Abstract
Objective:
To evaluate the association between in vitro fertilization (IVF) and ischemic placental disease (IPD), stratified by gestational age.
Design:
We performed a secondary analysis of a retrospective cohort study of deliveries.
Setting:
Deliveries were performed over fifteen years at a single tertiary hospital.
Patients:
We included all parturients who had a live born infant or an intrauterine fetal demise (IUFD).
Intervention:
We compared pregnancies resulting from IVF cycles to non-IVF pregnancies.
Main Outcome Measures:
The primary outcomes were preterm and term IPD (preeclampsia, placental abruption, small-for-gestational age infant (SGA), or an intrauterine fetal demise (IUFD) due to placental insufficiency).
Results:
Of the 69,084 deliveries during the study period, 3,763 (5.4%) were conceived with IVF. The incidence of preterm delivery was 32.6% in IVF pregnancies and 10.8% in non-IVF pregnancies. Multiple gestations were more common in IVF pregnancies. Compared to non-IVF pregnancies, IVF pregnancies were more likely to develop both preterm and term IPD, even after adjustment for maternal age and parity. The risk of preterm IPD was 4 times higher (95% CI 3.7–4.4) in persons who underwent IVF compared to persons who did not undergo IVF. Among parturients who delivered at ≥37 weeks of gestation, IVF pregnancies had 1.7 times the risk of term IPD (95% CI: 1.6–1.9) compared to non-IVF pregnancies.
Conclusion:
IVF was strongly associated with preterm IPD. We found a similar, but attenuated, association between IVF and term IPD. The stronger association with preterm IPD suggests an association between IVF and placental insufficiency.
Keywords: Abruption, Assisted Reproductive Technology, Intrauterine fetal demise, Intrauterine growth restriction, Placental insufficiency
Capsule:
In vitro fertilization is associated with development of ischemic placental disease, with a stronger association between IVF and preterm IPD than between IVF and term IPD.
Introduction
Ischemic placental disease (IPD) is a term used to describe conditions that commonly are thought to occur due to placental insufficiency (1). The exact pathophysiology of the associated conditions, which include preeclampsia, abruption, and intrauterine growth restriction (IUGR), remains unknown, but inadequate remodeling of spiral arteries in early pregnancy (2) likely contributes to a shared etiology. In vitro fertilization (IVF) is associated with preeclampsia, abruption, and IUGR individually, and also when these conditions are studied collectively as IPD (3–5). This association may be due to coexistent factors, such as advanced maternal age, multiple gestation or obesity; the underlying reason for infertility (6), such as polycystic ovarian syndrome or premature ovarian insufficiency; or the peri-conception environment, which may contribute to poor placentation (7,8).
While preeclampsia, abruption, and IUGR can develop at any time in pregnancy, those more likely to be due to placental insufficiency result in preterm delivery (9,10). Given that preterm birth associated with IPD is more consistent with placental insufficiency than term birth associated with IPD (9,11), a stronger association between IVF and preterm IPD will provide more evidence of IVF as a risk factor for poor placentation.
We hypothesized that IVF is more strongly associated with IPD resulting in preterm birth than IPD resulting in term birth. Exploration of a gestational-age dependent association between IVF and IPD will contribute to the growing body of work evaluating the association between IVF and placental insufficiency.
Material and Methods
We performed a secondary analysis of a cohort study of deliveries in order to evaluate the effect of gestational age on the risk of IPD among pregnancies conceived with IVF relative to pregnancies conceived without IVF. The original cohort was developed specifically to evaluate the association between IVF and IPD and consisted of singleton and multiple gestations that resulted in deliveries from January 1, 2000 to June 1, 2015 at an academic, tertiary-care hospital. Detailed methods have been published previously (5). Briefly, we included all pregnancies that resulted in a delivery of either a live born infant or an intrauterine fetal demise (IUFD) greater than or equal to 20 weeks of gestation at our institution. We identified IVF pregnancies from medical records at our Division of Reproductive Endocrinology and Infertility and through birth certificate data from the Massachusetts Department of Public Health. We included both autologous and donor IVF cycles. Autologous IVF cycles used a person’s own oocytes for the IVF cycle, and donor IVF cycles used donated oocytes. Sperm could be either from the partner or donated. Non-IVF pregnancies were those delivered at our institution during the study period that were not conceived with IVF based on records from Boston IVF, Division of Reproductive Endocrinology and Infertility at Beth Israel Deaconess Medical Center or birth certificate data. We abstracted IVF information, including the oocyte source, from electronic medical records and birth certificate data.
Persons who were treated at Boston IVF underwent standard ovarian stimulation protocols, monitoring, and oocyte retrieval. In general, the fresh embryo transfer took place 3 or 5 days after the oocyte retrieval. The number of embryos transferred reflected national guidelines, with some variation according to treating physician’s specification, embryo quality and relevant patient history. Cryopreservation was generally performed 3 or 5 days after oocyte retrieval and included only embryos that were deemed viable by morphologic criteria. During the study period, vitrification was introduced in August 2011, with all freezing performed at the blastocyst stage by July 2013. The majority of frozen embryo transfers occurred after a programmed hormone replacement cycle, though some transfers occurred after a natural cycle. For cycles using donor oocytes at Boston IVF, it was the standard practice to transfer the embryo after a programmed hormone replacement cycle. IVF protocol details for IVF pregnancies identified through birth certificates were unavailable.
We abstracted demographic characteristics, obstetric history and delivery outcomes from electronic medical records. We obtained information regarding diabetes and smoking prior to pregnancy from birth certificate data.
The primary outcomes included preterm and term IPD (presence of preeclampsia, placental abruption, small for gestational age (SGA) neonate) or an IUFD due to placental insufficiency. We defined preterm IPD as IPD or IUFD from placental insufficiency in pregnancies that resulted in a delivery <37 weeks of gestation. Term IPD was defined as IPD or IUFD from placental insufficiency in pregnancies that resulted in a delivery ≥37 weeks of gestation.
We identified preeclampsia, placental abruption, and IUFD with ICD-9 codes and performed a medical record review to verify these outcomes, as described previously (5). Preeclampsia was defined using current criteria from the American College of Obstetrics and Gynecology (12). To determine whether placental insufficiency led to an IUFD, we reviewed autopsy, pathology, and clinician notes for documented evidence that included the term “placental insufficiency” or a similar phrase. We used SGA as a proxy of IUGR, which has been commonly done in other studies (6,9,11). We defined SGA as <10th percentile using published U.S. growth curves stratified by sex and gestational age (13). These curves are based on all U.S. births in a given period; the racial and ethnic composition of those births was similar to that in our study cohort. Multiple gestation pregnancies were defined as SGA if at least one infant was SGA. Secondary outcomes included each of the components of IPD separately, in addition to IPD with severe SGA, defined as <3rd percentile.
We used generalized estimating equations with an independent correlation matrix, accounting for multiple deliveries for the same woman, to estimate risk ratios (RR) and 95% confidence intervals (CI). To estimate the risk of preterm IPD, we included the full cohort. To estimate the risk of term IPD, we included only those pregnancies that were delivered ≥37 weeks of gestation because only those pregnancies were at risk for term IPD. We adjusted all models for maternal age and also considered race/ethnicity, gravidity, parity, smoking prior to pregnancy, diabetes prior to pregnancy, and year of delivery as potential confounders. We tested covariates individually for inclusion in the model and retained those that had an appreciable effect on the risk ratio. Observations with missing data were dropped from the regression analyses given that less than 1% of the covariates tested were missing.
To further investigate the gestational age variation of IPD by IVF versus non-IVF pregnancies, we plotted the cumulative incidence of IPD and its components.
To address limitations in the data, we performed sensitivity analyses. First, given the known higher incidence of multiple gestations among IVF pregnancies and the increased risk of preterm birth among multiple gestations, we evaluated the outcomes restricting to singleton gestations. Second, given our previously presented data on the increased incidence of IPD among donor IVF pregnancies (5), we stratified our analysis by oocyte source. We additionally plotted the cumulative incidence of IPD and its components, stratified by donor IVF, autologous IVF, and non-IVF pregnancies.
The Beth Israel Deaconess Medical Center institutional review board approved this study.
Results
Of the 69,084 deliveries during the study period, 3,763 (5.4%) were conceived with IVF. Those who conceived with IVF were older and more likely to be Caucasian, married, nulliparous, and have a graduate degree or higher (Table 1). The incidence of preterm delivery was 32.6% in IVF pregnancies and 10.8% in non-IVF pregnancies. Multiple gestations were more common in IVF pregnancies (Table 1). The incidence of preterm delivery among multiple gestations was 68.3% among IVF pregnancies and 70.3% among non-IVF pregnancies.
Table 1:
Patient and delivery characteristics in IVF and non-IVF pregnancies
| Characteristics | IVF n = 3763 | Non-IVF n=65321 |
|---|---|---|
| Demographics | ||
| Maternal age at conception (years) | 35.9 (32.8–39.2) | 31.9 (28.6–35.0) |
| Race | ||
| Caucasian | 3098 (82.3) | 40194 (61.5) |
| African American | 143 (3.8) | 7915 (12.1) |
| Hispanic | 68 (1.8) | 3997 (6.1) |
| Asian | 286 (7.6) | 9939 (15.2) |
| Other | 164 (4.4) | 3034 (4.6) |
| Not reported/missing | 4 (0.1) | 242 (0.4) |
| Marital status | ||
| Married or partnered | 3508 (93.2) | 52745 (80.7) |
| Single | 218 (5.8) | 11254 (17.2) |
| Divorced, separated, widowed | 30 (0.8) | 761 (1.2) |
| Missing | 7 (0.2) | 561 (0.9) |
| Highest level of education achieved | ||
| High school or less | 308 (8.2) | 14163 (21.7) |
| College or associate’s degree | 1751 (46.5) | 27087 (41.5) |
| Graduate degree or higher | 1598 (42.5) | 19515 (29.9) |
| Missing | 106 (2.8) | 4556 (7.0) |
| Insurance | ||
| Public | 52 (1.4) | 10053 (15.4) |
| Private, other | 3711 (98.6) | 55267 (84.6) |
| Gravidity | ||
| 1 | 2211 (58.8) | 23710 (36.3) |
| 2 | 791 (21.0) | 20949 (32.1) |
| 3+ | 761 (20.2) | 20656 (31.6) |
| Missing | 0 (0.0) | 6 (0.01) |
| Parity | ||
| 0 | 2498 (66.4) | 30400 (46.5) |
| 1 | 1058 (28.1) | 23555 (36.1) |
| 2+ | 207 (5.5) | 11366 (17.4) |
| Diabetes prior to pregnancy | 81 (2.2) | 1028 (1.6) |
| Smoking prior to pregnancy | 48 (1.3) | 2147 (3.3) |
| Gestations | ||
| Singleton | 2547 (67.7) | 63898 (97.8) |
| Multiple | 1216 (32.3) | 1423 (2.2) |
| Delivery characteristics | ||
| Gestational age at delivery (weeks) | 38.0 (36.0–39.0) | 39.0 (38.0–40.0) |
| Preterm delivery | 1227 (32.6) | 7040 (10.8) |
| Intrauterine fetal demise | 12 (0.3) | 287 (0.4) |
| Mode of delivery | ||
| Vaginala | 1273 (33.8) | 41012 (62.8) |
| Cesarean | 2287 (60.8) | 23906 (36.6) |
| Vaginal and cesareanb | 199 (5.3) | 359 (0.5) |
| Missing | 4 (0.1) | 44 (0.1) |
| Birth weight (grams) | ||
| Singletons | 3290 (2920–3625) | 3360 (3025–3680) |
| Multiplesc | 2323 (1825–2710) | 2290 (1810–2645) |
Data presented as median (interquartile range) or n (%)
Includes dilation and evacuation for IUFDs
Some parturients who had multiples delivered both vaginally and via cesarean
Birthweights combined for all babies and averaged
Compared to non-IVF pregnancies, IVF pregnancies were more likely to develop both preterm and term IPD, even after adjustment for maternal age and parity. Persons who underwent IVF had 4 times the risk of preterm IPD (95% CI: 3.7–4.4) compared to the non-IVF group (Table 2). Among parturients who delivered ≥37 weeks of gestation, the IVF group had 1.7 times the risk of term IPD compared to the non-IVF group (95% CI: 1.6–1.9) (Table 3). While IVF pregnancies were significantly more likely to result in preterm and term preeclampsia, abruption, and SGA than non-IVF pregnancies, the risks of these outcomes were notably higher in the preterm period. When restricting IPD to severe SGA (<3rd percentile), the risks of both preterm and term IPD, as well as SGA <3rd percentile, remained significantly higher for IVF pregnancies (Table 2) compared with non-IVF pregnancies.
Table 2:
Risk of preterm and term ischemic placental disease and its components in IVF compared with non-IVF pregnancies
| Cohort at risk for preterm IPD n = 69,084 | Cohort at risk for term IPD n = 60,797 | |||
|---|---|---|---|---|
| Outcome | IVF n=3763 | Non-IVF n=65321 | IVF n=2536 | Non-IVF n=58281 |
| SGA <10th percentile | ||||
| IPD/IUFD | 580 (15.4) | 2434 (3.7) | 478 (18.8) | 6339 (10.9) |
| RR (95% CI) | 4.1 (3.8–4.5) | 1.0 | 1.7 (1.6–1.9) | 1.0 |
| aRR (95% CI) | 4.0 (3.7–4.4)a | 1.0 | 1.7 (1.6–1.9)a | 1.0 |
| Preeclampsia | 273 (7.3) | 1160 (1.8) | 96 (3.8) | 1194 (2.0) |
| RR (95% CI) | 4.1 (3.6–4.6) | 1.0 | 1.8 (1.5–2.3) | 1.0 |
| aRR (95% CI) | 3.5 (3.1–4.1)a | 1.0 | 1.5 (1.2–1.9)a | 1.0 |
| Abruption | 97 (2.6) | 509 (0.8) | 26 (1.0) | 334 (0.6) |
| RR (95% CI) | 3.3 (2.7–4.1) | 1.0 | 1.8 (1.2–2.7) | 1.0 |
| aRR (95% CI) | 3.7 (2.9–4.6)b | 1.0 | 1.6 (1.05–2.4)b | 1.0 |
| SGA | 342 (9.1) | 1134 (1.7) | 385 (15.2) | 5073 (8.7) |
| RR (95% CI) | 5.2 (4.7–5.9) | 1.0 | 1.7 (1.6–1.9) | 1.0 |
| aRR (95% CI) | 5.2 (4.5–5.9)a | 1.0 | 1.8 (1.7–2.0)a | 1.0 |
| SGA <3rd percentile | ||||
| IPD/IUFD | 417 (11.1) | 1863 (2.9) | 234 (9.2) | 2589 (4.4) |
| RR (95% CI) | 3.9 (3.5–4.3) | 1.0 | 2.1 (1.8–2.4) | 1.0 |
| aRR (95% CI) | 4.0 (3.6–4.5)b | 1.0 | 1.9 (1.7–2.2)a | 1.0 |
| SGA | 89 (2.4) | 268 (0.4) | 119 (4.7) | 1165 (2.0) |
| RR (95% CI) | 5.8 (4.5–7.3) | 1.0 | 2.3 (2.0–2.8) | 1.0 |
| aRR (95% CI) | 6.3 (4.9–8.1)b | 1.0 | 2.5 (2.0–3.0)a | 1.0 |
Data presented as n (%), crude risk ratio (RR), adjusted risk ratio (aRR) and 95% confidence interval (CI)
IPD, ischemic placental disease; IUFD, intrauterine fetal demise; SGA, small for gestational age
Adjusted for maternal age, parity
Adjusted for maternal age
Table 3:
Risk of preterm and term ischemic placental disease and its components in IVF compared with non-IVF pregnancies among singleton pregnancies
| Cohort at risk for preterm IPD n = 66,445 | Cohort at risk for term IPD n = 60,010 | |||
|---|---|---|---|---|
| Outcome | IVF n=2547 | Non-IVF n=63898 | IVF n=2151 | Non-IVF n=57859 |
| SGA <10th percentile | ||||
| IPD/IUFD | 140 (5.5) | 1971 (3.1) | 268 (12.5) | 6101 (10.5) |
| RR (95% CI) | 1.8 (1.5–2.1) | 1.0 | 1.2 (1.1–1.3) | 1.0 |
| aRR (95% CI) | 1.9 (1.6–2.3)a | 1.0 | 1.2 (1.1–1.4)b | 1.0 |
| Preeclampsia | 73 (2.9) | 983 (1.5) | 68 (3.2) | 1169 (2.0) |
| RR (95% CI) | 1.9 (1.5–2.4) | 1.0 | 1.6 (1.2–2.0) | 1.0 |
| aRR (95% CI) | 1.7 (1.3–2.1)b | 1.0 | 1.3 (1.00–1.7)b | 1.0 |
| Abruption | 46 (1.8) | 491 (0.8) | 22 (1.0) | 334 (0.6) |
| RR (95% CI) | 2.4 (1.7–3.2) | 1.0 | 1.8 (1.2–2.7) | 1.0 |
| aRR (95% CI) | 2.7 (2.0–3.7)a | 1.0 | 1.6 (1.00–2.4)a | 1.0 |
| SGA | 63 (2.5) | 791 (1.2) | 192 (8.9) | 4846 (8.4) |
| RR (95% CI) | 2.0 (1.6–2.6) | 1.0 | 1.1 (0.93–1.2) | 1.0 |
| aRR (95% CI) | 2.0 (1.5–2.6)b | 1.0 | 1.1 (0.99–1.3)b | 1.0 |
| SGA <3rd percentile | ||||
| IPD/IUFD | 122 (4.8) | 1600 (2.5) | 134 (6.2) | 2490 (4.3) |
| RR (95% CI) | 1.9 (1.6–2.3) | 1.0 | 1.4 (1.2–1.7) | 1.0 |
| aRR (95% CI) | 2.0 (1.7–2.5)a | 1.0 | 1.3 (1.1–1.6)b | 1.0 |
| SGA | 12 (0.5) | 180 (0.3) | 48 (2.2) | 1085 (1.9) |
| RR (95% CI) | 1.7 (0.9–3.0) | 1.0 | 1.2 (0.89–1.6) | 1.0 |
| aRR (95% CI) | 1.9 (1.1–3.5)a | 1.0 | 1.3 (0.99–1.8)c | 1.0 |
Data presented as n (%), crude risk ratio (RR) and 95% confidence interval (CI)
IPD, ischemic placental disease; IUFD, intrauterine fetal demise; SGA, small for gestational age
Adjusted for maternal age
Adjusted for maternal age and parity
Adjusted for maternal age, parity, race
We plotted the cumulative incidence of IPD, as well as the individual components of IPD, for each week of gestational age stratified by IVF and non-IVF pregnancies (Figure 1 and Supplementary figure 1). The curves for IVF and non-IVF pregnancies diverge just after 30 weeks of gestation and the cumulative incidence of IPD remains higher for IVF pregnancies at each subsequent week of gestation; the same is true for each component of IPD. Notably, the slope of the curve increases most steeply for the IVF group between 35–37 weeks, suggesting that most of the development of IPD in preterm gestations is occurring in the late preterm period.
Figure 1:
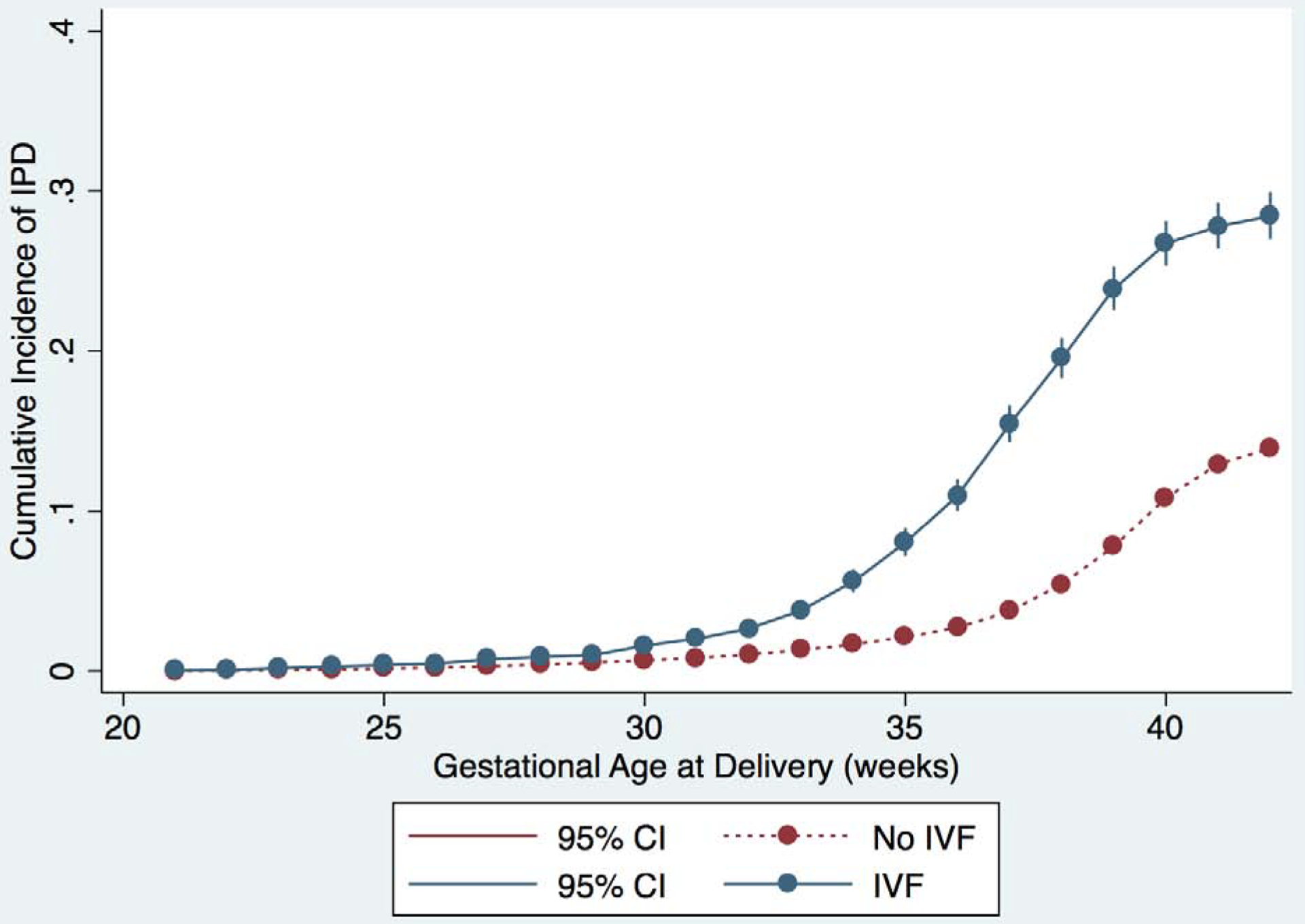
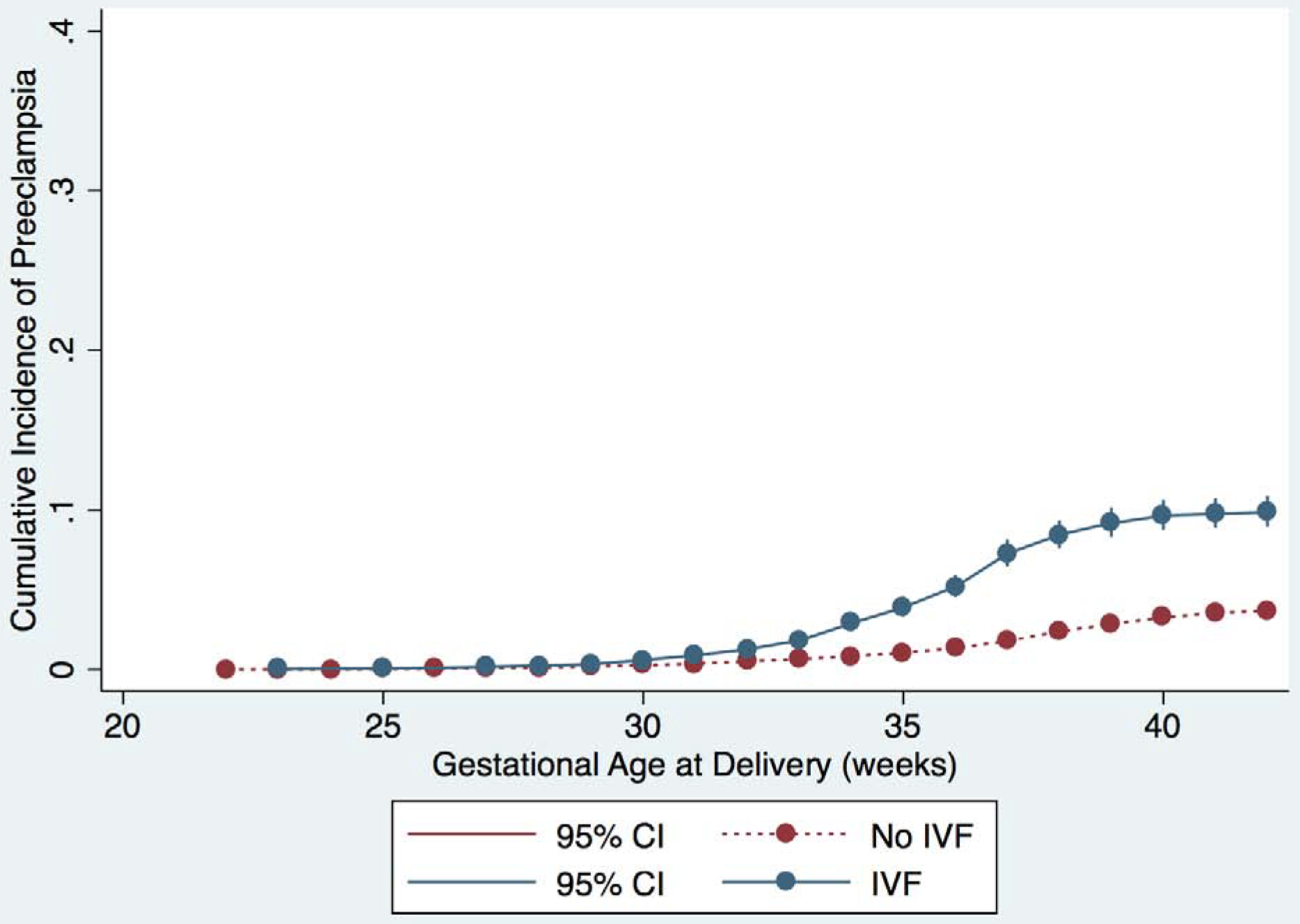
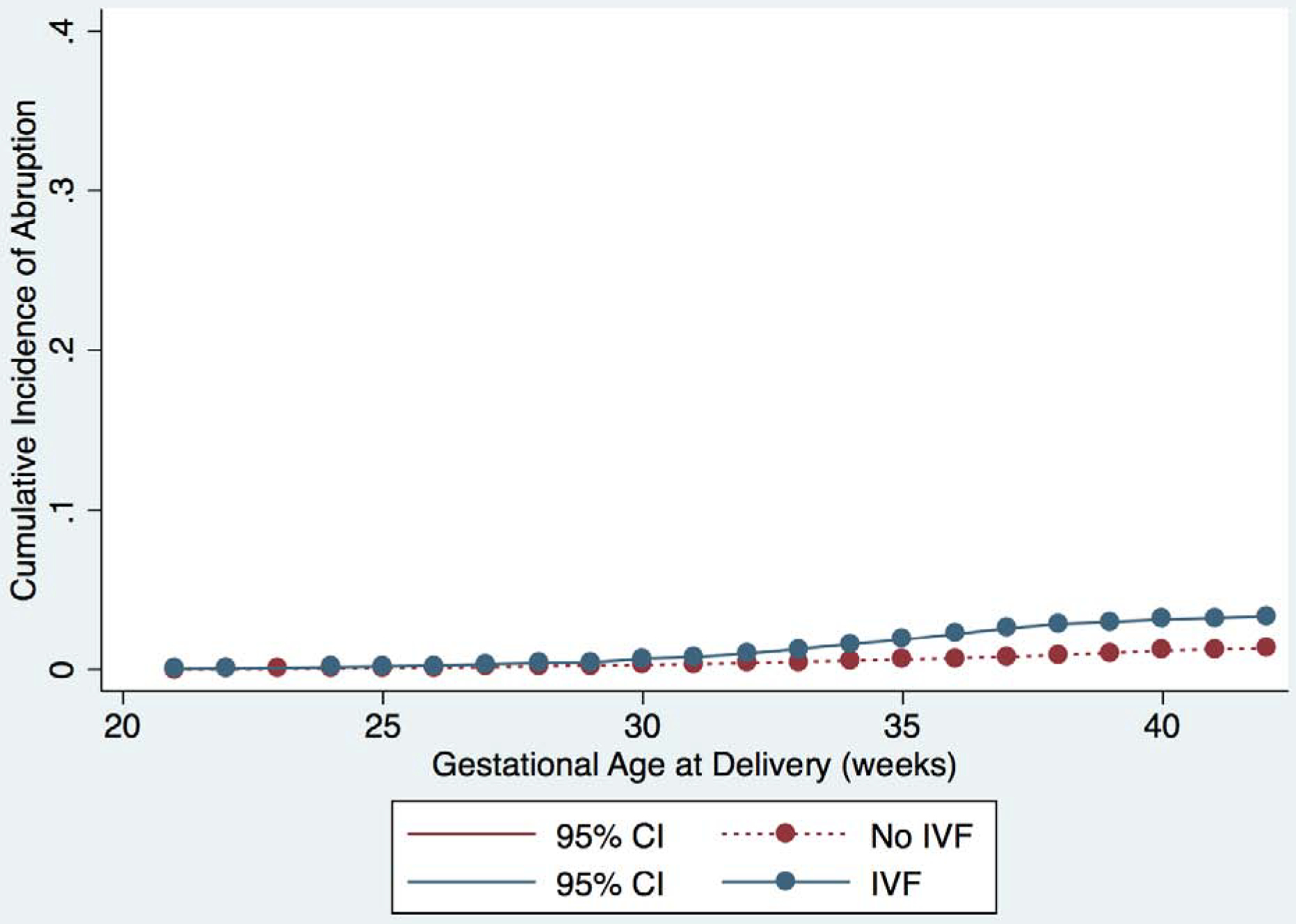
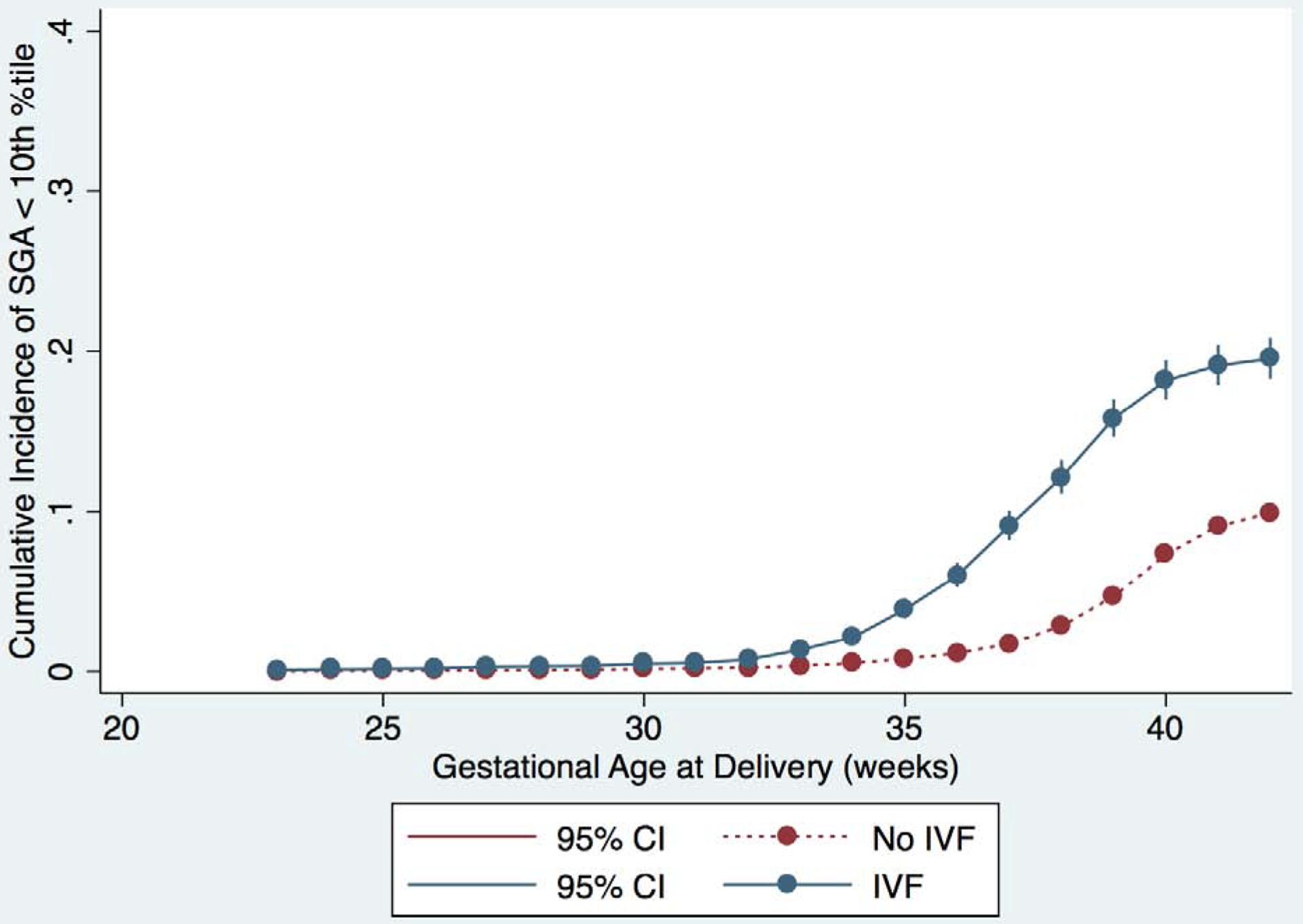
Cumulative incidence of outcomes for IVF (blue) and non-IVF (red) pregnancies. Curves represent incidence of the primary and secondary outcomes at each gestational age, and outcomes are represented separately in each graph as follows: A) IPD or IUFD from placental insufficiency, B) preeclampsia, C) abruption, and D) SGA <10th percentile. 95% confidence intervals are shown in brackets on each curve.
In the sensitivity analysis restricting to singleton gestations, the risk of preterm IPD remained significantly elevated among IVF pregnancies, although the effect size was attenuated (age-adjusted RR 1.9, 95% CI: 1.6–2.3) from what was seen for the full cohort (Table 3). Likewise, among term deliveries, the age- and parity-adjusted risk of term IPD was higher in IVF than non-IVF pregnancies (RR 1.2, 95% CI: 1.1–1.4). A similar pattern was seen for all components of IPD and when the definition of IPD was restricted to SGA <3rd percentile (Table 3).
When analyzing the donor and autologous IVF pregnancies separately, both donor and autologous IVF groups had significantly elevated risks of preterm IPD compared to non-IVF pregnancies, with a stronger effect observed among donor IVF pregnancies for all outcomes except SGA <3rd percentile at term (Supplementary table 1). The risk of preterm IPD was consistently higher than the risk ratios for term outcomes (Supplementary table 1). We observed similar patterns for the components of IPD and when IPD was restricted to SGA <3rd percentile. We were unable to adjust all of the models for covariates due to small sample sizes. We plotted cumulative incidence of IPD, as well as the individual components of IPD, for each week of gestational age stratified by donor IVF, autologous IVF and non-IVF pregnancies (Supplementary Figure 2). The cumulative incidence of IPD for both autologous and donor IVF pregnancies was higher than non-IVF pregnancies at all gestational ages >30 weeks, similar to the overall curves comparing IVF and non-IVF pregnancies. Donor IVF pregnancies demonstrated the highest cumulative incidence of IPD throughout gestation. Similar results were seen for each of the components of IPD.
Discussion
We found that pregnancies conceived with IVF, compared with those conceived without IVF, were at higher risk of both preterm and term IPD, even when restricting to singleton gestations. The association was stronger between IVF and preterm IPD than it was between IVF and term IPD.
Relative to IPD that occurs at term, IPD that occurs before 37 weeks of gestation more frequently has multiple simultaneous clinical sequelae, including preeclampsia, abruption and SGA (11), and also tends to be more homogenous (9). These properties allow for evaluation of a potential shared mechanism. Given the higher risk of preterm IPD among IVF pregnancies, IPD that occurs in IVF pregnancies likely is more related to placental insufficiency than disparate mechanisms.
Grouping preeclampsia, IUGR, and abruption as IPD facilitates evaluation of potential risk factors (10), such as IVF (3). Previous work has not consistently demonstrated an association between IVF and preeclampsia, SGA, or abruption (14). Watanabe et al. found no difference in the incidence of early-onset versus late-onset preeclampsia between IVF and non-IVF pregnancies (15), although the study was small and early onset preeclampsia was <32 weeks of gestation. Our study, which highlights the increased risk of not only preeclampsia, but also abruption, SGA, and IPD as a whole, particularly in the preterm period, provides support for placental insufficiency as a mechanism. It remains unclear if factors unique to the IVF procedure mediate this effect, or if the differences are due to unmeasured differences between those persons who require IVF for conception and those who do not (3).
Multiple gestation is one potential effect modifier of the relationship between IVF and preterm IPD (16). In our study and in others (17), it is notable that the incidence of preterm delivery is higher among IVF pregnancies, although this can largely be attributed to multiple gestations (18). Nevertheless, a subset may be due to indicated preterm birth in the setting of IPD (19), particularly as the increased rate of preterm birth has been observed in singletons as well (4). Oberg et al performed a study to disentangle the role of multiple gestation in the relationship between IVF and pregnancy outcomes; while they found that multiple gestation could explain much of the increased risk of preeclampsia in this population, it did not explain an increased risk of abruption (18). Neither IUFD nor small for gestational age were evaluated as outcomes in that study. When we restricted to singleton pregnancies, the higher risk of IPD in IVF pregnancies, though attenuated, persisted in both preterm and term deliveries, suggesting a direct effect of IVF on IPD. Thus, while multiple gestations likely play a role, it does not fully explain the stronger association of IVF with preterm IPD identified in this study.
Use of donor oocytes also may explain some of the increased risk of IPD among IVF pregnancies. Increased risks of preterm delivery (20) and preeclampsia (21) have been observed in donor oocyte cycles, and our other work in this cohort demonstrated an increased risk of IPD among pregnancies from donor oocyte cycles (5). Although our sample size of donor oocyte cycles was small, we consistently found that, compared to pregnancies conceived without IVF, both donor and autologous IVF pregnancies had a higher risk of IPD and its components. This was particularly true in the preterm period, indicating that the relationship between IVF and IPD is not driven solely by donor oocyte use.
Strengths of this study include assessment of IPD as a group of disorders, which allowed us to investigate a possible shared biologic mechanism. Moreover, while this study used billing codes to identify outcomes, these outcomes were verified through review of the medical record. In addition, we had a large sample size, which allowed us to investigate the association of IVF with not only IPD as a whole, but also the components of IPD.
Our study has several limitations. First, we did not have baseline data on some maternal comorbidities, such as chronic hypertension and obesity, which may be confounders (22,23). Second, other than whether they were conceived with donor or autologous oocytes, we did not have cycle information for all of the pregnancies conceived with IVF; thus, we cannot account for the contribution of cycle parameters, such as programmed versus natural cycle or use of frozen-thawed embryo transfers, to the development of IPD. Third, we defined preterm IPD by timing of delivery rather than diagnosis; this is clinically relevant given that more severely affected pregnancies necessitate delivery. Fourth, we used SGA as a proxy for IUGR, though it may reflect constitutional small size; thus, we also used SGA <3rd percentile as an outcome. A fifth limitation is our inability to include pregnancies that were lost prior to 20 weeks of gestation. Preeclampsia cannot be diagnosed prior to 20 weeks and we do not have information about pregnancy losses before this time. This necessary restriction creates the possibility for selection bias. In order for this potential selection bias to explain away the results from this study, the risk of IPD would need to be greater among pregnancies lost in the non-IVF group than in the IVF group, which is not likely given higher risks of loss overall among IVF pregnancies. It is more likely that the risk of IPD would be greater among pregnancies lost in the IVF group, biasing our estimate towards the null. For the outcome of term IPD, our restriction to pregnancies that delivered after 37 weeks of gestation also may impose a selection bias; however, pregnancies that deliver prior to 37 weeks of gestation are not eligible for term IPD. Again, this type of selection bias, also referred to as collider bias, likely would bias our results towards the null given that the IVF pregnancies are more likely to result in preterm birth and thus would be more likely to be excluded from the group restricted to term deliveries. The restrictions described and their potential for selections bias are recognized as potential problems in perinatal research(24). Finally, our study included deliveries at one center in Massachusetts, where state law mandates insurance coverage of infertility services. Though this could limit generalizability, persons who conceived via IVF in our study were more likely to be Caucasian, have private insurance and have a higher level of education than those who conceived without IVF, which is what one would expect in a state without mandated insurance. In at least one other study, insurance coverage did not alter the demographic characteristics of those who sought IVF treatment (25).
Conclusions
Our findings support the hypothesis that IVF is associated with placental insufficiency, given that the association of IVF with preterm IPD was consistently stronger than the association between IVF and term IPD. However, this mechanism of placental insufficiency needs to be examined further in other studies, ideally with the use of biomarkers for poor placentation. Our results may help clinicians provide anticipatory guidance for parturients with IVF pregnancies, although reassuringly most of the preterm occurrence of IPD was late preterm. Future research may further elucidate the contribution of multiple gestations, components of the IVF procedure and endometrial preparation, and medical comorbidities, thereby improving our understanding of the pathophysiology of IPD.
Supplementary Material
Supplementary Figure 1: Cumulative incidence of outcomes for IVF (blue) and non-IVF (red) pregnancies. Curves represent incidence of the primary and secondary outcomes at each gestational age, and outcomes are represented separately in each graph as follows: A) IPD or IUFD from placental insufficiency, restricting to just those outcomes including SGA <3rd percentile and B) SGA <3rd percentile. 95% confidence intervals are shown in brackets on each curve.
Supplemental Figure 2: Cumulative incidence of outcomes for donor IVF (blue), autologous IVF (brown) and non-IVF (red) pregnancies. Curves represent incidence of the primary and secondary outcomes at each gestational age, and outcomes are represented separately in each graph as follows: A) IPD or IUFD from placental insufficiency, B) preeclampsia, C) abruption, and D) SGA <10th percentile. 95% confidence intervals are shown in brackets on each curve.
Acknowledgements
Funding: AM received funding from NIH T32 HD052458 - Boston University Reproductive, Perinatal and Pediatric Epidemiology Training Program. This work was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol 2006;195(6):1557–63. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol 2014;38(3):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroener L, Wang ET, Pisarska MD. Predisposing Factors to Abnormal First Trimester Placentation and the Impact on Fetal Outcomes. Semin Reprod Med 2016;34(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Pregnancy, birth, and infant outcomes by maternal fertility status: the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol 2017;217(3):327.e1–327.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modest AM, Johnson KM, Karumanchi SA, Resetkova N, Young BC, Fox MP, et al. Risk of ischemic placental disease is increased following in vitro fertilization with oocyte donation: a retrospective cohort study. J Assist Reprod Genet 2019;36(9):1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjaerven R, Gunnell D, et al. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet 2008;372(9640):737–43. [DOI] [PubMed] [Google Scholar]
- 7.Farhi J, Ben-Haroush A, Haroush AB, Andrawus N, Pinkas H, Sapir O, et al. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online 2010;21(3):331–7. [DOI] [PubMed] [Google Scholar]
- 8.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol 2011;118(4):863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananth CV, Vintzileos AM. Ischemic placental disease: epidemiology and risk factors. Eur J Obstet Gynecol Reprod Biol 2011;159(1):77–82. [DOI] [PubMed] [Google Scholar]
- 10.Parker SE, Werler MM. Epidemiology of ischemic placental disease: a focus on preterm gestations. Semin Perinatol 2014;38(3):133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananth CV, Smulian JC, Vintzileos AM. Ischemic placental disease: maternal versus fetal clinical presentations by gestational age. J Matern Fetal Neonatal Med 2010;23(8):887–93. [DOI] [PubMed] [Google Scholar]
- 12.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol 2019;133(1):e1–25. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L-M, Walker MC, Cao H-L, Yang Q, Duan T, Kingdom JCP. Assisted Reproductive Technology and Placenta-Mediated Adverse Pregnancy Outcomes: Obstetrics & Gynecology 2009;114(4):818–24. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe N, Fujiwara T, Suzuki T, Jwa SC, Taniguchi K, Yamanobe Y, et al. Is in vitro fertilization associated with preeclampsia? A propensity score matched study. BMC Pregnancy and Childbirth [Internet] 2014. [cited 2018 Apr 2];14(1). Available from: http://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-14-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin J-B, Sheng X-Q, Wang H, Chen G-C, Yang J, Yu H, et al. Worldwide prevalence of adverse pregnancy outcomes associated with in vitro fertilization/intracytoplasmic sperm injection among multiple births: a systematic review and meta-analysis based on cohort studies. Arch Gynecol Obstet 2017;295(3):577–97. [DOI] [PubMed] [Google Scholar]
- 17.Barda G, Gluck O, Mizrachi Y, Bar J. A comparison of maternal and perinatal outcome between in vitro fertilization and spontaneous dichorionic-diamniotic twin pregnancies. J Matern Fetal Neonatal Med 2017;30(24):2974–7. [DOI] [PubMed] [Google Scholar]
- 18.Oberg AS, VanderWeele TJ, Almqvist C, Hernandez-Diaz S. Pregnancy complications following fertility treatment-disentangling the role of multiple gestation. Int J Epidemiol 2018;47(4):1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okby R, Harlev A, Sacks KN, Sergienko R, Sheiner E. Preeclampsia acts differently in in vitro fertilization versus spontaneous twins. Arch Gynecol Obstet 2018;297(3):653–8. [DOI] [PubMed] [Google Scholar]
- 20.Dude AM, Yeh JS, Muasher SJ. Donor oocytes are associated with preterm birth when compared to fresh autologous in vitro fertilization cycles in singleton pregnancies. Fertil Steril 2016;106(3):660–5. [DOI] [PubMed] [Google Scholar]
- 21.Levron Y, Dviri M, Segol I, Yerushalmi GM, Hourvitz A, Orvieto R, et al. The ‘immunologic theory’ of preeclampsia revisited: a lesson from donor oocyte gestations. American Journal of Obstetrics and Gynecology 2014;211(4):383.e1–383.e5. [DOI] [PubMed] [Google Scholar]
- 22.Reismullerova L, Holoman K, Polackova-Borosova M, Luha J. Polycystic ovary syndrome - a risk factor of pre-eclampsia after in vitro fertilisation. Bratisl Lek Listy 2015;116(5):311–5. [DOI] [PubMed] [Google Scholar]
- 23.Dayan N, Pilote L, Opatrny L, Basso O, Messerlian C, El-Messidi A, et al. Combined impact of high body mass index and in vitro fertilization on preeclampsia risk: a hospital-based cohort study. Obesity (Silver Spring) 2015;23(1):200–6. [DOI] [PubMed] [Google Scholar]
- 24.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol 2015;44(1):345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitler M, Schmidt L. Health disparities and infertility: impacts of state-level insurance mandates. Fertil Steril 2006;85(4):858–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Cumulative incidence of outcomes for IVF (blue) and non-IVF (red) pregnancies. Curves represent incidence of the primary and secondary outcomes at each gestational age, and outcomes are represented separately in each graph as follows: A) IPD or IUFD from placental insufficiency, restricting to just those outcomes including SGA <3rd percentile and B) SGA <3rd percentile. 95% confidence intervals are shown in brackets on each curve.
Supplemental Figure 2: Cumulative incidence of outcomes for donor IVF (blue), autologous IVF (brown) and non-IVF (red) pregnancies. Curves represent incidence of the primary and secondary outcomes at each gestational age, and outcomes are represented separately in each graph as follows: A) IPD or IUFD from placental insufficiency, B) preeclampsia, C) abruption, and D) SGA <10th percentile. 95% confidence intervals are shown in brackets on each curve.


