Abstract
Tumor metastasis comprises a series of coordinated events that culminate in dissemination of cancer cells to distant sites within the body: representing the greatest challenge impeding effective therapy of cancer and the leading cause of cancer-associated morbidity. Cancer cells exploit multiple genes and pathways to colonize to distant organs. These pathways are integrated and regulated at different levels by cellular- and extracellular-associated factors. Defining the genes and pathways that govern metastasis can provide new targets for therapeutic intervention. Melanoma differentiation associated gene-9 (mda-9) (also known as Syntenin-1 and SDCBP (Syndecan binding protein)) was identified by subtraction hybridization as a novel gene displaying differential temporal expression during differentiation of melanoma. MDA-9/Syntenin is an established Syndecan binding protein that functions as an adaptor protein. Expression of MDA-9/Syntenin is elevated at an RNA and protein level in a wide-range of cancers including melanoma, glioblastoma, neuroblastoma, and prostate breast and liver cancer. Expression is increased significantly in metastatic cancer cells as compared with non-metastatic cancer cells or normal cells, which make it an attractive target in treating cancer metastasis. In this review, we focus on the role and regulation of mda-9 in cancer progression and metastasis.
Keywords: mda-9, Syntenin, PDZ domain, Metastasis, Cancer
1. Introduction
Cancer represents a diverse collection of diseases in which transformed cells display uncontrolled proliferation and often invade nearby tissues (metastasis). Metastatic tumors can be distinguished from less aggressive and benign tumors, which do not spread and are curable [1]. The metastatic process involves the detachment of cancer cells from a primary tumor, invasion into the circulation and/or surrounding tissues and establishment of micrometastases in distant tissues/organs [2]. In aggressive forms of cancer characterized by metastasis, tumor cells must survive immune clearance and invade, proliferate and establish new blood vessels (undergo angiogenesis) in distant sites [3]. There has been significant recent progress in deciphering the molecular underpinnings of cancer progression, resulting in enhanced therapies culminating in increased patient survival. Technological advances have led to enhanced disease diagnosis at an early stage through molecular screening [4]. Targeted therapeutic strategies and immunotherapy have recsently resulted in profound clinical responses in specific subsets of patients [5,6]. Despite these advances, metastasis still remains a leading cause of morbidity and mortality in ~90% of patients with solid tumors and a detailed understanding of mechanism(s) of cancer metastasis development and molecules involved in this process is required to develop rationally-designed effective therapies.
Cancer is a multigene and multifactor disorder where many differentially regulated genes orchestrate disease pathogenesis, either in a positive or a negative manner [7]. In an earlier study, we applied a subtraction hybridization technique to terminally differentiating human melanoma cells [8, 23]. This experimental strategy involved treatment of metastatic human melanoma cells with recombinant human interferon beta and mezerein (MEZ) [8, 53], which resulted in irreversible growth arrest, and terminal cell differentiation. These changes in melanoma physiology were associated with changes in the expression of several novel genes that were designated melanoma differentiation-associated (mda) genes [8]. Some noteworthy mda genes are described in Table 1.
Table 1:
Selected melanoma differentiation associated (mda) genes with relevant anti- or pro-cancer and immune functions
| Gene | Functions | References |
|---|---|---|
| mda-5/IFIH1 | MDA-5 has RNA-dependent ATPase activity. Over expression of the mda-5 gene in cancer cells inhibits proliferation. MDA-5 also promotes anti-tumor immunity. | [9,10] |
| mda-6/P21/WAF1/CIP1 | MDA-6 is the cyclin-dependent kinase inhibitor (p21) that induces cell cycle arrest. | [11,12] |
| mda-7/IL-24 | Inhibits cancer-specific cell growth, induces toxic autophagy and cell death, activates the immune system to target cancer cells for destruction. | [8, 13–16] |
| mda-9/syntenin | Promotes cancer cell invasion, angiogenesis and metastasis, a “pro-metastatic gene” (Focus of this Review) | [17] |
Melanoma differentiation associated gene-9 (mda-9), also known as Syntenin-1 or Syndecan binding protein (SDCBP), is a member of the PDZ family of proteins (postsynaptic density protein PSD95/SAP90, drosophila large tumor suppressor DLGA, and zonula occludens 1 ZO-1) [18–20]. mda-9 was originally identified as a differentially regulated gene during induction of terminal differentiation of metastatic human melanoma. mda-9/syntenin was identified as a differentially expressed gene in highly aggressive HO-1 human melanoma cells treated with IFN-β and the protein kinase C activating, anti-leukemic drug Mezerein [21–23]. mda-9/syntenin is a major contributor to several important biological functions in cancers, i.e., invasion and metastasis, predominantly during advanced disease stages [17]. The expression level of MDA-9/Syntenin is higher in cancer cells as compared to normal cells [17,24,25]. Also, its expression is elevated even further in metastatic cancers or progressed disease stages as compared to benign initial phases of the disease [17]. MDA-9/Syntenin functions by interacting with an array of regulatory proteins predominantly via the conserved PDZ domains thereby serving as a scaffold or adaptor protein [26,27]. Based on bioinformatics analyses (http://cancergenome.nih.gov/), it has been shown that mda-9/syntenin is over expressed in many cancers [28,29]. Overexpression of mda-9/syntenin is evident in breast cancer [30], prostate cancer [31,25], metastatic melanoma [32–35], glioblastoma [36–39], head and neck cancers [40], gastric [41], liver cancer [42], urothelial carcinoma [43], and small cell lung cancer [44]. Immunohistochemical analyses confirm a significant increase in MDA-9/Syntenin protein expression in metastatic melanoma and other cancers [45]. Over expression of mda-9/syntenin increases migration and invasion of cancer cells, which correlates with a polarized distribution of actin and Epithelial Mesenchymal Transition (EMT) [46,47]. Genetic silencing or knockdown of mda-9/syntenin leads to a decrease in invasion of cancer cells [26]. Additionally, our group developed a specific pharmacological inhibitor of MDA-9/Syntenin, PDZ1i, which results in a decrease in cancer metastasis [39,31,48]. Experimental evidence suggests that mda-9/syntenin functions as a positive regulator of cancer metastasis, promoting disease aggressiveness [32]. In this review, we provide comprehensive information about the role of mda-9/syntenin in the metastatic disease process and also briefly discuss our current assessment of the various signaling pathways involved in this regulation.
2. MDA-9/Syntenin, Gene and Protein
The 2100-bp mda-9/syntenin gene is located at 8q12, the open reading frame (ORF) is 894-bp encoding a 298-amino acid protein with a 33k-Da predicted molecular mass [26]. This gene is highly conserved across species from mouse, rat, xenopus, and zebrafish [26]. The MDA-9/Syntenin protein has four domains, N-terminal domain (1–109-aa), PDZ1 domain (110–193-aa), PDZ2 domain (194–274-aa), and C-terminal domain (275–298-aa) (Fig. 1) [17]. Other than influencing the structure and stability of the MDA-9/Syntenin protein, the functions of the N- and C-terminal domains are not well understood. The two MDA-9/Syntenin PDZ domains are interlinked by 4-amino acids. In general, PDZ proteins control diverse cellular and physiological functions [8]. The PDZ1 and PDZ2 domains of MDA9/Syntenin share only 26% homology [17]. Crystal structures of the two MDA-9/Syntenin PDZ domains are similar and are arranged in a head-to-tail fashion [17] (Fig. 1). MDA-9/Syntenin acts as a scaffold protein by interacting with structurally diverse signaling proteins and regulates major signal transduction pathways in different cancers. The MDA-9/Syntenin PDZ2 domain binds and interacts with c-Src [49], whereas the PDZ1 domain binds to TGF-β [30], EGFR [39], and IGF-1R [25]. In prostate cancer MDA-9/Syntenin interacts with IGF-1R [25] and regulates prostate cancer metastasis. In glioblastoma, EGFR is the principal mediator of the function of MDA-9/Syntenin [39]. MDA-9/Syntenin regulates TGF-β which in turn regulates EMT and metastasis in breast cancer [30].
Figure 1:
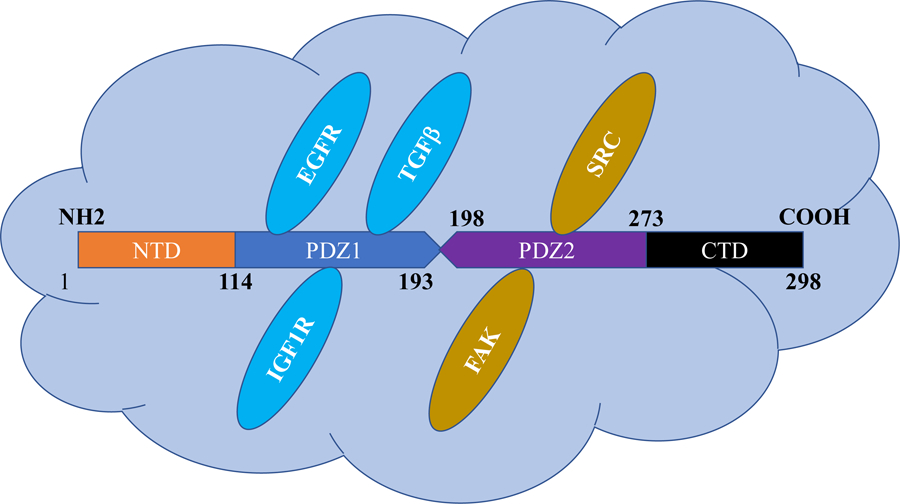
Protein structure of MDA-9/Syntenin: MDA-9/Syntenin protein is composed of 298-amino acids and contains four domains as shown in this figure. PDZ1 and PDZ2 domains are the signaling domains and the predominant binding domains for interacting protein partners. These two domains are surrounded by the NTD (N-terminal domain) and CTD (C-terminal domain).
The MDA-9/Syntenin protein has 3 isoforms, isoform 1 is 298-aa and is well-characterized both structurally and functionally [13]. Isoform 2 is 292-aa and lacks 12–17-aa from isoform 1. Isoform 3 is 297-aa and lacks the amino acid at position 87. The function of isoforms 2 and 3 have not been thoroughly investigated. MDA-9/Syntenin is conserved through evolution with sequence homology in Chimpanzee, Rhesus monkey, dog, cow, mouse, rat, chicken, zebrafish, and mosquito (atlasgeneticsoncology.org). Syntenin-2, another PDZ-domain containing protein shows 70% homology with MDA-9/Syntenin [17]. Syntenin-2 is the binding partner of Syndecan-2 and has two isoforms, (full length) and 2β (shorter version lacking 85-aa residues) [46]. Like MDA-9/Syntenin, Syntenin-2 interacts with PIP2 and ALIX and plays a role in cell division, nuclear PIP2 organization and cell survival [50]. The cellular function and physiology of Syntenin-2 is not well understood and requires further study.
3. Role and regulation of MDA-9/Syntenin in cancer
The cellular and biological functions of MDA-9/Syntenin have been studied in detail in cancer disease progression [17] and exosome biogenesis [51,52]. However, genetic regulation and the transcriptional regulation of MDA-9/Syntenin requires further analysis. Early studies demonstrate that IFN-gamma induces expression of MDA-9/Syntenin [53]. Extensive studies have shown that mda-9/syntenin is upregulated in cancer as compared to adjacent normal cells [25], however, the increased expression is not due to genomic amplification or hypomethylation [29]. Inhibition of PKCα suppresses MDA-9/Syntenin expression [24]. Additionally, MDA-9/Syntenin activates PKCα [47]. These results indicate a potential feedback loop between MDA-9/Syntenin and PKCα that requires further validation and mechanistic studies.
Raf kinase inhibitor protein (RKIP) is down regulated in melanoma and a negative correlation exists between RKIP and mda-9/syntenin [35]. RKIP binds to MDA-9/Syntenin to block its interaction with SRC/FAK complexes, ultimately deregulating MDA-9/Syntenin-induced invasion and decreasing metastasis [35]. Developing approaches to upregulate RKIP in melanoma might provide a means of mitigating the effects of MDA-9/Syntenin and suppressing its cancer-promoting activities.
MicroRNAs or miRNAs are noncoding small RNAs that regulate gene expression posttranscriptionally [54]. miR-216b [55] is expressed at significantly lower levels in breast cancer specimens as compared to adjacent normal tissues and targets mda-9/syntenin in breast cancer [55]. miR-216b over expression leads to a deregulation in MDA-9/Syntenin, ultimately negatively affecting invasion and metastasis [55]. This suggests that targeted overexpression of miR-216b in breast cancers might provide a therapeutic strategy for inhibiting MDA-9/Syntenin-promoted breast cancer aggressiveness. Bioinformatic analysis identified mda-9/syntenin as a transcriptional target of miR-139–3p [56]. This was validated in glioma cell lines, which showed that miR-139–3p regulates cell proliferation, migration, invasion and tumor growth [56] by down regulating mda-9/syntenin. The effects of overexpression of miR-139–3p was reversed by co-transfection with mda-9/syntenin. Another miRNA that has been shown to regulate mda-9/syntenin is miR-155 [57]. miR-155 is a negative regulator of the blood-brain barrier (BBB) [57]. In neurodegenerative disorders, permeability of the BBB is increased [57]. miR-155 regulates BBB through regulation of focal adhesion organization [57]. Ectopic expression of miR-155 causes a down regulation of mda-9/syntenin and increased paracellular permeability [57]. This also results in delocalization of ZO-1 and Vinculin in the cellular borders emphasizing the role of miR-155-MDA-9/Syntenin axis in cell permeability [57].
Post-translational modification of mda-9/syntenin has not been studied in detail. Two possible protein kinase C phosphorylation sites at amino acid 171 and 189 [58], and five casein kinase phosphorylation sites (at aa 6, 60, 97, 189 and 289) are predicted from earlier studies. Seven possible tyrosine phosphorylation sites are also predicted [17]. The validation and biological functions of these predicted phosphorylation sites require further experimentation.
5. MDA-9/Syntenin, Clinical Relevance with Tumor Metastasis
a. Melanoma
Metastatic malignant melanoma is the major cause of patient death from melanoma [59]. Although some remarkable therapeutic responses have been observed in a relatively small subset of patients with metastatic melanoma using immunotherapy (checkpoint inhibitors) [60], there is a dire need for new diagnostics for early detection and additional novel therapeutic approaches for metastatic melanoma that target the disease at a molecular level [61]. Metastasis-associated proteins were investigated by analyzing the proteomic profiles of primary cutaneous melanomas relative to matched lymph node metastases [62]. This approach minimized heterogeneity among tumor samples that might arise from different patients. Results of two-dimensional gel electrophoresis (2-DE) followed by proteomic analysis revealed that MDA-9/Syntenin is a differentially expressed protein [62]. In this array, another protein that was found to be overexpressed is GRP78. This increased expression of MDA-9/Syntenin and GRP78 was also observed in exosomes from metastatic melanoma patients [62]. Studies have shown that MDA-9/Syntenin protein physically interacts with GRP78, the pathophysiology of which requires further investigation [62]. This study predicted that MDA-9/Syntenin could provide a biomarker for melanoma metastasis [62]. MDA-9/Syntenin in the microenvironment also significantly affects melanoma growth and metastasis [63]. The growth of subcutaneously implanted B16 tumors and lung metastases in MDA-9/Syntenin knockout mice is suppressed [63]. Also, a deficiency of MDA-9/Syntenin in the microenvironment of the lungs suppresses tumor growth by regulating IL-17A (Interleukin 17A) expression and deregulates the recruitment of myeloid derived suppressor cells (MDSCs) and Th17 cells as compared to wild type mice [63]. Melanocyte-specific pten loss and BrafV600E mutations cause spontaneous melanoma development in mice and loss of MDA-9/Syntenin expression in this model significantly delays tumor initiation and suppresses cancer metastasis to the lymph nodes and lungs [63].
MDA-9/Syntenin influences cancer cell migration and invasion by regulating diverse signaling pathways, including FAK, Src and mitogen-activated protein kinase (MAPK) [34]], which results in NF-κB activation [34]. Inhibition of c-Src or mda-9/syntenin with small interfering RNA, deregulates NF-κB activation [34]. Deletion or point mutations in the binding domain of PDZ that prevent MDA-9/Syntenin binding with Src reveal that both PDZ domains, with PDZ2 being the active module, are required for activating downstream pathways, including p38 MAPK and NF-κB [34]. MAPK and NF-κB induction in turn activate membrane-type matrix metalloproteinase-1, which then activates MMP-2 and MMP-9 promoting migration and invasion of melanoma cancer cells [33]. MDA-9/Syntenin interacts with c-Src as a complex and cooperates with NF-κB to enhance migration and invasion of human melanoma cells [34]. The interaction of MDA-9/Syntenin with c-Src correlates with an increase in FAK/c-Src complexes and subsequent c-Src activation [34]. Pharmacological inhibition of c-Src or genetic knockdown of c-Src or mda-9/syntenin reduces the migration and anchorage-independent growth of melanoma cells and tumor cell dissemination in a human melanoma metastasis model in vivo [34]. These findings highlight MDA-9/Syntenin and its interacting partners as potential therapeutic targets for cancer metastasis [34].
Analysis of cutaneous melanomas and melanoma metastases using subtractive suppression hybridization reveals mda-9/syntenin as a differentially expressed gene [64]. Over expression of MDA-9/Syntenin protein is detected in melanoma metastases from patients by immunohistochemistry analysis [64]. Also, metastases contain a higher percentage of tumor cells positive for MDA-9/Syntenin as compared to non-metastasizing melanomas (Fig. 2). Gastric cancer tissues have also been shown to have higher mda-9/syntenin mRNA expression than their normal counterparts [64].
Figure 2:
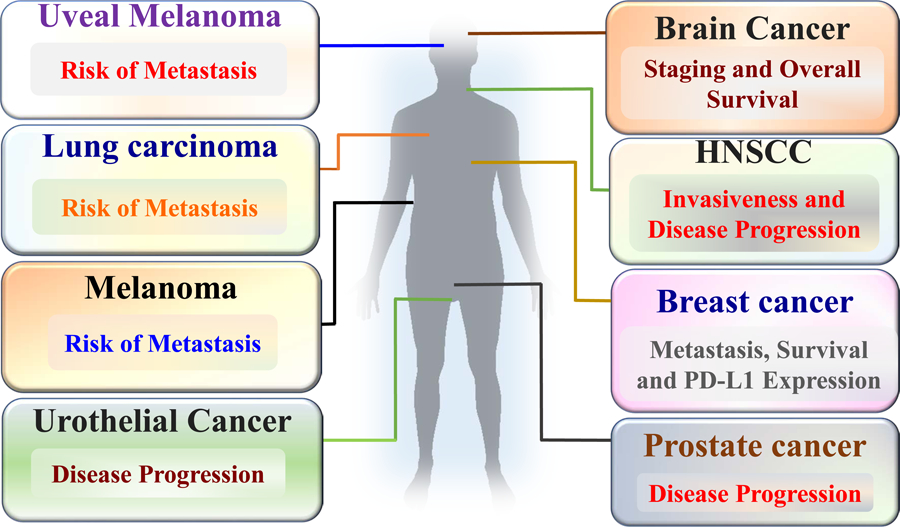
Clinical relevance of MDA-9/Syntenin in a wide spectrum of cancers: Expression analysis in patient’s samples and in vivo studies in animal models signify the role of MDA-9/Syntenin in cancer progression, risk of metastasis and overall survival (Figure adapted from Das SK et al, Advances in Cancer Research, Volume 144, 2019, Pages 137–191).
Uveal melanoma is an aggressive cancer that metastasizes to the liver in approximately 50% of patients, with a high lethality rate [45]. Analysis of expression profiles of primary human uveal melanomas confirm high expression of the mda-9/syntenin gene, which is even higher in patients with recurrence [45]. High expression of the mda-9/syntenin gene correlates positively with metastatic progression in datasets of uveal melanoma patients [45].
Tumor arrays and cancer cell line analyses indicate an inverse correlation between expression of MDA-9/Syntenin and RKIP during different stages of melanoma progression [35] (Fig. 2). MDA-9/Syntenin negatively regulates RKIP transcriptionally [35]. Furthermore, MDA-9/Syntenin interacts with RKIP physically suppressing FAK and c-Src phosphorylation [35]. Phosphorylation of FAK and Src are two of the initiator signaling cascades that regulate the MDA-9/Syntenin-mediated metastatic phenotype [35]. Over expression of RKIP in melanoma cells rescues the cells from MDA-9/Syntenin-mediated increase in invasion, and metastasis [35]. All of these studies document a role of MDA-9/Syntenin in melanoma metastasis and hence support the relevance of MDA-9/Syntenin as a therapeutic target for metastatic melanomas [63].
b. Breast cancer
Breast cancer is a consequence of the uncontrolled growth of transformed breast cells. Approximately 12% (1 in 8) of U.S. women will have breast cancer during their lifetime. In 2019, approximately 268,600 new cases of breast cancer were expected to be diagnosed in U.S. women with 62,930 new cases of non-invasive breast cancer. Although breast cancer is rare in men, it still can occur in approximately 1 in 1,000 men. Immunohistochemistry evaluation of normal and cancer breast tissues confirm positive MDA-9/Syntenin expression in 80.6% (n = 160) of breast cancer tissue samples, as compared to 13% (n = 23) of normal breast samples with a significant statistical index [65]. The expression of MDA-9/Syntenin is even higher in ER-negative breast cancer cells. MDA-9/Syntenin expression decreases following 17-β estradiol treatment in MCF-7 cells, which are estrogen-responsive. MDA-9/Syntenin regulates EMT in breast cancer by regulating the small GTPases, RhoA and Cdc42, via TGFβ1. MDA-9/Syntenin interacts with TGFβ1 via its PDZ1 domain [30]. MDA-9/Syntenin regulates lung metastasis of breast cancer cells and TGFβ1 is one of the mediators in this regulation [30].
miR-216b directly down regulates mda-9/syntenin expression transcriptionally and hence is a tumor suppressive miRNA [55]. miR-216b is down-regulated in advanced cases of metastatic breast cancer. Overexpression of miR-216b results in proliferation arrest, decreased migration and invasion in cell lines by down regulating mda-9/syntenin [55]. Analysis of breast cancer patient samples confirms a negative correlation between the expression of miR-216b and mda-9/syntenin [55].
Integrins are aberrantly expressed in cancer [66] and play differential roles in cancer progression and metastasis [66]. Another study showed that overexpression of mda-9/syntenin induces lung metastasis of breast cancer cells, and this process is mediated by integrin β1 and ERK1/2 [67].
c. Glioblastoma
MDA-9/Syntenin is highly over expressed in human GBM cell lines and GBM patient samples [39]. mda-9/syntenin expression increases with tumor stage and correlates with lower survival and poor response to chemo- and radio-therapy [39]. Knockdown of MDA-9/Syntenin sensitizes GBM cells to radiation and reduces post-radiation invasion gains [39]. Knockdown of MDA-9/Syntenin down-regulates radiation-induced Src and EGFRvIII signaling in GBM [39]. Genetic and pharmacological knockdown of MDA-9/Syntenin reduces invasion and metastasis of GBM cells following radiation treatment [39]. A chemical inhibitor of MDA-9/Syntenin activity, 113B7 or PDZ1i, developed and characterized by us [39], inhibits the binding of MDA-9/Syntenin to EGFRvIII.
d. Small cell lung cancer
Small cell lung cancer or SCLC is highly aggressive in nature with increased invasiveness and metastatic potential [68]. mda-9/syntenin expression is higher in small cell lung carcinomas than other neuroendocrine cancers, predicting that mda-9/syntenin expression may regulate aggressive forms of lung cancer [44]. As observed in other cancers, in SCLC mda-9/syntenin overexpression is associated with more advanced stage of the disease [44]. In vitro assays with SCLC cell lines also demonstrate similar changes in invasion and migration of cancer cells following mda-9/syntenin manipulation regulated by MMPs, p38 MAPK and PI3K/AKT, rather than NF-κB [44].
Urothelial cell carcinoma (UCC) is another cancer that progresses rapidly from initial early stage disease to invasive tumors [43]. MDA-9/Syntenin is over expressed in UCC patient samples [43] and EGFR signaling is one of the key pathways regulated by MDA-9/Syntenin, which is similar to GBM [43]. Overexpression of mda-9/syntenin in a noncancerous UCC cell line increases its proliferation and invasion of the cell line converting it into a cancerous state [43]. Alteration of β-catenin expression, and EMT proteins, Ecadherin, vimentin, claudin-1, and ZO-1, expression are observed in UCC [43] and MDA-9/Syntenin knockdown reverses these protein changes [43].
e. Head and neck squamous cell carcinoma
Elevated levels of MDA-9/Syntenin expression have also been reported in head and neck squamous cell carcinoma (HNSCC) tumors [40]. MDA-9/Syntenin expression is correlated with the stage and grade of the disease, and with lymph node metastases [40]. In vitro assays following knockdown of MDA-9/Syntenin in undifferentiated HNSCC cell lines induces squamous epithelial cell differentiation [40]. Knockdown of MDA-9/Syntenin leads to a disruption in angiogenesis and impaired tumor growth [40]. SPRR1B and VEGFR1 are key molecular targets of MDA-9/Syntenin in HNSCC differentiation and angiogenesis [40].
f. Prostate Cancer
Prostate cancer results from uncontrolled proliferation of epithelial cells in the prostate. Five year survival of patients with benign prostate cancer is almost 100%, whereas survival decreases when prostate cancer cells metastasize to distant organs [69]. Moreover, metastasis is the primary cause of death in prostate cancer patients [69]. Metastatic prostate cancer cells show increased expression of the mda-9/syntenin transcript and protein [31]. Analysis of Prostate cancer tissue sections indicates a strong correlation between expression of MDA-9/Syntenin protein and prostate cancer disease stage or Gleason stage [25]. MDA-9/Syntenin activates STAT3 phosphorylation, which in turn leads to an aberrant regulation in the expression pattern of MMP2 and MMP9, which regulates migration and invasion of cancer cells. IGFBP2 has been shown as a mediator of this regulation [25]. Also, MDA-9/Syntenin regulates angiogenesis in prostate cancer by upregulating angiogenic factors IGFBP2, IL6, IL8, and VEGFA, as also observed in other cancers [25].
5. Mediators of Metastasis
a. FAK/c-JNK and p38 pathway
MDA-9/Syntenin increases phosphorylation of focal adhesion kinase, c-Jun-NH2-kinase, and p38 MAPK [32]. Forced expression of MDA-9/Syntenin increases invasiveness and motility of cells and promotes cytoskeletal changes and intracellular signaling [32]. The organization of focal contacts is complex with several proteins involved, i.e., Vinculin, α-actinin, and Talins and Tyrosine kinases of the SRC family and FAK (Fig. 3) [32]. MDA-9/Syntenin colocalizes with FAK in melanoma cells, induces phosphorylation of FAK with total FAK protein levels remaining unchanged [32]. Because FAK activates the MAPK pathway, mda-9/syntenin induces phosphorylation of ERK, JNK, Akt, and p38 [32]. This activation causes migration and dissemination of cancer cells and also leads to the formation of focal adhesion complexes [32]. High levels of p38 contribute to a selective advantage promoting lung metastasis of melanoma cells [32].
Figure 3:

Role of MDA-9/Syntenin in different steps of metastasis: MDA-9/Syntenin regulates adhesion of cancer cells by regulating FAK/SRC signaling, MMPs, and EGFR, migration and invasion by Syndecans, β-Catenin, and MMPs and EMT mostly by E-Cadherin, ZO-1, and Snail. Multiple factors are regulated by MDA-9/Syntenin in angiogenesis, i.e., VEGFA, IGFBP2, and IL8.
b. STAT3:
STAT3 (signal transducer and activator of transcription 3) is a transcription factor, which is latent and predominantly located in the cytoplasm [70]. Tyrosine phosphorylation at amino acid position 705 causes activation, and nuclear translocation of STAT3. Constitutive activation of STAT3 in cancer leads to tumor growth and metastasis [71]. Protein kinases, oncogenes, and viruses activate STAT3 to cause cellular transformation [72]. STAT3 activation by SRC causes the transformation of NIH3T3 cells [73]. Targeting STAT3 blocks invasion and metastasis by regulating the matrix metalloproteinases (MMPs) [74]. Constitutively active STAT3 binds to the MMP2 promoter and transactivates MMP2 [74]. Also, in a similar manner STAT3 upregulates MMP9 and MMP1 (Fig. 4) [74]. STAT3 regulates cell motility and cellular migration, which has been validated by siRNA-mediated knockdown of STAT3 [74]. STAT3 also contributes to the survival of tumor cells in the circulation by regulating the levels of IL6 and TNFα and blocking NK (natural killer) cell activity [75]. MDA-9/Syntenin downregulates phosphorylation of STAT3 in prostate cancer and other cancers, thereby regulating the overall functions of STAT3 (Fig. 4) [25].
Figure 4:

MDA-9/Syntenin interacts with a broad spectrum of protein partners and regulates diverse signaling cascades: Following interaction with FAK/SRC complex MDA-9/Syntenin regulates p38MAPK, ultimately regulating MMPs. MDA-9/Syntenin also regulates VEGFA and MMPs via regulating STAT3. MDA-9/Syntenin interacts with EGFR which deregulates the GSK3β/β-Catenin pathway. Also, it interacts with ILK complex regulating IGFBP2 mediated by HIF1α. In addition, MDA-9/Syntenin regulates the TGFβ pathway.
c. NF-κB pathway
NF-κB transcription factor controls transcription of DNA, cytokine production, and survival of cells [76]. NF-κB plays diverse roles in infections, cancer, inflammation, and autoimmune diseases [76]. NF-κB controls cell proliferation and survival by regulating a plethora of genes [76]. Also, genes that regulate NF-κB expression are abnormally expressed in cancer, both in the frameworks of metastasis and immune evasion [77]. NF-κB consists of subunits of homodimer or heterodimer of the Rel family proteins [78]. These proteins in normal cells are inactive through binding to IκBα [78]. In a deregulated state, IκBα is phosphorylated and degraded, releasing NF-κB proteins that translocate into the nucleus where the protein complexes target expression of many oncogenes [78]. MDA-9/Syntenin induces NF-κB, leading to an increase in cancer cell migration and invasion [33]. This also leads to aberrant cell growth in an anchorage-independent manner [33]. MDA-9/Syntenin-mediated NF-κB regulation is through Src- and p38 MAPK-dependent pathways [33]. This activation enhances the expression of MMP2/MMP9 and positively regulates invasion (Fig. 4) [33]. MMPs are zinc-dependent endopeptidases, which degrade the indispensable components of the extracellular matrix (ECM) [79]. They play pivotal roles in normal development, i.e., during growth, uterine cycling, post-partum involution and tissue repair. Also from a different perspective, MMPs with excessive degradation of ECM cause Rheumatoid arthritis, osteoarthritis, atherosclerotic plaque rupture, periodontitis, autoimmune disorders of the skin, dermal photoaging, tumor metastasis and invasion [79].
d. Integrins:
Integrins are the most common transmembrane surface receptors on cells that assist cellextracellular matrix (ECM) adhesion [66]. They consist of an alpha subunit and beta subunit. There are 24 different integrins with 18 different types of alpha and 8 beta subunits in interacting in different combinations [66]. They are referred to as αVβ1, αVβ3, etc. Upon ligand stimulation, integrins activate various downstream signaling pathways that mediate cellular signals regulating cell cycle, cytoskeleton reorganization, survival of cells, healing and wound repair, and tumorigenesis.
Integrins are cell adhesion molecules that are crucial contributors to metastasis. Experimental evidence has identified several integrins that might play a role in cancer progression [66]. αVβ3, αVβ5, αVβ1, αVβ4, α4β1, and αVβ6 are the integrins that correlate with disease progression. In NSCLC the importance of integrins in development and progression of cancer is well studied. αVβ5, β1, β3 are prognostic markers of lymph node metastasis and recurrence and these integrins are over expressed in tumors [66]. A frequent association of bone metastasis with advanced prostate cancer is governed by integrinmediated interaction of metastatic cancer cells and the bone microenvironment. αVβ3 helps in attachment to bone microenvironment and subsequent growth of the tumor. Activated αVβ3 rescues circulating cancer cells and platelets enhancing their survival [80]. αVβ3 supports adhesion and migration to the bone matrix. Osteopontin is a secreted glycoprotein that is over expressed in a number of different cancers and promotes adhesion, migration, and metastasis by binding to αVβ3 and CD44 antigen.
Silencing of mda-9/syntenin genetically or pharmacologically down-regulates the integrins α6 and β4 [48]. A decreased level of α6 and β4 causes a deregulation in the activity of cofilins and matrix metalloproteinases leading to an inhibition in cell migration and invasion of neuroblastoma cancer cells [48]. This study further showed that overexpression of α6 and β4 in mda-9/syntenin silenced cells rescued the migration and invasion of these cancer cells. These studies support the hypothesis that mda-9/syntenin is a critical regulator of integrins in cancer [48].
e. Anoikis resistance, protective autophagy and EGFR
MDA-9/Syntenin expression correlates with protective autophagy and anoikis resistance in glioblastoma stem cells (GSCs) [37]. Silencing of mda-9/syntenin causes the autophagic death of GSCs. MDA-9/Syntenin-mediated autophagic regulation is dependent on the phosphorylation status of Bcl2 and EGFR signaling [37]. Also, MDA-9/Syntenin-mediated autophagic regulation is mediated by modification of FAK and PKC signaling. Phosphorylation of EGFR and Bcl2 is regulated dynamically by FAK and PKC, which in turn regulate the autophagic regulator molecules ATG5, LC3B, and LAMP1 [37]. Silencing of MDA-9/Syntenin deregulates this process leading to toxic autophagy under anoikis conditions [37].
PDZ1i, a MDA-9/Syntenin pharmacological inhibitor, was identified by NMR guided fragment-based drug-design [39] and evaluated therapeutically in prostate cancer [21], GBM [39] and Neuroblastoma [48] animal models. PDZ1i blocks the interaction of MDA-9/Syntenin and EGFRvIII. EGFRvIII is an oncogene, whose expression is increased with radiation, a widely used therapy for Glioblastoma [39]. Both pharmacological (PDZ1i), and genetic (mda-9/syntenin knockdown) targeting of MDA-9/Syntenin inhibits the invasion of cancer cells. The interesting and relevant outcome of this approach is that this therapy doesn’t harm normal cells. Also, PDZ1i sensitizes cancer cells to radiotherapy by modulating FAK and MMPs [39].
Overexpression of MDA-9/Syntenin in liver cancer cells increases their proliferation [42]. This is mediated by EGFR signaling. Specifically, use of EGFR siRNA in mda-9/syntenin overexpressed cells attenuated cell proliferation highlighting EGFR as a critical modulator of MDA-9/Syntenin-mediated proliferation of liver cancer cells [42].
Another cancer where mda-9/syntenin and EGFR signaling has been studied is UCC. MDA-9/Syntenin induces proliferation and invasion of UCC cell lines [43]. MDA-9/Syntenin interacts with EGFR in UCC cell lines and primary tumors [43]. Also, overexpression of MDA-9/Syntenin enhances EGFR, PI3K, Akt, and SRC expression. Thus, EGFR signaling is important for MDA-9/Syntenin-induced invasion and metastasis of cancer cells in various cancer contexts.
f. Exosomes:
Interest in exosomes is growing due to their critical roles in cellular signaling [81]. Exosomes are small-sized membranous vesicles secreted into body fluids [82]. In tumors, exosomes carry functional noncoding RNAs, miRNAs, RNAs, and proteins that alter the micro-environment promoting tumor growth [83]. Exosomes are one of the mediators of cancer metastasis that regulate the pathophysiology of the disease and act as transporters of biomolecules [84], both locally and systemically in cancer cells and cancer-associated cells. Proteomic analyses with melanoma exosomal proteins indicate differential expression of GRP78, HAPLN1, Syntenin-1 (which is MDA-9/Syntenin), Annexin A1 and A2 in advanced stages of metastasis [85]. Normal melanocytes become invasive when treated with melanoma cell-derived exosomes [85]. Biogenesis of exosomes or their secreted vesicles requires further clarification [86]. Syndecans and their adaptor protein, MDA-9/Syntenin, regulate the biogenesis of exosomes [84]. MDA-9/Syntenin physically interacts with ALIX supporting the intraluminal budding from the endosomal membranes [50]. MDA-9/Syntenin regulates Ang2 in exosomes, hence, also controlling angiogenesis [87]. Oncogenic Src also regulates the biogenesis of exosomes and induces its secretion [84]. Exosomes derived from Src-depleted cells show a significant decrease in syntenin-1 (MDA-9/Syntenin), ALIX, and CD63 compared with exosomes secreted by control cells [84]. Exosomes derived from syntenin-1 (MDA-9/Syntenin) knockout but Src-overexpressed cells fail to stimulate the migration of HUVEC cells suggesting that Src functions on exosomes specifically through the syntenin-1 (MDA-9/Syntenin) pathway [61].
g. EMT
Epithelial cells lose their cell polarity, gain invasive and migratory properties to convert into mesenchymal cells by the process termed EMT [88]. EMT is essential for cancer metastasis besides their roles in numerous developmental processes including mesoderm formation and neural tube formation, wound healing, and organ fibrosis [88].
The TGFβ receptor phosphorylates Smad2 and Smad3 [89]. These two factors form a trimer, transfer into the nucleus, and interact/activate multiple transcription factors that again activate mesenchymal genes or suppress epithelial genes [89]. TGFβ can also work in Smad-independent pathways through Rho GTPases [90]. MDA-9/Syntenin interacts with TGFβ-R1 and not R2 [30]. There is a correlation in the expression pattern of MDA-9/Syntenin and p-Smad2 in clinical samples (Fig. 4) [17]. Knockdown of MDA-9/Syntenin enhances the expression of E-cadherin [30]. MDA-9/Syntenin upregulates Slug thereby mediating suppression of E-cadherin to induce EMT (Fig. 4) [91]. MDA-9/Syntenin interacts with Slug, further recruiting HDACs and then translocate into the nucleus [91]. Also, mda-9/syntenin and slug expression patterns positively correlate in existing databases of clinical samples [91].
h. Angiogenesis:
An essential component of tumor progression and metastasis is the formation and recruitment of new blood vessels, i.e., angiogenesis [92]. Blood vessels provide nutrients and other essential materials to tumor cells. Also, these vessels are the main site for the tumor cells to exit the primary tumor site and enter the circulation [92]. High vascularity in tumors is a prognostic marker for the aggressiveness and metastatic potential of the tumor. If tumor blood vessels decrease after treatment, tumor regression is often evident. Based on this consideration, a number of angiogenic factors and angiogenic inhibitors are being studied [92]. The primary angiogenic factor is fibroblast growth factor (FGF) followed by vascular endothelial growth factor (VEGF) and others [93]. These factors are produced by host cells, i.e., macrophages, mast cells, and lymphocytes, or the tumor cells themselves. Many tumor cells are shed into the blood stream, most of which are cleared by the immune system. In this regard, the chances of metastasis development may be directly related to the number of tumor cells being shed into the bloodstream.
Several studies describe a role of angiogenic factors in tumor relapse, metastasis, and poor prognosis. Renal cancer patients with increased levels of FGF show poor survival [94], which is similar to VEGF in breast cancer patients. In support of this study, tumors producing multiple angiogenic factors result in excessive growth of primary tumors. Similar results have been shown in lung cancer, prostate cancer, ovarian cancer, and head and neck cancer [95].
Many of the chemotherapeutic agents, i.e., taxol, tamoxifen, and Adriamycin, block angiogenesis [96]. Also, chemotherapy when combined with an angiogenic inhibitor produces a synergistic effect. Some well-described angiogenesis inhibitors are angiostatin, endostatin, thalidomide, and TNP-470 [96]. Many of these newer drugs were studied in animal models and have not yet reached the final stage of entry into the clinic. The other problem with anti-angiogenic therapies is that they do not guarantee complete elimination of the tumor, but rather reduce the size of the tumor. Since inhibition of mda-9/syntenin blocks angiogenesis (Fig. 4) [97], targeting MDA-9/Syntenin or its downstream pathways may help provide a therapeutically beneficial endpoint.
6. Limitations, Conclusions and Future Prospective
Current strategies commonly used in cancer therapeutics include targeted cancer therapies, immunotherapies, and combinatorial therapies. Targeted cancer therapy is dependent-on targeting a specific gene or protein, or a specific pathway that is deregulated in a specific cancer (this is essentially a personalized approach to therapy). For this strategy to achieve its objective it is critical to identify specific genes or proteins that are differentially regulated in normal vs. cancer cells. MDA-9/Syntenin is differentially expressed, i.e., highly overexpressed in cancer, and induces metastases to distant organ sites [97]. Also recent studies shows that MDA-9/Syntenin regulates chemoresistance of cancer stem cells [98]. Therefore, in principle, MDA-9/Syntenin is an attractive molecular target for developing cancer therapies. Based on the oncogenic and/or the pro-metastatic nature of MDA-9/Syntenin, this gene and its protein can also be used as a biomarker for disease aggressiveness. MDA-9/Syntenin is a scaffold protein and the majority of its functions are dependent on its interaction with protein partners thereby regulating multiple distinct pathways in cancer metastasis. Further in-depth studies of MDA-9/Syntenin in the context of its partner selection and functional roles in different cancer contexts are required to fully comprehend the mechanisms of action and consequences of this multifunctional molecule.
As described above, mda-9/syntenin regulates exosome biogenesis [84], and therefore might provide a target for the inhibition of exosome-mediated tumor development, which is an area that requires further experimental confirmation. The tumor microenvironment is also a key regulator of the successful growth and metastasis of cancer cells [99]. Host mda-9/syntenin regulates the ability of subcutaneously implanted B16 tumor cells to grow and metastasize to the lungs [63]. The role of mda-9/syntenin in the tumor microenvironment remains enigmatic, where this gene is ubiquitously expressed in organs that serve as metastatic niches, e.g., lung, liver, lymph nodes, and brain [17]. Environmental mda-9/syntenin has been found to play a critical role in melanoma growth and metastasis [63]. To fully understand this relationship will require expanded research. Another area that requires additional scrutiny, which is relevant to therapeutic applications, is mda-9/syntenin-mediated immune regulation that activates the immune system against cancer.
Based on the structure and known functions of MDA-9/Syntenin, a small molecule pharmacological inhibitor named PDZ1i has been synthesized and used therapeutically in different cancer cell lines and in in vivo mouse models of cancer [97,100]. The absorption, distribution, metabolism and excretion (ADME) and pharmacokinetic properties of PDZ1i are promising, however, further work is needed to potentially move this novel agent forward from the laboratory into the clinic. PDZ1i potently inhibits cancer cell invasion without inducing tumor cell toxicity, providing a novel paradigm for cancer therapy, “anti-invasive therapy”. However, when PDZ1i is combined with an additional chemotherapeutic agent or radiation, “anti-invasion therapy” is converted into “cytotoxic therapy”, resulting in the induction of apoptosis and/or toxic autophagy selectively in cancer cells. These observations suggest that combinatorial therapies with PDZ1i may hold promise for more effective therapeutic outcomes in the treatment of primary and metastatic cancers.
Acknowledgments
Funding information: We thank the members of the Fisher and Sarkar laboratories for their contributions to our understanding of MDA-9/Syntenin-1/SDCBP that serve as a basis for this review. The present studies were supported in part by NCI R01 CA244993 (DS and PBF), the National Foundation for Cancer Research (NFCR) (PBF) and a sponsored research agreement from InVaMet Therapeutics, Inc. (IVMT) (SKD). DS is the Harrison Foundation Distinguished Professor in Cancer Research. PBF holds the Thelma Newmeyer Corman Chair in Oncology.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interests: PBF is co-founder, CEO and has an ownership interest in InVaMet Therapeutics, Inc. (IVMT). VCU and the Sanford Burnham Prebys Medical Discovery Institute also have an equity interest in IVMT. SD is recipient of a Sponsored Research Agreement between VCU and IVMT.
References:
- 1.Proverbs-Singh T, Feldman JL, Morris MJ, Autio KA, & Traina TA (2015). Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr Relat Cancer, 22(3), R87–R106, doi: 10.1530/ERC-14-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Zijl F, Krupitza G, & Mikulits W (2011). Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res, 728(1–2), 23–34, doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seyfried TN, & Huysentruyt LC (2013). On the origin of cancer metastasis. Crit Rev Oncog, 18(1–2), 43–73, doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holloway CM, Easson A, Escallon J, Leong WL, Quan ML, Reedjik M, et al. (2010). Technology as a force for improved diagnosis and treatment of breast disease. Can J Surg, 53(4), 268–277. [PMC free article] [PubMed] [Google Scholar]
- 5.Castella M, Fernandez de Larrea C, & Martin-Antonio B (2018). Immunotherapy: A Novel Era of Promising Treatments for Multiple Myeloma. Int J Mol Sci, 19(11), doi: 10.3390/ijms19113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Y, Kumar AB, Finnes H, Markovic SN, Park S, Dronca RS, et al. (2018). Combining Immune Checkpoint Inhibitors With Conventional Cancer Therapy. Front Immunol, 9, 1739, doi: 10.3389/fimmu.2018.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavel J (2007). Progress in the epidemiological understanding of gene-environment interactions in major diseases: cancer. C R Biol, 330(4), 306–317, doi: 10.1016/j.crvi.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, & Fisher PB (1996). The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U S A, 93(17), 9160–9165, doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, & Fisher PB (2002). mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci U S A, 99(2), 637–642, doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Wang H, Li X, Guo C, Yuan F, Fisher PB, et al. (2016). Activation of the MDA-5-IPS-1 Viral Sensing Pathway Induces Cancer Cell Death and Type I IFN-Dependent Antitumor Immunity. Cancer Res, 76(8), 2166–2176, doi: 10.1158/0008-5472.CAN-15-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamloo B, & Usluer S (2019). p21 in Cancer Research. Cancers (Basel), 11(8), doi: 10.3390/cancers11081178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freemerman AJ, Vrana JA, Tombes RM, Jiang H, Chellappan SP, Fisher PB, et al. (1997). Effects of antisense p21 (WAF1/CIP1/MDA6) expression on the induction of differentiation and drug-mediated apoptosis in human myeloid leukemia cells (HL-60). Leukemia, 11(4), 504–513, doi: 10.1038/sj.leu.2400625. [DOI] [PubMed] [Google Scholar]
- 13.Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, et al. (2014). MDA-7/IL-24: multifunctional cancer killing cytokine. Adv Exp Med Biol, 818, 127–153, doi: 10.1007/978-1-4471-6458-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menezes ME, Bhoopathi P, Pradhan AK, Emdad L, Das SK, Guo C, et al. (2018). Role of MDA-7/IL-24 a Multifunction Protein in Human Diseases. Adv Cancer Res, 138, 143–182, doi: 10.1016/bs.acr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan AK, Bhoopathi P, Talukdar S, Scheunemann D, Sarkar D, Cavenee WK, et al. (2019). MDA-7/IL-24 regulates the miRNA processing enzyme DICER through downregulation of MITF. Proc Natl Acad Sci U S A, 116(12), 5687–5692, doi: 10.1073/pnas.1819869116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradhan AK, Talukdar S, Bhoopathi P, Shen XN, Emdad L, Das SK, et al. (2017). mda-7/IL-24 Mediates Cancer Cell-Specific Death via Regulation of miR-221 and the Beclin-1 Axis. Cancer Res, 77(4), 949–959, doi: 10.1158/0008-5472.CAN-16-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das SK, Sarkar D, Emdad L, & Fisher PB (2019). MDA-9/Syntenin: An emerging global molecular target regulating cancer invasion and metastasis. Adv Cancer Res, 144, 137–191, doi: 10.1016/bs.acr.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Kang BS, Cooper DR, Devedjiev Y, Derewenda U, & Derewenda ZS (2003). Molecular roots of degenerate specificity in syntenin’s PDZ2 domain: reassessment of the PDZ recognition paradigm. Structure, 11(7), 845–853, doi: 10.1016/s0969-2126(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 19.Kang BS, Cooper DR, Jelen F, Devedjiev Y, Derewenda U, Dauter Z, et al. (2003). PDZ tandem of human syntenin: crystal structure and functional properties. Structure, 11(4), 459–468, doi: 10.1016/s0969-2126(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, & Zheng JJ (2010). PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal, 8, 8, doi: 10.1186/1478-811X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang F, Adelman J, Jiang H, Goldstein NI, & Fisher PB (1999). Identification and temporal expression pattern of genes modulated during irreversible growth arrest and terminal differentiation in human melanoma cells. Oncogene, 18(23), 3546–3552, doi: 10.1038/sj.onc.1202715. [DOI] [PubMed] [Google Scholar]
- 22.Lin JJ, Jiang H, & Fisher PB (1998). Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene, 207(2), 105–110, doi: 10.1016/s0378-1119(97)00562-3. [DOI] [PubMed] [Google Scholar]
- 23.Huang F, Adelman J, Jiang H, Goldstein NI, & Fisher PB (1999). Differentiation induction subtraction hybridization (DISH): a strategy for cloning genes displaying differential expression during growth arrest and terminal differentiation. Gene, 236(1), 125–131, doi: 10.1016/s0378-1119(99)00244-9. [DOI] [PubMed] [Google Scholar]
- 24.Kegelman TP, Das SK, Emdad L, Hu B, Menezes ME, Bhoopathi P, et al. (2015). Targeting tumor invasion: the roles of MDA-9/Syntenin. Expert Opin Ther Targets, 19(1), 97–112, doi: 10.1517/14728222.2014.959495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das SK, Pradhan AK, Bhoopathi P, Talukdar S, Shen XN, Sarkar D, et al. (2018). The MDA-9/Syntenin/IGF1R/STAT3 Axis Directs Prostate Cancer Invasion. Cancer Res, 78(11), 2852–2863, doi: 10.1158/0008-5472.CAN-17-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Li S, Wang K, & Wan X (2019). A PDZ Protein MDA-9/Syntenin: As a Target for Cancer Therapy. Comput Struct Biotechnol J, 17, 136–141, doi: 10.1016/j.csbj.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar D, Boukerche H, Su ZZ, & Fisher PB (2008). mda-9/Syntenin: more than just a simple adapter protein when it comes to cancer metastasis. Cancer Res, 68(9), 3087–3093, doi: 10.1158/0008-5472.CAN-07-6210. [DOI] [PubMed] [Google Scholar]
- 28.Das SK, Bhutia SK, Kegelman TP, Peachy L, Oyesanya RA, Dasgupta S, et al. (2012). MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci (Landmark Ed), 17, 1–15, doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- 29.Bacolod MD, Das SK, Sokhi UK, Bradley S, Fenstermacher DA, Pellecchia M, et al. (2015). Examination of Epigenetic and other Molecular Factors Associated with mda-9/Syntenin Dysregulation in Cancer Through Integrated Analyses of Public Genomic Datasets. Adv Cancer Res, 127, 49–121, doi: 10.1016/bs.acr.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menezes ME, Shen XN, Das SK, Emdad L, Sarkar D, & Fisher PB (2016). MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor beta1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget, 7(49), 80175–80189, doi: 10.18632/oncotarget.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das SK, Kegelman TP, Pradhan AK, Shen XN, Bhoopathi P, Talukdar S, et al. (2019). Suppression of Prostate Cancer Pathogenesis Using an MDA-9/Syntenin (SDCBP) PDZ1 Small-Molecule Inhibitor. Mol Cancer Ther, 18(11), 1997–2007, doi: 10.1158/1535-7163.MCT-18-1019. [DOI] [PubMed] [Google Scholar]
- 32.Boukerche H, Su ZZ, Emdad L, Baril P, Balme B, Thomas L, et al. (2005). mda-9/Syntenin: a positive regulator of melanoma metastasis. Cancer Res, 65(23), 10901–10911, doi: 10.1158/0008-5472.CAN-05-1614. [DOI] [PubMed] [Google Scholar]
- 33.Boukerche H, Su ZZ, Emdad L, Sarkar D, & Fisher PB (2007). mda-9/Syntenin regulates the metastatic phenotype in human melanoma cells by activating nuclear factor-kappaB. Cancer Res, 67(4), 1812–1822, doi: 10.1158/0008-5472.CAN-06-3875. [DOI] [PubMed] [Google Scholar]
- 34.Boukerche H, Su ZZ, Prevot C, Sarkar D, & Fisher PB (2008). mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc Natl Acad Sci U S A, 105(41), 15914–15919, doi: 10.1073/pnas.0808171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das SK, Bhutia SK, Sokhi UK, Azab B, Su ZZ, Boukerche H, et al. (2012). Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res, 72(23), 6217–6226, doi: 10.1158/0008-5472.CAN-12-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talukdar S, Pradhan AK, Bhoopathi P, Shen XN, August LA, Windle JJ, et al. (2018). Regulation of protective autophagy in anoikis-resistant glioma stem cells by SDCBP/MDA-9/Syntenin. Autophagy, 14(10), 1845–1846, doi: 10.1080/15548627.2018.1502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talukdar S, Pradhan AK, Bhoopathi P, Shen XN, August LA, Windle JJ, et al. (2018). MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc Natl Acad Sci U S A, 115(22), 5768–5773, doi: 10.1073/pnas.1721650115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das SK, Sarkar D, Cavenee WK, Emdad L, & Fisher PB (2019). Rethinking Glioblastoma Therapy: MDA-9/Syntenin Targeted Small Molecule. ACS Chem Neurosci, 10(3), 1121–1123, doi: 10.1021/acschemneuro.9b00016. [DOI] [PubMed] [Google Scholar]
- 39.Kegelman TP, Wu B, Das SK, Talukdar S, Beckta JM, Hu B, et al. (2017). Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proc Natl Acad Sci U S A, 114(2), 370–375, doi: 10.1073/pnas.1616100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oyesanya RA, Bhatia S, Menezes ME, Dumur CI, Singh KP, Bae S, et al. (2014). MDA-9/Syntenin regulates differentiation and angiogenesis programs in head and neck squamous cell carcinoma. Oncoscience, 1(11), 725–737, doi: 10.18632/oncoscience.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koo TH, Lee JJ, Kim EM, Kim KW, Kim HD, & Lee JH (2002). Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene, 21(26), 4080–4088, doi: 10.1038/sj.onc.1205514. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Zhang X, Lv Y, Xiang J, & Shi J (2014). Overexpression of syntenin enhances hepatoma cell proliferation and invasion: potential roles in human hepatoma. Oncol Rep, 32(6), 2810–2816, doi: 10.3892/or.2014.3498. [DOI] [PubMed] [Google Scholar]
- 43.Dasgupta S, Menezes ME, Das SK, Emdad L, Janjic A, Bhatia S, et al. (2013). Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin Cancer Res, 19(17), 4621–4633, doi: 10.1158/1078-0432.CCR-13-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim WY, Jang JY, Jeon YK, Chung DH, Kim YG, & Kim CW (2014). Syntenin increases the invasiveness of small cell lung cancer cells by activating p38, AKT, focal adhesion kinase and SP1. Exp Mol Med, 46, e90, doi: 10.1038/emm.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangemi R, Mirisola V, Barisione G, Fabbi M, Brizzolara A, Lanza F, et al. (2012). Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression. PLoS One, 7(1), e29989, doi: 10.1371/journal.pone.0029989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, et al. (1997). Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci U S A, 94(25), 13683–13688, doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirbec H, Martin S, & Henley JM (2005). Syntenin is involved in the developmental regulation of neuronal membrane architecture. Mol Cell Neurosci, 28(4), 737–746, doi: 10.1016/j.mcn.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Bhoopathi P, Pradhan AK, Bacolod MD, Emdad L, Sarkar D, Das SK, et al. (2019). Regulation of neuroblastoma migration, invasion, and in vivo metastasis by genetic and pharmacological manipulation of MDA-9/Syntenin. Oncogene, 38(41), 6781–6793, doi: 10.1038/s41388-019-0920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boukerche H, Aissaoui H, Prevost C, Hirbec H, Das SK, Su ZZ, et al. (2010). Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene, 29(21), 3054–3066, doi: 10.1038/onc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, et al. (2014). Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun, 5, 3477, doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 51.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol, 14(7), 677–685, doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 52.Hurley JH, & Odorizzi G (2012). Get on the exosome bus with ALIX. Nat Cell Biol, 14(7), 654–655, doi: 10.1038/ncb2530. [DOI] [PubMed] [Google Scholar]
- 53.Fisher PB, Prignoli DR, Hermo H Jr., Weinstein IB, & Pestka S (1985). Effects of combined treatment with interferon and mezerein on melanogenesis and growth in human melanoma cells. J Interferon Res, 5(1), 11–22, doi: 10.1089/jir.1985.5.11. [DOI] [PubMed] [Google Scholar]
- 54.Pradhan AK, Emdad L, Das SK, Sarkar D, & Fisher PB (2017). The Enigma of miRNA Regulation in Cancer. Adv Cancer Res, 135, 25–52, doi: 10.1016/bs.acr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Jana S, Sengupta S, Biswas S, Chatterjee A, Roy H, & Bhattacharyya A (2017). miR-216b suppresses breast cancer growth and metastasis by targeting SDCBP. Biochem Biophys Res Commun, 482(1), 126–133, doi: 10.1016/j.bbrc.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Tian W, Wu W, Li X, Rui X, & Wu Y (2019). MiRNA-139–3p inhibits the proliferation, invasion, and migration of human glioma cells by targeting MDA-9/syntenin. Biochem Biophys Res Commun, 508(1), 295–301, doi: 10.1016/j.bbrc.2018.11.144. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, et al. (2014). MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J, 28(6), 2551–2565, doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 58.Hwangbo C, Kim J, Lee JJ, & Lee JH (2010). Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase Calpha and the PDZ adapter protein mda-9/Syntenin. Cancer Res, 70(4), 1645–1655, doi: 10.1158/0008-5472.CAN-09-2447. [DOI] [PubMed] [Google Scholar]
- 59.Sandru A, Voinea S, Panaitescu E, & Blidaru A (2014). Survival rates of patients with metastatic malignant melanoma. J Med Life, 7(4), 572–576. [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss SA, Wolchok JD, & Sznol M (2019). Immunotherapy of Melanoma: Facts and Hopes. Clin Cancer Res, 25(17), 5191–5201, doi: 10.1158/1078-0432.CCR-18-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, & Sheikh MS (2014). Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol Cell Pharmacol, 6(3), 228. [PMC free article] [PubMed] [Google Scholar]
- 62.Guan M, Chen X, Ma Y, Tang L, Guan L, Ren X, et al. (2015). MDA-9 and GRP78 as potential diagnostic biomarkers for early detection of melanoma metastasis. Tumour Biol, 36(4), 2973–2982, doi: 10.1007/s13277-014-2930-9. [DOI] [PubMed] [Google Scholar]
- 63.Das SK, Guo C, Pradhan AK, Bhoopathi P, Talukdar S, Shen XN, et al. (2016). Knockout of MDA-9/Syntenin (SDCBP) expression in the microenvironment dampens tumor-supporting inflammation and inhibits melanoma metastasis. Oncotarget, 7(30), 46848–46861, doi: 10.18632/oncotarget.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helmke BM, Polychronidis M, Benner A, Thome M, Arribas J, & Deichmann M (2004). Melanoma metastasis is associated with enhanced expression of the syntenin gene. Oncol Rep, 12(2), 221–228. [PubMed] [Google Scholar]
- 65.Qian XL, Li YQ, Yu B, Gu F, Liu FF, Li WD, et al. (2013). Syndecan binding protein (SDCBP) is overexpressed in estrogen receptor negative breast cancers, and is a potential promoter for tumor proliferation. PLoS One, 8(3), e60046, doi: 10.1371/journal.pone.0060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desgrosellier JS, & Cheresh DA (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer, 10(1), 9–22, doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Hong Q, Shi P, Liu Z, Luo J, & Shao Z (2013). Elevated expression of syntenin in breast cancer is correlated with lymph node metastasis and poor patient survival. Breast Cancer Res, 15(3), R50, doi: 10.1186/bcr3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demedts IK, Vermaelen KY, & van Meerbeeck JP (2010). Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J, 35(1), 202–215, doi: 10.1183/09031936.00105009. [DOI] [PubMed] [Google Scholar]
- 69.Madu CO, & Lu Y (2010). Novel diagnostic biomarkers for prostate cancer. J Cancer, 1, 150–177, doi: 10.7150/jca.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitchell TJ, & John S (2005). Signal transducer and activator of transcription (STAT) signalling and T-cell lymphomas. Immunology, 114(3), 301–312, doi: 10.1111/j.1365-2567.2005.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamran MZ, Patil P, & Gude RP (2013). Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int, 2013, 421821, doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. (2009). Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci, 1171, 59–76, doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai YT, Su YH, Fang SS, Huang TN, Qiu Y, Jou YS, et al. (2000). Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol, 20(6), 2043–2054, doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. (2004). Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene, 23(20), 3550–3560, doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 75.Cacalano NA (2016). Regulation of Natural Killer Cell Function by STAT3. Front Immunol, 7, 128, doi: 10.3389/fimmu.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu T, Zhang L, Joo D, & Sun SC (2017). NF-kappaB signaling in inflammation. Signal Transduct Target Ther, 2, doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vlahopoulos SA (2017). Aberrant control of NF-kappaB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode. Cancer Biol Med, 14(3), 254–270, doi: 10.20892/j.issn.2095-3941.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huxford T, & Ghosh G (2009). A structural guide to proteins of the NF-kappaB signaling module. Cold Spring Harb Perspect Biol, 1(3), a000075, doi: 10.1101/cshperspect.a000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gialeli C, Theocharis AD, & Karamanos NK (2011). Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J, 278(1), 16–27, doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 80.Foss A, Munoz-Sagredo L, Sleeman J, & Thiele W (2019). The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clin Exp Metastasis, doi: 10.1007/s10585-019-10009-y. [DOI] [PubMed]
- 81.Wu M, Wang G, Hu W, Yao Y, & Yu XF (2019). Emerging roles and therapeutic value of exosomes in cancer metastasis. Mol Cancer, 18(1), 53, doi: 10.1186/s12943-019-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Liu Y, Liu H, & Tang WH (2019). Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci, 9, 19, doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, et al. (2018). Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer, 17(1), 82, doi: 10.1186/s12943-018-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imjeti NS, Menck K, Egea-Jimenez AL, Lecointre C, Lembo F, Bouguenina H, et al. (2017). Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc Natl Acad Sci U S A, 114(47), 12495–12500, doi: 10.1073/pnas.1713433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, et al. (2012). Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One, 7(10), e46874, doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McAndrews KM, & Kalluri R (2019). Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer, 18(1), 52, doi: 10.1186/s12943-019-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ju R, Zhuang ZW, Zhang J, Lanahan AA, Kyriakides T, Sessa WC, et al. (2014). Angiopoietin-2 secretion by endothelial cell exosomes: regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. J Biol Chem, 289(1), 510–519, doi: 10.1074/jbc.M113.506899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mittal V (2018). Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol, 13, 395–412, doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 89.Xu J, Lamouille S, & Derynck R (2009). TGF-beta-induced epithelial to mesenchymal transition. Cell Res, 19(2), 156–172, doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang YE (2017). Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harb Perspect Biol, 9(2), doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang LK, Pan SH, Chang YL, Hung PF, Kao SH, Wang WL, et al. (2016). MDA-9/Syntenin-Slug transcriptional complex promote epithelial-mesenchymal transition and invasion/metastasis in lung adenocarcinoma. Oncotarget, 7(1), 386–401, doi: 10.18632/oncotarget.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zetter BR (1998). Angiogenesis and tumor metastasis. Annu Rev Med, 49, 407–424, doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 93.Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, et al. (2003). Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol, 162(6), 1913–1926, doi: 10.1016/S0002-9440(10)64325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akl MR, Nagpal P, Ayoub NM, Tai B, Prabhu SA, Capac CM, et al. (2016). Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget, 7(28), 44735–44762, doi: 10.18632/oncotarget.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishida N, Yano H, Nishida T, Kamura T, & Kojiro M (2006). Angiogenesis in cancer. Vasc Health Risk Manag, 2(3), 213–219, doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.El-Kenawi AE, & El-Remessy AB (2013). Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br J Pharmacol, 170(4), 712–729, doi: 10.1111/bph.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Das SK, Bhutia SK, Azab B, Kegelman TP, Peachy L, Santhekadur PK, et al. (2013). MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res, 73(2), 844–854, doi: 10.1158/0008-5472.CAN-12-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Talukdar S, Das SK, Pradhan AK, Emdad L, Windle JJ, Sarkar D, et al. (2019). MDA-9/Syntenin (SDCBP) Is a Critical Regulator of Chemoresistance, Survival and Stemness in Prostate Cancer Stem Cells. Cancers (Basel), 12(1), doi: 10.3390/cancers12010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mbeunkui F, & Johann DJ Jr. (2009). Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol, 63(4), 571–582, doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Das SK, Maji S, Wechman SL, Bhoopathi P, Pradhan AK, Talukdar S, et al. (2020). MDA-9/Syntenin (SDCBP): Novel gene and therapeutic target for cancer metastasis. Pharmacol Res, 155, 104695, doi: 10.1016/j.phrs.2020.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]


