Abstract
Purpose
Prior studies of menopausal hormone therapy (MHT) and ovarian cancer survival have been limited by lack of hormone regimen detail and insufficient sample sizes. To address these limitations, a comprehensive analysis of 6,419 post-menopausal women with pathologically confirmed ovarian carcinoma was conducted to examine the association between MHT use prior to diagnosis and survival.
Methods
Data from 15 studies in the Ovarian Cancer Association Consortium were included. MHT use was examined by type (estrogen-only (ET) or estrogen+progestin (EPT)), duration, and recency of use relative to diagnosis. Cox proportional hazards models were used to estimate the association between hormone therapy use and survival. Logistic regression and mediation analysis was used to explore the relationship between MHT use and residual disease following debulking surgery.
Results
Use of ET or EPT for at least five years prior to diagnosis was associated with better ovarian cancer survival (hazard ratio, 0.80; 95% CI, 0.74 to 0.87). Among women with advanced stage, high-grade serous carcinoma, those who used MHT were less likely to have any macroscopic residual disease at the time of primary debulking surgery (p for trend <0.01 for duration of MHT use). Residual disease mediated some (17%) of the relationship between MHT and survival.
Conclusions
Pre-diagnosis MHT use for 5+ years was a favorable prognostic factor for women with ovarian cancer. This large study is consistent with prior smaller studies, and further work is needed to understand the underlying mechanism.
INTRODUCTION
Invasive epithelial ovarian cancers including ovarian, fallopian tube and primary peritoneal cancer (hereafter referred to as ovarian cancer) collectively account for more deaths than any other cancer of the female reproductive system in the United States, with a five-year survival rate of less than 50%1. There is clear evidence that menopausal estrogen-alone hormone therapy (ET) is associated with an increased risk of developing ovarian cancer2–4, but the relationship between menopausal estrogen plus progestin therapy (EPT) and risk of ovarian cancer is less clear3. Further, the relationship between menopausal hormone therapy (MHT) use and survival may not be the same as the relationship with risk.
Pre-diagnosis MHT use and ovarian cancer survival has been examined in nine population-based studies5–13. Most observed a modestly inverse association, with hazard ratios ranging from 0.2311 to 1.112 (Table S1), but protection was statistically significant in only one study (MHT use >5 years: HR, 0.79; 95% CI, 0.55 to 0.90)5. These studies were subject to one or more of the following important limitations: they (1) lacked information about duration of use; (2) did not distinguish between types of MHT use before diagnosis (i.e., ET and/or EPT); (3) had follow-up times of only a few years; (4) had an insufficient sample size to stratify by ovarian cancer histotype; and (5) lacked information about residual disease after debulking surgery. Many women use MHT for only a short period of time, thus missing duration information is an important weakness that may have masked effects in prior studies14. Rigorously evaluating the association between pre-diagnosis MHT use and ovarian cancer survival by hormone type, duration, survival time, residual disease and cancer histotype is essential to advance our understanding of disease prognosis.
In the present analysis from the Ovarian Cancer Association Consortium (OCAC), we followed 6,419 women with ovarian cancer for up to 26 years and investigated the association between pre-diagnosis MHT use and survival. We investigated duration, type and timing of MHT use in each of the main histological subtypes. A particularly important prognostic factor in ovarian cancer survival is residual disease after initial debulking surgery. Therefore, we also considered the potential relationship of MHT use with residual disease after surgery.
METHODS
Institutional Review Board or comparable ethics approval was received by each study and informed consent was provided by all women.
Study populations and exclusion criteria
OCAC is an international, multidisciplinary collaboration of ovarian cancer research teams (http://ocac.ccge.medschl.cam.ac.uk/). Post-menopausal women (as defined in each study) with pathologically confirmed ovarian carcinoma and survival time available (n=10,120) were considered for our analyses. We were interested a priori in the potential of a duration effect of MHT use and thus three studies (n=2121) were excluded. Therefore, this analysis used pooled ovarian cancer survival data from population-based (n=14) and clinic-based (n=1) OCAC studies (Table S2) conducted in the United States (n=9), Europe (n=4), and Australia (n=2). Women from these studies missing MHT duration were excluded. Only those with invasive tumors, high-grade serous, low-grade serous, mucinous, endometrioid, or clear cell carcinomas, were eligible (i.e. mixed cell, undifferentiated, and non-epithelial cancers were excluded; n=1,260). Women missing data for stage at diagnosis (n=282), race/ethnicity (n=25), or time from diagnosis to interview/study enrolment (n=13) were also excluded. There was no upper or lower age limit exclusion beyond the impact of excluding women who were pre-menopausal at diagnosis. Our final analytic sample was 6,419 ovarian cancer patients. (Figure 1). Survival times and proportion of deaths were comparable between women excluded and those included.
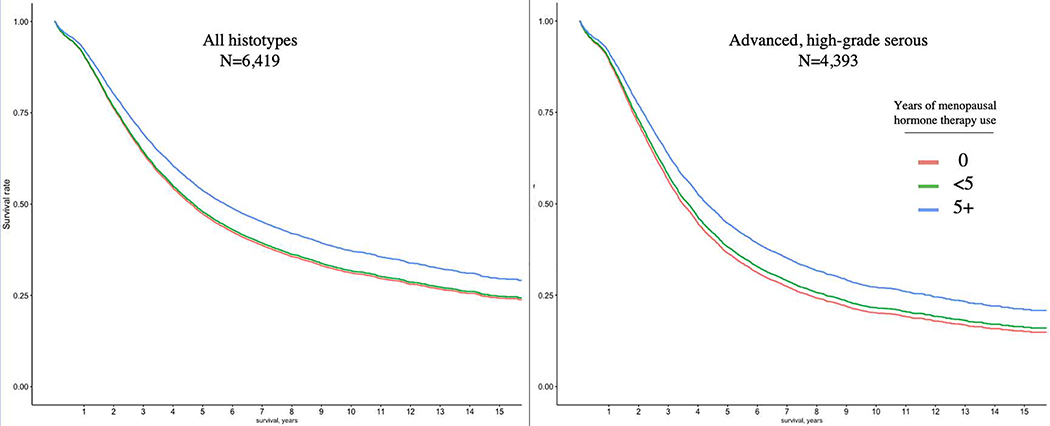
Figure 1: Overall survival stratified by years of menopausal hormone therapy use.
Adjusted survival curves among all women with ovarian cancer (n=6,419) and among women with advanced stage, high-grade serous cancer (n=4,393). The adjusted survival curves are generated from the hazard ratios estimated from a cox proportional hazards model of menopausal hormone therapy use and are adjusted for age at diagnosis, race/ethnicity, histotype (left panel only), stage at diagnosis, and OCAC site.
Exposure and covariate assessment
Participants provided information on their history of MHT use prior to diagnosis via phone or in-person interviews (n=10 study sites) or self-completed questionnaires (n=5 study sites) (Table S2) at the time of study enrollment. MHT use was categorized as exclusive use of ET, exclusive use of EPT, use of both therapies, or use of unknown type. First, exclusive ET use was examined based on (1) total duration of ET use (never (reference category), >0 to <5, 5 to <10, 10+ years) and (2) recency of ET use (within the year prior to diagnosis, 1 to <5, 5+ years prior to diagnosis). There was no additional duration effect observed after 5 years and so the categories 5 to <10 and 10+ years were combined into one. Exclusive EPT use was examined in the same manner. The reference group for both the ET and EPT analyses was never use of any type of MHT. Next, total duration of any type of MHT use prior to diagnosis was examined (ET, EPT, both, or unknown type) with the same approach. BMI (kg/m2) categories were assigned according to World Health Organization15 definitions (underweight, BMI<18.5; normal weight, 18.5≤BMI<25.0; overweight, 25≤BMI<30; obese, BMI≥30), using the values reported for adult BMI one to five years prior to diagnosis. Duration of combined oral contraceptive use was coded as never, <1, 1 to <5, 5 to <10, or 10+ years. Parity was coded as 0, 1, or 2+ pregnancies. Education level was coded as less than high school, high school graduate, some college, college graduate, or graduate school. Stage was recorded as local (with no lymph node involvement), regional (direct extension and/or local lymph node involvement), and advanced (distant sites and/or distant lymph nodes involved)16. For all patients, the standard of care is assumed to have been a platinum-based regimen.
Outcome assessment
Overall survival was recorded as either length of time (in days) from diagnosis to death or to date of last follow-up (for censored patients). Follow-up is largely done via linkage with national death databases All survival models incorporated left truncation time, accounting for the difference between date of diagnosis and date of patient interview, though there was little variability in delay to patient interview and so accounting for left truncation did not affect results. Women were typically enrolled within the first sixth months following diagnosis (median 152 days). For a subset of women there was information on duration of progression-free survival (n=2,239) and presence/absence and size of residual disease after debulking surgery (n=2,056) (Table S2).
Statistical analysis
Overall survival models
Cox proportional hazards models with left truncation and right censoring were used to estimate the association (hazard ratio; HR, and associated Wald-type confidence intervals) of each hormone therapy exposure on ovarian cancer survival. The exposures were modeled as categories of duration of use and recency of use, as detailed above. Exclusive use of ET or exclusive use of EPT were first examined separately to determine their association with survival. Because the hazards for the two types of hormone therapies were not statistically different and showed a similar magnitude, we combined types as an “any HT use” variable including unknown types of MHT, as these would have been either ET or EPT.
Important a priori variables included in all models were age at diagnosis (continuous), race/ethnicity, surgical stage at diagnosis, and OCAC study site. Sensitivity analyses adjusting for age in five- and ten-year categories did not materially alter the HR estimates for MHT. Education level adjustment in sensitivity analyses also did not influence HR estimates. The possible confounding effects of additional exposures prior to diagnosis were examined, but none affected the association between MHT duration and survival (Table S5).
Separate models for each histotype were also fitted to estimate HRs for MHT duration. The adjusted survival curves presented (overall, and high-grade serous) allow for visualization of survival curves based on the Cox proportional hazard results.
Discrete windows of clinical interest and progression-free survival
We tested discrete windows of clinical interest following diagnosis. Although the proportional hazards assumptions were not violated for MHT use prior to diagnosis in the Cox proportional hazards models, an additional model was fit allowing the data to be split into time intervals after diagnosis. This allowed us to assess subtle variation in HR estimates at all time points after diagnosis. To assess the specificity of the protective effect of MHT, Cox proportional hazards model was fit for time to progression, treating progression of disease as the event of interest. Although ovarian cancer-specific mortality was not assessed, nearly all deaths within the first five years following diagnosis are related to ovarian cancer thus our time interval analysis provides insight into this question.
Residual disease in women with advanced stage, high-grade serous cancer
Among women with advanced stage (stage III or IV), high-grade serous carcinoma (HGSC, n=903), we examined possible mechanisms underlying the MHT-survival association, namely the association of MHT use with residual disease. We used logistic regression, investigating MHT use in those with and without macroscopic residual disease following primary debulking surgery. Mediation analysis was used to examine whether the relationship between MHT use and survival was mediated by residual disease. In this analysis, the first step (mediator) model was residual disease regressed on MHT use and the covariates age, stage, histotype, education level, and race. The second step (outcome) model was modeled as survival regression on residual disease, MHT use, and the same set of covariates. Finally, mediation was assessed using 2,000 simulations to estimate the average causal mediated effect, the average causal direct effect, the total effect, and the proportion mediated, using the generalizable approach to causal mediation outlined by Imai et al.17. All statistical analysis was performed in R18.
RESULTS
The analytic sample included 6,419 post-menopausal women from 15 sites in the OCAC (Figure S1; Table S2). A majority of the women had HGSC (68.4%) and most had advanced stage disease at diagnosis (67.7%; Table 1). Exclusive EPT use (18.5%) was more common than exclusive ET use (14.2%). Most women (58.9%) did not use either type and 212 (3.3%) used both ET and EPT (Table 1).
Table 1:
Demographic and clinical characteristics of women with ovarian cancer from the Ovarian Cancer Association Consortium (OCAC) included in the survival analysis.
| Pre-diagnosis MHT use duration | ||||
|---|---|---|---|---|
| Overall1 | Never | <5 years | 5+ years | |
| N | 6419 | 3784 | 1183 | 1452 |
| Hormone therapy use (%) | ||||
| None | 3784 (58.9) | 3784 (100.0) | 0 (0.0) | 0 (0.0) |
| ET only | 909 (14.2) | 0 (0.0) | 379 (32.0) | 530 (36.5) |
| EPT only | 1188 (18.5) | 0 (0.0) | 561 (47.4) | 627 (43.2) |
| ET and EPT | 212 (3.3) | 0 (0.0) | 62 (5.2) | 150 (10.3) |
| Unknown +/− ET/EPT | 326 (5.1) | 0 (0.0) | 181 (15.3) | 145 (10.0) |
| Age at dx. (mean (SD)) | 62.67 (8.71) | 62.36 (9.33) | 60.78 (8.16) | 65.00 (6.75) |
| Education (%) | ||||
| Less than high school | 1135 (20.7) | 760 (23.7) | 177 (17.4) | 198 (15.9) |
| High school graduate | 1567 (28.6) | 948 (29.6) | 272 (26.7) | 347 (27.8) |
| Some college | 1325 (24.2) | 745 (23.2) | 265 (26.0) | 315 (25.3) |
| College graduate | 799 (14.6) | 400 (12.5) | 174 (17.1) | 225 (18.1) |
| Graduate school | 646 (11.8) | 353 (11.0) | 132 (12.9) | 161 (12.9) |
| Race / ethnicity (%) | ||||
| Non-Hispanic white | 5679 (88.5) | 3308 (87.4) | 1042 (88.1) | 1329 (91.5) |
| Hispanic white | 198 (3.1) | 126 (3.3) | 45 (3.8) | 27 (1.9) |
| Black | 101 (1.6) | 72 (1.9) | 15 (1.3) | 14 (1.0) |
| Asian | 249 (3.9) | 146 (3.9) | 51 (4.3) | 52 (3.6) |
| Other | 192 (3.0) | 132 (3.5) | 30 (2.5) | 30 (2.1) |
| Histotype (%) | ||||
| Low-grade serous | 245 (3.8) | 134 (3.5) | 47 (4.0) | 64 (4.4) |
| High-grade serous | 4393 (68.4) | 2504 (66.2) | 820 (69.3) | 1069 (73.6) |
| Mucinous | 373 (5.8) | 255 (6.7) | 65 (5.5) | 53 (3.7) |
| Endometrioid | 925 (14.4) | 552 (14.6) | 168 (14.2) | 205 (14.1) |
| Clear cell | 483 (7.5) | 339 (9.0) | 83 (7.0) | 61 (4.2) |
| Stage (%) | ||||
| Local (FIGO I) | 947 (14.8) | 616 (16.3) | 173 (14.6) | 158 (10.9) |
| Regional (FIGO II) | 1126 (17.5) | 684 (18.1) | 211 (17.8) | 231 (15.9) |
| Advanced (FIGO III/IV) | 4346 (67.7) | 2484 (65.6) | 799 (67.5) | 1063 (73.2) |
| BMI category (%) | ||||
| Underweight | 117 (2.0) | 71 (2.1) | 18 (1.6) | 28 (2.0) |
| Normal weight | 2684 (45.7) | 1424 (42.0) | 515 (45.9) | 745 (54.5) |
| Overweight | 1754 (29.9) | 1026 (30.3) | 339 (30.2) | 389 (28.5) |
| Obese | 1320 (22.5) | 866 (25.6) | 249 (22.2) | 205 (15.0) |
| Family2 cancer history (%) | ||||
| Breast cancer | 1098 (17.6) | 690 (18.9) | 195 (16.8) | 213 (15.0) |
| Ovarian cancer | 329 (5.3) | 203 (5.6) | 61 (5.3) | 65 (4.6) |
| Combined oral contraceptive use (%) | ||||
| Never | 3127 (49.2) | 2030 (54.2) | 451 (38.6) | 646 (44.9) |
| <1 year | 590 (9.3) | 313 (8.4) | 142 (12.1) | 135 (9.4) |
| 1 to <5 years | 1209 (19.0) | 659 (17.6) | 265 (22.7) | 285 (19.8) |
| 5 to <10 years | 773 (12.2) | 390 (10.4) | 176 (15.1) | 207 (14.4) |
| 10+ years | 656 (10.3) | 356 (9.5) | 135 (11.5) | 165 (11.5) |
| Parity (%) | ||||
| 0 births | 1223 (19.1) | 738 (19.6) | 232 (19.6) | 253 (17.4) |
| 1 birth | 858 (13.4) | 525 (13.9) | 157 (13.3) | 176 (12.1) |
| 2+ births | 4324 (67.5) | 2508 (66.5) | 794 (67.1) | 1022 (70.4) |
| Smoking (%) | ||||
| Never | 2910 (52.9) | 1803 (55.5) | 495 (48.8) | 612 (49.4) |
| Current | 700 (12.7) | 445 (13.7) | 126 (12.4) | 129 (10.4) |
| Former | 1891 (34.4) | 998 (30.7) | 394 (38.8) | 499 (40.2) |
The total N for certain variables reported does not total to 6,419 because of missing data. These included variables that were not confounders and thus not needed for covariate adjustment in final models, such as family history of cancer, education, and smoking.
First-degree family members, i.e. sister or mother.
The median survival time was 5.4 years after diagnosis. ET and EPT use for at least five years were both associated with longer survival (Table 2). For exclusive ET users, lower mortality was observed for use of 5+ years (HR, 0.85; 95% CI, 0.75 to 0.96). For exclusive EPT users, the HR for use for 5+ years was similar (HR, 0.79; 95% CI, 0.70 to 0.89). Because the magnitudes of effect for ET and EPT were similar, all MHT types were combined for subsequent analyses. Significantly better survival was observed for those who had used any type of MHT for at least 5 years (HR, 0.80; 95% CI, 0.74 to 0.87) (Table 2). This corresponds to a median survival of 5.75 years among women who had used MHT for 5+ years and 4.6 years for those who has not used any.
Table 2:
Hazards ratios for menopausal hormone therapy (MHT) use before diagnosis of ovarian cancer among women with invasive epithelial ovarian cancer in the Ovarian Cancer Association Consortium (OCAC).
| Estrogen alone (ET) | Estrogen-progestin combined therapy (EPT) | Any menopausal hormone therapy | ||||
|---|---|---|---|---|---|---|
| MHT use | Na | HR (95% CI)b | N | HR (95% CI) | N | HR (95% CI) |
| None (ref) | 3,784 | 1.0 | 3,784 | 1.0 | 3,784 | 1.0 |
| <5 years | 379 | 0.99 (0.86, 1.15) | 561 | 1.01 (0.89, 1.14) | 1,183 | 0.97 (0.88, 1.06) |
| 5+ years | 530 | 0.85 (0.75, 0.96) | 627 | 0.79 (0.70, 0.89) | 1,452 | 0.80 (0.74, 0.87) |
The three analyses have different total N’s because the exclusive ET analysis excluded women who had ever used EPT, and the exclusive EPT analysis excluded women who had ever used ET. Users of unknown type were also excluded from this analysis.
Hazard ratios (HRs) are adjusted for age at diagnosis and race/ethnicity, and stratified by histotype, stage at diagnosis, and OCAC site.
An adjusted survival curve illustrates the apparent protective benefit of MHT use was restricted to women with 5+ years use compared to those who did not use MHT and that no benefit was observed for <5 years of use (Figure 1). Recency of MHT use did not affect the hazard ratio estimates. The association observed for all histotypes combined was also similar for individual histotypes, with the exception of endometrioid carcinomas, but was only statistically significant for HGSC (HR, 0.78; 95% CI, 0.71 to 0.86) (Table S3a). Progression-free survival (time from diagnosis to first recurrence, documented by clinical, biochemical (e.g. serum CA125 levels) or radiological disease progression) was also better in those who had used MHT (Table S4).
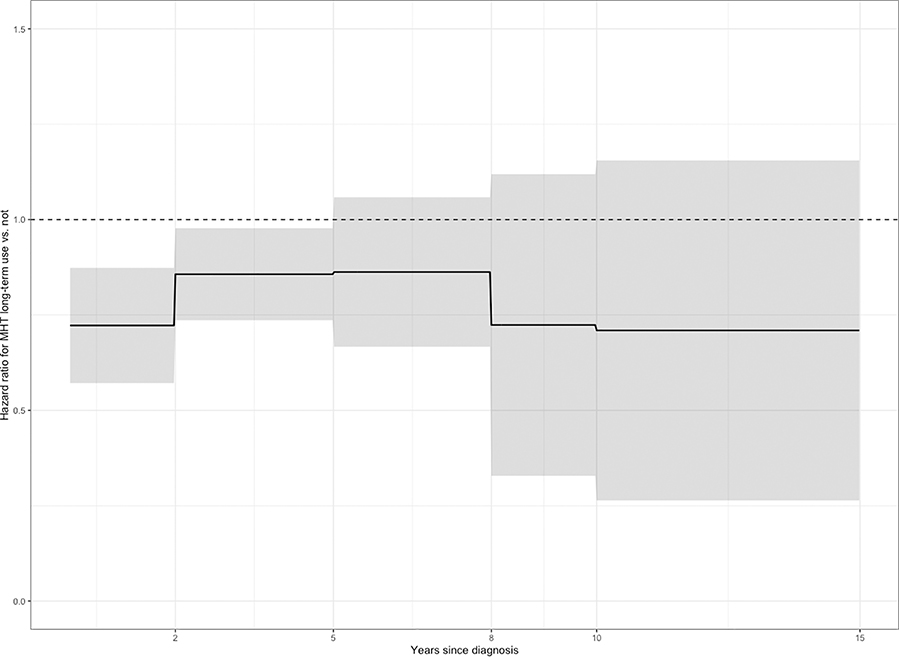
Time-varying HRs were also estimated. Although the proportional hazards assumptions were not violated for the survival model of MHT use, the additional analyses allowed for finer estimation of the protective association during particular windows of interest after diagnosis. The estimated effect was protective in all time intervals. MHT use was associated with reduced risk of death significantly in the first two years after diagnosis (HR, 0.72; 95% CI, 0.62 to 0.84) and in years 2 through 5 after diagnosis (HR, 0.86; 95% CI, 0.76 to 0.97) (Figure 2).
Figure 2: Estimated time-varying hazard ratio (HR) and 95% confidence intervals (CIs) for use of menopausal hormone therapy (5+ years) relative to no use.
In a Cox proportional hazard model allowing for interaction of the effect of menopausal hormone therapy use with time since diagnosis, the estimated effect is protective at all time points. Menopausal hormone therapy use is significantly protective in the first two years after diagnosis (HR = 0.72; 95% CI = 0.62, 0.84) and in years 2 through 5 after diagnosis (HR = 0.86; 95% CI = 0.76, 0.97).
Stratification by stage at diagnosis for HGSC showed a positive association with prognosis at advanced stages (III/IV) (Table S3b). Among women with advanced stage HGSC, MHT use was associated with improved survival both in the women with and those without residual disease (Figure S2 and Table S6). MHT use prior to diagnosis was associated with lower likelihood of residual disease at the time of debulking surgery among women with advanced stage HGSC. Of women with local (stage I, n=180) and regional (stage II, n=343) disease, only 2 women (2%) and 18 women (5.2%) respectively had residual disease after surgery, thus we cannot estimate ORs for MHT use in these strata. Among those with advanced disease (stage III/IV), MHT use was associated with significantly lower odds of having macroscopic residual disease relative to no macroscopic disease in an MHT duration-dependent manner (p for trend = 0.009), adjusted for age at diagnosis (Table 3). Adjusting for OCAC site and race/ethnicity did not alter the trend. Residual disease partially mediated the relationship between long-term (5+ years) MHT use and survival. Among women with advanced HGSC, the proportion mediated was 0.17 (p=0.04).
Table 3:
Odds ratios for macroscopic residual disease based on use of menopausal hormone therapy (MHT) use before diagnosis of ovarian cancer, among women with advanced, high-grade serous carcinoma in the Ovarian Cancer Association Consortium (OCAC).
| Residual disease | ORa (95% CI) | p for trend | ||
|---|---|---|---|---|
| MHT use | N | |||
| None (ref) | 859 | 574 (66%) | 1.0 | |
| <5 years | 239 | 146 (61%) | 0.79 (0.58, 1.06) | |
| 5+ years | 290 | 171 (59%) | 0.71 (0.54, 0.93) | 0.009 |
ORs are adjusted for age at diagnosis. Adjusting for OCAC site and race/ethnicity did not alter the trend for inverse association.
DISCUSSION
In this study, pre-diagnosis MHT use for at least five years was associated with better ovarian cancer survival, regardless of MHT type (ET or EPT) and recency of use relative to diagnosis. Other studies reported ever use of MHT to be associated with improved survival (Table S1), but this is the first study to report on the effect of duration and recency of MHT use, type of MHT use, histotype, and residual disease after debulking surgery on survival outcomes.
Women with advanced HGSC who had used MHT prior to diagnosis were less likely to have macroscopic disease following primary debulking surgery. We estimated that about 17% of the survival improvement associated with MHT use could be due to the higher proportion of MHT users with no residual disease. The mechanism of the effect of MHT on residual disease is unclear. At least one previous study has noted that MHT use was associated with optimal debulking status19. One possibility is that MHT use prior to diagnosis alters the pattern of metastatic spread, such that the disease is easier to access or less adhesive to surrounding tissues. It has been reported that tumor tissue from sub-optimally debulked patients expressed molecular signatures consistent with increased stromal activation and lymphovascular invasion20. A predictive gene expression signature, developed for likelihood of optimal debulking, suggested that there may be a subset of tumors for which the TGF-ß activated pathway stimulates epithelial to mesenchymal transition and activation of tumor associated fibroblasts21, both of which would contribute to spread of tumor and difficulty in debulking.
Prior studies have established a complex relationship between hormonal exposures, including hormone therapy22,23, and inflammation that depends on multiple factors including the formula, dose, route of delivery, and other immune stimuli. MHT use may result in an anti-inflammatory milieu that is beneficial for resection. Particularly at high concentrations, estrogen has anti-inflammatory properties24,25 in some tissues. Furthermore, evidence supports a mutually dependent relationship between inflammation and angiogenesis26. Immune cells stimulated during inflammatory reactions secrete cytokines such as IL-6, TNF-α and CXCR2 that promote neovascularization and thus potentially contribute to tumor establishment and growth. On the other hand, an anti-inflammatory environment would prevent this sequence. Mechanistic studies are needed to understand the relationship between MHT use and ease of debulking. Mechanistic studies are also needed to investigate whether it is primarily women with estrogen-receptor negative cancers who are driving the protective association with MHT use; indeed, the current literature suggests avoiding MHT in women with estrogen-sensitive histologic subtypes27. This may explain why the endometrioid subtype findings deviated from the other histotypes.
Pre-diagnosis use, as previously discussed and as demonstrated by this current study, appears to offer a survival benefit to women with ovarian cancer5,7–9,11,13,19 (Table S1). The existing literature on post-diagnosis MHT use and ovarian cancer survival includes several population-based cohort studies6,8,28–30 and two small randomized controlled trials31,32. The population-based studies were largely inconclusive, but they all suggest reduced mortality in post-diagnosis MHT users6,8,28–30,33,34.
Two randomized trials have indicated survival benefits of hormone therapy use31,32 after surgical debulking of the ovarian tumor. A clinical trial in 199932 randomized women with ovarian cancer of any histotype to either conjugated estrogen or to no supplementation after debulking surgery. The women who received estrogen therapy had non-significantly longer disease-free intervals and better overall survival. In a second study, Eeles et al.31 randomized women who had been diagnosed with ovarian cancer within the previous 9 months to receive hormone therapy or none. The study observed a statistically significant beneficial effect of hormone therapy on overall survival (HR, 0.63; 95% CI, 0.44 to 0.90), but this likely reflects some of the general benefits of MHT on survival as this is not an ovarian cancer-specific survival estimate. However, no specific hormonal regimen was used, as individual clinicians had control over type, dose and duration.
Limitations of our results include the self-reported exposure measures. However, prior studies have documented good correlation between self-report of hormone use and prescription records35. Although our analysis was restricted to women who were classified as postmenopausal at diagnosis, some may have used MHT before menopause occurred. To address this issue, we conducted a sensitivity analysis restricting the exposure to MHT use after the age of 50, as a proxy for post-menopausal use, and the results did not change. An additional limitation was the lack of information on MHT use post-diagnosis. We cannot exclude the possibility that pre-diagnosis use predicts post-diagnosis use, conferring part of the survival benefit. Likewise, there was not a large enough sample of women with chemotherapy information to conduct analyses on any differential effects of MHT based on chemotherapy treatment. However, regardless of prior exposures and medical history, the standard of care for the vast majority of women is a platinum-based regimen. Additionally, we assumed that the chemotherapy ordered for women who had been on MHT was comparable to that for women who were never on MHT; however, because treatment data were not available, we cannot rule out that women who were previously on MHT were better able to tolerate the full dose of chemotherapy. Finally, use of MHT could serve as a proxy for overall adherence to medical recommendations and treatment and access to specialist surgical practices. However, controlling for education, which was expected to correlate with these characteristics, did not affect results.
We observed that the association of MHT use for five or more years prior to diagnosis was protective against death at all points after diagnosis. Since the cause of death during the first five years is most commonly ovarian cancer-specific and not from other causes, this suggests that the association of survival with MHT use is at least in part due to cancer-specific protection. We also offer evidence that the relationship is partially mediated by the relationship between MHT use and optimal surgical cytoreduction.
The findings presented here, taken in context with the other literature on the topic (Table S1), suggest that MHT is beneficial with respect to ovarian cancer survival, particularly among women with HGSC. These findings are helpful to understand the biology of the disease, and ultimately our goal is to help women diagnosed with ovarian cancer to live both longer and with a higher quality of life. Post-menopausal symptoms, including severe vasomotor symptoms for some women, can negatively impact quality of life. It is well known that an early onset of menopause increases risks for overall mortality, particularly cardiovascular mortality, and thus ovarian cancer patients who undergo surgical menopause prior to natural menopause would likely benefit from MHT36. Whether MHT is cardioprotective in women who are postmenopausal depends on timing of initiation in relation to onset of menopause, with women initiating MHT within 4–6 years of onset of menopause showing decreased risk for CVD. Thus, there are many ovarian cancer patients who would receive cardioprotective effects from MHT37,38. There are also important benefits of MHT use in postmenopausal women in terms of reduced risk of hip fracture39 and reduced risk for colorectal cancer40. Therefore, clinician and patient confidence in using MHT offers great potential benefit to women with ovarian cancer. A large randomized clinical trial would help determine the impact of MHT on survival and quality of life for women living with ovarian cancer. Such a future trial could incorporate detailed mechanistic studies to better understand how MHT influences survival. Despite remaining questions, the current evidence should allow providers to at least discuss MHT use with ovarian cancer patients, with shared decision making regarding the benefits and limitations of therapy.
Supplementary Material
Highlights.
Using menopausal hormone therapy prior to diagnosis extends ovarian cancer survival.
Estrogen alone and estrogen+progestin are associated with better survival.
Women who used hormone therapy have less residual disease after surgery.
Acknowledgements
We are grateful to the family and friends of Kathryn Sladek Smith for their generous support of the Ovarian Cancer Association Consortium through their donations to the Ovarian Cancer Research Fund. We thank the study participants, doctors, nurses, clinical and scientific collaborators, health care providers and health information sources who have contributed to the many studies contributing to this manuscript.
Acknowledgements for individual studies: AUS: The AOCS also acknowledges the cooperation of the participating institutions in Australia, and the contribution of the study nurses, research assistants and all clinical and scientific collaborators. The complete AOCS Study Group can be found at www.aocstudy.org. We would like to thank all of the women who participated in this research program; CON: The cooperation of the 32 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged. This study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in this study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data; GER: The German Ovarian Cancer Study (GER) thank Ursula Eilber for competent technical assistance; OPL: Members of the OPAL Study Group (http://opalstudy.qimrberghofer.edu.au/); UKO: We particularly thank I. Jacobs, M.Widschwendter, E. Wozniak, A. Ryan, J. Ford and N. Balogun for their contribution to the study.
OCAC Funding
The Ovarian Cancer Association Consortium is supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07).
Funding for individual studies: AUS: The Australian Ovarian Cancer Study (AOCS) was supported by the U.S. Army Medical Research and Materiel Command (DAMD17-01-1-0729), National Health & Medical Research Council of Australia (199600, 400413 and 400281), Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania and Cancer Foundation of Western Australia (Multi-State Applications 191, 211 and 182). AOCS gratefully acknowledges additional support from Ovarian Cancer Australia and the Peter MacCallum Foundation; CON: National Institutes of Health (R01-CA063678, R01-CA074850; R01-CA080742); DOV: National Institutes of Health R01-CA112523 and R01-CA87538; GER: German Federal Ministry of Education and Research, Programme of Clinical Biomedical Research (01 GB 9401) and the German Cancer Research Center (DKFZ); HAW: U.S. National Institutes of Health (R01-CA58598, N01-CN-55424 and N01-PC-67001); HOP: University of Pittsburgh School of Medicine Dean’s Faculty Advancement Award (F. Modugno), Department of Defense (DAMD17-02-1-0669) and NCI (K07-CA080668, R01-CA95023, P50-CA159981 MO1-RR000056 R01-CA126841); MAL: Funding for this study was provided by research grant R01- CA61107 from the National Cancer Institute, Bethesda, MD, research grant 94 222 52 from the Danish Cancer Society, Copenhagen, Denmark; and the Mermaid I project; MAY: National Institutes of Health (R01-CA122443, P30-CA15083, P50-CA136393); Mayo Foundation; Minnesota Ovarian Cancer Alliance; Fred C. and Katherine B. Andersen Foundation; NEC: National Institutes of Health R01-CA54419 and P50-CA105009 and Department of Defense W81XWH-10-1-02802; NJO: National Cancer Institute (NIH-K07 CA095666, R01-CA83918, NIH-K22-CA138563, and P30-CA072720) and the Cancer Institute of New Jersey; OPL: National Health and Medical Research Council (NHMRC) of Australia (APP1025142, APP1120431) and Brisbane Women’s Club; POL: Intramural Research Program of the National Cancer Institute; UCI: NIH R01-CA058860 and the Lon V Smith Foundation grant LVS-39420; UKO: The UKOPS study was funded by The Eve Appeal (The Oak Foundation) with investigators supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre and MRC core funding (MR_UU_12023); USC: P01CA17054, P30CA14089, R01CA61132, N01PC67010, R03CA113148, R03CA115195, N01CN025403, and California Cancer Research Program (00-01389V-20170, 2II0200);
Footnotes
Conflict of Interest Statement
Usha Menon has stocks in Abcodia awarded to her by UCL. The rest of the authors declare no potential conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cronin KA, Lake AJ, Scott S, et al. : Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 124:2785–2800, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beral V, Million Women Study C, Bull D, et al. : Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 369:1703–10, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Group On Epidemiological Studies Of Ovarian C, Beral V, Gaitskell K, et al. : Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet 385:1835–42, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AW, Ness RB, Roman LD, et al. : Association Between Menopausal Estrogen-Only Therapy and Ovarian Carcinoma Risk. Obstet Gynecol 127:828–36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafrir AL, Babic A, Tamimi RM, et al. : Reproductive and hormonal factors in relation to survival and platinum resistance among ovarian cancer cases. Br J Cancer 115:1391–1399, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power L, Lefas G, Lambert P, et al. : Hormone Use After Nonserous Epithelial Ovarian Cancer: Overall and Disease-Free Survival. Obstet Gynecol 127:837–47, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Rosen B, Fan I, et al. : Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer 116:964–971, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascarenhas C, Lambe M, Bellocco R, et al. : Use of hormone replacement therapy before and after ovarian cancer diagnosis and ovarian cancer survival. Int J Cancer 119:2907–15, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Nagle CM, Bain CJ, Green AC, et al. : The influence of reproductive and hormonal factors on ovarian cancer survival. Int J Gynecol Cancer 18:407–13, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Felix AS, Bunch K, Yang HP, et al. : Menopausal hormone therapy and mortality among women diagnosed with ovarian cancer in the NIH-AARP Diet and Health Study. Gynecol Oncol Rep 13:13–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Holman CD: Tubal ligation and survival of ovarian cancer patients. J Obstet Gynaecol Res 38:40–7, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Wernli KJ, Newcomb PA, Hampton JM, et al. : Hormone therapy and ovarian cancer: incidence and survival. Cancer Causes Control 19:605–13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besevic J, Gunter MJ, Fortner RT, et al. : Reproductive factors and epithelial ovarian cancer survival in the EPIC cohort study. Br J Cancer 113:1622–31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madalinska JB, van Beurden M, Bleiker EM, et al. : The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorectomy. J Clin Oncol 24:3576–82, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Organization WH: BMI Classification, Global Database on Body Mass Index, 2006
- 16.Bhatla N, Denny L: FIGO Cancer Report 2018. Int J Gynaecol Obstet 143 Suppl 2:2–3, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Keele L, Tingley D: A general approach to causal mediation analysis. Psychol Methods 15:309–34, 2010 [DOI] [PubMed] [Google Scholar]
- 18.R Core Team: R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing, 2019 [Google Scholar]
- 19.Hein A, Thiel FC, Bayer CM, et al. : Hormone replacement therapy and prognosis in ovarian cancer patients. Eur J Cancer Prev 22:52–8, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Beach JA, Agadjanian H, et al. : Suboptimal cytoreduction in ovarian carcinoma is associated with molecular pathways characteristic of increased stromal activation. Gynecol Oncol 139:394–400, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riester M, Wei W, Waldron L, et al. : Risk prediction for late-stage ovarian cancer by meta-analysis of 1525 patient samples. J Natl Cancer Inst 106, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiadou P, Sbarouni E: Effect of hormone replacement therapy on inflammatory biomarkers. Adv Clin Chem 47:59–93, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Lamon-Fava S, Posfai B, Schaefer EJ: Effect of hormonal replacement therapy on C-reactive protein and cell-adhesion molecules in postmenopausal women. Am J Cardiol 91:252–4, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Martin-Millan M, Castaneda S: Estrogens, osteoarthritis and inflammation. Joint Bone Spine 80:368–73, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Straub RH: The complex role of estrogens in inflammation. Endocr Rev 28:521–74, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ono M: Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci 99:1501–6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris BS, Bishop KC, Kuller JA, et al. : Hormonal management of menopausal symptoms in women with a history of gynecologic malignancy. Menopause, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Eeles RA, Tan S, Wiltshaw E, et al. : Hormone replacement therapy and survival after surgery for ovarian cancer. BMJ 302:259–62, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen Y, Huang H, Huang H, et al. : The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 16:673–81, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Pan Z, Gao K, et al. : Impact of post-operative hormone replacement therapy on life quality and prognosis in patients with ovarian malignancy. Oncol Lett 3:244–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eeles RA, Morden JP, Gore M, et al. : Adjuvant Hormone Therapy May Improve Survival in Epithelial Ovarian Cancer: Results of the AHT Randomized Trial. J Clin Oncol 33:4138–44, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Guidozzi F, Daponte A: Estrogen replacement therapy for ovarian carcinoma survivors: A randomized controlled trial. Cancer 86:1013–8, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Bebar S, Ursic-Vrscaj M: Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 21:192–6, 2000 [PubMed] [Google Scholar]
- 34.Ursic-Vrscaj M, Bebar S, Zakelj MP: Hormone replacement therapy after invasive ovarian serous cystadenocarcinoma treatment: the effect on survival. Menopause 8:70–5, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Sandini L, Pentti K, Tuppurainen M, et al. : Agreement of self-reported estrogen use with prescription data: an analysis of women from the Kuopio Osteoporosis Risk Factor and Prevention Study. Menopause 15:282–9, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Hu FB, Grodstein F, Hennekens CH, et al. : Age at natural menopause and risk of cardiovascular disease. Arch Intern Med 159:1061–6, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Hodis HN, Mack WJ, Henderson VW, et al. : Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med 374:1221–31, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grodstein F, Manson JE, Stampfer MJ: Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 15:35–44, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Manson JE, Chlebowski RT, Stefanick ML, et al. : Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 310:1353–68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossouw JE, Anderson GL, Prentice RL, et al. : Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–33, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.