Abstract
Early life adversity (ELA) increases risk for negative health outcomes, with sex disparities in prevalence and form of ELA experienced and risk for neuropsychiatric pathology. ELA comes in many forms (e.g. parental neglect/loss, limited access to resources) but whether disparate forms of ELA have common effects on outcomes, and if males and females are equally affected, remains unknown. Epidemiological studies often fail to accurately account for differences in type, timing, and duration of adversity experienced. Rodent models allow precise control of many of these variables. However, differences in the form of ELA, and species, strain, housing, and testing paradigms used, may contribute to differences in outcomes leading to questions of whether differences are the result of the form of ELA or these other variables. Here, we directly compared two mouse models of ELA, maternal separation (MS) and limited bedding (LB) in males and females on development of the body, motor and visual milestones, stress physiology, and anxiety-like behavior. LB affected timing of early milestones, somatic growth, and stress physiology in both sexes, yet only females showed later anxiety-like behaviors. MS rearing affected males and females similarly in early milestone development, yet only males showed changes in stress physiology and anxiety-like outcomes. These studies provide a platform to directly compare MS and LB models within one lab. The current work advances our understanding of the unique features of ELA that shape early neurodevelopmental events and risk for later pathology, increasing the translational relevance of these ELA models.
Keywords: early life adversity, development, motor milestone, visual development, Hypothalamic-pituitary-adrenal axis
Introduction:
Early experiences play a significant role in guiding the timing and trajectory of neuronal and behavioral maturation. Attentive parental care can buffer against deleterious effects of stress (Iwata et al., 2007; Nawaz et al., 2018) and is associated with improved cognitive functioning, including improved performance on learning and memory measures (Del Arco et al., 2007; Hullinger et al., 2015). Conversely, diminished quality or quantity of care, abuse, or reduced access to resources experiences (e.g. early life adversity) and enriching experiences have been associated with increased risk for psychopathology (Dong et al., 2003; Dube et al., 2001; Felitti et al., 1998; Nelson et al., 2011), altered brain maturation (Gee et al., 2013; Honeycutt et al., 2020), impaired performance on learning tasks (Goodwill et al., 2018), delayed sexual maturation (Manzano Nieves et al., 2019), and impaired regulation of the stress response (Sanchez, 2006; Sanchez et al., 2010). Early life adversity (ELA) is highly prevalent, however the type, timing, and duration of these experiences are often unique across individuals (Anda et al., 2006). In addition, sex and genetic background can contribute to differences in later risk for negative outcomes (Bath et al., 2020).. Thus, it is important to better understand how unique types of adversity may shape neural and behavioral developmental trajectories. Controlling for these key variables will advance our understanding of the contribution of those experiences to vulnerability or resilience to pathological outcomes later in life. Given the rapid development and precise control of genetic and environmental parameters, rodent models of ELA provide an important testbed to determine the impact of different forms of adversity on brain and behavioral maturation.
Here, the effects of two commonly employed forms of ELA in rodents, limited bedding (LB) and maternal separation (MS), were compared, with regard to their effects on the trajectory of multiple measures of sensory, motor and affective development, as well as neural maturation in male and female mice. These models of ELA have provided evidence for increased risk for later life negative outcomes, that may be the consequence of altered trajectory of basic neurodevelopmental programs and/or changes in the timing of regional brain development. The consequences of altered development could have lasting consequences on core systems involved in sensory, motor, learning, and emotional regulation (Bath et al., 2016; Ganguly and Brenhouse, 2015; Gee et al., 2013; Manzano Nieves et al., 2019). The two models were designed to parallel forms of adversity experienced by humans, which have been shown to differentially affect outcomes (Fisher et al., 2010). The LB model was designed to simulate stress associated with loss of resources, such as extreme poverty, homeless, or refugee settings. The MS model was designed to simulate stress associated with loss or absence of parental care, such as institutional rearing and parental separation. Although each of these forms of ELA ostensibly alter dam:pup interactions, the type of adversity experienced are different and may have unique consequences on developmental processes (Peña et al., 2019). Further, as these procedures are often carried out by different labs, variability in protocol administration, testing context, animal care, species, and colony composition makes comparison or generalization of results across the different paradigms difficult. Here, the two ELA manipulations were carried out within the same lab, using the same breeding colony of mice, the same investigator, and same testing apparatus, to allow for direct comparison of the effects of each of these experiences on early neurodevelopment. In addition, because adversity has been shown to affect males and females differently, both males and females were included and analyzed in parallel (Coley et al., 2019; Ganguly et al., 2019; Gobinath et al., 2015; Goodwill et al., 2018; Goodwill et al., 2018; Honeycutt et al., 2020; Oyola and Handa, 2017).
The impact of early experience had unique consequences on different systems, that depended on both the type of ELA and sex of the animal. Measures of early motor development were assessed by tracking righting reflex behavior and showed a developmental delay in response to both forms of ELA, with males and females being similarly delayed. For measures of early visual development (assessed by the timing of eye opening), both forms of ELA resulted in a delayed trajectory in males and females. However, the effects of LB were more robust than those observed for MS. For both somatic development (assessed by weight gain) and the maturation of HPA reactivity (assessed by basal corticosterone levels), the effects of LB were similar across sex, yet MS rearing led to sex-specific effects with males being more affected than females. Finally, effects of ELA on anxiety-like outcomes (assessed by light-dark box behavior) were found in LB females (with effects throughout development) and in MS males (with effects emerging in adulthood). This work directly compared, within a lab, the early developmental changes in two rodent models of ELA within one study and provides compelling evidence for sex- and experience-dependent effects of ELA. Overall, this work advances our understanding of the types of developmental mechanisms impacted in response to early adversity and highlights the potential unique contribution of each form of ELA on development and behavioral outcomes.
Methods
Mice and housing
C57BL/6N mice were bred in house and maintained on a 12h:12h light cycle (lights on at 7AM) with ad libitum access to food and water. Mice were housed in 31x12x14 cm cages with cob bedding and 4x4 cm cotton nestlet. Litters were composed of both male and female pups and ranged from 3 to 8 pups per litter. A total of 113 litters were used for the studies described here. Virgin dams were not used for the current studies. Pups were weaned and sex segregated at postnatal day 21 (PD). All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee and consistent with the guide for the care and use of animals in research.
Limited bedding (LB) manipulation
An experienced breeder female and male were pair-housed in a standard home cage with bedding and a 4x4 cm cotton nestlet for nest building. Four days following the birth of a litter (PD4), the dam and pups were transferred from their standard home cage, to a cage with a wire mesh floor, no bedding, and a 3x4 cm cotton nestlet (Fig. 1B). Mice remained in the LB housing conditions for seven days. On PD11, the dam and pups were returned to their standard housing conditions where they remained until weaning at PD21.
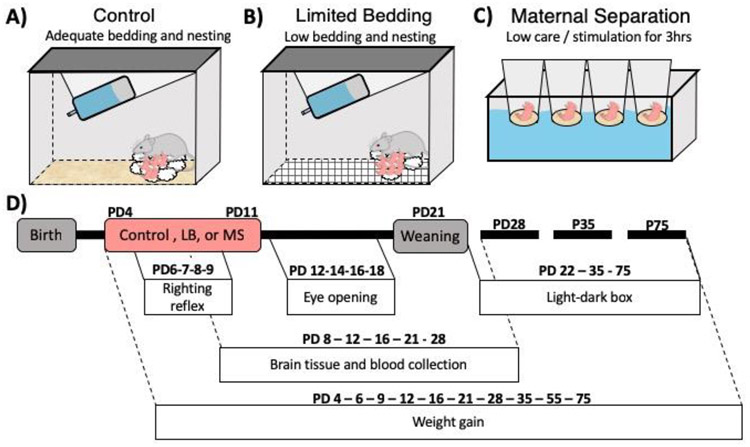
Figure 1-. Experimental Design.
At birth, litters were assigned to one of three treatment groups. Control reared litters were maintained in standard housing conditions with adequate bedding and 4x4 cm cotton nestlet (A). Limited bedding reared pups and dam were placed in a cage with a wire mesh floor, no bedding, and a 3x4 cm cotton nestlet from PD4-PD11 (B). Maternal separation reared pups and dam were housed in a standard home cage with bedding and 4x4 cotton nestlet. From PD4-PD11, maternal separation pups were removed daily from their home cage for 3 hours a day into cups placed in a water bath maintained at 35-37°C (C). A variety of early developmental outcomes were measured (D). Weight gain was measured from PD4-PD75. Righting reflex was tracked daily from PD6-PD9 to assess the trajectory of motor milestone development. Eye opening was tracked daily from PD12-PD19 to assess the trajectory of visual development. Brain tissue and blood were collected at PD8, PD12, PD16, PD21 and PD28 to assess effects of ELA on the timing of motor cortex maturation and on the trajectory of basal corticosterone levels. Finally, anxiety-like behaviors were assessed via light-dark box task at PD22, PD35, PD75.
Maternal separation (MS) manipulation
A breeder female and male were housed in a standard home cage with cob bedding and a 4x4 cm cotton nestlet. From PD4 to PD11, the pups were removed daily from their home cage for three hours per day between the hours of ~9AM and ~12PM (Fig. 1C). During this separation, the pups were placed in individual cups that contained soiled home cage bedding and that were secured in cup holders in a circulating water bath maintained at 35-37°C to support pup thermoregulation.
Developmental assays
The trajectory of early developmental outcomes were measured in both male and female control, LB, and MS pups to assess the impact of early life experience on these measures (see Fig. 1D for timeline of data collection).
Weight gain:
Total weight of each animal was measured and recorded at PD4, PD6, PD9, PD12, PD16, PD21, PD28, PD35, PD55, and PD75. Measurements were taken by briefly removing the pup from the dam, placing it in a weighing cup, and then returning it to the dam within ~1 minute of starting the manipulation.
Eye opening:
To assess ELA effects on visual development, timing of eye opening was assessed beginning at PD12 and repeated every day until PD19. To do this, mice were individually identified by tail markings, briefly removed from the nest and visually inspected for presence of open eyes. Eye opening scores for individual animals were recorded as follows: both eyes closed = 0; half eye open = 0.5; both eyes half open =1; one eye fully open and another eye fully closed = 1; one eye fully open and another eye half open = 1.5; two eyes fully open = 2. Following assessment pups were rapidly returned to the nest. Completion of this procedure for a single litter took an average of ~1-2 minutes.
Righting reflex:
Pups were tested in the righting reflex task to assess the impact of ELA on the trajectory of motor development. Righting reflex behavior was measured daily from PD6 to PD9. For the task, a single pup was removed from the litter, placed on a flat surface on its back and their behavior was digitally recorded. The amount of time to right itself to a standing position (all four paws in contact with the ground) was measured. Pups were given a maximum of 60 seconds to successfully right themselves or were returned to the prone position. Following a successful right, or 60 seconds, mice were tested on the task again. Each pup was subjected to five consecutive trials on each day and data from individual pups were tracked independently to assess change in performance over time. Following testing, each pup was returned to the litter and the next pup was tested. The average time to right across the five trials for each day was recorded for each subject.
Light-dark box:
Effects of ELA on development of a single form of anxiety-like behavior was assessed using the light-dark box task. The light-dark box apparatus was a 58x22 cm box that was divided in two equal sides connected by a small 5x5 cm opening. The dark side of the box was completely covered and unilluminated (~10 lux) and the light side was open and illuminated with approximately 2,000 lux. At the start of the trial, the mouse was placed in the dark side of the box. Noldus Ethovision XT 11 was used to track the latency, duration, and frequency of entries to the light-side during the seven-minute task.
Physiological and Neurodevelopmental Assays
Corticosterone ELISA:
Basal corticosterone (CORT) levels were assessed at several time points across early development to determine the impact of the different forms of ELA on HPA-axis development. All collection procedures occurred between 08:30-09:30AM when baseline CORT is at its lowest (Kakihana and Moore, 1976). Trunk blood was collected by rapid decapitation at PD8, PD12, PD16, PD21 and PD28. To eliminate cohort effects or dramatic reduction in litter size, a maximum of two pups/sex were taken per litter at each developmental time point, and each litter was maintained at ~70% of initial size to eliminate potential stress associated with diminishing litter size over development. This led to a random sampling of pups from ~3-5 litters/timepoint. After collection, blood was allowed to clot for one hour at room temperature and was then centrifuged at 10,000rpm for ten minutes. Serum (~50-150μl) was collected and immediately stored at −20°C for later analysis. Total CORT levels from serum (25x diluted) were determined using a competition-based ELISA (AssayMax, Corticosterone ELISA kit, AssayPro, St. Charles, MO; CAT: EC3001-1) using the manufacturer's protocol. This kit reports a sensitivity of up to 0.3ng/mL, with a 5-7% intra-assay reliability, and <2% cross reactivity with steroid and stress related hormones. All samples across groups were run on a single plate with duplicates run across plates. ELISA plates were read to determine optical density on an OpsysMR plate reader (Dynex Technologies) at 450nm. The mean optical density for each sample was calculated based on a standard curve, generated from the standardized CORT dilutions (100ng/ml, 25ng/ml, 6.25ng/ml, 1.563ng/ml, 1.563ng/ml, 0.391ng/ml, 0ng/ml) and a curve was created using a four parameter logistic regression fit method to obtain the concentration of CORT (ng/ml) within each sample. Each sex was run on its own 96-well plate and quantified using the standard curve within that plate.
Brain tissue extraction, RNA purification, and cDNA synthesis:
Brain punches were collected from the motor cortex in order to assess the maturational profile of this region during the period that righting reflex and locomotor activity was developing. The same cohort of mice was used for trunk blood and brain collection. The full brain was immediately extracted after blood collection and placed on dry ice. Brain punches were hand-dissected using Allen Brain Atlas as a guide(“Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact - 5th Edition,” n.d.). Motor cortex punches were homogenized in RNAzol (Molecular Research Center, Cincinnati, OH) and stored at −20°C until processing. Total RNA was isolated using the manufacturer's protocol. Briefly, DNA and protein were precipitated using isopropanol and RNA was precipitated using 75% ethanol. After precipitation, the RNA pellet was washed 3x in 75% ethanol, solubilized in 30μl of RNAsecure resuspension solution (Thermofisher Scientific, CAT: AM7010) and stored in −20°C until further processing. Single stranded cDNA was obtained using New England Biolabs MmULV protocols (NEB, Ipswitch, MA). cDNA was solubilized in 25μl of 10 mM Tris-HCl, pH 7.5. 1 mM EDTA.
Realtime qPCR:
Predesigned and pre-validated Taqman assays from Applied Biosystems (Life Technologies, Norwalk, CT) were run in multiplex with the housekeeping gene (18s rRNA). For each plate, relative gene expression of doublecortin (dcx) (corrected for 18-s as loading control) was calculated based upon a standard curve created from pooled and serially diluted cDNA samples. Each sex was run on its own 382-well plate and quantified relative to the standard curve made from the cDNA samples within that plate. All gene expression profiling was completed using CFX384 RT-qPCR system (Biorad, Hercules, CA).
Statistics:
A 3-way ANOVA (treatment x age x sex) was used to assess interaction effects in weight gain, eye opening, and righting reflex. Subsequent 2-way ANOVAs were then carried out in males and females separately to test for main effects of treatment and age and their interactions on developmental measures of weight gain, eye opening, righting reflex. Due to methodological constraints on the number of samples we could include on each reverse transcription, qPCR, and ELISA plate, we did not feel confident in making direct comparisons of results between the sexes for gene expression, and basal corticosterone levels. Observed differences could be due to true sex differences, or technical differences due to the need to carry out assessment of male and female samples at separate time points and on different plates. Thus, a 2-way ANOVA was used to assess males and females separately for those measures. For measures of anxiety-like behavior, a 2-way ANOVA was used at each developmental time point separately (juvenile, adolescent, adult) to test for main effects of treatment, sex, and their interaction. Significant main effects (alpha = 0.05) were followed up with post-hoc tests using Tukey’s LSD to correct for multiple comparisons. Effect sizes were estimated by calculating the value of Partial η2 (SSeffect/SStotal+SSerror).
Results:
ELA alters the trajectory of weight gain:
A 3-way ANOVA revealed a significant treatment x sex x age interaction (F(18,5006) = 4.074, p < 0.001, partial η2 = 0.015), in addition to a significant main effect of treatment (F(2, 5006) = 376.051, p < 0.001, η2 = 0.132), sex (F(1, 5006) = 586.519, p < 0.001, η2 = 0.106), and age (F(9, 5006) = 9519.335 p < 0.001, η2 = 0.945). Follow-up 2-way ANOVA’s were analyzed per treatment group to assess effects of sex. A significant main effect of sex was observed for weight gain in all treatment groups (2-way sex x age ANOVA; Control: F(1, 1688) = 207.4, p < 0.001, η2= 0.006; MS: F(1, 1175) = 221.8, p < 0.001, η2 = 0.009; LB: F(1, 1926) = 126.0, p < 0.001, η2 = 0.004). As the focus of this work was to assess the impact of ELA treatment on outcomes, for clarity of presentation, the data was separated by sex and analyses were carried out separately to determine LB and MS effects on weight gain.
Males:
A significant main effect of treatment was observed on weight gain in males (2-way ANOVA, F(2, 2361) = 200.4, p < 0.001, η2 = 0.009), and both an effect of age (F(9, 2361) = 4313, p < 0.001, η2 = 0. 922) and a treatment x age interaction (F(18, 2361) = 12.75, p>0.001, η2 = 0.005; Fig.2A) were found. Both LB and MS groups were significantly different from controls (p < 0.001, and p = 0.010; respectively) and each other (p < 0.001; Fig.2A). Importantly, no effect of group was found for weight at PD4, before the ELA manipulation began (p>0.05). Consistent with previous results 16,23, LB mice showed a reduction in weight compared to control pups, an effect that emerged two days after the initiation of LB (PD6, p = 0.048; Fig. 2A) and persisted throughout development (p < 0.05 at all timepoints). In contrast to LB, MS and control mice did not statistically differ from each other in weight from PD4-PD28 (p < 0.05; Fig.2A). However, MS mice showed a momentary increase in weight compared to controls at PD35 and PD55 (p < 0.001 and p = 0.029, respectively), with weight returning to control levels by PD75 (p = 0.341; Fig.2A). Here, the type of adversity had differing effects on the trajectory of weight gain, with LB mice showing reduced weight throughout development and MS mice having increased weight compared to controls during late adolescence.
Figure 2-. ELA in the form of LB, but not MS, affects physical growth.
Plots of the mean weight for male (A) and female mice (B) reared in control (gray-closed), MS (green-open) or LB (blue-closed) conditions. Insets on the right represent the same data as graphs on the left between ages P35-75. Red highlighter on x-axis indicates ELA being experienced. Green * indicates significant difference between MS-Control at that time point. Blue * indicates significant difference between LB-Control at that time point. n/group= 65-75, all plots depict mean and standard error of the mean. *Tukey’s LSD p<0.05
Females:
Consistent with male weight gain results, for females a main effect of treatment was found for weight gain (2-way ANOVA, F(2, 2598) = 162.2, p < 0.001, η2 = 0.006; Fig.2B). A significant effect of age (F(9, 2598) = 4749, p < 0.001, η2 = 0.907) and a treatment x age interaction (F(18, 2598) = 8.05, p < 0.001, η2 = 0.003; Fig.2B) were also found. As with males, LB mice showed a reduction in weight compared to control mice (p<0.001), however MS mice did not differ in weight compared to controls (p=0.931). While, LB and MS groups did not differ in weight from controls at P4, immediately prior to experimental manipulation (PD4, p > 0.05), female LB mice weighed significantly less than both control and MS reared two days after initiation of LB (PD6 p = 0.02) and persisted across all developmental time points measured (p < 0.05 at all timepoints; Fig.1B). However, unlike the male results, the weight of MS female mice did not differ from controls (p = 0.931; Fig.2B), and no effects of MS rearing were observed for any time point measured (p > 0.05 at all time points). Thus, the type of adversity experienced, and sex of the animal impacted trajectories of weight gain compared with control reared mice.
ELA impacts maturational timing of visual development
Eye opening:
At birth, the eyes of mouse pups are closed and remain closed during the ~first week and a half of life. Eye opening represents an important milestone, triggering significant organizational effects on visual cortex maturation to support later vision, stereopsis, navigation and interaction with the environment. To assess the effect of ELA on the trajectory of eye opening, the degree of eye opening (see methods) was scored from PD12 until all animals showed complete eye opening (e.g. PD19). A 3-way repeated measures ANOVA (age x treatment x sex) revealed a between-subject effect of treatment (F(2, 409) = 59.016, p < 0.001, η2 = 0.224), and of sex F(1, 409) = 3.383, p = 0.018, η2 = 0.014) and a within-subject effect of age (F(3.628, 1483) = 1567.051, p < 0.001, η2 = 0.793), age x treatment F(7.256, 1483) = 19.689, p < 0.001, η2 = 0.088), age x sex (F(3.628, 1483) = 3.958, p < 0.001, η2=0.010), but not an age x treatment x sex interaction (F(7.256, 1483) 0.097, p = 0.687, η2 = 0.003). As the focus of this work was to assess the impact of ELA treatment on outcomes, data are presented for males and females separately and further analysis was carried out to determine LB and MS effects on eye opening in males and females separately.
Males:
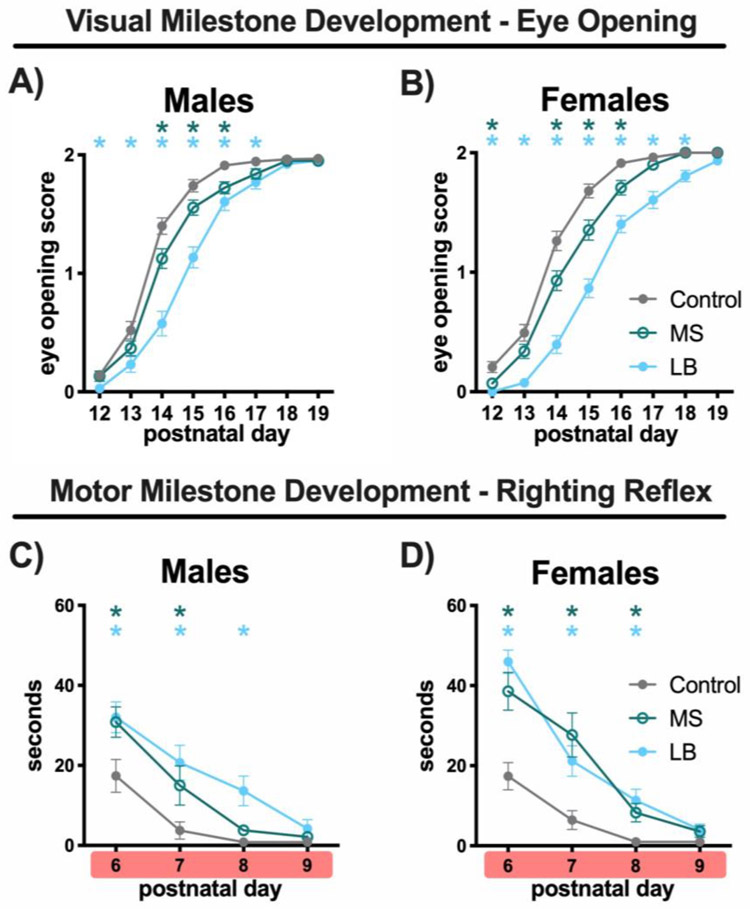
In males, a main effect of treatment on eye opening score was observed (2-way ANOVA, F(7, 1407) = 465.8, p < 0.001, η2 = 0.019). An effect of age (2-way ANOVA, F(3.592, 718.4) = 749.1, p < 0.01, η2 = 0.659) and a treatment x age interaction (2-way ANOVA, F(14, 1400) = 9.354, p < 0.001, η2 = .016; Fig.3A) were also observed. Interestingly, the trajectory of eye opening differed for each group. Eye opening in both LB and MS mice were significantly different from controls (p < 0.001 and p = 0.032, respectively) and LB mice were also significantly different from MS mice (p = 0.003; Fig.3A). Post-hoc analysis at each PD showed that LB mice had lower (e.g. delayed) eye-opening scores compared to controls at all time points from PD12-PD17 (p < 0.05) and lower scores compared to MS from PD14-PD15 (p < 0.05; Fig.3A). In addition, MS mice showed reduced eye-opening scores compared to controls on PD14 (p = 0.034) and PD16 (p = 0.011; Fig.3A). No significant difference between the groups were seen at PD19 (p > 0.05; Fig.3A), when all mice had reached completion of eye opening. Both forms of adversity resulted in delayed eye opening in males, with LB manipulation showing a more robust effect on this measure.
Figure 3-. Both forms of ELA delay the maturation of motor behavior and signs of visual development.
Plots of mean eye-opening scores measured on PD12-PD19 for male (A) and female mice (B) reared in control (gray-closed), MS (green-open) or LB (blue-closed) conditions. Plots of the mean duration of time (s) it took mice to right themselves on PD6-PD9 in male (C) and female mice (D) reared in control (gray-closed), MS (green-open) or LB (blue-closed) conditions. Red highlighter on x-axis indicates ELA being experienced. Green * indicates significant difference between MS-Control at that time point. Blue * indicates significant difference between LB-Control at that time point. Eye opening: 65-80/group, sampled from 23-28 litters. Eye opening: 21-23/ group, sampled from 5-7 litters. All plots depict mean and standard error of the mean. * Tukey’s LSD p<0.05
Females:
The effect of ELA on the trajectory of eye-opening in females was similar to those observed in males. Eye opening scores revealed a main effect of treatment (2-way ANOVA, F(2, 209) = 47.65, p < 0.001, η2 = 0.044), age (F(3.546, 741) = 824.5, p < 0.001, η2 = 0.669) and treatment x age interaction (F(14, 1463) = 11.11, p < 0.001, η2 = 0.018; Fig.3B). Again, the type of ELA resulted in different developmental trajectories, with LB and MS mice both being significantly different from control (p < 0.001, p = 0.004, respectively) and each other (p < 0.001; Fig.3B). LB mice had lower eye-opening scores compared to controls at all time points from PD12-PD18 (p < 0.05) and compared to MS from PD12-PD18 (p < 0.05; Fig.3B). Additionally, MS mice had lower eye-opening scores compared to controls at PD12 (p = 0.029) and from PD14-PD16 (p < 0.05; Fig3B). No significant differences between the groups were seen at PD19 (p > 0.05; Fig.3B), when all mice had completed eye opening. These results suggest that ELA delayed eye opening in females and the type of adversity determined the degree of delay.
ELA impacts behavioral measures of motor maturation
Righting reflex:
As in humans, motor development in mice can be assessed by tracking the trajectory of motor milestones or emerging expertise in motor development. In mice, proficiency in the ability to right from a supine to a prone position (righting reflex) improves significantly during the first week of life. The maturation of this behavior is crucial for the pup to begin interacting with the environment and seek parental care when needed. To assess the impact of ELA on the trajectory of righting reflex maturation, the duration of time it took mice to right themselves was measured daily from PD6 to PD9. A 3-way repeated measures ANOVA (age x treatment x sex) revealed a between-subject effect of treatment (F(2, 129) = 17.648, p < 0.001, η2 = 0.215), and of sex (F(1, 129) = 3.726, p = 0.028, η2 = 0.028) and a within-subject effect of age (F(23, 296.8) = 132.365, p < 0.001, η2 = 0.506), age x treatment (F(4.603, 296.8) = 6.417 p < 0.001, η2 = 0.090), but not age x sex (F(2.30,296.8) = 1.417, p = 0.236, η2 = 0.011), or age x treatment x sex interaction (F(4.603, 296.8) = 1.322, p = 0.258, η2 = 0.020). Next, we assessed the impact of ELA on righting reflex in males and females separately.
Males:
For males, a main effect of treatment (2-way ANOVA, F(2, 64) = 6.669, p < 0.002, η2 = 0.057), age (F(2.84, 146.5) = 448, p < 0.001, η2 = 0.267), and a treatment x age interaction (F(6, 192) = 2.171, p = 0.47, η2 = 0.025; Fig.3C) were found. Post-hoc tests showed that both LB and MS rearing groups were significantly different from controls (p < 0.01) but not from each other (p = 0.803; Fig.3C). Post-hoc comparisons within age were analyzed to assess the effect of ELA on righting behavior across early development. LB male mice took longer to right compared to controls at PD6 (p = 0.052), PD7 (p = 0.019), and PD8 (p = 0.012; Fig.3C). By PD9, both LB and control male mice were significantly faster to right than prior time points and no longer different from controls (LB vs Control, p = 0.271; Fig.3C). MS mice showed a similar trajectory. At PD6, MS mice showed a trend toward a longer righting reflex time compared to controls (p = 0.060;) and remained slower than controls at PD7 (p = 0.01), and PD8 (p = 0.014; Fig.3C). By PD9, MS mice also showed a rapid righting reflex and were not different from controls (p = 0.142; Fig.3C).
Females:
In females, a main effect of treatment (2-way ANOVA, F(2, 66) = 11.73, p < 0.001, η2 = 0.096), age (F(2, 329) = 96.95, p < 0.001, η2 = 0.347) and treatment x age interaction (F(6, 198) = 6.365, p < 0.001, η2 = 0.045; Fig.3D) were found. Righting reflex in both LB (p < 0.001) and MS (p < 0.001) reared groups differed from control reared mice but did not differ from each other (LB vs MS; p = 0.943; Fig.3D). Both LB and MS reared mice took longer to right themselves at PD6 (p < 0.001 and p = 0.010, respectively), PD7 (p = 0.001 and p = 0.011, respectively) and PD8 (p = 0.01 and p = 0.053, respectively; Fig.3D). At PD9, mice showed rapid righting reflex and were not different from controls (LB: 0.163, MS: 0.243 Fig.3D).
Type of ELA alters expression of marker of immature neurons in the motor cortex
As motor cortex matures, there is a decrease in the expression of markers of immature neurons. To determine the effects of ELA on the maturation of motor cortex, gene expression for a marker of immature neurons (doublecortin- dcx) was measured at multiple time points from PD12-PD21.
Males:
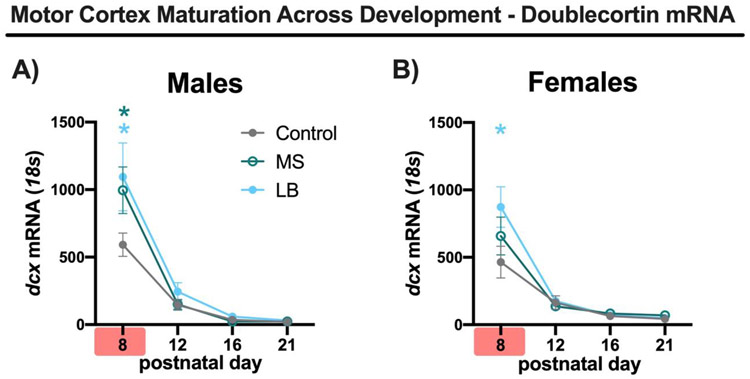
In males, there was a main effect of treatment (2-way ANOVA, F(2, 60) = 2.984, p = 0.053, η2 = 0.023) and age (F(3, 60) = 61.97, p = < 0.001, η2 = 0.717) for dcx expression. In addition, a trending treatment x age interaction was observed (F(6, 60) = 2.029, p = 0.075, η2 = 0.047; Fig.4A). Subsequent analysis of dcx expression at each PD showed that both LB and MS mice had increased expression of dcx compared to controls at PD8 (p < 0.001 and p = 0.003, respectively) and that LB did not differ from MS levels (p = 0.734; Fig.4A). In addition, no detectable differences in dcx expression were seen at PD12, PD16, and PD21. Dcx expression was nearly undetectable at PD12, PD16, and PD21.
Figure 4-. ELA alters the maturation of the motor cortex.
Plots of mean doublecortin mRNA expression relative to control gene (18s) at PD8, PD12, PD16, PD21 for male (A) and female mice (B) reared in control (gray-closed), MS (green-open) or LB (blue-closed) conditions. Red highlighter on x-axis indicates ELA being experienced. Green * indicates significant difference between MS-Control at that time point. Blue * indicates significant difference between LB-Control at that time point. n/group=5-6, all plots depict mean and standard error of the mean. * Tukey’s LSD p<0.05
Females:
In females, there was a trend level main effect of treatment on dcx expression (2-way ANOVA, F(2,60) = 2.740, p = 0.072, η2 = 0.021), a main effect of age (2-way ANOVA, F(3, 60) = 54.83, p < 0.001, η2 = 0.653) and a tending treatment x age interaction (2-way ANOVA, F(6, 60) = 2.105, p = 0.065, η2 = 0.050; Fig.4B). As age specific effects were anticipated, with diminishing differences between groups as animals aged, planned post-hoc analyses were performed. LB female mice had higher levels of dcx expression at PD8 compared to controls (p < 0.001) while MS were not significantly different compared to controls at PD8 (p = 0.133; Fig.4B). In addition, no effect of treatment on dcx level was found at PD12, PD16, and PD21.
Type of ELA drives differential developmental patterns of basal stress hormone levels
Corticosterone:
The HPA-axis undergoes a protracted development, with multiple reports showing a hyporesponsive period during early development resulting from a diminished ability to synthesize or secrete CORT between P2-10/14 in rodents (Sapolsky and Meaney, 1986; Schmidt et al., 2003). As neural and adrenal tissues mature, HPA-axis begins to show the prototypical response to stressors, as evidenced by elevated basal and stress-induced levels of CORT in the brain and periphery. Here, the effects of ELA on the trajectory of basal CORT levels in blood serum were assessed as an indirect measure of HPA maturation.
Males:
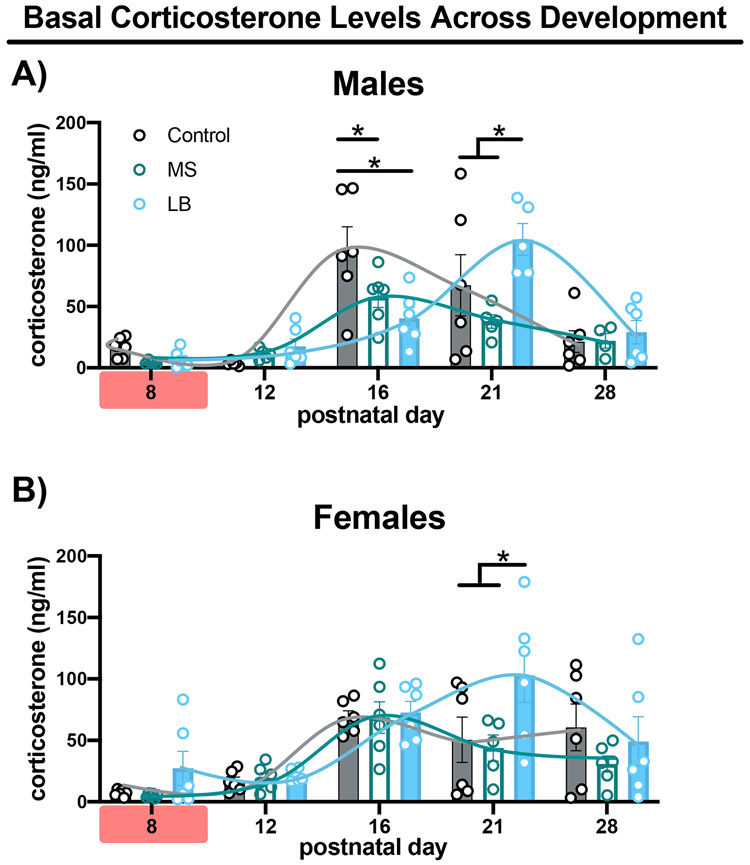
Baseline CORT levels in males showed a trending effect of treatment (2-way ANOVA, F(2, 68) = 2.509, p = 0.088, η2 = 0.026), an effect of age (F(4, 68) = 22.01, p < 0.001, η2 = 0.464) and a treatment x age interaction (F(8, 68) = 3.626, p = 0.001, η2 = 0.153; Fig.5A). CORT levels were nearly absent in all treatment groups at PD8 and PD12. At P16, CORT levels in controls showed a dramatic increase and were elevated compared to LB and MS mice at this age (p < 0.001 and p = 0.029, respectively; Fig.5A). Importantly, LB male mice showed a similar increase in CORT at PD21 and had elevated CORT levels compared to both control (p = 0.048) and MS reared mice (p < 0.001; Fig.5A). Based upon this data, control mice showed maturational elevations in circulating basal CORT around PI6, with LB males showing a delayed elevation in developmental CORT expression and MS animals showing a general blunting in developmental elevations of CORT at the time points measured (inset).
Figure 5-. Type of ELA alters basal corticosterone levels.
Bar graphs depict mean corticosterone levels at PD8, PD12, PD16, PD21, PD28 in male (A) and female (B) mice reared in control (gray-filled), MS (green-unfilled) or LB (blue-filled) conditions. Curves represent summary data. Red highlighter on x-axis indicates ELA being experienced. All bars depict mean, standard error of the mean and individual data points. * Tukey’s LSD p<0.05
Females:
For baseline CORT levels in females, a main effect of treatment (2-way ANOVA, F(2, 74) = 3.835, p = 0.026, η2 = 0.051) and age (F(4, 74) = 13.44, p < 0.001, η2 = 0.364) were found, but no treatment x age interaction (F(8, 74) = 1.403, p = 0.209, η2 = 0.076; Fig.5B). As with males, CORT levels in females were low at PD8 and PD12. LB rearing led to elevated basal CORT levels at PD21 compared to both control (p < 0.001) and MS mice (p = 0.004; Fig.5B). No other differences were seen between treatment groups at PD8, PD12, PD16, or PD28 (p > 0.2; Fig5B).
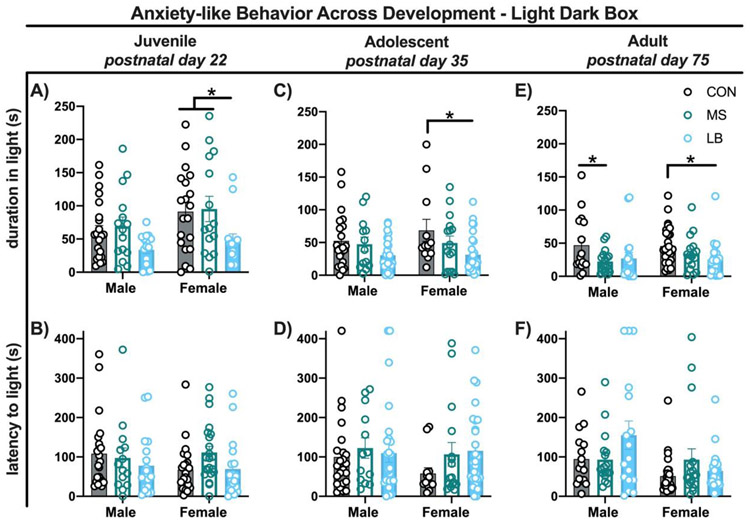
Type of ELA contributed to sex differences in risk for expression of anxiety-like behavior Light-dark box:
To determine if the type of adversity experienced conferred risk for development of anxiety-like behavior, separate groups of mice were tested in the light-dark box task at three developmental time points (juvenile (PD22), adolescent (PD35), and adult (PD75). Analysis of effects of treatment were carried out separately at each developmental timepoint.
Juvenile:
An overall main effect of treatment was found for time spent in the light-side of the box (2-way ANOVA, F(2, 97) = 5.921, p = 0.004, η2 = 0.101). In addition, an effect of sex (F(1, 97) = 4.967, p = 0.028, η2 = 0.043) but no treatment x sex interaction (F(2, 97) = 0.238, p = 0.788, η2 = 0.004; Fig.6A) were found. Post-hoc analysis revealed that LB female mice spent less time in the light side compared to both controls (p=0.043) and MS female mice (p=0.038), while LB male mice did not (p=0.245 and p=0.146, respectively; Fig.6A). In addition, MS and control mice (both males and females) spent an equivalent amount of time in the light (p>0.05; Fig.6A). Further, for the latency to enter the light side, no effect of treatment (2-way ANOVA, F(2, 111) = 1.366, p = 0.259, η2 = 0.023), sex (F(1, 11) = 0.6450, p = 0.4236, η2 = 0.005) or interaction (F(2, 111) = 1.222, p = 0.299, η2 = 0.021; Fig.6B) were found.
Figure 6-. ELA in the form of LB, but not MS, results in anxiety-like behaviors throughout development.
Bar graph depicting mean duration and latency to the light side at PD22 (A-B), P35 (C-D_ and PD75 (E-F) in male and female mice reared in control (gray-fdled), MS (green-unfdled) or LB (blue-filled) conditions. All bars depict mean, standard error of the mean and individual data points. * Tukey’s LSD p<0.05
Adolescent:
Analysis of anxiety-like behavior in adolescent mice revealed a similar overall pattern to those observed in juvenile mice. A main effect of treatment was found (2-way ANOVA, F(2, 110) = 6.320, p = 0.002, η2 = 0.102) but no main effect of sex (F(1, 110) = 0.7247, p = 0.396, η2 = 0.005) or treatment x sex interaction (F(2, 110) = 0.431, p = 0.651, η2 = 0.007; Fig.6C). Again, LB female mice, but not males (p = 0.107) spent decreased time in the light compared to control (p=0.015; Fig.6C). In addition, MS male (p = 0.914) and MS female (p = 0.391; Fig.6C) mice spent an equivalent amount of time in the light side compared to their control counterparts. There were no treatment effects on the latency to enter the light-side of the box (2-way ANOVA, F(2, 112) = 1.370, p = 0.258, η2 = 0.023), no effects of sex (F(1, 112) = 0.779, p = 0.379, η2 = 0.006), and no treatment x sex interaction (F(2, 112) = 0568, p = 0.568, η2 = 0.009; Fig.6D).
Adult:
For duration of time spent on the light side of the box for adults, a main effect of treatment (F(2, 112) = 5.315, p = 0.006, η2 = 0.085) was found, but no effect of sex (F(1, 112) = 0.2666, p = 0.606, η2 = 0.002) or treatment x sex interaction (F(2, 112) = 0.565, p = 0.569, η2 = 0.009; Fig.6E). Consistent with both juvenile and adolescent ages, LB female mice (but not male p = 0.142) spent less time in the light side compared to controls (p = 0.048; Fig.6E). Interestingly, while MS male mice did not differ from controls in the duration spent in the light in either juvenile or adolescent ages, a difference emerged in adults, with MS males spending decreased time in the light compared to control males (p = 0.055; Fig.6E). There were no treatment differences in the latency to enter the light-side of the box (2-way ANOVA, F(2, 113) = 1.813, p = 0.168, η2 = 0.027), or treatment x sex interaction (F(2, 113) = 2.535, p = 0.838, η2 = 0.040; Fig.6F). For latency to enter the light side, there was a trending effect of sex (F(1, 113) = 7.700, p = 0.007, η2 = 0.059 Fig.6F), with no observed significant post-hoc effects.
Discussion
Early experiences provide critical signals to support both neural and behavioral development, which serve as building blocks for continued maturation. ELA can come in many forms, including alterations in the quality, quantity, and reliability of care, loss of resources, and physiological stress signals provided by the parent or environment. To causally assess the effects of different forms of early life adversity on maturation, we used two common rodent models of ELA, limited bedding and maternal separation, to respectively model loss of care and resource restriction. Both models had unique effects on weight gain, eye opening, motor behavior maturation, neural maturation, and basal CORT levels, with several of these effects being sex and/or model dependent (see Table 1 for summary).
Table 1-. Summary of results.
Type of ELA confers different developmental and behavioral outcomes in a sex-dependent manner.
| Maternal Separation | Limited Bedding | |
|---|---|---|
| Weight gain | PD35-55 increased ♂ | PD6-75 decreased ♂♀ |
| Eye opening | PD 14-16 decreased ♂ PD 12-16 decreased ♀ |
PD 12-17 decreased ♂ PD 12-18 decreased ♀ |
| Righting reflex | PD6-7 decreased ♂ PD6-8 decreased ♀ |
PD6-8 decreased ♂ ♀ |
| Motor cortex maturation (qPCR- dcx) | PD8 increased DCX ♂ | PD8 increased DCX ♂ ♀ |
| Basal corticosterone | PD16 decreased ♂ | PD16 decreased ♂ PD21 increased ♂ ♀ |
| Light-dark box–Juvenile | Reduced duration in light ♀ | |
| Light-dark box–Adolescence | Reduced duration in light ♀ | |
| Light-dark box–Adult | Reduced duration in light ♂ | Reduced duration in light ♀ |
ELA in the form of LB, but not MS, affected physical growth
In the current report, LB and MS differed in their effects on physical development. LB resulted in a significant decrease in body weight across all ages measured and MS resulted in no change in early weight gain, and a modest increase in body weight relative to controls shortly following weaning in males. These results are consistent with prior work in the LB model, where LB rearing impacted physical growth (Bath et al., 2016; Manzano Nieves et al., 2019; Rice et al., 2008), and work from others where MS did not impact weight gain (George et al., 2010). However, effects of weight gain in MS are heterogeneous and may depend upon the strain and specific method of ELA implemented. Shorter protocols shown no effect on weight (George et al., 2010; Roque et al., 2014) while longer protocols lead to reduced weight gain in MS pups (de Almeida Magalhães et al., 2018). Further, for MS in rats, reductions in weight during the separation period has been shown to recover shortly after weaning (Coley et al., 2019; Mesquita et al., 2007).
While both ELA models impact dam-pup interactions and the care of the offspring (Bailoo et al., 2014; Gallo et al., 2019; Ivy et al., 2008), differences in the design and execution of each manipulation could account for the disparate effects on weight gain in the present study. First, the LB paradigm chronically restricts access to bedding throughout the full seven-day manipulation, while MS separates pups from mom for 3 hours per day for 7 days. In this case, for MS, the effects of separation on access to care may be transient and compensated for during the 21 hours per day of normal housing conditions (Bailoo et al., 2014), leading to less robust effects. The effects of LB on pup-dam interactions, however are chronic, occur during the full period of manipulation, and result in fragmented care (Gallo et al., 2019; Ivy et al., 2008). Alternatively, differences between the models may be the result of the impact of the form of adversity on maternal physiology, with the stress of LB housing impacting maternal health, milk production, and hormones associated with milk ejection, effects that may not occur in the MS model. Further study of the effects of the two forms of ELA on these processes may provide important insights into ELA effects on basic physiological processes over development, and additional challenges faced by pups in varied ELA conditions.
Both forms of ELA delay signs of visual development
Early sensory experience drives dramatic changes in synaptic organization of the cerebral cortex (Prusky et al., 2008; Sirevaag and Greenough, 1988; Wiesel and Hubel, 1963). For the visual system, a significant component of visual development occurs postnatally and relies, at least partially, upon input from the environment For example, an accelerated development of the visual system has been reported both in pups reared in enriched environments who received increased levels of licking (Sale et al., 2004), and in pups who received artificial tactile stimulation in the absence of a dam (Smart et al., 1990). Understanding how ELA impacts the timing of sensory, and underlying neural development, can provide insights into possible mismatches in the unfolding of neurodevelopmental programs and timing of key experiences. Here, we found that both forms of ELA resulted in delayed timing of eye opening of males and females, with the degree of delay depending on the form of adversity. LB and MS rearing led to a significant delay in the initiation and the completion of eye opening compared to controls. Further, the effects of LB rearing on eye opening were more profound than the effects observed in MS mice. Based on these observations, it is possible that LB rearing is a more potent paradigm of adversity than MS rearing, due to the chronicity of effects on maternal care (Walker et al., 2017). Our current findings in MS reared mice contradict previous findings in other rodent models of MS rearing, where MS resulted in either no changes in eye opening date (Farkas et al., 2009) or accelerated eye opening after MS rearing (Mesquita et al., 2007). The divergence from prior reports may be due to inconsistency in the implementation of MS protocols, methods of data collection, and specific strains or species used. Prior reports using daily three-hour MS from PD1-14 in rats showed no differences in terms of final day of eye opening, however, the trajectory of eye opening was not measured (Farkas et al., 2009). Eye opening does not occur abruptly, and the trajectory of development provides useful information regarding the rate of maturation. Interestingly, a group utilizing a six-hour MS paradigm from PD2-PD15 in rats found an acceleration in eye opening, with eye opening onset occurring earlier in MS males and females compared to controls (Mesquita et al., 2007). In that report, the authors propose that glucocorticoid levels resulting from the prolonged separation may serve to stimulate growth and maturation. Alternatively, in some MS paradigms, there is an uptick in maternal care following daily reunion of pups with the dam (Macrí et al., 2004). The increased care upon reunion in some iterations of this model could contribute to the disparate outcomes. Notably, in a separate line of work, an enriched early rearing environment has been associated with precocious eye opening, which was associated with increased maternal licking (Sale et al., 2004) and precocious expression of brain derived neurotrophic factor (BDNF) in the visual cortex (Cancedda, 2004). Thus, the results from LB and MS rearing, show how subtle differences in the way that maternal care and resources that are provided can profoundly impact key milestones that are important for basic sensory and cortical development.
Both forms of ELA delay maturation of motor behavior
Motor experiences during postnatal life are crucial drivers of normative maturation and produce long-lasting changes in the brain (Kolb and Gibb, 2011). Righting reflex requires the recruitment of muscular and motor systems and is also dependent on successful coordination between the left and right side of the body (Feather-Schussler and Ferguson, 2016). This fine motor coordination is an important milestone for the rodent pup as it allows them to begin to engage with their environment in richer ways and changes their ability to interact with the dam. Rodents raised in enriched housing have been shown to engage in increased motor activity compared with those reared in standard caging. Enrichment reared animals have increased cortical thickness, dendritic branching and spine density in regions measured (Sirevaag and Greenough, 1988). Consistent with other work in MS mice (Farkas et al., 2009; Mesquita et al., 2007), ELA in the form of MS, as well as LB rearing, drove significant delays in motor development in both males and females. Thus, like visual development, motor development is highly sensitive to the early rearing environment. Interestingly, delays in motor function were apparent as early as two days after the initiation of ELA manipulations. Thus, motor development appears to be highly sensitive to these forms of ELA during this period of rapid development. Importantly, abnormalities in the development of motor milestones in infants have been linked to other neurodevelopmental disorders, including autism (Leonard et al., 2014), impaired social communication. (Bhat et al., 2012), and delayed language development (Iverson, 2010). Thus, ELA effects on the timing of neurodevelopmental and physical development have the potential to compound effects of other genetic and environmental drivers of risk and potentially impact the expression or severity of symptom development in other conditions.
ELA alters maturation of the motor cortex
ELA has been associated with significant effects on the timing of neural maturation (Bath et al., 2016), as well as on morphometry of regional brain structure measured in adolescence and adulthood (Bagot et al., 2009; Brunson, 2005; Champagne et al., 2008; Chen et al., 2008; Chen and Baram, 2016; Gee et al., 2013; Joëls and Baram, 2009; Wang et al., 2011). To date, studies have principally focused on later maturing cortical regions, such as the prefrontal cortex (Pascual and Zamora-León, 2007), and subcortical structures, including hippocampus (Oomen et al., 2010) and amygdala (Danielewicz and Hess, 2014). Here, we have found early emerging and significant effects of ELA on the timing of motor development. The motor cortex is one of the earliest maturing cortical regions (Gao et al., 2015), and could provide additional and important insights into the effects of ELA on the timing of regional brain development. Here, dcx gene expression (a marker found in immature neurons) was measured as a proxy of neural maturation, with motor cortex showing adversity- and sex- dependent effects. When measured across development, there is a general decline in dcx expression, indicative of the process of maturation (more mature and fewer immature cells). At PD8, LB and MS reared males had increased dcx expression compared with controls (indicative of a later developmental decline in expression of this gene). However, for females, only LB rearing was associated with increased dcx expression at early time points. While we observed a general trend toward higher dcx levels in ELA conditions at early time points, the less robust effects in females may have been the result of sex differences in the timing of this developmental process. It is possible that motor cortex maturation in females occurs slightly earlier than males and that analysis of dcx at PD6 (two days earlier) might have revealed a more robust difference between MS females and controls, matching the results found in males. Despite this, the current results suggest that ELA leads to a delay in one marker of motor cortex maturation, which may drive, or be the result of, motor behavioral deficits observed in ELA reared animals. While dcx expression is a useful marker for immature neurons undergoing differentiation, it does not index the multitude of additional processes comprising cortical development. For a more complete picture of motor cortex development, other morphological, cellular and behavioral variables would need to be measured. Future studies will be needed to test for markers of proliferation, division, migration, death, etc. and for morphological changes in motor cortex.
Importantly, while these data suggest a delay in brain maturation after ELA, other research has shown accelerated neuronal development of other regions such as the hippocampus (Bath et al., 2016) and amygdala (Gee et al., 2013; Honeycutt et al., 2020), which were associated with accelerated development of fear learning, and altered emotional regulation. Thus, we propose a mixed model of adversity’s effect on neurobehavioral maturation, where resources are being allocated to support precocious maturation of some brain regions (limbic and paralimbic structures) while other regions are delayed or possibly failing to develop completely (motor cortex). Alterations in the timing of maturation is likely an adaptive process and provides short-term advantages by promoting investment in processes and structures that will support successful survival within and adaptation to the rearing environment.
ELA alters developmental changes in basal corticosterone levels
In rodents, the adrenals continue to develop during the early postnatal period, resulting in the stress hyporesponsive period, indexed by low CORT levels and a diminished or absent CORT response to stress (Schmidt et al., 2003). Interestingly, despite low basal levels of CORT, the developing nervous system is sensitive to changes in circulating CORT levels. For example, repeated administration of exogenous CORT through drinking water or injection can change the maturational profile of the brain through effects on cell proliferation and cell death (Gould et al., 1991; Gould et al., 1991). Administration of drugs that suppress CORT synthesis have been shown to prevent normal dendritic spine turnover (Liston and Gan, 2011). Activation of CORT receptors, including mineralocorticoid and glucocorticoid receptors, have been associated with changes in transcription of genes involved in spine plasticity, growth factors (including BDNF) (Schaaf et al., 1997), and synaptic transmission (Datson et al., 2001). Thus, developmental changes in levels of CORT can play a major role in regulating postnatal development of the central nervous system. Here, we tested the effects of ELA on developmental changes in plasma CORT levels of pups.
The type of ELA experienced, and the sex of the mouse, led to distinct effects on basal CORT levels over early development. In males and females reared in control conditions, CORT levels were low at PD8 and PD12 and then rose by PD16, consistent with our previous work (Bath et al., 2016). Although, a mature hormonal response to a stressor has been shown to occur as early as PD12 (Schmidt et al., 2003) or even PD9 (Gilles et al., 1996), male mice reared in LB conditions showed what appeared to be a delayed maturational profile, with the developmental increase in CORT levels occurring at PD21, five days later than control males. Conversely, while female LB mice were not different from controls at PD16, LB females showed elevated levels of basal CORT compared to controls at PD21. The present results are consistent with previous reports in rats and indicate that male pups exposed to LB show reduced CORT levels early in development and a delayed rise in basal CORT levels, while females show elevated CORT levels at PD21 (Moussaoui et al., 2017, 2016). In contrast to LB, ELA in the form of MS resulted in no changes in females and lower basal CORT levels in males at PD16, with no subsequent elevation at PD21. These results are consistent with other work in mice, with groups showing no effects on basal CORT after repeated MS (Horii- Hayashi et al., 2013). Notably, this work showed elevated CORT levels after a 3h separation at both PD14 and PD21, suggesting that repeated MS does not habituate nor sensitize to separation-induced elevations in CORT. Likewise, other studies in rats have shown that MS pups were able to mount a stress response to restraint during the stress hypo-responsive period (Dent et al., 2000; Vazquez et al., 2006). A recent study in rats compared basal CORT levels in male and female pups at PD21 who had experienced LB and MS rearing and found parallel ELA-specific results with LB rearing leading to the most robust changes in basal CORT levels (Moussaoui et al., 2017).
One theory is that the sex- and adversity- specific changes in basal CORT levels are likely the result of changes in the quality of maternal care (Francis et al., 1999). As a consequence of LB rearing, maternal care becomes fragmented, unpredictable, and, in some cases, abusive (Gallo et al., 2019; Ivy et al., 2008; Walker et al., 2017). Changes in maternal care have been proposed to alter normative development of the HPA axis and systems involved in stress regulation (Gunnar and Quevedo, 2007), and tactile stimulation with feeding of neonates reversing the effects of MS (van Oers et al., 1998). In contrast to LB, MS rearing leads to higher maternal care upon reunion and may act as a buffer to protect against HPA-axis dysregulation (Daskalakis et al., 2011; Gee et al., 2014; Sanchez et al., 2015). Alternatively, the timing of the HPA axis development may also be the consequence of exposure to exogenous CORT, delivered through the breastmilk of the stressed dam (Ivy et al., 2008). While, prior work has shown that LB rearing drives a robust CORT response in the dam during the light-cycle, others fail to show this increase during the dark-cycle (Opala et al., 2019). Divergent results may reflect circadian rhythm-dependent changes in CORT response in LB dams, disruptions in synchronization of circadian rhythms of CORT secretion, or true differences in basal levels. Exposure to exogenous CORT has been shown to stimulate regional brain development (Gould et al., 1991) and levels of CORT receptor expression in the brain (Thompson et al., 2004). More work will be needed to better understand whether altered timing of HPA development is either the result of the psychological and physical stress of LB rearing, or receipt of chemical signals of stress from the lactating dam.
Experiencing adversity early in life has lasting consequences for HPA axis function and likely mediates vulnerabilities to developing psychiatric disorders such as anxiety (van Bodegom et al., 2017). Females have been shown to be particularly vulnerable to stress-associated pathology due to HPA-axis reprogramming (Carpenter et al., 2017), are more likely to develop anxiety-like phenotypes after experiencing ELA (Kalinichev et al., 2002) and show increased rates of anxiety (Reynolds et al., 2015). Supporting these and other reports (Kanatsou et al., 2016), LB rearing led to anxiety-like behaviors in the light dark box in females, but not males, an effect that was present throughout development. It is possible that these effects may also be sensitive to the type of task used to assess anxiety-like behavior, as we had not previously observed an anxiety-like phenotype in LB reared animal on the elevated plus, elevated zero, or open field task (Goodwill et al., 2018). MS rearing resulted in a reversed pattern of effects, with males being affected only at adult time point, but not females. This sex-specific consequence of MS is in agreement with others and has been linked to changes in HPA axis programming (Papaioannou et al., 2002) and is potentially explained by sex-specific differences in maternal care during MS rearing (Moore and Morelli, 1979). Further characterization of the relationship between developmental changes in CORT and expression of behavioral markers of pathology may elucidate possible mechanisms of disease and stress-associated vulnerability and resilience.
Conclusions
In summary, a better understanding of the effects of ELA on early physical, behavioral, and neural developmental trajectories can provide important new insights into the way circuit development adapts to the early environment. Specifically, ELA supports a shift in the timing of physical weight gain, early visual maturation, motor development, and the maturation of a stress associated marker. Using two different forms of ELA, in both male and female mice, we find that the presence and degree of ELA effects depend upon the sex- and type of adversity experienced. These results highlight the complex nature of ELA as it relates to the developing organism and likely reflect adaptations of the system in response to the early caregiving environment. That said, additional research will be needed to further isolate the developmental mechanisms that are impacted by different adverse experiences and how males and females adapt differently to these early experiences. Such findings will not only reveal targets for earlier medical and therapeutic interventions but will also provide critical groundwork for individualized medicine.
Highlights:
Early life adversity in the form of maternal separation and limited bedding differentially alter trajectories of growth and motor milestone attainment.
The type of early life adversity experienced determines severity of effects on somatic growth, eye opening and basal stress hormone levels.
Both maternal separation and limited bedding lead to similar impairments in early motor behaviors.
Limited bedding, but not maternal separation, results in female-specific vulnerability to anxiety-like behaviors.
Maternal separation leads to adult-emergence of anxiety-like behaviors in males.
Acknowledgements:
This work was supported by grants from the National Institute of Health RO1-MH115914 1098 (KGB) and RO1-MH115049 (KGB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare no competing interests.
References:
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH, 2006. The enduring effects of abuse and related adverse experiences in childhood. Eur. Arch. Psychiatry Clin. Neurosci 256, 174–186. 10.1007/s00406-005-0624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joëls M, 2009. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem 92, 292–300. 10.1016/j.nlm.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Bailoo JD, Jordan RL, Garza XJ, Tyler AN, 2014. Brief and long periods of maternal separation affect maternal behavior and offspring behavioral development in C57BL/6 mice. Dev. Psychobiol 56, 674–685. 10.1002/dev.21135 [DOI] [PubMed] [Google Scholar]
- Bath KG, Manzano-Nieves G, Goodwill H, 2016. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav 82, 64–71. 10.1016/j.yhbeh.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ, 2012. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav. Dev 35, 838–846. 10.1016/j.infbeh.2012.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, 2005. Mechanisms of Late-Onset Cognitive Decline after Early-Life Stress. J. Neurosci 25, 9328–9338. 10.1523/JNEUROSCI.2281-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, 2004. Acceleration of Visual System Development by Environmental Enrichment. J. Neurosci 24, 4840–4848. 10.1523/JNEUROSCI.0845-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter T, Grecian SM, Reynolds RM, 2017. Sex differences in early-life programming of the hypothalamic–pituitary–adrenal axis in humans suggest increased vulnerability in females: a systematic review. J. Dev. Orig. Health Dis 8, 244–255. 10.1017/S204017441600074X [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H, 2008. Maternal Care and Hippocampal Plasticity: Evidence for Experience-Dependent Structural Plasticity, Altered Synaptic Functioning, and Differential Responsiveness to Glucocorticoids and Stress. J. Neurosci 28, 6037–6045. 10.1523/JNEUROSCI.0526-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Baram TZ, 2016. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41, 197–206. 10.1038/npp.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dubé CM, Rice CJ, Baram TZ, 2008. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. Off. J. Soc. Neurosci 28, 2903–2911. 10.1523/JNEUROSCI.0225-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley EJL, Demaestri C, Ganguly P, Honeycutt JA, Peterzell S, Rose N, Ahmed N, Holschbach M, Trivedi M, Brenhouse HC, 2019. Cross-Generational Transmission of Early Life Stress Effects on HPA Regulators and Bdnf Are Mediated by Sex, Lineage, and Upbringing. Front. Behav. Neurosci 13 10.3389/fnbeh.2019.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielewicz J, Hess G, 2014. Early life stress alters synaptic modification range in the rat lateral amygdala. Behav. Brain Res 265, 32–37. 10.1016/j.bbr.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Claessens SEF, Laboyrie JJL, Enthoven L, Oitzl MS, Champagne DL, de Kloet ER, 2011. The newborn rat’s stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors in context dependent fashion. Horm. Behav 60, 165–176. 10.1016/j.yhbeh.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E, 2001. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur. J. Neurosci 14, 675–689. 10.1046/j.0953-816x.2001.01685.x [DOI] [PubMed] [Google Scholar]
- de Almeida Magalhães T, Correia D, de Carvalho LM, Damasceno S, Brunialti Godard AL, 2018. Maternal separation affects expression of stress response genes and increases vulnerability to ethanol consumption. Brain Behav. 8, e00841 10.1002/brb3.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Garrido P, de Bias M, Mora F, 2007. Stress, prefrontal cortex and environmental enrichment: Studies on dopamine and acetylcholine release and working memory performance in rats. Behav. Brain Res 176, 267–273. 10.1016/j.bbr.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S, 2000. Stress-Induced Alterations in Corticotropin-Releasing Hormone and Vasopressin Gene Expression in the Paraventricular Nucleus during Ontogeny. Neuroendocrinology 71, 333–342. 10.1159/000054554 [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Dube SR, Giles WH, Felitti VJ, 2003. The relationship of exposure to childhood sexual abuse to other forms of abuse, neglect, and household dysfunction during childhood. Child Abuse Negl. 27, 625–639. 10.1016/s0145-2134(03)00105-4 [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH, 2001. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 286, 3089–3096. 10.1001/jama.286.24.3089 [DOI] [PubMed] [Google Scholar]
- Farkas J, Reglodi D, Gaszner B, Szogyi D, Horvath G, Lubics A, Tamas A, Frank F, Besirevic D, Kiss P, 2009. Effects of maternal separation on the neurobehavioral development of newborn Wistar rats. Brain Res. Bull 79, 208–214. 10.1016/j.brainresbull.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Feather-Schussler DN, Ferguson TS, 2016. A Battery of Motor Tests in a Neonatal Mouse Model of Cerebral Palsy. J. Vis. Exp. JoVE 10.3791/53569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. Am. J. Prev. Med 14, 245–258. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Fisher HL, Jones PB, Fearon P, Craig TK, Dazzan P, Morgan K, Hutchinson G, Doody GA, McGuffin P, Leff J, Murray RM, Morgan C, 2010. The varying impact of type, timing and frequency of exposure to childhood adversity on its association with adult psychotic disorder. Psychol. Med 40, 1967–1978. 10.1017/S0033291710000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ, 1999. Maternal Care, Gene Expression, and the Development of Individual Differences in Stress Reactivity. Ann. N. Y. Acad. Sci 896, 66–84. 10.1111/j.1749-6632.1999.tb08106.x [DOI] [PubMed] [Google Scholar]
- Gallo M, Shleifer DG, Godoy LD, Ofray D, Olaniyan A, Campbell T, Bath KG, 2019. Limited Bedding and Nesting Induces Maternal Behavior Resembling Both Hypervigilance and Abuse. Front. Behav. Neurosci 13 10.3389/fnbeh.2019.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, Brenhouse HC, 2015. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev. Cogn. Neurosci 11, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, Honeycutt JA, Rowe JR, Demaestri C, Brenhouse HC, 2019. Effects of early life stress on cocaine conditioning and AMPA receptor composition are sex-specific and driven by TNF. Brain. Behav. Immun 10.1016/j.bbi.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W, 2015. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cereb. Cortex N. Y. NY 25, 2919–2928. 10.1093/cercor/bhu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, Tottenham N, 2014. Maternal Buffering of Human Amygdala-Prefrontal Circuitry During Childhood but Not During Adolescence. Psychol. Sci 25, 2067–2078. 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N, 2013. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci 110, 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George ED, Bordner KA, Elwafi HM, Simen AA, 2010. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 11, 123 10.1186/1471-2202-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ, 1996. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr. Neurol 15, 114–119. 10.1016/0887-8994(96)00153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobinath AR, Mahmoud R, Galea LAM, 2015. Influence of sex and stress exposure across the lifespan on endophenotypes of depression: focus on behavior, glucocorticoids, and hippocampus. Front. Neurosci 8 10.3389/fnins.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, Gallo M, Lee H-I, Oyerinde E, Thomas Serre, Bath KG, 2018a. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology, 10.1038/s41386-018-0195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, Stevenson RJ, Theyel BB, Moore CI, Connors BW, Bath KG, 2018b. Early Life Stress Drives Sex-Selective Impairment in Reversal Learning by Affecting Parvalbumin Intemeurons in Orbitofrontal Cortex of Mice 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould Elizabeth, Woolley CS, Cameron HA, Daniels DC, McEwen BS, 1991. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J. Comp. Neurol 313, 486–493. 10.1002/cne.903130309 [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS, 1991. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J. Comp. Neurol 313, 479–485. 10.1002/cne.903130308 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM, 2007. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability, in: De Kloet ER, Oitzl MS, Vermetten E (Eds.), Progress in Brain Research, Stress Hormones and Post Traumatic Stress Disorder Basic Studies and Clinical Perspectives. Elsevier, pp. 137–149. 10.1016/S0079-6123(07)67010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt JA, Demaestri C, Peterzell S, Silveri MM, Cai X, Kulkarni P, Cunningham MG, Ferris CF, Brenhouse HC, 2020. Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. eLife 9, e52651 10.7554/eLife.52651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii- Hayashi N, Sasagawa T, Matsunaga W, Matsusue Y, Azuma C, Nishi M, 2013. Developmental Changes in Desensitisation of c-Fos Expression Induced by Repeated Maternal Separation in Pre-Weaned Mice. J. Neuroendocrinol 25, 158–167. 10.1111/j.1365-2826.2012.02377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullinger R, O’Riordan K, Burger C, 2015. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol. Learn. Mem 125, 126–134. 10.1016/j.nlm.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, 2010. Developing language in a developing body: the relationship between motor development and language development. J. Child Lang 37, 229–261. 10.1017/S0305000909990432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ, 2008. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154, 1132–1142. 10.1016/j.neuroscience.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata E, Kikusui T, Takeuchi Y, Mori Y, 2007. Fostering and environmental enrichment ameliorate anxious behavior induced by early weaning in Balb/c mice. Physiol. Behav 91, 318–324. 10.1016/j.physbeh.2007.03.013 [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ, 2009. The neuro-symphony of stress. Nat. Rev. Neurosci 10, 459–466. 10.1038/nrn2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakihana R, Moore JA, 1976. Circadian rhythm of corticosterone in mice: The effect of chronic consumption of alcohol. Psychopharmacologia 46, 301–305. 10.1007/BF00421118 [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG, 2002. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long–Evans rats. Pharmacol. Biochem. Behav 73, 131–140. 10.1016/S0091-3057(02)00781-5 [DOI] [PubMed] [Google Scholar]
- Kanatsou S, Ter Horst JP, Harris AP, Seckl JR, Krugers HJ, Joëls M, 2016. Effects of Mineralocorticoid Receptor Overexpression on Anxiety and Memory after Early Life Stress in Female Mice. Front. Behav. Neurosci 9 10.3389/fnbeh.2015.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R, 2011. Brain Plasticity and Behaviour in the Developing Brain. J. Can. Acad. Child Adolesc. Psychiatry 20, 265–276. [PMC free article] [PubMed] [Google Scholar]
- Leonard HC, Bedford R, Charman T, Elsabbagh M, Johnson MH, Hill EL, 2014. Motor development in children at risk of autism: A follow-up study of infant siblings. Autism 18, 281–291. 10.1177/1362361312470037 [DOI] [PubMed] [Google Scholar]
- Liston C, Gan W-B, 2011. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci 108, 16074–16079. 10.1073/pnas.1110444108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrí S, Mason GJ, Würbel H, 2004. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur. J. Neurosci 20, 1017–1024. 10.1111/j.1460-9568.2004.03541.x [DOI] [PubMed] [Google Scholar]
- Manzano Nieves G, Schilit Nitenson A, Lee H-I, Gallo M, Aguilar Z, Johnsen A, Bravo M, Bath KG, 2019. Early Life Stress Delays Sexual Maturation in Female Mice. Front. Mol. Neurosci 12 10.3389/fnmol.2019.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita AR, Pêgo JM, Summavielle T, Maciel P, Almeida OFX, Sousa N, 2007. Neurodevelopment milestone abnormalities in rats exposed to stress in early life. Neuroscience 147, 1022–1033. 10.1016/j.neuroscience.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA, 1979. Mother rats interact differently with male and female offspring. J. Comp. Physiol. Psychol 93, 677–684. 10.1037/h0077599 [DOI] [PubMed] [Google Scholar]
- Moussaoui N, Jacobs JP, Larauche M, Biraud M, Million M, Mayer E, Taché Y, 2017. Chronic Early-life Stress in Rat Pups Alters Basal Corticosterone, Intestinal Permeability, and Fecal Microbiota at Weaning: Influence of Sex. J. Neurogastroenterol. Motil 23, 135–143. 10.5056/jnm16105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui N, Larauche M, Biraud M, Molet J, Million M, Mayer E, Taché Y, 2016. Limited Nesting Stress Alters Maternal Behavior and In Vivo Intestinal Permeability in Male Wistar Pup Rats. PloS One 11, e0155037 10.1371/journal.pone.0155037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck EFG, Oosterink JE, Yam K-Y, de Vries LP, Schierbeek H, van Goudoever JB, Verkaik-Schakel R-N, Plantinga JA, Plosch T, Lucassen PJ, Korosi A, 2017. Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 31, 505–518. 10.1096/fj.201600834R [DOI] [PubMed] [Google Scholar]
- Nawaz A, Batool Z, Shazad S, Rafiq S, Afzal A, Haider S, 2018. Physical enrichment enhances memory function by regulating stress hormone and brain acetylcholinesterase activity in rats exposed to restraint stress. Life Sci. 207, 42–49. 10.1016/j.lfs.2018.05.049 [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bos K, Gunnar MR, Sonuga- Barke EJS, 2011. V. the Neurobiological Toll of Early Human Deprivation. Monogr. Soc. Res. Child Dev 76, 127–146. 10.1111/j.1540-5834.2011.00630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, Hasselt F.N. van, Manders EMM, Joëls M, Lucassen PJ, Krugers H, 2010. Severe Early Life Stress Hampers Spatial Learning and Neurogenesis, but Improves Hippocampal Synaptic Plasticity and Emotional Learning under High-Stress Conditions in Adulthood. J. Neurosci 30, 6635–6645. 10.1523/JNEUROSCI.0247-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opala EA, Verlezza S, Long H, Rusu D, Woodside B, Walker C-D, 2019. Experience of Adversity during a First Lactation Modifies Prefrontal Cortex Morphology in Primiparous Female Rats: Lack of Long Term Effects on a Subsequent Lactation. Neuroscience 417, 95–106. 10.1016/j.neuroscience.2019.08.022 [DOI] [PubMed] [Google Scholar]
- Orso R, Creutzberg KC, Wearick-Silva LE, Wendt Viola T, Tractenberg SG, Benetti F, Grassi-Oliveira R, 2019. How Early Life Stress Impact Maternal Care: A Systematic Review of Rodent Studies. Front. Behav. Neurosci 13 10.3389/fnbeh.2019.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, Handa RJ, 2017. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: sex differences in regulation of stress responsivity. Stress Amst. Neth 20, 476–494. 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A, Gerozissis K, Prokopiou A, Bolaris S, Stylianopoulou F, 2002. Sex differences in the effects of neonatal handling on the animal’s response to stress and the vulnerability for depressive behaviour. Behav. Brain Res 129, 131–139. 10.1016/S0166-4328(01)00334-5 [DOI] [PubMed] [Google Scholar]
- Pascual R, Zamora-León SP, 2007. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiol. Exp. (Warsz.) 67, 471–479. [DOI] [PubMed] [Google Scholar]
- Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact - 5th Edition [WWW Document], n.d. URL https://www.elsevier.com/books/paxinos-and-franklins-the-mouse-brain-in-stereotaxic-coordinates-compact/franklin/978-0-12-816159-3 (accessed 1.25.20). [Google Scholar]
- Peña CJ, Nestler EJ, Bagot RC, 2019. Environmental Programming of Susceptibility and Resilience to Stress in Adulthood in Male Mice. Front. Behav. Neurosci 13 10.3389/fnbeh.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Silver BD, Tschetter WW, Alam NM, Douglas RM, 2008. Experience-dependent plasticity from eye opening enables lasting, visual cortex-dependent enhancement of motion vision. J. Neurosci. Off. J. Soc. Neurosci 28, 9817–9827. 10.1523/JNEUROSCI.1940-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Pietrzak RH, El-Gabalawy R, Mackenzie CS, Sareen J, 2015. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry 14, 74–81. 10.1002/wps.20193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ, 2008. A Novel Mouse Model for Acute and Long-Lasting Consequences of Early Life Stress. Endocrinology 149, 4892–4900. 10.1210/en.2008-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M, 2014. The Behavioral and Immunological Impact of Maternal Separation: A Matter of Timing. Front. Behav. Neurosci 8 10.3389/fnbeh.2014.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Putignano E, Cancedda L, Landi S, Cirulli F, Berardi N, Maffei L, 2004. Enriched environment and acceleration of visual system development. Neuropharmacology, The Cytoskeleton and Synaptic Function 47, 649–660. 10.1016/j.neuropharm.2004.07.008 [DOI] [PubMed] [Google Scholar]
- Sanchez MM, 2006. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm. Behav 50, 623–631. 10.1016/j.yhbeh.2006.06.012 [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D, 2010. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev. Psychopathol 22, 45–53. 10.1017/S0954579409990253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, Howell BR, 2015. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc. Neurosci 10, 512–526. 10.1080/17470919.2015.1087426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ, 1986. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. Rev 11, 65–76. 10.1016/0165-0173(86)90010-X [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E, 1997. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J. Neurosci. Res 48, 334–341. [PubMed] [Google Scholar]
- Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS, 2003. The postnatal development of the hypothalamic–pituitary–adrenal axis in the mouse. Int. J. Dev. Neurosci. 21, 125–132. 10.1016/S0736-5748(03)00030-3 [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT, 1988. A multivariate statistical summary of synaptic plasticity measures in rats exposed to complex, social and individual environments. Brain Res. 441, 386–392. 10.1016/0006-8993(88)91420-5 [DOI] [PubMed] [Google Scholar]
- Smart JL, McMahon AC, Massey RF, Akbar G-NK, Warren MA, 1990. Evidence of non-maternally mediated acceleration of eye-opening in ‘enriched’ artificially reared rat pups. Dev. Brain Res 56, 141–143. 10.1016/0165-3806(90)90174-W [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB, 2004. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav. Brain Res 149, 209–215. 10.1016/S0166-4328(03)00216-X [DOI] [PubMed] [Google Scholar]
- van Bodegom M, Homberg JR, Henckens MJAG, 2017. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell. Neurosci 11 10.3389/fncel.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Whelan T, Levine S, 1998. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J. Neurosci. Off. J. Soc. Neurosci 18, 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, López JF, Levine S, 2006. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 1121, 83–94. 10.1016/j.brainres.2006.08.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Morris MJ, Raineki C, Roth TL, Sullivan RM, Taché Y, Baram TZ, 2017. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress 20, 421–448. 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-D, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W, Holsboer F, Deussing JM, Baram TZ, Stewart MG, Müller MB, Schmidt MV, 2011. Forebrain CRF1 Modulates Early-Life Stress-Programmed Cognitive Deficits. J. Neurosci 31, 13625–13634. 10.1523/JNEUROSCI.2259-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, 1963. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J. Neurophysiol 26, 1003–1017. 10.1152/jn.1963.26.6.1003 [DOI] [PubMed] [Google Scholar]