Abstract
Since the FDA approval of two Chimeric Antigen Receptor (CAR) T cell therapies against CD19+ malignancies, there has been significant interest in adapting CAR technology to other diseases. As such, the ability to simultaneously monitor manufacturing criteria and functional characteristics of multiple CAR T cell products by a single instrument would likely accelerate the development of candidate therapies. Here, we demonstrate that image-based cytometry yields high-throughput measurements of CAR T cell proliferation and size, and captures the kinetics of in vitro antigen-specific CAR T cell-mediated killing. The data acquired and analyzed by the image cytometer are congruent with results derived from conventional technologies when tested contemporaneously. Moreover, the use of bright-field and fluorescence microscopy by the image cytometer provides kinetic measurements and rapid data acquisition, which are direct advantages over industry standard instruments. Together, image cytometry enables fast, reproducible measurements of CAR T cell manufacturing criteria and effector function, which can greatly facilitate the evaluation of novel CARs with therapeutic potential.
Keywords: Image cytometry, Chimeric Antigen Receptor (CAR) T cells, Cell-mediated cytotoxicity, CAR T cell manufacturing, Bioluminescence (BLI)-based cytotoxicity assay
1. Introduction
Adoptive immunotherapy involves the transfer of immunocompetent cells for the treatment of pathologies with the goal to replace, restore or augment the biological function of the native immune response (Maldini et al., 2018). To date, the adoptive transfer of genetically-modified, autologous Chimeric Antigen Receptor (CAR) T cells have significantly improved the clinical outcome of patients with treatment refractory B cell malignancies (Maude et al., 2014; Porter et al., 2015), culminating in two FDA-licensed CAR T cell therapies. CARs redirect T cell responses through surface expression of an extracellular antigen-binding domain, typically an antibody-derived single-chain variable fragment, fused to an intracellular domain comprising the TCR signal transduction domain (CD3-ζ) and one or more costimulatory domains (Gross et al., 1989; van der Stegen et al., 2015). Given the modular nature of CARs, each domain can be switched permitting the generation of engineered T cells with new specificities and functions. Due to recent success of CD19-targeted CAR T cell therapies many researchers are investigating novel CARs for other disease indications; however, preclinical evaluation including the simultaneous manufacturing and functional testing of multiple CAR T cell products can be time-consuming and labor-intensive (Levine et al., 2017). As such, the ability to measure these criteria with a single instrument will likely accelerate the development and evaluation of novel immunotherapies.
The adoptive transfer of CAR T cells relies on the large scale ex vivo activation and expansion of genetically-engineered T cells, generating >1 × 109 CAR T cells for re-infusion into patients (Hay and Turtle, 2017). This process involves activating purified, patient blood-derived T cells by ligating CD3 and CD28 surface receptors through monoclonal antibodies conjugated to beads (Levine et al., 1998), or by co-culture with artificial antigen-presenting cells (Maus et al., 2002; Thomas et al., 2002). Activated T cells can be transduced with a viral vector containing the CAR, and then grow logarithmically in culture for 1 to 2 weeks prior to adoptive transfer into patients. Throughout the expansion phase, CAR T cells are routinely counted using automated cell counters, which have largely replaced manual hemocytometers, given their ability to accurately quantify cell number and size (Vembadi et al., 2019). During this time, the culture volume is continuously adjusted to lower the cell density and remove the buildup of waste products such as lactate and ammonia (Somerville et al., 2012), which improves cell growth and viability. In addition to measuring the growth rate, CAR T cells are screened for phenotypic characteristics such as memory distribution and functionality, including antigen-specific cytokine release and target cell killing. Collectively, these readouts represent critical measures of quality control that need to be defined in order to ensure consistent manufacturing of a uniform CAR T cell product (Levine et al., 2017).
Here, we have developed an efficient image-based cytometry method using the Celigo Image Cytometer (Nexcelom Bioscience LLC.) to overcome limitations of conventional instruments used to measure CAR T cell expansion criteria and effector function during the manufacturing process (Chan et al., 2019; Magnotti et al., 2020). We validated this novel technique against a commonly used cell counting method and a bioluminescence-based cytotoxicity assay by generating distinct HIV-specific CAR T cell populations from multiple donors, and then we assessed the in vitro growth kinetics and cytotoxic potential of these CAR T cells against antigen-presenting target cells. Notably, image cytometry permitted the simultaneous evaluation of distinct CAR T cell populations, which enabled us to directly compare the growth rate and functionality of these cells. Image cytometry provided a sensitive, kinetic and high-throughput method to assess the physiological functions of CAR T cells, which could improve the efficiency for identifying high-quality CAR T cell products with therapeutic potential.
2. Materials and methods
2.1. Ethics statement
De-identified, purified CD3+, CD8+ and CD4+ T cells from human donors were obtained by the University of Pennsylvania Human Immunology Core/CFAR Immunology Core under an institutional review board (IRB)-approved protocol.
2.2. Plasmid construction
The amino acid sequences for the HIV-specific CD4-based CAR constructs containing the following intracellular domains: CD3-ζ, 4–1BB/CD3-ζ, CD28/CD3-ζ, CD28/4–1BB/CD3-ζ, CD27/CD3-ζ, OX40/CD3-ζ and ICOS/CD3-ζ have previously been described (Leibman et al., 2017). In this study, each CAR was amplified from their original plasmid with the following primer: 5’-CACGTCCTAGGATGGCCTTACCAGTG and 5’-GTGGTCGACTTATGCGCTCCTGCTGAAC and cloned into pTRPE plasmid using the AvrII and SalI restriction enzyme sites. In this orientation, the CAR sequence is downstream of GFP or iRFP670 and a T2A sequence intervenes to permit expression of both proteins.
2.3. Lentivirus production and transfection
To generate the lentiviral particles containing each HIV-specific CAR, expression vectors encoding VSV glycoprotein, HIV Rev, HIV Gag and Pol (pTRPE pVSV-g, pTRPE.Rev, and pTRPE g/p, respectively) were synthesized by DNA 2.0 or ATUM (Newark, CA) and transfected into HEK293T cells with a pTRPE transfer vector encoding the CAR using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) per manufacturer’s protocol, and as previously described (Richardson et al., 2008). Transfected HEK293T cell supernatant was collected 24 hours later, filtered through a 0.45 μm nylon syringe filter and concentrated by ultracentrifugation for 2.5 hours at 25,000 RPM at 4°C. The supernatant was aspirated and the virus pellet was resuspended in 800 μL total volume of RPMI 1640, and subsequently, four 200 μL aliquots were stored at −80°C for each CAR T cell construct.
2.4. Preparation of CAR T cells
Purified CD4+ and CD8+ T cells (RosetteSep, StemCell Technologies, Vancouver, Canada) were obtained by the University of Pennsylvania Human Immunology Core/CFAR Immunology Core from apheresis products of three de-identified healthy human donors (Donor 1, 2, 3). CD4+ T cells were used in the experiments to address expansion kinetics (Fig. 1) and cell size (Fig. 2), while CD8+ T cells were used in the cytotoxicity assays (Fig. 3 and Fig. 4). To generate CAR T cells, all T cell subsets were cultured at 1×106 cells mL−1 in a 24-well flat bottom plate (Corning) in complete RPMI 1640 with 10% FCS (Seradigm), 1% (v/v) Penicillin-Streptomycin (100 U mL−1), 2 mM GlutaMax and 25 mM HEPES buffer (Life Technologies). T cell expansion medium was supplemented with 100 U mL−1 recombinant human IL-2 (Clinigen). T cells were stimulated with αCD3/CD28 Dynabeads (Life Technologies) at a 3:1 bead-to-cell ratio at 37°C, 5% CO2 and 95% humidity incubation conditions. Approximately 18 hours after stimulation, half of the medium was removed and replaced with 200 μL of concentrated lentiviral supernatant containing the appropriate HIV-specific CAR. 5 days post-stimulation, the Dynabeads were removed from cell culture by magnetic separation. T cells counts (cells mL−1) and size measurements (cell area, μm2) were measured daily using both the Celigo Image Cytometer (Nexcelom Bioscience LLC, Lawrence, MA) and Multisizer 3 Coulter Counter (Beckman Coulter, Brea, CA) as described below. After de-beading, the cells were propagated in a 6-well flat bottom plate (Corning) and the cell concentration was adjusted to 0.5×106 cells mL−1 with complete RPMI supplemented with IL-2 (100 U mL−1) every second day (i.e. day 5, 7 and 9 post-stimulation).
Figure 1. Image-based cytometry reliably measures the kinetics and magnitude of in vitro CAR T cell expansion.
A) Bright-field image and segmentation of individual T cells (blue) seeded in a 96-well microwell plate using the Celigo Image Cytometer. B) Triplicate serial dilutions of CD3+ T cells were counted by the image cytometer and Multisizer 3 and mean cell densities were plotted against theoretical values. Slope of each line is denoted as “m”. C) Cell density values measured by the image cytometer and Multisizer 3 from (B) were plotted against each other. Spearman correlation test was used to calculate significance. D) CD4+ T cells were activated with αCD3/CD28 Dynabeads and 24-hours later were transduced with a lentivirus encoding an HIV-specific CAR containing the indicated intracellular domain (ICD). Population doublings were calculated by measuring the total cell number in culture at the indicated time-points using the image cytometer (square) and Multisizer 3 (circle). E) Comparison of paired CAR T cell population doublings on day 10 post-activation measured by the image cytometer and Multisizer 3. Wilcoxon matched pairs sign rank test was used to calculate significance (NS, P>0.05). The comparison of CAR T cell expansion kinetics using the image cytometer and Multisizer 3 were repeated in two independent donors.
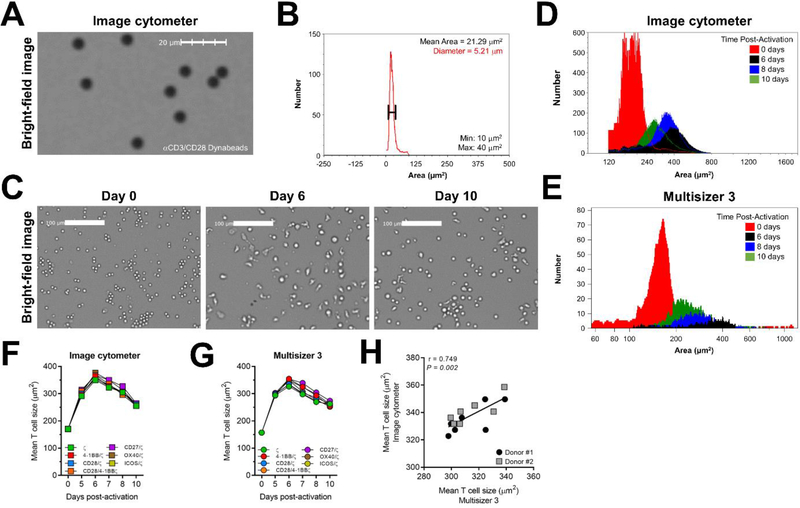
Figure 2. Image-based cytometry quantifies changes in CAR T cell size post-activation.
A) Bright-field image of αCD3/CD28 Dynabeads seeded in 96-well microwell plate. B) Histogram of αCD3/CD28 Dynabeads imaged in (A) shows average bead area and diameter within min (10 μm2) and max (40 μm2) limits. C) Bright-field image of CAR T cells on days 0, 6 and 10 post-activation. D) Histogram of CAR T cell area at days 0 (red), 6 (black), 8 (blue) and 10 (green) post-activation by image cytometry and E) Multisizer 3. Data are representative of 2 donors. F) Longitudinal cell size calculated as cell surface area (μm2) of the indicated CAR T cell types measured by image cytometry and G) Multisizer 3. Data are representative of 2 donors. H) Correlation of cell size values from the image cytometer plotted against Multisizer 3. Each symbol represents a unique CAR T cell type 7 days post-activation. Spearman correlation test was used to calculate significance. The longitudinal comparison of CAR T cell size using the image cytometer and Multisizer 3 were repeated in two independent donors.
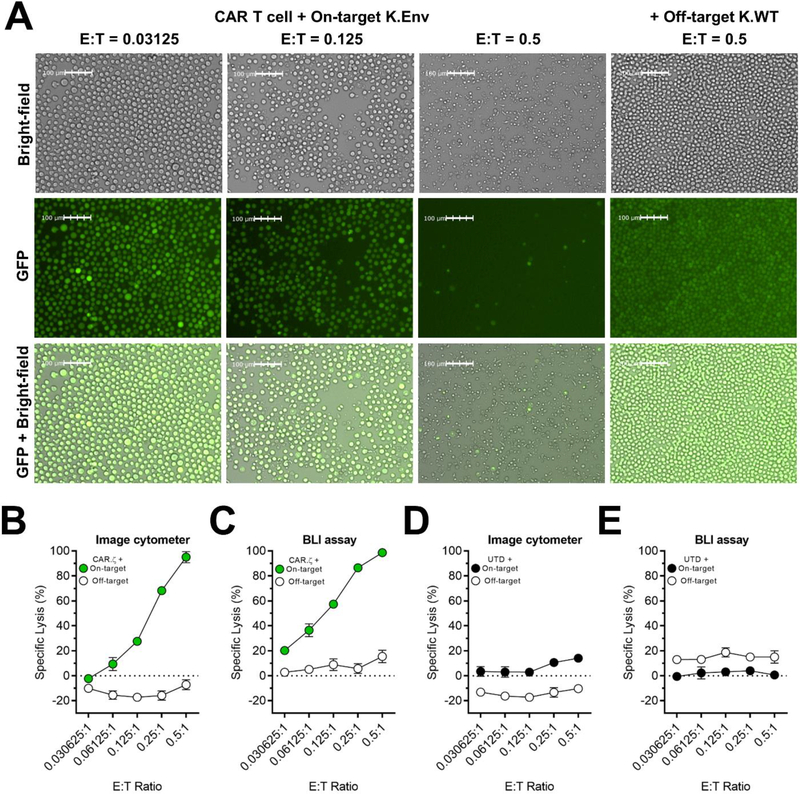
Figure 3. In vitro HIV-specific CAR T cell-mediated killing assessed by image-based cytometry.
HIV-specific CD8+ CAR T cells expressing the CD3ζ intracellular domain were mixed in triplicate with on-target K562 HIVYU2 GFP160+ cells (K.Env) or off-target K562 wild-type cells (K.WT) at 0.5, 0.25, 0.125, 0.061 and 0.0306:1 effector-to-target (E:T) ratios. Target cells stably expressed GFP linked to Click Beetle Green luciferase by an intervening T2A sequence. Cell lysis was determined by successive imaging of target cells using image cytometry and bioluminescence (BLI). A) Bright-field and fluorescence microscopy images of GFP+ K.Env cells and K.WT cells 72-hours after co-culture acquired by the image cytometer. B) Specific lysis of on-target and off-target cells cultured with CAR T cells and measured by image cytometry and C) BLI. D) Specific lysis of on-target and off-target cells cultured with UTD T cells and measured with image cytometry and E) BLI. Specific lysis values were calculated as described for each method in Materials and Methods. Symbols represent mean and error bars show ± SD. The comparison of CAR T cell-mediated cytotoxicity using image cytometry and BLI were repeated in 3 independent donors. All data are representative of 3 donors.
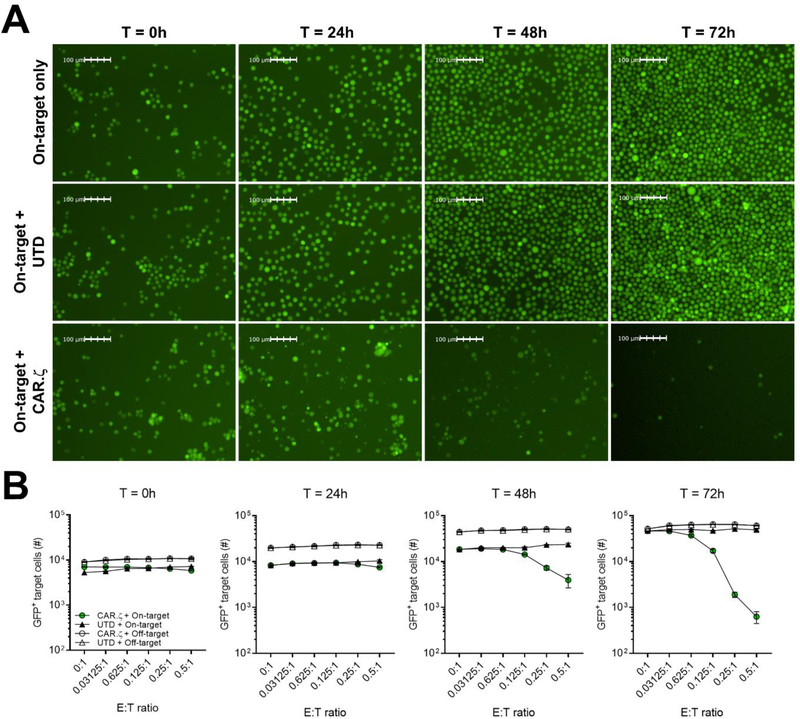
Figure 4. Image-based cytometry captures the kinetics of in vitro CAR T cell-mediated cytolysis.
A) Fluorescence microscopy images of on-target GFP+ K.Env cells alone, or mixed with HIV-specific CD8+ CAR or UTD T cells at 0.5:1 E:T ratio at the indicated time points after co-culture acquired by the image cytometer. B) Enumeration of on-target cells and off-target GFP+ K.WT cells mixed with CD8+ CAR or UTD T cells at different E:T ratios at the indicated time points after co-culture. Symbols represent average of triplicate values and error bars show ± SD. The comparison of CAR T cell-mediated cytotoxicity using image cytometry and BLI were repeated in 3 independent donors. All data are representative of 3 donors.
2.5. Preparation of K562 target cells
The K562 cell line stably transduced to express the HIVYU2 GP160 Envelope protein (K562.Env) was a kind gift from Aimee Payne (University of Pennsylvania, Philadelphia, PA) and wild-type K562 cell line (K562.WT) (ATCC CCL-243) were maintained in complete RPMI 1640 at a density of 0.1×106 cells mL−1. Both K562 cell lines were transduced with a lentiviral vector encoding GFP coupled to Click Beetle Green (CBG) luciferase by an intervening T2A sequence. The K562 cell lines were single-cell sorted on the Aria II (BD Bioscience) based on GFP expression. For each cell line, an individual single-cell clone was selected based on uniform GFP expression and subsequently propagated for use in the cytotoxicity experiments.
2.6. T cell dilution series
Approximately, 4×106 total T cells were transferred into a single well of a 96-well flat bottom plate (Corning #3596) in 200 μL of 1X PBS (Corning). Subsequently, 7 two-fold serial dilutions were made by carrying over 100 μL of liquid containing cells. After the dilution series, 100 μL of 1X PBS was added to all wells for a final volume of 200 μL per well. The dilution series spanned 107 to 0.156×107 cells mL−1 and was performed in triplicate. Cell concentration and average cell size were determined by the Image Cytometer and Multisizer 3 by mixing each well of the dilution series by manual pipetting and then transferring 10 μL of culture to a 96-well flat bottom plate (Image Cytometer) and a coulter counter vial (Multisizer 3). The cell concentration values from each instrument were compared to theoretical values representing the 7 data points that comprise the ideal cell concentrations of the dilution series in the absence of error that may be induced by manual pipetting.
2.7. T cell proliferation and size measurement using image cytometry
The Celigo Image Cytometer (Nexcelom Bioscience) utilizes one bright-field (BF) and four fluorescent (FL) imaging channels, blue (EX377/50 nm, EM470/22 nm), green (EX 483/32 nm, EM 536/40 nm), red (EX 531/40 nm, EM 629/53 nm), and far red (EX 628/40 nm, EM 688/31 nm) for high-throughput cell-based assays (David et al., 2017; Mazor et al., 2017; Fantini et al., 2018; Fantini et al., 2019; Rosen et al., 2019). The target and effector cells seeded in standard multi-well microplates are auto-focused, imaged, and analyzed using the Celigo software (version 5.1). The software consists of five major steps START, SCAN, ANALYZE, GATE, and RESULTS, where the users can enter general information, setup imaging/analysis parameters, perform imaging/analysis of cells, and view/export images and results.
The Celigo Application “Target 1” was used to measure beads with specific diameter in the bright-field channel at an exposure time of ~2,800 μs. The Celigo Application “Target 1 + Mask” was employed for measuring proliferation by directly counting total T cells in the bright-field channel in each well over time. Both Target 1 and Mask channels were set to bright-field illumination with exposure times 1 and ~3,250 μs, respectively. The Mask channel allowed the dilation of the outline diameter to improve size measurement of T cells. In addition, a specific image analysis template was used from FCS Express 6 (De Novo Software, Pasadena, CA). The counted T cell data (.ice files) were exported from the image cytometer software and imported into the FCS Express (De Novo Software) template that automatically generated size histograms, cell count, cell concentration, diameter (μm), minimum and maximum cell size (μm2), and average cell size (μm2). The average cell size values were multiplied by a factor of 4 to account for the surface area of spherical objects.
2.8. T cell proliferation and size measurement using the Multisizer 3
40 μL of cell culture was transferred into individual Accuvette ST vials (Beckman Coulter) containing 20 mL of ISOTON II Diluent (Beckman Coulter). Vials were loaded onto the Multisizer 3 one at a time and cell concentration (cells mL−1) was based on volumetric sampling (500 μL) under the following conditions: 70 μm aperture, 1600 μA current, and a gain setting of 2. Cell size was determined by applying a manual gate to all samples (range 150 μm2 to 1500 μm2). The cell concentration and average cell size was determined by the Beckman Coulter Particle Characterization software (v3.51) using the ‘Statistics’ function. Similarly, αCD3/CD28 Dynabeads beads at 4.5 μm diameter (Gibco) were counted and analyzed following the procedure described above.
2.9. CAR T cell-mediated cytotoxicity assay
Untransduced (UTD) and CAR CD8+ T cells that expressed the CD3-ζ endodomain coupled with iRFP670 were manufactured as described above. Prior to setting up the assay, CAR transduction efficiency was normalized across all donors based on positivity for iRFP670 expression detected by flow cytometry (BD LSR Fortessa). For each donor, 2×104 CAR T cells were seeded in complete RPMI, and then five 2-fold serial dilutions were performed in a 96-well black polystyrene microplate (Corning #3603). 1×104 K562.Env.CBG.GFP or K562.WT.CBG.GFP cells were added to each well with final E:T ratios of 0.500, 0.250, 0.125, 0.061, and 0.031:1. Target cells were also cultured in the absence of CAR T cells to account for spontaneous death, while wells containing complete RPMI only were included as a measure for maximal killing (used for BLI-based assay). Three technical replicates were performed for each donor. Plates were cultured at 37°C, 5% CO2, and 95% humidity incubation conditions. The cytotoxicity measurements were analyzed at 0, 24, 48 and 72 h using the image cytometer.
2.10. Cytotoxicity measurement using image cytometry
The Celigo application “Target 1” was used for CAR T cell-mediated cytotoxicity assay, which utilized only the green fluorescence channel. Green fluorescent images were acquired with exposure times ranging from ~15,000 to 30,000 μs. The image cytometer was used to directly count the number of GFP+ target cells in the wells with different donors and E:T ratios over time in triplicate for each condition. Since GFP fluorescence is significantly reduced as the target cells are killed during co-culture due to leakage, direct counting of the bright GFP+ cells represented viable cells in the well. Specific lysis was calculated by the following: % Specific Lysis = 100 × (# cells in no effector wells - # cells in treatment well) / (# cells in no Effectors – 0 (max killing). Maximal killing represents total target cell lysis, as such this value is equal to 0. This calculation was performed to align with the normalized RLU data from BLI-based assay.
2.11. Cytotoxicity measurement using BLI microplate reader
Immediately after imaging the plate with the image cytometer at the 72 hour time point, D-Luciferin potassium salt was added to each well producing a final concentration of 15 μg mL−1 (GoldBio) and incubated at 37°C for 10 min. Luminescence was measured for 1 second with a luminometer (Synergy H4 Hybrid Microplate Reader) as relative light units (RLU). Triplicate wells were averaged and the percent specific lysis was calculated with the following equation (Karimi et al., 2014) specific lysis (%) = 100 × [(spontaneous death RLU - treatment RLU)/(spontaneous death RLU - maximal killing RLU)]. Maximal killing represents the RLU measurement of wells containing complete RPMI in the absence of target and effector cells.
2.12. Statistical analysis
All statistical analysis was performed using GraphPad Prism, version 7 (San Diego, CA). Comparison of means from matched samples was performed using Wilcoxon matched pairs signed rank test. Bivariate correlations were performed using Spearman’s rank correlation.
3. Results
3.1. Image-based cytometry measures CAR T cell expansion criteria during manufacturing
Image cytometry uses the bright-field channel and auto-focus feature to identify individual cells that are seeded in a microwell plate (Fig. 1A), after which the well can be imaged and analyzed using the Celigo software to calculate cell density. We validated this cell counting method by comparing the image cytometer to the Multisizer 3 (Beckman Coulter), an automated cell counter and particle sizing instrument frequently used in the hematology field that employs the coulter principle (Bessman et al., 1982; Fernyhough et al., 2003). To do so, we prepared a triplicate series of two-fold T cell dilutions from which cell counting measurements were made by each instrument. The cell concentrations measured by the image cytometer and Multisizer 3 maintained a high degree of linearity over the indicated range of dilutions compared to the theoretical values (Fig. 1B), and were strongly correlated with each other (r = 0.9996; Fig. 1C).
The image cytometer was then used to monitor the cell density of multiple CAR T cell products, using a manufacturing process similar to one being employed in clinical trials (Fesnak et al., 2016; Wang and Riviere, 2016). We generated seven distinct CAR T cell products per donor by transducing αCD3/CD28 bead-activated CD4+ T cells with single lentiviruses containing an HIV-specific CAR derived from the full-length CD4 extracellular region fused to an intracellular domain (ICD) comprising the TCR CD3-ζ (ζ) chain and one or more costimulatory domains, including 4–1BB, CD28, CD28/4–1BB, CD27, OX40 or ICOS (Supplementary Fig. 1). Beginning 5 days after the initial stimulation, we calculated the number of population doublings from baseline (day 0) using the cell density values measured by the image cytometer and Multisizer 3. The expansion curves of each CAR T cell type generated by the image cytometer were nearly identical to the Multisizer 3 (Fig. 1D), and no statistical differences were observed between the two methods comparing total population doublings after 10 days of culture (Fig. 1E). Notably, we did not observe any substantial differences in proliferation among the CAR T cell populations with the exception of CAR T cells expressing the CD27/ζ ICD, which consistently exhibited fewer population doublings (Fig. 1D,E).
Following cognate antigen stimulation, T cell size increases due to the accumulation of biomass prior to cellular division (Zangle et al., 2013). As such, we simultaneously quantified the size of expanding CAR T cells throughout manufacturing as a surrogate readout of their activation state. However, to first validate the image cytometry-based object sizing protocol, we measured the diameter of αCD3/CD28 Dynabeads. The image cytometer detected the beads using bright-field microscopy (Fig. 2A), and calculated an average bead diameter of 5.2 μm (Fig. 2B), which approximates the manufacturer (Gibco) listed width of 4.5 μm. This method was then applied to CAR T cells during the manufacturing process, and we observed that CAR T cell size (area, μm2) peaked 6 days after the initial stimulation before gradually contracting to a smaller, ‘rested’ size on day 10 (Fig. 2C–E). The cell areas of each CAR T cell population were roughly similar during the expansion phase (Fig. 2F,G and Supplementary Fig. 2), which was expected given their comparable proliferation kinetics (Fig. 1E). Moreover, the cell areas calculated by each method strongly correlated with one another (Fig. 2H). Together, the data acquired by image cytometry were directly comparable to conventional technologies and supported the use of this method to evaluate CAR T cell growth kinetics during the manufacturing process.
3.2. Image fluorescence microscopy captures the kinetics of in vitro CAR T cell-mediated killing
To align with traditional assays used to quantify the in vitro functional potency of CAR T cells, we determined if the image cytometer could detect antigen-specific effector function. To do so, we set up a cytotoxicity assay using untransduced (UTD) and HIV-specific CD8+ CAR T cells expressing the CD3-ζ ICD cultured at different effector-to-target (E:T) ratios with on-target K562 cells expressing the HIVYU2 GP160 protein (K.Env) or off-target, wild-type K562 cells (K.WT). For this assay, we stably transduced these target cell lines with a lentiviral vector containing GFP linked to click beetle green luciferase by an intervening T2A sequence. The inclusion of GFP enabled the enumeration of GFP+ target cells by using the image cytometer, followed by the sequential detection of bioluminescence (BLI) from luciferase activity using the same set of effector CAR T cells and K562 target cells. As such, we were able to directly compare the extent of target cell killing measured by image cytometry to a standard BLI-based assay routinely used to characterize cytotoxic potential (Karimi et al., 2014; Posey et al., 2016).
The image cytometer accurately detected GFP+ target cells utilizing the bright-field and green fluorescent channels, and we observed substantial CAR T cell-mediated reductions of on-target GFP+ K.Env cells (Fig. 3A), but not off-target GFP+ K.WT cells (Supplementary Fig. 3). We then calculated specific lysis values using the image cytometer and BLI-based assay at all E:T ratios 72-hours after co-culture. Both methods showed that CAR T cells exhibited dose-dependent cytotoxicity at E:T ratios <1; this effect was HIV-specific as only limited off-target cytolysis occurred (Fig. 3B and Supplementary Fig. 4A). In contrast, UTD T cells induced minimal killing as we observed less than 20% target cell lysis across all E:T ratios (Fig. 3D,E and Supplementary Fig. 4B). Although the same cultures were analyzed by both methods, the specific lysis values obtained by the BLI-based assay were marginally greater than the image cytometer (Supplementary Fig. 4C), suggesting there may be slight differences in sensitivity. However, the BLI-based assay also measured greater off-target CAR T cell-mediated killing, likely resulting from background luciferase signals (Fig. 3C and Supplementary Fig. 4A). It is important to note that both methods measured negative specific lysis, which indicated target cell growth in the control conditions, evidenced by the accumulation of target cells in the absence of CAR T cells (Fig. 4A and Supplementary Fig. 5B).
Notably, image cytometry captured the kinetics of CAR T cell-mediated killing by enumerating GFP+ target cells without further manipulation unlike end-point BLI-based assays or the Chromium-51 release assay (Karimi et al., 2014). We imaged the cells at 0, 24, 48 and 72-hours after co-culture and observed marked CAR T cell-mediated reductions of on-target GFP+ K.Env cells beginning at 48-hours (Fig. 4A), and cell lysis increased through 72-hours after co-culture indicating that CAR T cells were capable of sustained cytotoxic function (Fig. 4B and Supplementary Fig. 5A). Importantly, we observed limited off-target toxicity as GFP+ K.WT cells continued to grow unabated when cultured with CAR T cells at the indicated E:T ratios (Fig. 4B, and Supplementary Fig. 5A,B). Taken together, these data demonstrate that image cytometry is capable of quantifying in vitro antigen-specific CAR T cell-induced cytotoxicity, and that the sensitivity of this method is comparable to conventional assays that assess effector CAR T cell function.
4. Discussion
The ability to functionally characterize multiple CAR T cell products in a high-throughput and comparative manner can expedite the discovery of novel CARs with therapeutic potential. Current methods to measure CAR T cell proliferation as well as cytotoxic potential during the manufacturing process can be time-consuming and requires the use of multiple equipment. The image-based cytometry method described herein was designed to eliminate these issues. In our study, we noted several advantages of using image cytometry compared to conventional instruments. For instance, the average time to scan, image and count T cells seeded in a 96-well microplate by the image cytometer was 5 seconds per well compared to >30 seconds by the Multisizer 3 from Beckman Coulter. Moreover, the bright-field and fluorescent images acquired by the Celigo Image Cytometer led to the direct enumeration of multiple cell types without manipulating the culture system, which can serve to bolster readouts from assays that employ indirect, end-point assays to measure T cell proliferation (i.e. CFSE dilution) (Quah and Parish, 2012) or cytotoxicity (Nacasaki Silvestre et al., 2020). Moreover, this platform is well-suited for future automation and adaption to additional assays to better CAR T cell manufacturing as more information becomes available regarding which parameters of T cell manufacturing correlate with clinical benefit (Garfall et al., 2019).
We first validated the image cytometer to measure the cell density of multiple CAR T cell products during the manufacturing process compared to the Multisizer 3. The cell concentration values from both instruments were nearly identical over the course of expansion (10 days), validating that image-based cytometry identified and accurately counted CAR T cells. Importantly, these data enabled us to expand CAR T cells at an optimal density by adding new expansion medium to the culture, which also serves to dilute the waste products that accumulate from cellular metabolism (Almeida et al., 2016). Interestingly, despite each CAR T cell type expressing a unique intracellular costimulatory domain, which are known to impart distinct functional properties (Weinkove et al., 2019), the CAR T cells exhibited similar expansion kinetics over 10 days in culture. This contrasts data from the cancer field demonstrating that some scFv-based CARs expressing distinct costimulatory signals exhibit differential expansion kinetics during ex vivo manufacturing (Kawalekar et al., 2016; Guedan et al., 2018), suggesting that the HIV-specific CAR mediates limited ligand-independent signaling, a property that may improve resistance to exhaustion (Long et al., 2015).
Furthermore, we measured the cell surface area of expanding CAR T cells as a surrogate readout of cellular activation (Zangle et al., 2013). Both instruments detected peak CAR T cell size 6 days post-activation (>350 μm2) before contracting to a rested size on day 10 (<250 μm2). However, it is important to note that each instrument uses a unique method to calculate cell size. For instance, the Multisizer 3 determines cell area by detecting the impedance of an electrical current measured as a voltage or current pulse. This transient change in impedance results as cells pass through the aperture a volume of electrolyte solution is displaced from the sensing zone equivalent to the volume of the cell (Hurley, 1970). In contrast, the image cytometer calculates cell size by measuring the finite pixels of a cell imaged in 2-dimensional space contained within the segmentation boundary, and then factors in the resolution of the acquired image. This method produces a cross-sectional area, but for CAR T cells and other spherical objects this measurement can be easily adjusted to account for 3-dimensional area. Nevertheless, the cell sizes from both instruments strongly correlated with one another, indicating that image cytometry captured the pattern of cell size dynamics during CAR T cell manufacturing.
Effector cell-mediated cytotoxicity assays are commonly used to detect the in vitro function of CAR-modified and other pathogen-specific T cells (Nacasaki Silvestre et al., 2020). These assays generally measure target cell death by 3 mechanisms: 1) the release of Chromium-51 (radioactivity) (Anurathapan et al., 2014), lactate dehydrogenase (enzymatic reaction) (Smith et al., 2011), or calcein (fluorescence) (Lichtenfels et al., 1994) from dead cells into the culture supernatant; 2) the loss of viable target cells by flow cytometry (Karawajew et al., 1994; Hermans et al., 2004); and 3) cell-associated luciferase activity (Karimi et al., 2014). All three categories of assays typically provide a fixed end-point measurement of cytotoxicity and consumption of samples. End-point assays necessitate that replicate experiments need to be performed to capture the kinetics of T cell killing, which may require a large input of both effector and target cells. However, we demonstrated that an image cytometry-based approach led to the reliable assessment of cytotoxic CAR T cell function over time. Notably, the image-cytometer can identify and enumerate a variety of target cell types through size exclusion or fluorescent labelling without using additional reagents, and as such, we measured the kinetics of target cell lysis by directly counting GFP+ target cells without disturbing or sacrificing cell samples. In contrast, the BLI-based assay provided an indirect measurement of antigen-specific killing at a single time point (72-hours after co-culture). Moreover, the image cytometry-based method utilizes cell counts within the same well at 0-hours to normalize the specific lysis values at 24, 48 and 72-hours after co-culture. In this way, the reduction of GFP+ target cells reference their respective wells instead of separate control wells, thereby negating the potential variability inherent to pipetting or differences in target cell input between treatment and control wells. As such, the ability to assess and quantify cytotoxicity in a stringent, kinetic manner is a distinct advantage of image cytometry.
In summary, with the advancements in camera, optics, and image analysis technologies, image cytometry has taken root in providing quantitative results to various aspects of biological assays (Chan et al., 2012; Kuksin et al., 2016; Chan et al., 2019; Magnotti et al., 2020). For CAR T cell therapy, conventional methods evaluating manufacturing criteria and effector function have resulted in the development of potent cellular products capable of inducing durable remissions for certain malignancies (Maude et al., 2014; Porter et al., 2015), but newer technologies are perhaps needed to rapidly advance the development and evaluation of preclinical candidate therapies. Here, image cytometry provided a novel, efficient, and high-throughput characterization of CAR T cells during the manufacturing process, and offered several direct advantages over industry-standard equipment and routine assays. The findings described herein highlight the utility of image-based cytometry to assess multiple attributes of CAR T cells, and show the potential of this method to expedite the discovery and validation of novel CAR T cell products.
Supplementary Material
Acknowledgments
We thank Phillip Rommel (University of Pennsylvania) for his support in conducting the cytotoxicity assays, and Christoph Ellebrecht and Aimee Payne (University of Pennsylvania) for providing the K562 cell line transduced with HIVYU2 GFP160. This work was supported by UM1AI126620 (JLR) co-funded by NIH, NIAID, NIMH, NINDS, and NIDA. CRM is supported by a T32 grant (AI00763). We also thank the Penn Center for AIDS Research (P30-AI045008) for providing human T cells, and helpful comments and suggestions from the Riley lab.
Footnotes
Conflicts of interest
JLR has filed a patent describing the construction of these HIV-specific CARs. JLR co-founded Tmunity Therapeutics that has the rights to license the CAR technology described herein. JLR holds an equity interest in Tmunity. AL, LLC, and TS are employees of Nexcelom Bioscience LLC. and declare competing financial interests. The research in this manuscript is for reporting on a biological detection method using the Celigo Image Cytometer, a product of Nexcelom Bioscience LLC. Authors declare no other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida L, Lochner M, Berod L and Sparwasser T, 2016, Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol 28, 514–524. [DOI] [PubMed] [Google Scholar]
- Anurathapan U, Chan RC, Hindi HF, Mucharla R, Bajgain P, Hayes BC, Fisher WE, Heslop HE, Rooney CM, Brenner MK, Leen AM and Vera JF, 2014, Kinetics of tumor destruction by chimeric antigen receptor-modified T cells. Mol Ther 22, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman JD, Williams LJ and Gilmer PR Jr., 1982, Platelet size in health and hematologic disease. Am J Clin Pathol 78, 150–3. [DOI] [PubMed] [Google Scholar]
- Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, Kuksin D, Lin B and Qiu J, 2012, A novel image-based cytometry method for autophagy detection in living cells. Autophagy 8, 1371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LL, Wucherpfennig KW and de Andrade LF, 2019, Visualization and quantification of NK cell-mediated cytotoxicity over extended time periods by image cytometry. J Immunol Methods 469, 47–51. [DOI] [PubMed] [Google Scholar]
- David JM, Dominguez C, McCampbell KK, Gulley James L., Schlom J. and Palena C, 2017, A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology 6, e1349589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini M, David JM, Saric O, Dubeykovskiy A, Cui Y, Mavroukakis SA, Bristol A, Annunziata CM, Tsang KY and Arlen a.P.M., 2018, Preclinical Characterization of a Novel Monoclonal Antibody NEO-201 for the Treatment of Human Carcinomas. Frontiers in Immunology 8, 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini M, David JM, Wong HC, Annunziata CM, Arlen PM and Tsang KY, 2019, An IL-15 Superagonist, ALT-803, Enhances Antibody-Dependent Cell-Mediated Cytotoxicity Elicited by the Monoclonal Antibody NEO-201 Against Human Carcinoma Cells. Cancer Biotherapy and Radiopharmaceuticals 34, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernyhough ME, Helterline DL, Vierck JL, Hill RA and Dodson MV, 2003, Coulter counter use in the enumeration of muscle and fat stem cells. Methods Cell Sci 25, 221–5. [DOI] [PubMed] [Google Scholar]
- Fesnak AD, June CH and Levine BL, 2016, Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16, 566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfall AL, Dancy EK, Cohen AD, Hwang WT, Fraietta JA, Davis MM, Levine BL, Siegel DL, Stadtmauer EA, Vogl DT, Waxman A, Rapoport AP, Milone MC, June CH and Melenhorst JJ, 2019, T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv 3, 2812–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Waks T and Eshhar Z, 1989, Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 86, 10024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedan S, Posey AD Jr., Shaw C, Wing A, Da T, Patel PR, McGettigan SE, Casado-Medrano V, Kawalekar OU, Uribe-Herranz M, Song D, Melenhorst JJ, Lacey SF, Scholler J, Keith B, Young RM and June CH, 2018, Enhancing CAR T cell persistence through ICOS and 4–1BB costimulation. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay KA and Turtle CJ, 2017, Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies. Drugs 77, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans IF, Silk JD, Yang J, Palmowski MJ, Gileadi U, McCarthy C, Salio M, Ronchese F and Cerundolo V, 2004, The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods 285, 25–40. [DOI] [PubMed] [Google Scholar]
- Hurley J, 1970, Sizing particles with a Coulter counter. Biophys J 10, 74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karawajew L, Jung G, Wolf H, Micheel B and Ganzel K, 1994, A flow cytometric long-term cytotoxicity assay. J Immunol Methods 177, 119–30. [DOI] [PubMed] [Google Scholar]
- Karimi MA, Lee E, Bachmann MH, Salicioni AM, Behrens EM, Kambayashi T and Baldwin CL, 2014, Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay. PLoS One 9, e89357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr., Patel PR, Guedan S, Scholler J, Keith B, Snyder NW, Blair IA, Milone MC and June CH, 2016, Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity 44, 380–90. [DOI] [PubMed] [Google Scholar]
- Kuksin D, Kuksin CA, Qiu J and Chan LL, 2016, Cellometer image cytometry as a complementary tool to flow cytometry for verifying gated cell populations. Anal Biochem 503, 1–7. [DOI] [PubMed] [Google Scholar]
- Leibman RS, Richardson MW, Ellebrecht CT, Maldini CR, Glover JA, Secreto AJ, Kulikovskaya I, Lacey SF, Akkina SR, Yi Y, Shaheen F, Wang J, Dufendach KA, Holmes MC, Collman RG, Payne AS and Riley JL, 2017, Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog 13, e1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Cotte J, Small CC, Carroll RG, Riley JL, Bernstein WB, Van Epps DE, Hardwick RA and June CH, 1998, Large-scale production of CD4+ T cells from HIV-1-infected donors after CD3/CD28 costimulation. J Hematother 7, 437–48. [DOI] [PubMed] [Google Scholar]
- Levine BL, Miskin J, Wonnacott K and Keir C, 2017, Global Manufacturing of CAR T Cell Therapy. Mol Ther Methods Clin Dev 4, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenfels R, Biddison WE, Schulz H, Vogt AB and Martin R, 1994, CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods 172, 227–39. [DOI] [PubMed] [Google Scholar]
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, Kaplan RN, Patterson GH, Fry TJ, Orentas RJ and Mackall CL, 2015, 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21, 581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotti EL, Chan LL-Y, Zhu Q and Marasco WA, 2020, A high-throughput chemotaxis detection method for CCR4+ T cell migration inhibition using image cytometry. Journal of Immunological Methods 112747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldini CR, Ellis GI and Riley JL, 2018, CAR T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol 18, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL and Grupp SA, 2014, Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371, 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL and June CH, 2002, Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB . Nat Biotechnol 20, 143–8. [DOI] [PubMed] [Google Scholar]
- Mazor Y, Sachsenmeier KF, Yang C, Hansen A, Filderman J, Mulgrew K, Wu H and Dall’Acqua WF, 2017, Enhanced tumor-targeting selectivity by modulating bispecific antibody binding affinity and format valence. Scientific Reports 7, 40098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacasaki Silvestre R, Moco PD and Picanco-Castro V, 2020, Determination of Cytotoxic Potential of CAR-T Cells in Co-cultivation Assays . Methods Mol Biol 2086, 213–222. [DOI] [PubMed] [Google Scholar]
- Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ and June CH, 2015, Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7, 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey AD Jr., Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM, Cogdill AP, Chen TJ, Song D, Scholler J, Kranz DM, Feldman MD, Young R, Keith B, Schreiber H, Clausen H, Johnson LA and June CH, 2016, Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 44, 1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah BJ and Parish CR, 2012, New and improved methods for measuring lymphocyte proliferation in vitro and in vivo using CFSE-like fluorescent dyes. J Immunol Methods 379, 1–14. [DOI] [PubMed] [Google Scholar]
- Richardson MW, Carroll RG, Stremlau M, Korokhov N, Humeau LM, Silvestri G, Sodroski J and Riley JL, 2008, Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. J Virol 82, 11117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O, Chan LL-Y, Abiona OM, Gough P, Wang L, Shi W, Zhang Y, Wang N, Kong W-P, McLellan JS, Graham BS and Corbett KS, 2019, A high-throughput inhibition assay to study MERS-CoV antibody interactions using image cytometry. Journal of Virological Methods 265, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Wunder MB, Norris DA and Shellman YG, 2011, A simple protocol for using a LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS One 6, e26908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RP, Devillier L, Parkhurst MR, Rosenberg SA and Dudley ME, 2012, Clinical scale rapid expansion of lymphocytes for adoptive cell transfer therapy in the WAVE(R) bioreactor. J Transl Med 10, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AK, Maus MV, Shalaby WS, June CH and Riley JL, 2002, A cell-based artificial antigen-presenting cell coated with anti-CD3 and CD28 antibodies enables rapid expansion and long-term growth of CD4 T lymphocytes. Clin Immunol 105, 259–72. [DOI] [PubMed] [Google Scholar]
- van der Stegen SJ, Hamieh M and Sadelain M, 2015, The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 14, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembadi A, Menachery A and Qasaimeh MA, 2019, Cell Cytometry: Review and Perspective on Biotechnological Advances. Front Bioeng Biotechnol 7, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X and Riviere I, 2016, Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics 3, 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinkove R, George P, Dasyam N and McLellan AD, 2019, Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology 8, e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangle TA, Burnes D, Mathis C, Witte ON and Teitell MA, 2013, Quantifying biomass changes of single CD8+ T cells during antigen specific cytotoxicity. PLoS One 8, e68916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.