Abstract
Antiphospholipid syndrome (APS) is one of the more common acquired causes of hypercoagulability. Its major presentations are thrombotic (arterial, venous or microvascular) and pregnancy morbidity (miscarriages, late intrauterine fetal demise, and severe pre-eclampsia). Classification criteria include three different antiphospholipid antibodies: lupus anticoagulant; anticardiolipin; and anti-beta 2 glycoprotein I. Management includes both preventive strategies (low dose aspirin, hydroxychloroquine) and long-term anticoagulation after thrombosis.
1. Introduction
Antiphospholipid syndrome (APS) is one of the more common acquired causes of hypercoagulability. Its major presentations are thrombotic (arterial, venous or microvascular) and pregnancy morbidity (miscarriages, late intrauterine fetal demise, and severe pre-eclampsia). Classification criteria include three different antiphospholipid antibodies: lupus anticoagulant; anticardiolipin; and anti-beta 2 glycoprotein I. Management includes both preventive strategies (low dose aspirin, hydroxychloroquine) and long-term anticoagulation after thrombosis.
2. Antibodies and Assays
There are three antiphospholipid antibodies listed in the classification criteria, but “non-criteria” antibodies exist as well (Tables Table 1). The most important antiphospholipid antibody is the lupus anticoagulant, as it is most strongly associated with both thrombotic1,2 and adverse pregnancy outcomes3. Lupus anticoagulant assays are functional, using plasma. Ideally the plasma must be platelet poor. International Society on Thrombosis and Haemostasis (ISTH) criteria4 for determination of a lupus anticoagulant include three steps. The first step is a sensitive screening assay. As lupus anticoagulants are heterogeneous, a battery of screening assays is preferred, usually including the dilute Russell Viper Venom time and a sensitive partial thromboplastin time (APTT). The next step is a mixing study to conclude that the prolongation of the screening time is not due to a factor deficiency. Although the mixing step is usually “one to one”, some lupus anticoagulants can correct with a one-to-one mix with normal plasma. Thus, a two-to-one mix can also be done. The final, or confirmatory step, is to prove that the inhibitor is phospholipid dependent.
Table 1 –
Antiphospholipid Antibodies
| Criteria Antiphospholipid Antibodies | Non-Criteria Antiphospholipid Antibodies |
|---|---|
| Lupus anticoagulant Anticardiolipin Anti-beta 2 glycoprotein I |
Anti-phosphatidylserine/prothrombin (aPS/PT) Domain-specific anti-beta 2 glycoprotein I Annexin A5 IgA isotopes |
Limitations of the lupus anticoagulant assay include the handling of “anticoagulated” patients. Heparin can be removed from the plasma before the assay. A few laboratories have validated their confirmatory values in patients treated with warfarin (as the mixing step does replace factors 2, 7, 9, 10). It is now possible to do valid lupus anticoagulant testing on direct oral anticoagulants (DOACs), using kits such as DOAC STOP5,6.
The other antiphospholipid antibodies are ascertained based on ELISA assays. The two most commonly done are anticardiolipin and anti-beta 2 glycoprotein 1. Although these assays are available as IgG, IgM and IgA isotypes, most of the data on IgA comes from the United States where the IgA essays are done routinely.
In addition to the classic antibodies that are mentioned in the classification criteria, there are other antiphospholipid antibodies, sometimes called “non-criteria” antibodies. As anti-phosphatidylserine/prothrombin is performed by ELISA assay, it is valid in anticoagulated patients. Anti-phosphatidylserine/prothrombin is strongly associated with the presence of the lupus anticoagulant and with thrombosis7,8. A strong case can be made that it should be elevated to the level of a “criteria” antiphospholipid antibody.
3. Mechanism of Action: Thrombosis and/or Inflammation
Targets of Lupus Anticoagulants
Because antiphospholipid antibodies are heterogeneous, they might have multiple mechanisms of action. The lupus anticoagulant, for example, can be split into two subtypes, those which target beta 2 glycoprotein 1 and those which target prothrombin9.
Most lupus anticoagulants increase the risk of thrombosis. However, pediatric patients with a lupus anticoagulant that targets prothrombin can develop a hypoprothrombinemia state and then present with bleeding, rather than thrombosis10.
Some lupus anticoagulants act via annexin A5. By blocking (putting “holes”) in the annexin shield, the phospholipid bilayer is exposed and vulnerable to clot (including the placenta)11.
Thrombocytopenia
Antiphospholipid antibodies bind to beta 2 glycoprotein I receptors on platelets, leading to activation and aggregation12,13. Thrombocytopenia, which is present in about a third of patients with antiphospholipid syndrome14, is due to binding and activation of platelets15. Antiphospholipid syndrome is one of several examples (including thrombotic thrombocytopenic purpura, diffuse intravascular coagulation, heparin induced thrombocytopenia, and paroxysmal nocturnal hemoglobinuria) in which a pro-thrombotic state occurs in the setting of thrombocytopenia.
Inflammatory Targets
Inflammatory manifestations of antiphospholipid antibodies include some of the non-thrombotic neurologic syndromes associated with antiphospholipid antibodies (including chorea and longitudinal myelitis)16,17. Cardiac valvulitis is a mixture of an inflammatory effect of antiphospholipid antibodies binding to the mitral and aortic valves as well as superimposed thrombosis with fibrin deposition18. Antiphospholipid syndrome nephropathy, however, may be a microvascular complication rather than inflammatory19.
Molecular Events in APS
Antiphospholipid antibodies bind to receptors on endothelial cells leading to eNOS inhibition, impairing nitric oxide production and release20,21. This leads to endothelial dysfunction22, likely through the transcription factors KLF2 and KLF4, critical regulators of eNOS and endothelial cells23. There can later be endothelial cell proliferation and intimal hyperplasia24–28.
Different molecular mechanisms may underlie the two major subsets of APS, obstetric APS and thrombotic APS. Gene expression profiling of monocytes exposed to IgG from thrombotic APS found upregulation of genes associated with cell response to stress, regulation of MAPK signaling pathway and cell communication. The obstetric APS analysis found genes involved in cell adhesion, extracellular matrix and embryonic and skeletal development29.
In thrombotic APS, neutrophils have spontaneous neutrophil extracellular trap (NET) release with products then incorporated into thrombi. Surface adenosine receptors trigger cyclicAMP in neutrophils, regulating NETosis. Selective agonism of the adenosine A2A receptor and dipyridamole (which increases extracellular adenosine) suppress antiphospholipid induced NETosis30. PSGL-1, a neutrophil protein that mediates adhesion to the endothelium, is a regulator of the pro-thrombotic neutrophil functions31. APS neutrophils also have upregulation of CD64, CEACAM1, beta-2 glycoprotein and activated MAC-1, explaining their increased adhesion32.
In obstetric APS, complement activation products and TNFα, among others, contribute to fetal loss33. There is impairment of cell adhesion molecules in the trophoblast and decidua, as well as defects in endometrial differentiation. Compromise of the mitochondria in obstetric APS may increase the risk of pre-eclampsia34.
Role of Complement
Animal models have been particularly helpful in tying together both thrombotic and obstetric APS through the single mechanism of complement activation. In a pregnancy model, obstetric APS required complement activation and was blocked by complement inhibition35. The benefit of prophylactic doses of heparin in the model could be explained by heparin blocking complement activation36. In a different animal model for thrombotic APS37, blockade of complement prevented thrombosis from infusion of antiphospholipid antibodies.
4. Classification Criteria
There have been multiple classification criteria for antiphospholipid syndrome, with the most recent, that of Miyakis et al38 called the Sydney classification criteria (Table 2). Although it is often stated that the classification criteria are not to be used for diagnosis, in the antiphospholipid field they are often used to confirm diagnosis. The Sydney classification criteria divide the criteria into clinical and laboratory. The first clinical criterion is thrombosis. Thrombosis subsets include: venous (usually deep vein thrombosis or pulmonary emboli); arterial (usually stroke or myocardial infarction); and microvascular (which would include catastrophic antiphospholipid syndrome). The second criterion, pregnancy morbidity, is further subdivided into late fetal loss (the most classic), recurrent early miscarriage, and severe preeclampsia or HELLP syndrome38.
Table 2:
Sydney Classification Criteria for Antiphospholipid Syndrome
| Clinical Criterion |
Clinical criteria must be present within 5 years of the positive aPL assays
|
| Laboratory Criterion |
|
Gaps in the classification criteria include the non-thrombotic (or “non-criteria”) manifestations of antiphospholipid antibodies. These include neurologic manifestations, particularly chorea and longitudinal myelitis. Cardiac valvulitis can be both inflammatory and thrombotic (with fibrin deposition). Hematologic manifestations include thrombocytopenia. APS nephropathy is microvascular, usually manifesting as hypertension and proteinuria19,39. Livedo racemosa and skin ulcers are often considered as a non-criteria manifestations, as well.
The laboratory criteria include lupus anticoagulant, anticardiolipin (IgG or IgM) and anti-beta 2 glycoprotein I (IgG or IgM). The criteria require that the laboratory test be positive twice over a three-month period of time. Only 59% meeting the earlier 1999 Sapporo criteria met the 2006 Sydney criteria40. The Sydney criteria also required that other causes of thrombosis must be excluded in older patients and that clinical criteria must be present within five years of the positive antiphospholipid assays.
In the clinic, a clinical treatment decision often must be based on a single set of laboratory tests, as waiting to repeat the tests in three months would leave a patient potentially insufficiently treated. In addition, the criteria do not account for the fact that, in particular in systemic lupus erythematosus, there is fluctuation over time in antiphospholipid antibodies41. Finally, there is confusion, as the cut-offs for anticardiolipin and anti-beta 2 glycoprotein in the classification criteria (cut-off of 20) are different from a term for high risk patients, “triple positivity” that chose 40 as the “high risk” cut-off42. In SLE, patients with a geometric mean titer of IgG anticardiolipin greater than 20 did have a significantly elevated rated of thrombosis (rate ratio 1.8, p = 0.0052)43.
The classification criteria do include the IgM isotype. In SLE, however, IgM anticardiolipin is not associated with lifetime risk of thrombosis43.
The classification criteria do not include the IgA isotype. Multiple studies have shown that the IgA isotype is associated with thrombosis44–46, and is one of the most common isotypes in SLE43.
5. Epidemiology
Prevalence of Antiphospholipid Antibodies
Antiphospholipid antibodies, especially at lower titers, are common in the general population. In young healthy subjects, 1% to 5% may have lupus anticoagulant or anticardiolipin47. In older subjects, anticardiolipin and anti-beta 2 glycoprotein I were found in 12%48.
Infection Induced Antiphospholipid Antibodies
Viral infections can induce antiphospholipid antibodies, particularly human immunodeficiency virus (HIV), hepatitis B and hepatitis C49. Bacterial infections, including leprosy and syphilis, can induce antiphospholipid antibodies as well49. These infection-induced antiphospholipid antibodies are generally not associated with thrombotic antiphospholipid syndrome. Malignancies can also lead to antiphospholipid antibodies50,51.
Prevalence of Antiphospholipid Antibodies in Systemic Lupus Erythematosus
In antiphospholipid syndrome, about half of the patients have systemic lupus erythematosus or another autoimmune disease. This has been termed “secondary” antiphospholipid syndrome. In systemic lupus erythematosus, about 30–40% of patients will have antiphospholipid antibodies if they are looked for serially and longitudinally43. In the Hopkins Lupus Cohort, in 2,534 patients, 26% have had lupus anticoagulant, 47% anticardiolipin and 28% anti-beta 2 glycoprotein I52. In SLE, the lupus anticoagulant is more common in men (40% vs 24.5%)53 and in Caucasians than in African-Americans (28% vs 23%)52. In an SLE patient with the lupus anticoagulant at baseline, the risk of venous thromboembolism in the next 20 years is 42%54.
Antiphospholipid Syndrome Incidence and Prevalence
General population studies of incidence/prevalence of antiphospholipid syndrome are rare. One estimate of 280,000 antiphospholipid syndrome events per annum in the U.S. was based on assumptions of 6% pregnancy morbidity, 13.5% stroke, 11% myocardial infarction and 9.5% deep venous thrombosis being due to antiphospholipid syndrome55. In a second study in Minnesota, the 2006 Sydney criteria or a diagnosis of APS by physician consensus were used. Laboratory results came from a centralized laboratory. Incidence rates (adjusted to the 2010 Caucasian population), and prevalence estimates from the incidence rates were then estimated. The annual incidence was 21 per 100,000 population and prevalence 50 per 100,000 population. Eighteen percent had SLE56.
6. Thrombotic Antiphospholipid Syndrome
Antiphospholipid antibodies are more strongly associated with stroke in patients under the age of 5057–59. In myocardial infarction the titers of antiphospholipid antibodies were elevated in those less than 50 years of age60. Up to 20% of cases of deep vein thrombosis are associated with antiphospholipid antibodies61.
7. Obstetric Antiphospholipid Syndrome
There are three kinds of pregnancy morbidity listed in the classification criteria of antiphospholipid syndrome. The first, recurrent early miscarriage, is nonspecific. In the general obstetric population, 10–15% of pregnancies end in early loss. One to two percent of women will have recurrent early miscarriage. Most women with recurrent early miscarriage will not have a known cause. In 25% to 60% of recurrent early miscarriage one partner will have an abnormal karyotype62. Most may have a successful pregnancy without any specific treatment63.
Late losses (fetal death) occur in 1–2% in the general obstetric population64. The frequency of early miscarriage (<10 weeks gestation) or late fetal death in untreated obstetric APS is unknown. The frequency of live birth is 70–80% in trials of treated obstetric APS (although the trials mostly included recurrent early miscarriage and would not meet the stringency of current APS criteria)65,66.
Severe preeclampsia can happen idiopathically and particularly in SLE is problematic, as active lupus and renal disease can also contribute to severe preeclampsia67.
8. Catastrophic Antiphospholipid Syndrome
The third type of thrombosis in the classification criteria is microvascular, which describes catastrophic antiphospholipid syndrome. This is a devastating, but very rare, form of APS occurring in only about 1% of total APS patients. A recent review of the International CAPS registry found a mortality of 37%. Forty-eight percent of catastrophic antiphospholipid syndrome patients will have primary antiphospholipid syndrome, 40% SLE, and 12% other predisposing causes68.
Characteristics of Catastrophic Antiphospholipid Syndrome
Thrombotic APS usually presents with deep venous thrombosis, pulmonary emboli, stroke, or fetal loss. Catastrophic antiphospholipid syndrome, however, presents with renal involvement in 73%, pulmonary 60% (such as acute respiratory distress syndrome), cerebral 56% (including encephalopathy), cardiac 50% and skin 47% (such as cutaneous necrosis)69.
Triggers of Catastrophic Antiphospholipid Syndrome
Triggers of catastrophic antiphospholipid syndrome include withdrawal or non-adherence with anticoagulation, malignancies, drugs, surgery, trauma, infection, SLE flare, or pregnancy/post-partum state70. Often, however, no predisposing factor is found. Infection was more often a trigger in younger patients and malignancy in older patients69.
Criteria for catastrophic antiphospholipid syndrome have been developed and require involvement of three or more organs (or systems or tissues), manifestations that have developed within one week, histopathology of small vessel occlusion, and confirmation of antiphospholipid antibodies71.
Catastrophic antiphospholipid syndrome can occur both in a patient who already has antiphospholipid syndrome and in a de novo way. Interestingly, although it can recur, recurrences are very rare72.
9. Natural History of Antiphospholipid Antibodies in SLE
Antiphospholipid antibodies tend to fluctuate in SLE. This is true for both lupus anticoagulant and anticardiolipin41,43. This is not surprising, as many auto-antibodies, including anti-dsDNA, also fluctuate in SLE. Antibodies that tend not to fluctuate in SLE are those that are made primarily by plasma cells, such as anti-Smith, anti RNP, anti-Ro and anti-La.
The fact that antiphospholipid antibodies tend to fluctuate in SLE has not been addressed in the classification criteria. It makes it particularly problematic to make a classification or diagnosis of APS in SLE and to make treatment decisions. Examples of fluctuations in antiphospholipid bodies in SLE patients are shown in Figure 1.
Figure 1 -.
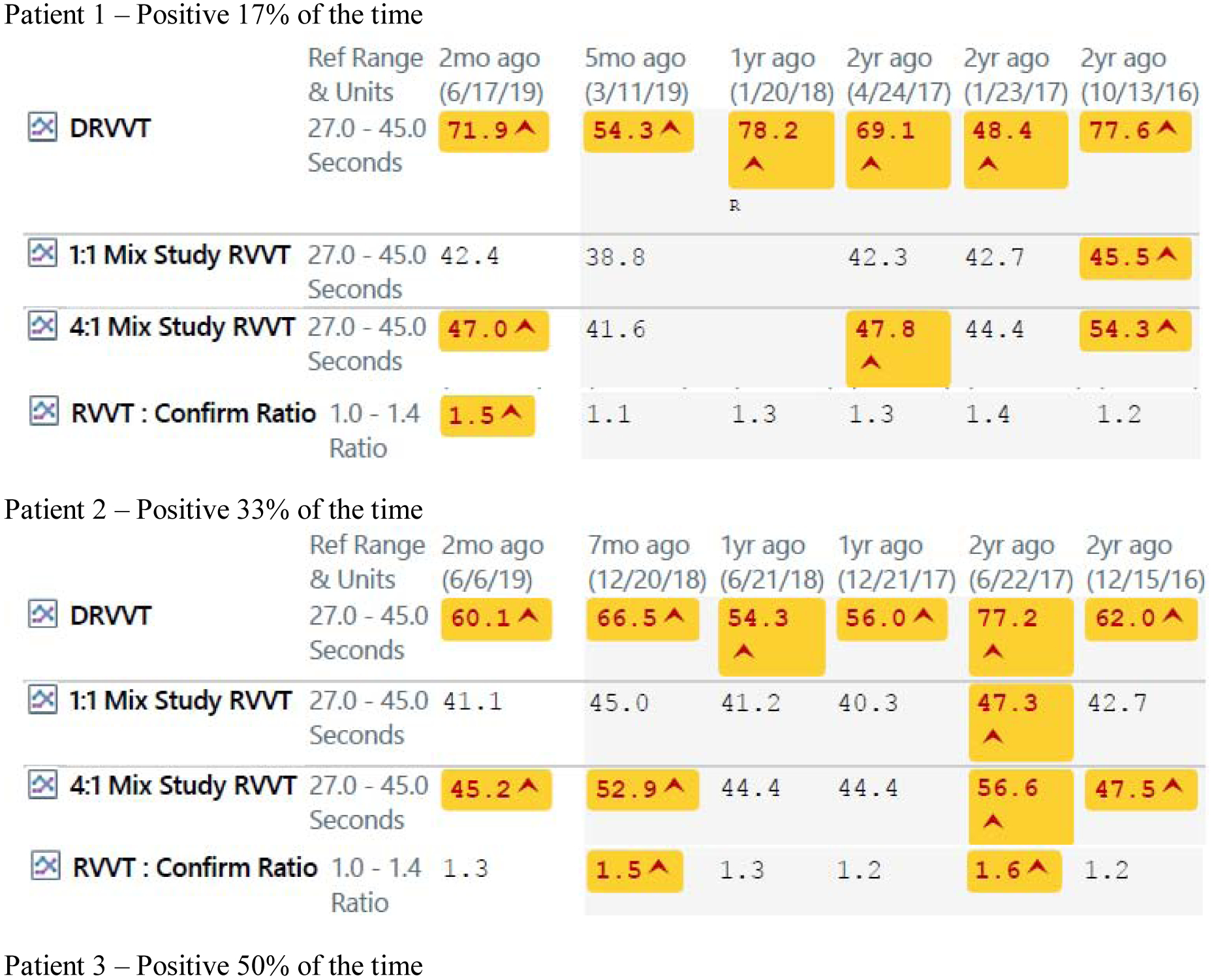
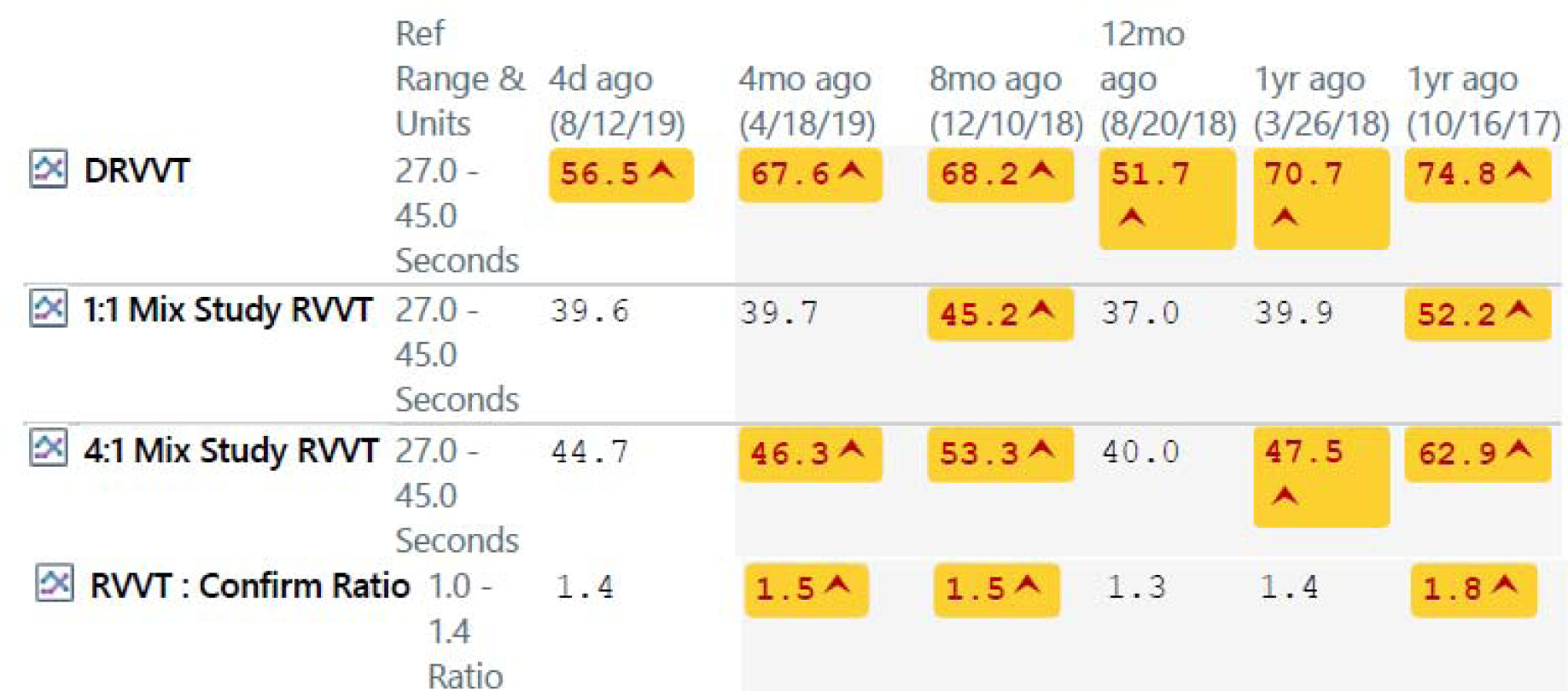
Fluctuations in Antiphospholipid Antibodies
10. Risk Stratification for Thrombosis in APS, including in SLE
Multifactorial pathogenesis – “multiple hits” – is accepted in APS. Clinical situations that increase risk include hypertension, surgery, pregnancy and post-partum period. The Global Antiphospholipid Syndrome Score (GAPSS) added points for hyperlipidemia and for arterial hypertension for this reason73. Drugs that increase hypercoagulability, such as estrogen and thalidomide, can act as “second hits”, as well.
Laboratory risk stratification includes lupus anticoagulant, IgG over IgM isotype, higher IgG titers, and persistence of antiphospholipid antibodies for 6 months or longer74,75. One risk score, the Antiphospholipid Score, or aPLS, added a weighted system of points for multiple different lupus anticoagulant, anticardiolipin, anti-beta 2 glycoprotein I and anti-phosphatidylserine/prothrombin assays76.
A different laboratory risk stratification called “triple positivity”, is defined as the presence of lupus anticoagulant, high titer (>40) anticardiolipin, and high titer (>40) anti-beta 2 glycoprotein I. “Triple positivity” has been defined as all present at one visit42. “Triple positivity” required that the anticardiolipin and anti-beta 2 glycoprotein I be the same isotype77–79.
“Triple positivity”, however, was not validated in the Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus (PROMISSE) study of pregnancy morbidity due to antiphospholipid antibodies. Only the lupus anticoagulant explained the risk of adverse pregnancy outcomes in PROMISSE80. Similarly, in SLE, it is the lupus anticoagulant that explains risk (the IgM isotype, for example, is not associated with thrombosis)43.
Immunothrombosis
In SLE the lupus anticoagulant is the most important autoantibody43,81. By itself, the lupus anticoagulant explains most of the thrombosis risk that can be attributed to antiphospholipid antibodies. Although in the non-SLE population the approach called “triple positivity” has been taken to assign risk77–79, triple positivity (positive for lupus anticoagulant, anticardiolipin and anti-beta 2 glycoprotein I) is not validated in SLE. In SLE the situation is more complicated because of the role of immunothrombosis82.
Immunothrombosis refers to the multiple interactions of complement, platelets and coagulation. Plasmin cleave C5 to C5a. C5a increases tissue factor and catalyzes Factor X. C3a and C5b lead to platelet activation, increase tissue factor, activate endothelial cells, increase von Willebrand factor and expose P selectin. C5b-9 increases exposure of prothrombinase assembly sites on platelets82,83.
Platelets are also involved in the thrombosis risk in SLE. This was shown initially by the Manzi group that found that a complement split product, C4d, bound to platelets, was associated with thrombosis, both arterial and venous, in SLE patients. They found that it was also associated with stroke and with stroke severity in the general population84,85.
Complementopathies
The field of “complementopathies” includes diseases, such as paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome, that lead to thrombosis and involve complement activation. Thrombosis in complementopathies can be blocked by a complement inhibitor such as anti-C5a83. In SLE, it has been possible to show the role of immunothrombosis in two ways, both by a reduction in C3 being associated with thrombosis and by complement split products bound to platelets86.
Hopkins SLE Thrombosis Risk Equation
A simple three variable model, the Hopkins SLE thrombosis risk equation, that includes low C3, C4d bound to platelets, and lupus anticoagulant, is highly associated with thrombosis occurring within the last five years86. The greater the number of risk factors, the greater the association with thrombosis. The Hopkins SLE thrombosis risk equation performed better than “triple positivity” in receiver operating characteristics analysis86.
11. Complement Mutations in Catastrophic Antiphospholipid Syndrome
A modified Ham assay (complement-dependent cell killing) and corresponding C5b-9 deposition were found in 86% of patients with catastrophic antiphospholipid syndrome, 36% of APS, but only 7% of SLE. Anti-beta 2 glycoprotein I antibodies induced C5b-9 deposition (which was blocked by anti-C5 but not by factor D inhibitor), indicative of classical (but not alternate) complement participation. Complement mutations have now been shown to be a risk factor, if not the major risk factor, for catastrophic antiphospholipid syndrome. Sixty percent of catastrophic antiphospholipid syndrome had germline variants in complement regulatory genes87.
12. Treatment
Preventive Treatment
In SLE patients with the lupus anticoagulant at baseline, there is a 42% risk of venous thrombosis in the next 20 years54. There are multiple potential candidates for prophylactic treatment. The most obvious one is low dose aspirin. Low dose aspirin was studied in a nested case control study within the Physicians Health Study and was not found to be preventive of deep venous thrombosis / pulmonary embolus in male physicians88. It was also examined in a randomized clinical trial. The clinical trial was likely underpowered, but did not show a protective role of low dose aspirin89. A more recent analysis of five cohort studies did find a protective effect of aspirin against arterial but not venous thrombosis90.
Hydroxychloroquine has been widely studied in SLE patients and in multiple studies has been shown to reduce the risk of thrombosis91,92. It has a good safety profile, but because of the rare risk of retinopathy it is necessary to do retina safety screening at baseline, five years, and then yearly93. The benefit of hydroxychloroquine is likely due to multiple actions, including an anti-platelet role, but it may also reduce antiphospholipid antibody titers91,94. The combination of low dose aspirin and hydroxychloroquine reduced thrombosis risk in SLE patients, but the study did not include primary APS95.
Potential Prophylactic Treatments
Vitamin D is a potential prophylactic drug against thrombosis. It reduces activation of tissue factor by antiphospholipid antibodies96. It has been shown in oncology to reduce the risk of thrombosis in patients with prostate cancer97. Subjects with low vitamin D have a higher risk of thrombosis both in SLE and in antiphospholipid syndrome96,98.
Statins have been studied in patients with the antiphospholipid antibodies and shown to reduce some inflammatory mediators and tissue factor99. In addition, statins have been shown to have an antithrombotic benefit in large trials of statins in the general population100.
Thrombosis Treatment
Degree of Anticoagulation
Once a patient has had a thrombotic event, regardless of whether venous or arterial, the preferred therapy is warfarin. Initially there was some controversy about the degree of anticoagulation necessary101, but two subsequent randomized clinical trials by Fenazzi et al102 and Crowther et al103 found that an INR target between two and three was adequate.
Choice of Anticoagulant
Heparin, given acutely at the time of the thrombotic event, can have two benefits: first, blocking complement activation (even if just a prophylactic dose) and second, as an anticoagulant36. However, heparin is rarely continued long-term due to concerns about osteopenia104.
With the advent of direct oral anticoagulants (DOACs), there was great interest in using DOACs instead of warfarin. However, two clinical trials have shown that APS patients should not be given DOACs as a first choice therapy. One randomized clinical trial, Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS), enrolled patients who were “triple positive” for antiphospholipid antibodies. These patients had an increased risk of arterial thrombosis if randomized to the trial DOAC (rivaroxaban)105. The second trial, Astro-APS, enrolled based on a clinical diagnosis of APS. It was interrupted due to safety concerns (arterial thrombosis) and then not completed. The study design of Astro-APS was randomization to apixaban versus warfarin. Those APS patients on apixaban had an increased risk of arterial events, even when the apixaban dose was doubled106.
Duration of Treatment
Given the high risk of recurrence in APS, anticoagulation is recommended long-term. The risk of recurrence when stopped has been particularly high in SLE (24%)107,108.
Treatment of Thrombocytopenia
Thrombocytopenia is usually mild in APS. When severe, it is treated with corticosteroids, intravenous immunoglobulin (cautiously, as this can cause hypercoagulability), and rituximab109. Thrombopoietin mimetic agents may increase the risk of thrombosis110.
Treatment of Catastrophic Antiphospholipid Syndrome
The treatment of catastrophic antiphospholipid syndrome has classically been the “triple therapy” of intravenous heparin, intravenous methylprednisolone pulse and plasmapheresis (or intravenous immunoglobulin)71. There has been some improvement in the very high mortality from 50% to 37%69. However, a large percentage of catastrophic antiphospholipid syndrome patients do not respond to triple therapy. Rituximab has had some benefit in case series111,112 and benefit for some non-criteria manifestations (such as skin ulcers and cognitive dysfunctions)109. Eculizumab, the anti-C5 monoclonal antibody approved for atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria, has been successful in case reports113–115.
Treatment in Pregnancy
If a woman has had a past normal pregnancy, no pregnancies, or has had only one early miscarriage, and has antiphospholipid antibodies, it is appropriate to prescribe a baby aspirin. The role of baby aspirin includes reduction in the risk of preeclampsia116. If the woman with antiphospholipid antibodies has had one late fetal demise, multiple early losses, or a history of severe preeclampsia or HELLP, then prophylactic heparin as well as low dose aspirin is recommended based on clinical trials117 and a meta-analysis118. The pathogenesis of pregnancy complications occurs very early during the pregnancy and during the time of implantation. This has led to the practice of starting the prophylactic heparin as soon as pregnancy is confirmed. It is important to dose LMW heparin twice daily to obtain “24 hour” coverage for the placenta. If the woman has had a past thrombotic event, then the recommendation is full dose heparin and low dose aspirin (the latter theoretically increases the risk of bleeding but reduces the risk of pre-eclampsia)119. An ongoing trial will evaluate the benefit of an anti-TNF biologic that does not cross the placenta in women with a past history of pregnancy losses in spite of heparin and aspirin (ClinicalTrials.gov number NCT03152058).
13. Conclusion
Antiphospholipid syndrome is defined as thrombotic, obstetric, or microvascular (catastrophic antiphospholipid syndrome). There is likely a fourth subset of inflammatory APS that includes non-thrombotic manifestations in the neurologic (chorea, longitudinal myelitis), hematologic (thrombocytopenia), and cardiac (valvulitis) systems. Antiphospholipid syndrome is one of the most important acquired causes of hypercoagulability.
Acknowledgements
All authors have read the journal’s policy on disclosure of potential conflicts of interest and disclose no financial or personal relationship with organizations that could potentially be perceived as influencing the described research. All authors have read the journal’s authorship statement.
Abbreviations
- aPLS
Antiphospholipid Score
- APS
Antiphospholipid syndrome
- APTT
Partial thromboplastin time
- CAPS
Catastrophic Antiphospholipid Syndrome
- DOAC
Direct oral anticoagulant
- ELISA
Enzyme-linked immunosorbent assay
- eNOS
Endothelial nitric oxide synthase
- GAPSS
Global Antiphospholipid Syndrome Score
- HELLP
Hemolysis, Elevated Liver enzymes, Low Platelets
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- ISTH
International Society on Thrombosis and Haemostasis
- LMW
Low molecular weight
- NET
Neutrophil extracellular trap
- PROMISSE
Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus
- RVVT
Russell Viper Venom Time
- SLE
Systemic Lupus Erythematosus
- TNF
Tumor necrosis factor
- TRAPS
Trial on Rivaroxaban in AntiPhospholipid Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petri M, Rheinschmidt M, Whiting-O’Keefe Q, Hellmann D, Corash L. The frequency of lupus anticoagulant in systemic lupus erythematosus. A study of sixty consecutive patients by activated partial thromboplastin time, Russell viper venom time, and anticardiolipin antibody level. Ann Intern Med. 1987;106(4):524–531. doi: 10.7326/0003-4819-106-4-524 [DOI] [PubMed] [Google Scholar]
- 2.Wahl D, Guillemin F, de Maistre E, Perret C, Lecompte T, Thibaut G. Risk for venous thrombosis related to antiphospholipid antibodies in systemic lupus erythematosus—A meta-analysis. Lupus. 1997;6(5):467–473. doi: 10.1177/096120339700600510 [DOI] [PubMed] [Google Scholar]
- 3.Lockshin MD, Kim M, Laskin CA, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum. 2012;64(7):2311–2318. doi: 10.1002/art.34402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. 2009;7(10):1737–1740. doi: 10.1111/j.1538-7836.2009.03555.x [DOI] [PubMed] [Google Scholar]
- 5.Favaloro EJ, Gilmore G, Arunachalam S, Mohammed S, Baker R. Neutralising rivaroxaban induced interference in laboratory testing for lupus anticoagulant (LA): A comparative study using DOAC Stop and andexanet alfa. Thromb Res. 2019;180:10–19. doi: 10.1016/j.thromres.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 6.Ząbczyk M, Kopytek M, Natorska J, Undas A. The effect of DOAC-Stop on lupus anticoagulant testing in plasma samples of venous thromboembolism patients receiving direct oral anticoagulants. Clin Chem Lab Med. 2019;57(9):1374–1381. doi: 10.1515/cclm-2018-1197 [DOI] [PubMed] [Google Scholar]
- 7.Akhter E, Shums Z, Norman GL, Binder W, Fang H, Petri M. Utility of antiphosphatidylserine/prothrombin and IgA antiphospholipid assays in systemic lupus erythematosus. J Rheumatol. 2013;40(3):282–286. doi: 10.3899/jrheum.120084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolaccini ML, Atsumi T, Koike T, Hughes GR V, Khamashta MA. Antiprothrombin antibodies detected in two different assay systems. Prevalence and clinical significance in systemic lupus erythematosus. Thromb Haemost. 2005;93(2):289–297. doi: 10.1160/TH04-06-0382 [DOI] [PubMed] [Google Scholar]
- 9.Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RF. Lupus anticoagulant IgG’s (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb Haemost. 1991;66(6):629–632. http://www.ncbi.nlm.nih.gov/pubmed/1796407. Accessed February 18, 2020. [PubMed] [Google Scholar]
- 10.Sarker T, Roy S, Hollon W, Rajpurkar M. Lupus anticoagulant acquired hypoprothrombinemia syndrome in childhood: two distinct patterns and review of the literature. Haemophilia. 2015;21(6):754–760. doi: 10.1111/hae.12669 [DOI] [PubMed] [Google Scholar]
- 11.Rand JH, Wu X-X, Quinn AS, et al. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers. Am J Pathol. 2003;163(3):1193–1200. doi: 10.1016/S0002-9440(10)63479-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Gao F, Lu D, et al. Anti-β2 glycoprotein I antibodies in complex with β2 glycoprotein I induce platelet activation via two receptors: apolipoprotein E receptor 2′ and glycoprotein I bα. Front Med. 2016;10(1):76–84. doi: 10.1007/s11684-015-0426-7 [DOI] [PubMed] [Google Scholar]
- 13.Bontadi A, Ruffatti A, Falcinelli E, et al. Platelet and endothelial activation in catastrophic and quiescent antiphospholipid syndrome. Thromb Haemost. 2013;109(05):901–908. doi: 10.1160/TH12-03-0212 [DOI] [PubMed] [Google Scholar]
- 14.Cuadrado MJ, Mujic F, Munoz E, Khamashta MA, Hughes GR V. Thrombocytopenia in the antiphospholipid syndrome. Ann Rheum Dis. 1997;56(3):194–196. doi: 10.1136/ard.56.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli M, Finazzi G, Barbui T. Thrombocytopenia in the antiphospholipid syndrome. Br J Haematol. 1996;93(1):1–5. doi: 10.1046/j.1365-2141.1996.390969.x [DOI] [PubMed] [Google Scholar]
- 16.Sciascia S, Amigo M-C, Roccatello D, Khamashta M. Diagnosing antiphospholipid syndrome: “extra-criteria” manifestations and technical advances. Nat Rev Rheumatol. 2017;13(9):548–560. doi: 10.1038/nrrheum.2017.124 [DOI] [PubMed] [Google Scholar]
- 17.Levine S, Brey R. Neurological aspects of antiphospholipid antibody syndrome. Lupus. 1996;5(5):347–353. doi: 10.1177/096120339600500503 [DOI] [PubMed] [Google Scholar]
- 18.Doria A, Shoenfeld Y, Pauletto P. Premature coronary disease in systemic lupus. N Engl J Med. 2004;350(15):1571–1575; author reply 1571–5. http://www.ncbi.nlm.nih.gov/pubmed/15074008. Accessed February 17, 2020. [PubMed] [Google Scholar]
- 19.Tektonidou MG, Sotsiou F, Nakopoulou L, Vlachoyiannopoulos PG, Moutsopoulos HM. Antiphospholipid syndrome nephropathy in patients with systemic lupus erythematosus and antiphospholipid antibodies: Prevalence, clinical associations, and long-term outcome. Arthritis Rheum. 2004;50(8):2569–2579. doi: 10.1002/art.20433 [DOI] [PubMed] [Google Scholar]
- 20.Mineo C, Shaul PW. New insights into the molecular basis of the antiphospholipid syndrome. Drug Discov Today Dis Mech. 2011;8(1–2):e47–e52. doi: 10.1016/j.ddmec.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramesh S, Morrell CN, Tarango C, et al. Antiphospholipid antibodies promote leukocyteendothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J Clin Invest. 2011;121(1):120–131. doi: 10.1172/JCI39828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velásquez M, Rojas M, Abrahams VM, Escudero C, Cadavid ÁP. Mechanisms of endothelial dysfunction in antiphospholipid syndrome: association with clinical manifestations. Front Physiol. 2018;9:1840. doi: 10.3389/fphys.2018.01840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen KL, Hamik A, Jain MK, McCrae KR. Endothelial cell activation by antiphospholipid antibodies is modulated by Krüppel-like transcription factors. Blood. 2011;117(23):6383–6391. doi: 10.1182/blood-2010-10-313072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canaud G, Bienaimé F, Tabarin F, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371(4):303–312. doi: 10.1056/NEJMoa1312890 [DOI] [PubMed] [Google Scholar]
- 25.Canaud G, Legendre C, Terzi F. AKT/mTORC pathway in antiphospholipid-related vasculopathy: a new player in the game. Lupus. 2015;24(3):227–230. doi: 10.1177/0961203315569336 [DOI] [PubMed] [Google Scholar]
- 26.Alarcón-Segovia D, Cardiel MH, Reyes E. Antiphospholipid arterial vasculopathy. J Rheumatol. 1989;16(6):762–767. http://www.ncbi.nlm.nih.gov/pubmed/2778758. Accessed February 17, 2020. [PubMed] [Google Scholar]
- 27.Hughson MD, Mccarty GA, Brumback RA. Spectrum of vascular pathology affecting patients with the antiphospholipid syndrome. Hum Pathol. 1995;26(7):716–724. doi: 10.1016/0046-8177(95)90218-X [DOI] [PubMed] [Google Scholar]
- 28.Long BR, Leya F. The role of antiphospholipid syndrome in cardiovascular disease. Hematol Oncol Clin North Am. 2008;22(1):79–94. doi: 10.1016/j.hoc.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 29.Ripoll VM, Pregnolato F, Mazza S, et al. Gene expression profiling identifies distinct molecular signatures in thrombotic and obstetric antiphospholipid syndrome. J Autoimmun. 2018;93:114–123. doi: 10.1016/j.jaut.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali RA, Gandhi AA, Meng H, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun. 2019;10(1):1916. doi: 10.1038/s41467-019-09801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight JS, Meng H, Coit P, et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI insight. 2017;2(18). doi: 10.1172/jci.insight.93897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sule G, Kelley WJ, Gockman K, et al. Increased Adhesive Potential of Antiphospholipid Syndrome Neutrophils Mediated by β2 Integrin Mac-1. Arthritis Rheumatol (Hoboken, NJ). 2020;72(1):114–124. doi: 10.1002/art.41057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MY, Guerra MM, Kaplowitz E, et al. Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies. Ann Rheum Dis. 2018;77(4):549–555. doi: 10.1136/annrheumdis-2017-212224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong M, Johansson C, Xiao F, et al. Antiphospholipid antibodies increase the levels of mitochondrial DNA in placental extracellular vesicles: Alarmin-g for preeclampsia. Sci Rep. 2017;7(1):16556. doi: 10.1038/s41598-017-16448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurman JM, Kraus DM, Girardi G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42(1):87–97. doi: 10.1016/J.MOLIMM.2004.07.043 [DOI] [PubMed] [Google Scholar]
- 36.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody–induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–1226. doi: 10.1038/nm1121 [DOI] [PubMed] [Google Scholar]
- 37.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52(7):2120–2124. doi: 10.1002/art.21157 [DOI] [PubMed] [Google Scholar]
- 38.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 39.Hughson MD, Nadasdy T, McCarty GA, Sholer C, Min KW, Silva F. Renal thrombotic microangiopathy in patients with systemic lupus erythematosus and the antiphospholipid syndrome. Am J Kidney Dis. 1992;20(2):150–158. doi: 10.1016/s0272-6386(12)80543-9 [DOI] [PubMed] [Google Scholar]
- 40.Kaul M, Erkan D, Sammaritano L, Lockshin MD. Assessment of the 2006 revised antiphospholipid syndrome classification criteria. Ann Rheum Dis. 2007;66(7):927–930. doi: 10.1136/ard.2006.067314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Out HJ, de Groot PG, Hasselaar P, dan Vliet M, Derksen RH. Fluctuations of anticardiolipin antibody levels in patients with systemic lupus erythematosus: a prospective study. Ann Rheum Dis. 1989;48(12):1023–1028. doi: 10.1136/ard.48.12.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pengo V, Biasiolo A, Pegoraro C, Cucchini U, Noventa F, Iliceto S. Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb Haemost. 2005;93(06):1147–1152. doi: 10.1160/TH04-12-0839 [DOI] [PubMed] [Google Scholar]
- 43.Domingues V, Magder LS, Petri M. Assessment of the independent associations of IgG, IgM and IgA isotypes of anticardiolipin with thrombosis in SLE. Lupus Sci Med. 2016;3(1):e000107. doi: 10.1136/lupus-2015-000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pericleous C, Ferreira I, Borghi O, et al. Measuring IgA anti-β2-glycoprotein I and IgG/IgA anti-domain I antibodies adds value to current serological assays for the antiphospholipid syndrome Kuwana M, ed. PLoS One. 2016;11(6):e0156407. doi: 10.1371/journal.pone.0156407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tebo AE, Willis R, Jaskowski TD, et al. Clinical significance and correlations between anti-β2 glycoprotein I IgA assays in antiphospholipid syndrome and/or systemic lupus erythematosus. Clin Chim Acta. 2016;460:107–113. doi: 10.1016/J.CCA.2016.06.025 [DOI] [PubMed] [Google Scholar]
- 46.Sweiss NJ, Bo R, Kapadia R, et al. IgA anti-beta2-glycoprotein I autoantibodies are associated with an increased risk of thromboembolic events in patients with systemic lupus erythematosus Unutmaz D, ed. PLoS One. 2010;5(8):e12280. doi: 10.1371/journal.pone.0012280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petri M Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15(2):145–151. doi: 10.1006/JAUT.2000.0409 [DOI] [PubMed] [Google Scholar]
- 48.Brey RL, Abbott RD, Curb JD, et al. beta(2)-Glycoprotein 1-dependent anticardiolipin antibodies and risk of ischemic stroke and myocardial infarction: the honolulu heart program. Stroke. 2001;32(8):1701–1706. doi: 10.1161/01.str.32.8.1701 [DOI] [PubMed] [Google Scholar]
- 49.Sène D, Piette J-C, Cacoub P. Antiphospholipid antibodies, antiphospholipid syndrome and infections. Autoimmun Rev. 2008;7(4):272–277. doi: 10.1016/j.autrev.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 50.Gurgey A, Balta G, Gumruk F, Altay C. Analysis of some clinical and laboratory aspects of adolescent patients with thrombosis. Blood Coagul Fibrinolysis. 2004;15(8):657–662. doi: 10.1097/00001721-200412000-00005 [DOI] [PubMed] [Google Scholar]
- 51.Unal S, Varan A, Yalçin B, Büyükpamukçu M, Gürgey A. Evaluation of thrombotic children with malignancy. Ann Hematol. 2005;84(6):395–399. doi: 10.1007/s00277-005-1004-x [DOI] [PubMed] [Google Scholar]
- 52.Petri M Update on anti-phospholipid antibodies in SLE: the Hopkins’ Lupus Cohort. Lupus. 2010;19(4):419–423. doi: 10.1177/0961203309360541 [DOI] [PubMed] [Google Scholar]
- 53.Tan TC, Fang H, Magder LS, Petri MA. Differences between male and female systemic lupus erythematosus in a multiethnic population. J Rheumatol. 2012;39(4):759–769. doi: 10.3899/jrheum.111061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somers E, Magder LS, Petri M. Antiphospholipid antibodies and incidence of venous thrombosis in a cohort of patients with systemic lupus erythematosus. J Rheumatol. 2002;29(12):2531–2536. http://www.ncbi.nlm.nih.gov/pubmed/12465147. Accessed August 22, 2018. [PubMed] [Google Scholar]
- 55.Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, de Jesus GR, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: A critical review of the literature. Arthritis Care Res (Hoboken). 2013;65(11):1869–1873. doi: 10.1002/acr.22066 [DOI] [PubMed] [Google Scholar]
- 56.Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: A population-based study. Arthritis Rheumatol. 2019;71(9):1545–1552. doi: 10.1002/art.40901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brey RL, Hart RG, Sherman DG, Tegeler CH. Antiphospholipid antibodies and cerebral ischemia in young people. Neurology. 1990;40(8):1190–1196. doi: 10.1212/wnl.40.8.1190 [DOI] [PubMed] [Google Scholar]
- 58.Nencini P, Baruffi MC, Abbate R, Massai G, Amaducci L, Inzitari D. Lupus anticoagulant and anticardiolipin antibodies in young adults with cerebral ischemia. Stroke. 1992;23(2):189–193. doi: 10.1161/01.str.23.2.189 [DOI] [PubMed] [Google Scholar]
- 59.Kristensen B, Malm J, Carlberg B, et al. Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in northern Sweden. Stroke. 1997;28(9):1702–1709. doi: 10.1161/01.str.28.9.1702 [DOI] [PubMed] [Google Scholar]
- 60.Adler Y, Finkelstein Y, Zandeman-Goddard G, et al. The presence of antiphospholipid antibodies in acute myocardial infarction. Lupus. 1995;4(4):309–313. doi: 10.1177/096120339500400413 [DOI] [PubMed] [Google Scholar]
- 61.Farmer-Boatwright MK, Roubey RAS. Venous thrombosis in the antiphospholipid syndrome. Arterioscler Thromb Vasc Biol. 2009;29(3):321–325. doi: 10.1161/ATVBAHA.108.182204 [DOI] [PubMed] [Google Scholar]
- 62.Suzumori N, Sugiura-Ogasawara M. Genetic factors as a cause of miscarriage. Curr Med Chem. 2010;17(29):3431–3437. doi: 10.2174/092986710793176302 [DOI] [PubMed] [Google Scholar]
- 63.Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod. 1999;14(11):2868–2871. doi: 10.1093/humrep/14.11.2868 [DOI] [PubMed] [Google Scholar]
- 64.Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387(10018):587–603. doi: 10.1016/S0140-6736(15)00837-5 [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Sun D. Pregnancy outcomes in patients with primary antiphospholipid syndrome: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98(20):e15733. doi: 10.1097/MD.0000000000015733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tektonidou MG, Andreoli L, Limper M, Tincani A, Ward MM. Management of thrombotic and obstetric antiphospholipid syndrome: A systematic literature review informing the EULAR recommendations for the management of antiphospholipid syndrome in adults. RMD Open. 2019;5(1):e000924. doi: 10.1136/rmdopen-2019-000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maynard S, Guerrier G, Duffy M. Pregnancy in women with systemic lupus and lupus nephritis. Adv Chronic Kidney Dis. 2019;26(5):330–337. doi: 10.1053/j.ackd.2019.08.013 [DOI] [PubMed] [Google Scholar]
- 68.Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome. Clinical and laboratory features of 50 patients. Medicine (Baltimore). 1998;77(3):195–207. http://www.ncbi.nlm.nih.gov/pubmed/9653431. Accessed April 25, 2019. [DOI] [PubMed] [Google Scholar]
- 69.Rodríguez-Pintó I, Moitinho M, Santacreu I, et al. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun Rev. 2016;15(12):1120–1124. doi: 10.1016/j.autrev.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 70.Asherson RA. Multiorgan failure and antiphospholipid antibodies: the catastrophic antiphospholipid (Asherson’s) syndrome. Immunobiology. 2005;210(10):727–733. doi: 10.1016/j.imbio.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 71.Asherson RA, Cervera R, de Groot PG, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12(7):530–534. doi: 10.1191/0961203303lu394oa [DOI] [PubMed] [Google Scholar]
- 72.Bucciarelli S, Erkan D, Espinosa G, Cervera R. Catastrophic Antiphospholipid Syndrome: Treatment, prognosis, and the risk of relapse. Clin Rev Allergy Immunol. 2009;36(2–3):80–84. doi: 10.1007/s12016-008-8107-9 [DOI] [PubMed] [Google Scholar]
- 73.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology (Oxford). 2013;52(8):1397–1403. doi: 10.1093/rheumatology/kes388 [DOI] [PubMed] [Google Scholar]
- 74.Ginsberg JS, Brill-Edwards P, Johnston M, et al. Relationship of antiphospholipid antibodies to pregnancy loss in patients with systemic lupus erythematosus: a cross-sectional study. Blood. 1992;80(4):975–980. http://www.ncbi.nlm.nih.gov/pubmed/1498338. Accessed January 22, 2020. [PubMed] [Google Scholar]
- 75.Long AA, Ginsberg JS, Brill-Edwards P, et al. The relationship of antiphospholipid antibodies to thromboembolic disease in systemic lupus erythematosus: a cross-sectional study. Thromb Haemost. 1991;66(5):520–524. http://www.ncbi.nlm.nih.gov/pubmed/1803614. Accessed January 22, 2020. [PubMed] [Google Scholar]
- 76.Otomo K, Atsumi T, Amengual O, et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum. 2012;64(2):504–512. doi: 10.1002/art.33340 [DOI] [PubMed] [Google Scholar]
- 77.Pengo V, Denas G. Diagnostics and treatment of thrombotic antiphospholipid syndrome (APS): A personal perspective. Thromb Res. 2018;169:35–40. doi: 10.1016/j.thromres.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 78.Pengo V, Banzato A, Bison E, et al. Laboratory testing for antiphospholipid syndrome. Int J Lab Hematol. 2016;38:27–31. doi: 10.1111/ijlh.12507 [DOI] [PubMed] [Google Scholar]
- 79.Pengo V, Ruffatti A, Del Ross T, et al. Confirmation of initial antiphospholipid antibody positivity depends on the antiphospholipid antibody profile. J Thromb Haemost. 2013;11(8):1527–1531. doi: 10.1111/jth.12264 [DOI] [PubMed] [Google Scholar]
- 80.Yelnik CM, Laskin CA, Porter TF, et al. Lupus anticoagulant is the main predictor of adverse pregnancy outcomes in aPL-positive patients: validation of PROMISSE study results. Lupus Sci Med. 2016;3(1):e000131. doi: 10.1136/lupus-2015-000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petri M The frequency of lupus anticoagulant in systemic lupus erythematosus. Ann Intern Med. 1987;106(4):524. doi: 10.7326/0003-4819-106-4-524 [DOI] [PubMed] [Google Scholar]
- 82.Keragala CB, Draxler DF, McQuilten ZK, Medcalf RL. Haemostasis and innate immunity - a complementary relationship. Br J Haematol. 2018;180(6):782–798. doi: 10.1111/bjh.15062 [DOI] [PubMed] [Google Scholar]
- 83.Chaturvedi S, Brodsky RA, McCrae KR. Complement in the pathophysiology of the antiphospholipid syndrome. Front Immunol. 2019;10:449. doi: 10.3389/fimmu.2019.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kao AH, McBurney CA, Sattar A, et al. Relation of platelet C4d with all-cause mortality and ischemic stroke in patients with systemic lupus erythematosus. Transl Stroke Res. 2014;5(4):510–518. doi: 10.1007/s12975-013-0295-9 [DOI] [PubMed] [Google Scholar]
- 85.Mehta N, Uchino K, Fakhran S, et al. Platelet C4d Is associated with acute ischemic stroke and stroke severity. Stroke. 2008;39(12):3236–3241. doi: 10.1161/STROKEAHA.108.514687 [DOI] [PubMed] [Google Scholar]
- 86.Petri MA, Conklin J, O’Malley T, Dervieux T. Platelet-bound C4d, low C3 and lupus anticoagulant associate with thrombosis in SLE. Lupus Sci Med. 2019;6(1):e000318. doi: 10.1136/lupus-2019-000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaturvedi S, Braunstein EM, Yuan X, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020;135(4):239–251. doi: 10.1182/blood.2019003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ginsburg KS, Liang MH, Newcomer L, et al. Anticardiolipin antibodies and the risk for ischemic stroke and venous thrombosis. Ann Intern Med. 1992;117(12):997–1002. doi: 10.7326/0003-4819-117-12-997 [DOI] [PubMed] [Google Scholar]
- 89.Erkan D, Harrison MJ, Levy R, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: A randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody–positive individuals. Arthritis Rheum. 2007;56(7):2382–2391. doi: 10.1002/art.22663 [DOI] [PubMed] [Google Scholar]
- 90.Arnaud L, Mathian A, Devilliers H, et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun Rev. 2015;14(3):192–200. doi: 10.1016/j.autrev.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 91.Petri M Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody–positive patients. Curr Rheumatol Rep. 2011;13(1):77–80. doi: 10.1007/s11926-010-0141-y [DOI] [PubMed] [Google Scholar]
- 92.Pierangeli S, Harris E. In Vivo models of thrombosis for the antiphospholipid syndrome. Lupus. 1996;5(5):451–455. doi: 10.1177/096120339600500524 [DOI] [PubMed] [Google Scholar]
- 93.Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123(6):1386–1394. doi: 10.1016/j.ophtha.2016.01.058 [DOI] [PubMed] [Google Scholar]
- 94.Nuri E, Taraborelli M, Andreoli L, et al. Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. Immunol Res. 2017;65(1):17–24. doi: 10.1007/s12026-016-8812-z [DOI] [PubMed] [Google Scholar]
- 95.Fasano S, Pierro L, Pantano I, Iudici M, Valentini G. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J Rheumatol. 2017;44(7):1032–1038. doi: 10.3899/jrheum.161351 [DOI] [PubMed] [Google Scholar]
- 96.Agmon-Levin N, Blank M, Zandman-Goddard G, et al. Vitamin D: an instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann Rheum Dis. 2011;70(1):145–150. doi: 10.1136/ard.2010.134817 [DOI] [PubMed] [Google Scholar]
- 97.Beer TM, Venner PM, Ryan CW, et al. High dose calcitriol may reduce thrombosis in cancer patients. Br J Haematol. 2006;135(3):392–394. doi: 10.1111/j.1365-2141.2006.06322.x [DOI] [PubMed] [Google Scholar]
- 98.Lertratanakul A, Wu P, Dyer A, et al. 25-Hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: Data from a large international inception cohort. Arthritis Care Res (Hoboken). 2014;66(8):1167–1176. doi: 10.1002/acr.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Erkan D, Willis R, Murthy VL, et al. A prospective open-label pilot study of fluvastatin on proinflammatory and prothrombotic biomarkers in antiphospholipid antibody positive patients. Ann Rheum Dis. 2014;73(6):1176–1180. doi: 10.1136/annrheumdis-2013-203622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360(18):1851–1861. doi: 10.1056/NEJMoa0900241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GRV. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332(15):993–997. doi: 10.1056/NEJM199504133321504 [DOI] [PubMed] [Google Scholar]
- 102.Finazzi G, Brancaccio V, Moia M, et al. Natural history and risk factors for thrombosis in 360 patients with antiphospholipid antibodies: a four-year prospective study from the Italian Registry. Am J Med. 1996;100(5):530–536. doi: 10.1016/s0002-9343(96)00060-5 [DOI] [PubMed] [Google Scholar]
- 103.Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349(12):1133–1138. doi: 10.1056/NEJMoa035241 [DOI] [PubMed] [Google Scholar]
- 104.Dadwal G, Schulte-Huxel T, Kolb G. Effect of antithrombotic drugs on bone health. Z Gerontol Geriatr. August 2019. doi: 10.1007/s00391-019-01590-8 [DOI] [PubMed] [Google Scholar]
- 105.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132(13):1365–1371. doi: 10.1182/blood-2018-04-848333 [DOI] [PubMed] [Google Scholar]
- 106.Woller SC, Stevens SM, Kaplan DA, et al. Apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome. Clin Appl Thromb. 2016;22(3):239–247. doi: 10.1177/1076029615615960 [DOI] [PubMed] [Google Scholar]
- 107.Comarmond C, Jego P, Veyssier-Belot C, et al. Cessation of oral anticoagulants in antiphospholipid syndrome. Lupus. 2017;26(12):1291–1296. doi: 10.1177/0961203317699285 [DOI] [PubMed] [Google Scholar]
- 108.Yelnik CM, Urbanski G, Drumez E, et al. Anticoagulation withdrawal in antiphospholipid syndrome: a retrospective matched-control study. Lupus. 2018;27(3):357–364. doi: 10.1177/0961203317721751 [DOI] [PubMed] [Google Scholar]
- 109.Erkan D, Vega J, Ramón G, Kozora E, Lockshin MD. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis Rheum. 2013;65(2):464–471. doi: 10.1002/art.37759 [DOI] [PubMed] [Google Scholar]
- 110.LaMoreaux B, Barbar-Smiley F, Ardoin S, Madhoun H. Two cases of thrombosis in patients with antiphospholipid antibodies during treatment of immune thrombocytopenia with romiplostim, a thrombopoietin receptor agonist. Semin Arthritis Rheum. 2016;45(4):e10–e12. doi: 10.1016/j.semarthrit.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 111.Sukara G, Baresic M, Sentic M, Brcic L, Anic B. Catastrophic antiphospholipid syndrome associated with systemic lupus erythematosus treated with rituximab: case report and a review of the literature. Acta Reumatol Port. 2015;40(2):169–175. http://www.ncbi.nlm.nih.gov/pubmed/25782914. Accessed February 19, 2020. [PubMed] [Google Scholar]
- 112.Berman H, Rodríguez-Pintó I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: Descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun Rev. 2013;12(11):1085–1090. doi: 10.1016/j.autrev.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 113.Tinti MG, Carnevale V, Inglese M, et al. Eculizumab in refractory catastrophic antiphospholipid syndrome: a case report and systematic review of the literature. Clin Exp Med. 2019;19(3):281–288. doi: 10.1007/s10238-019-00565-8 [DOI] [PubMed] [Google Scholar]
- 114.Rovere-Querini P, Canti V, Erra R, et al. Eculizumab in a pregnant patient with laboratory onset of catastrophic antiphospholipid syndrome. Medicine (Baltimore). 2018;97(40):e12584. doi: 10.1097/MD.0000000000012584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Espinosa G, Rodríguez-Pintó I, Cervera R. Catastrophic antiphospholipid syndrome: an update. Panminerva Med. 2017;59(3):254–268. doi: 10.23736/S0031-0808.17.03324-9 [DOI] [PubMed] [Google Scholar]
- 116.Imperiale TF, Petrulis AS. A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease. JAMA. 1991;266(2):260–264. http://www.ncbi.nlm.nih.gov/pubmed/1829118. Accessed February 19, 2020. [PubMed] [Google Scholar]
- 117.Cowchock FS, Reece EA, Balaban D, Branch DW, Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol. 1992;166(5):1318–1323. http://www.ncbi.nlm.nih.gov/pubmed/1595785. Accessed December 6, 2017. [DOI] [PubMed] [Google Scholar]
- 118.Liu X, Qiu Y, Yu ED, et al. Comparison of therapeutic interventions for recurrent pregnancy loss in association with antiphospholipid syndrome: A systematic review and network meta-analysis. Am J Reprod Immunol. January 2020:e13219. doi: 10.1111/aji.13219 [DOI] [PubMed] [Google Scholar]
- 119.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2019;2019(10). doi: 10.1002/14651858.CD004659.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]


