Abstract
Gentle tactile stimuli, such as insects crawling on the skin, can cause itching sensation called mechanical itch. Recent studies have begun to shed light on the neural mechanisms of mechanical itch. Interestingly, the neural pathway for mechanical itch is apparently different from that for chemical itch triggered by the activation of pruriceptors with various mediators. Mechanical itch dysesthesia is frequently seen in patients with chronic itch. Mechanisms of this dysesthesia are plausibly involved in central sensitization. In this review, we summarize the current knowledge of mechanical itch under normal and pathological conditions.
Keywords: Chemical itch, sensitization, disinhibition, GRPR, LTMR
1. Introduction
Itch is an unpleasant cutaneous sensation that can be induced by chemical substances, referred to as chemical itch or by gentle touch and pressure, referred to as mechanical itch. In human psychophysics, mechanical itch is assessed by using a wide range of mechanical stimuli, such as cotton wisp, brush, and von Frey filaments, which activate low threshold mechanoreceptors (LTMRs).1 In the last decades, the mechanisms underlying chemical itch have been well studied. However, very little was known about the mechanisms behind mechanical itch partially due to the lack of animal models for mechanical itch. Recently, we proposed a mouse model for studying mechanical itch (touch-evoked itch).2 In this model, very weak von Frey filament is applied to the murine rostral back to elicit itch-related behavior (scratching). Since its first publication, this model, with some modifications, has been widely used to investigate the molecular and cellular mechanisms underlying mechanical itch.
Sensitization of mechanical itch is frequently observed under pathological conditions.3–5 The normally non-itchy mechanical stimuli near a site of itch or to the skin of patients with chronic itch can elicit itch, a phenomenon known as itchy skin or alloknesis.1,6 Likewise, touch-evoked itch is frequently observed in the mouse models of chronic itch (e.g., dry skin, atopic dermatitis, and psoriasis).7–9 Mechanical itch caused by innocuous mechanical stimuli such as from clothing often represents a bothersome clinical problem that reduces the quality of life in patients with chronic itch.10,11 Recent studies have provided new insight into the mechanisms of mechanical itch sensitization.7,9,12,13
Hyperknesis is defined as an increased sensitivity of itch induced by either chemical or pinprick stimuli.1 Punctate pricking stimuli immediately below the mechanical pain threshold often produce mild itch in healthy subjects.14,15 The same stimuli can elicit increased itch in patients with chronic itch. Punctate stimuli-evoked itch is thought to be mediated by Aδ-/polymodal C-fibers, based on the onset of reaction.16–18 Since light tactile stimuli-evoked itch is mediated by Aβ-fibers as described in the next section, punctate stimuli-evoked itch is conceivably transmitted via a pathway distinct from that of itch evoked by light tactile stimuli. This review uses the term “mechanical itch” to describe itch evoked by light tactile stimuli. This is generally in accordance with the recent papers.13,19,20
In this review, we will summarize the current knowledge about the newly revealed neural pathway for mechanical itch (Fig. 1) and the molecular mechanisms of mechanical itch sensitization (Fig. 2).
Fig. 1.
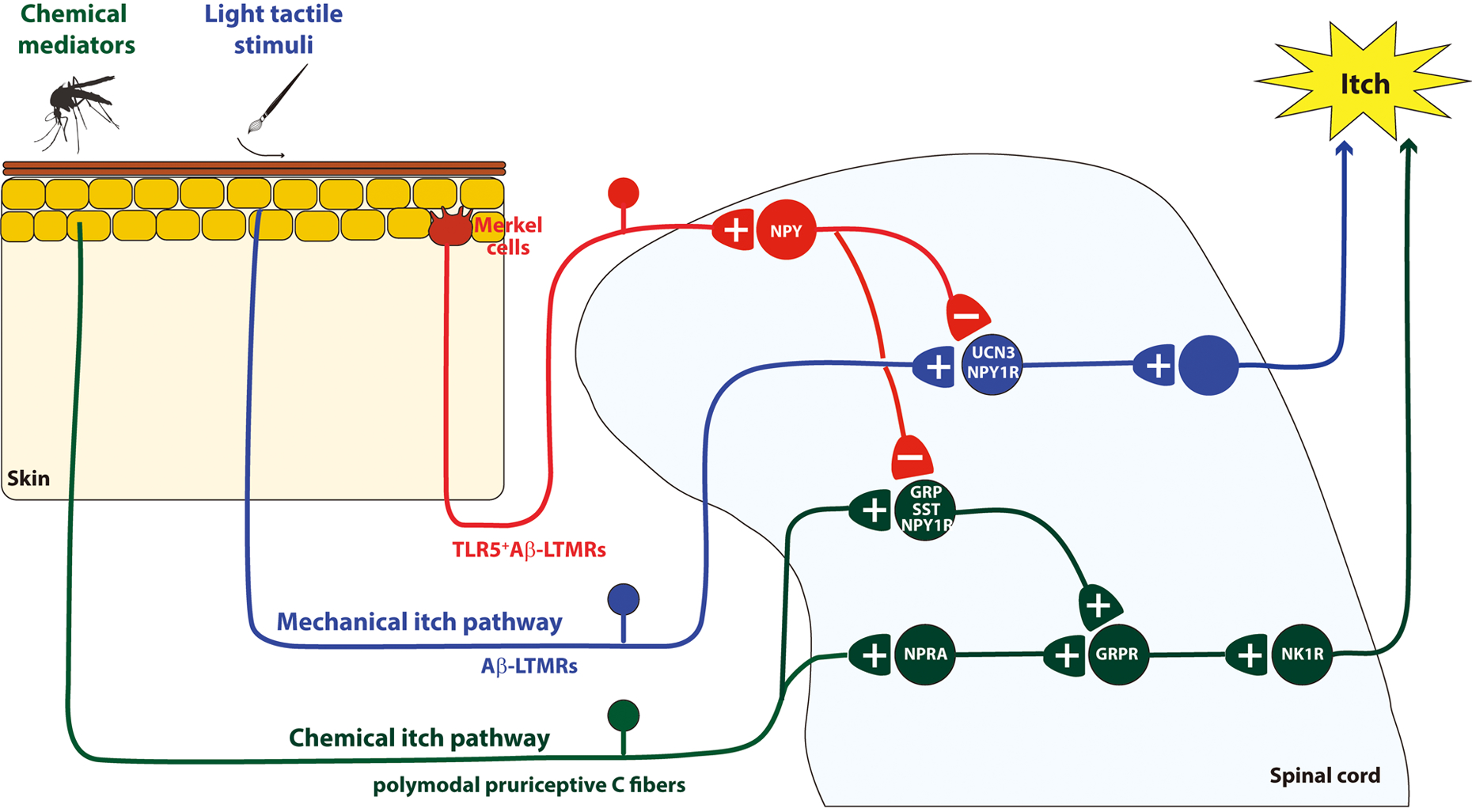
Schematic diagram of neural circuits for mechanical and chemical itch. +, - denote excitatory and inhibitory synapses, respectively. GRP, Gastrin Releasing Peptide; GRPR, Gastrin Releasing Peptide Receptor; LTMRs, Low-threshold mechanoreceptors; NK1R, Neurokinin 1 Receptor; NPRA, Natriuretic Peptide Receptor A; NPY, Neuropeptide Y; NPY1R, NPY1 receptor; SST, somatostatin; TLR5, Toll Like Receptor 5.
Fig. 2.
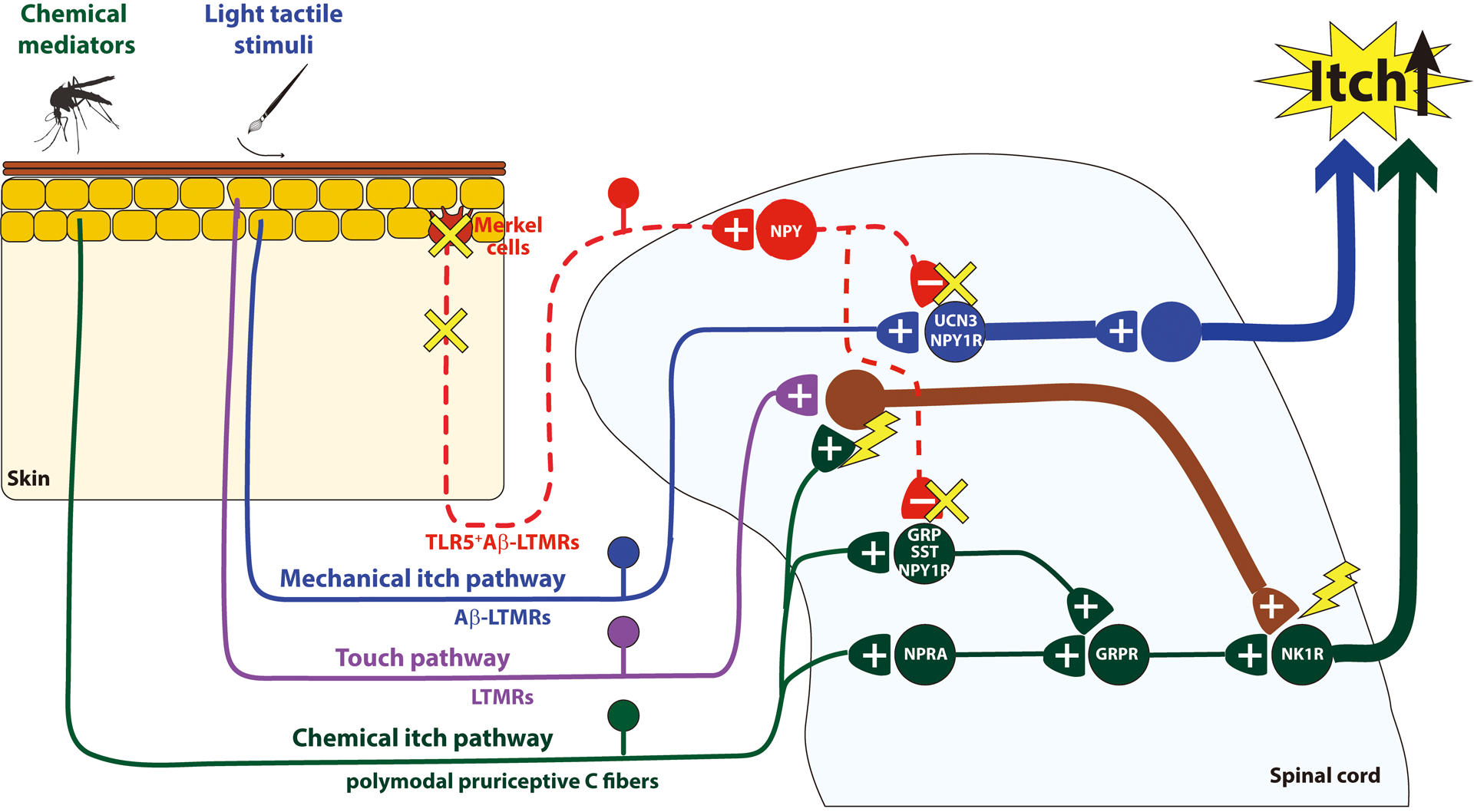
Schematic diagram of mechanisms underlying mechanical itch sensitization. Sustained activation of polymodal pruriceptive C fibers (green) sensitizes excitatory interneurons that receive convergent input from LTMRs (purple). Consequently, NK1R-expressing projection neurons (green) respond to light tactile stimuli, leading to mechanical itch sensitization. Alternatively, the disinhibition of NPY inhibitory circuits (red) may contribute to mechanical itch sensitization. +, - denote excitatory and inhibitory synapses, respectively. GRP, Gastrin Releasing Peptide; GRPR, Gastrin Releasing Peptide Receptor; LTMRs, Low-threshold mechanoreceptors; NK1R, Neurokinin 1 Receptor; NPRA, Natriuretic Peptide Receptor A; NPY, Neuropeptide Y; NPY1R, NPY1 receptor; SST, somatostatin; TLR5, Toll Like Receptor 5.
2. Excitatory mechanical itch pathway
2-1. Sensory Neurons Mediating Mechanical Itch
Very low-intensity of mechanical stimuli can elicit itch in humans and scratching in mice.13,14,19,21 These innocuous mechanical stimuli activate cutaneous Aβ-, Aδ-, and/or C- LTMRs.22 The role of LTMRs in mechanical itch has been assessed in mouse models. Touch-evoked scratching is usually observed immediately after the stimulus, implying a role of Aβ-LTMRs in mechanical itch (Fig. 1). This idea is further supported by a recent study using the targeted delivery of a sodium channel blocker, QX-314. Aβ-fibers can be silenced by a localized co-injection of QX-314 and Toll-like receptor5 (TLR5) agonist, flagellin.23 Activation of TLR5 with flagellin leads to the selective entry of QX-314 into Aβ-fibers and subsequent blockade of sodium currents in those fibers.13,23 Following Aβ-fiber silencing, scratching induced by weak (0.7mN) von Frey filament stimulation was attenuated in naïve mice.13 Moreover, co-treatment of flagellin and QX-314 also reduced touch-evoked scratching in histamine-sensitized mice and mice with atopic dermatitis-like skin inflammation. Taken together, these data suggest that TLR5-expressing Aβ-LTMRs transmit mechanical itch. In contrast, C-LTMRs are likely dispensable for mechanical itch.13 C-LTMRs are a small subset of Nav1.8-expressing neurons. Mice depleted of Nav1.8-expressing neurons showed normal touch-evoked scratching.13
2-2. Excitatory Spinal Interneurons Mediating Mechanical Itch
Within the spinal cord, excitatory interneurons that receive input from LTMRs are located in laminae IIi-IV.24,25 A subpopulation of excitatory interneurons that express the neuropeptide urocortin 3 (Ucn3) was distributed in the laminae IIi to the dorsal laminae III.13 Ucn3-expressing interneurons in the dorsal spinal cord were categorized into two types based on their location and type of sensory inputs.13 Type 1 Ucn3-expressing interneurons are located in laminae vIIi-dIII and receive monosynaptic inputs from Aβ-LTMRs. Type 2 Ucn3-expressing interneurons are located in laminae IIo-dIIi and receive sensory inputs mainly from Aδ- and/or C-LTMRs. Considering the contribution of TLR5-expressing Aβ-LTMRs to mechanical itch, the type 1 Ucn3-expressing interneurons play a functional role in mechanical itch (Fig. 1). This concept was further supported by the inhibition of touch-evoked scratching by ablation or chemogenetic silencing of Ucn3-expressing neurons in the spinal cord. Interestingly, the ablation of Ucn3-expressing neurons did not affect touch, pain, or temperature sensations, implying that these neurons may represent a unique subpopulation of excitatory interneurons that predominantly mediate mechanical itch.
It has also been reported that neuropeptide Y1 receptor (NPY1R)-expressing interneurons are excitatory and involved in mechanical itch (Fig. 1).20,26 NPY1R-expressing neurons are located within laminae I-VI and X in the rat lumbar spinal cord.27 In the spinal cord of NPY1RCre knock-in mice, NPY1RCre neurons are distributed throughout laminae I-IV and enriched in laminae IIi and III. The NPY1RCre neurons receive input from cutaneous LTMRs, including Aδ- and Aβ-LTMRs. Ablation of spinal NPY1RCre neurons reduced touch-evoked scratching. Moreover, designer receptors exclusively activated by designer drugs-based chemogenetic silencing of the spinal NPY1RCre neurons also reduced touch-evoked scratching, while chemogenetic activation of the same neurons increased spontaneous and touch-evoked scratching. These data suggest that NPY1R-expressing interneurons receive input from LTMRs and contribute to the transmission of mechanical itch. Interestingly, only ~15% of Ucn3-expressing interneurons expressed NPY1R in the dorsal spinal cord.13 In the future, it will be highly worthwhile to unravel the functional relationship between NPY1R- and Ucn3-expressing excitatory interneurons.
In addition to the role in mechanical itch, NPY1R-expressing neurons are presumably implicated in the transmission of general light touch and possibly pain. NPY1R-expressing neurons could be morphologically divided into at least seven distinct populations in the rat spinal cord.27 Recent single-cell RNA sequencing study classified the spinal excitatory neurons into 15 clusters, and Npy1r were detected in three clusters: Glut2, Glut8, and Glut9.28 Considering that NPY1R-expressing neurons comprise a heterogeneous population, these neurons are implicated in multiple spinal functions. Some NPY1RCre neurons expressed makers of excitatory neurons that regulate mechano-transmission, such as retinoid-related orphan receptor α+ and cMaf+ neurons29,30. NPY1RCre neurons-ablated mice or mice lacking NPY1R in dorsal horn neurons exhibited decreased sensitivity to von Frey hair stimulation of the hindpaw glabrous skin, suggesting their role in light touch. On the other hand, the majority of NPY1R-expressing neurons in lamina II contained somatostatin (SST) , which is involved in mechanical pain.26,31,32 Transcriptional profiling of SST interneurons revealed that Npy1r was enriched in the SST dorsal horn population.33 Moreover, neuropeptide Y (NPY)-saporin treatment reduced NPY1R-expressing spinal neurons and decreased inflammatory pain as well as mechanical hypersensitivity in a rat model of neuropathic pain.26,33,34 Therefore, the neurons co-expressing SST and NPY1R may regulate mechanical hypersensitivity in neuropathy. Further studies are required to identify molecular markers for a population of NPY1R-expressing neurons that predominantly mediate mechanical itch.
2-3. Spinal Projection Neurons Mediating Mechanical Itch
Spinal projection neurons mediating mechanical itch have not been identified yet. Nociceptive and pruritic information are transmitted from the spinal cord to the brain through the anterolateral tract (ALT).35–37 ALT projection neurons are concentrated in lamina I and scattered throughout lamina III-IV. The majority of lamina I projection neurons express the neurokinin 1 receptor (NK1R).35,38 The primary ligand for NK1R is substance P, and intrathecal injection of substance P-saporin conjugate (SP-SAP) can ablate spinal NK1R.39 Ablation of spinal NK1R by intrathecal injection of SP-SAP reduced chemical itch in rodents.20,40 While NK1R and NPY1RCre expression partially overlapped, the same SP-SAP treatment failed to attenuate touch-evoked scratching in wild-type mice or NPY1RCre neuron-activated mice.20 Therefore, neither NK1R+ projection neurons nor NK1R+ NPY1RCre neurons are presumably required for mechanical itch under physiological conditions (Fig. 1).
3. Inhibitory Pathway for Mechanical Itch
3-1. Merkel Cells
In the epidermis, Merkel cells and Aβ slowly adapting (SA)-LTMRs together compose touch-domes, which detect and transmit touch signals (Fig. 1).22,41,42 Piezo2 channels, Piezo-type mechanosensitive ion channel component 2, expressed by Merkel cells and Aβ SA-LTMRs are required for the detection of touch stimuli.41,42 A recent study has addressed the involvement of Merkel cells in mechanical itch.7 Genetic ablation of Merkel cells or Piezo2 channels expressed by Merkel cells elicited touch-evoked scratching, implying that mechanical itch is constitutively suppressed by Merkel cells or Piezo2 channels expressed by Merkel cells under physiological conditions. Interestingly, the ablation of Merkel cells did not affect chemical itch induced by histamine and chloroquine or mechanical and thermal pain. Thus, Merkel cells unlikely regulate either chemical itch, or mechanical and thermal pain. Previous studies have reported that Merkel cells undergo turnover under physiological conditions.43,44 How the turnover of Merkel cells affects mechanical itch is still an open question.
3-2. Sensory Neurons Modulating Mechanical Itch
Recently, we have reported that TLR5-expressing Aβ-fibers are involved in the inhibition of mechanical itch (Fig. 1).12 Following the silencing of TLR5-expressing Aβ-fibers by co-injection of flagellin and QX-314 to the shaved rostral back skin, mechanical stimuli adjacent to the site of co-injection elicited scratching. Importantly, the same stimuli to the injection site failed to elicit scratching, suggesting that TLR5-expressing Aβ-fibers in the site of injection also contribute to transmitting mechanical itch. Distinct subsets of TLR5-expressing Aβ-fibers may be responsible for transmitting and gating mechanical itch. The previous study has shown that Aβ-fibers can inhibit the responses of dorsal horn neurons evoked by stimulating separate neighboring Aβ-fibers.45 Taken together, co-injection of flagellin and QX-314 likely silences TLR5-expressing Aβ-fibers at the injection site without affecting TLR5-expressing Aβ-fibers adjacent to the injection site and disinhibits dorsal horn neurons receiving mechanical itch inputs from neighboring TLR5-expressing Aβ-fibers adjacent to the injection site. This interpretation is also consistent with the observation that mechanical stimuli to peri-affected skin, but not directly to pathological skin elicited scratching in mice with dry skin or psoriasis that exhibit loss of or reduced activity of Aβ-fibers.2,7,12,46
3-3. Spinal Inhibitory Interneurons for Mechanical Itch
NPY is expressed by inhibitory interneurons that are localized in laminae I-IV of the spinal cord.19,47,48 NPY-expressing neurons account for ~15% or ~45% of the inhibitory neurons in the dorsal horn of rats or mice, respectively. They constitute a different population from the neuronal nitric oxide synthase-, galanin-, or parvalbumin-expressing inhibitory interneurons and receive inputs from Aβ-, Aδ-, and C-LTMRs as well as C-nociceptors. Ablation or silencing of NPY-expressing neurons in the spinal cord increased touch-evoked scratching.19,32 Conversely, chemogenetic activation of NPY-expressing neurons decreased touch-evoked scratching.20 Therefore, NPY-expressing inhibitory interneurons appear to gate transmission of mechanical itch in the spinal cord. In addition to the involvement of mechanical itch, NPY-expressing neurons also slightly regulate mechanical pain and gentle touch.20
NPY-expressing inhibitory interneurons regulate both Ucn3- and NPY1R-expressing excitatory interneurons (Fig. 1).13,20 The cell bodies of Ucn3- and NPY1R-expressing neurons displayed synaptic contacts from NPY-expressing neurons. NPY silences NPY1R-expressing neurons through Gi-signaling. The depletion of the NPY1R in dorsal horn neurons disinhibited NPY1R-expressing neurons and increased touch-evoked scratching. Additionally, chemogenetic activation of NPY-expressing inhibitory interneurons decreased touch-evoked scratching, and the NPY1R antagonist canceled this inhibition. These results suggest that NPY is released from NPY-expressing inhibitory interneurons to regulate NPY1R-expressing excitatory interneurons. On the other hand, optogenetic activation of NPY-expressing neurons induced the release of gamma aminobutyric acid (GABA) and/or glycine to regulate Ucn3-expressing excitatory interneurons.13 However, whether GABA and/or glycine regulates Ucn3-expressing excitatory interneurons under physiological conditions needs to be tested.
4. Parallel Pathways for Transmitting Mechanical and Chemical Itch
Mechanical and chemical itch are apparently transmitted by separate neural pathways (Fig. 1).19 Chemical itch is mediated by polymodal pruriceptive fibers via a wide array of receptors in the peripheral nervous system, while Aβ-LTMR fibers mediate mechanical itch.6,13 In the spinal cord, gastrin-releasing peptide receptor (GRPR)-expressing excitatory interneurons play a central role in chemical itch. By contrast, GRPR-expressing excitatory interneurons are dispensable for mechanical itch. In line with this concept, the portion of Ucn3- and NPY1R-expressing excitatory interneurons are involved in the transmission of mechanical itch, but not chemical itch.13,20 Ablation of Ucn3- or NPY1R-expressing excitatory interneurons diminished mechanically-evoked scratching without affecting pruritogens-evoked scratching. However, NPY1R-expressing excitatory interneurons are also likely slightly involved in chemical itch. Some of them co-expressed somatostatin and gastrin-releasing peptide (GRP) observed in excitatory interneurons for chemical itch.20 In accordance with this finding, a recent study has demonstrated that intrathecal injection of NPY or NPY1R agonist LP-NPY reduced the duration of histamine- and compound 48/80-induced scratching, without affecting the frequency of scratching.49 Moreover, the reduction of scratching duration was abolished by injection of the selective NPY1R antagonist BIBO3304. Thus, the NPY1R is apparently involved in chemical itch as well as mechanical itch. NPY1R-expressing excitatory interneurons that co-express somatostatin and GRP appear to mediate chemical itch under the control of NPY-expressing inhibitory interneurons.
5. Sensitization of Mechanical Itch
5-1. Hypersensitivity of Itch Pathway Neurons
Here we propose a potential mechanism underlying mechanical itch sensitization based on the recent findings as well as the central sensitization theory that represents hyperexcitability of spinal neurons caused by excessive C-fiber inputs or a decrease in inhibitory transmission (Fig. 2).50 Ongoing inputs from primary sensory neurons mediating chemical itch produce hypersensitivity of excitatory interneurons that are involved in feedforward activation of projection neurons and receive inputs from primary sensory neurons mediating light tactile stimuli. Consequently, the projection neurons that normally mediate chemical itch acquire the increased responsiveness to light tactile stimuli. Previous studies indicate that ongoing inputs from pruriceptive primary sensory neurons are required for the development of alloknesis.51,52 Human subjects with allergic contact dermatitis (ACD) showed persistent itch and mechanically-evoked itch in the surrounding skin. The mechanically-evoked itch was reversely blocked by numbing the ACD site with cold. Moreover, ACD caused an increase in the incidence of spontaneous activity of Mas-related G-protein coupled receptor member A3 (MrgprA3)-expressing sensory neurons that represent a subset of pruriceptive sensory neurons in mice.53 Taken together, ongoing inputs from MrgprA3-expressing sensory neurons are likely responsible for the development of alloknesis. It has been reported that pruriceptive projection neurons acquire the enhanced responsiveness to light tactile stimuli after pruritic responses.54,55 In nonhuman primates, spinothalamic tract neurons developed enhanced responses to innocuous mechanical stimuli after a response to histamine. Likewise, intrathecal application of morphine enhanced the responses to innocuous mechanical stimuli in rat trigeminothalamic tract neurons.56 In line with these findings, NK1R+ spinal projection neurons are responsible for mechanical itch in a mouse model of atopic dermatitis, while they are dispensable for mechanical itch under the physiological conditions.9,20
Direct activation of LTMRs may contribute to mechanical itch sensitization. It has been demonstrated that hydrogen sulfide donor induces mechanical itch via the Cav3.2 T-type calcium channel.57 Considering that Cav3.2 is exclusively expressed by Aδ- and C-LTMRs58, hypersensitivity of Aδ- and C-LTMRs may contribute to the sensitization of mechanical itch under certain conditions. However, the Cav3.2 inhibitor failed to inhibit touch-evoked scratching in a mouse model of type I diabetes.59 Further research should be undertaken to investigate whether hydrogen sulfide, Cav3.2, Aδ-LTMRs, and C-LTMRs contribute to mechanical itch sensitization under chronic itch conditions.
5-2. Disinhibition of Neural Pathway for Mechanical Itch
While it is still uncertain whether disinhibition of itch pathways contributes to itch sensitization in chronic itch patients, dysfunction of the inhibitory pathway for chemical itch was observed in the patients with atopic dermatitis.60 Likewise, recent mouse studies suggest dysfunction of the inhibitory pathway for mechanical itch under the chronic itch condition and its possible role in sensitization of mechanical itch (Fig. 2). As mentioned in section 3-1, a recent study demonstrated that Merkel cells are involved in the inhibition of mechanical itch.7 Interestingly, the number of Merkel cells was decreased in mice with dry skin or aged mice that displayed touch-evoked scratching. Moreover, chemogenetic activation of Merkel cells inhibited touch-evoked scratching via the increase in the firing rate of Aβ-LTMRs in mice with dry skin. Thus, dysfunction of Merkel cell-Aβ-LTMRs signaling appears to contribute to mechanical itch in aged skin or dry skin. However, this finding is apparently contrary to previous observations of the increased number of Merkel cells in the skin of the patients with psoriasis or prurigo nodularis.61,62 Further studies are required to confirm whether Merkel cells contribute to the disinhibition of mechanical itch in the pathological condition.
We have recently demonstrated that TLR5-expressing Aβ-fibers contribute to inhibition of mechanical itch. The number of TLR5-expressing Aβ-fibers was decreased in the epidermis of mice with psoriasis.12 This finding also accords with previous observations, which showed that myelinated nerve fibers exhibited demyelination in the lesional skin of patients with atopic dermatitis.63,64 It is interesting to investigate whether the Aβ-fibers are impaired in the skin of the patients with other itch conditions.
Impaired Aβ-fibers presumably contribute to the reduced activity of inhibitory interneurons that signal mechanical itch. While there is no direct evidence demonstrating that inhibitory interneurons are suppressed under chronic itch conditions, a recent study demonstrated that inhibitory inputs to Ucn3-expressing excitatory interneurons are decreased in mice with atopic dermatitis.13
While the disinhibition of the mechanical itch pathway causes spontaneous itch, it is unknown how itch signals are generated under the condition of disinhibition. Pan et al., demonstrated that Aβ-LTMRs are responsible for mediating mechanical itch.13 Therefore, it seems fair to assume that skin stretch or skin movement excites Aβ-LTMRs to elicit mechanical itch.24 Future studies are required to understand the generation mechanisms of mechanical itch by identifying subtype of Aβ-LTMRs that mediate mechanical itch.
6. Conclusion
In this review, we highlight new insights into the neural mechanisms of mechanical itch. Despite significant progress in the study of the mechanisms of mechanical itch, several questions remain to be answered: (i) which molecules stimulate the inhibitory pathway for mechanical itch?; (ii) which molecules develop mechanical itch sensitization?; (iii) which projection neurons conduct mechanical itch signals?; (iv) do the mechanisms of mechanical itch differ in glabrous and hairy skins?; (v) which diseases cause chronic itch via mechanical itch sensitization? Insights from future studies could pave the way for new treatments for chronic itch.
Acknowledgements
TA is supported by a grant from the National Institutes of Health (R01AR074062).
Footnotes
Conflict of interest
The authors have declared no conflicting interests.
References
- 1.Andersen HH, Akiyama T, Nattkemper LA, van Laarhoven A, Elberling J, Yosipovitch G, Arendt-Nielsen L. Alloknesis and hyperknesis-mechanisms, assessment methodology, and clinical implications of itch sensitization. Pain. 2018;159(7):1185–1197. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis). J Invest Dermatol. 2012;132(7):1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126(1–3):16–23. [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren CF, Hagermark O, Bergstrom R. Patients’ perception of itch induced by histamine, compound 48/80 and wool fibres in atopic dermatitis. Acta Derm Venereol. 1991;71(6):488–494. [PubMed] [Google Scholar]
- 5.Andersen HH, Elberling J, Solvsten H, Yosipovitch G, Arendt-Nielsen L. Nonhistaminergic and mechanical itch sensitization in atopic dermatitis. Pain. 2017;158(9):1780–1791. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng J, Luo J, Yang P, Du J, Kim BS, Hu H. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science. 2018;360(6388):530–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, Akiyama T. Mouse model of imiquimod-induced psoriatic itch. Pain. 2016;157(11):2536–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama T, Nguyen T, Curtis E, Nishida K, Devireddy J, Carstens MI, C. E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain. 2015;156:1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7(7):535–547. [DOI] [PubMed] [Google Scholar]
- 11.Hundley JL, Carroll CL, Lang W, Snively B, Yosipovitch G, Feldman SR, Jorizzo JL. Cutaneous symptoms of dermatomyositis significantly impact patients’ quality of life. J Am Acad Dermatol. 2006;54(2):217–220. [DOI] [PubMed] [Google Scholar]
- 12.Sakai K, Akiyama T. Disinhibition of Touch-Evoked Itch in a Mouse Model of Psoriasis. J Invest Dermatol. 2019;139(6):1407–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan H, Fatima M, Li A, Lee H, Cai W, Horwitz L, Hor CC, Zaher N, Cin M, Slade H, Huang T, Xu XZS, Duan B. Identification of a Spinal Circuit for Mechanical and Persistent Spontaneous Itch. Neuron. 2019;103(6):1135–1149 e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen HH, Elberling J, Lo Vecchio S, Arendt-Nielsen L. Topography of itch: evidence of distinct coding for pruriception in the trigeminal nerve. Itch. 2017;2(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyer G, Ulmer FJ, Schmitz J, Handwerker HO. Histamine-induced itch and alloknesis (itchy skin) in atopic eczema patients and controls. Acta Derm Venereol. 1995;75(5):348–352. [DOI] [PubMed] [Google Scholar]
- 16.Andersen HH, Marker JB, Hoeck EA, Elberling J, Arendt-Nielsen L. Antipruritic effect of pretreatment with topical capsaicin 8% on histamine- and cowhage-evoked itch in healthy volunteers: a randomized, vehicle-controlled, proof-of-concept trial. Br J Dermatol. 2017;177(1):107–116. [DOI] [PubMed] [Google Scholar]
- 17.Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62(2):212–217. [DOI] [PubMed] [Google Scholar]
- 18.Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19(22):10184–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science. 2015;350(6260):550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acton D, Ren X, Di Costanzo S, Dalet A, Bourane S, Bertocchi I, Eva C, Goulding M. Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch. Cell Rep. 2019;28(3):625–639 e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuoka M, Miyachi Y, Ikoma A. Mechanically evoked itch in humans. Pain. 2013;154(6):897–904. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346(6212):950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nature medicine. 2015;21(11):1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79(4):618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell. 2017;168(1–2):295–310 e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson TS, Fu W, Donahue RR, Corder GF, Hokfelt T, Wiley RG, Taylor BK. Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci Rep. 2019;9(1):7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brumovsky P, Hofstetter C, Olson L, Ohning G, Villar M, Hokfelt T. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience. 2006;138(4):1361–1376. [DOI] [PubMed] [Google Scholar]
- 28.Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerstrom MC, Linnarsson S, Ernfors P. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci. 2018;21(6):869–880. [DOI] [PubMed] [Google Scholar]
- 29.Wende H, Lechner SG, Cheret C, Bourane S, Kolanczyk ME, Pattyn A, Reuter K, Munier FL, Carroll P, Lewin GR, Birchmeier C. The transcription factor c-Maf controls touch receptor development and function. Science. 2012;335(6074):1373–1376. [DOI] [PubMed] [Google Scholar]
- 30.Bourane S, Grossmann KS, Britz O, Dalet A, Del Barrio MG, Stam FJ, Garcia-Campmany L, Koch S, Goulding M. Identification of a spinal circuit for light touch and fine motor control. Cell. 2015;160(3):503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamessian A, Young M, Qadri Y, Berta T, Ji RR, Van de Ven T. Transcriptional Profiling of Somatostatin Interneurons in the Spinal Dorsal Horn. Sci Rep. 2018;8(1):6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159(6):1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiley RG, Lemons LL, R.H.t. Kline. Neuropeptide Y receptor-expressing dorsal horn neurons: role in nocifensive reflex responses to heat and formalin. Neuroscience. 2009;161(1):139–147. [DOI] [PubMed] [Google Scholar]
- 34.Lemons LL, Wiley RG. Neuropeptide Y receptor-expressing dorsal horn neurons: role in nocifensive reflex and operant responses to aversive cold after CFA inflammation. Neuroscience. 2012;216:158–166. [DOI] [PubMed] [Google Scholar]
- 35.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27(37):10007–10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiyama T, Curtis E, Nguyen T, Carstens MI, Carstens E. Anatomical evidence of pruriceptive trigeminothalamic and trigeminoparabrachial projection neurons in mice. J Comp Neurol. 2016;524(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cameron D, Polgar E, Gutierrez-Mecinas M, Gomez-Lima M, Watanabe M, Todd AJ. The organisation of spinoparabrachial neurons in the mouse. Pain. 2015;156(10):2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278(5336):275–279. [DOI] [PubMed] [Google Scholar]
- 40.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21(4):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509(7502):617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509(7502):622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doucet YS, Woo SH, Ruiz ME, Owens DM. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep. 2013;3(6):1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall KL, Clary RC, Baba Y, Orlowsky RL, Gerling GJ, Lumpkin EA. Touch Receptors Undergo Rapid Remodeling in Healthy Skin. Cell Rep. 2016;17(7):1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woolf CJ, Wall PD. Chronic peripheral nerve section diminishes the primary afferent A-fibre mediated inhibition of rat dorsal horn neurones. Brain Res. 1982;242(1):77–85. [DOI] [PubMed] [Google Scholar]
- 46.Iwanaga T, Tominaga M, Hirata Y, Matsuda H, Shimanuki T, Ogawa H, Takamori K. Effects of Film Dressings on Itch Hypersensitivity Using Murine Dry Skin Models. Acta Derm Venereol. 2018;98(9):902–903. [DOI] [PubMed] [Google Scholar]
- 47.Polgar E, Sardella TC, Watanabe M, Todd AJ. Quantitative study of NPY-expressing GABAergic neurons and axons in rat spinal dorsal horn. J Comp Neurol. 2011;519(6):1007–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwagaki N, Ganley RP, Dickie AC, Polgar E, Hughes DI, Del Rio P, Revina Y, Watanabe M, Todd AJ, Riddell JS. A combined electrophysiological and morphological study of neuropeptide Y-expressing inhibitory interneurons in the spinal dorsal horn of the mouse. Pain. 2016;157(3):598–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao T, Ma H, Xu B, Bergman J, Larhammar D, Lagerstrom MC. The Neuropeptide Y System Regulates Both Mechanical and Histaminergic Itch. J Invest Dermatol. 2018;138(11):2405–2411. [DOI] [PubMed] [Google Scholar]
- 50.Schmelz M. Sensitization for Itch In: Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. Boca Raton (FL)2014. [Google Scholar]
- 51.Pall PS, Hurwitz OE, King BA, LaMotte RH. Psychophysical measurements of itch and nociceptive sensations in an experimental model of allergic contact dermatitis. J Pain. 2015;16(8):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res. 1991;8(3):271–279. [DOI] [PubMed] [Google Scholar]
- 53.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, LaMotte RH. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014;137(Pt 4):1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ Jr. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108(6):1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ Jr. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91(1):213–222. [DOI] [PubMed] [Google Scholar]
- 56.Moser HR, Giesler GJ Jr. Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci. 2013;33(14):6093–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang XL, Tian B, Huang Y, Peng XY, Chen LH, Li JC, Liu T. Hydrogen sulfide-induced itch requires activation of Cav3.2 T-type calcium channel in mice. Sci Rep. 2015;5:16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francois A, Schuetter N, Laffray S, Sanguesa J, Pizzoccaro A, Dubel S, Mantilleri A, Nargeot J, Noel J, Wood JN, Moqrich A, Pongs O, Bourinet E. The Low-Threshold Calcium Channel Cav3.2 Determines Low-Threshold Mechanoreceptor Function. Cell Rep. 2015;10(3):370–382. [DOI] [PubMed] [Google Scholar]
- 59.Cheng RX, Feng Y, Liu D, Wang ZH, Zhang JT, Chen LH, Su CJ, Wang B, Huang Y, Ji RR, Hu J, Liu T. The role of Nav1.7 and methylglyoxal-mediated activation of TRPA1 in itch and hypoalgesia in a murine model of type 1 diabetes. Theranostics. 2019;9(15):4287–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishiuji Y, Coghill RC, Patel TS, Dawn A, Fountain J, Oshiro Y, Yosipovitch G. Repetitive scratching and noxious heat do not inhibit histamine-induced itch in atopic dermatitis. Br J Dermatol. 2008;158(1):78–83. [DOI] [PubMed] [Google Scholar]
- 61.Wollina U, Mahrle G. Epidermal Merkel cells in psoriatic lesions: immunohistochemical investigations on neuroendocrine antigen expression. J Dermatol Sci. 1992;3(3):145–150. [DOI] [PubMed] [Google Scholar]
- 62.Nahass GT, Penneys NS. Merkel cells and prurigo nodularis. J Am Acad Dermatol. 1994;31(1):86–88. [DOI] [PubMed] [Google Scholar]
- 63.Sugiura H, Maeda T, Uehara M. Mast cell invasion of peripheral nerve in skin lesions of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1992;176:74–76. [PubMed] [Google Scholar]
- 64.Mihm MC Jr., Soter NA, Dvorak HF, Austen KF. The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol. 1976;67(3):305–312. [DOI] [PubMed] [Google Scholar]


