri B, Farivar R and Hess RF (2011). The perceptual consequences of interocular suppression in amblyopia. Invest Ophthalmol Vis Sci 52(12): 9011–9017. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Sengpiel F (2018) Animal models of amblyopia. Visual Neuroscience doi: 10.1017/S0952523817000244. [DOI] [PubMed] [Google Scholar]
- Lunghi C, Sframeli AT, Lepri A, Lisi D, Sale A & Morrone MC (2019). A new counterintuitive training for adult amblyopia. Annals of Clinical and Translational Neurology; 6(2): 274–284. doi: 10.1002/acn3.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manh VM, Holmes JM, Lazar EL, Kraker RT, Wallace DK, Kulp MT, Galvin JA, Shah BK, Davis PL and Pediatric G Eye Disease Investigator (2018). A Randomized Trial of a Binocular iPad Game Versus Part-Time Patching in Children Aged 13 to 16 Years With Amblyopia. Am J Ophthalmol 186: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri B, Thompson B and Hess RF (2008). Measurement of suprathreshold binocular interactions in amblyopia. Vision Res 48(28): 2775–2784. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Otero-Millan J, & Macknik SL (2013). The impact of microsaccades on vision: towards a unified theory of saccadic function. Nat Rev Neurosci, 14 (2), 83–96. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL and Tytla ME (1983) Contrast sensitivity in cases of unilateral congenital cataract. Invest. Ophthal. Visual Sci, (Suppl.), 24, 21. [Google Scholar]
- Maurer D, Lewis TL, Brent HP, & Levin AV (1999) Rapid improvement in the acuity of infants after visual input. Science, 286, 108–10. [DOI] [PubMed] [Google Scholar]
- Maurer D and McKee SP (2018). Classification and diversity of amblyopia. Vis Neurosci 35: E012. [DOI] [PubMed] [Google Scholar]
- Mckee SP, Levi DM, & Movshon JA (2003). The pattern of visual deficits in amblyopia. Journal of Vision, 3, 380–405. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Schor CM and Movshon JA (2016). Saccadic latency in amblyopia. J Vis 16(5): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmoth DR, Finlay AL, Morgan MJ and Grant S (2009). Grasping deficits and adaptations in adults with stereo vision losses. Invest Ophthalmol Vis Sci 50(8): 3711–3720. [DOI] [PubMed] [Google Scholar]
- Melmoth DR, Storoni M, Todd G, Finlay AL and Grant S (2007). Dissociation between vergence and binocular disparity cues in the control of prehension. Exp Brain Res 183(3): 283–298. [DOI] [PubMed] [Google Scholar]
- Mendola JD, Lam J, Rosenstein M, Lewis LB and Shmuel A (2018). Partial correlation analysis reveals abnormal retinotopically organized functional connectivity of visual areas in amblyopia. Neuroimage Clin 18: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Bansal A and Prakash P (1997). Randot stereoacuity at various binocular combinations of Snellen acuity. Indian J Ophthalmol 45(3): 169–171. [PubMed] [Google Scholar]
- Mohindra I, Jacobson SG, Thomas J and Held R (1979) Development of amblyopia in infants. Trans. Ophthal. Soc. U.K, 99, 344–346. [PubMed] [Google Scholar]
- Mollon JD, & Danilova MV (1996) Three remarks on perceptual learning. Spatial Vision, 10, 51–58. [DOI] [PubMed] [Google Scholar]
- Morishita H & Hensch TK (2008). Critical period revisited: impact on vision. Curr Opin Neurobiol, 18, 101–7. [DOI] [PubMed] [Google Scholar]
- Movshon JA and Van Sluyters RC (1981). Visual neural development. Annu Rev Psychol 32: 477–522. [DOI] [PubMed] [Google Scholar]
- Muckli L, Kiess S, Tonhausen N, Singer W, Goebel R and Sireteanu R (2006). Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Res 46(4): 506–526. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Chandrakumar M, Goltz HC and Wong AM (2012a). Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior, I: saccadic eye movements. Invest Ophthalmol Vis Sci 53(12): 7458–7468. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Kennedy SA, Colpa L, Chandrakumar M, Goltz HC and Wong AM (2012b). Effects of induced monocular blur versus anisometropic amblyopia on saccades, reaching, and eye-hand coordination. Invest Ophthalmol Vis Sci 53(8): 4354–4362. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Appelbaum LG, Ales JM, Cottereau BR, Rossion B. The steady-state visual evoked potential in vision research: A review. (2015) Journal of Vision. 15(6):4. doi: 10.1167/15.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR, & Freeman RD (1980). Profile of the sensitive period for monocular deprivation in kittens. Experimental Brain Research, 39, 17–21. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group 2005. Randomized Trial of Treatment of Amblyopia in Children Aged 7 to 17 Years. Am. J. Ophthalmol, 143, 1634–42. [Google Scholar]
- Pediatric Eye Disease Investigator Group Writing, R. C Rutstein P, Quinn GE, Lazar EL, Beck RW, Bonsall DJ, Cotter SA, Crouch ER, Holmes JM, Hoover DL, Leske DA, Lorenzana IJ, Repka MX and Suh DW (2010). A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology 117(5): 998–1004 e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MEF and Simmers AJ (2019). ‘It’s too late’. Is it really? Considerations for amblyopia treatment in older children. Ther Adv Ophthalmol 11: 2515841419857379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popple AV and Levi DM (2008). The attentional blink in amblyopia. J Vis 8(13): 12 11–19. [DOI] [PubMed] [Google Scholar]
- Portela-Camino JA, Martín-González S, Ruiz-Alcocer J, Illarramendi-Mendicute I, Garrido-Mercado R. (2018). A Random Dot Computer Video Game Improves Stereopsis. Optom Vis Sci. 95(6):523–535. doi: 10.1097/OPX.0000000000001222. [DOI] [PubMed] [Google Scholar]
- Rahi JS, Logan S, Borja MC, Timms C, Russell-Eggitt I, & Taylor D (2002). Prediction of improved vision in the amblyopic eye after visual loss in the non-amblyopic eye. Lancet, 360, 621–622. [DOI] [PubMed] [Google Scholar]
- Ranson A, Cheetham CE, Fox K, & Sengpiel F (2012). Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proceedings of the National Academy of Sciences of the United States of America, 109, 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Wallace DK, Beck RW, Kraker RT, Birch EE, Cotter SA, Donahue S, Everett DF, Hertle RW, Holmes JM, Quinn GE, Scheiman MM, Weakley DR and Pediatric G Eye Disease Investigator (2005). Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol 123(2): 149–157. [DOI] [PubMed] [Google Scholar]
- Repka MX, Cotter SA, Beck RW, Kraker RT, Birch EE, Everett DF, Hertle RW, Holmes JM, Quinn GE, Sala NA, Scheiman MM, Stager DR Sr., Wallace DK and Pediatric G Eye Disease Investigator (2004). A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology 111(11): 2076–2085. [DOI] [PubMed] [Google Scholar]
- Repka MX, Beck RW, Holmes JM, Birch EE, Chandler DL, Cotter SA, Hertle RW, Kraker RT, Moke PS, Quinn GE, Scheiman MM and Pediatric G Eye Disease Investigator (2003). A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol 121(5): 603–611. [DOI] [PubMed] [Google Scholar]
- Revell MJ. 1971 Strabismus: A History Orthoptic Techniques: London, Barrie and Jenkins. [Google Scholar]
- Rokem A, & Silver MA (2010). Cholinergic enhancement augments magnitude and specificity of visual perceptual learning in healthy humans. Curr Biol, 20, 1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokem A, & Silver MA (2013). The benefits of cholinergic enhancement during perceptual learning are long-lasting. Front Comput Neurosci, 7, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucci M, & Victor JD (2015). The unsteady eye: an information-processing stage, not a bug. Trends Neurosci, 38 (4), 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R & Maffei L (2008) Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci, 10, 679–81. [DOI] [PubMed] [Google Scholar]
- Scholes C, McGraw PV, Nystrom M, & Roach NW (2015). Fixational eye movements predict visual sensitivity. Proc Biol Sci, 282 (1817), 20151568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor C & Hallmark W (1978). Slow control of eye position in strabismic amblyopia. Investigative Ophthalmology & Visual Science, 17, 577–581. [PubMed] [Google Scholar]
- Secen J, Culham J, Ho C and Giaschi D (2011). Neural correlates of the multiple-object tracking deficit in amblyopia. Vision Res 51(23–24): 2517–2527. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Blakemore C, Kind PC and Harrad R (1994). Interocular suppression in the visual cortex of strabismic cats. J Neurosci 14(11 Pt 2): 6855–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT and Movshon JA (1996). A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci 16(4): 1486–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Levi DM and Klein SA (2000). Undercounting features and missing features: evidence for a high-level deficit in strabismic amblyopia. Nat Neurosci 3(5): 496–501. [DOI] [PubMed] [Google Scholar]
- Shooner C, Hallum LE, Kumbhani RD, Garcia-Marin V, Kelly JG, Majaj NJ, Movshon JA and Kiorpes L (2017). Asymmetric Dichoptic Masking in Visual Cortex of Amblyopic Macaque Monkeys. J Neurosci 37(36): 8734–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrand J and Abrahamsson M (1996). [Can we identify risk groups for the development of amblyopia and strabismus?]. Klin Monbl Augenheilkd 208(1): 23–26. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Brewer AA, Schmid MC, Tolias AS, Schuz A, Augath M, Inhoffen W, Wandell BA and Logothetis NK (2005). Lack of long-term cortical reorganization after macaque retinal lesions. Nature 435(7040): 300–307. [DOI] [PubMed] [Google Scholar]
- Smith E. L. d., Levi DM, Manny RE, Harwerth RS and White JM (1985). The relationship between binocular rivalry and strabismic suppression. Invest Ophthalmol Vis Sci 26(1): 80–87. [PubMed] [Google Scholar]
- Spiegel DP, Byblow WD, Hess RF, and Thompson B (2013). Anodal Transcranial Direct Current Stimulation Transiently Improves Contrast Sensitivity and Normalizes Visual Cortex Activation in Individuals With Amblyopia. Neurorehabil Neural Repair 27 (8), 760–9 [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Fielder AR, Stephens DA and Cooperative M (2004a). Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol 88(12): 1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Stephens DA and Fielder AR (2004b). Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci 45(9): 3048–3054. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ and Cooperative M (2007). Modeling dose-response in amblyopia: toward a child-specific treatment plan. Invest Ophthalmol Vis Sci 48(6): 2589–2594. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Jost RM and Birch EE (2013). A quantitative study of fixation stability in amblyopia. Invest Ophthalmol Vis Sci 54(3): 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle CM, Melmoth DR, Finlay AL, Sloper JJ and Grant S (2011). Eye-hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci 52(3): 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Zhang B, Shen G, Wensveen J, Smith EL 3rd, Nishimoto S, Ohzawa I and Chino YM (2014). Early monocular defocus disrupts the normal development of receptive-field structure in V2 neurons of macaque monkeys. J Neurosci 34(41): 13840–13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller DY, & Movshon JA (1986) Visual Development. Vision Research, 26, 1483–1506. [DOI] [PubMed] [Google Scholar]
- Thompson B, Mansouri B, Koski L & Hess RF 2008. Brain Plasticity in the Adult: Modulation of Function in Amblyopia with rTMS. Curr. Biol 18, 1067–1071. [DOI] [PubMed] [Google Scholar]
- Tsirlin I, Colpa L, Goltz HC and Wong AM (2015). Behavioral Training as New Treatment for Adult Amblyopia: A Meta-Analysis and Systematic Review. Invest Ophthalmol Vis Sci 56(6): 4061–4075. [DOI] [PubMed] [Google Scholar]
- Tytla ME and Labow-Daily LS (1981). Evaluation of the CAM treatment for amblyopia: a controlled study. Invest Ophthalmol Vis Sci 20(3): 400–406. [PubMed] [Google Scholar]
- Vedamurthy I, Knill DC, Huang SJ, Yung A, Ding J, Kwon OS, Bavelier D and Levi DM (2016). Recovering stereo vision by squashing virtual bugs in a virtual reality environment. Philos Trans R Soc Lond B Biol Sci 371(1697). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedamurthy I, Nahum M, Bavelier D and Levi DM (2015). Mechanisms of recovery of visual function in adult amblyopia through a tailored action video game. Sci Rep 5: 8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedamurthy I, Nahum M, Huang SJ, Zheng F, Bayliss J, Bavelier D and Levi DM (2015). A dichoptic custom-made action video game as a treatment for adult amblyopia. Vision Res 114: 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese P, McKee SP and Levi DM (2019). Attention deficits in Amblyopia. Curr Opin Psychol 29: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castrén E & Maffei L (2008) Science, 320, 385–8. [DOI] [PubMed] [Google Scholar]
- Vedamurthy I, Knill DC, Huang SJ, Yung A, Ding J, Kwon OS, Bavelier D & Levi DM (2016). Recovering stereo vision by squashing virtual bugs in a virtual reality environment. Philos Trans R Soc Lond B Biol Sci 371(1697). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecken EP, & Brabant P, (1984) Prognosis for vision in amblyopia after the loss of the good eye. Archives in Ophthalmology, 102, 220–4. [DOI] [PubMed] [Google Scholar]
- von Noorden GK (1965). Occlusion Therapy in Amblyopia. Arch. Ophthal, 73, 776–781. [DOI] [PubMed] [Google Scholar]
- von Noorden GK and Crawford MLJ (1979) The sensitive period. Trans Opthalmol Soc UK, 99: 442–446. [PubMed] [Google Scholar]
- von Noorden GK (1981) New clinical aspects of stimulus deprivation amblyopia. Am. J. Ophthal, 92, 416–421. [DOI] [PubMed] [Google Scholar]
- von Noorden GK (1990). Binocular Vision and Ocular Motility. St. Louis, C.V. Mosby [Google Scholar]
- Wallace DK, Pediatric G Eye Disease Investigator, Edwards AR, Cotter SA, Beck RW, Arnold RW, Astle WF, Barnhardt CN, Birch EE, Donahue SP, Everett DF, Felius J, Holmes JM, Kraker RT, Melia M, Repka MX, Sala NA, Silbert DI and Weise KK (2006). A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology 113(6): 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SP, Li QX and Li S (2020). Systematic Evaluation of Levodopa Effect on Visual Improvement in Amblyopia: A Meta-analysis. Clin Neuropharmacol 43(1): 20–25. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang B, Tao X, Wensveen JM, Smith ELR and Chino YM (2017). Noisy Spiking in Visual Area V2 of Amblyopic Monkeys. J Neurosci 37(4): 922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA & Brown B (2008). Effect of amblyopia on self-esteem in children. Optom Vis Sci, 85, 1074–81. [DOI] [PubMed] [Google Scholar]
- Westheimer G and McKee SP (1980). Stereoscopic acuity with defocused and spatially filtered retinal images. Journal of the Optical Society of America 70: 772–778. [DOI] [PubMed] [Google Scholar]
- Wiesel TN (1982) Postnatal development of the visual cortex and the influence of environment. Nature, 299, 583–591. [DOI] [PubMed] [Google Scholar]
- Wilmer JB and Backus BT (2009). Genetic and environmental contributions to strabismus and phoria: evidence from twins. Vision Res 49(20): 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Kee-You AM, Wei H & Hou C (2020). Feature Counting Under Dichoptic Viewing in Anisometropic and Strabismic Amblyopia. Trans Vis Sci Tech 9(6):13, 10.1167/tvst.9.6.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff G, Hiscox F, Thompson JR, & Smith LK (1994). Factors affecting the outcome of children treated for amblyopia. Eye (London), 8(Pt 6), 627–631. [DOI] [PubMed] [Google Scholar]
- Worth CA (1903) Squint: Its causes, pathology and treatment. The Blakiston Co., Philadelphia. [Google Scholar]
- Xi J, Jia WL, Feng LX, Lu ZL, & Huang CB (2014). Perceptual learning improves stereoacuity in amblyopia. Investigative Ophthalmology & Visual Science, 55(4), 2384–2391 [April 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tao X, Wensveen JM, Harwerth RS, Smith EL 3rd, Chino YM. (2011). Effects of brief daily periods of unrestricted vision during early monocular form deprivation on development of visual area 2. Invest Ophthalmol Vis Sci. 2011 September 14;52(10):7222–31. doi: 10.1167/iovs.11-7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Clavagnier S & Hess RF (2013). Short-term monocular deprivation strengthens the patched eye’s contribution to binocular combination. Journal of Vision, vol. 13, (5). [DOI] [PubMed] [Google Scholar]
- Zhou J, Reynaud A & Hess RF (2017). Aerobic exercise effects on ocular dominance plasticity with a phase combination task in human adults. Neural Plasticity, Article ID 4780876, 7 pages, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, et al. , (2019). Inverse Occlusion: A Binocularly Motivated Treatment for Amblyopia. Neural Plasticity Article ID 5157628, 12 pages 10.1155/2019/5157628 [DOI] [PMC free article] [PubMed] [Google Scholar]


